Abstract
Paclitaxel, a natural secondary metabolite isolated and purified from the bark of the Taxus tree, is considered one of the most successful natural anticancer drugs due to its low toxicity, high potency and broad-spectrum anticancer activity. Taxus trees are scarce and slow-growing, and with extremely low paclitaxel content, the contradiction between supply and demand in the market is becoming more and more intense. Therefore, researchers have tried to obtain paclitaxel by various methods such as chemical synthesis, artificial culture, microbial fermentation and tissue cell culture to meet the clinical demand for this drug. This paper provides a comprehensive overview of paclitaxel extraction, combination therapy, total synthesis, semi-synthesis and biosynthesis in recent years and provides an outlook, aiming to provide a theoretical basis and reference for further research on the production and application of paclitaxel in the future.
1. Introduction
Nature has been the source of medicinal products for thousands of years, with many drugs derived from plants. As a model for drug discovery from natural products, paclitaxel (registered as Taxol® by BMS (New York, NY, USA)) is one of the most successful anticancer drugs of the past 50 years. The global paclitaxel market was valued at US$4.51 billion in 2021 and is expected to reach over US$11.16 billion by 2030 [1]. Paclitaxel was first isolated and purified from the bark of Taxus brevifolia, which is a rare and slow-growing evergreen found in the old-growth forests of the Pacific Northwest (also known as the yew tree), and its structure was characterized by Wani et al. in 1971 [2]. Paclitaxel possesses a highly oxygenated tetracyclic skeleton with a bridged bicyclo [5.3.1] undecane ring system (Figure 1). The anti-tumor activity of paclitaxel is mainly due to the C13 side chain, A ring, oxetane ring and C2 benzoyl group [3]. Paclitaxel exists in the form of a white crystalline powder that is highly lipophilic and thus very insoluble in water.
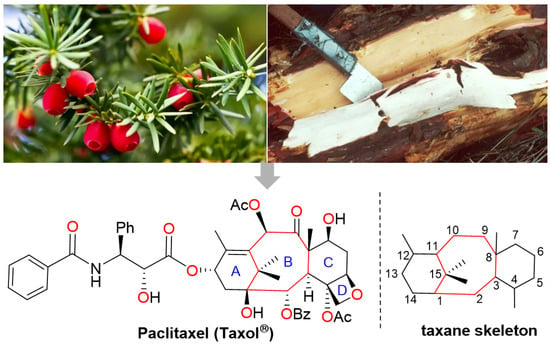
Figure 1.
The structure of paclitaxel.
The anticancer activity of paclitaxel was demonstrated in the mouse melanoma B16 model in 1976 [4]. Subsequently, Horwitz et al. found that paclitaxel inhibited cancer cell proliferation by stabilizing microtubules, especially in melanoma cells and ovarian cancer cells [5]. Paclitaxel was initially approved by the U.S. Food and Drug Administration (FDA) in 1992 for the treatment of ovarian cancer and in 1994 for the treatment of metastatic breast cancer [6]. In subsequent years, it has also been approved to treat non-small cell lung carcinoma, AIDS-related Kaposi’s sarcoma and cancers of the lung, bladder, esophagus, prostate and pancreas, either alone or in combination with other anticancer drugs [7,8]. It has been clinically proven that paclitaxel has good anti-tumor effects, especially for ovarian cancer, uterine cancer and breast cancer, which have a high incidence of occurrence [9].
Acquiring adequate supply has been a major challenge throughout the development of paclitaxel. Economically synthesizing paclitaxel is very complex, and isolating it from natural sources is cumbersome. According to a report from CEC China Pharmaceuticals Ltd. (Shanghai, China), 10,000 kg of leaves and bark from Taxus chinensis are required to isolate 1 kg of paclitaxel. Even in the most productive species, Taxus brevifolia, the paclitaxel content is only 0.01–0.05% [10]. Therefore, direct extraction methods cannot support the large-scale production of paclitaxel and have raised considerable environmental concerns. With ongoing efforts dedicated to paclitaxel production, at least two promising approaches have been developed to address supply and ecological challenges. The first large-scale approach involves the semi-synthesis of paclitaxel. The method was derived to extract 10-deacetylbaccatin III (10-DAB) or baccatin III from renewable twigs of Taxus species, which is subsequently converted to paclitaxel [11]. Another method for large-scale production of paclitaxel is cell fermentation using Taxus cell suspension cultures. At the moment, Python Biotech is the largest producer of paclitaxel by this method [1]. Although many advances have been made in the production of paclitaxel over the years, there are still several drawbacks to the current approaches, and the problems of supply shortages and high costs of production still cannot be ignored.
In recent years, synthetic biology technology has brought a green and sustainable strategy for the large-scale production of structurally complex and rare natural products through artificially building and optimizing biosynthetic pathways of target compounds in microbial chassis cells. Therefore, analyzing the biosynthetic pathway of paclitaxel and constructing this biosynthetic pathway in microorganisms using synthetic biology methods may be a new route to overcome the paclitaxel supply problem. Despite some progress in this field [12], there are still gaps in our understanding of paclitaxel biosynthesis pathway and its regulatory mechanisms that hinder paclitaxel production using biology methods. Our goal in writing this review was to provide a comprehensive review of paclitaxel extraction, total synthesis, semi-synthesis and biosynthesis methods. We have emphasized the evolution of each approach and highlighted the merits and demerits of each. Ultimately, we hope to provide a theoretical basis and reference for further research on the production and utilization of paclitaxel.
2. Anticancer Mechanism and Clinical Applications
Unlike traditional anticancer drugs, paclitaxel neither affects the synthesis of DNA and RNA in cancer cells nor damages DNA molecules, and its mechanism of action is mainly to promote the polymerization of tubulin [13,14]. Tubulin, the basic structural unit of intracellular microtubules, is a heterodimer formed by the polymerization of α-tubulin and β-tubulin molecules. Paclitaxel selectively binds to β-tubulin and promotes the polymerization and assembly of tubulin, which depletes intracellular tubulin, prevents spindle formation, leads to mitotic arrest in G2/M phase, terminates cell division and ultimately leads to cancer cell death (Figure 2) [15].
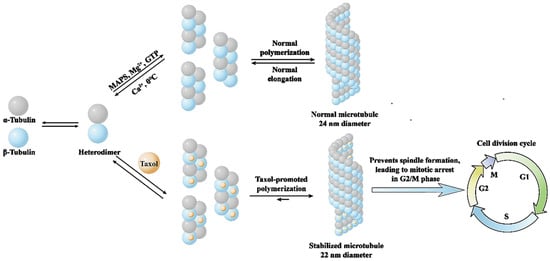
Figure 2.
Mechanism of action of paclitaxel on microtubulin.
Paclitaxel also induces the expression of genes and cytokines that inhibit tumor cell growth and apoptosis. Paclitaxel inhibits regulatory cells (Tregs) and tumor-associated macrophages (TAMs), stimulates anti-tumor immunity and leads to the release of pro-apoptotic molecules such as Fas L, TNF-related apoptosis-inducing ligand (TRAIL) and cytokines such as TNF-α and IFN-γ [16,17]. It can also induce apoptosis by activating signaling pathways. Paclitaxel activates phosphatase and tensin homologs deleted on chromosome ten (PTEN) and inhibits PI3K/Akt expression and phosphorylation by generating an excess of ROS and promoting miR-22 overexpression [18]. Another study showed that paclitaxel could upregulate miR-145 and directly inhibit the expression of Akt, thereby inducing apoptosis [19,20].
Paclitaxel may inhibit tumor cell growth by inducing autophagy, but this effect is dependent on the type of cells and the concentration of paclitaxel. One study found that the treatment of MDA-MB-231 cells with paclitaxel (24 μM) induced autophagy but showed no significant inhibition, and combined treatment of cancer cells with paclitaxel and Bridgestone induced the significant inhibition of cancer cells [21]. Other anti-tumor mechanisms of paclitaxel are cellular pyroptosis, senescence and ferroptosis [22,23,24,25,26,27].
The main paclitaxel-like compounds currently in clinical use are paclitaxel, docetaxel (registered as Taxotere® by Sanofi-Aventis (Paris, France)) and cabazitaxel (registered as JEVTANA® by Sanofi-Aventis) (Figure 3) [28]. Docetaxel is structurally similar to paclitaxel, with the difference being that the C10 position is a hydroxyl group instead of an acetyl group and the C3′ position is a Boc group instead of a benzoyl group. In 1996, docetaxel was marketed for the treatment of breast cancer, colon cancer and NSCLC [29]. It binds microtubulin better than paclitaxel and exhibits better solubility, bioavailability and anti-tumor activity. Cabazitaxel, another paclitaxel-based anti-tumor agent, was approved by the FDA in 2010 for the treatment of advanced prostate cancer and can be used in combination with prednisone to treat hormone-refractory metastatic prostate cancer [30,31]. In addition, the new drug larotaxel has completed phase III clinical evaluation for breast cancer alone, and the ternary ring in its structure is thought to minimize P-glycoprotein recognition, potentially overcoming multidrug resistance mechanisms and crossing the blood–brain barrier [32]. Conmotaxel is an access to the paclitaxel structure to inhibit NOD2-mediated inflammatory signaling pathway, which can enhance the therapeutic effect of paclitaxel and inhibit tumor metastasis and has received clinical approval [33] (Figure 3).
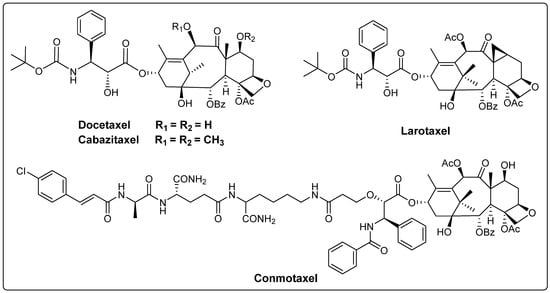
Figure 3.
The structures of paclitaxel-like compounds.
Paclitaxel-based antineoplastic drugs are mostly used as first-line anticancer drugs, often in combination with other anticancer drugs (Table 1 and Table 2). Numerous clinical evaluations have found that the combination of paclitaxel and platinum-based drugs for the treatment of advanced solid tumors has the advantages of high survival rate and good tolerance, safety and efficacy for esophageal, ovarian epithelial, cervical and gastric cancers [34,35,36]. In addition to its combination with chemotherapeutic drugs, paclitaxel is often used as a radiotherapy sensitizer in the treatment of squamous cell carcinomas such as intermediate-to-advanced head and neck cancer and nasopharyngeal carcinoma, and it participates in radiotherapy to minimize the duration of treatment with acceptable tolerability and good local control [37,38].

Table 1.
Clinical combinations of paclitaxel.

Table 2.
Ongoing clinical trials of paclitaxel in combination with other drugs.
In recent years, paclitaxel has also also often used in combination with other natural drugs, as in the case of co-administration with resveratrol against hepatocellular carcinoma, laryngeal carcinoma and gastric carcinoma, which can improve the anti-tumor activity of the drugs used alone and reduce the dosage and side effects of the two drugs alone [39]. It was shown that paclitaxel elevated the expression of caspase-3, caspase-8, Bax (Bcl-2 assaciated X protein), p53, Fas (factor associated suicide), Fas L (factor associated suicide ligand), cIAP-2 (cIap, cellular inhibitor of apoptosis), NF-кB and epidermal growth factor receptor (EGFR) mRNAs and proteins in HepG2 human liver cancer cells, and resveratrol enhanced the changes in the expression of these mRNAs [40]. The combination of paclitaxel and curcumin reversed multidrug resistance of paclitaxel and inhibited cancer cell growth [41,42]. Furthermore, combination therapy with the two improved the anti-glioma efficacy and helped reduce the side effects of cytotoxic treatment [43]. Curcumin can enhance the anticancer effect of paclitaxel in ovarian cancer by modulating the miR-9-5p/BRCA1 axis [44]. When used in combination for the treatment of lung cancer, curcumin enhanced the growth inhibition of lung cancer H1299 cells and showed a significantly lower IC50 value than that of paclitaxel alone [45].
3. Sources and Production Methods of Paclitaxel
3.1. Extraction from Taxus Plants
Direct extraction of paclitaxel from Taxus plants has always been the main method for paclitaxel preparation. However, it is not realistic to supply paclitaxel by extraction from wild natural resources due to its extremely low concentration and the slow growth of yew trees. To meet the increased demand for clinical use and to preserve the wild Taxus species, artificial cultivation has been utilized to alleviate the shortage of paclitaxel. For example, two seedling bases were established in Sichuan Province, China, including the Bei-chuan and Hong-ya bases. The yew seedlings from Bei-chuan and Hong-ya bases have been introduced to other provinces in China. To date, more than 150 yew forest farms have been established in various provinces of China, some of which can provide suitable active pharmaceutical ingredients [28].
Typically, for the extraction of paclitaxel or its precursor 10-deacetylbaccatin III (10-DAB), the branches and/or needles are harvested to keep the plant alive. This method can prevent the destruction of wild resources and achieve the sustainable use of resources. Paclitaxel is easily soluble in organic solvents, so ethanol, methanol, chloroform, ethyl acetate-acetone and ionic liquids can be used to extract paclitaxel from the plant. In recent years, microwave-assisted solvent extraction (MASE), ultrasound-assisted extraction (USAE), supercritical CO2 extraction and pressurized solvent extraction (PLE) have been widely used for the extraction of paclitaxel [46,47,48,49,50,51]. These methods can reduce the amount of solvent and operating time required and increase the purity and yield of paclitaxel compared to that achieved with conventional extraction methods. For example, Min et al. utilized the synergistic effect of ultrasound and negative pressure cavitation extraction (NPCE) to achieve more than 99% extraction of paclitaxel at an ultrasound power of 380 W and a vacuum of 260 mm Hg, with an extraction time of only 3 min [52].
Currently, artificial propagation of Taxus seedlings is considered one of the most efficient methods for obtaining paclitaxel and its chemical semi-synthetic precursors. Meanwhile, the extraction technology for paclitaxel is improving. However, these do not fully address the inadequate supply of paclitaxel.
3.2. Total Synthesis
Total synthesis is ideal for addressing the clinical supply of paclitaxel. However, the complex structure is a major obstacle to total synthesis of paclitaxel. Paclitaxel possesses a highly oxygenated [6-8-6-4] core with 11 stereocenters. Moreover, the unique bicyclo [5.3.1] undecane ring system, densely aligned oxygen functionalities and four flanking acyl groups all contribute to heightening the challenge of its chemical construction. In 1994, Nicolaou et al. and Holton et al. reported the first total synthesis of paclitaxel, and subsequently, various total synthesis methods were reported [53,54,55]. So far, eleven total syntheses and three formal syntheses, as well as over 60 synthetic model studies of paclitaxel, have been completed by more than 60 research groups worldwide [56,57]. The eight-membered ring synthetic strategy for each total synthesis is summarized in Figure 4, and the starting materials, key steps and total steps for each total synthesis are summarized in Table 3. In the synthesis of paclitaxel (Figure 4), bonding to close the required eight-membered ring usually takes place at the top of the planar structure of the molecule. In particular, the top C9–C10 bond disconnections (Nicolaou, Kuwajima and Takaihashi) and the C10–C11 bond disconnections (Danishefsky, Kishi, Chida and Nakada) are the most used (7 out of 14 syntheses). It is noteworthy that both Holton and Wender used Grob-type fragmentation to construct the A and B rings; Mukaiyama’s total synthetic approach was based on an intramolecular aldol cyclization employing SmI2 to synthesize the eight-membered ring at the C3–C8 site; Baran utilized the type II IMDA reaction to form the A and B rings through the formation of C1–C15 and C13–C14 bonds; and Li and Inoue utilized SmI2–pinacol coupling to form the eight-membered ring through the formation of C1–C2 bonds. These successful methods for the total synthesis of paclitaxel are landmarks in the field of organic chemistry. In general, the pathway of the full chemical synthesis method for paclitaxel is too long and there are too many synthetic steps. Not only are expensive chemical reagents required, but the reaction conditions are also difficult to control and the yield is low (e.g., the overall yield of Li’s 21-step synthetic route was 0.118%), which is not suitable for industrial production. Further efforts are needed to reduce the synthesis steps and improve the yield of paclitaxel total synthesis.
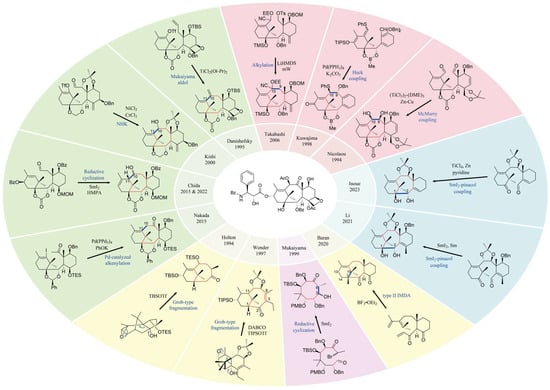
Figure 4.
Summary of the eight-membered ring synthetic strategy of paclitaxel [53,54,55,56,58,59,60,61,62,63,64,65,66,67,68,69,70].

Table 3.
Summary of the total synthetic route of paclitaxel.
3.3. Semi-Synthesis
From the 1960s to the 1980s, paclitaxel could only be isolated from the bark of the yew tree in very low yields. In 1988, Dr. Denis first obtained 10-deacetylbaccation III (10-DAB) from yew needles and used it for the semi-synthesis of paclitaxel with a 53% yield [71]. Subsequently, Prof. Holton and Prof. Potier patented the semi-synthesis of paclitaxel from baccatin III. The US Bristol Myers Squibb (BMS) company received approval from the FDA to produce paclitaxel using Prof. Holton’s patent for the semi-synthesis of paclitaxel from baccatin III and decided to discontinue the extraction of paclitaxel from the bark of the yew tree at the end of 1994.
The chemical semi-synthesis of paclitaxel is the main source of paclitaxel in the current market, accounting for approximately 80% of the market share. Because of the relatively high content of 10-DAB and baccatin III in the needles and twigs of Taxus, studies on the semi-synthesis of paclitaxel mainly focus on these two substances. More than twenty routes for the semi-synthesis of paclitaxel have been reported [72], and three different types of side chains, including linear phenylisoserine, β-lactam tetracyclic and oxazolidine pentacyclic, are primarily utilized to react with 7-triethylsilyl baccatin III (7-TES-baccatin III), which is then deprotected to produce paclitaxel. It should be noted that the side chain C2′-OH is prone to epimerization during chemical synthesis, which not only greatly affects the yield of paclitaxel but also directly pushes up the production cost of paclitaxel. The semi-synthesis method of paclitaxel by BMS [73], common types of paclitaxel semi-synthetic side chains and advantages and disadvantages of different side-chain syntheses are shown in Figure 5. In addition, Borah et al. comprehensively summarized the methods for the synthesis of C-13 chiral side chains in 2007, including asymmetric epoxidation routes, enol–imine condensation, the Diels–Alder reaction, β-lactams and the use of asymmetric catalysts (Figure 6, Figure 7 and Figure 8) [74,75].
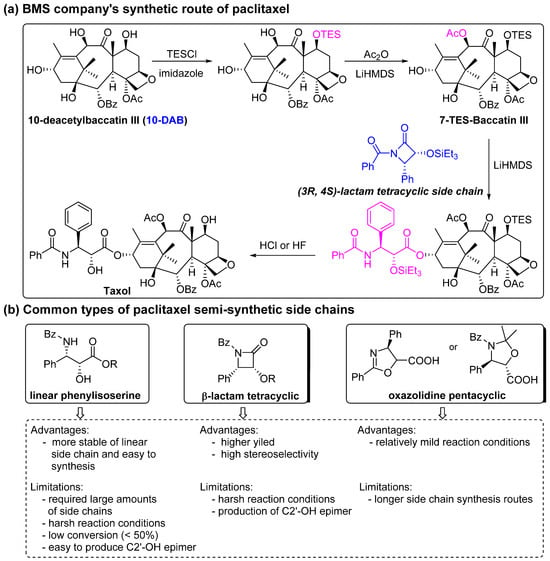
Figure 5.
BMS company’s synthetic route for paclitaxel and common types of paclitaxel semi-synthetic side chains [57,73].
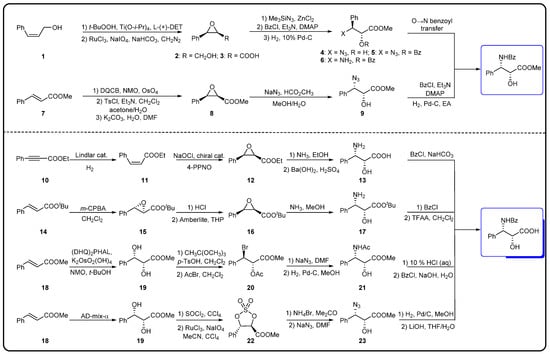
Figure 6.
Synthesis of linear phenylisoserine side chains [74].
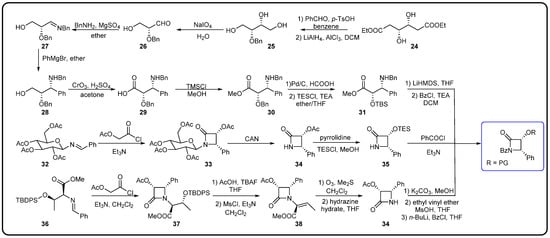
Figure 7.
Synthesis of β-lactam tetracyclic side chains [74].
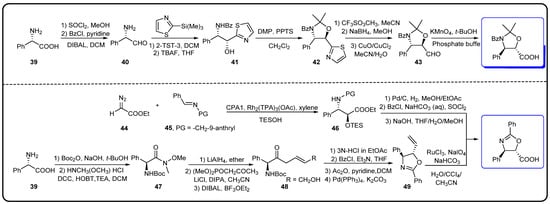
Figure 8.
Synthesis of oxazolidine pentacyclic side chains [74,75].
In addition to 10-DAB and baccatin III, which are the two commonly used semi-synthetic raw materials, other natural precursors such as 10-deacetyl-7-xylosyltaxanes and 10-deacetyl paclitaxel-7-xyloside have also been reported to be converted to paclitaxel. In the cultivated Taxus, 10-deacetyl-7-xylosyltaxanes (a mixture of 10-deacetyl-7-xylosyltaxols A, B and C), which is 10–30 times more abundant than paclitaxel, is usually transformed to 10-DAB or discarded as waste [76]. In 2020, Xue et al. found that these compounds can be converted to paclitaxel through a three-step reaction of redox, acetylation and deacetylation with a purity of 99.52% with 67.6% total yield (Figure 9a). This synthetic process circumvented the use of 10-DAB precursors and expensive chiral side chains, resulting in lower costs, fewer reaction steps and significantly higher yields [77]. 10-Deacetyl paclitaxel-7-xyloside (XDT) was isolated from the bark of Taxus brevifolia and has a structure similar to that of paclitaxel. After hydrolysis of the xylose moiety at the C-7 site, paclitaxel can be obtained by a three-step reaction of TES protection, acetylation and deprotection of TES (Figure 9b) [78]. Compared to 10-DAB or baccatin III precursors for semi-synthetic methods, this precursor contains a C-13 side chain and has a simple synthesis procedure.
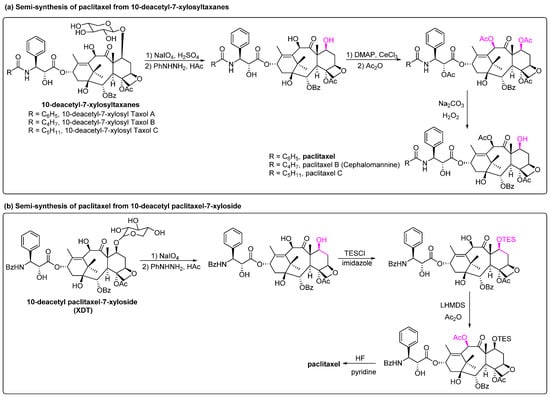
Figure 9.
Semi-synthesis of paclitaxel from 10-deacetyl-7-xylosyltaxanes and 10-deacetyl paclitaxel-7-xyloside [77,78].
3.4. Tissue and Cell Culture
Plant cells are totipotent, and the induction and regulation of paclitaxel synthesis in yew cells is a current research hotspot for paclitaxel drug development. Plant cell culture can completely alleviate the dependence on the Taxus plant and mitigate the effects of survival conditions such as temperature on paclitaxel yield. Moreover, it not only avoids the complex transgenic manipulations but also avoids the introduction of exogenous genes that produce cytotoxicity. Currently, two companies, Phyton Biotech (U.S.) and Samyang Genex (Korea), supply paclitaxel extracted from cultured plant cells, accounting for about 10% of the paclitaxel market share.
Christen et al. first discovered that the cell cultures of Taxus brevifolia could produce paclitaxel in 1989, a finding that was patented two years later [79]. The production of paclitaxel can reach 1–3 mg/L within 2 to 4 weeks. Currently, more than ten Taxus species or variants have been found to produce paclitaxel and paclitaxel-like compounds. However, paclitaxel was found in low and often unstable yields in cultured Taxus cells, which hampered large-scale production. Therefore, various factors affecting cell suspension culture such as Taxus species, culture conditions, phytohormones and inducers have been widely studied [1,80,81,82]. For example, significantly increased amounts of paclitaxel (28 to 110 mg/L) were observed in cell cultures of Taxus species by adding methyl jasmonate [83]. Wang et al. screened three stable, high-yielding cell lines from Taxus cuspidate, and they are promising candidate sources for the large-scale production of paclitaxel [84].
In addition to different Taxus species, other natural sources for paclitaxel production have also been explored, such as Corylus avellana, the hazelnut tree. Although the concentration of paclitaxel in C. avellana is 10 times lower than that in yews [85], C. avellana cells grow faster, and the paclitaxel content of cell suspension cultures of C. avellana as well as the excretion of paclitaxel in the culture medium could be increased by treatments with inducers such as methyl jasmonate. Gallego et al. found that treatment of C. avellana cells with methyl jasmonate (100 μM) and coronarin (1 μM) exciton increased the paclitaxel content in cell suspension cultures 3-fold and 27-fold, respectively [86]. The cell wall of C. palmarum was the most effective fungal inducer of paclitaxel synthesis in C. avellana cell medium. The combination of the cell wall of C. palmarum and methyl-β-cyclodextrin (50 mM) as an inducer increased the total production of paclitaxel in C. avellana cell medium 5.8-fold (402.4 μg/L), of which 78.6% (316.5 μg/L) was secreted into the medium [87].
3.5. Paclitaxel-Producing Endophytic Fungi
Endophytic fungi are present in plants and co-evolve with their host plants. They produce biologically active secondary metabolites that are identical or similar to those made by the host plants. Thus, endophytic fungi of plants can be a new platform for the commercial production of bioactive metabolites. In 1993, the endophytic fungus Taxomyces andreanae from T. brevifolia was discovered by Stierle et al. to produce paclitaxel in vitro [88]. Since then, more and more researchers have been engaged in isolating and characterizing paclitaxel-producing endophytic fungi [89,90,91]. To date, more than 20 genera of endophytic fungi have been identified in Taxus species and non-Taxus species such as sycamore and ginkgo (Table 4) [92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141]. In addition to plants, paclitaxel-producing endophytic fungi can also be isolated from animals. In 2015, Gu et al. first isolated Pestalotiopsis hannanensis from the scalp of Ailuropoda melanoleuca, a giant panda with skin disease, which produced paclitaxel at a yield of 1466.87 μg/L [142]. The production of paclitaxel from endophytic fungi by microbial fermentation is a sustainable way of obtaining paclitaxel. This method is characterized by simple medium formulation, controlled fermentation conditions and mature technology for large-scale production. However, the content of paclitaxel obtained in this way is generally low. Therefore, there is still a long way to go for the industrial production of paclitaxel using endophytic fungi in the future.

Table 4.
Endophytic fungi producing paclitaxel from different hosts and their yields.
In recent years, optimization of the fermentation culture is one of the important ways to increase paclitaxel production by endophytic fungi through complementation with a variety of substances including carbon sources, nitrogen sources, precursors, elicitors and metabolic bypass inhibitors.
Garyali et al. isolated the endophytic fungus Fusarium redolens from Himalayan yew plants and demonstrated its ability to produce paclitaxel. The results showed that sucrose and NH4NO3 were the best carbon and nitrogen sources for paclitaxel production. The yield of paclitaxel synthesized by Fusarium redolens increased from 66 to 198 μg/L with the addition of NH4NO3 (6.25 g/L), MgSO4·7H2O (0.63 g/L) and NaOAc (1.25 g/L) to the medium, which was three times higher than the yield in unoptimized medium [112,143]. Furthermore, addition of early precursors (isopentenyl pyrophosphate (IPP) and geranylgeranylpyrophosphate (GGPP)) of the terpene pathway to cell cultures of the endophytic fungus Paraconiothyrium SSM001 plants stimulated terpene production, with a 3-fold and 5-fold increase in the production of paclitaxel compared to controls [144]. Qiao et al. isolated a strain of A. aculeatinus from Taxus bark and confirmed that the endophytic fungus A. aculeatinus Tax-6 was able to produce paclitaxel in potato dextrose agar liquid medium. Since sodium acetate is an important precursor of paclitaxel, Cu2+ can enhance the activity of oxidase, thereby catalyzing the formation of paclitaxel, and salicylic acid can act as an induction signal. The introduction of Cu2+ (0.1 mg/L), salicylic acid (10 mg/L) and NaOAc (8 g/L) to the medium increased the yield of paclitaxel from 334.92 μg/L to 1337.56 μg/L g/L [145].
In addition, co-culture of B. subtilis and A. flavipes can regulate paclitaxel biosynthesis in A. flavipes by modulating chromatin remodeling, resulting in an approximately 1.6-fold increase in paclitaxel production [146]. When fluconazole (1.0 μg/mL) was co-cultured with A. flavipes, paclitaxel production was increased 5-fold [110,147]. When salicylic acid and P. microspora were co-cultured, the yield of paclitaxel was 625.47 μg/L, which was 45 times higher than that of the control group. This is due to the fact that salicylic acid enhances the lipid peroxidation reaction in P. microspora mycelia, and the production of peroxides stimulates oxidative stress, which induces the activation of 3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMGR) proteins by regulatory proteins and eventually triggers the expression of GGPSG to stimulate the isoprenoid biosynthetic pathway, leading to improved biosynthesis in P. microspora [148].
The pH of the culture medium also has an effect on the yield of paclitaxel. El-Sayed et al. isolated Penicillium chrysogenum strains from the inter-root region of Glycine max, a legume that can produce paclitaxel, and investigated the effect of initial pH on the growth and paclitaxel production of P. chrysogenum. The results showed that paclitaxel production reached a maximum of 200–220 μg/L at a pH of 7–8 at a temperature of 30 °C and an agitation rate of 120 rpm [149]. Yang et al. isolated and characterized Alternaria alternata MF5 to produce paclitaxel. The results showed that the production of paclitaxel started at 12 h (1.193 mg/L, pH = 6.21) and reached a maximum value at 60 h (pH = 4.96), and the production gradually decreased after 60 h. A pH of 4.8–5.2 is the optimal pH for rpm production [150]. However, Abdel-Fatah et al. optimized the yield of paclitaxel production from Aspergillus flavus by CCD design and found that the maximum yield of paclitaxel (302.72 μg/L) was achieved at pH 6.0 when other conditions were the same [151].
Temperature also effects fungal growth and paclitaxel synthesis. For example, the maximum radial growth of Fusarium solani was achieved at 30 °C [152]. The yield of paclitaxel is also influenced by light. Under natural conditions, plant tissues provide protection to endophytic fungi, so the fungi do not need to produce pigments and instead use their metabolic resources to produce paclitaxel against fungal pathogens of the host plant. However, once the fungus is released and exposed to light, the endophytic fungus shifts its metabolic resources from the synthetic paclitaxel pathway to the production of defensive pigments. Thus, paclitaxel production by endophytic fungi increases when plant host conditions are simulated [153,154].
4. Synthetic Biology Studies of Paclitaxel
In recent years, with the successful application of synthetic biology in the synthesis of natural products, synthetic biology research on paclitaxel has also attracted much attention, and the work in this area mainly focuses on the analysis of the biosynthetic pathways of paclitaxel and the construction and optimization of the precursor cell factory of paclitaxel.
4.1. Biosynthetic Pathways
Paclitaxel has a complex molecular structure, and its biosynthetic pathway is equally complicated. Until today, the biosynthetic pathway of paclitaxel is not yet fully understood, as several steps remain undetermined and several enzymes remain unknown. The pathway is postulated to involve 19 steps and is divided into three parts: (1) synthesis of paclitaxel precursor 10-DAB or baccatin III from GGPP, a precursor of diterpene compounds; (2) synthesis of the phenyl-isoserine side chain; (3) acylation linkage of the side chain and the C-13 position of baccatin III to form paclitaxel by hydroxylation and benzoylation (Figure 10) [155].
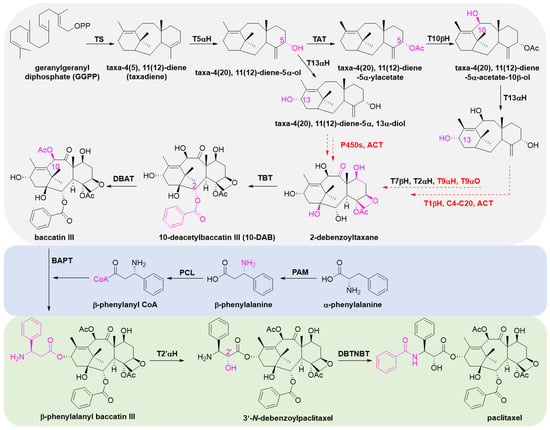
Figure 10.
The biosynthetic pathway of paclitaxel. Enzymes in red are not yet characterized, and steps in red dotted arrows are not yet fully elucidated. Enzyme abbreviations: TS, taxadiene synthase; T5αH, taxane-5α-hydroxylase; TAT, taxane-5α-ol-O-acetyltransferase; T10βH, taxane-10β-hydroxylase; T13αH, taxane-13α-hydroxylase; T1βH, taxane 1β-hydroxylase; T9αH, taxane 9α-hydroxylase; T9αO, taxane 9α-dioxygenase; T2αH, taxane 2α-hydroxylase; T7βH, taxane 7β-hydroxylase; C4-C20, C4-C20 epoxidase; TBT, taxane-2α-O-benzoyl transferase; DBAT, 10-deacetylbaccatin III-10-O-acetyltransferase; PAM, phenylalanine aminomutase; PCL, phenylalanine-CoA ligase; BAPT, C-13 phenylpropanoyl-CoA transferase; T2′αH, taxane 2′α-hydroxylase; DBTNBT, debenzoyl taxol N-benzoyl transferase; ACT, acyl-CoA transferase.
There is a large number and variety of enzymes involved in the first stage, which is key to the formation of paclitaxel. Taxadiene synthase (TS) catalyzes the first and committed step to cyclize GGPPS to taxa-4(5),11(12)-diene (taxadiene). After the formation of the backbone, further hydroxylation at the C1, C2, C5, C7, C9, C10 and C13 sites is followed by acylation, carbonylation, epoxidation and benzoylation, ultimately resulting in the formation of baccatin III, the precursor compound of paclitaxel. Among the enzymes, five CYP450s [taxane 5α-hydroxylase (T5αH), taxane 2α-hydroxylase (T2αH), taxane 7β-hydroxylase (T7βH), taxane 10β-hydroxylase (T10βH) and taxane 13α-hydroxylase (T13αH)] responsible for catalyzing the hydroxylation of C-2, C-5, C-7, C-10 and C-13 sites have been cloned and identified. Enzymes that remain unidentified are the CYP450s taxane 1β-hydroxylase (T1βH), taxane 9α-hydroxylase (T9αH), taxane 9α-oxidase (T9αO) and C4,5 epoxidase.
The synthesis of the C13 side chain is a key factor in ensuring the anticancer activity of paclitaxel [13] and is accomplished through a two-step reaction: α-phenylalanine isomerizes to form β-phenylalanine catalyzed by phenylalanine aminomutase (PAM), and then β-phenylalanine-CoA forms with acetyl coenzyme A in the presence of phenylalanine-CoA ligase (PCL) [156].
Subsequently, β-phenylalanyl-CoA is catalyzed by C-13 phenylpropanoyl-CoA transferase (BAPT) to form β-phenylalanyl baccatin III. The latter is further hydroxylated to form 3′-N-debenzoyltaxol by the action of taxane 2′α-hydroxylase (T2′αH), which was recently isolated from mining the T. baccata transcriptome [157]. Finally, paclitaxel was obtained by the benzoylation of the nitrogen atom at the C3′ site of the side chain under the catalysis of debenzoyl taxol N-benzoyl transferase (DBTNBT).
4.2. Ab Initio Biosynthesis of Paclitaxel by Heterologous Systems
The production of paclitaxel in more amenable, fast-growing, heterologous hosts is a truly sustainable green pathway, as there is no dependence on Taxus species at all. In recent years, significant advances were conducted to develop heterologous systems for paclitaxel biosynthesis, leading to the accumulation of paclitaxel intermediates. Recent achievements in different hosts are summarized in Table 5.

Table 5.
Heterologous production of taxane metabolites in different platforms.
In 2001, Huang et al. realized the first heterologous synthesis of taxadiene by co-expression of 1-deoxy-D-xylulose 5-phosphate synthase (DXS), isopentenyl pyrophosphate isomerase (IDI), geranylgeranyl diphosphate synthase (GGPPS) and TS in a single strain of E. coli with an unoptimized yield of 1.3 mg per liter of cell culture [160]. In 2010, Ajikumar et al. reported a multivariate modular approach to metabolic pathway engineering by which the biosynthetic pathway of paclitaxel was divided into two parts: a natural mevalonate (MEP) pathway leading to IPP and dimethylallyl pyrophosphate (DMAPP), and a downstream terpene synthesis route. By optimally balancing the two blocks, the yield of paclitaxel in engineered E. coli was eventually successfully increased 15,000-fold to ~1 g/L. And with the subsequent introduction of T5αH and the Taxus CYP450 reductase, taxadiene-5α-ol was heterologously synthesized with a yield of 58 mg/L [158]. E. coli is an excellent host, but P450s are hard to express in E. coli due to the lack of an endomembrane system. In 2016, Biggs et al. achieved efficient expression of T5αH in E. coli by optimizing P450 expression, N-terminal modification and reductase chaperone interaction, with an oxygenated taxane yield of 570 ± 45 mg/L [159].
S. cerevisiae is also a common chassis for the heterologous synthesis of natural products. S. cerevisiae produces a functional type II P450 monooxygenase with an intact intracellular membrane system that ensures the co-expression of hydroxylase genes associated with paclitaxel biosynthesis [179]. Therefore, S. cerevisiae is more suitable and feasible for paclitaxel intermediate expression. In 2008, Engels et al. efficiently synthesized taxadiene in S. cerevisiae by establishing an adequate supply of GGPP and significantly increased TS expression through codon optimization. Finally, taxadiene (8.7 mg/L) and geranyl geraniol (33.1 mg/L) were obtained, which was the first demonstration of such enhanced taxadiene levels in S. cerevisiae, indicating that taxadiene levels could be further increased [164]. Ding et al. constructed a pathway for paclitaxel biosynthesis by overexpressing ERG20 and tHMGR genes in S. cerevisiae and introducing TS genes, and paclitaxel yield reached 72.8 mg/L [165]. Zhou et al. reported a co-culture method for the production of oxygenated taxanes using E. coli and S. cerevisiae [161]. E. coli was used for taxadiene production, whereas S. cerevisiae was employed for acetylation and CYP450-oxygenation chemistry. This study combined the strengths of E. coli and S. cerevisiae and demonstrated the feasibility of microbial consortia to rebuild the metabolite pathway.
Plant systems are safer and more economical than microbial systems. In 2019, Li et al. utilized chloroplastic metabolic engineering to express TS, T5αH and cytochrome P450 reductase in Nicotiana benthamiana and successfully obtained taxadiene and taxadiene-5α-ol with yields of 56.6 μg/g and 1.3 μg/g, respectively [174]. This study shows that tobacco is a potential heterologous plant platform for the production of paclitaxel and lays the foundation for further synthesis of oxygenated taxanes. The use of plant systems for the synthesis of plant-derived natural products has theoretical advantages, such as its ability to produce secondary metabolites from sunlight and natural carbon dioxide. However, the superiority of plant systems over microbial systems in terms of culture conditions, difficulty of genetic manipulation and mass cultivation is not prominent. Therefore, the realization of heterologous synthesis of paclitaxel or its key precursors in plant chassis remains a long way to go.
4.3. Semi-Synthesis by Microbial Systems
Paclitaxel analogues are structurally similar to paclitaxel and can be converted to paclitaxel in just a few steps. Among them, XDT is the most abundant paclitaxel analogue in the bark of the yew tree. The amount of XDT (0.4% of dry weight) is much higher than that of paclitaxel. While XDT is not an intermediate in the paclitaxel biosynthetic pathway, it can be transformed to paclitaxel via deglycosylation and acetylation. However, it is often discarded in the process of extracting paclitaxel, resulting in the waste of resources and potential environmental pollution. In 2017, Li et al. improved the catalytic efficiency of 10-deacetylbaccatin III-10-Oacetyltransferase (DBAT) of Taxus by mutagenesis and then combined DBAT with a β-xylosidase to obtain an in vitro one-pot conversion of XDT to paclitaxel yielding 0.64 mg/mL paclitaxel in 50 mL at 15 h (Figure 11) [180]. This approach shows a promising, eco-friendly alternative for paclitaxel production from an abundant analogue. However, the precursors currently used are still of plant origin, and the paclitaxel supply issue is essentially not yet fully solved.
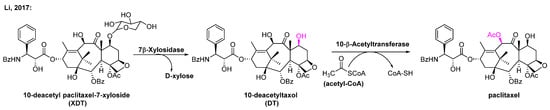
Figure 11.
One-pot reaction system for the bioconversion of XDT to paclitaxel. The system contained a specific 7-β-xlyosidase, the improved 10-β-acetyltransferase, the substrate XDT and the acetyl group donor acetyl-CoA [180].
In summary, paclitaxel has been extensively studied in the past decades both from the biosynthetic and chemosynthetic standpoints. Although these methods continue to evolve, there are still inevitable problems that limit their capabilities and drive up the price of paclitaxel (Table 6). Therefore, the development of efficient methods for the production of paclitaxel is highly desirable.

Table 6.
The production methods of paclitaxel and their advantages and disadvantages.
5. Conclusions
As the tumor incidence rate around the world is increasing, malignant tumors such as lung, breast and ovarian cancers have also become more prevalent, and affected patients are the main users of paclitaxel. On the other hand, paclitaxel has been found to have other medical uses beyond anticancer drugs. For instance, paclitaxel gel, a topical formulation of paclitaxel for the treatment of rheumatoid arthritis, has been developed and marketed in the United States. In addition, paclitaxel has been used as a coating agent for vascular stents in medicinal devices. Newly developed uses of paclitaxel have further boosted the demand for paclitaxel crude drugs on the international market.
Given this information, there has been a worldwide effort to address the availability of paclitaxel over the past several decades. Although chemical semi-synthesis and direct extraction of paclitaxel from the nursery cultivation of Taxus species are the main sources for the clinical supply of paclitaxel, they are still dependent on plant material, and the problem of paclitaxel supply is not inherently fully solved. Total synthesis research showed that it was feasible to prepare paclitaxel in the laboratory and laid the foundation for future approaches to paclitaxel. However, it remained within the realm of academic research. The isolation of paclitaxel from endophytic fungi by microbial fermentation is considered a sustainable method for obtaining paclitaxel, but no breakthroughs have been made. Tissue and cell cultures represent an alternative and environmentally sustainable source of paclitaxel. To increase paclitaxel yield, efforts have been made to optimize culture conditions, screen highly productive cell lines and induce secondary metabolite pathways. Future perspectives should be concentrated on the simultaneous use of empirical and rational approaches.
Synthetic biology methods have been widely used for biosynthetic research on paclitaxel. Currently paclitaxel precursors such as taxadiene have been synthesized heterologously in microbial and plant systems, but further studies are needed to understand the missing pathway enzymes and regulatory mechanisms. With the sequencing of the Taxus genome, as well as progress in enzyme engineering, the biotechnological production of paclitaxel will no longer be a dream in the near future.
Author Contributions
Conceptualization, F.L. and Q.M.; investigation, Y.L.; resources, G.H.; data curation, Q.W. and F.Z.; writing—original draft preparation, S.Z. and T.Y.; writing—review and editing, F.L. and S.Z.; supervision, G.H. and Q.M.; project administration, Q.M.; funding acquisition, Q.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shandong Provincial Natural Science Foundation, China (No. ZR2022QB148) and the National Natural Science Foundation of China (No. 81473104).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are included in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perez-Matas, E.; Hanano, A.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazon, J. Insights into the control of taxane metabolism: Molecular, cellular, and metabolic changes induced by elicitation in Taxus baccata cell suspensions. Front. Plant Sci. 2022, 13, 942433. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Zefirova, O.N.; Nurieva, E.V.; Ryzhov, A.N.; Zyk, N.V.; Zefirov, N.S. Taxol: Synthesis, Bioactive Conformations, and Structure-Activity Relationships in Its Analogs. Russ. J. Org. Chem. 2005, 41, 315–351. [Google Scholar] [CrossRef]
- Expósito, O.; Bonfill, M.; Moyano, E.; Onrubia, M.; Mirjalili, M.H.; Cusidó, R.M.; Palazón, J. Biotechnological production of taxol and related taxoids: Current state and prospects. Anticancer Agents Med. Chem. 2009, 9, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Schiff, P.B.; Fant, J.; Horwitz, S.B. Promotion of microtubule assembly in vitro by taxol. Nature 1979, 277, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Khanna, C.; Rosenberg, M.; Vail, D.M. A Review of Paclitaxel and Novel Formulations Including Those Suitable for Use in Dogs. J. Vet. Intern. Med. 2015, 29, 1006–1012. [Google Scholar] [CrossRef]
- Jeong, I.S.D.; Mo, H.; Nguyen, A.; Chong, E.G.; Tsai, H.H.C.; Moyers, J.; Kim, M.; Lacy, C.; Shah, V.; Lau, E.; et al. Primary chemoradiation with cisplatin versus cetuximab for locally advanced head and neck cancer: A retrospective cohort study. Exp. Hematol. Oncol. 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Oi, H.; Matsuda, T.; Kimura, T.; Morise, M.; Yamano, Y.; Yokoyama, T.; Kataoka, K.; Kondoh, Y. Weekly nanoparticle albumin-bound paclitaxel and paclitaxel for relapsed small cell lung cancer: A retrospective observational study. Medicine 2022, 101, e28863. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Mao, J.-W.; Tan, X.-L. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar] [CrossRef]
- Wheeler, N.C.; Jech, K.; Masters, S.; Brobst, S.W.; Alvarado, A.B.; Hoover, A.J.; Snader, K.M. Effects of Genetic, Epigenetic, and Environmental Factors on Taxol Content in Taxus brevifolia and Related Species. J. Nat. Prod. 1992, 55, 432–440. [Google Scholar] [CrossRef]
- Croteau, R.; Ketchum, R.E.B.; Long, R.M.; Kaspera, R.; Wildung, M.R. Taxol Biosynthesis and Molecular Genetics. Phytochem. Rev. 2006, 5, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Mutanda, I.; Li, J.; Xu, F.; Wang, Y. Recent Advances in Metabolic Engineering, Protein Engineering, and Transcriptome-Guided Insights toward Synthetic Production of Taxol. Front. Bioeng. Biotechnol. 2021, 9, 632269. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Joo, H.-G. Protective effects of fucoidan on the cell death of spleen cells induced by taxol. J. Prev. Vet. Med. 2015, 39, 185–188. (In Chinese) [Google Scholar] [CrossRef]
- Veerabhadrappa, B.; Subramanian, S.; Sudharshan, S.J.; Dyavaiah, M. Evaluating the genetic basiss of anti-cancer property of Taxol in Saccharomyces cerevisiae model. FEMS Microbiol. Lett. 2021, 368, fnab077. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-P.H.; Horwitz, S.B. Taxol®: The First Microtubule Stabilizing Agent. Int. J. Mol. Sci. 2017, 18, 1733. [Google Scholar] [CrossRef]
- Feng, R.; Morine, Y.; Ikemoto, T.; Imura, S.; Iwahashi, S.; Saito, Y.; Shimada, M. Nab-paclitaxel interrupts cancer-stromal interaction through C-X-C motif chemokine 10-mediated interleukin-6 downregulation in vitro. Cancer Sci. 2018, 109, 2509–2519. [Google Scholar] [CrossRef]
- Muraro, E.; Comaro, E.; Talamini, R.; Turchet, E.; Miolo, G.; Scalone, S.; Militello, L.; Lombardi, D.; Spazzapan, S.; Perin, T.; et al. Improved Natural Killer cell activity and retained anti-tumor CD8+ T cell responses contribute to the induction of a pathological complete response in HER2-positive breast cancer patients undergoing neoadjuvant chemotherapy. J. Transl. Med. 2015, 13, 204. [Google Scholar] [CrossRef]
- Wang, S.Q.; Wang, C.; Chang, L.M.; Zhou, K.R.; Wang, J.W.; Ke, Y.; Yang, D.X.; Shi, H.G.; Wang, R.; Shi, X.L.; et al. Geridonin and paclitaxel act synergistically to inhibit the proliferation of gastric cancer cells through ROS-mediated regulation of the PTEN/PI3K/Akt pathway. Oncotarget 2016, 7, 72990–73002. [Google Scholar] [CrossRef]
- Papadopoulos, E.I.; Scorilas, A. Cisplatin and Paclitaxel Alter the Expression Pattern of miR-143/145 and miR-183/96/182 Clusters in T24 Bladder Cancer Cells. Clin. Transl. Sci. 2015, 8, 668–675. [Google Scholar] [CrossRef]
- Xin, Z.; Tong, Z.; Tan, J.; Liu, C. MicroRNA-145-5p aggravates cell apoptosis and oxidative stress in tongue squamous cell carcinoma. Exp. Ther. Med. 2021, 21, 373. [Google Scholar] [CrossRef]
- Lee, Y.; Na, J.; Lee, M.S.; Cha, E.Y.; Sul, J.Y.; Park, J.B.; Lee, J.S. Combination of pristimerin and paclitaxel additively induces autophagy in human breast cancer cells via ERK1/2 regulation. Mol. Med. Rep. 2018, 18, 4281–4288. [Google Scholar] [CrossRef]
- Prencipe, M.; Fitzpatrick, P.; Gorman, S.; Mosetto, M.; Klinger, R.; Furlong, F.; Harrison, M.; O’Connor, D.; Roninson, I.B.; O’Sullivan, J.; et al. Cellular senescence induced by aberrant MAD2 levels impacts on paclitaxel responsiveness in vitro. Br. J. Cancer 2009, 101, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ren, J.J.; Liang, C.; Wang, F.; Li, W.; Li, X.F. GSDME influences sensitivity of breast cancer MCF-7 cells to paclitaxel by regulating cell pyroptosis. Chin. J. Cancer Biother 2019, 26, 146–151. (In Chinese) [Google Scholar]
- Weiner-Gorzel, K.; Dempsey, E.; Milewska, M.; McGoldrick, A.; Toh, V.; Walsh, A.; Lindsay, S.; Gubbins, L.; Cannon, A.; Sharpe, D.; et al. Overexpression of the microRNA miR-433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells. Cancer Med. 2015, 4, 745–758. [Google Scholar] [CrossRef]
- You, J.H.; Lee, J.; Roh, J.-L. PGRMC1-dependent lipophagy promotes ferroptosis in paclitaxel-tolerant persister cancer cells. J. Exp. Clin. Cancer Res. 2021, 40, 350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Li, C.G.; Wang, Y.F.; Xu, L.H.; He, X.H.; Zeng, Q.Z.; Zeng, C.Y.; Mai, F.Y.; Hu, B.; Ouyang, D.Y. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis 2019, 24, 312–325. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Liu, R.; Ning, T.; Yang, H.; Liu, D.; Zhang, Q.; Lin, D.; Ge, S.; Bai, M.; et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 2020, 19, 43. [Google Scholar] [CrossRef]
- Liu, W.C.; Gong, T.; Zhu, P. Advances in exploring alternative Taxol sources. RSC Adv. 2016, 6, 48800–48809. [Google Scholar] [CrossRef]
- Ojima, I.; Lichtenthal, B.; Lee, S.; Wang, C.; Wang, X. Taxane anticancer agents: A patent perspective. Expert Opin. Ther. Pat. 2016, 26, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.X.; Yu, L.J.; Bo, S.J. Paclitaxel: A Landmark Natural Anticancer Drug. World Sci. Technol. Mod. Tradit. Chin. Med. Mater. Medica 2017, 19, 941–949. (In Chinese) [Google Scholar]
- Ni, Z.Y.; Li, Y.; Wang, Y.F.; Wang, S.M.; Dong, M.; Shi, Q.W. Cancer-Fighting Molecules-Taxol and its Analogs. Curr. Org. Chem. 2012, 16, 2038–2052. [Google Scholar] [CrossRef]
- Ren, S.; Wang, Y.; Wang, J.; Gao, D.; Zhang, M.; Ding, N.; Li, Y. Synthesis and biological evaluation of novel larotaxel analogues. Eur. J. Med. Chem. 2018, 156, 692–710. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, S.; Wang, C.; Li, Z.; Ma, Y.; Liu, G. Antagonizing NOD2 Signaling with Conjugates of Paclitaxel and Muramyl Dipeptide Derivatives Sensitizes Paclitaxel Therapy and Significantly Prevents Tumor Metastasis. J. Med. Chem. 2017, 60, 1219–1224. [Google Scholar] [CrossRef]
- Ferrandina, G.; Corrado, G.; Vitrano, G.; Gallotta, V.; Palluzzi, E.; Distefano, M.; Scambia, G. Dose-dense paclitaxel/carboplatin as neo-adjuvant chemotherapy followed by radical surgery in locally advanced cervical cancer: A prospective phase II study. Cancer Chemother. Pharmacol. 2019, 83, 431–438. [Google Scholar] [CrossRef]
- Park, S.-A.; Kim, L.K.; Park, H.M.; Kim, H.J.; Heo, T.-H. Inhibition of GP130/STAT3 and EMT by combined bazedoxifene and paclitaxel treatment in ovarian cancer. Oncol. Rep. 2022, 47, 52. [Google Scholar] [CrossRef]
- Yan, X.; Sheng, X.; Chi, Z.; Si, L.; Cui, C.; Kong, Y.; Tang, B.; Mao, L.; Wang, X.; Lian, B.; et al. Randomized Phase II Study of Bevacizumab in Combination with Carboplatin Plus Paclitaxel in Patients with Previously Untreated Advanced Mucosal Melanoma. J. Clin. Oncol. 2021, 39, 881–889. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, B.; Zhou, Z.; Meng, L.; Sun, Z.; Xu, Y.; Xu, Q.; Yuan, A.; Yu, L.; Qian, H.; et al. Enhancement of radiotherapy efficacy by pleiotropic liposomes encapsulated paclitaxel and perfluorotributylamine. Drug Deliv. 2017, 24, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.A.R.; Hassan, A.A.; Ibrahim, N.Y.; Toeama, A.A.M. 217P—Concurrent paclitaxel and radiotherapy for node positive breast cancer. Ann. Oncol. 2016, 27, vi65. [Google Scholar] [CrossRef]
- Hussain, T.; Paranthaman, S.; Rizvi, S.M.; Moin, A.; Gowda, D.V.; Subaiea, G.M.; Ansari, M.; Alanazi, A.S. Fabrication and Characterization of Paclitaxel and Resveratrol Loaded Soluplus Polymeric Nanoparticles for Improved BBB Penetration for Glioma Management. Polymers 2021, 13, 3210. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, M.; Qu, Z.; Zhou, J.; Zhang, Q. Resveratrol enhances anticancer effects of paclitaxel in HepG2 human liver cancer cells. BMC Complement. Altern. Med. 2017, 17, 477–488. [Google Scholar] [CrossRef]
- Calaf, G.M.; Ponce-Cusi, R.; Carrión, F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol. Rep. 2018, 40, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Pu, X.; Zhao, L. Preclinical studies for the combination of paclitaxel and curcumin in cancer therapy (Review). Oncol. Rep. 2017, 37, 3159–3166. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Molonia, M.S.; Bashllari, R.; Muscarà, C.; Ferlazzo, G.; Costa, G.; Saija, A.; Cimino, F.; Speciale, A. Curcumin potentiates the antitumor activity of Paclitaxel in rat glioma C6 cells. Phytomedicine 2019, 55, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, Z.; Zhu, T.; Lu, W.; Fu, Y. Curcumin enhances the anti-cancer efficacy of paclitaxel in ovarian cancer by regulating the miR-9-5p/BRCA1 axis. Front. Pharmacol. 2022, 13, 1014933. [Google Scholar] [CrossRef]
- Boztas, A.O.; Karakuzu, O.; Galante, G.; Ugur, Z.; Kocabas, F.; Altuntas, C.Z.; Yazaydin, A.O. Synergistic interaction of paclitaxel and curcumin with cyclodextrin polymer complexation in human cancer cells. Mol. Pharm. 2013, 10, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, F.; Kikuchi, Y.; Ohira, T.; Yatagai, M. Accelerated Solvent Extraction of Paclitaxel and Related Compounds from the Bark of Taxus cuspidata. J. Nat. Prod. 1999, 62, 244–247. [Google Scholar] [CrossRef]
- McPartland, T.J.; Patil, R.A.; Malone, M.F.; Roberts, S.C. Liquid–liquid extraction for recovery of paclitaxel from plant cell culture: Solvent evaluation and use of extractants for partitioning and selectivity. Biotechnol. Prog. 2012, 28, 990–997. [Google Scholar] [CrossRef]
- Tan, Z.; Li, Q.; Wang, C.; Zhou, W.; Yang, Y.; Wang, H.; Yi, Y.; Li, F. Ultrasonic Assisted Extraction of Paclitaxel from Taxus × media Using Ionic Liquids as Adjuvants: Optimization of the Process by Response Surface Methodology. Molecules 2017, 22, 1483. [Google Scholar] [CrossRef]
- Min, H.-S.; Kim, J.-H. Process synthesis and optimization for the isolation and purification of paclitaxel from Taxus chinensis. Korean J. Chem. Eng. 2022, 39, 3389–3398. [Google Scholar] [CrossRef]
- Shin, E.-J.; Kim, J.-H. Ultrasound- and Negative Pressure-assisted Fractional Precipitation of Paclitaxel from Taxus chinensis. Biotechnol. Bioproc. Eng. 2023, 28, 336–344. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Kim, J.-H. Effect of Gas Bubbles on the Recovery Efficiency of Paclitaxel from Biomass of Taxus chinensis in Ultrasonic Extraction. Biotechnol. Bioproc. Eng. 2023, 28, 545–553. [Google Scholar] [CrossRef]
- Min, H.-S.; Kim, H.-G.; Kim, J.-H. Ultrasound-negative pressure cavitation extraction of paclitaxel from Taxus chinensis. Korean J. Chem. Eng. 2022, 39, 398–407. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Nantermet, P.G.; Ueno, H.; Guy, R.K.; Couladouros, E.A.; Sorensen, E.J. Total Synthesis of Taxol. 1. Retrosynthesis, Degradation, and Reconstitution. J. Am. Chem. Soc. 1995, 117, 624–633. [Google Scholar] [CrossRef]
- Holton, R.A.; Somoza, C.; Kim, H.B.; Liang, F.; Biediger, R.J.; Boatman, P.D.; Shindo, M.; Smith, C.C.; Kim, S. First total synthesis of taxol. 1. Functionalization of the B ring. J. Am. Chem. Soc. 1994, 116, 1597–1598. [Google Scholar] [CrossRef]
- Holton, R.A.; Kim, H.B.; Somoza, C.; Liang, F.; Biediger, R.J.; Boatman, P.D.; Shindo, M.; Smith, C.C.; Kim, S. First total synthesis of taxol. 2. Completion of the C and D rings. J. Am. Chem. Soc. 1994, 116, 1599–1600. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, J.; Li, W.-D.Z. Diverse strategic approaches en route to Taxol total synthesis. Chin. Chem. Lett. 2022, 33, 4957–4968. [Google Scholar] [CrossRef]
- Min, L.; Han, J.-C.; Zhang, W.; Gu, C.-C.; Zou, Y.-P.; Li, C.-C. Strategies and Lessons Learned from Total Synthesis of Taxol. Chem. Rev. 2023, 123, 4934–4971. [Google Scholar] [CrossRef] [PubMed]
- Danishefsky, S.J.; Masters, J.J.; Young, W.B.; Link, J.T.; Snyder, L.B.; Magee, T.V.; Jung, D.K.; Isaacs, R.C.A.; Bornmann, W.G.; Alaimo, C.A.; et al. Total Synthesis of Baccatin III and Taxol. J. Am. Chem. Soc. 1996, 118, 2843–2859. [Google Scholar] [CrossRef]
- Wender, P.A.; Badham, N.F.; Conway, S.P.; Floreancig, P.E.; Glass, T.E.; Gränicher, C.; Houze, J.B.; Jänichen, J.; Lee, D.; Marquess, D.G.; et al. The Pinene Path to Taxanes. 5. Stereocontrolled Synthesis of a Versatile Taxane Precursor. J. Am. Chem. Soc. 1997, 119, 2755–2756. [Google Scholar] [CrossRef]
- Wender, P.A.; Badham, N.F.; Conway, S.P.; Floreancig, P.E.; Glass, T.E.; Houze, J.B.; Krauss, N.E.; Lee, D.; Marquess, D.G.; McGrane, P.L.; et al. The Pinene Path to Taxanes. 6. A Concise Stereocontrolled Synthesis of Taxol. J. Am. Chem. Soc. 1997, 119, 2757–2758. [Google Scholar] [CrossRef]
- Kusama, H.; Hara, R.; Kawahara, S.; Nishimori, T.; Kashima, H.; Nakamura, N.; Morihira, K.; Kuwajima, I. Enantioselective Total Synthesis of (−)-Taxol. J. Am. Chem. Soc. 2000, 122, 3811–3820. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Shiina, I.; Iwadare, H.; Saitoh, M.; Nishimura, T.; Ohkawa, N.; Sakoh, H.; Nishimura, K.; Tani, Y.-i.; Hasegawa, M.; et al. Asymmetric Total Synthesis of Taxol\R. Chem. Eur. J. 1999, 5, 121–161. [Google Scholar] [CrossRef]
- Doi, T.; Fuse, S.; Miyamoto, S.; Nakai, K.; Sasuga, D.; Takahashi, T. A Formal Total Synthesis of Taxol Aided by an Automated Synthesizer. Chem. Asian J. 2006, 1, 370–383. [Google Scholar] [CrossRef]
- Hirai, S.; Utsugi, M.; Iwamoto, M.; Nakada, M. Formal Total Synthesis of (−)-Taxol through Pd-Catalyzed Eight-Membered Carbocyclic Ring Formation. Chem. Eur. J. 2015, 21, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, K.; Tanaka, Y.; Sato, A.C.; Kodama, K.; Yamazaki, H.; Ishimoto, T.; Nozaki, Y.; Iwaki, Y.M.; Yuki, Y.; Umei, K.; et al. Synthesis of Paclitaxel. 1. Synthesis of the ABC Ring of Paclitaxel by SmI2-Mediated Cyclization. Org. Lett. 2015, 17, 2570–2573. [Google Scholar] [CrossRef]
- Fukaya, K.; Kodama, K.; Tanaka, Y.; Yamazaki, H.; Sugai, T.; Yamaguchi, Y.; Watanabe, A.; Oishi, T.; Sato, T.; Chida, N. Synthesis of Paclitaxel. 2. Construction of the ABCD Ring and Formal Synthesis. Org. Lett. 2015, 17, 2574–2577. [Google Scholar] [CrossRef]
- Kanda, Y.; Nakamura, H.; Umemiya, S.; Puthukanoori, R.K.; Murthy Appala, V.R.; Gaddamanugu, G.K.; Paraselli, B.R.; Baran, P.S. Two-Phase Synthesis of Taxol. J. Am. Chem. Soc. 2020, 142, 10526–10533. [Google Scholar] [CrossRef]
- Hu, Y.J.; Gu, C.C.; Wang, X.F.; Min, L.; Li, C.C. Asymmetric Total Synthesis of Taxol. J. Am. Chem. Soc. 2021, 143, 17862–17870. [Google Scholar] [CrossRef]
- Iiyama, S.; Fukaya, K.; Yamaguchi, Y.; Watanabe, A.; Yamamoto, H.; Mochizuki, S.; Saio, R.; Noguchi, T.; Oishi, T.; Sato, T.; et al. Total Synthesis of Paclitaxel. Org. Lett. 2022, 24, 202–206. [Google Scholar] [CrossRef]
- Imamura, Y.; Takaoka, K.; Komori, Y.; Nagatomo, M.; Inoue, M. Total Synthesis of Taxol Enabled by Inter- and Intramolecular Radical Coupling Reactions. Angew. Chem. Int. Ed. 2023, 62, e202219114. [Google Scholar] [CrossRef]
- Denis, J.N.; Greene, A.E.; Guenard, D.; Gueritte-Voegelein, F.; Mangatal, L.; Potier, P. Highly efficient, practical approach to natural taxol. J. Am. Chem. Soc. 1988, 110, 5917–5919. [Google Scholar] [CrossRef]
- Deng, X.M.; Cao, H.; Shen, H.W.; Zhong, G.X.; Xia, C.N. Overview of the semisynthesis of paclitaxel and its derivatives. Zhejiang Chem. 2018, 49, 1–8. (In Chinese) [Google Scholar]
- Yan, J.-Q. Chiral synthesis of the Taxol side chain and semi synthesis of Taxol. Fine Spec. Chem. 2005, 13, 1–6+11. (In Chinese) [Google Scholar]
- Borah, C.J.; Boruwa, J.; Barua, C.N. Synthesis of the C-13 Side-Chain of Taxol. Curr. Org. Synth. 2007, 4, 175–199. [Google Scholar] [CrossRef]
- Shi, T.; Li, X.; Li, Y.; Fu, X.; Wu, L.; Wu, D.; Hu, W. An asymmetric catalytic multi-component reaction enabled the green synthesis of isoserine derivatives and semi-synthesis of paclitaxel. Green Synth. Catal. 2023, 4, 58–63. [Google Scholar] [CrossRef]
- Zhang, H.; Gan, H.; Wu, Z.; Meng, Z.; Dou, G. Study on biotransformation of 7-xylosyl-10deacetyltaxol. Chin. J. New Drugs 2013, 22, 1029–1033. (In Chinese) [Google Scholar]
- Xue, B.; Zhao, J.; Fan, Y.; Chen, S.; Li, W.; Chen, J.; Li, Z.; Wang, H.; Kong, H. Synthesis of Taxol and Docetaxel by Using 10-Deacetyl-7-xylosyltaxanes. Chem. Biodivers. 2020, 17, e1900631. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V. Semi-synthesis of paclitaxel from naturally occurring glycosidic precursors. J. Heterocycl. Chem. 1997, 34, 675–680. [Google Scholar] [CrossRef]
- Christen, A.A.; Gibson, D.M.; Bland, J. Production of Taxol or Taxol-Like Compounds in Cell Culture. U.S. Patent 5,019,504, 28 May 1991. [Google Scholar]
- Bamneshin, M.; Mirjalili, M.H.; Naghavi, M.R.; Cusido, R.M.; Palazón, J. Gene expression pattern and taxane biosynthesis in a cell suspension culture of Taxus baccata L. subjected to light and a phenylalanine ammonia lyase (PAL) inhibitor. J. Photochem. Photobiol. B Biol. 2022, 234, 112532. [Google Scholar] [CrossRef] [PubMed]
- Golinejad, S.; Mirjalili, M.H.; Rezadoost, H.; Ghorbanpour, M. Molecular, biochemical, and metabolic changes induced by gold nanoparticles in Taxus baccata L. cell culture. Ind. Crops Prod. 2023, 192, 115988. [Google Scholar] [CrossRef]
- Hazrati, R.; Zare, N.; Asghari, R.; Sheikhzadeh, P.; Johari-Ahar, M. Biologically synthesized CuO nanoparticles induce physiological, metabolic, and molecular changes in the hazel cell cultures. Appl. Microbiol. Biotechnol. 2022, 106, 6017–6031. [Google Scholar] [CrossRef] [PubMed]
- Yukimune, Y.; Tabata, H.; Higashi, Y.; Hara, Y. Methyl jasmonate-induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat. Biotechnol. 1996, 14, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, H.; Li, T.; Li, C.; Zhou, Y.; Zhong, X. The selection and stability analysis of stable and high Taxol-producing cell lines from Taxus cuspidata. J. For. Res. 2018, 29, 65–71. [Google Scholar] [CrossRef]
- Hoffman, A.; Khan, W.; Worapong, J.; Strobel, G.; Griffin, D.; Arbogast, B.; Barofsky, B.; Boone, D.; Ning, R.B.; Zheng, P.; et al. Bioprospecting for taxol in angiosperm plant extracts: Using high performance liquid chromatography–thermospray mass spectrometry to detect the anticancer agent and its related metabolites in filbert trees. Spectroscopy 1998, 13, 22–32. [Google Scholar]
- Gallego, A.; Imseng, N.; Bonfill, M.; Cusido, R.M.; Palazon, J.; Eibl, R.; Moyano, E. Development of a hazel cell culture-based paclitaxel and baccatin III production process on a benchtop scale. J. Biotechnol. 2015, 195, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, S.; Moieni, A.; Safaie, N.; Sabet, M.S.; Salehi, M. Fungal Cell Wall and Methyl-β–Cyclodextrin Synergistically Enhance Paclitaxel Biosynthesis and Secretion in Corylus avellana Cell Suspension Culture. Sci. Rep. 2020, 10, 5427. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; El-Sayed, M.T.; Rady, A.; Zein, N.; Enan, G.; Shindia, A.; El-Hefnawy, S.; Sitohy, M.; Sitohy, B. Exploiting the Biosynthetic Potency of Taxol from Fungal Endophytes of Conifers Plants; Genome Mining and Metabolic Manipulation. Molecules 2020, 25, 3000. [Google Scholar] [CrossRef]
- Subban, K.; Kempken, F. Insights into Taxol® biosynthesis by endophytic fungi. Appl. Microbiol. Biotechnol. 2023, 107, 6151–6162. [Google Scholar] [CrossRef]
- Shankar Naik, B. Developments in taxol production through endophytic fungal biotechnology: A review. Orient. Pharm. Exp. Med. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Strobel, G.; Yang, X.; Sears, J.; Kramer, R.; Sidhu, R.S.; Hess, W.M. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology 1996, 142, 435–440. [Google Scholar] [CrossRef]
- Tian, R.P.; Yang, Q.; Zhou, G.L. Taxonomic study on a taxol producing fungus isolated from the bark of Taxus chinensis var. mairei. J. Wuhan Bot. Res. 2006, 24, 541–545. [Google Scholar]
- Strobel, G.A.; Hess, W.M.; Ford, E.; Sidhu, R.S.; Yang, X. Taxol from fungal endophytes and the issue of biodiversity. J. Ind. Microbiol. 1996, 17, 417–423. [Google Scholar] [CrossRef]
- Fu, Y.; Li, X.; Yuan, X.; Zhang, Z.; Wei, W.; Xu, C.; Song, J.; Gu, C. Alternaria alternata F3, a Novel Taxol-Producing Endophytic Fungus Isolated from the Fruits of Taxus cuspidata: Isolation, Characterization, Taxol Yield Improvement, and Antitumor Activity. Appl. Biochem. Biotechnol. 2023, 195. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Zhang, Z.; Wang, Y.; Su, Y.; Zhang, R. Screening endophytic fungus to produce taxol from Taxus Yunnanensis. Biotechnol. 2003, 13, 10–11. (In Chinese) [Google Scholar]
- Zhang, P.; Zhou, P.-P.; Yu, L.-J. An Endophytic Taxol-Producing Fungus from Taxus media, Cladosporium cladosporioides MD2. Curr. Microbiol. 2009, 59, 227–232. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, P.-P.; Yu, L.-J. An endophytic taxol-producing fungus from Taxus × media, Aspergillus candidus MD3. FEBS Microbiol. Lett. 2009, 293, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, B.; Thakur, V.; Thakur, A.; Thakur, N.; Pandey, D.; Chand, D. Hyper-production of taxol from Aspergillus fumigatus, an endophytic fungus isolated from Taxus sp. of the Northern Himalayan region. Biotechnol. Rep. 2019, 24, e00395. [Google Scholar] [CrossRef]
- Zhao, K.; Ping, W.X.; Li, Q. Aspergillus niger var. taxi, a new species variant of taxol-producing fungus isolated from Taxus cuspidata in China. J. Appl. Microbiol. 2009, 107, 1202–1207. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, J.J.; Zang, G.G. Screening of taxol-producing endophytic fungi and regulation of fermentation conditions. J. Cent. South Univ. Nat. Sci. 2004, 35, 65–69. [Google Scholar]
- Zhou, X.; Zhu, H.; Liu, L.; Lin, J.; Tang, K. A review: Recent advances and future prospects of taxol-producing endophytic fungi. Appl. Microbiol. Biotechnol. 2010, 86, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hu, C.H. Separation and identification of a new Taxus chinensis var. mairei endophytic fungus (Bionectria sp.) and the activity of its metabolites. J. Southwest Univ. Nat. Sci. Ed. 2007, 29, 131–135. [Google Scholar]
- Venkatachalam, R.; Subban, K.; Paul, M.J. Taxol from Botryodiplodia theobromae (BT 115)-an endophytic fungus of Taxus baccata. J. Biotechnol. 2008, 136, 189–190. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, L.; Jin, Y.; Wei, H.; Ping, W.; Zhou, D. Isolation of a taxol-producing endophytic fungus and inhibiting effect of the fungus metabolites on HeLa cell. Mycosystema 2008, 27, 735–744. (In Chinese) [Google Scholar]
- Hu, K.; Tan, F.; Tang, K.; Zhu, S.; Wang, W. Isolation and screening of endophytic fungi synthesizing taxol from Taxus chinensis var. mairei. J. Southwest China Normal Univ. Nat. Sci. Ed. 2006, 31, 134–137. [Google Scholar]
- Wang, Y.; Ma, X.Y.; Ping, W.; Zhou, D. A new species of Botrytis. J. Fungal. Res. 2006, 4, 62–64. (In Chinese) [Google Scholar]
- Wang, Y.; Tang, K. A new endophytic taxol- and baccatin III-producing fungus isolated from Taxus chinensis var. mairei. Afr. J. Biotechnol. 2011, 10, 16379–16386. [Google Scholar]
- Li, C.T.; Li, Y.; Wang, Q.; Sung, C. Taxol production by Fusarium Arthrosporioides isolated from yew, Taxus cuspidata. J. Med. Biochem. 2008, 27, 454–458. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Ali, D.M.I.; Yassin, M.A.; Zayed, R.A.; Ali, G.S. Sterol inhibitor “Fluconazole” enhance the Taxol yield and molecular expression of its encoding genes cluster from Aspergillus flavipes. Process Biochem. 2019, 76, 55–67. [Google Scholar] [CrossRef]
- Dai, W.L.; Tao, W.Y. Preliminary study on fermentation conditions of taxol-producing endophytic fungus. Chem. Ind. Eng. Prog. 2008, 27, 883–886. (In Chinese) [Google Scholar]
- Garyali, S.; Kumar, A.; Reddy, M.S. Taxol production by an endophytic fungus Fusarium redolens, isolated from Himalayan Yew. J. Microbiol. Biotechnol. 2013, 23, 1372–1380. [Google Scholar] [CrossRef]
- Deng, B.W.; Liu, K.H.; Chen, W.Q.; Ding, X.W.; Xie, X.C. Fusarium solani, Tax-3, a new endophytic taxol-producing fungus from Taxus chinensis. World J. Microbiol. Biotechnol. 2009, 25, 139–143. [Google Scholar] [CrossRef]
- Lu, L.Y.; Qin, Z.; Xu, J.K.; Li, X.S. Production of taxol by an endophytic fungus isolated from Taxus chinensis var. Mairei. Chin. Med. Biotechnol. 2010, 5, 202–207. (In Chinese) [Google Scholar]
- Cheng, L.; Ma, Q.M.; Tao, G.J.; Tao, W.Y.; Wang, R.M.; Yang, J.; Guo, X.L. Systematic identification of a paclitaxel-producing endofungus. Ind. Microbiol. 2007, 37, 23–30. (In Chinese) [Google Scholar]
- Xiong, Z.Q.; Yang, Y.Y.; Zhao, N. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglo jap yew, Taxus × media. BMC Microbiol. 2013, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.Y.; Zhang, P.; Liu, B.; Xu, M.; Zhou, P.P.; Yu, L.J. Isolation and identification of a taxol-producing endophytic fungus Z58. Chin. J. Biochem. Mol. Biol. 2012, 28, 1141–1146. (In Chinese) [Google Scholar]
- Liu, W.H.; Yao, B.; Zhu, S.Q. Advances in studies on biosynthetic pathway of taxol precursor and its correlative biotechnology. Chin. Tradit. Herb Drugs 2009, 40, 1327–1331. [Google Scholar]
- Miao, Z.; Wang, Y.; Yu, X.; Guo, B.; Tang, K. New endophytic taxane production fungus from T. chinensis. Appl. Biochem. Microbiol. 2009, 45, 81–86. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, B.; Xu, M.; Ao, M.Z.; Fu, C.H.; Yu, L.J.; Gu, Y.C. Isolation and identification of a taxol-producing endophytic fungus O60B1. Hubei Agric. Sci. 2012, 51, 5315–5317. (In Chinese) [Google Scholar]
- Zhou, D.; Zhao, K.; Ping, W. Study on the mutagenesis of protoplasts from taxol-producing fungus Nodulisporium sylviforme. J. Am. Sci. 2005, 1, 55–62. [Google Scholar]
- Zhao, K.; Sun, L.X.; Wang, X. Screening of high taxol producing fungi by mutagenesis and construction of subtracted cDNA library by Suppression substracted hybridization for differentially expressed genes. Acta. Microbiol. Sin. 2011, 51, 923–933. [Google Scholar]
- Guo, B.H.; Wang, Y.C.; Zhou, X.W. An endophytic taxol-producing fungus BT2 isolated from Taxus chinensis var. mairei. Afr. J. Biotechnol. 2006, 5, 875–877. [Google Scholar]
- Zhang, P.; Liu, B.; Zhou, B.B.; Wang, C.L.; Jiang, C.; Yu, L.J. Isolation and identification of a taxol-producing endophytic fungus YN6. Chin. J. Biochem. Mol. Biol. 2011, 27, 961–967. (In Chinese) [Google Scholar]
- Gangadevi, V.; Muthumary, J. Taxol production by Pestalotiopsis terminaliae, an endophytic fungus of Terminalia arjuna (arjun tree). Biotechnol. Appl. Biochem. 2009, 52, 9–15. [Google Scholar] [CrossRef]
- Kumaran, R.S.; Hur, B.K. Screening of species of the endophytic fungus Phomopsis for the production of the anticancer drug taxol. Biotechnol. Appl. Biochem. 2009, 54, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Q.; Zhang, Z.J.; Zhang, P.; Wang, C.L.; Liu, B.; Liu, T.T.; Fu, C.H.; Yu, L.J. Isolation and identification of a taxol-producing endophytic fungus identified from Taxus media. Agric. Sci. Technol. 2010, 11, 38–40+68. (In Chinese) [Google Scholar]
- Mirjalili, M.H.; Farzaneh, M.; Bonfill, M.; Rezadoost, H.; Ghassempour, A. Isolation and characterization of Stemphylium sedicola SBU-16 as a new endophytic taxol-producing fungus from Taxus baccata grown in Iran. FEMS Microbiol. Lett. 2012, 328, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Staniek, A.; Woerdenbag, H.J.; Kayser, O. Taxomyces andreanae: A presumed paclitaxel producer demystified. Planta. Med. 2009, 75, 1561–1566. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, G.L.; Lu, H.Y. Taxol from Tubercularia sp. Strain TF5, an endophytic fungus of Taxus mairei. FEMS Microbiol. Lett. 2000, 193, 249–253. [Google Scholar] [CrossRef]
- Bi, J.; Ji, Y.; Pan, J.; Yu, Y.; Chen, H.; Zhu, X. A new taxol-producing fungus (Pestalotiopsis malicola) and evidence for taxol as a transient product in the culture. Afr. J. Biotechnol. 2011, 10, 6647–6654. [Google Scholar]
- Strobel, G.A.; Hess, W.M.; Li, J.Y.; Ford, E.; Sears, J.; Sidhu, R.S.; Summerell, B. Pestalotiopsis guepinii, a taxol-producing endophyte of the Wollemi pine, Wollemia nobilis. Aust. J. Bot. 1997, 45, 1073–1082. [Google Scholar] [CrossRef]
- Li, J.Y.; Strobel, G.; Sidhu, R. Endophytic taxol-producing fungi from bald cypress, Taxodium distichum. Microbiology 1996, 142, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, H.; Barrero, R.A.; Zhang, B.; Sun, G.; Wilson, I.W.; Xie, F.; Walker, K.D.; Parks, J.W.; Bruce, R. Genome sequencing and analysis of the paclitaxel-producing endophytic fungus Penicillium aurantiogriseum NRRL 62431. BMC Genom. 2014, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, R.S.; Choi, Y.K.; Lee, S.; Jeon, H.J.; Jung, H.; Kim, H.J. Isolation of taxol, an anticancer drug produced by the endophytic fungus, Phoma betae. Afr. J. Biotechnol. 2012, 11, 950–960. [Google Scholar]
- Kumaran, R.S.; Muthumary, J.; Hur, B.K. Production of taxol from Phyllosticta spinarum, an endophytic fungus of Cupressus sp. Eng. Life Sci. 2008, 8, 438–446. [Google Scholar] [CrossRef]
- Sun, D.F.; Ran, X.Q.; Wang, J.F. Isolation and identifcation of a taxol-producing endophytic fungus from Podocarpus. Acta. Microbiol. Sin. 2008, 48, 589–595. [Google Scholar]
- Gangadevi, V.; Murugan, M.; Muthumary, J. Taxol determination from Pestalotiopsis pauciseta, a fungal endophyte of a medicinal plant. Chin. J. Biotechnol. 2008, 24, 1433–1438. [Google Scholar] [CrossRef]
- Gangadevi, V.; Muthumary, J. A novel endophytic taxolproducing fungus Chaetomella raphigera isolated from a medicinal plant, Terminalia arjuna. Appl. Biochem. Biotechnol. 2009, 158, 675–684. [Google Scholar] [CrossRef]
- Kumaran, R.S.; Muthumary, J.; Hur, B.K. Isolation and identifcation of an anticancer drug, taxol from Phyllosticta tabernaemontanae, a leaf spot fungus of an angiosperm Wrightia tinctoria. J. Microbiol. 2009, 47, 40–49. [Google Scholar] [CrossRef]
- Gokul Raj, K.; Manikandan, R.; Arulvasu, C.; Pandi, M. Anti-proliferative effect of fungal taxol extracted from Cladosporium oxysporum against human pathogenic bacteria and human colon cancer cell line HCT 15. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 667–674. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Ma, X.; Wang, C.; Yue, G.; Zhang, Y.; Zhang, Y.; Li, S.; Ling, S.; Liu, X.; et al. Greater Taxol Yield of Fungus Pestalotiopsis hainanensis from Dermatitic Scurf of the Giant Panda (Ailuropoda melanoleuca). Appl. Biochem. Biotechnol. 2015, 175, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Garyali, S.; Kumar, A.; Reddy, M.S. Enhancement of taxol production from endophytic fungus Fusarium redolens. Biotechnol. Bioproc. E. 2014, 19, 908–915. [Google Scholar] [CrossRef]
- Soliman, S.S.M.; Mosa, K.A.; El-Keblawy, A.A.; Husseiny, M.I. Exogenous and endogenous increase in fungal GGPP increased fungal Taxol production. Appl. Microbiol. Biotechnol. 2017, 101, 7523–7533. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Ling, F.; Yu, L.; Huang, Y.; Wang, T. Enhancing taxol production in a novel endophytic fungus, Aspergillus aculeatinus Tax-6, isolated from Taxus chinensis var. mairei. Fungal Biol. 2017, 121, 1037–1044. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Shindia, A.A.; AbouZeid, A.; Koura, A.; Hassanein, S.E.; Ahmed, R.M. Triggering the biosynthetic machinery of Taxol by Aspergillus flavipes via cocultivation with Bacillus subtilis: Proteomic analyses emphasize the chromatin remodeling upon fungal-bacterial interaction. Environ. Sci. Pollut. Res. 2021, 28, 39866–39881. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; Fathalla, M.; Yassin, M.A.; Zein, N.; Morsy, S.; Sitohy, M.; Sitohy, B. Conjugation of Aspergillus flavipes Taxol with Porphyrin Increases the Anticancer Activity of Taxol and Ameliorates Its Cytotoxic Effects. Molecules 2020, 25, 263. [Google Scholar] [CrossRef]
- Subban, K.; Subramani, R.; Srinivasan, V.P.M.; Johnpaul, M.; Chelliah, J. Salicylic acid as an effective elicitor for improved taxol production in endophytic fungus Pestalotiopsis microspora. PLoS ONE 2019, 14, e0212736. [Google Scholar] [CrossRef]
- El-Sayed, A.; Enan, G.; Al-Mohammadi, A.-R.; Moustafa, A.H.; El-Gazzar, N. Detection, Purification and Elucidation of Chemical Structure and Antiproliferative Activity of Taxol Produced by Penicillium chrysogenum. Molecules 2020, 25, 4822. [Google Scholar] [CrossRef]
- Yang, N.; Pan, X.W.; Chen, G.J.; Sarsaiya, S.; Yu, J.; Fan, X.K.; Jin, L.L.; Zhang, B.H.; Shi, J.; Chen, J.S. Fermentation Engineering for Enhanced Paclitaxel Production by Taxus Media Endophytic Fungus MF-5 (Alternaria sp.). J. Biobased Mater. Biol. 2018, 12, 545–550. [Google Scholar] [CrossRef]
- Abdel-Fatah, S.S.; El-Sherbiny, G.M.; Khalaf, M.; Baz, A.F.E.; El-Sayed, A.S.A.; El-Batal, A.I. Boosting the Anticancer Activity of Aspergillus flavus “endophyte of Jojoba” Taxol via Conjugation with Gold Nanoparticles Mediated by γ-Irradiation. Appl. Biochem. Biotechnol. 2022, 194, 3558–3581. [Google Scholar] [CrossRef]
- Chakravarthi, B.V.S.K.; Singh, S.; Kamalraj, S.; Gupta, V.K.; Jayabaskaran, C. Evaluation of spore inoculum and confirmation of pathway genetic blueprint of T13αH and DBAT from a Taxol-producing endophytic fungus. Sci. Rep. 2020, 10, 21139. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.S.; Greenwood, J.S.; Bombarely, A.; Mueller, L.A.; Tsao, R.; Mosser, D.D.; Raizada, M.N. An Endophyte Constructs Fungicide-Containing Extracellular Barriers for Its Host Plant. Curr. Biol. 2015, 25, 2570–2576. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.S.M.; Raizada, M.N. Darkness: A Crucial Factor in Fungal Taxol Production. Front. Microbiol. 2018, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, L.; Zhuang, W.; Zhang, F.; Shu, X.; Wang, N.; Wang, Z. Recent Research Progress in Taxol Biosynthetic Pathway and Acylation Reactions Mediated by Taxus Acyltransferases. Molecules 2021, 26, 2855. [Google Scholar] [CrossRef]
- Muchiri, R.; Walker, K.D. Taxol Biosynthesis: Tyrocidine Synthetase A Catalyzes the Production of Phenylisoserinyl CoA and Other Amino Phenylpropanoyl Thioesters. Cell Chem. Biol. 2012, 19, 679–685. [Google Scholar] [CrossRef][Green Version]
- Walker, K.; Fujisaki, S.; Long, R.; Croteau, R. Molecular cloning and heterologous expression of the C-13 phenylpropanoid side chain-CoA acyltransferase that functions in Taxol biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 12715–12720. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Xiao, W.-H.; Tyo, K.E.J.; Wang, Y.; Simeon, F.; Leonard, E.; Mucha, O.; Phon, T.H.; Pfeifer, B.; Stephanopoulos, G. Isoprenoid Pathway Optimization for Taxol Precursor Overproduction in Escherichia coli. Science 2010, 330, 70–74. [Google Scholar] [CrossRef]
- Biggs, B.W.; Lim, C.G.; Sagliani, K.; Shankar, S.; Stephanopoulos, G.; De Mey, M.; Ajikumar, P.K. Overcoming heterologous protein interdependency to optimize P450-mediated Taxol precursor synthesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2016, 113, 3209–3214. [Google Scholar] [CrossRef]
- Huang, Q.; Roessner, C.A.; Croteau, R.; Scott, A.I. Engineering Escherichia coli for the synthesis of taxadiene, a key intermediate in the biosynthesis of taxol. Bioorg. Med. Chem. 2001, 9, 2237–2242. [Google Scholar] [CrossRef]
- Zhou, K.; Qiao, K.; Edgar, S.; Stephanopoulos, G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat. Biotechnol. 2015, 33, 377–383. [Google Scholar] [CrossRef]
- Abdallah, I.I.; Pramastya, H.; van Merkerk, R.; Sukrasno; Quax, W.J. Metabolic Engineering of Bacillus subtilis toward Taxadiene Biosynthesis as the First Committed Step for Taxol Production. Front. Microbiol. 2019, 10, 218. [Google Scholar] [CrossRef]
- Dejong, J.M.; Liu, Y.; Bollon, A.P.; Long, R.M.; Jennewein, S.; Williams, D.; Croteau, R.B. Genetic engineering of taxol biosynthetic genes in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2006, 93, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Engels, B.; Dahm, P.; Jennewein, S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab. Eng. 2008, 10, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.-z.; Yan, H.-f.; Li, L.-f.; Zhai, F.; Shang, L.-q.; Yin, Z.; Yuan, Y.-j. Biosynthesis of Taxadiene in Saccharomyces cerevisiae: Selection of Geranylgeranyl Diphosphate Synthase Directed by a Computer-Aided Docking Strategy. PLoS ONE 2014, 9, e109348. [Google Scholar] [CrossRef] [PubMed]
- Reider Apel, A.; d’Espaux, L.; Wehrs, M.; Sachs, D.; Li, R.A.; Tong, G.J.; Garber, M.; Nnadi, O.; Zhuang, W.; Hillson, N.J.; et al. A Cas9-based toolkit to program gene expression in Saccharomyces cerevisiae. Nucleic Acids Res. 2017, 45, 496–508. [Google Scholar] [CrossRef]
- Nowrouzi, B.; Li, R.A.; Walls, L.E.; d’Espaux, L.; Malcı, K.; Liang, L.; Jonguitud-Borrego, N.; Lerma-Escalera, A.I.; Morones-Ramirez, J.R.; Keasling, J.D.; et al. Enhanced production of taxadiene in Saccharomyces cerevisiae. Microb. Cell Fact. 2020, 19, 200. [Google Scholar] [CrossRef]
- Walls, L.E.; Malcı, K.; Nowrouzi, B.; Li, R.A.; d’Espaux, L.; Wong, J.; Dennis, J.A.; Semião, A.J.C.; Wallace, S.; Martinez, J.L.; et al. Optimizing the biosynthesis of oxygenated and acetylated Taxol precursors in Saccharomyces cerevisiae using advanced bioprocessing strategies. Biotechnol. Bioeng. 2021, 118, 279–293. [Google Scholar] [CrossRef]
- Walls, L.E.; Martinez, J.L.; Rios-Solis, L. Enhancing Saccharomyces cerevisiae Taxane Biosynthesis and Overcoming Nutritional Stress-Induced Pseudohyphal Growth. Microorganisms 2022, 10, 163. [Google Scholar] [CrossRef]
- Besumbes, O.; Sauret-Güeto, S.; Phillips, M.A.; Imperial, S.; Rodríguez-Concepción, M.; Boronat, A. Metabolic engineering of isoprenoid biosynthesis in Arabidopsis for the production of taxadiene, the first committed precursor of Taxol. Biotechnol. Bioeng. 2004, 88, 168–175. [Google Scholar] [CrossRef]
- Kovacs, K.; Zhang, L.; Linforth, R.S.; Whittaker, B.; Hayes, C.J.; Fray, R.G. Redirection of carotenoid metabolism for the efficient production of taxadiene [taxa-4(5),11(12)-diene] in transgenic tomato fruit. Transgenic Res. 2007, 16, 121–126. [Google Scholar] [CrossRef]
- Rontein, D.; Onillon, S.; Herbette, G.; Lesot, A.; Werck-Reichhart, D.; Sallaud, C.; Tissier, A. CYP725A4 from yew catalyzes complex structural rearrangement of taxa-4(5),11(12)-diene into the cyclic ether 5(12)-oxa-3(11)-cyclotaxane. J. Biol. Chem. 2008, 283, 6067–6075. [Google Scholar] [CrossRef]
- Hasan, M.M.; Kim, H.-S.; Jeon, J.-H.; Kim, S.H.; Moon, B.; Song, J.-Y.; Shim, S.H.; Baek, K.-H. Metabolic engineering of Nicotiana benthamiana for the increased production of taxadiene. Plant Cell Rep. 2014, 33, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.; Li, X.; Shi, H.; Wang, F.; Zhang, Y.; Li, X. Combinatorial Engineering of Mevalonate Pathway and Diterpenoid Synthases in Escherichia coli for cis-Abienol Production. J. Agric. Food Chem. 2019, 67, 6523–6531. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xu, W.; Huang, W.; Wang, B.; Li, S.; Zhang, J.; Chang, L. Importation of taxadiene synthase into chloroplast improves taxadiene production in tobacco. Planta 2021, 253, 107. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.; Shim, S.H.; Kim, S.H.; Kim, O.T.; Lee, S.W.; Kwon, S.Y.; Baek, K.H. Production of taxadiene from cultured ginseng roots transformed with taxadiene synthase gene. BMB Rep. 2012, 45, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Anterola, A.; Shanle, E.; Perroud, P.F.; Quatrano, R. Production of taxa-4(5),11(12)-diene by transgenic Physcomitrella patens. Transgenic Res. 2009, 18, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Yuan, Y.; Tao, H.; Shi, X.; Zhong, X.; Han, Y.; Fu, S.; Fang, C.; Deng, Z.; Liu, T. Production of taxadiene by engineering of mevalonate pathway in Escherichia coli and endophytic fungus Alternaria alternata TPF6. Biotechnol. J. 2017, 12, 1–12. [Google Scholar] [CrossRef]
- Kirby, J.; Keasling, J.D. Biosynthesis of Plant Isoprenoids: Perspectives for Microbial Engineering. Annu. Rev. Plant Biol. 2009, 60, 335–355. [Google Scholar] [CrossRef]
- Li, B.J.; Wang, H.; Gong, T.; Chen, J.-J.; Chen, T.-J.; Yang, J.-L.; Zhu, P. Improving 10-deacetyl baccatin III-10-beta-O-acetyltransferase catalytic fitness for Taxol production. Nat. Commun. 2017, 8, 15544. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).