Resveratrol, ε-Viniferin, and Vitisin B from Vine: Comparison of Their In Vitro Antioxidant Activities and Study of Their Interactions
Abstract
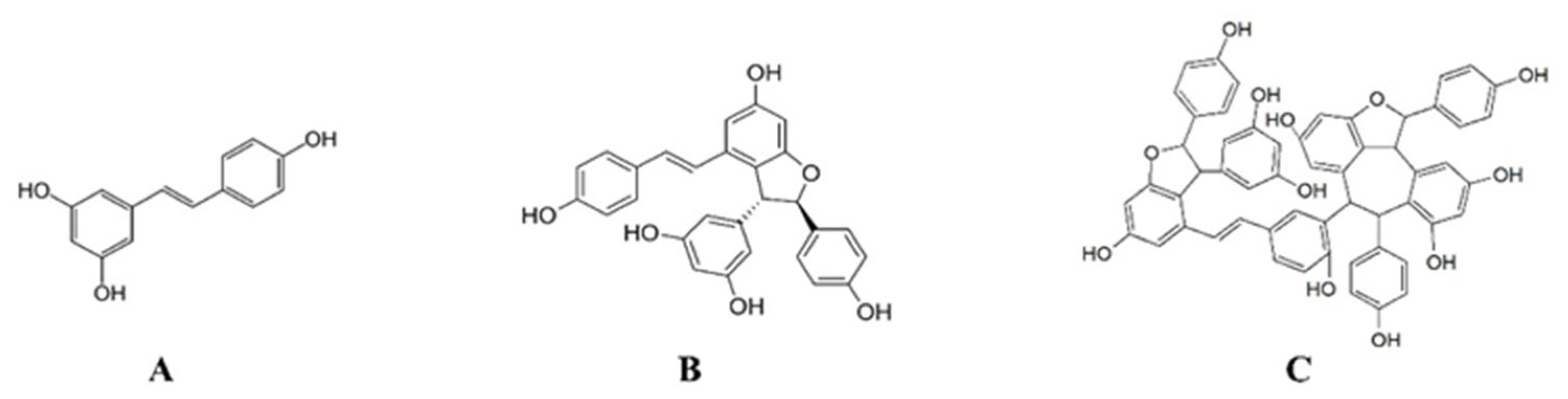
:1. Introduction
2. Results
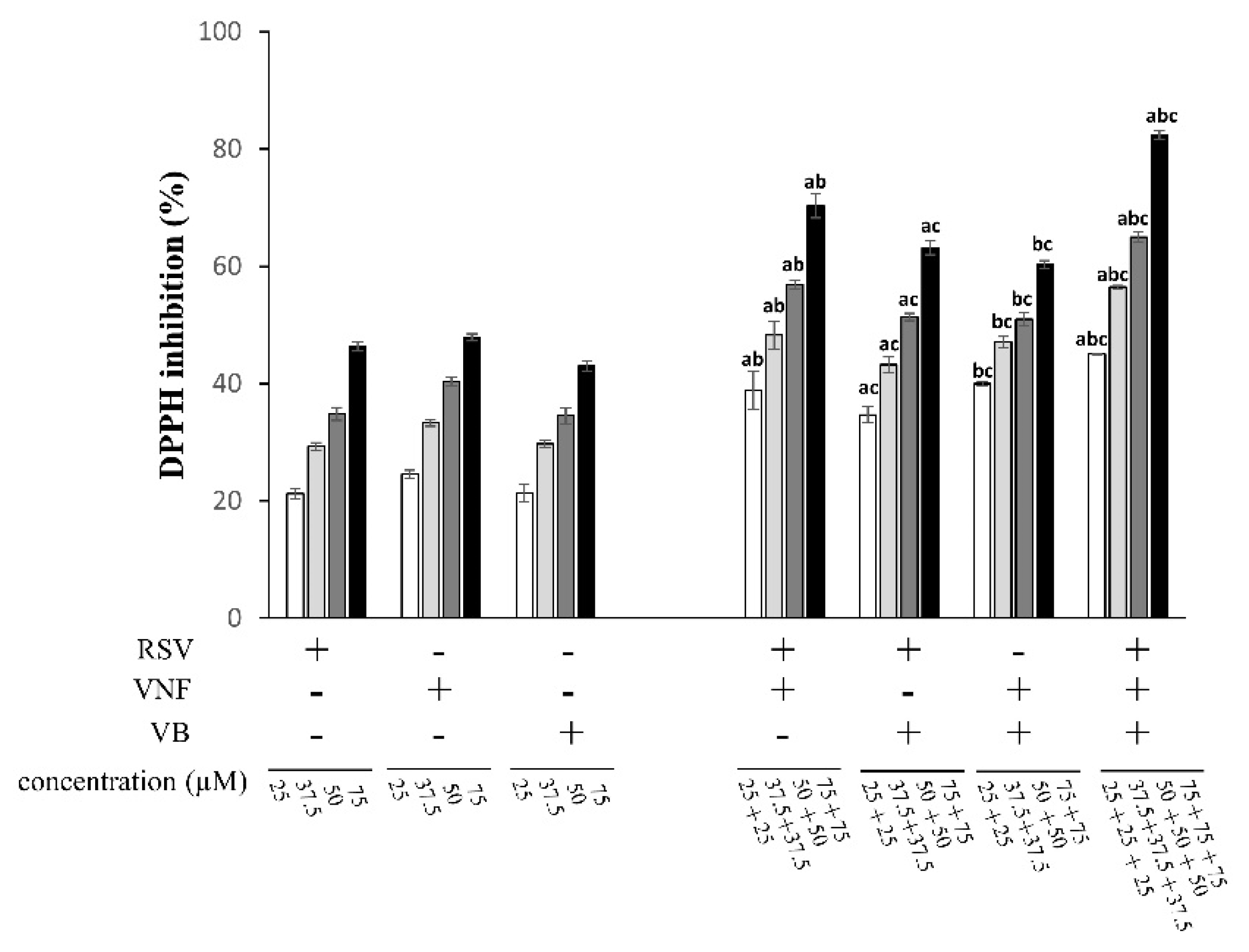
2.1. Determination of Antioxidant Activities with the DPPH-Scavenging Assay
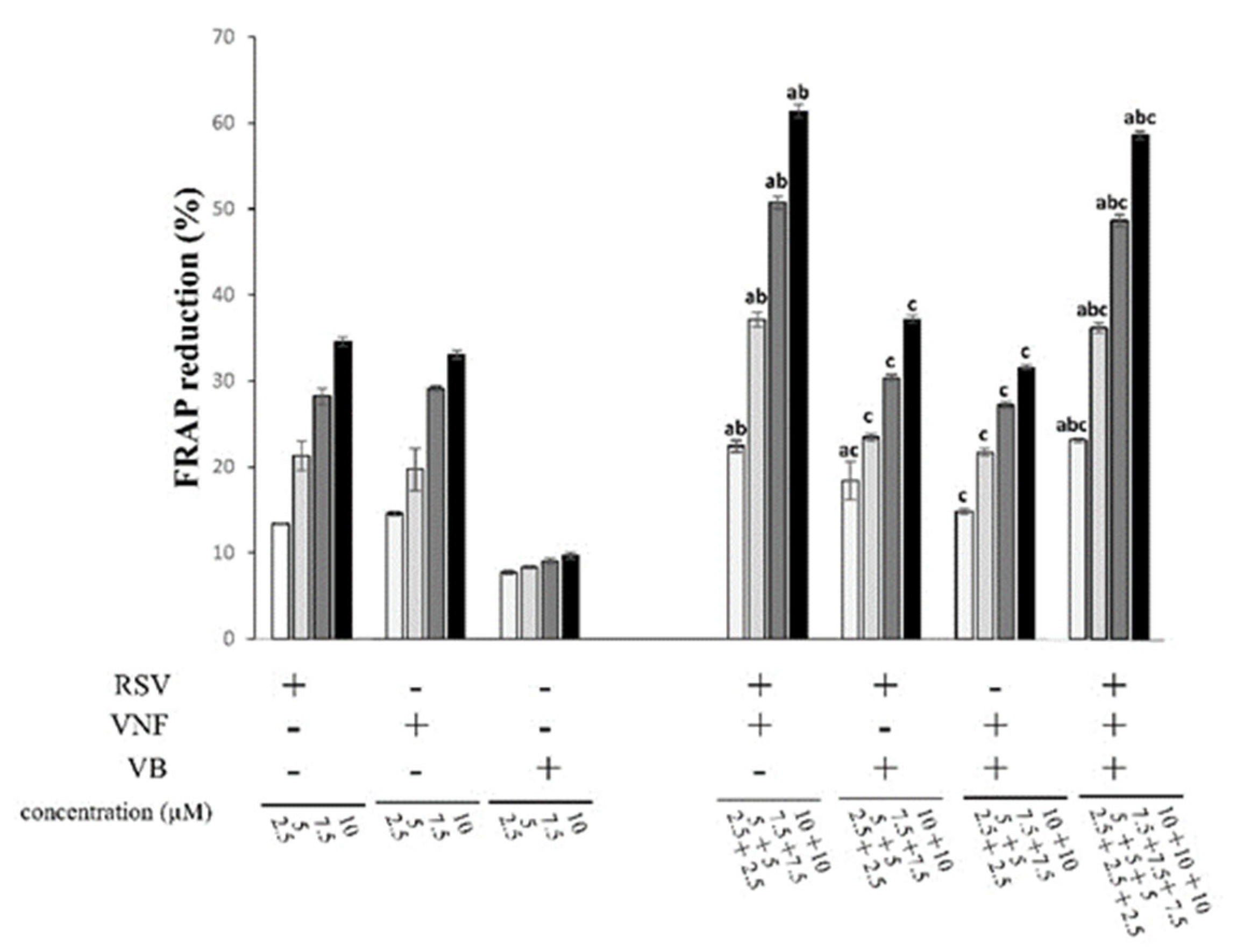
2.2. Determination of Antioxidant Activities with the FRAP Assay
2.3. Determination of Antioxidant Activity with NO-Scavenging Assay
2.4. Comparison of the Antioxidant Activities between the Combination RSV + VNF and the Standardized Stilbene-Enriched Vine Extract
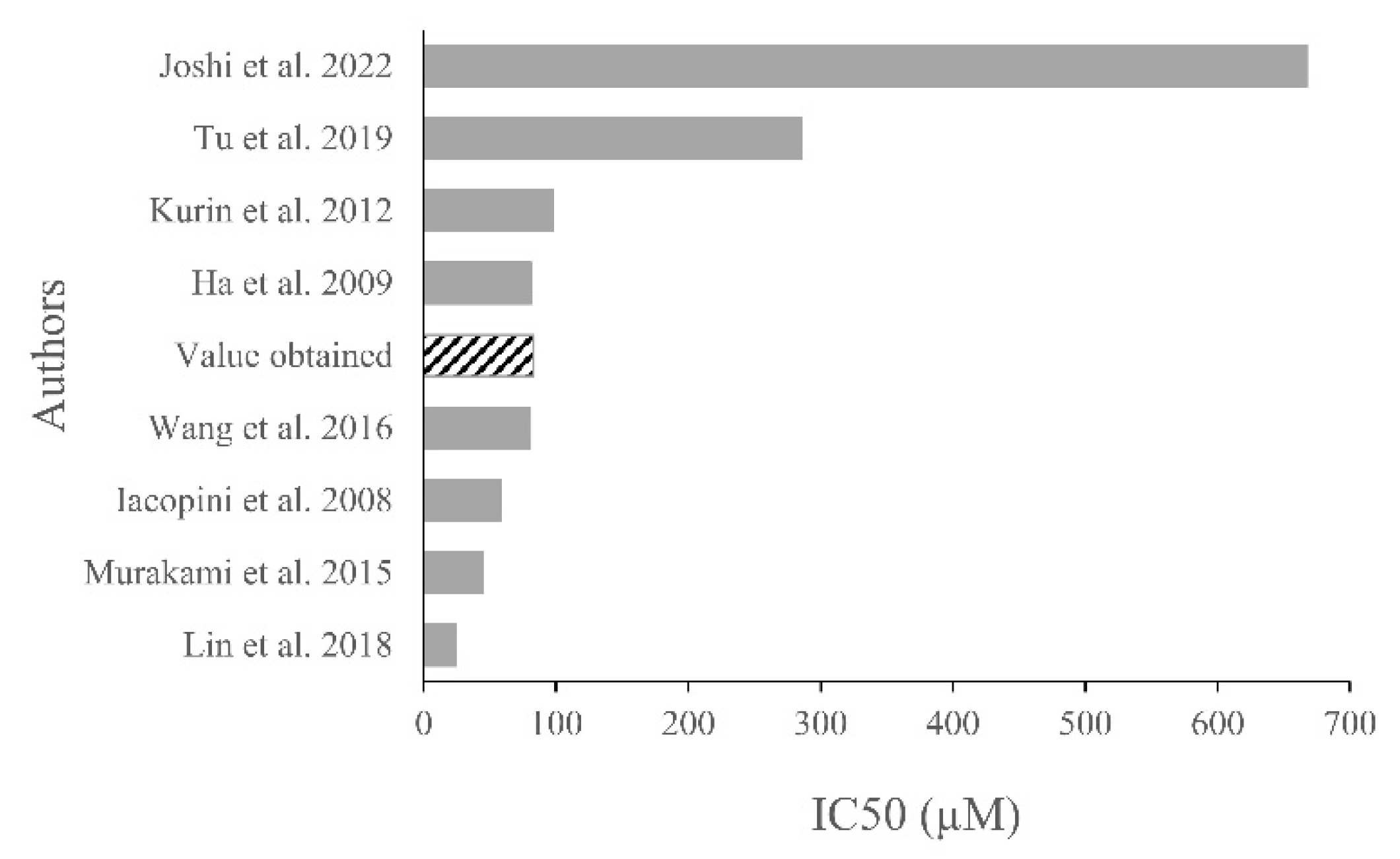
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. DPPH-Scavenging Assay
4.3. FRAP Assay
4.4. NO-Scavenging Assay
4.5. Statistical Analysis and Determination of the Interactions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.; Wu, X. Diet Antioxidant Capacity: Relationships to Oxidative Stress and Health. Am. J. Biomed. Sci. 2013, 5, 126–139. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Kurin, E.; Mučaji, P.; Nagy, M. In Vitro Antioxidant Activities of Three Red Wine Polyphenols and Their Mixtures: An Interaction Study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological Aspects about in Vitro Evaluation of Antioxidant Properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Aftab, N.; Vieira, A. Antioxidant Activities of Curcumin and Combinations of This Curcuminoid with Other Phytochemicals. Phytother. Res. 2010, 24, 500–502. [Google Scholar] [CrossRef]
- Beaumont, P.; Courtois, A.; Atgié, C.; Richard, T.; Krisa, S. In the Shadow of Resveratrol: Biological Activities of Epsilon-Viniferin. J. Physiol. Biochem. 2022, 78, 465–484. [Google Scholar] [CrossRef]
- Nassra, M.; Krisa, S.; Papastamoulis, Y.; Kapche, G.D.; Bisson, J.; André, C.; Konsman, J.-P.; Schmitter, J.-M.; Mérillon, J.-M.; Waffo-Téguo, P. Inhibitory Activity of Plant Stilbenoids against Nitric Oxide Production by Lipopolysaccharide-Activated Microglia. Planta Med. 2013, 79, 966–970. [Google Scholar] [CrossRef]
- Liu, W.-B.; Hu, L.; Hu, Q.; Chen, N.-N.; Yang, Q.-S.; Wang, F.-F. New Resveratrol Oligomer Derivatives from the Roots of Rheum Lhasaense. Molecules 2013, 18, 7093–7102. [Google Scholar] [CrossRef]
- Biais, B.; Krisa, S.; Cluzet, S.; Da Costa, G.; Waffo-Teguo, P.; Mérillon, J.-M.; Richard, T. Antioxidant and Cytoprotective Activities of Grapevine Stilbenes. J. Agric. Food Chem. 2017, 65, 4952–4960. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.T.; Kim, H.; Thuong, P.T.; Ngoc, T.M.; Lee, I.; Hung, N.D.; Bae, K. Antioxidant and Lipoxygenase Inhibitory Activity of Oligostilbenes from the Leaf and Stem of Vitis Amurensis. J. Ethnopharmacol. 2009, 125, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Chen, Y.; Zou, J.; Li, X. Biotransformation of Resveratrol: New Prenylated Trans-Resveratrol Synthesized by Aspergillus sp. SCSIOW2. Molecules 2016, 21, 883. [Google Scholar] [CrossRef]
- Joshi, T.; Deepa, P.R.; Sharma, P.K. Effect of Different Proportions of Phenolics on Antioxidant Potential: Pointers for Bioactive Synergy/Antagonism in Foods and Nutraceuticals. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2022, 92, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, X.; Chen, B.; Wei, G.; Chen, D. E-Configuration Improves Antioxidant and Cytoprotective Capacities of Resveratrols. Molecules 2018, 23, 1790. [Google Scholar] [CrossRef]
- Tu, J.; Shi, D.; Wen, L.; Jiang, Y.; Zhao, Y.; Yang, J.; Liu, H.; Liu, G.; Yang, B. Identification of Moracin N in Mulberry Leaf and Evaluation of Antioxidant Activity. Food Chem. Toxicol. 2019, 132, 110730. [Google Scholar] [CrossRef]
- Agbadua, O.G.; Kúsz, N.; Berkecz, R.; Gáti, T.; Tóth, G.; Hunyadi, A. Oxidized Resveratrol Metabolites as Potent Antioxidants and Xanthine Oxidase Inhibitors. Antioxidants 2022, 11, 1832. [Google Scholar] [CrossRef]
- Skroza, D.; Generalić Mekinić, I.; Svilović, S.; Šimat, V.; Katalinić, V. Investigation of the Potential Synergistic Effect of Resveratrol with Other Phenolic Compounds: A Case of Binary Phenolic Mixtures. J. Food Compos. Anal. 2015, 38, 13–18. [Google Scholar] [CrossRef]
- Man-Ying Chan, M.; Mattiacci, J.A.; Hwang, H.S.; Shah, A.; Fong, D. Synergy between Ethanol and Grape Polyphenols, Quercetin, and Resveratrol, in the Inhibition of the Inducible Nitric Oxide Synthase Pathway. Biochem. Pharmacol. 2000, 60, 1539–1548. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, Epicatechin, Quercetin, Rutin and Resveratrol in Red Grape: Content, in Vitro Antioxidant Activity and Interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Abraham, S.K.; Eckhardt, A.; Oli, R.G.; Stopper, H. Analysis of in Vitro Chemoprevention of Genotoxic Damage by Phytochemicals, as Single Agents or as Combinations. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2012, 744, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Peyrat-Maillard, M.N.; Cuvelier, M.E.; Berset, C. Antioxidant Activity of Phenolic Compounds in 2,2′-Azobis (2-Amidinopropane) Dihydrochloride (AAPH)-Induced Oxidation: Synergistic and Antagonistic Effects. J. Am. Oil Chem. Soc. 2003, 80, 1007–1012. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant Properties of Phenolic Compounds: H-Atom versus Electron Transfer Mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Murakami, Y.; Kawata, A.; Ito, S.; Katayama, T.; Fujisawa, S. The Radical Scavenging Activity and Cytotoxicity of Resveratrol, Orcinol and 4-Allylphenol and Their Inhibitory Effects on Cox-2 Gene Expression and Νf-ĸb Activation in RAW264.7 Cells Stimulated with Porphyromonas Gingivalis-Fimbriae. In Vivo 2015, 29, 341–350. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]



| DPPH | FRAP | NO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 (µM) | CI | Interaction | IC50 (µM) | CI | Interaction | IC50 (µM) | CI | Interaction | |
| RSV | 81.92 ± 9.17 | 13.36 ± 0.91 | 200.68 ± 15.40 | ||||||
| VNF | 80.12 ± 13.79 | 28.81 ± 4.15 | 338.35 ± 89.47 | ||||||
| VB | 129.14 ± 26.13 | ND | 368.80 ± 14.20 | ||||||
| RSV + VNF | 71.55 ± 7.26 | 0.89 ± 0.06 | Ad | 13.28 ± 1.44 | 0.70 ± 0.12 | Sy | 259.78 ± 40.56 | 1.03 ± 0.01 | Ad |
| RSV + VB | 87.82 ± 19.05 | 0.88 ± 0.06 | Ad | ND | 1.36 ± 0.34 | An | 243.07 ± 52.03 | 0.87 ± 0.13 | Ad |
| VNF + VB | 89.07 ± 11.41 | 0.91 ± 0.05 | Ad | ND | 0.96 ± 0.20 | Ad | ND | 0.99 ± 0.17 | Ad |
| RSV + VNF + VB | 90.42 ± 10.99 | 0.98 ± 0.04 | Ad | 21.61 ± 1.94 | 0.81 ± 0.06 | Sy | 216.24 ± 80.03 | 0.72 ± 0.18 | Sy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sy, B.; Krisa, S.; Richard, T.; Courtois, A. Resveratrol, ε-Viniferin, and Vitisin B from Vine: Comparison of Their In Vitro Antioxidant Activities and Study of Their Interactions. Molecules 2023, 28, 7521. https://doi.org/10.3390/molecules28227521
Sy B, Krisa S, Richard T, Courtois A. Resveratrol, ε-Viniferin, and Vitisin B from Vine: Comparison of Their In Vitro Antioxidant Activities and Study of Their Interactions. Molecules. 2023; 28(22):7521. https://doi.org/10.3390/molecules28227521
Chicago/Turabian StyleSy, Biranty, Stéphanie Krisa, Tristan Richard, and Arnaud Courtois. 2023. "Resveratrol, ε-Viniferin, and Vitisin B from Vine: Comparison of Their In Vitro Antioxidant Activities and Study of Their Interactions" Molecules 28, no. 22: 7521. https://doi.org/10.3390/molecules28227521
APA StyleSy, B., Krisa, S., Richard, T., & Courtois, A. (2023). Resveratrol, ε-Viniferin, and Vitisin B from Vine: Comparison of Their In Vitro Antioxidant Activities and Study of Their Interactions. Molecules, 28(22), 7521. https://doi.org/10.3390/molecules28227521







