Abstract
Chanterelles are one of the most highly valued wild edible mushroom genera worldwide. This work aimed to investigate the nutritional characteristics and volatile compounds’ profile of Cantharellus alborufescens for the first time. Proximate analysis was performed according to the Association of Official Agricultural Chemists, while the mineral contents and the volatile compounds were determined using ICP-MS and GC-MS, respectively. C. alborufescens had an average of 25.8% protein, 5.5% fat, 12.7% ash, and 55.9% carbohydrates, including 11.4% fiber per dw of mushroom. Further analyses of the fat and protein contents revealed high amounts of polyunsaturated fatty acids as well as monosodium glutamate-like amino acids. Linoleic acid (42.0% of fat) and oleic acid (28.6% of fat) were the major fatty acids, while leucine (1.2%) and lysine (0.9%) were the most abundant essential amino acids. The results showed that C. alborufescens contained 3.1 µg/g vitamin D2 and 4.9 mg/g vitamin E per dw, as well as notable quantities of macro- and microelements, such as potassium, calcium, magnesium, and iron. GC-MS analysis revealed various volatile compounds such as acetaldehyde, n-hexanal, 3-methylbutanal, 1-octen-3-ol, etc. In conclusion, this study supports the use of C. alborufescens as a food rich in fiber and vitamin E, with a suitable amount of protein and other nutrients.
1. Introduction
Edible mushrooms are becoming increasingly popular as a source of food for humans, especially for vegetarians and vegans. Mushrooms are the natural and non-animal source of vitamin D and contain higher amounts of proteins than most vegetables [1]. In addition to high protein and low fat contents, mushrooms also have excellent aroma and flavor properties that make them ideal components of low-calorie diets [2]. It is estimated that there are approximately 20,000 species of mushrooms on Earth; among them, more than 3000 are edible, and 200 of those are collected from the wild [3]. Chanterelles are amongst the most highly valued mushrooms, and they are harvested in the wild. In the past, chanterelles from all over the world were summarized under the scientific name Cantharellus cibarius Fr., which referred to nearly all prized edible yellow mushrooms with a base and a cap with radial wrinkles on the underside. In the previous decades, with the progress of molecular biology, the differences between various populations of this taxon in different geographical areas were revealed and several species were newly described [4]. So far, the nutritional and medicinal properties of only a few of these chanterelle species, in particular, of Cantharellus cibarius, have been studied [5,6,7,8,9,10,11]. For example, chanterelles are reported to have high levels of bioavailable vitamin D2, which is critical for bone health [12]. The pleasant smell and taste of chanterelles are partly attributed to volatile compounds, including saturated and unsaturated aldehydes and ketones [13].
Chanterelles are widely distributed in various parts of the world, including Europe, Asia, Africa, and Central America [14]. Cantharellus alborufescens (Malençon) Papetti & S. Alberti (Cantharellaceae) grows in calcareous soils and its distribution is mostly associated with the occurrence of Mediterranean evergreen oaks in Europe. Parad et al. (2018) also reported this species from the forests of northern Iran [15]. There are no reports on the chemical composition and nutritional value of C. alborufescens. Therefore, the study at hand aimed to evaluate the dietary characteristics as well as the volatile constituents of this species. Moreover, the analysis of the mineral content of C. alborufescens was performed.
2. Results and Discussion
2.1. Proximate Analysis
The proximate analysis was conducted in triplicate and the results are shown in Table 1. According to the results, C. alborufescens has an average of 90.5% moisture, 25.8% protein, 5.5% fat, 12.7% ash, and 55.9% carbohydrates, including 11.4% fiber, and around 380 kcal of energy per 100 g of dry sample.

Table 1.
Nutrient content of C. alborufescens (g/100 g dw).
Based on the results, the carbohydrates and crude protein content comprise the largest part of the nutrient content of C. alborufescens, while the fat content is low, making it suitable for healthy and calorie-restricted diets [16]. Meanwhile, Cantharellus alborufescens contains essential fatty acids (Section 2.2, Table 2) that can be physiologically active in a variety of ways. The protein content of this mushroom is on average more than a quarter of its dry weight (25.8%).

Table 2.
Fatty acid content of C. alborufescens (g/100 g fat).
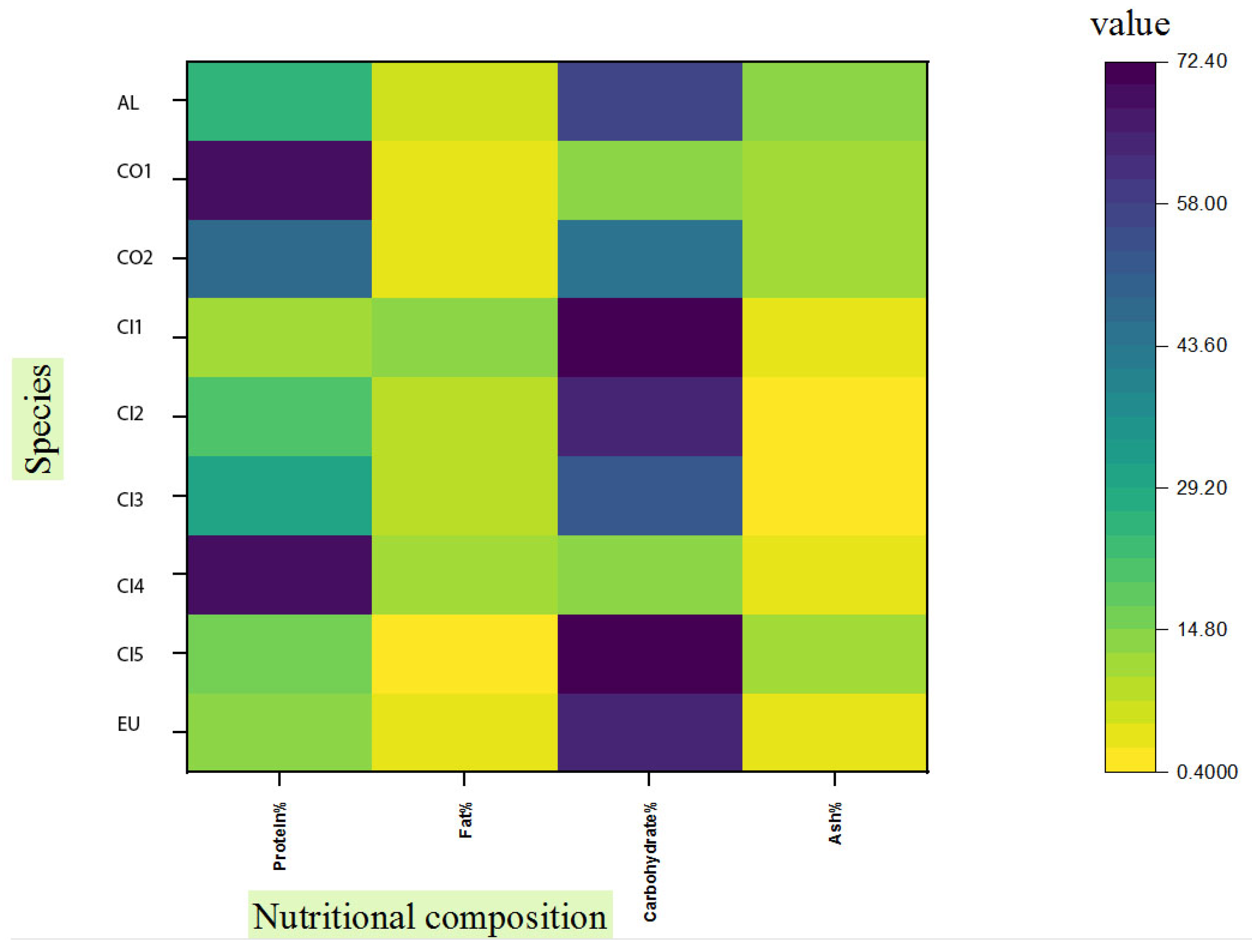
In Figure 1, we summarize nutritional information on chanterelle mushrooms. Most of the data available in the literature belong to Cantharellus cibarius. (The values may vary for the same species due to various factors such as the stage of development and environmental conditions, especially soil and climate characteristics [17]). Despite the fact that C. alborufescens has a high carbohydrate content compared to closely related species, its protein content is only average. Among mushroom species, total carbohydrate content ranges from 35% to 70% dw, including digestible and indigestible carbohydrates. Non-digestible carbohydrates include beta-glucans, mannans, and non-starch polysaccharides (NSPs). Humans can benefit from these non-digestible carbohydrates as dietary fibers [18]. Fiber-rich foods contain at least 6 g of fiber per 100 g [19], and C. alborufescens has an average content of 11.4 g fiber per 100 g dry weight.
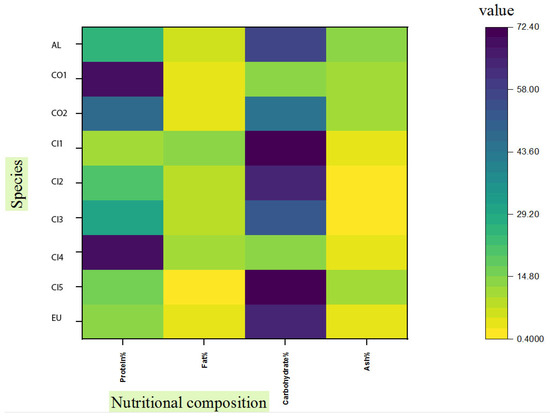
Figure 1.
Comparison of nutrient contents (protein, fat, carbohydrate and ash percentages) in different chanterelles: (AL) C. alborufescens; (CO 1–2) C. cornucopioides; (CI 1–5) C. cibarius; (EU) ‘Ero ububa, purple Cantharellus’. Sources of data except for C. alborufescens (this study) can be found in the following references: [5,6,8,9,10,20].
According to Figure 1, the ash content in C. alborufescens (12.7%) is higher than in other chanterelles and it is related to the presence of nutritionally important minerals (Section 2.5). The physiological and chemical characteristics of soil, along with the species of mushrooms, determine the concentrations of trace elements in the fruiting bodies [21]. Since the majority of mushroom carbohydrates are non-digestible, these polysaccharides cannot be a source of energy for humans. Nevertheless, C. alborufescens represents a food source with a good amount of protein, fiber, and minerals as well as a low fat content.
2.2. Fatty Acid Composition
The results obtained from the analyses of fatty acid contents of C. alborufescens are shown in Table 2. In general, the major fatty acid found in this mushroom was linoleic acid (42.0% of fat), followed by oleic acid (28.6% of fat), and palmitic acid (18.2% of fat). Besides these, eleven additional fatty acids were detected (Table 2). The amount of unsaturated fatty acids in C. alborufescens is higher than for the saturated ones, which is in agreement with results from related species [6,9]. Due to the high amount of linoleic acid in this mushroom, the polyunsaturated fatty acid (PUFA) content is 1.5 times higher than the saturated fatty acid (SFA) content. Since PUFAs in the diet can reduce low-density lipoprotein cholesterol (LDL-C), a high PUFA/SFA index indicates a positive effect on cardiovascular health (CVH) [22]. Linoleic acid is believed to be a precursor of compounds that contribute to mushroom flavor; C. alborufescens can be considered as a good food source for this essential fatty acid [2].
2.3. Amino Acid Composition
In this study, 16 amino acids were detected in C. alborufescens and the results are shown in Table 3. As part of an overall increase in protein and nitrogen intake, consumers need minor quantities of basic amino acids from biologically available sources. Mushrooms are good sources for both essential and non-essential amino acids [23,24]. The most abundant essential amino acids in C. alborufescens were leucine and lysine with 1.2% and 0.9%, respectively. Beluhan et al. (2011) found that lysine and threonine are the most prevalent amino acids in C. cibarius (5.74 and 8.98 mg/g dw) [5]. Generally, the amounts of non-essential amino acids in C. alborufescens were higher than those for essential ones, resulting in a 0.39 ratio. Glutamic acid was present in the highest amount (3.80%), which may especially contribute to the savory flavor of this mushroom.

Table 3.
Amino acid content of C. alborufescens.
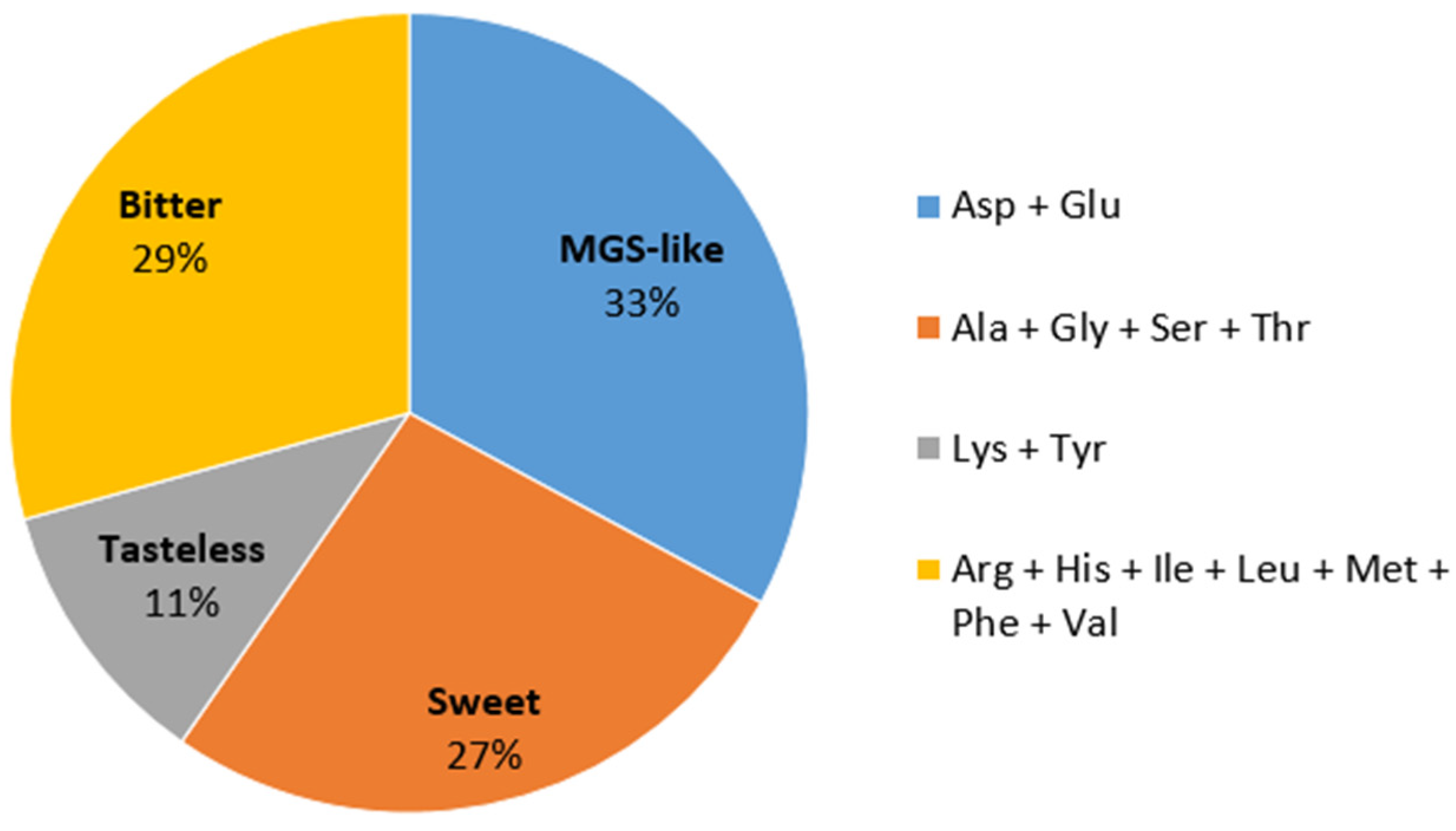
Amino acids are reported to play a role in mushroom taste, which is crucial to consumers’ acceptance. As shown in Figure 2, contents of free amino acids based on taste characteristics can be divided into four groups [25,26]. Monosodium glutamate-like (MGS-like) and sweet components are involved in the pleasant taste of mushrooms. In C. alborufescens, MGS-like amino acids contributed the highest amounts, followed by bitter amino acids. Apparently, the sweet tasting compounds mask the taste of the bitter amino acids well. According to the FAO/WHO (1973), every adult human needs 14 mg and 12 mg/kg body weight of the essential amino acids leucine and lysine per day, respectively. Considering the average weight of an adult to be 70 kg, each person needs 980 mg of leucine and 840 mg of lysine on average per day. By consuming 30 g of dried C. alborufescens per day, 360 mg of leucine and 273 mg of lysine can be obtained, corresponding to more than 30% of the daily requirement.
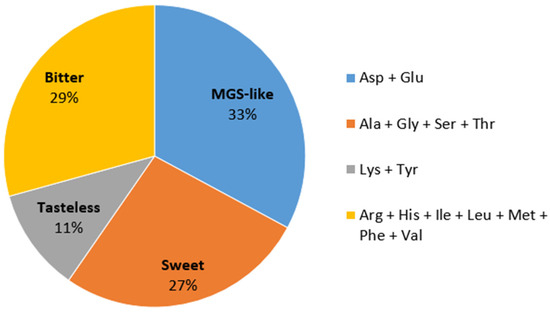
Figure 2.
Amino acid contents in C. alborufescens based on taste characteristics: monosodium glutamate-like (MGS-like), bitter, sweet and tasteless. The percentage of each flavor is shown in the pie chart. Ala: alanine, Arg: arginine, Asp: aspartic acid, Glu: glutamic acid, Gly: glycine, His: histidine, Ile: isoleucine, Leu: leucine, Lys: lysine, Met: methionine, Phe: phenylalanine, Ser: serine, Thr: threonine, Tyr: tyrosine, Val: valine.
2.4. Vitamin Content
Our results showed that C. alborufescens contains 3.1 µg/g dw of vitamin D2 (ergocalciferol) and 4.9 mg/g dw of vitamin E (alpha-tocopherol). The amount of vitamin D2 in C. alborufescens in our study is higher than the values reported for C. cibarius (0.5, 0.84, and 1.5 µg/g dw) in the literature [12,27,28]. Phillips et al. (2011) studied the vitamin D2 and sterol contents of ten species of mushrooms, including white button, shiitake, and oyster. Among them, C. cibarius contained the highest amount of vitamin D2 (0.5 µg/g dw), which is probably explained by the morphology and the growth condition for the chanterelle mushrooms (more exposure to sunlight on the gills), combined with a relatively high natural vitamin D2 content [12,27].
Few papers have been published on the tocopherol content of chanterelles. Barros et al. (2008) reported 0.12 µg/g dw of alpha-tocopherol in C. cibarius [6]. Natural antioxidants like vitamin E play an important role in human health. The biologically active form of vitamin E is alpha-tocopherol [29]. According to the daily uptake amounts reported by the FDA in March 2020, we should consume 20 µg of vitamin D and 15 mg of alpha-tocopherol per day. Accordingly, a daily consumption of 30 g of fresh C. alborufescens can provide approximately the required content of vitamin E and half of the vitamin D needed by the human body.
2.5. Mineral Content
The concentrations of minerals in C. alborufescens are shown in the Table 4 and Table 5. The results show that the order of mineral contents is K > P > Ca > Mg > Na > Fe > Zn > Cu > Mn > Cr and the order of heavy metal contents is Pb > As > Cd > Hg. Potassium constituted the most abundant element and Na was the fifth. The Na/K ratio was thus less than 0.066. According to WHO recommendations, sodium intake should not exceed 2000 mg daily and potassium intake should not exceed 3510 mg daily, resulting in a Na/K ratio of ≤1.0, which is optimal for cardiovascular health [30].

Table 4.
Major and trace element concentrations of C. alborufescens (mg/kg dry weight).

Table 5.
Heavy metal contents of C. alborufescens (mg/kg dw).
The mineral contents of C. alborufescens compared to the daily values (DVs) reported by the FDA in March 2020 are shown in Table 4 (considering that each person consumes 30 g of dried mushrooms daily). According to the results, C. alborufescens can be a good source of minerals, especially potassium and iron. The updated DVs from the FDA for Cu and Cr have been reduced compared to the original values. Now, if we compare the amounts of these minerals in the mushroom with the original amounts (2.00 and 0.12 mg, respectively), consuming 300 g of fresh C. alborufescens per day will be harmless to humans, but according to the updated values, both the copper and the chromium contents will exceed the recommended limits.
In order to determine the heavy metals in C. alborufescens, the Health Risk Index (HRI) was calculated (Table 5). HRI > 1 for any metal in food indicates that the consumer population faces a health risk. In this case, the concentrations of Pb and Cd are at safe levels, but for As and Hg (and, in particular, for As), HRIs exceed the levels considered safe, which means they require monitoring. Mushroom fruiting bodies have the capacity to absorb and bioaccumulate metals and metalloids from the substrate via their mycelia. Therefore, it can be assumed that a high level of arsenic might result either from bioaccumulation (where it is preferentially absorbed from the soil with low levels of arsenic) or from “normal uptake” from soil with a high level of arsenic [31].
In the areas contaminated with heavy metals, ion bioaccumulation can occur in the mushroom fruiting bodies. Therefore, it is important to investigate the element’s contents in edible mushrooms. The study by Grzybek and Janczy in 1990 showed that the amounts of lead and cadmium in C. cibarius were lower than in other edible mushrooms collected at the same site [32]. Generally, heavy metal bioaccumulation by mushrooms is complex and is influenced by both environmental as well as intrinsic factors. Different species behave differently according to their physiological requirements [33]. Since we do not know the amounts of As and Hg in the mushroom substrate in the present study, a more comprehensive investigation of the heavy metal bioaccumulation in C. alborufescens seems warranted.
2.6. Volatile Compound Profile
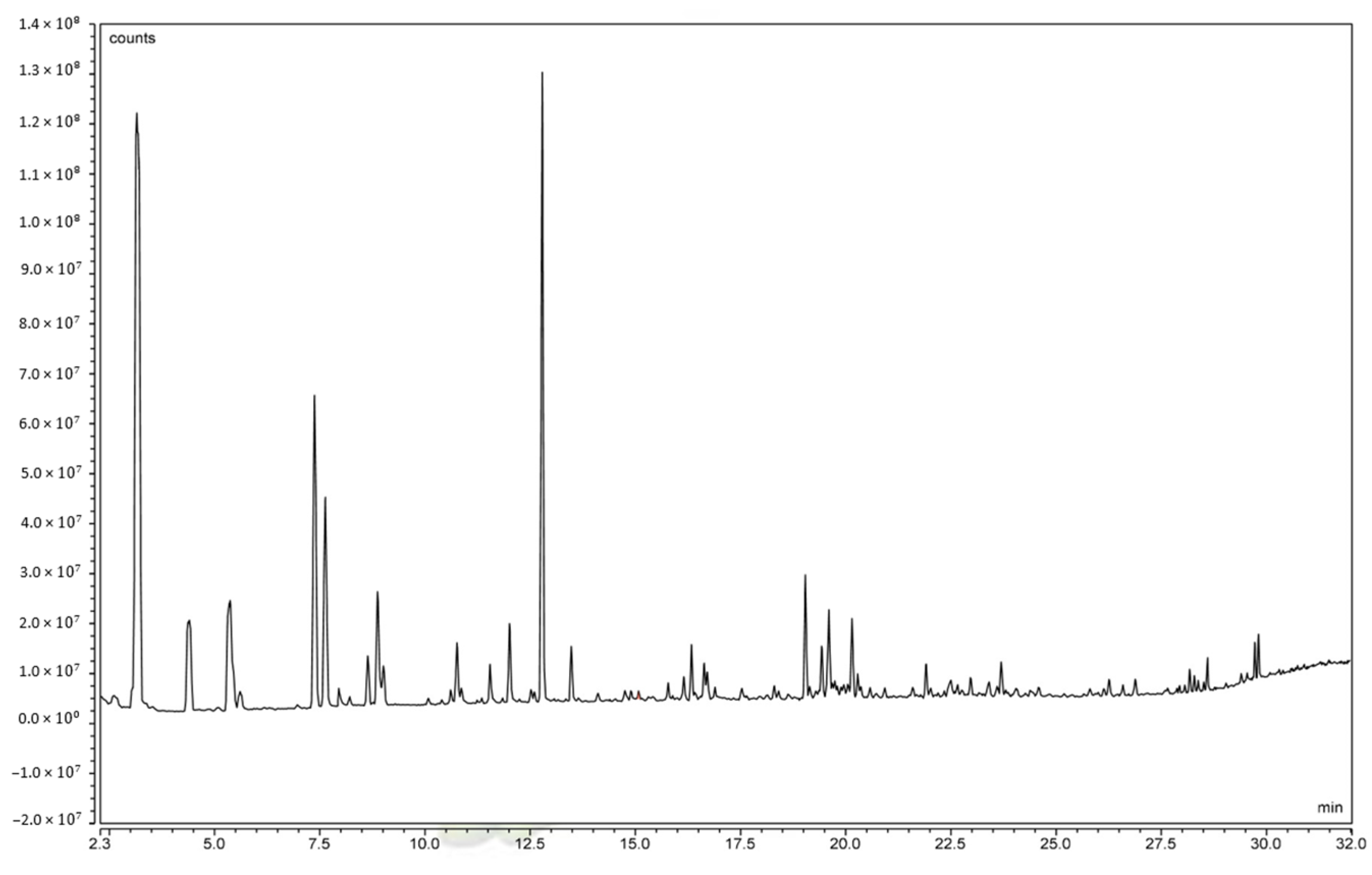
An analysis of volatile and subsequent odor-determining constituents of C. alborufescens is shown in Table 6 and the GC-MS chromatogram is shown in Figure 3. The 30 most abundant constituents with similarity indices (SIs) of more than 750 are listed in Table 6. Contents are given relative to all constituents (total content—ToC) and to all constituents excluding water (water-free content—WfC).

Table 6.
Composition of the volatile constituents identified via headspace GC-MS analysis and concentrations relative to the compounds.
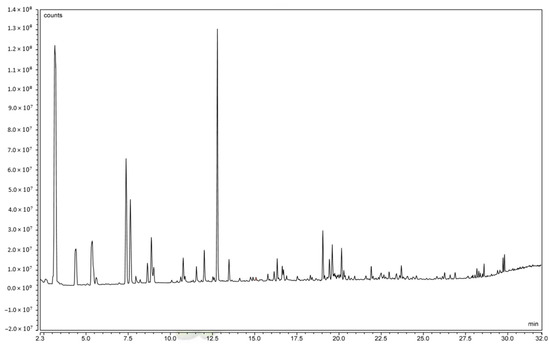
Figure 3.
Headspace GC-MS chromatogram (time vs. abundance) of C. alborufescens.
Of the 30 most abundant volatile constituents found in C. alborufescens (Table 6), 13 compounds belong to the class of aldehydes and 6 to the class of ketones. In addition, alcohols, carboxyl esters, and nitrogen constituents were identified. Also, in terms of quantity, aldehydes represent the major compound class, with acetaldehyde, n-hexanal, 3-methylbutanal, 2-methylbutanal, and n-butanal showing water-free contents of more than 5%, only surpassed by acetone with an amount of around 28%. Aisala et al. (2019) reported volatile compounds in the Cantharellus cibarius and Craterellus tubaeformis species using the headspace solid-phase micro-extraction method. Their results generally agree with this research and show the effective role of aldehydes and ketones in the odor of mushrooms. Although, acetaldehyde was not reported in their species, and the amount of acetone was reported to be very low compared to C. alborufescens, while the highest amount for a compound in C. cibarius was n-hexanal [13].
Two of the main compounds responsible for ‘mushroom-like flavor’ in many species are 1-octen-3-ol and 1-octen-3-one [34]. The amount of 1-octen-3-ol alcohol (1.58 WfC%) in C. alborufescens is not significant; it may be be influenced by various factors such as the maturity stage, drying temperature, analysis method, and the duration of mushroom storage, in addition to the type of species [8,35]. Among N-containing compounds in C. alborufescens, there are three pyrazine derivatives. Rychen et al. (2016) conducted comprehensive research on the use of 22 compounds of pyrazine derivatives (including methylpyrazine, ethylpyrazine, and dimethylpyrazine) as a food flavoring for different animals; they reported that if these compounds are used in feeds at the highest level proposed, there is no safety concern for the consumer [36]. Among the volatile compounds, an aziridine derivative (methylpyrazine) was also detected here. It has been proven that the toxicity of aziridine derivatives depends on their structure and activity [37]. Aziridines have been reported to cause changes in behavior and neurotransmission and to affect the movement, but there should be no reason for concern at the concentrations detected [38].
3. Materials and Methods
3.1. Mushroom Samples
Fruiting bodies were collected from Nur Forest Park, Mazandaran Province, Iran, N 36.579904°, E 52.045385°, in October 2021, and were deposited in the Iranian Cryptogamic Herbarium (ICH), Iranian Research Organization for Science and Technology (IROST), Tehran, Iran. The identification of the specimens was performed by MG as described earlier [15]. Fruiting bodies were lyophilized and then stored at −80 °C until further analysis.
3.2. Proximate Analysis
Moisture, crude protein, crude fat, fiber, ash, and carbohydrate content were determined according to the Association of Official Agricultural Chemists (1995) method [39]. The moisture content was determined by heating the fresh sample at 105 °C until a constant weight was reached. The crude protein content was estimated using the Kjeldahl method. Total fat was obtained with a Soxhlet extractor using petroleum ether as the solvent. After defatting, concentrated sulfuric acid and sodium hydroxide were used to estimate crude fiber. The samples were placed in a furnace at 600 ± 15 °C to determine the ash content. The following formulas were used to calculate total carbohydrates and energy [7]:
Total carbohydrates percentage = 100 − (moisture + protein + fat + ash) %
Total energy (kcal) = 4 × (g protein + g carbohydrate) + 9 × (g fat)
3.3. Fatty Acid Composition
Fatty acids were identified according to a modification of ISO 12966-1 at the Behesht Aein Laboratory Complex, Tehran, Iran [40]. Fatty acid methyl esters (FAMEs) (after Soxhlet extraction) were obtained through the ISO 12966-2: 2017 protocol using methanolic CH3NaO and H2SO4 (0.2 and 1 mol/L, respectively) [41]. The fatty acid profile was analyzed on an YL6500 GC instrument (YOUNGIN Chromass, Anyang-si, Republic of Korea) with a flame ionization detector (FID) and TR-CN100 Teknokroma column. The oven temperature program was as follows: temperature change from 120 °C to 240 °C with a speed of 4 °C per minute, then held at 240 °C for seven minutes. The carrier gas (hydrogen) flow rate was one mL/min with column head pressure of 220 kPa and a split ratio of 1:100. Then, 1 µL of the sample was injected into the GC at 250 °C (injector temperature). Identification of FAMEs was performed by comparing their retention times with those of known standards.
3.4. Amino Acid Composition
Amino acids were analyzed at the Behesht Aein Laboratory Complex, Tehran, Iran, according to an in-house method, including three steps: (1) Acid hydrolysis of the sample to release amino acids with HCl 6N/1% phenol. (2) Amino acid derivatization with phenyl isocyanate. (3) Isolation of the derivative obtained from the preparation stage using HPLC. The YL9100 HPLC instrument (YOUNGIN Chromass, Republic of Korea) with a UV detector and software YL Clarity (version 8.1) was used. The analytical experiment was performed under the following conditions: flow rate: 1.0 mL/min, injection volume: 20 μL, absorbance at 254 nm, analytical column: C18, 5 μm, 4.6 × 250 mm, guard column: C18, 5 μm with guard column holder, column temp: 40 °C. A standard L-amino acid kit containing 22 amino acids plus glycine (Merck, Darmstadt, Germany) was used for identification. To calculate the percentage of each amino acid, the calibration curve was plotted.
3.5. Vitamin Content
Vitamins D2 and E (alpha-tocopherol) were determined according to national standard protocols retrieved from DIN EN 12821:2009 and DIN EN 12822: 2012 standards at the Behesht Aein Laboratory Complex, Tehran, Iran [42,43]. Methanol (50 mL), antioxidants such as ascorbic acid (0.25 g), and KOH (5 mL of 50 g/100 mL solution) were used for saponification of 5 g of mushroom powder at 80 °C for 30 min. Extraction of tocopherols and vitamin D was carried out using n-hexane. For identification and quantification of vitamins, the YL9100 HPLC instrument (YOUNGIN Chromass, Republic of Korea) was used with the mobile phase consisting of methanol and water (97:3), and a flow rate of 2 mL/min. Vitamin D was measured at wavelength 265 nm and vitamin E at wavelength 292 nm, with a C18 column (250 × 4 mm, 5 μm). Compounds were identified by comparing the retention times of individual peaks in the chromatograms and the standard solutions.
3.6. Mineral Content
The concentrations of Na, K, Fe, Ca, P, Mg, Mn, Cu, Zn, Cr, Pb, Cd, As, and Hg in the mushroom samples were analyzed using an inductively coupled plasma-mass spectrometer (Agilent Technologies Inc.-7500 ICP-MS, Santa Clara, CA, USA). For sample preparation, 5 mL of HNO3 was added to 0.1 g of mushroom powder. Via indirect heating, the sample was completely dissolved in acid and the filtered solution reached a volume of 15 mL with pure water. The dilution factor was included in the final result. The ICP-MS measurements were performed using the instrumental conditions as follows: 1200 W radio frequency generator power with 24 MHz resonance frequency, argon as nebulizer and auxiliary gas with 0.8 (L/min) flow rate, argon plasma flow rate of 12.2 (L/min), 260 total (S) sample uptake time, CCD solid-state detector, and a modified Lichte spray chamber cyclonic. An internal standard of IV-STOCK-8 and calibration solution of IV-STOCK-24 (Merck KGaA, Darmstadt, Germany) was used for calibration and quality control [44]. To calculate the daily intake (DI) of toxic heavy metals (Pb, Cd, As, Hg) and their health risk index (HRI) in mushroom, the following formulas were used:
DI = Mean concentration of toxic element in mushroom (mg/kg dw) ×
Daily mushroom consumption (g/day)/Average body weight (kg)
Daily mushroom consumption (g/day)/Average body weight (kg)
HRI = DI/Oral reference dose (RfDₒ)
In our study, a person’s average body weight was taken as 70 kg, and each person was assumed to consume on average 30 g dry of mushrooms per day. RfDₒ represents the extent of exposure to oral contaminants during the life, and for Cd, Hg, As, and Pb, they were 0.5, 0.3, 0.3, and 4 μg/kg/day, respectively [45,46,47].
3.7. Analysis of Volatile Compound Profile
The volatile compound profile was determined with a headspace GC-MS analyzer. A Trace 1310 gas chromatograph equipped with split/splitless (SSL) and programmable temperature vaporizer inlets and a TSQ Duo mass spectrometer (ThermoFisher Scientific, Waltham, MA, USA) was used. A Thermo Fisher TG-624SilMS (30 m × 0.25 mm × 1.4 µm) column was used for GC analyses. Incubation of the sample vial at 90 °C for 30 min was carried out by directly weighing 100 mg of partially dried and ground material into the headspace vial. Through a heated syringe, 1000 µL of headspace were injected into the GC-MS. According to the GC program, 35 °C was held for 1 min, followed by 5 °C/min to 120 °C, and 30 °C/min to 300 °C and 1 min. The MS parameters were as follows: 43–300 m/z scan with MS Source at 280 °C. MS spectra were matched with spectra from the Adams and NIST databases.
3.8. Statistical Analysis
The experiments were performed in triplicate and the obtained results were expressed as mean ± standard deviation (SD). Data visualization was carried out using the OriginPro program, version 2023b.
4. Conclusions
This study revealed the nutritional composition and odor-contributing volatile compound profiles of C. alborufescens. The findings showed the crude protein content of this mushroom is more than 25% (% dw). MGS-like amino acids form the highest amount of its amino acid content, which plays an important role in the pleasant taste of this mushroom as a non-volatile compound. GC/MS analysis showed various volatile organic compounds (VOCs), predominantly aldehydes and ketones as odor-contributing substances. In compliance with the literature on other chanterelles, our study showed that C. alborufescens has a high ash content, related to the high presence of macro- and microelements such as potassium, calcium, and iron. All minerals were at safe levels except mercury and arsenic, which may pose a risk for humans (exceeding the recommended values).
Notably, despite the relatively low fat content of this mushroom, it has a significant amount of fat-soluble vitamins E and D and an adequate amount of linoleic acid. Overall, this study suggests that C. alborufescens provides an applicable amount of protein, fiber, and minerals in a low-fat form. Also, research has shown that edible mushrooms can be used as an additive to improve the nutritional profile of food due to their valuable nutritional contents [48]. Due to the high vitamin E content of this mushroom, more studies are suggested in the field, which should use C. alborufescens as an antioxidant additive in food.
Author Contributions
M.G.-N. developed the original idea and the general outline of the study, M.M. designed the study, performed experiments, and provided the first draft; T.S. and M.G.-N. performed part of the experiments; M.M. and S.S.Ç. wrote the manuscript; M.J. reviewed the final draft; M.G.-N., S.S.Ç. and C.Z. edited and reviewed the draft and final draft; all authors contributed to the interpretation of results and final drafting. All authors have read and agreed to the published version of the manuscript.
Funding
Iran National Science Foundation (INSF) project no. 4000655.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Xun, W.; Wang, G.; Zhang, Y.; Liao, G.; Ge, C. Analysis of flavor-related compounds in four edible wild mushroom soups. Microchem. J. 2020, 159, 105548. [Google Scholar] [CrossRef]
- Sun, L.-B.; Zhang, Z.-Y.; Xin, G.; Sun, B.-X.; Bao, X.-J.; Wei, Y.-Y.; Zhao, X.-M.; Xu, H.-R. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Olariaga, I.; Moreno, G.; Manjón, J.L.; Salcedo, I.; Hofstetter, V.; Rodríguez, D.; Buyck, B. Cantharellus (Cantharellales, Basidiomycota) revisited in Europe through a multigene phylogeny. Fungal Divers. 2017, 83, 263–292. [Google Scholar] [CrossRef]
- Beluhan, S.; Ranogajec, A. Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 2011, 124, 1076–1082. [Google Scholar] [CrossRef]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef]
- Barros, L.; Venturini, B.A.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C. Chemical composition and biological properties of Portuguese wild mushrooms: A comprehensive study. J. Agric. Food Chem. 2008, 56, 3856–3862. [Google Scholar] [CrossRef]
- Fogarasi, M.; Socaci, S.A.; Dulf, F.V.; Diaconeasa, Z.M.; Fărcaș, A.C.; Tofană, M.; Semeniuc, C.A. Bioactive compounds and volatile profiles of five Transylvanian wild edible mushrooms. Molecules 2018, 23, 3272. [Google Scholar] [CrossRef]
- Ugbogu, E.A.; Emmanuel, O.; Ude, V.C.; Ijioma, S.N.; Ugbogu, O.C.; Akubugwo, E.I. Nutritional composition and toxicity profile of Cantharellus species (Purple Mushroom) in rats. Sci. Afr. 2020, 8, e00375. [Google Scholar] [CrossRef]
- Bak, K.H.; Bauer, S.; Rattner, J.; Wagner, M.; Ludewig, M. Nutritional properties, microbial and sensory quality, and formation of biogenic amines in wild-grown mushrooms (Cantharellus cibarius & Boletus edulis) from Austrian local markets. Food Chem. Adv. 2023, 2, 100193. [Google Scholar]
- Nasiry, D.; Khalatbary, A.R.; Ebrahimzadeh, M.A. Anti-inflammatory and wound-healing potential of golden chanterelle mushroom, Cantharellus cibarius (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.M.; Ruggio, D.M.; Horst, R.L.; Minor, B.; Simon, R.R.; Feeney, M.J.; Byrdwell, W.C.; Haytowitz, D.B. Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. J. Agric. Food Chem. 2011, 59, 7841–7853. [Google Scholar] [CrossRef] [PubMed]
- Aisala, H.; Sola, J.; Hopia, A.; Linderborg, K.M.; Sandell, M. Odor-contributing volatile compounds of wild edible Nordic mushrooms analyzed with HS–SPME–GC–MS and HS–SPME–GC–O/FID. Food Chem. 2019, 283, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Pilz, D. Ecology and Management of Commercially Harvested Chanterelle Mushrooms; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2003; Volume 576.
- Parad, G.A.; Ghobad-Nejhad, M.; Tabari, M.; Yousefzadeh, H.; Esmaeilzadeh, O.; Tedersoo, L.; Buyck, B. Cantharellus alborufescens and C. ferruginascens (Cantharellaceae, Basidiomycota) new to Iran. Cryptogam. Mycol. 2018, 39, 299–310. [Google Scholar] [CrossRef]
- González, A.; Nobre, C.; Simões, L.S.; Cruz, M.; Loredo, A.; Rodríguez-Jasso, R.M.; Contreras, J.; Texeira, J.; Belmares, R. Evaluation of functional and nutritional potential of a protein concentrate from Pleurotus ostreatus mushroom. Food Chem. 2021, 346, 128884. [Google Scholar] [CrossRef]
- Ao, T.; Deb, C.R. Nutritional and antioxidant potential of some wild edible mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef]
- Cheung, P.C. The nutritional and health benefits of mushrooms. Nutr. Bull. 2010, 35, 292–299. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, W.; Yang, Z.; Li, J.; Zeng, X. Study on nutrients, non-volatile compounds, volatile compounds and antioxidant capacity of oyster mushroom cultivated with corn distillers’ grains. LWT 2023, 183, 114967. [Google Scholar] [CrossRef]
- Thonart, P.; Bouzouita, N. Chemical composition and non-volatile components of three wild edible mushrooms collected from northwest Tunisia. Mediterr. J. Chem. 2016, 5, 434–441. [Google Scholar]
- Mleczek, M.; Szostek, M.; Siwulski, M.; Budka, A.; Kalač, P.; Budzyńska, S.; Kuczyńska-Kippen, N.; Niedzielski, P. Road traffic and abiotic parameters of underlying soils determine the mineral composition and nutritive value of the mushroom Macrolepiota procera (Scop.) Singer. Chemosphere 2022, 303, 135213. [Google Scholar] [CrossRef]
- Gałgowska, M.; Pietrzak-Fiećko, R. Evaluation of the nutritional and health values of selected Polish mushrooms considering fatty acid profiles and lipid indices. Molecules 2022, 27, 6193. [Google Scholar] [CrossRef]
- Kıvrak, İ.; Kıvrak, Ş.; Harmandar, M. Free amino acid profiling in the giant puffball mushroom (Calvatia gigantea) using UPLC–MS/MS. Food Chem. 2014, 158, 88–92. [Google Scholar] [CrossRef]
- Lee, K.J.; Yun, I.J.; Kim, K.H.; Lim, S.H.; Ham, H.J.; Eum, W.S.; Joo, J.H. Amino acid and fatty acid compositions of Agrocybe chaxingu, an edible mushroom. J. Food Compos. Anal. 2011, 24, 175–178. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lin, H.-C.; Mau, J.-L. Non-volatile taste components of several commercial mushrooms. Food Chem. 2001, 72, 465–471. [Google Scholar] [CrossRef]
- Mau, J.-L.; Lin, H.-C.; Chen, C.-C. Non-volatile components of several medicinal mushrooms. Food Res. Int. 2001, 34, 521–526. [Google Scholar] [CrossRef]
- Mattila, P.; Lampi, A.-M.; Ronkainen, R.; Toivo, J.; Piironen, V. Sterol and vitamin D2 contents in some wild and cultivated mushrooms. Food Chem. 2002, 76, 293–298. [Google Scholar] [CrossRef]
- Teichmann, A.; Dutta, P.C.; Staffas, A.; Jägerstad, M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation. LWT-Food Sci. Technol. 2007, 40, 815–822. [Google Scholar] [CrossRef]
- Engin, K.N. Alpha-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855. [Google Scholar] [PubMed]
- Vulin, M.; Magušić, L.; Metzger, A.-M.; Muller, A.; Drenjančević, I.; Jukić, I.; Šijanović, S.; Lukić, M.; Stanojević, L.; Davidović Cvetko, E. Sodium-to-potassium ratio as an indicator of diet quality in healthy pregnant women. Nutrients 2022, 14, 5052. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Alonso, J.; García, M. Total contents of arsenic and associated health risks in edible mushrooms, mushroom supplements and growth substrates from Galicia (NW Spain). Food Chem. Toxicol. 2014, 73, 44–50. [Google Scholar] [CrossRef]
- Grzybek, J.; Janczy, B. Quantitative estimatation of lead, cadmium. and nickel contents by means of atomic absorption spectroscopy in fruitbodies of some macromycetes in Poland. Acta Mycol. 1990, 26, 17–23. [Google Scholar] [CrossRef][Green Version]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.H.; Claver, I.P.; Zhu, K.X.; Peng, W.; Zhou, H.M. Effect of different cooking methods on the flavour constituents of mushroom (Agaricus bisporus (Lange) Sing) soup. Int. J. Food Sci. Technol. 2011, 46, 1100–1108. [Google Scholar] [CrossRef]
- Liu, Q.; Bau, T.; Jin, R.; Cui, X.; Zhang, Y.; Kong, W. Comparison of different drying techniques for shiitake mushroom (Lentinus edodes): Changes in volatile compounds, taste properties, and texture qualities. LWT 2022, 164, 113651. [Google Scholar] [CrossRef]
- Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; Kolar, B.; et al. Safety and efficacy of pyrazine derivatives including saturated ones belonging to chemical group 24 when used as flavourings for all animal species. EFSA J. 2017, 15, e04671. [Google Scholar]
- Ismail, F.M.; Levitsky, D.O.; Dembitsky, V.M. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, J.; Kassa, J.; Fusek, J.; Kuca, K.; Jun, D. Other toxic chemicals as potential chemical warfare agents. In Handbook of Toxicology of Chemical Warfare Agents; Elsevier: Amsterdam, The Netherlands, 2015; pp. 337–345. [Google Scholar]
- Cunniff, P. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- ISO 12966-1; 2014-Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 12966-2: 2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2017.
- DIN EN 12821:2009; Foodstuffs-Determination of Vitamin D by High Performance Liquid Chromatography—Measurement of Cholecalciferol (D3) or Ergocalciferol (D2). European Union: Luxembourg, 2009.
- DIN EN 12822: 2012; Foodstuffs—Determination of Vitamin E by High Performance Liquid Chromatography—Measurement of α-, β-, γ- and δ-Tocopherols. European Union: Luxembourg, 2012.
- Sami, A.S.; Suat, E.; Alkis, I.; Karakus, Y.; Guler, S. The role of trace element, mineral, vitamin and total antioxidant status in women with habitual abortion. J. Matern. -Fetal Neonatal Med. 2021, 34, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Nag, R.; Cummins, E. Human health risk assessment of lead (Pb) through the environmental-food pathway. Sci. Total Environ. 2022, 810, 151168. [Google Scholar] [CrossRef]
- Liu, S.; Fu, Y.; Shi, M.; Wang, H.; Guo, J. Pollution level and risk assessment of lead, cadmium, mercury, and arsenic in edible mushrooms from Jilin Province, China. J. Food Sci. 2021, 86, 3374–3383. [Google Scholar] [CrossRef]
- Širić, I.; Kumar, P.; Eid, E.M.; Bachheti, A.; Kos, I.; Bedeković, D.; Mioč, B.; Humar, M. Occurrence and health risk assessment of cadmium accumulation in three Tricholoma mushroom species collected from wild habitats of Central and Coastal Croatia. J. Fungi 2022, 8, 685. [Google Scholar] [CrossRef]
- Rangel-Vargas, E.; Rodriguez, J.A.; Domínguez, R.; Lorenzo, J.M.; Sosa, M.E.; Andrés, S.C.; Rosmini, M.; Pérez-Alvarez, J.A.; Teixeira, A.; Santos, E.M. Edible mushrooms as a natural source of food ingredient/additive replacer. Foods 2021, 10, 2687. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).