Abstract
Cadmium (Cd) pollution in wastewater has become an increasingly widespread concern worldwide. Studies on Cd (II) removal using phosphate-adsorbed sorbents are limited. This study aimed to elucidate the behaviors and mechanisms of Cd (II) sorption on phosphate-loaded Ca-Mn-impregnated biochar (Ps-CMBC). The Cd (II) sorption on Ps-CMBC reached equilibrium within 2 h and exhibited a higher sorption efficiency than biochar and CMBC. Additionally, the Langmuir isotherm could better describe the Cd (II) adsorption on the sorbents. P75-CMBC had a maximum Cd (II) sorption capability of 70.13 mg·g−1 when fitted by the Langmuir isotherm model, which was approximately 3.18 and 2.86 times greater than those of biochar and CMBC, respectively. Higher pH (5–7) had minimal effect on Cd (II) sorption capacity. The results of characterization analyses, such as SEM-EDS, FTIR, and XPS, suggested that there was a considerable difference in the sorption mechanisms of Cd (II) among the sorbents. The primary sorption mechanisms for biochar, CMBC, and Ps-CMBC included electrostatic attraction and surface complexation; additionally, for Ps-CMBC, Cd (II)-π interactions and coordination of Cd (II) with P=O were critical mechanisms for Cd (II) removal. The results of this study demonstrate that phosphate-loaded CMBC can be used as an effective treatment for heavy metal pollution in aqueous media.
1. Introduction
In recent years, contamination with heavy metals, such as cadmium (Cd), arsenic (As), lead (Pb), and stibium (Sb), has attracted worldwide concern, due to their high toxicity and non-biodegradability [1,2]. Cd, one of the most toxic heavy metals, is discharged into the environment via paints, waste batteries, mining, and other industrial processes; moreover, Cd can easily migrate and enrich agricultural crops [3,4]. Furthermore, Cd can cause serious health problems in wildlife and humans via the food chain. For example, long-term exposure to Cd can result in damage to both the bones and kidneys [5,6]. The US Environmental Protection Agency (EPA) has established Cd limit standards for drinking water as being less than 5 μg·L−1 [7]. Thus, it is imperative to develop an appropriate technique for efficient and selective Cd (II) removal from wastewater.
Chemical methods such as membrane filtration, adsorption, and ion exchange are applied to control Cd (II) pollution in aqueous media. Among them, adsorption has gained interest, owing to its simple operation, low cost, high efficiency, and economic feasibility for practical applications [8,9]. In recent years, carbon-rich sorbent materials, including activated carbon, carbon fibers, graphene nanomaterials, and other carbon-based materials, have gained attention [10,11]. However, the high cost of these advanced materials may hinder their practical application in heavy metal remediation. Owing to the developed micropore structure, extensive specific surface area, and multiple functional groups, biochar has received increasing attention as an eco-friendly, cost-effective, and carbon-based material [12]. It has been demonstrated that the synthesis of biochar is conducive to carbon sequestration, waste management, environmental remediation, and improvement in soil properties, owing to its high pH and rich matter content [13,14]. Heavy metal ions could be adsorbed onto biochar via electrostatic attraction due to negative charges on the surface of biochar [15]. In addition, the raw materials used to prepare biochar may contain traces of heavy metals. So, the environmental risks of biochar synthesized via pyrolysis also require attention [16]. Xu [17] found that the heavy metal risk of kitchen waste biochar decreased as pyrolysis temperature increased from 300 to 600 °C, and the biochar pyrolyzed at 500 °C was the most suitable for the eco-friendly, efficient and cost-saving remediation of Cd (II)-polluted water. A previous study has shown that the contaminant adsorption ability of pristine biochar is limited because of its poor selectivity and non-ideal physicochemical property [5]. Thus, biochar modification using other reagents is needed to further improve its Cd (II) adsorption performance. Several researchers have prepared new functional absorbent materials by adding chemical reagents such as HCl, magnesium oxide, sulfur, iron, and manganese to modify raw biochar [1,12,18]. In addition, a two-step process of pyrolysis and activation with different chemical reagents is usually applied to achieve a more efficient adsorption capacity for heavy metal contaminants [19,20]. Previous studies have confirmed that phosphate-containing materials exhibit excellent performance in heavy metal removal from solutions using phosphate-modified biochar [21,22]. Peng [5] synthesized phosphorus-modified biochar using phosphoric acid-modified pine wood chip biochar and achieved a larger surface area, a higher amount of oxygen-containing functional groups, and better sorption capacity of Cu (II) and Cd (II) compared to virgin biochar. Phosphorus-modified biochar prepared with rape straw as the raw material and orthophosphate as the modifying reagent showed outstanding performance for Pb sorption in wastewater [22]. Zhang [16] applied a P-modified biochar to heavy-metal-contaminated soil and found that higher ash and P retention on the P-modified biochar endorsed the conversion of Cu and Cd into more stable forms, and greatly reduced the extraction of Cd and Cu from soil. In addition, uranium (VI) removal from a solution using phosphate-functionalized bamboo biochar has also been reported [23]. Phosphorus-modified biochar has outstanding removal performance for heavy metals, which may be due to the phosphate combining with heavy metal ions to form mineral precipitates, while the heavy metal ions could interact with the active functional groups to generate more stable metalorganics [24]. Studies focused on Cd (II) sorption via phosphate-loaded Ca-Mn-impregnated biochar (Ps-CMBC; prepared via the phosphate sorption process on CMBC) are limited. Our previous studies prepared Ca-Mn-impregnated biochar by modifying biochar with CaCl2 and KMnO4, which increased the adsorption active sites and microporous structure of CMBC and showed that CMBC had excellent sorption performance in phosphate removal from solution, and that CMBC possesses stable properties and strong buffering capacity [25]. Thus, we hypothesized that Ps-CMBC may have more capacity for adsorption of Cd due to the presence of P in the biochar.
In this study, Ps-CMBC was prepared via a phosphate adsorption process on Ca-Mn-impregnated biochar (CMBC). Moreover, a series of Cd adsorption experiments and characterization analyses of Ps-CMBC were performed. The aims of this study were to (1) explore the basic properties of Ps-CMBC, (2) evaluate the Cd (II) sorption behavior of Ps-CMBC, and (3) determine the possible sorption mechanisms of Cd (II) on Ps-CMBC via characterization.
2. Results and Discussion
2.1. General Properties of Ps-CMBC
The results of space pore structure parameters, including the specific surface area, pore volume, and average pore diameter of Ps-CMBC, are listed in Table 1. The SSAs of sorbents for P25-CMBC, P50-CMBC, and P75-CMBC were 81.63, 75.15, and 66.59 m2·g−1, respectively. The average pore diameter and pore volume decreased with the increase in P loading content (Table 1), which may be due to partial blockage in the biochar or the collapse of the pore structure due to P-Ca precipitation and complexation reactions during the process of phosphate sorption on CMBC.

Table 1.
The basic physiochemical properties of raw biochar Ca/Mn-impregnated biochar, and P-sorbed biochar.
Table 1 summarizes that all the Ps-CMBC were alkaline, with the highest pH of 9.27 (P50-CMBC) and the lowest pH of 9.03 (P25-CMBC). This might be due to carbonates, the increase in ash contents generated, and the accumulation of alkaline inorganic matter at higher pyrolysis temperatures [26]. In addition, the presence of carboxyl and hydroperoxide functional groups on the surface of Ps-CMBC decreased with increasing temperature during the pyrolysis process [27,28]. Generally, the high aromatic structure of biochar may form more stable heavy metal complexing bonds, which could improve the sorption performance and reduce the risk of heavy metals [29]. The H/C ratio value may be applied to assess the degree of carbonization, which can indicate the degree of aromatization of biochar [30]. The H/C values of P25-CMBC, P50-CMBC, and P75-CMBC were 0.049, 0.050, and 0.051, respectively, indicating that orthophosphate improved the aromaticity of biochar [22].
2.2. Kinetics Experiment
The time required for reaching adsorption equilibrium is an important factor in assessing sorbent efficiency. The adsorption capacity of Cd (II) on biochar with increasing contact time is listed in Figure 1. The Cd (II) sorption on Ps-CMBC reached equilibrium within 2 h, which was less than the time required by biochar and CMBC (approximately 8 h). This result suggests that phosphate loading may accelerate Cd sorption. So, 24 h was chosen as the sorption time in the subsequent batch experiments to ensure that adsorption equilibrium was achieved. The Cd adsorption rate of biochar increased rapidly in the first 1–2 h, which is called the rapid initial phase. In the second (slow) phase, the adsorption rate declined from 2 h to 6 h, as the mass of the sorption sites was occupied [12].
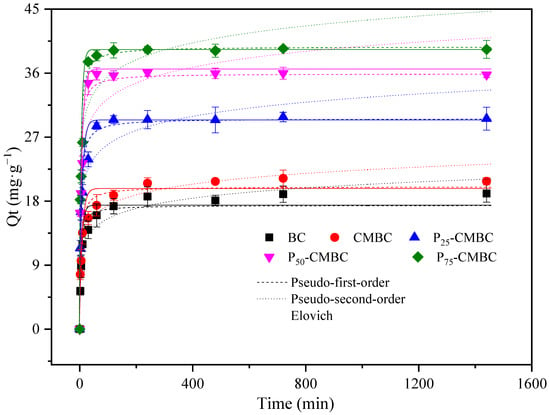
Figure 1.
Adsorption kinetics of Cd on different adsorbents.
To further evaluate the Cd (II) removal efficiency of different sorbents, three models (Supplementary Materials, Equations (S1)–(S3) have been used for fitting the kinetics curve [31].
Table 2 presents the corresponding parameters of the kinetic fitting models. In general, the theoretical adsorption amounts were in good agreement with the equilibrium experimental results. The pseudo-second-order model better fitted to kinetics data with respect to high correlation coefficients, implying that the reaction speed of Cd (II) removal by biochar is mainly dominated by chemisorption actions such as ion exchange and precipitation [12]. Moreover, the pseudo-second-order model also defines the processes of outer liquid film diffusion, surface sorption, and intra-particle diffusion actions [32].

Table 2.
The kinetic parameters for Cd sorption on various adsorbents.
2.3. Isotherm Experiments
As shown in Figure 2, the adsorption capacity of Cd (II) on biochar, especially for Ps-CMBC, increased rapidly when increasing the initial content of Cd (II) in the solution. The Cd equilibrium adsorption capacity on various sorbents can be expressed as follows: biochar < CMBC < P25-CMBC < P50-CMBC < P75-CMBC.
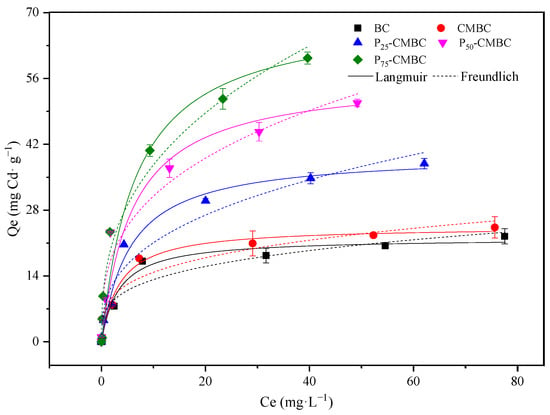
Figure 2.
Adsorption isotherms of Cd onto various adsorbents.
To further elucidate the mechanism and sorption performance, Langmuir and Freundlich models were used, and the corresponding models can be seen in Supplementary Materials, Equations (S4)–(S6).
According to the fitting parameters, the Langmuir model fitted better than the Freundlich model (Table 3), indicating that Cd (II) sorption on biochar may be a monolayer coverage [31]. The corresponding values of maximum adsorption capacity fitted by the Langmuir model (Qm) of biochar, CMBC, P25-CMBC, P50-CMBC, and P75-CMBC are 22.05, 24.51, 40.01, 56.70, and 70.13 mg·g−1, respectively. It is clear that Ps-CMBC exhibited a much higher adsorption capacity than the raw biochar, and the Qm of P25-CMBC, P50-CMBC, and P75-CMBC increased 1.82, 2.57, and 3.18 times, respectively, compared to biochar. These results suggest that the phosphate-sorbed method can greatly enhance the Cd (II) sorption capacity of biochar. A previous study showed that the P-loaded method has good prospects in the commercial field for improving Cd(II) adsorption performance [33].

Table 3.
Langmuir and Freundlich equation parameters of Cd adsorption onto different adsorbents.
The maximum Cd sorption capacity of P75-CMBC (Qm: 70.1 mg·g−1) fitted via Langmuir was higher than other modified biochars like commercial active carbon (12.6 mg·g−1) [34] and synthetic hydroxyapatite nanoparticles (61.7 mg·g−1) [35]. In addition, Qm is close to the biochars after HCl treatment (68.2 mg·g−1) [12] while smaller than hydroxyapatite-modified sludge-based biochar (114.7 mg·g−1) [36]. These results show that P-loaded Ca-Mn-impregnated biochar has a great potential as a sorbent for Cd(II) removal.
2.4. Zeta Potential of Biochar
The zeta potential is a crucial index of the dispersion stability of colloids. The sign (positive and negative) and the absolute value of the zeta potential indicate the level of electrostatic attraction or repulsion between charged particles in the dispersion [37]. Figure 3 shows that the zeta potential of different sorbents declined with increasing pH value, which may result from the various degrees of protonation of carboxyl and hydroxyl groups under different pH values. The oxygen-containing functional groups could be deprotonated under alkaline conditions, leading to a decrease in the zeta potential. The pHzpc value of P75-CMBC (pH 5.08) was lower than that of biochar (pH 5.53) and CMBC (pH 6.41). These results may be due to phosphate loading on the surface of the biochar, which increased the negative charge and reduced the zeta potential of the biochar’s surface effectively. Therefore, phosphate loading favors the adsorption of heavy metals from solutions [38].
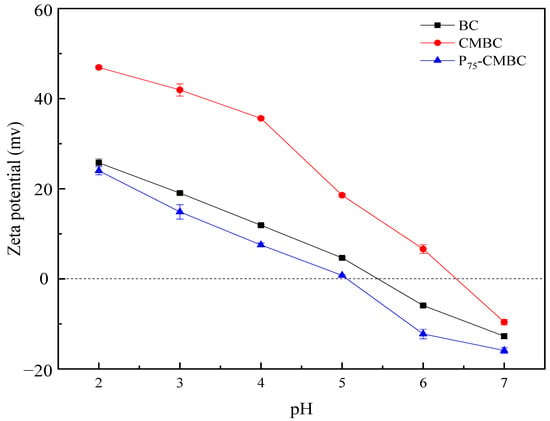
Figure 3.
Zeta potential of BC, CMBC, and P75-CMBC.
2.5. Effect of pH
The initial pH value is a crucial factor in Cd adsorption performance because it can change the surface charge of the sorbent, the level of ionization, and the sorbate speciation [39]. As presented in Figure S1, the sorption performance of Cd on adsorbents is strongly related to the pH of the initial solution (3–7). As the pH of the solution changed from 3 to 7, the sorption amount of Cd was greatly enhanced, and a relatively constant Cd adsorption capacity was maintained at pH > 5.0. These results are consistent with those of previous studies [23,40]. In general, as the pH value of the solution increases, the deprotonation of biochar results in an increase in negative charges, which is conducive to enhancing the adsorption capacity of heavy metals via electrostatic action or precipitation. At a lower pH value (3) of the solution, the surface of the biochar was protonated and the positive charge on the surface of the biochar increased, which tended to repel the adsorption of positively charged Cd (II) ions for electrostatic repulsion [7]. Additionally, redundant protonation of the sorbent surface promotes competition between numerous H+ binding sites with heavy metal ions at low pH levels [41]. Under acidic conditions, we can predict that cadmium will detach from the material’s surface after initially binding to it, allowing for the material to be effectively recycled.
2.6. Adsorption Thermodynamics
To further assess the stability and feasibility of Cd adsorption on the adsorbents, the effects of temperature on Cd sorption were explored at 288.15, 298.15, and 308.15 K. As shown in Figure S2, the Cd sorption amounts improved with increasing reaction temperature (288.15, 298.15, and 308.15 K). This implies that higher temperatures have a positive effect on the Cd sorption capacity.
Three thermodynamic parameters, such as the standard entropy change (ΔS0), standard enthalpy change (ΔH0), and Gibbs free energy (ΔG0), were calculated using Supplementary Materials, Equations (S7)–(S9), respectively.
The parameter ΔG0 could estimate the thermodynamic potential and the level of spontaneity of the sorption. When the ΔH0 value is positive, the sorption process is endothermic, whereas it is negative for an exothermic process. Figure S2f shows a linear plot of 1/T versus ln (KL), and all the corresponding thermodynamic parameters are listed in Table 4. The negative values of ΔG0 suggest that the Cd sorption process is spontaneous. For instance, P75-CMBC showed a drop in ΔG0 value from −22.72 kJ·mol−1 to −25.79 kJ·mol−1 with increasing temperature from 15 to 35 °C. These results show that the spontaneity increases with increasing temperatures. The values of ΔH0 (8.07–18.66 kJ·mol−1) were positive for Cd sorption, indicating that the sorption process on the sorbents was endothermic, and the biochar would possess higher sorption capacity with the increasing temperature. Additionally, the positive values of ΔS0 (0.108, 0.124, and 0.143 kJ·mol−1·K−1 for the sorption on biochar, CMBC, and P75-CMBC, respectively) imply the spontaneity of Cd sorption. This result demonstrates that there was a great disorder of the adsorbate/adsorbent system when Cd was migrated from the aqueous phase to the biochar’s surface [42].

Table 4.
Thermodynamic parameters for Cd adsorption on different adsorbents.
2.7. The Surface Characterization
The SEM images show the surface morphology of P75-CMBC (after Cd sorption) at different resolutions. Figure S3 shows an abundant pore structure. In addition, the SEM images also show the phenomenon of pore obstruction, which could be due to the crumble of the internal pore structure of the biochar. SEM-EDS investigation after Cd sorption confirmed phosphate loading by the intense P peaks present in P75-CMBC (wt.%: 4.74); Cd was also observed by EDS, demonstrating that Cd was successfully adsorbed on P-sorbed-biochar (wt%: 7.46). Other constituent elements, including C, O, Mn, and Ca, are shown in Figure S3.
The biochar surface’s functional groups had a crucial effect on its adsorption performance. The FTIR spectroscopy results of P75-CMBC before and after Cd adsorption are listed in Figure 4. The broad and strong peaks of the biochar observed at 3427–3430 cm−1 were related to hydroxyl band (-OH) [43]. The peaks observed at 2923–2926 cm−1 corresponded to the stretching of -CH2 vibrations, which is a characteristic peak of aromatic or aliphatic compounds [33]. The peaks observed at 1591–1614 cm−1 could be attributed to the stretching vibration of the C=C or C=O group of carboxyl [44]. A previous study showed that the aromatic structure serves as a π-donor during heavy metal adsorption. Functional groups such as C=C are important for Cd (II) sorption because they can interact with Cd (II) ions via Cd (II)-π interactions [5]. The bands located at approximately 1094.5 cm−1 may be ascribed to the vibration of C-O-C, or the ionization of P+-O− in phosphate esters, and the vibration of P-O-P chains [45]. These results suggest that the P (P=O or P=OOH) loaded on the biochar may form compounds with heavy metal ions. This plays a crucial role in upgrading the sorption capacity of heavy metals. The weak peaks at approximately 802 cm−1 may be attributed to the stretching vibrations of C-H. Moreover, the peaks under 600 cm−1 could be attributed to the stretching vibrations of the metal halogen.
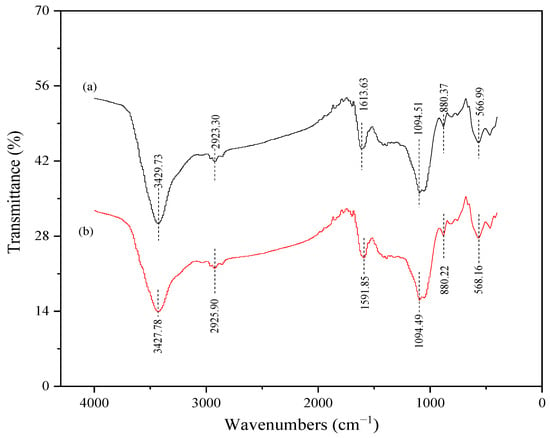
Figure 4.
Fourier transform infrared spectrum (FTIR) of P75-CMBC before (a) and after (b) Cd (II) sorption.
Figure 5 shows the results of the XPS analysis. The peaks of C1s, O1s, Ca2p, N1s, Mn2p, Mg1s, and P2p can be observed in all spectra of P-loaded biochar, implying that P was successfully loaded onto the modified biochar (Figure 5A). In addition, a new peak at approximately 413.6 eV after Cd (II) sorption was observed, and the surface atomic Cd content of P75-CMBC was 1.82%. This suggests that Cd (II) was successfully adsorbed onto the surface of Ps-CMBC. The XPS spectrum of C1s before and after Cd adsorption is shown in Figure 5B. Before Cd sorption, five peaks were observed at the following bond energies: C-C/C-H (284.3 eV, 57.3%), C=C (284.9 eV, 20.3%), C-OH/C-N (285.6 eV, 9.1%), C=O (286.7 eV, 7.2%), and O=C-OH (289.6 eV, 6.4%) [17]. After Cd sorption, the corresponding functional groups occupied 54.7%, 23.5%, 7.3%, 10.6%, and 4.1%, respectively. The decrease in O=C-OH and C-OH suggests that the process of Cd (II) sorption involves the depletion of carboxyl and hydroxyl groups on the surface of biochar. In addition, augmentation of the C=O functional groups indicated that the O atom may act as an electron donor during the uptake of Cd (II) [12]. For the P 2p spectrum of P-loaded biochar, the pristine peak could be fitted well by two peaks at about 133.2 eV and 134.3 eV, before and after Cd adsorption, respectively (Figure 5C). This indicates that P may combine with O atoms to form P-O and P=O groups during the phosphate adsorption process [23]. Additionally, the corresponding groups were considerably weakened after Cd adsorption, implying that chemical reactions between Cd (II) and P compounds may have occurred on the surface of P75-CMBC [16].
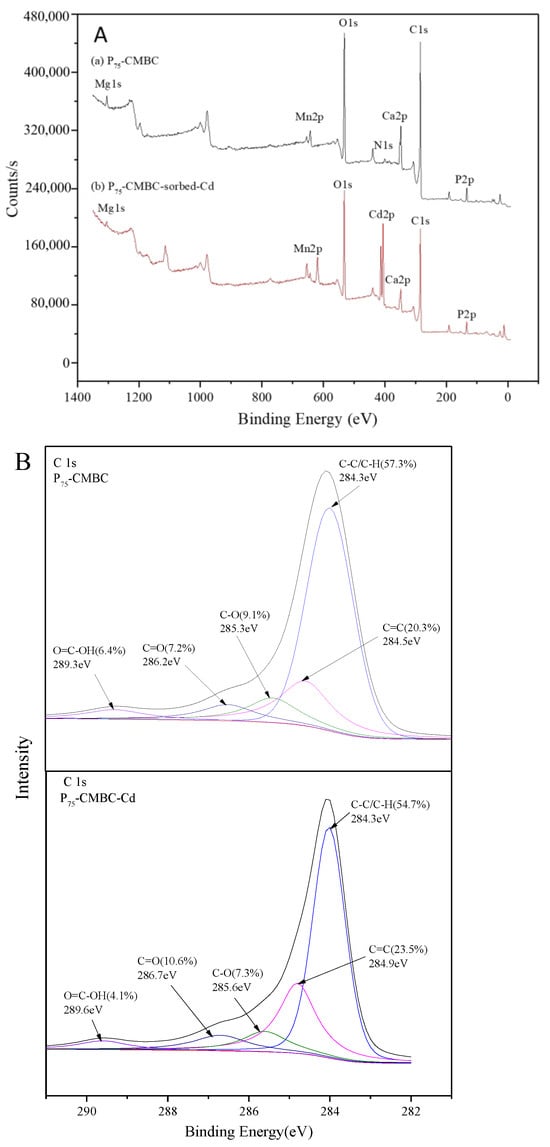
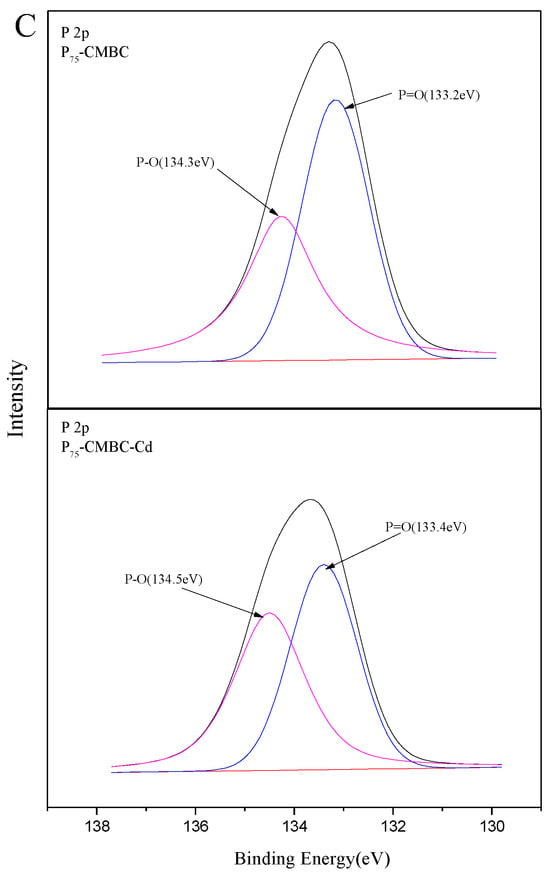
Figure 5.
The XPS spectra of P75-CMBC before or after Cd (II) sorption: (A) XPS spectra of P75-CMBC before or after Cd (II) sorption. (B) C 1s XPS spectra of P75-CMBC before and after Cd (II) adsorption. (C) P 2p XPS spectra of P75-CMBC before and after phosphate adsorption.
Thus, the sorption mechanisms of Cd (II) on Ps-CMBC involve the following: (1) Electrostatic attraction occurs between the positively charged Cd (II) in solution and negatively charged surface of Ps-CMBC. (2) The P (P=O or P=OOH) loaded on the biochar forms complex compounds with Cd (II). (3) The electronic structure of P-loaded biochar provides a cation–π bond between Cd (II) and a π-electron donor. (4) The coordination of Cd (II) with O atoms of P=O/P=OOH occurs via filling the empty orbital of Cd (II) with P=O groups.
3. Materials and Methods
3.1. Reagents and Analysis Equipment
All chemical reagents used in this study, including calcium chloride (CaCl2), potassium permanganate (KMnO4), sodium dihydrogen phosphate (NaH2PO4), cadmium nitrate tetrahydrate (Cd (NO3)2·4H2O), sodium nitrate (NaNO3), hydrochloric acid (HCl), and sodium hydroxide (NaOH), were of analytical grade; all solutions were prepared and biochar washing was carried out with deionized (DI) water. The Cd (II) was determined using an atomic absorbance spectrometer via the flame method.
3.2. Ca-Mn-Impregnated Biochar (CMBC) Preparation
The CMBC was synthesized via a two-stage pyrolysis and impregnation process. Firstly, corn straw powder was pyrolyzed in a muffle furnace for 1 h at 600 °C under a sufficient N2 environment. The obtained biochar was washed and dried to obtain pristine biochar. For biochar modification, a 40 mL solution containing 20 mL CaCl2 (25.53 g·L−1) and 20 mL KMnO4 (7900 mg·L−1) was prepared for impregnation of 5 g of biochar, and the mixture was stirred on a magnetic stirrer followed by water bath drying. Then, the CMBC was obtained by pyrolyzing the dried mixture at 600 °C. Finally, Ps-CMBC was prepared via the phosphate adsorption process on CMBC. Phosphate sorption experiments were conducted by adding 2 g of CMBC to 1 L beakers containing 500 mL of P solutions (the sorbent dosage was 4 g·L−1; initial phosphate concentration was 25, 50, or 75 mg·L−1). The mixture stirring was performed at 25 ± 0.5 °C and 300 rmp. The mixture was then filtered and the Ps-CMBC was dried to a constant weight at 60 °C in an oven. The Ps-CMBCs prepared with initial phosphate concentrations of 25, 50, and 75 mg P·L−1 can be denoted as P25-CMBC, P50-CMBC, and P75-CMBC, respectively.
3.3. Characterization
An elemental analyzer (Elementar VARIOEL cube, Langenselbold, Germany) was used to measure the total elemental compositions, such as those of S, N, O, H, and C. The pH of biochar was determined with a pH meter (Orion Star A215, Thermo Scientific, Waltham, MA, USA). The Brunauer–Emmett–Teller (BET) method was used to determine specific surface area (SSA), pore volume, and pore diameter distribution. The zeta potential of prepared materials was investigated using a Malvern Zetasizer NanoZSE (Nano ZS90, Malvern, UK).
The elemental composition and surface morphology of the biochar were analyzed using a scanning electron microscope coupled with an energy-dispersive spectrometer (SEM-EDS) analyzer (ZEISS, Gemini SEM 500, Oberkochen, Germany). The surface elemental content (C, O, Ca, Mn, P, and Cd) and the binding energy on the biochar surface were characterized via X-ray photoelectron spectroscopy (XPS; PHI 5000 Versa Probe, Chigasaki, Japan). The functional groups on the surface of biochar were characterized via Fourier-transform infrared spectroscopy (FTIR; Thermo Scientific Nicolet iS5, Waltham, MA, USA).
3.4. Adsorption Experiments
The Cd stock solution (1000 mg·L−1) was prepared using Cd(NO3)2·4H2O, and 85 mg·L−1 NaNO3 was used as the background electrolyte. The solution of the required initial concentration was obtained by diluting the stock solution. Batch sorption studies were conducted to investigate the effects of temperature, solution pH, initial Cd2+ concentration, and contact time on the Cd2+ sorption efficiency and performance. The solution pH values were adjusted using 4000 mg·L−1 NaOH or 6300 mg·L−1 HNO3 solutions. All sorption experiments were conducted three times, and standard deviation error bars were obtained from three replicates.
3.4.1. Kinetics Experiment
The sorption kinetics of Cd2+ were studied by adding 0.25 g of biochar to a 500 mL beaker containing 250 mL Cd2+ solution (50 mg·L−1) followed by stirring at 300 rpm, and the temperature was set at 25 ± 0.5 °C for different sampling times (0, 2, 5, 10, 30, 1, 2, 4, 8, 12, and 24 h). A 0.5 mL solution was filtered and the remaining Cd2+ concentration in the filtrate was measured using an atomic absorption spectrometer (AAS; Beijing Haiguang Instruments, Co., Ltd., Beijing, China), using the flame method, where the sensitivity was 0.05 mg·L−1 and a minimum detectable limit of 0.05 mg·L−1 was used for Cd. The Cd sorption amounts at time t were calculated using Equation (1):
where the Cd sorption amount at time t is represented by Qt (mg·g −1), Co (mg·L−1) and Ct (mg·L−1) are the initial Cd2+ concentrations at time t (min), respectively, V (mL) represents the volume of Cd solution, and the mass of adsorbents is represented by m (g).
3.4.2. Isotherms Experiment
Adsorbents of 1 g·L−1 (0.02 g of biochar in 20 mL of Cd solution) were taken in 40 mL brown glass vials containing 20 mL of different initial Cd concentrations (0, 1, 5, 10, 25, 50, 75, and 100 mg Cd·L−1). The vials were shaken (end-to-end) for 24 h at a temperature of 25 ± 0.5 °C at 120 rpm. At sorption equilibrium, the mixtures were completely filtered through a 0.22 μm membrane and the remaining Cd2+ concentration was measured using an AAS. The Cd sorption capacity at equilibrium Qe (mg·g−1) was calculated using Equation (2):
where the Cd sorption capacity at equilibrium is Qe (mg·g−1), the equilibrium and initial concentrations of Cd are represented by Ce (mg·L−1) and Co (mg·L−1), respectively, m (g) is the mass of the adsorbent and V (mL) is the volume of the solution.
3.4.3. Effect of pH on Cd Adsorption
The influence of solution pH was determined with various initial pH values (3, 4, 5, 6, and 7) of 20 mL Cd solution (0, 1, 5, 10, 25, 50, 75, and 100 mg Cd·L−1). Adsorbents (0.02 g) were added in the solution of Cd (1 g·L−1) and shaken for 24 h at 120 rpm (end-to-end).
3.4.4. Adsorption Thermodynamics
For thermodynamic analyses, adsorption was carried out at temperatures of 15, 25, and 35 °C. The sorption process was followed by an isothermal sorption method: 0.1 g of prepared materials was added into 40 mL brown glass vials that contained 20 mL of various initial Cd concentrations (0, 1, 5, 10, 25, 50, 75, and 100 mg Cd·L−1). The equilibrium Cd concentrations were then determined.
3.5. Data Analysis
All experimental data are the average of triplicate measurements. Statistical analysis was performed using Microsoft Excel 2010, and the results are presented as mean ± standard deviation and were plotted using Origin 2016 software for Windows (Origin Lab, Northampton, MA, USA).
4. Conclusions
In this study, P-loaded biochar was prepared to elucidate the sorption mechanisms of Cd from aqueous solutions. Ps-CMBC showed higher sorption efficiency for a shorter time, compared to biochar and CMBC, and exhibited outstanding Cd (II) adsorption performance, with an equilibrium sorption capacity of 70.13 mg·g−1, higher than those of biochar (22.05 mg·g−1) and CMBC (24.51 mg·g−1). Higher pH (5–7) had relatively lower effects on the Cd (II) sorption capacity. The primary mechanisms for Cd adsorption on Ps-CMBC were surface complexation, electrostatic attraction, Cd (II)-π interactions, and the coordination of Cd (II) with P=O. In summary, P-sorbed biochar has great potential for Cd (II) adsorption.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28227553/s1, Figure S1: Effects of initial pH (3–7) on Cd adsorption of different materials. (A) BC; (B) CMBC; (C) P75-CMBC; Figure S2: Cd sorption of different materials at different temperatures. BC (a), CMBC (b), P25-CMBC (c) and P50-CMBC (d), P75-CMBC (e). Plots of lnb versus 1/T for different adsorbents (f); Figure S3: SEM images and EDS spectra of P75-CMBC after adsorbed Cd(II);
Author Contributions
C.Q. and C.W. Wang preformed the laboratory experiments. M.G. and Z.S. provided significant input on the experimental design and conceived of the idea of this study. C.Q. and M.G. provided financial means. Q.L. interpreted histological data. C.Q., C.W., M.G. and Z.S. analyzed the data and prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the key scientific and technological research topics of Tibet (XZ202001ZY0048N), the China Agriculture Research System of MOF and MARA (CARS-05-02A-13), and the STU Scientific Research Foundation for Talents (No. NTF19026).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.Y.; Ji, H.Y.; Lyu, H.H.; Liu, Y.X.; He, L.L.; You, L.C.; Zhou, C.H.; Yang, S.M. Simultaneous alleviation of Sb and Cd availability in contaminated soil and accumulation in Lolium multiflorum Lam. After amendment with Fe-Mn-modified biochar. J. Clean. Prod. 2019, 231, 556–564. [Google Scholar] [CrossRef]
- Wang, H.Y.; Gao, B.; Wang, S.S.; Fang, J.; Xue, Y.W.; Yang, K. Removal of Pb (II), Cu (II), and Cd (II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Yang, X.; Wang, X.P.; Wang, Z.X.; Li, M.; Zhao, F.J. Dietary cadmium intake from rice and vegetables and potential health risk: A case study in Xiangtan, southern China. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; He, F.F.; Shen, X.Y.; Hu, D.W.; Huang, Q. Pyrolyzed fabrication of N/P co-doped biochars from (NH4)3PO4-pretreated coffee shells and appraisement for remedying aqueous Cr (VI) contaminants. Bioresour. Technol. 2020, 315, 123840. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.B.; Gao, P.; Chu, G.; Pan, B.; Peng, J.H.; Xing, B.S. Enhanced adsorption of Cu (II) and Cd (II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef]
- Gong, X.M.; Huang, D.L.; Liu, Y.G.; Peng, Z.W.; Zeng, G.M.; Xu, P.; Cheng, M.; Wang, R.Z.; Wan, J. Remediation of contaminated soils by biotechnology with nanomaterials: Bio-behavior, applications, and perspectives. Crit. Rev. Biotechnol. 2017, 38, 455–468. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.; Ricchetti, L.; Pittman, C.U.; Anderson, R.; Mohan, D.; Mlsna, T. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem. Eng. J. 2018, 331, 480–491. [Google Scholar] [CrossRef]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash. J. Colloid Interface Sci. 2017, 513, 72–81. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, J.W.; Song, K.G.; Choi, K.; Lee, Y.J.; Jung, K.W. Adsorption and mechanistic study for phosphate removal by rice husk-derived biochar functionalized with Mg/Al-calcined layered double hydroxides via co-pyrolysis. Compos. Part B Eng. 2019, 176, 107209. [Google Scholar] [CrossRef]
- Guo, X.J.; Chen, R.R.; Liu, Q.; Liu, J.Y.; Zhang, H.S.; Yu, J.; Li, R.M.; Zhang, M.L.; Wang, J. Graphene oxide and silver ions coassisted zeolitic imidazolate framework for antifouling and Uranium enrichment from seawater. ACS. Sustain. Chem. Eng. 2019, 7, 6185–6195. [Google Scholar] [CrossRef]
- Kulakova, I.I.; Lisichkin, G.V. Prospects for using graphene nanomaterials: Sorbents, membranes, and gas sensors. Russ. J. Appl. Chem. 2021, 94, 1177–1188. [Google Scholar] [CrossRef]
- Wu, J.W.; Wang, T.; Zhang, Y.S.; Pan, W.P. The distribution of Pb (II)/Cd (II) adsorption mechanisms on biochars from aqueous solution: Considering the increased oxygen functional groups by HCl treatment. Bioresour. Technol. 2019, 291, 121859. [Google Scholar] [CrossRef]
- Leng, L.J.; Huang, H.J. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour. Technol. 2018, 270, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.A.; Lu, L.; He, H.J.; Li, J.X.; Zhu, Z.Q.; Zhu, Y.N. Applications of biochar and modified biochar in heavy metal contaminated soil: A descriptive review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Qiu, B.B.; Tao, X.D.; Wang, H.; Li, W.K.; Ding, X.; Chu, H.Q. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrol. 2021, 155, 105081. [Google Scholar] [CrossRef]
- Zhang, P.Z.; Zhang, X.X.; Li, Y.F.; Han, L.J. Influence of pyrolysis temperature on chemical speciation, leaching ability, and environmental risk of heavy metals in biochar derived from cow manure. Bioresour. Technol. 2020, 302, 122850. [Google Scholar] [CrossRef]
- Xu, C.B.; Tan, X.; Zhao, J.W.; Cao, J.M.; Ren, M.; Xiao, Y.; Lin, A.J. Optimization of biochar production based on environmental risk and remediation performance: Take kitchen waste for example. J. Hazard. Mater. 2021, 416, 125785. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.L.; Liu, W.J.; Zhang, S.; Jiang, H. Magnesium oxide embedded nitrogen self-doped biochar composites: Fast and high-efficiency adsorption of heavy metals in an aqueous solution. Environ. Sci. Technol. 2017, 51, 10081. [Google Scholar] [CrossRef]
- Li, X.Y.; Peng, P.Q.; Long, J.; Dong, X.; Jiang, K.; Hou, H.B. Plant-induced insoluble Cd mobilization and Cd redistribution among different rice cultivars. J. Clean. Prod. 2020, 256, 120494. [Google Scholar] [CrossRef]
- Lin, L.N.; Song, Z.G.; Khan, Z.H.; Liu, X.W.; Qiu, W.W. Enhanced As (III) removal from aqueous solution by Fe-Mn-La-impregnated biochar composites. Sci. Total. Environ. 2019, 315, 123840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shao, J.G.; Zhang, S.H.; Zhang, X.; Chen, H.P. Effect of phosphorus-modified biochars on immobilization of Cu (II), Cd (II), and As (V) in paddy soil. J. Hazard. Mater. 2019, 390, 121349. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.L.; Fu, Q.L.; Hu, H.Q.; Wang, Q.; Liu, Y.H.; Zhu, J. Highly-effective removal of Pb by co-pyrolysis biochar derived from rape straw and orthophosphate. J. Hazard. Mater. 2019, 371, 191–197. [Google Scholar] [CrossRef]
- Hu, R.; Xiao, J.; Wang, T.H.; Chen, G.C.; Chen, L.; Tian, X.Y. Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chem. Eng. J. 2020, 379, 122388. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Xiao, Z.H.; Zhang, G.L.; Wang, A.D.; Li, Z.H.; Liu, Y.H.; Wang, H.; Zeng, Q.R.; Liang, Y.S.; Zou, D.S. Speciation and bioavailability of heavy metals in pyrolytic biochar of swine and goat manures. J. Anal. Appl. Pyrol. 2018, 132, 82–93. [Google Scholar] [CrossRef]
- Wang, C.W.; Qiu, C.; Song, Z.G.; Gao, M.L. A novel Ca/Mn modified biochar recycles P from solution: Mechanisms and phosphate efficiency. Environ. Sci. Proc. Imp. 2022, 24, 474–485. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2012, 5, 104–115. [Google Scholar] [CrossRef]
- Tag, A.T.; Duman, G.; Ucar, S.; Yanik, J. Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J. Anal. Appl. Pyrol. 2016, 120, 200–206. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.T.; Xiao, Q.; Lu, S.G. Biochar derived from cadmium-contaminated rice straw at various pyrolysis temperatures: Cadmium immobilization mechanisms and environmental implication. Bioresour. Technol. 2020, 321, 124459. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Z.; Huang, D.L.; Liu, Y.G.; Zhang, C.; Lai, C.; Zeng, G.M.; Cheng, M.; Gong, X.M.; Wan, J.; Luo, H. Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour. Technol. 2018, 261, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X.D. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Chen, H.Y.; Li, W.Y.; Wang, J.J.; Xu, H.J.; Liu, Y.L.; Zhang, Z.; Li, Y.T.; Zhang, Y.L. Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: Selective adsorption and influence of dissolved organic matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Krukowska, J.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Joshi, P.; Manocha, S. Sorption of cadmium ions onto synthetic hydroxyapatite nanoparticles. Mater. Today 2017, 4, 10460–10464. [Google Scholar] [CrossRef]
- Chen, Y.N.; Li, M.L.; Li, Y.P.; Liu, Y.H.; Chen, Y.R.; Li, H.; Li, L.S.Z.; Xu, F.T.; Jiang, H.J.; Chen, L. Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+ and Cd2+: Adsorption behavior and mechanisms. Bioresour. Technol. 2021, 321, 124413. [Google Scholar] [CrossRef]
- Kirby, B.J.; Hasselbrink, E.F. Zeta potential of microfluidic substrates: 1. Theory, experimental techniques, and effects on separations. Electrophoresis 2010, 25, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.J.; Li, J.Y.; Yuan, J.H.; Xu, R.K. Adsorption of Cu (II) by biochars generated from three crop straws. Chem. Eng. J. 2011, 172, 828–834. [Google Scholar] [CrossRef]
- Wang, R.Z.; Huang, D.L.; Liu, Y.G.; Peng, Z.W.; Zeng, G.M.; Lai, C.; Xu, P.; Huang, C.; Zhang, C.; Gong, X.M. Selective removal of BPA from aqueous solution using molecularly imprinted polymers based on magnetic graphene oxide. RSC Adv. 2016, 6, 106201–106210. [Google Scholar] [CrossRef]
- Zhou, Q.W.; Liao, B.H.; Lin, L.N.; Qiu, W.W.; Song, Z.G. Adsorption of Cu (II) and Cd (II) from aqueous solutions by ferromanganese binary oxide-biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, X.B.; Wang, X.L.; Feng, K.; Su, J.C.; Dong, J.N. The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef]
- Yan, L.G.; Yang, K.; Shan, R.R.; Yan, T.; Wei, J.; Yu, S.J.; Yu, H.Q.; Du, B. Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core–shell Fe3O4@LDHs composites with easy magnetic separation assistance. J. Colloid Interface Sci. 2015, 448, 508–516. [Google Scholar] [CrossRef]
- Tan, Z.X.; Yuan, S.N.; Hong, M.F.; Zhang, L.M.; Huang, Q.Y. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater. 2019, 384, 121370. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhang, J.; Mu, Y.; Gao, J.H.; Feng, Y.L.; Liu, H.; Guo, Z.Z.; Zhang, C.L. Preparation and evaluation of activated carbon with different polycondensed phosphorus oxyacids (H3PO4, H4P2O7, H6P4O13 and C6H18O24P6) activation employing mushroom roots as precursor. J. Anal. Appl. Pyrol. 2014, 108, 41–46. [Google Scholar] [CrossRef]
- Tao, Q.; Chen, Y.X.; Zhao, J.W.; Li, B.; Li, Y.H.; Tao, S.Y.; Li, M.; Li, Q.Q.; Xu, Q.; Li, Y.D.; et al. Enhanced Cd removal from aqueous solution by biologically modified biochar derived from digestion residue of corn straw silage. Sci. Total. Environ. 2019, 674, 213–222. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).