Abstract
The nitrate ion (NO3−) is a typical pollutant in environmental samples, posing a threat to the aquatic ecosystem and human health. Therefore, rapid and accurate detection of NO3− is crucial for both the aquatic sciences and government regulations. Here we report the fabrication of an amino-functionalized, vertically ordered mesoporous silica film (NH2-VMSF) confining localized copper nanoparticles (CuNPs) for the electrochemical detection of NO3−. NH2-VMSF-carrying amino groups possess an ordered perpendicular nanochannel structure and ultrasmall nanopores, enabling the confined growth of CuNPs through the electrodeposition method. The resulting CuNPs/NH2-VMSF-modified indium tin oxide (ITO) electrode (CuNPs/NH2-VMSF/ITO) combines the electrocatalytic reduction ability of CuNPs and the electrostatic attraction capacity of NH2-VMSF towards NO3−. Thus, it is a rapid and sensitive electrochemical method for the determination of NO3− with a wide linear detection range of 5.0–1000 μM and a low detection limit of 2.3 μM. Direct electrochemical detection of NO3− in water samples (tap water, lake water, seawater, and rainwater) with acceptable recoveries ranging from 97.8% to 109% was performed, demonstrating that the proposed CuNPs/NH2-VMSF/ITO sensor has excellent reproducibility, regeneration, and anti-interference abilities.
1. Introduction
In the past few decades, nitrate ion (NO3−) pollution in water environments has become a serious global environmental issue [1]. The wide-spread use of nitrogen fertilizers and fossil fuels dramatically increases food production and the human population. However, the excess nitrogen on land and in the air has adverse effects on aquatic ecosystems and human health. Excessive use of nitrogen-containing fertilizers in agriculture and livestock farming, along with the uncontrolled discharge of wastewater into the groundwater, can result in the contamination of multiple aquatic environments [2]. Emissions of industrial gases are also a source of nitrate pollution, which can form nitric acid and further acidify lakes and streams through atmospheric deposition [3]. Increased nitrogen flowing into estuarine and nearshore waters contributes to the eutrophication of coastal waters. This phenomenon leads to a rise in the occurrence of harmful algal blooms, coastal hypoxia, and the degradation of habitats [4]. The ultimate oxidation product of inorganic nitrogen, NO3−, accounts for more than 65% of the dissolved nitrogen in seawater. Its status and fate are crucial to the marine biogeochemical cycles of carbon [5], whose coupling regulates the climate on Earth [6]. As for human health, high levels of NO3− intake in the human body induce methemoglobinemia, colorectal cancer, thyroid disease, and neural tube defects [7]. For these reasons, the World Health Organization, the Food and Agriculture Organization, and governments have all set strict limits on NO3− in drinking water [8,9]. Therefore, simple and sensitive methods for the quantitative analysis of NO3− in aquatic environments are urgently needed for the aquatic science community and governments.
Various methods for NO3− determination include the visible spectrophotometric method using color reagents [10], direct ultraviolet spectrophotometry [11], fluorescence [12], chemiluminescence [13], electrochemical sensors [14], high-performance liquid chromatography [15], and ion chromatography [16]. Electrochemical sensing has gained widespread popularity because it generally offers a fast response time, high sensitivity, space-saving designs, and cost-efficiency [17,18,19]. However, electrochemical process of NO3− at the common electrodes is rather slow and produces high overpotentials. To overcome this issue, researchers have focused on the exploitation of various nanomaterials, such as metal nanoparticles [20], carbon nanotubes/fibers [21], and graphene-based materials [22]. Copper nanoparticles (CuNPs) have been reported as an effective electrocatalytic material for NO3− and nitrite (NO2−) reduction and have been combined with other carbonaceous materials for the sensitive determination of NO3− and NO2− [23,24,25].
Recently, mesoporous materials integrated with various functional nanomaterials have shown great potential in the fields of adsorption, catalysis, and sensing [26,27,28,29,30,31]. Vertically ordered mesoporous silica film (VMSF, also referred to as silica nanochannel film) has opened a vast range of potential opportunities for the electrochemical analysis of complicated real samples in recent decades [32,33,34]. VMSFs consisting of vertically oriented, open nanochannels attached to the electrode ensure the accessible transport of analytes or probes to the VMSF/electrode interface [35,36,37]. In addition, VMSFs have the electrostatic accumulation capacity for the target analyte due to the silanol groups on the walls and tiny nanochannels; at the same time, the insulating silica membrane can impede the ingress of interfering substances to the underlying electrode surface via charge, size, and lipophilicity interactions, showing good sensitivity, reproducibility, and long-term stability in real media analysis [38,39,40]. To enhance analytical sensitivity, electrocatalytic and/or conductive nanomaterials, such as metal nanoparticles [41], graphene quantum dots [42], and graphene nanosheets [43,44,45,46], have been incorporated into the inner space or bottom of VMSFs. Metal nanoparticles (e.g., gold and platinum) have been synthesized within the tiny nanochannels of VMSFs for the construction of attractive electrochemical sensors. To the best of our knowledge, CuNPs confined in the silica nanochannels of VMSFs have not yet been reported.
In this study, we synthesized electrodeposited CuNPs using an amino-functionalized, vertically ordered mesoporous silica film (NH2-VMSF) as a hard template. Their electrochemical performance with regard to NO3− quantification in environmental water samples has been examined. Many uniform, tiny NH2-VMSF nanochannels favor the localized, stable growth of CuNPs, avoiding the use of any protective agent. The resulting CuNPs/NH2-VMSF-modified indium tin oxide (ITO) electrode, referred to as CuNPs/NH2-VMSF/ITO, exhibits superior analytical performance with respect to NO3− due to the electrocatalytic properties of the CuNPs and the electrostatic accumulation ability of NH2-VMSF. Moreover, the CuNPs/NH2-VMSF/ITO sensor we developed exhibits excellent reproducibility, regeneration, and anti-interference capabilities, which have been successfully employed to accurately measure the concentration of NO3− in diverse environmental water samples, including tap water, lake water, seawater, and rainwater.
2. Results and Discussion
2.1. Characterizations of NH2-VMSF/ITO and CuNPs/NH2-VMSF/ITO Electrodes
Figure 1 shows the schematic illustration of the preparation of a CuNPs/NH2-VMSF/ITO sensor and the electrochemical reduction detection of NO3−, which is divided into the following three sections: First, a binary film consisting of surfactant micelles (SM) and VMSF-bearing amino groups, abbreviated as SM@NH2-VMSF/ITO, was grown onto the patterned ITO electrode using the traditional electrochemical-assisted self-assembly (EASA) method [47,48] (Figure 1a). EASA combines electrochemical deposition and self-assembly techniques, inducing the self-assembly of SMs on the ITO electrode surface and the sol-gel process of the silane precursors within several seconds. SMs consisting of CTAB micelles are physically confined within the ultrasmall nanochannel space of the NH2-VMSF and can be excluded by simple solvent extraction [49]. The addition of (3-aminopropyl) triethoxysilane into the precursor solution can result in the silica nanochannels carrying amino groups [50]. The NH2-VMSF/ITO with an ultrasmall, open nanochannel array provided a confined nanospace for the stable synthesis of CuNPs with no protective agents. CuNPs were grown into the nanochannels of the NH2-VMSF using a controllable electrodeposition method to form the CuNPs/NH2-VMSF/ITO (Figure 1b), and their fabrication conditions (electrodeposition time) were also optimized. Not only could the CuNPs/NH2-VMSF/ITO enrich NO3− through electrostatic interaction between the amino groups of the NH2-VMSF and NO3−, but it also exhibited the capacity to electrocatalytically reduce NO3− via CuNPs. NO3− could enter into the nanochannels of the NH2-VMSF and be electrochemically reduced to NO2− in an acidic environment (Figure 1c), ultimately giving rise to the reductive peak and enabling the quantitative determination of NO3−. The anti-fouling properties of NH2-VMSF make the proposed CuNPs/NH2-VMSF/ITO sensor suitable for direct analysis of NO3− in practical water samples.
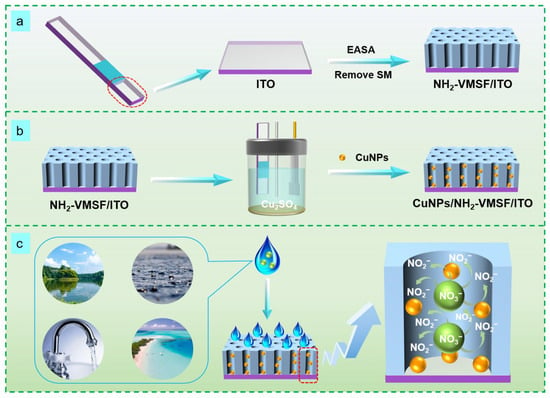
Figure 1.
Schematic diagram of the preparation of an NH2-VMSF/ITO sensor (a) and a CuNPs/NH2-VMSF/ITO sensor (b) and the electrochemical reduction detection of NO3− (c).
An NH2-VMSF layer grown on the ITO surface was investigated by transmission electron microscopy (TEM). As shown in Figure 2a, the NH2-VMSF, which was prepared using the electrochemically assisted self-assembly method with a high level of ordering, has a great deal of uniformly and hexagonally distributed nanopores (top-view TEM). The average pore diameter of the NH2-VMSF was in the range of 2~3 nm. Nanochannels oriented orthogonally to the NH2-VMSF surface were parallel to each other with a length of 92 nm (cross-sectional view SEM, Figure 2b). Modification of the NH2-VMSF layer with the permselective properties of the ITO was able to give rise to distinct electrochemical responses towards charged probes compared to a bare ITO electrode. Figure 2c,d shows the cyclic voltammetry (CV) curves of bare ITO, NH2-VMSF/ITO and SM@NH2-VMSF/ITO electrodes in a buffer solution containing either 50 μM Fe(CN)63− or 50 μM Ru(NH3)63+. As can be seen, both Fe(CN)63− and Ru(NH3)63+ were able to generate a pair of reversible redox peaks on the bare ITO (black curve). When insulating the NH2-VMSF with a SM inside the nanochannels, no Faradic current was measured at the SM@NH2-VMSF/ITO electrode (blue curve), because the templated SM molecules blocked access of charged hydrophilic probes; this further indicates that intact the NH2-VMSF homogeneously covers the whole ITO electrode surface. Effective exclusion of SMs from the nanochannels could be achieved using a HCl–ethanol solution to obtain the NH2-VMSF/ITO with recovered electrode accessibility [51]. Amino groups on the NH2-VMSF were exposed to the buffer solution and carried positive charges under the measured experimental conditions (pH = 7.0), leading to enhanced voltammetric currents for Fe(CN)63− (anodic peak current, Iox, 33.7 μA vs. 24.4 μA (bare ITO)), while decreasing signals for Ru(NH3)63+ (Iox, 33.7 μA vs. 24.4 μA (bare ITO)) at the NH2-VMSF/ITO electrode. The values of the peak-to-peak separation obtained at the NH2-VMSF/ITO electrode were slightly larger than those at the bare ITO, suggesting the transport of probes into the nanochannels of NH2-VMSF is effective. This anion-selective permeability of the NH2-VMSF/ITO is due to the protonation of amino groups on the channel walls of the NH2-VMSF and to pronounced electrostatic interaction within the tiny space [52].
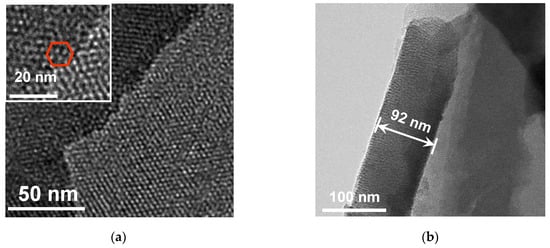
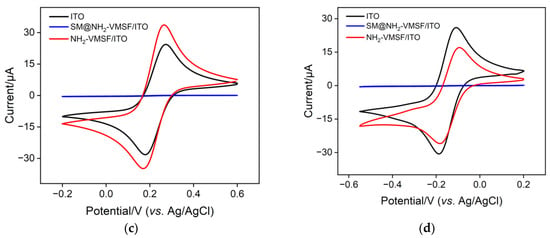
Figure 2.
(a) Top-view and (b) cross-sectional TEM images of the NH2-VMSF. The inset in (a) is the corresponding magnified image showing hexagonally distributed nanopores. CV responses of ITO (black), VMSF/ITO (red) and SM@VMSF/ITO (blue) to 50 μM Fe(CN)63− (c) and 50 μM Ru(NH3)63+ (d) at a scan rate of 50 mV/s. The supporting electrolytes for Fe(CN)63− and Ru(NH3)63+ are 0.05 M KHP.
The surface appearance of the NH2-VMSF before and after the confined growth of CuNPs for an electrodeposition time of 10 s and 15 s (abbreviated as CuNPs10s/NH2-VMSF/ITO and CuNPs15s/NH2-VMSF/ITO) was examined via scanning electron microscopy (SEM). As seen in Figure 3a–c, the CuNPs10s/NH2-VMSF/ITO gave rise to a smooth surface, which was similar to that of the NH2-VMSF/ITO, suggesting that electrodeposited CuNPs were inside the tiny nanochannels of the NH2-VMSF. However, numerous nanoparticles were observed at the surface of the CuNPs15s/NH2-VMSF/ITO (Figure 3c), which resulted from the formation of inhomogeneous, large CuNPs on the top surface of the NH2-VMSF when the electrodeposition time for the CuNPs was extended to 15 s (Figure 3d). Therefore, longer electrodeposition time can lead to the extended growth of CuNPs from the nanochannel to the surface of the NH2-VMSF/ITO. CuNPs on the surface of the NH2-VMSF/ITO are unstable and easily fall off the electrode surface, while those inside the silica nanochannels exhibit high stability due to the confinement effect. Considering the stability issue of the fabricated electrode, 10s was selected as the optimal electrodeposition time to guarantee the growth of CuNPs inside the nanochannels. Figure 3e shows CV curves for the NH2-VMSF/ITO and CuNPs/NH2-VMSF/ITO electrodes in a 0.1 M KCl solution. By comparison, the CuNPs/NH2-VMSF/ITO electrode exhibited the characteristic peaks of CuNPs, namely anodic peaks at −0.15 V and 0.10 V, corresponding to the oxidation of Cu(0) to Cu(I) and Cu(I) to Cu(II), and cathodic peaks at −0.09 V and −0.50 V, corresponding to the reduction of Cu(II) to Cu(I) and Cu(I) to Cu(0). Figure 3f shows the XPS spectrum of the CuNPs/NH2-VMSF/ITO, and the inset shows a magnified view of the peak of Cu 2p, demonstrating the presence of CuNPs. All of these results confirm the successful confinement of CuNPs inside the nanochannels of the NH2-VMSF via the electrodeposition procedure.
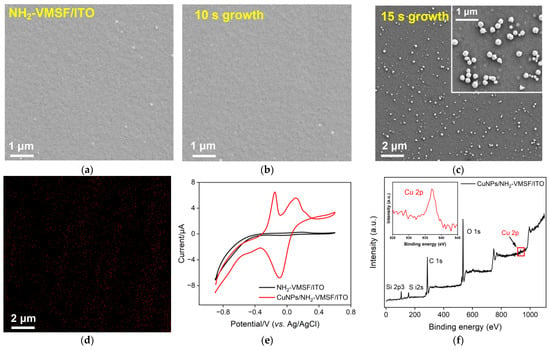
Figure 3.
Top-view SEM images of the NH2-VMSF/ITO before (a) and after the confined growth of CuNPs for an electrodeposition time of 10 s (b) and 15 s (c). (d) The Cu element mapping image of the CuNPs/NH2-VMSF/ITO from Figure 2c. (e) CV curves of the NH2-VMSF/ITO and CuNPs/NH2-VMSF/ITO electrodes in a 0.1 M KCl solution. (f) XPS spectra of the CuNPs/NH2-VMSF/ITO; the inset is a magnified view of the red box in the figure.
2.2. Electrocatalytic Reduction of NO3− Using CuNPs/NH2-VMSF/ITO
Figure 4a depicts the electrochemical reduction ability of the fabricated CuNPs/NH2-VMSF/ITO electrode towards NO3−. Upon the addition of 300 μM NO3− into a 0.1 M Na2SO4 (pH = 3.0) solution, an obvious cathodic peak was observed at the CuNPs/NH2-VMSF/ITO electrode, which was attributed to the electrocatalytic reduction of NO3− at the fabricated electrode. Figure 4b compares the CV and DPV responses of the CuNPs/NH2-VMSF/ITO, NH2-VMSF/ITO and bare ITO electrodes towards 300 μM NO3− in a 0.1 M Na2SO4 (pH = 3.0) solution. As shown, no cathodic peak signal was observed at the bare ITO electrode and a weak signal was obtained at the NH2-VMSF/ITO electrode due to the electrostatic interaction between the positively charged channel walls and the negatively charged NO3−. After electrodeposition of CuNPs into the nanochannels, the CuNPs/NH2-VMSF/ITO exhibited a significantly increased cathodic peak current for NO3−, which was attributed to the excellent electrocatalytic ability of CuNPs and the porous nanostructure of the NH2-VMSF for the growth of numerous CuNPs. Therefore, the inherent nanocatalytic properties of CuNPs and the electrostatic accumulation ability were combined to obtain a highly sensitive determination of NO3−, showing superior analytical performance.
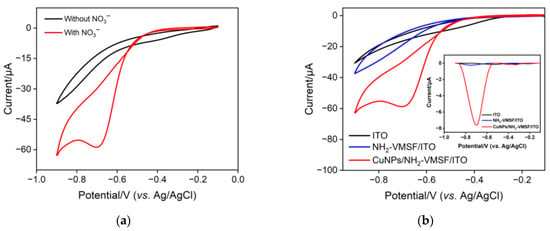
Figure 4.
(a) CV curves of a fabricated CuNPs/NH2-VMSF/ITO electrode in 0.1 M Na2SO4 (pH = 3.0) in the absence and presence of 300 μM NO3−. (b) CV responses of bare ITO, NH2-VMSF/ITO, and CuNPs/NH2-VMSF/ITO electrodes towards 300 μM NO3− in 0.1 M Na2SO4 (pH = 3.0). The inset in (b) shows the corresponding DPV curves for 300 μM NO3−.
The mechanism for the electrocatalytic reduction of NO3− on the CuNPs/NH2-VMSF/ITO electrode was investigated via CV and differential pulse voltammetry (DPV). Figure 5a shows the CV curves of 300 μM NO3− in 0.1 M Na2SO4 (pH = 3.0) at the CuNPs/NH2-VMSF/ITO under different scan rates. The cathodic peak current (Ipc) and cathodic peak (Epc) of 300 μM NO3− at the CuNPs/NH2-VMSF/ITO were extracted from Figure 5a and plotted as a function of scan rate (v) and natural logarithm (ln v), respectively. As shown in Figure 5b, the Ipc was linearly proportional to the v within the range of 60 to 220 mV/s, suggesting a surface-controlled electrocatalytic reduction process of NO3− at the CuNPs/NH2-VMSF/ITO. The relation between Epc and ln v was linear as shown in Figure 5c and can be expressed as the following equation:
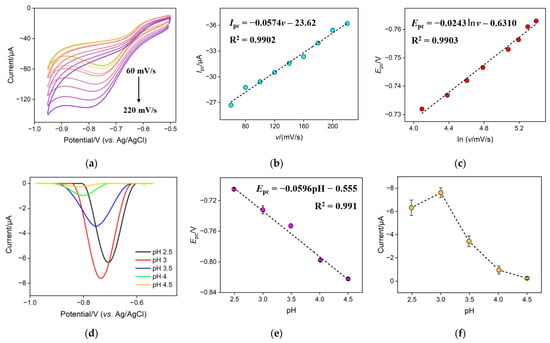
Figure 5.
(a) CV curves for 300 μM NO3− in 0.1 M Na2SO4 (pH = 3.0) at the CuNPs/NH2-VMSF/ITO under different scan rates (from top to bottom: 60, 80, 100, 120, 140, 160, 180, 200, and 220 mV/s). (b) The plot of the cathodic peak current (Ipc) obtained from (a) against scan rate (v). (c) The plot of the cathodic peak (Epc) obtained from (a) against the natural logarithm of the scan rate (lnv). (d) DPV responses of the CuNPs/NH2-VMSF/ITO for 300 μM NO3− in 0.1 M Na2SO4, adjusted to various pH values. The plots of the Epc (e) and cathodic peak current (f) obtained from (d) against the pH value.
The Laviron equation was used as follows to describe the relationship between Epc and ln v:
where , Ks, α, and n are the standard electrode potential, the standard heterogeneous rate constant, the transfer coefficient, and the number of electrons involved in the rate-determining step, respectively. Other symbols have their usual physical meanings: R, gas constant (8.314 J moL−1 K−1); T, absolute temperature (298 K); F, Faraday constant (96,485 C moL−1).
The following can be deduced from Equations (1) and (2):
According to Equation (3), the value of αn was calculated to be 1.05. Given a α of 0.5 in the completely irreversible electrochemical reaction, n was calculated to be 2.1, indicating that the reduction process of NO3− involves two electrons.
The slope of the fitting linear relationship between Epc and pH could be used to determine the ratio of electrons and protons participating in the electrochemical reaction on the electrode surface. Figure 5d shows the DPV curves of the CuNPs/NH2-VMSF/ITO electrode for 300 μM NO3− in 0.1 M Na2SO4, adjusted to various pH values. As the curves demonstrate, Epc became more negative as the pH increased. The good linear relationship shown in Figure 5e in the range of 2.5 to 4.5 can be expressed as follows:
Given that , where m is the number of protons and the other symbols are the same as above, the calculated m/n for the NO3− reduction process was 1.01, indicating equal involvement of protons and electrons in the electrochemical reduction of NO3−. Combining an n of 2 as obtained above, it could be inferred that the electrochemical reduction reaction of NO3− is a two-electron coupled, two-proton process, which can be shown as follows:
2.3. Influence of Experimental Conditions on Electrochemical Detection of NO3−
To obtain good analytical performance, the influence of the pH value of the Na2SO4 solution on the reduction of NO3− was investigated. Figure 5f shows the cathodic peak current for 300 μM NO3− in 0.1 M Na2SO4 at the CuNPs/NH2-VMSF/ITO at different pH values. The cathodic peak current initially increased with increasing pH, reaching a maximum at pH 3, and then decreased as the pH continued to increase. When the pH was less than 3, a hydrogen evolution reaction occurred at a less negative potential in strongly acidic media, which could affect the reduction of NO3−. When the pH was greater than 3, a decrease in the cathodic peak current of NO3− was found. This is because the hydrogen ions participate in the electrochemical reduction reaction of NO3− to NO2−. An increase in pH made the chemical equilibrium (Equation (5)) shift to the left, leading to the decreased cathodic peak current. Therefore, pH 3 was selected as the optimum condition.
Optimizing the amount of CuNPs inside the nanochannels of the NH2-VMSF was crucial to the effective accumulation of NO3− for achieving the highest sensitivity. The electrodeposition time of CuNPs can be used for determining the amount of CuNPs inside the nanochannels, which was studied in Figure 6a. As shown here, when the electrodeposition time of the CuNPs increased from 0 s to 10 s, the electrochemical reduction signal of NO3− gradually improved, because having more CuNPs inside the nanochannels enhanced the electrocatalytic capacity for NO3− reduction. But further increasing the electrodeposition time causes excessive aggregation of CuNPs on the outer surface of the NH2-VMSF channels. Therefore, the optimal electrodeposition time for the growth of CuNPs was achieved at 10 s. The inner walls of the NH2-VMSF channels are rich in amino groups, which carry positive charges in acidic environments and exhibit electrostatic adsorption towards NO3−. When the CuNPs/NH2-VMSF/ITO was magnetically stirred in a buffer solution containing 100 μM NO3−, the achieved cathodic peak current signal increased significantly from 0 to 6 min (Figure 6b), which was due to more NO3− having diffused to the underlying electrode surface through the nanochannels of the NH2-VMSF. After 6 min, the cathodic peak current of NO3− remained unchanged. Therefore, the accumulation time of 6 min was employed for the subsequent test.
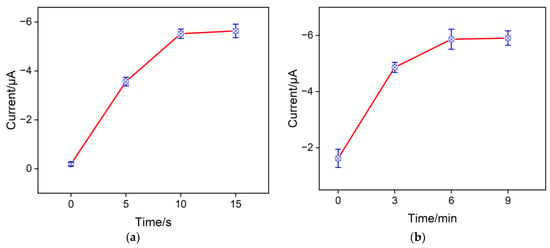
Figure 6.
Effects of electrodeposition time (a) on the growth of CuNPs and of mechanical stirring time (b) for the preconcentration of NO3− on the electrochemical responses for 100 μM NO3− at the CuNPs/NH2-VMSF/ITO in a Na2SO4 (0.1 M, pH = 3) solution.
2.4. Electroanalytical Performance of NO3− Using CuNPs/NH2-VMSF/ITO
The differential pulse voltammetry (DPV) technique was used to determine different concentrations of NO3− in a 0.1 M Na2SO4 solution using the CuNPs/NH2-VMSF/ITO electrode. Figure 7 shows the DPV signals and calibration curves of NO3− in the range of 5 μM−1 mM. As presented, the cathodic peak current of NO3− tested at the CuNPs/NH2-VMSF/ITO electrode grew linearly with concentration. The linear regression equation in the range of 5–100 μM can be expressed as I (μA) = 0.051 C (μM) + 0.29 (R2 = 0.996), and the other linear regression equation in the range of 100–1000 μM as I (μA) = 0.021 C (μM) + 3.77 (R2 = 0.998). The limit of detection (LOD) was estimated to be 2.3 μM at a signal-to-noise ratio of three using the formula of LOD = 3SD/k (where SD and k are the standard deviation of the blank solution and the slope of the calibration curve, respectively). Note that the LOD value is far below the concentration limit (806 μM) for NO3− in the water quality standard specified by World Health Organization. Moreover, the proposed CuNPs/NH2-VMSF/ITO has several advantages over other electrodes reported in the literature, such as a lower LOD, a wider dynamic linear range, and easy fabrication steps (Table 1).
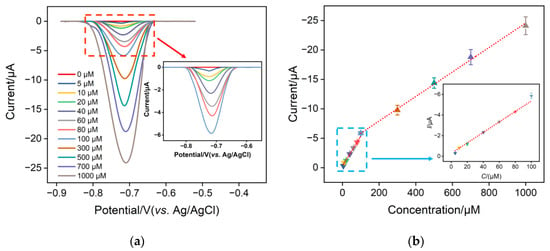
Figure 7.
(a) DPV responses of the CuNPs/NH2-VMSF/ITO to the successive addition of various concentrations of NO3− in a Na2SO4 (0.1 M, pH 3.0) solution. The concentrations of NO3− range from 5 μM to 1000 μM. (b) The cathodic peak current—concentration plot for the CuNPs/NH2-VMSF/ITO electrode with the addition of various concentrations of NO3− in a Na2SO4 (0.1 M, pH 3.0) solution. Insets in (a,b) represent the corresponding amplified curves in the low concentration range, and the error bars in (b) represent the standard deviations of three measurements.

Table 1.
Analytical results of several modified electrodes for the detection of NO3−.
2.5. Anti-Interference, Regeneration, Reproducibility, and Stability of CuNPs/NH2-VMSF/ITO
The selectivity of the prepared CuNPs/NH2-VMSF/ITO sensor for NO3− detection was evaluated in the presence of common interfering ions, such as 1 mM Na2+, K+, Ca2+, Mg2+, Na+, NO2−, Cl−, Br−, SO42−, PO43−, and SO32−. As shown in Figure 8a, a ten-fold concentration of these interfering ions produced minimal interference for the determination of 100 μM NO3− at the CuNPs/NH2-VMSF/ITO, indicating the good selectivity and anti-interference ability of the proposed sensor. To assess the electrode’s regeneration capacity, the same CuNPs/NH2-VMSF/ITO electrode was used to repeatedly measure 300 μM NO3−; the used electrode was washed with a 0.1 M HCl–ethanol solution for 5 min prior to testing. As shown in Figure 8b, no significant decrease in the current signal was observed at our fabricated sensor after five-time elution, demonstrating the excellent regeneration ability of the sensor. Five batches of CuNPs/NH2-VMSF/ITO electrodes were prepared under the same conditions and used to test 300 μM NO3− in order to examine the reproducibility of the electrode. The calculated relative standard deviation (RSD) of the measured results from the five electrodes was 1.8% (Figure 8c), confirming the high reproducibility of the CuNPs/NH2-VMSF/ITO electrode. Additionally, the stability of the fabricated CuNPs/NH2-VMSF/ITO electrode was studied by comparing the initial cathodic peak current of NO3− with that obtained after five days in storage. The data shown in Figure 8d prove the good stability of CuNPs/NH2-VMSF/ITO electrode under a nitrogen atmosphere compared with storage under an air atmosphere.
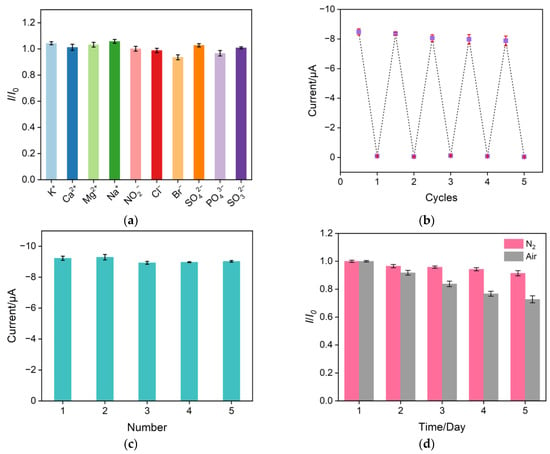
Figure 8.
(a) Cathodic peak current ratio obtained at the developed CuNPs/NH2-VMSF/ITO electrode for the detection of 100 μM NO3− before (I0) and after (I) the addition of 1 mM of various interfering substances to a Na2SO4 solution (0.1 M pH = 3). (b) DPV signals of 300 μM NO3− measured repeatedly after multiple elutions of the CuNPs/NH2-VMSF/ITO electrode. (c) DPV signals of 300 μM NO3− in a Na2SO4 solution (0.1 M pH = 3) measured using five different electrodes prepared in parallel. (d) DPV signals of the CuNPs/NH2-VMSF/ITO electrodes for the detection of 300 μM NO3− in a 0.1 M Na2SO4 (pH = 3) solution after storage for different numbers of days in air and nitrogen atmospheres, respectively. The error bars represent the standard deviations of three measurements.
2.6. Direct Analysis of NO3− in Water Samples
According to the previous reports, NH2-VMSF serves as a good anti-fouling protective layer on the electrode surface and has been used to design many electrochemical sensors in rather complicated real samples [38]. Environmental water samples including tap water, rainwater, lake water, and seawater were selected to validate the practical applicability of our fabricated CuNPs/NH2-VMSF/ITO electrode. The pH of these collected water samples was adjusted to 3 using sulfuric acid, and several known concentrations of NO3− were added. The CuNPs/NH2-VMSF/ITO electrode was then used to analyze the above samples using the DPV technique. Table 2 shows the quantitative results for NO3− in water samples using the standard addition method. As shown, our proposed CuNPs/NH2-VMSF/ITO electrode exhibited excellent recovery values ranging from 97.8% to 109%, demonstrating the good analytical performance of the developed sensor in real water samples.

Table 2.
Quantification of NO3− in real samples using CuNPs/NH2-VMSF/ITO electrode.
3. Materials and Methods
3.1. Chemicals and Instrumentations
All analytical grade chemicals and reagents in this study were used as received without further purification. Ultrapure water was obtained from the Millipore Milli-Q system (18 MΩ cm). Tetraethoxysilane (TEOS), cetyltrimethylammonium bromide (CTAB), and (3-aminopropyl) triethoxysilane (APTES) were purchased from Sigma-Aldrich. Potassium ferricyanide (K3[Fe(CN)6]), hexaammineruthenium (III) chloride ([Ru(NH3)6Cl3]), and acetone were ordered from Shanghai Aladdin Biochemical Technology Co., Ltd. Sodium sulfate (Na2SO4), sodium sulfite (Na2SO3), sodium nitrite (NaNO2), sodium phosphate (Na3PO4), sodium chloride (NaCl), potassium chloride (KCl), potassium bromide (KBr), potassium nitrate (KNO3), and copper (II) sulfate pentahydrate (CuSO4·5H2O) were bought from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China) Indium tin oxide (ITO) conductive glass (surface resistivity < 17 Ω/square, thickness of 100 ± 20 nm) were purchased from Zhuhai Kaivo Optoelectronics Technology Co., Ltd. (Zhuhai, China) The ITO glass was sonicated with 1 M aqueous sodium hydroxide for two hours, followed by acetone, ethanol, and deionized water sonication for ten minutes each. Finally, the ITO glass was dried at 60 °C prior to use.
The shape and thickness of the NH2-VMSF were determined using transmission electron microscopy (TEM, HT7700, Hitachi, Japan) and scanning electron microscopy (SEM, SU8010, Hitachi, Japan) at accelerating voltages of 200 kV and 5 kV, respectively. A PHI5300 electron spectrometer (PE Ltd., Boston, MA, USA) was used to conduct an X-ray photoelectron spectroscopy (XPS) examination with 250 W, 14 kV Mg K radiation. Electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), and differential pulse voltammetry (DPV) measurements were carried out using an Autolab electrochemical workstation (PGSTAT302N, Metrohm, Switzerland). Measurements of electrochemical reactions were performed using a conventional three-electrode system. A bare ITO or modified ITO (0.5 × 1 cm2), an Ag/AgCl (saturated with KCl solution), and a platinum wire were selected as the working electrode, the reference electrode, and the counter electrode, respectively.
3.2. Preparation of the NH2-VMSF/ITO Electrode
The NH2-VMSF was grown on a conductive ITO electrode using the electrochemically assisted self-assembly (EASA) method [35]. A mixed solution composed of 20 mL ethanol, 20 mL 0.1 M NaNO3, 13.6 mM “TEOS + APTES” (9:1 molar ratio), and 4.35 mM CTAB were first prepared. After adjusting the pH to 3.0 with hydrochloric acid (HCl, 3 M), the precursor solution was stirred for 2.5 h at room temperature. A clean ITO (0.5 × 1 cm2) was immersed into the precursor solution and electrodeposited at a constant current of −0.35 mA for 10 s. The resulting electrode was immediately removed from the growth solution, thoroughly rinsed with ultrapure water, dried under a nitrogen atmosphere, and aged overnight at 120 °C. During the preparation process, a surfactant micelle (SM) consisting of CTAB served as a template and remained in the nanochannels of the NH2-VMSF; this was given the designation SM@NH2-VMSF/ITO. SMs can be easily eliminated by stirring in 50 mL of an ethanol solution containing 0.1 M hydrochloric acid for 5 min. The resulting electrode was designated as the NH2-VMSF/ITO electrode.
3.3. Electrochemical Deposition of CuNPs
The electrochemical deposition of CuNPs into the NH2-VMSF was modified slightly according to reference [56] as follows: 0.5 mmol CuSO4·5H2O was added to 50 mL of a 0.1 M sulfuric acid solution and sonicated for 5 min to obtain the electrodeposition solution. Then, the NH2-VMSF/ITO/ITO electrode was placed in the above electrodeposition solution and was subjected to a constant potential of −0.6 V for 10 s using a platinum sheet as the counter electrode and Ag/AgCl as the reference electrode. Finally, the resulting electrode was rinsed with ultrapure water and blow-dried with nitrogen gas, yielding the CuNPs/NH2-VMSF/ITO electrode.
3.4. Detection of NO3−
Before the electrochemical test, the Na2SO4 solution (0.1 M and pH adjusted to 3.0 with sulfuric acid) was deoxygenated by bubbling nitrogen gas in the solution for 30 min. Various concentrations of NO3− were added to the above solution and determined using the CuNPs/NH2-VMSF/ITO electrode under a nitrogen atmosphere. The DPV parameters included a step potential of 0.005 V, a pulse amplitude of 0.05 V, an interpulse time of 0.2 s, and a pulse time of 0.05 s.
3.5. Actual Sample Testing
Tap water, rainwater, lake water, and seawater were selected as actual samples to verify the accuracy of the CuNPs/NH2-VMSF/ITO sensor using the standard addition method. Tap water, lake water, and rainwater in the samples were sourced locally (Hangzhou, China), and seawater produced in Qingdao (Shandong, China) was purchased from Taobao. The pH of these environmental water samples was adjusted to 3 using sulfuric acid (0.1 M) without dilution. Different concentrations of NO3− were added to the water samples and then determined using the CuNPs/NH2-VMSF/ITO sensor.
4. Conclusions
A simple and highly sensitive NO3− electrochemical sensor was developed based on an NH2-VMSF and CuNPs confined in the nanochannels. Physical confinement of CuNPs was achieved via a controllable one-step electrodeposition procedure. The immobilization of the CuNPs in the tiny nanochannels endows the electrode with the electrocatalytic capacity for reducing NO3− and conducting highly sensitive NO3− measurements. Not only can the NH2-VMSF serve as a hard template for the stable growth of CuNPs, but it can also provide the electrostatic accumulation capacity for the target NO3−. The detection limit for this kind of CuNPs/NH2-VMSF/ITO sensor is as low as 2.3 μM with a linear range extending from 5.0 μM to 1 mM for NO3− determinations. Furthermore, direct analysis of NO3− concentrations in various environmental samples using our proposed sensor was evaluated, revealing acceptable accuracy and great promise for fast NO3− monitoring in samples of polluted water such as sewage.
Author Contributions
Investigation, D.L. and H.J.; data curation, D.L., S.X. and J.W.; writing—original draft preparation, D.L.; validation, H.J. and J.W.; writing—review and editing, conceptualization and supervision, F.Y.; project administration, D.L. and F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was jointly funded by Science Foundation of Donghai Laboratory (Grant no. DH-2022KF0201), the project of the Donghai Laboratory (Grant no. DH-2022ZY0006), Scientific Research Fund of the Second Institute of Oceanography, MNR (QNYJ2203), Zhejiang Provincial Natural Science Foundation of China (LY21B050003), and the Fundamental Research Funds of Zhejiang Sci-Tech University (22062310-Y).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Sun, Z.; Liu, Y. An overview of in-situ remediation for nitrate in groundwater. Sci. Total Environ. 2022, 804, 149981. [Google Scholar] [CrossRef] [PubMed]
- Brimblecombe, P.; Stedman, D.H. Historical evidence for a dramatic increase in the nitrate component of acid rain. Nature 1982, 298, 460–462. [Google Scholar] [CrossRef]
- Howarth, R.W. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 2008, 8, 14–20. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Sigman, D.M.; Boyle, E.A. Glacial/interglacial variations in atmospheric carbon dioxide. Nature 2000, 407, 859–869. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; De Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; Van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- Abascal, E.; Gómez-Coma, L.; Ortiz, I.; Ortiz, A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2022, 810, 152233. [Google Scholar] [CrossRef]
- Yu, G.; Wang, J.; Liu, L.; Li, Y.; Zhang, Y.; Wang, S. The analysis of groundwater nitrate pollution and health risk assessment in rural areas of Yantai, China. BMC Public Health 2020, 20, 437. [Google Scholar] [CrossRef]
- Hansen, H.P.; Koroleff, F. Determination of nutrients. In Methods Seawater Analysis, 3rd; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Wiley: Weinheim, Germany, 1999; pp. 159–228. [Google Scholar]
- Johnson, K.S.; Coletti, L.J. In situ ultraviolet spectrophotometry for high resolution and long-term monitoring of nitrate, bromide and bisulfide in the ocean. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2002, 49, 1291–1305. [Google Scholar] [CrossRef]
- Strianese, M.; Milione, S.; Bertolasi, V.; Pellecchia, C. Iron and manganese pyridoxal-based complexes as fluorescent probes for nitrite and nitrate anions in aqueous solution. Inorg. Chem. 2013, 52, 11778–11786. [Google Scholar] [CrossRef] [PubMed]
- Kodamatani, H.; Kubo, S.; Takeuchi, A.; Kanzaki, R.; Tomiyasu, T. Sensitive detection of nitrite and nitrate in seawater by 222 nm UV-irradiated photochemical conversion to peroxynitrite and ion chromatography-luminol chemiluminescence system. Environ. Sci. Technol. 2023, 57, 5924–5933. [Google Scholar] [CrossRef] [PubMed]
- Kaminskaya, O.V.; Zakharova, E.A.; Slepchenko, G.B. Simultaneous voltammetric determination of nitrites and nitrates in waters. J. Anal. Chem. 2004, 59, 1091–1096. [Google Scholar] [CrossRef]
- Khan, M.R.; Wabaidur, S.M.; Alothman, Z.A.; Busquets, R.; Naushad, M. Method for the fast determination of bromate, nitrate and nitrite by ultra performance liquid chromatography–mass spectrometry and their monitoring in Saudi Arabian drinking water with chemometric data treatment. Talanta 2016, 152, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Hern, J.A.; Rutherford, G.K.; Vanloon, G.W. Determination of chloride, nitrate, sulphate and total sulphur in environmental samples by single-column ion chromatography. Talanta 1983, 30, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.F.; Anastasi, A.; Chandra, S. Electrochemical detection of nitrate, nitrite and ammonium for on-site water quality monitoring. Curr. Opin. Electrochem. 2022, 32, 100926. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Des. 2022, 215, 110506. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, H.; Chen, M.; Zhao, C.; Liu, Y.; Xi, F.; Luo, T. Functional nanostructure-loaded three-dimensional graphene foam as a non-enzymatic electrochemical sensor for reagentless glucose detection. RSC Adv. 2020, 10, 33739–33746. [Google Scholar] [CrossRef]
- Fox, C.M.; Breslin, C.B. Electrochemical formation of silver nanoparticles and their applications in the reduction and detection of nitrates at neutral pH. J. Appl. Electrochem. 2020, 50, 125–138. [Google Scholar] [CrossRef]
- Pan, D.; Lu, W.; Wu, S.; Zhang, H.; Qin, W. In situ spontaneous redox synthesis of carbon nanotubes/copper oxide nanocomposites and their preliminary application in electrocatalytic reduction of nitrate. Mater. Lett. 2012, 89, 333–335. [Google Scholar] [CrossRef]
- Marlinda, A.R.; An’amt, M.N.; Yusoff, N.; Sagadevan, S.; Wahab, Y.A.; Johan, M.R. Recent progress in nitrates and nitrites sensor with graphene-based nanocomposites as electrocatalysts. Trends Environ. Anal. Chem. 2022, 34, e00162. [Google Scholar] [CrossRef]
- Parveen, S.; Pathak, A.; Gupta, B.D. Fiber optic SPR nanosensor based on synergistic effects of CNT/Cu-nanoparticles composite for ultratrace sensing of nitrate. Sens. Actuators B Chem. 2017, 246, 910–919. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Bressi, V.; Espro, C.; Iannazzo, D.; Piperopoulos, E.; Neri, G. Electrochemical determination of nitrites and sulfites by using waste-derived nanobiochar. J. Electroanal. Chem. 2023, 928, 117071. [Google Scholar] [CrossRef]
- Saha, P.; Akter, R.; Shaheen Shah, S.; Mahfoz, W.; Aziz, M.A.; Saleh Ahammad, A.J. Gold nanomaterials and their composites as electrochemical sensing platforms for nitrite detection. Chem.-Asian J. 2022, 17, e202200823. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Duan, W.; Liu, X.; Xi, F.; Wu, J. Microneedle patch integrated with porous silicon confined dual nanozymes for synergistic and hyperthermia-enhanced nanocatalytic ferroptosis treatment of melanoma. Adv. Funct. Mater. 2023, 2023, 2308183. [Google Scholar] [CrossRef]
- Duan, W.; Jin, Y.; Cui, Y.; Xi, F.; Liu, X.; Wo, F.; Wu, J. A co-delivery platform for synergistic promotion of angiogenesis based on biodegradable, therapeutic and self-reporting luminescent porous silicon microparticles. Biomaterials 2021, 272, 120772. [Google Scholar] [CrossRef]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Ratiometric fluorescent nanohybrid for noninvasive and visual monitoring of sweat glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, S.; Li, Y.; Liu, J. Facile synthesis of iron and nitrogen Co-doped carbon dot nanozyme as highly efficient peroxidase mimics for visualized detection of metabolites. Molecules 2023, 28, 6064. [Google Scholar] [CrossRef]
- He, J.; Li, Z.; Zhao, R.; Lu, Y.; Shi, L.; Liu, J.; Dong, X.; Xi, F. Aqueous synthesis of amphiphilic graphene quantum dots and their application as surfactants for preparing of fluorescent polymer microspheres. Colloid Surf. A 2019, 563, 77–83. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, Z.; Li, Y.; Xi, F.; Liu, J. Magnetic nanozyme based on loading nitrogen-doped carbon dots on mesoporous Fe3O4 nanoparticles for the colorimetric detection of glucose. Molecules 2023, 28, 4573. [Google Scholar] [CrossRef]
- Walcarius, A. Electroinduced surfactant self-assembly driven to vertical growth of oriented mesoporous films. Acc. Chem. Res. 2021, 54, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, S.; Liu, J.; Xing, J. Homogeneous electrochemical aptamer sensor based on two-dimensional nanocomposite probe and nanochannel modified electrode for sensitive detection of carcinoembryonic antigen. Molecules 2023, 28, 5186. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Qiu, X.; Han, Q.; Xi, F.; Wang, Y.; Chen, J. Anti-biofouling electrochemical sensor based on the binary nanocomposite of silica nanochannel array and graphene for doxorubicin detection in human serum and urine samples. Molecules 2022, 27, 8640. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, C.; Xi, F. Disposable amperometric label-free immunosensor on chitosan-graphene-modified patterned ITO electrodes for prostate specific antigen. Molecules 2022, 27, 5895. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, T.; Zheng, Y.; Liu, J. Dual-mode sensing platform for cancer antigen 15-3 determination based on a silica nanochannel array using electrochemiluminescence and electrochemistry. Biosensors 2023, 13, 317. [Google Scholar] [CrossRef]
- Zhou, P.; Yao, L.; Chen, K.; Su, B. Silica nanochannel membranes for electrochemical analysis and molecular sieving: A comprehensive review. Crit. Rev. Anal. Chem. 2019, 50, 424–444. [Google Scholar] [CrossRef]
- Wang, K.; Yang, L.; Huang, H.; Lv, N.; Liu, J.; Liu, Y. Nanochannel array on electrochemically polarized screen printed carbon electrode for rapid and sensitive electrochemical determination of clozapine in human whole blood. Molecules 2022, 27, 2739. [Google Scholar] [CrossRef]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical sensor nanoarchitectonics for sensitive detection of uric acid in human whole blood based on screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef]
- Huang, L.; Su, R.; Xi, F. Sensitive detection of noradrenaline in human whole blood based on Au nanoparticles embedded vertically-ordered silica nanochannels modified pre-activated glassy carbon electrodes. Front. Chem. 2023, 11, 1126213. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Yan, F.; Lin, J. N-doped graphene quantum dots confined within silica nanochannels for enhanced electrochemical detection of doxorubicin. Molecules 2023, 28, 6443. [Google Scholar] [CrossRef]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ding, Y.; Su, R.; Lu, D.; Tang, H.; Xi, F. Silica nanochannel array film supported by ß-cyclodextrin-functionalized graphene modified gold film electrode for sensitive and direct electroanalysis of acetaminophen. Front. Chem. 2022, 9, 812086. [Google Scholar] [CrossRef]
- Zheng, W.; Su, R.; Lin, X.; Liu, J. Nanochannel array modified three-dimensional graphene electrode for sensitive electrochemical detection of 2,4,6-trichlorophenol and prochloraz. Front. Chem. 2022, 10, 954802. [Google Scholar] [CrossRef]
- Deng, X.; Lin, X.; Zhou, H.; Liu, J.; Tang, H. Equipment of vertically-ordered mesoporous silica film on electrochemically pretreated three-dimensional graphene electrodes for sensitive detection of methidazine in urine. Nanomaterials 2023, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Luo, X.; Xi, F. Probe-integrated electrochemical immunosensor based on electrostatic nanocage array for reagentless and sensitive detection of tumor biomarker. Front. Chem. 2023, 11, 1121450. [Google Scholar] [CrossRef]
- Ma, N.; Luo, X.; Wu, W.; Liu, J. Fabrication of a disposable electrochemical immunosensor based on nanochannel array modified electrodes and gated electrochemical signals for sensitive determination of C-reactive protein. Nanomaterials 2022, 12, 3981. [Google Scholar] [CrossRef]
- Zhou, X.; Han, Q.; Zhou, J.; Liu, C.; Liu, J. Reagentless electrochemical detection of tumor biomarker based on stable confinement of electrochemical probe in bipolar silica nanochannel film. Nanomaterials 2023, 13, 1645. [Google Scholar] [CrossRef]
- Su, R.; Tang, H.; Xi, F. Sensitive electrochemical detection of p-nitrophenol by pre-activated glassy carbon electrode integrated with silica nanochannel array film. Front. Chem. 2022, 10, 954748. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, T.; Tang, H.; Liu, J. Novel electrochemical and electrochemiluminescence dual-modality sensing platform for sensitive determination of antimicrobial peptides based on probe encapsulated liposome and nanochannel array electrode. Front. Nutr. 2022, 9, 962736. [Google Scholar] [CrossRef] [PubMed]
- Patella, B.; Russo, R.R.; O’Riordan, A.; Aiello, G.; Sunseri, C.; Inguanta, R. Copper nanowire array as highly selective electrochemical sensor of nitrate ions in water. Talanta 2021, 221, 121643. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.-P.N.; Brockgreitens, J.; Ahmed, S.; Abbas, A. Dual detection of nitrate and mercury in water using disposable electrochemical sensors. Biosens. Bioelectron. 2016, 85, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Amini, N.; Maleki, A.; Maleki, P. Electrochemical detection of nitrate ions via reduction of NO2− and oxidation of NO reactions based on Cu@TiO2 coreshell/nafion/polyalizarin immobilized electrode. Mater. Chem. Phys. 2021, 264, 124384. [Google Scholar] [CrossRef]
- Bagheri, H.; Hajian, A.; Rezaei, M.; Shirzadmehr, A. Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate. J. Hazard. Mater. 2017, 324, 762–772. [Google Scholar] [CrossRef]
- Wang, J.; Diao, P. Simultaneous detection of ammonia and nitrate using a modified electrode with two regions. Microchem. J. 2020, 154, 104649. [Google Scholar] [CrossRef]
- Essousi, H.; Barhoumi, H.; Bibani, M.; Ktari, N.; Wendler, F.; Al-Hamry, A.; Kanoun, O. Ion-imprinted electrochemical sensor based on copper nanoparticles-polyaniline matrix for nitrate detection. J. Sens. 2019, 2019, 4257125. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, M.; Li, S.; Ren, Y.; Qin, G. Copper wires with seamless 1D nanostructures: Preparation and electrochemical sensing performance. Mater. Lett. 2018, 211, 247–249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).