The Role of Polyphenolic Antioxidants from Tea and Rosemary in the Hydroxyl Radical Oxidation of N-Acetyl Alanine
Abstract
1. Introduction
2. Results and Discussion
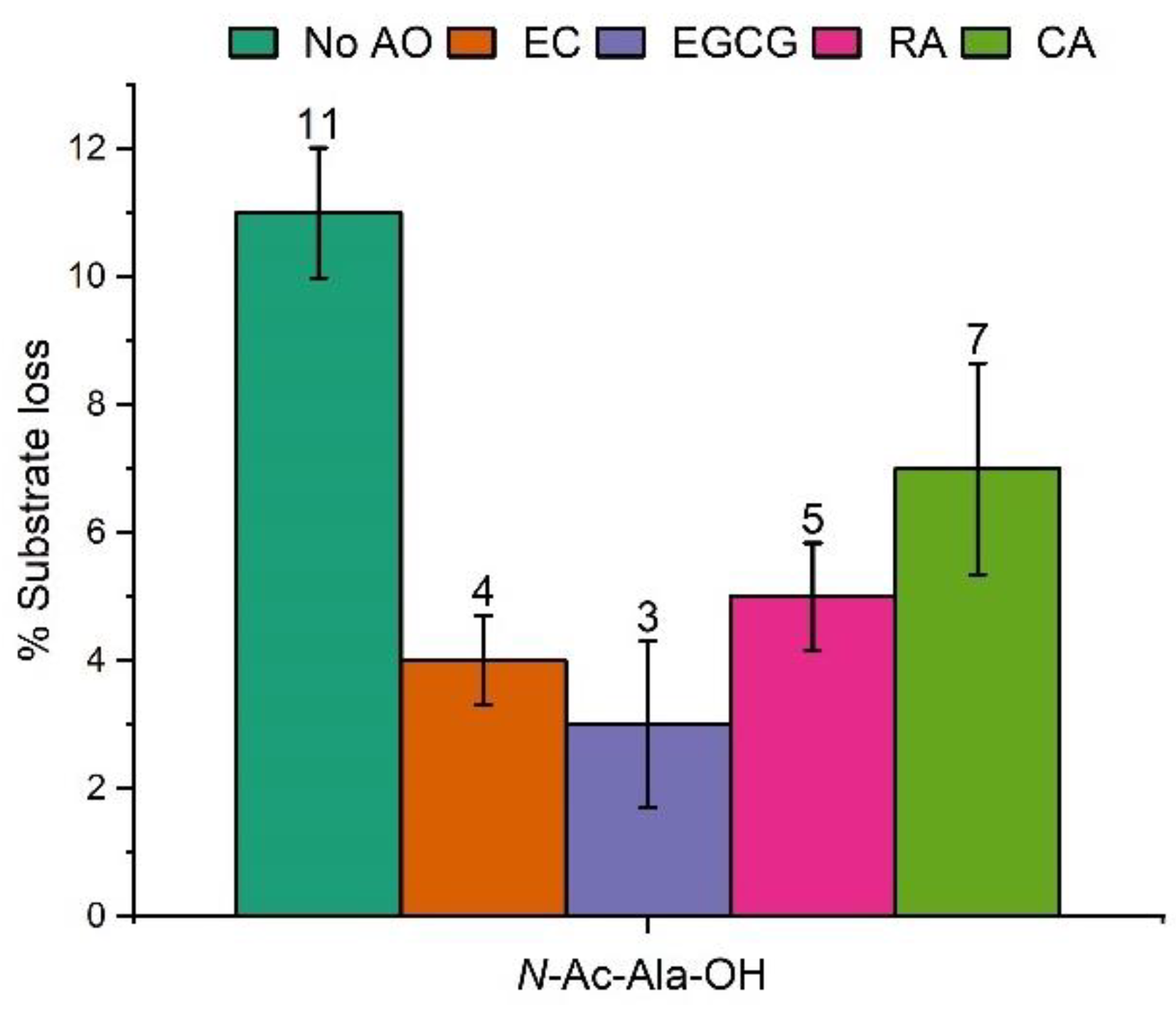
2.1. Hydroxyl Radical-Induced Oxidation of N-Ac-Ala-OH
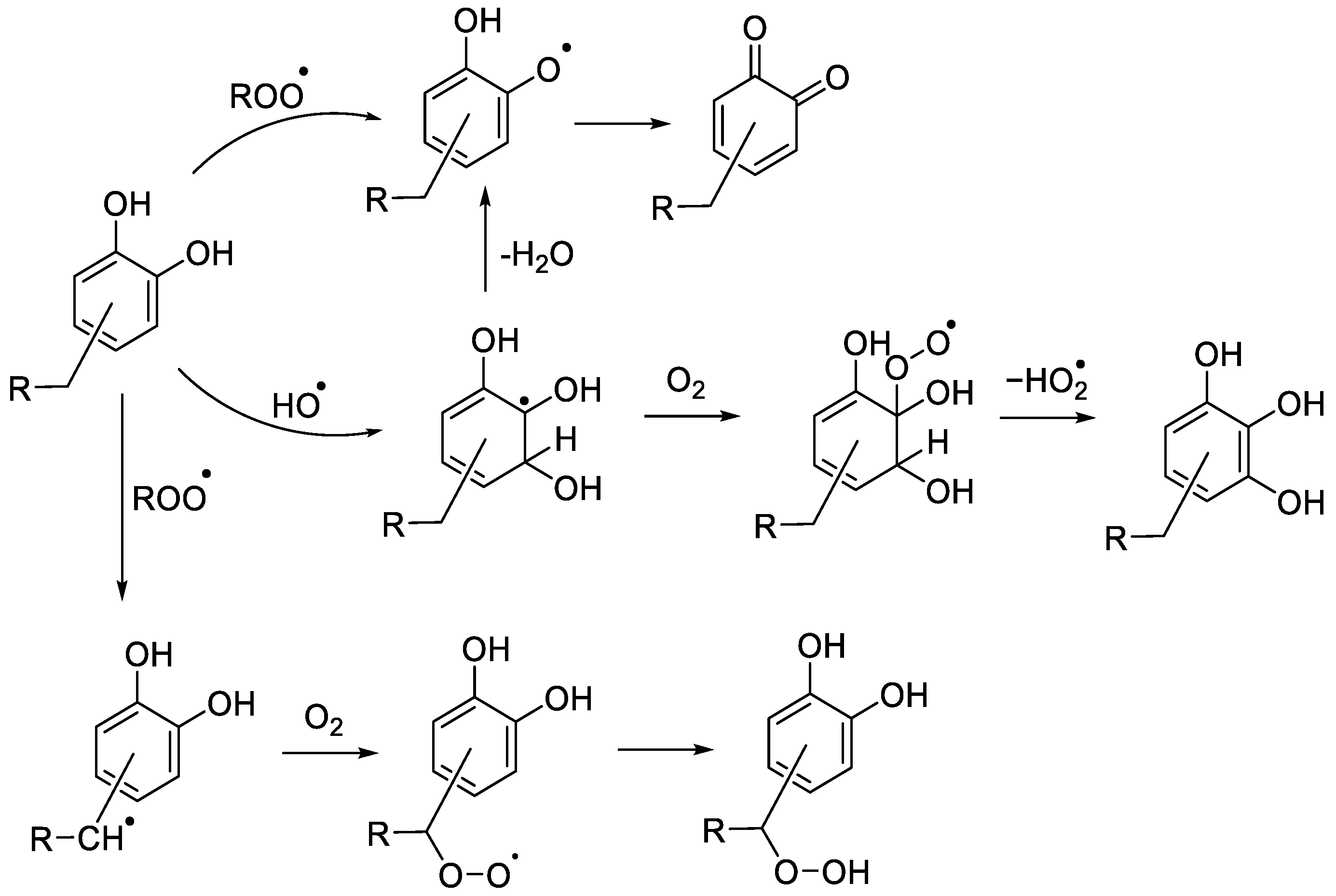
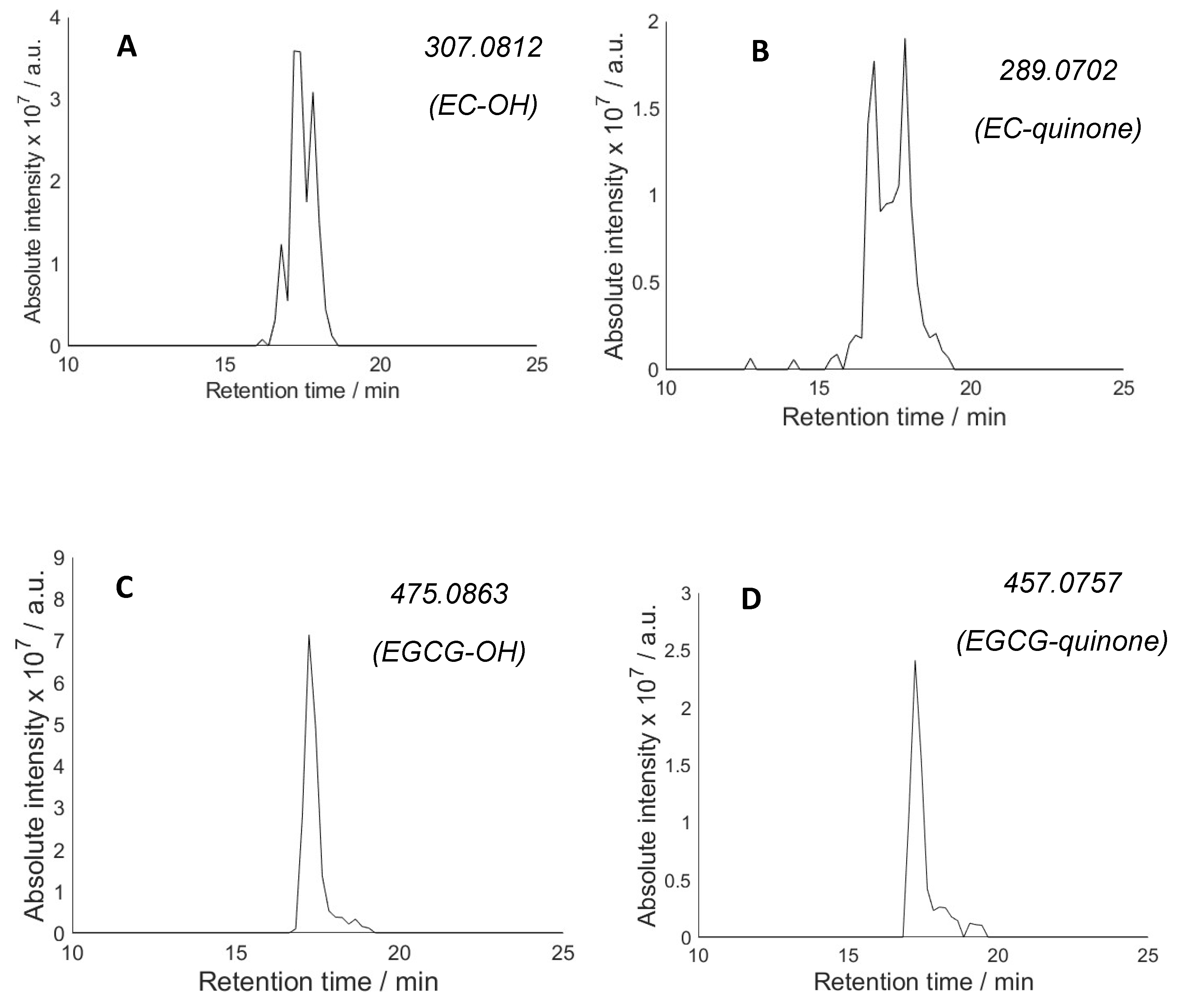
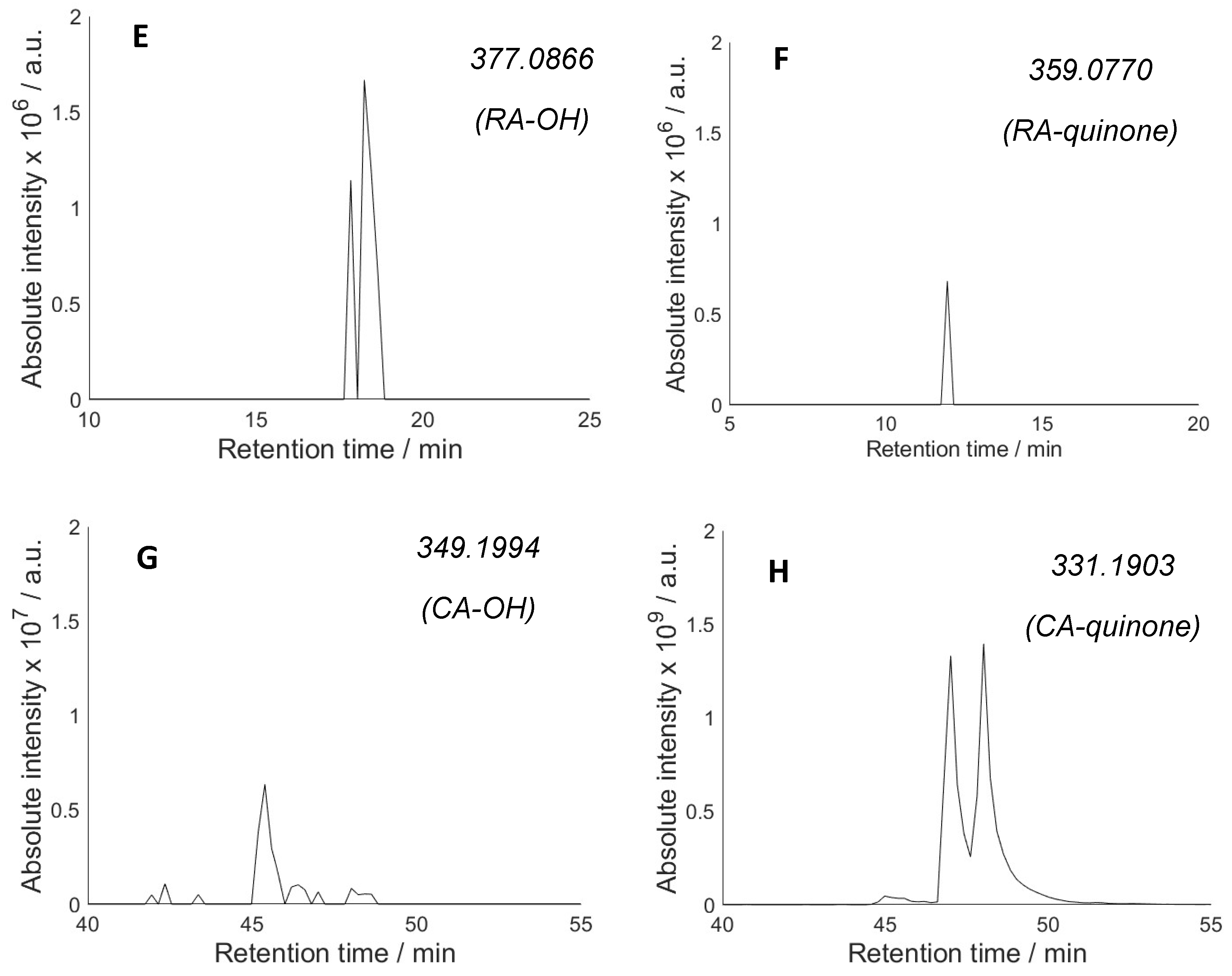
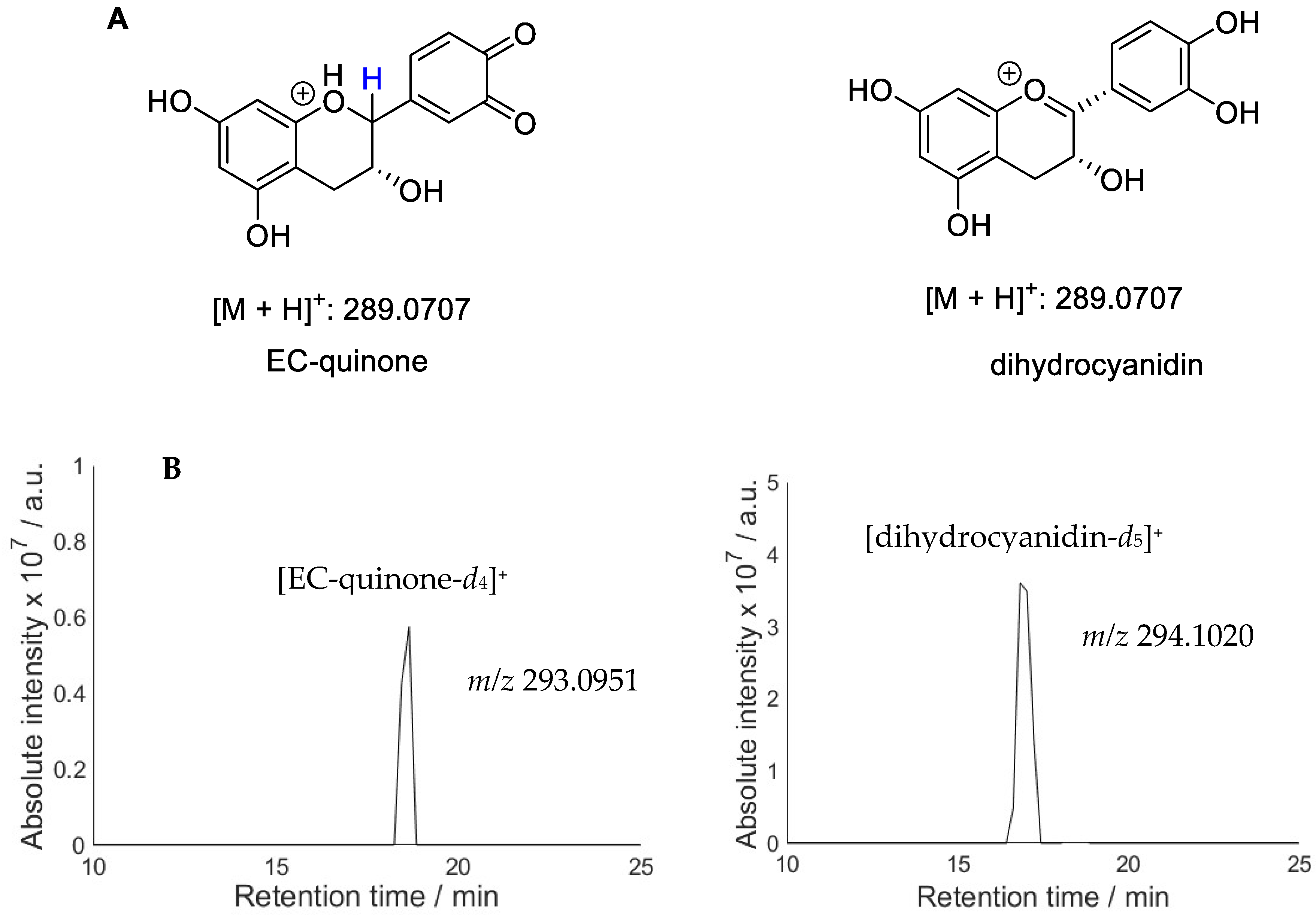
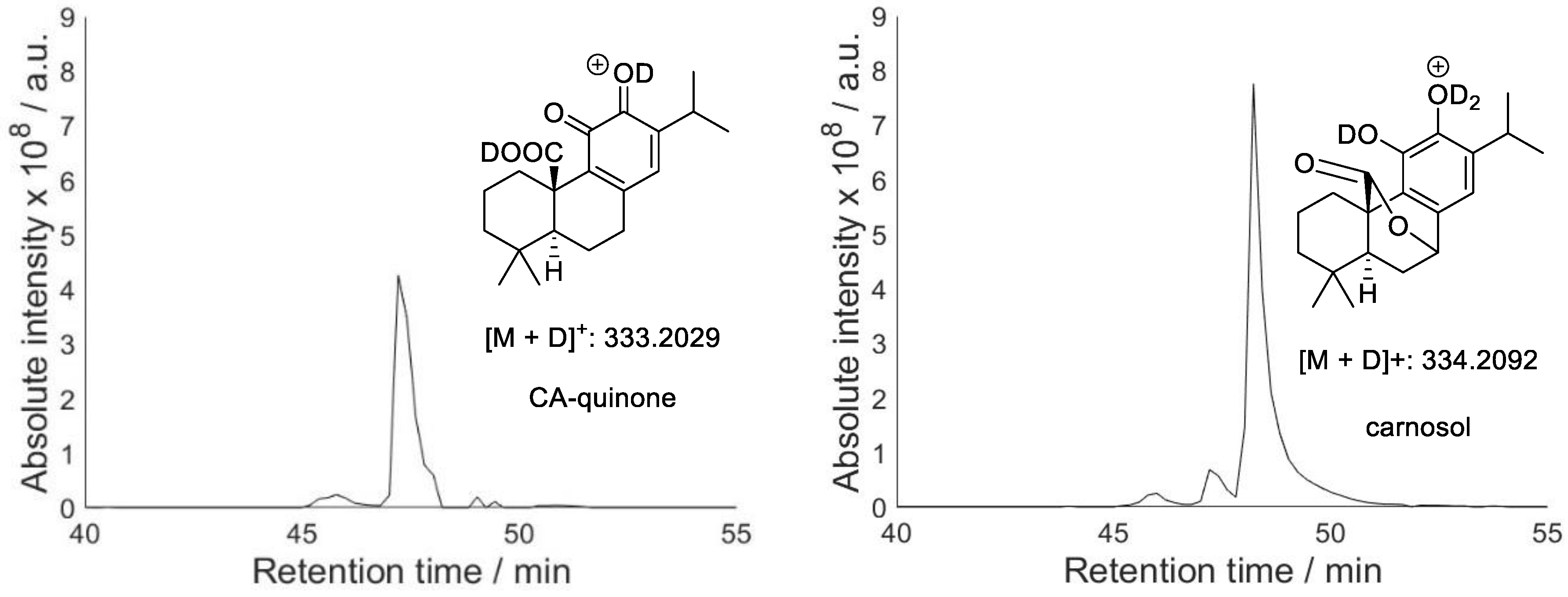
2.2. Hydroxylation of Antioxidants and Quinone Formation
2.3. Hydroperoxides of Antioxidants
2.4. Antioxidant Capacity
3. Materials and Methods
3.1. Chemicals
3.2. HO•-Mediated Oxidation of N-Ac-Ala-OH in the Presence of Antioxidants
3.3. LC-MS Analysis
3.4. N-Ac-Ala-OH LC-MS Calibration Curve
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Pokorný, J. Are natural antioxidants better—And safer—Than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Jukić, D.P.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant Activities of Antioxidants and their Impact on Health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: Implications for anticancer properties. Toxicol. In Vitro 2004, 18, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.Y.; Ou, N.; Lu, Q.-B. Antioxidant Induces DNA Damage, Cell Death and Mutagenicity in Human Lung and Skin Normal Cells. Sci. Rep. 2013, 3, 3169. [Google Scholar] [CrossRef]
- Gutiérrez-Del-río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394–e13416. [Google Scholar] [CrossRef]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2019, 8, 542. [Google Scholar] [CrossRef]
- Hong, K.H. Phenol compounds treated cotton and wool fabrics for developing multi-functional clothing materials. Fibers Polym. 2015, 16, 565–571. [Google Scholar] [CrossRef]
- Dario, M.F.; Pahl, R.; De Castro, J.R.; De Lima, F.S.; Kaneko, T.M.; Pinto, C.A.S.O.; Baby, A.R.; Velasco, M.V.R. Efficacy of Punica granatum L. hydroalcoholic extract on properties of dyed hair exposed to UVA radiation. J. Photochem. Photobiol. B Biol. 2013, 120, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Q.; Zhang, W.P.; Cui, J.J.; Wu, X.J. Effect of Honeysuckle Extract for Color Protection of Human Hair Exposed to Sunlight Radiation. Adv. Mater. Res. 2013, 821–822, 28–31. [Google Scholar] [CrossRef]
- Fernández, E.; Martínez-Teipel, B.; Armengol, R.; Barba, C.; Coderch, L. Efficacy of antioxidants in human hair. J. Photochem. Photobiol. B Biol. 2012, 117, 146–156. [Google Scholar] [CrossRef]
- Alboreadi, M.A.; Al-Najdawi, M.M.; Jarrar, Q.B.; Moshawih, S. Evaluation of hair growth properties of Topical Kombucha tea extracts. Adv. Trad. Med. 2022, 22, 155–161. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Ternes, W. Antioxidative constituents of Rosmarinus officinalis and Salvia officinalis. II. Isolation of carnosic acid and formation of other phenolic diterpenes. Z. Lebensm. Unters. Forsch. 1992, 195, 99–103. [Google Scholar] [CrossRef]
- Truong, D.H.; Ngo, T.C.; Nhung, N.T.A.; Quang, D.T.; Nguyen, T.L.A.; Khiri, D.; Taamalli, S.; Louis, F.; El Bakali, A.; Dao, D.Q. New insights into the competition between antioxidant activities and pro-oxidant risks of rosmarinic acid. RSC Adv. 2022, 12, 1499–1514. [Google Scholar] [CrossRef]
- Fujimoto, A.; Masuda, T. Antioxidation mechanism of rosmarinic acid, identification of an unstable quinone derivative by the addition of odourless thiol. Food Chem. 2012, 132, 901–906. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedrielli, P.; Pedulli, G.F.; Cabiddu, S.; Fattuoni, C. Bond Dissociation Energies of O−H Bonds in Substituted Phenols from Equilibration Studies. J. Org. Chem. 1996, 61, 9259–9263. [Google Scholar] [CrossRef]
- Mulder, P.; Korth, H.G.; Pratt, D.A.; DiLabio, G.A.; Valgimigli, L.; Pedulli, G.F.; Ingold, K.U. Critical re-evaluation of the O-H bond dissociation enthalpy in phenol. J. Phys. Chem. A 2005, 109, 2647–2655. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F.; Guerra, M. A Critical Evaluation of the Factors Determining the Effect of Intramolecular Hydrogen Bonding on the O−H Bond Dissociation Enthalpy of Catechol and of Flavonoid Antioxidants. Chem. Eur. J. 2004, 10, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, M.; Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Thermochemical Properties and Decomposition Pathways of Three Isomeric Semiquinone Radicals. J. Phys. Chem. A 2010, 114, 1098–1108. [Google Scholar] [CrossRef]
- Thavasi, V.; Leong, L.P.; Bettens, R.P.A. Investigation of the Influence of Hydroxy Groups on the Radical Scavenging Ability of Polyphenols. J. Phys. Chem. A 2006, 110, 4918–4923. [Google Scholar] [CrossRef] [PubMed]
- Roginsky, V.; Barsukova, T.; Loshadkin, D.; Pliss, E. Substituted p-hydroquinones as inhibitors of lipid peroxidation. Chem. Phys. Lipids 2003, 125, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Piskula, M.; Yao, Q. Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch. Biochem. Biophys. 1994, 308, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.J.; Ma, L.P.; Hou, L.F.; Zhou, B.; Yang, L.; Liu, Z.L. Antioxidant effects of green tea polyphenols on free radical initiated peroxidation of rat liver microsomes. Chem. Phys. Lipids 2002, 120, 109–117. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.P.; Zhou, B.; Yang, L.; Liu, Z.L. Antioxidative effects of green tea polyphenols on free radical initiated and photosensitized peroxidation of human low density lipoprotein. Chem. Phys. Lipids. 2000, 106, 53–63. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as Antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Masuda, T.; Inaba, Y.; Takeda, Y. Antioxidant mechanism of carnosic acid: Structural identification of two oxidation products. J. Agric. Food Chem. 2001, 49, 5560–5565. [Google Scholar] [CrossRef]
- Zhang, Y.; Smuts, J.P.; Dodbiba, E.; Rangarajan, R.; Lang, J.C.; Armstrong, D.W. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar] [CrossRef]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act through Different Mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Valcic, S.; Muders, A.; Jacobsen, N.E.; Liebler, D.C.; Timmermann, B.N. Antioxidant Chemistry of Green Tea Catechins. Identification of Products of the Reaction of (−)-Epigallocatechin Gallate with Peroxyl Radicals. Chem. Res. Toxicol. 1999, 12, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yamazaki, S.I.; Kano, K.; Ikeda, T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta Gen. Subj. 2002, 1569, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; De Bruijn, W.J.C.; Van Zadelhoff, A.; Lin, Z.; Vincken, J.P. Browning of Epicatechin (EC) and Epigallocatechin (EGC) by Auto-Oxidation. J. Agric. Food Chem. 2020, 68, 13879–13887. [Google Scholar] [CrossRef]
- Sang, S.; Tian, S.; Wang, H.; Stark, R.E.; Rosen, R.T.; Yang, C.S.; Ho, C.T. Chemical studies of the antioxidant mechanism of tea catechins: Radical reaction products of epicatechin with peroxyl radicals. Bioorganic Med. Chem. 2003, 11, 3371–3378. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Generation and propagation of radical reactions on proteins. Biochim. Biophys. Acta 2001, 1504, 125–219. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Krimmel, B.; Swoboda, F.; Solar, S.; Reznicek, G. OH-radical induced degradation of hydroxybenzoic- and hydroxycinnamic acids and formation of aromatic products—A gamma radiolysis study. Radiat. Phys. Chem. 2010, 79, 1247–1254. [Google Scholar] [CrossRef]
- Yue, J.; Side, Y.; Weizhen, L.; Nianyun, L.; Dayuan, Z. Reactions of tea polyphenol four constituents with hydroxyl radical: A pulse radiolytic study. Sci. China Chem. 1998, 41, 295–300. [Google Scholar]
- Vagkidis, N.; Li, L.; Marsh, J.M.; Chechik, V. Synergy of UV light and heat in peptide degradation. J. Photochem. Photobiol. A Chem. 2023, 439, 114627. [Google Scholar] [CrossRef]
- Niemeyer, E.D.; Brodbelt, J.S. Isomeric differentiation of green tea catechins using gas-phase hydrogen/deuterium exchange reactions. J. Am. Soc. Mass Spectrom. 2007, 18, 1749–1759. [Google Scholar] [CrossRef][Green Version]
- Kušić, H.; Koprivanac, N.; Božić, A.L.; Selanec, I. Photo-assisted Fenton type processes for the degradation of phenol: A kinetic study. J. Hazard. Mater. 2006, 136, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yoshihara, K.; Shinohara, H.; Kondo, M. Reactivity of Aliphatic Peptides toward Hydroxyl Radicals in Aqueous Solution. J. Radiat. Res. 1976, 17, 106–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kondo, K.; Kurihara, M.; Miyata, N.; Suzuki, T.; Toyoda, M. Mechanistic studies of catechins as antioxidants against radical oxidation. Arch. Biochem. Biophys. 1999, 362, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Kurihara, M.; Miyata, N.; Suzuki, T.; Toyoda, M. Scavenging mechanisms of (-)-epigallocatechin gallate and (-)-epicatechin gallate on peroxyl radicals and formation of superoxide during the inhibitory action. Free Radic. Biol. Med. 1999, 27, 855–863. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar]
- Gracanin, M.; Hawkins, C.L.; Pattison, D.I.; Davies, M.J. Singlet-oxygen-mediated amino acid and protein oxidation: Formation of tryptophan peroxides and decomposition products. Free Radic. Biol. Med. 2009, 47, 92–102. [Google Scholar] [CrossRef]
- Gay, C.; Gebicki, J.M. A Critical Evaluation of the Effect of Sorbitol on the Ferric–Xylenol Orange Hydroperoxide Assay. Anal. Biochem. 2000, 284, 217–220. [Google Scholar] [CrossRef]
- Dickinson, D.P.; Evans, S.W.; Grellier, M.; Kendall, H.; Perutz, R.N.; Procacci, B.; Sabo-Etienne, S.; Smart, K.A.; Whitwood, A.C. Photochemical Oxidative Addition of Germane and Diphenylgermane to Ruthenium Dihydride Complexes. Organometallics 2019, 38, 626–637. [Google Scholar] [CrossRef]
- Procacci, B.; Duckett, S.B.; George, M.W.; Hanson-Heine, M.W.D.; Horvath, R.; Perutz, R.N.; Sun, X.Z.; Vuong, K.Q.; Welch, J.A. Competing Pathways in the Photochemistry of Ru(H)2(CO)(PPh3)3. Organometallics 2018, 37, 855–868. [Google Scholar] [CrossRef]
- HPK125W—Hi-Tech Lamps, Inc. Available online: https://www.hi-techlamps.com/hpk125w/ (accessed on 23 February 2020).


| Time/min | % A | % B |
|---|---|---|
| 0 | 100 | 0 |
| 5 | 100 | 0 |
| 10 | 70 | 30 |
| 15 | 70 | 30 |
| 25 | 50 | 50 |
| 35 | 50 | 50 |
| 40 | 30 | 70 |
| 45 | 30 | 70 |
| 50 | 100 | 0 |
| 60 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagkidis, N.; Marsh, J.; Chechik, V. The Role of Polyphenolic Antioxidants from Tea and Rosemary in the Hydroxyl Radical Oxidation of N-Acetyl Alanine. Molecules 2023, 28, 7514. https://doi.org/10.3390/molecules28227514
Vagkidis N, Marsh J, Chechik V. The Role of Polyphenolic Antioxidants from Tea and Rosemary in the Hydroxyl Radical Oxidation of N-Acetyl Alanine. Molecules. 2023; 28(22):7514. https://doi.org/10.3390/molecules28227514
Chicago/Turabian StyleVagkidis, Nikolaos, Jennifer Marsh, and Victor Chechik. 2023. "The Role of Polyphenolic Antioxidants from Tea and Rosemary in the Hydroxyl Radical Oxidation of N-Acetyl Alanine" Molecules 28, no. 22: 7514. https://doi.org/10.3390/molecules28227514
APA StyleVagkidis, N., Marsh, J., & Chechik, V. (2023). The Role of Polyphenolic Antioxidants from Tea and Rosemary in the Hydroxyl Radical Oxidation of N-Acetyl Alanine. Molecules, 28(22), 7514. https://doi.org/10.3390/molecules28227514








