Lateral Flow Immunoassay Based on Quantum-Dot Nanobeads for Detection of Chloramphenicol in Aquatic Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Principle of QBs-LFIA
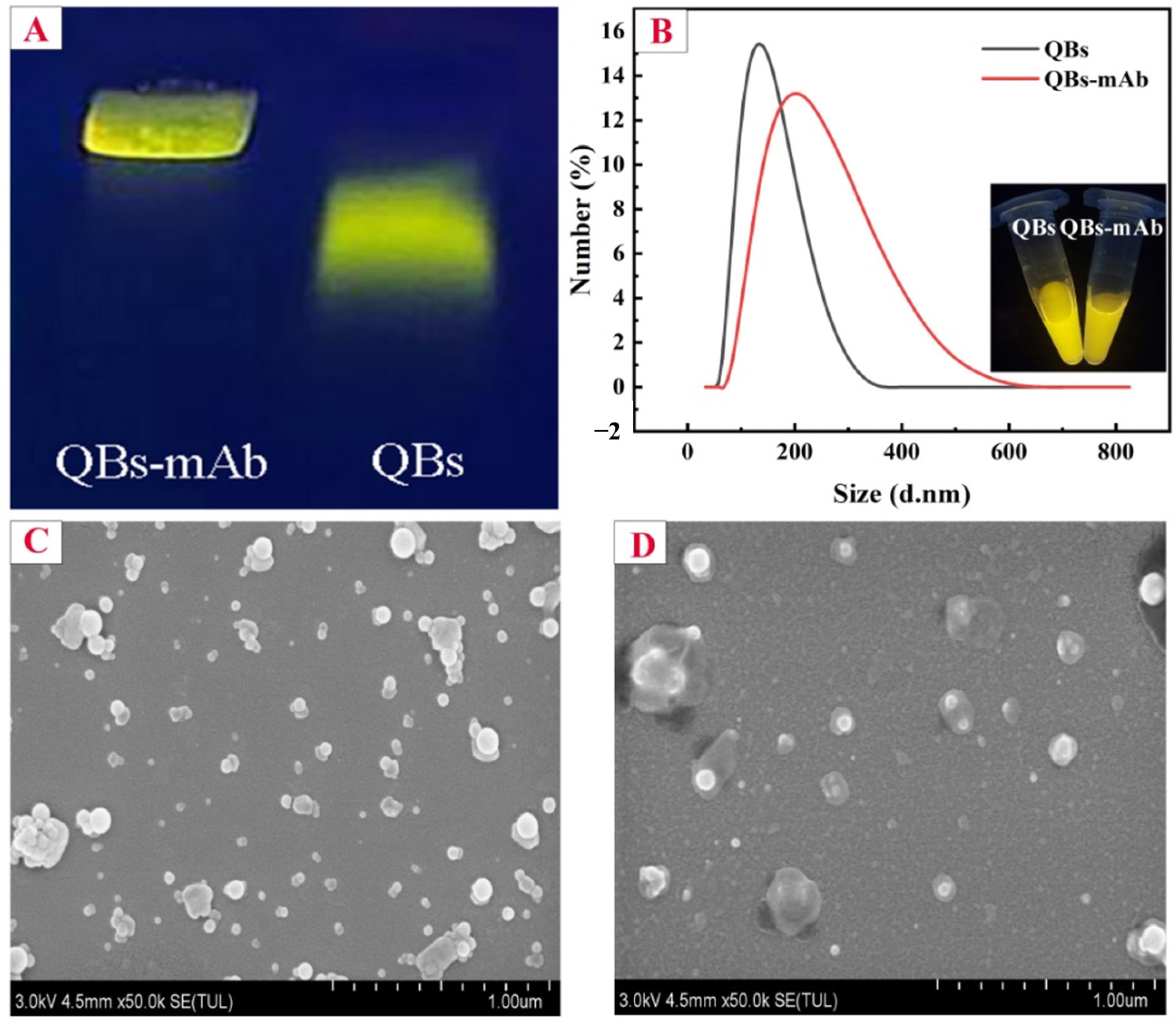
2.2. Conjugation of QBs and mAb
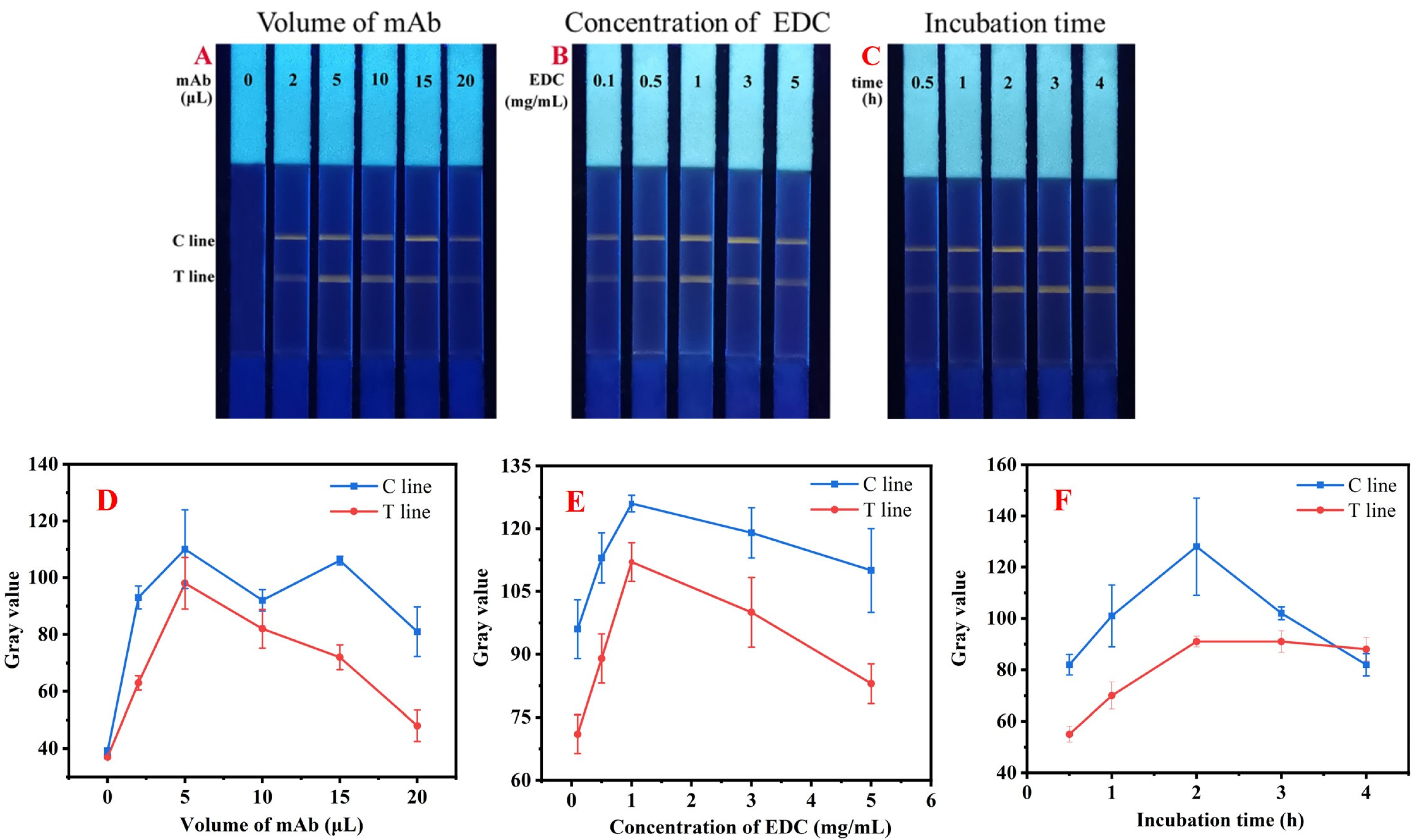
2.3. Optimization of QBs-mAb
2.4. Optimization of QBs-LFIA
2.5. Analytical Performance of QBs-LFIA
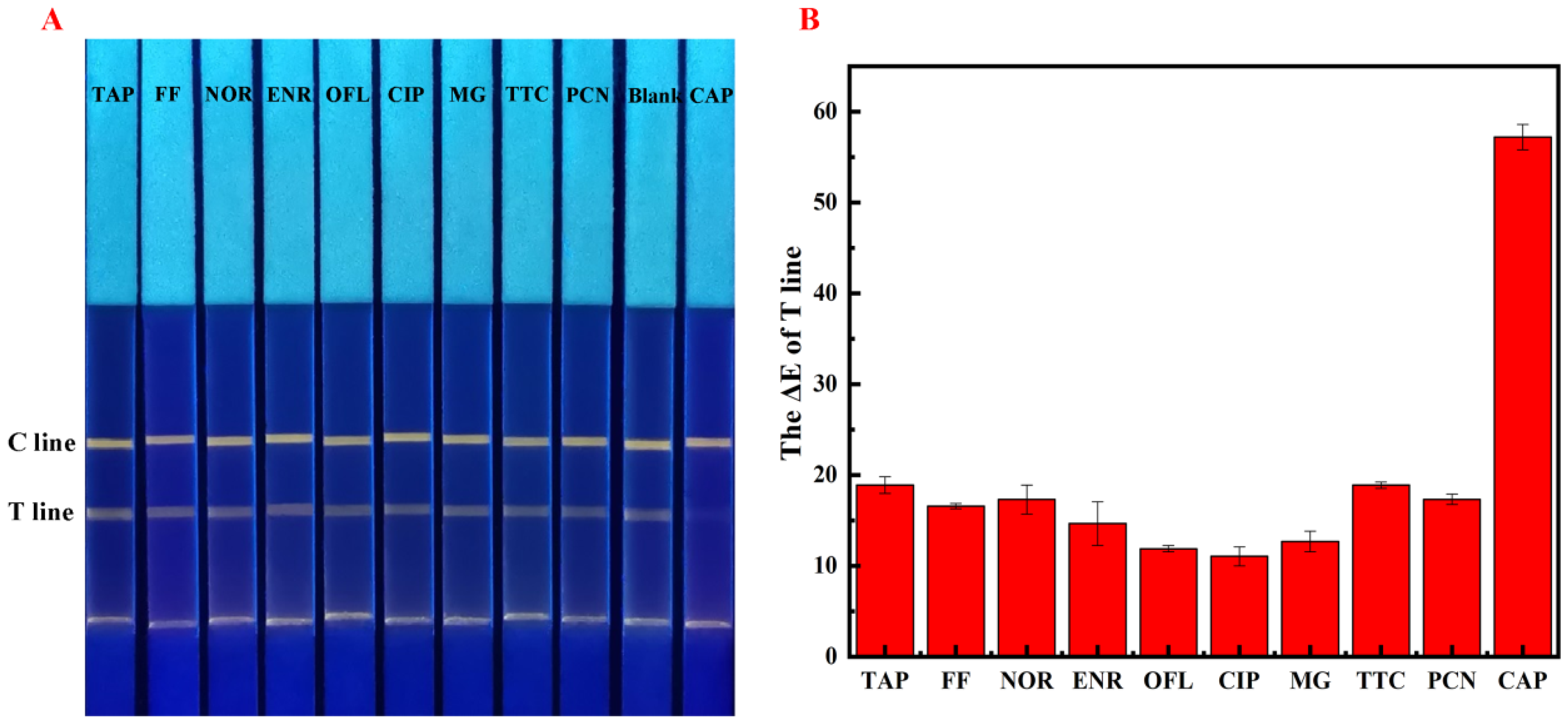
2.6. Determination of Specificity
2.7. Analysis of CAP in Real Samples
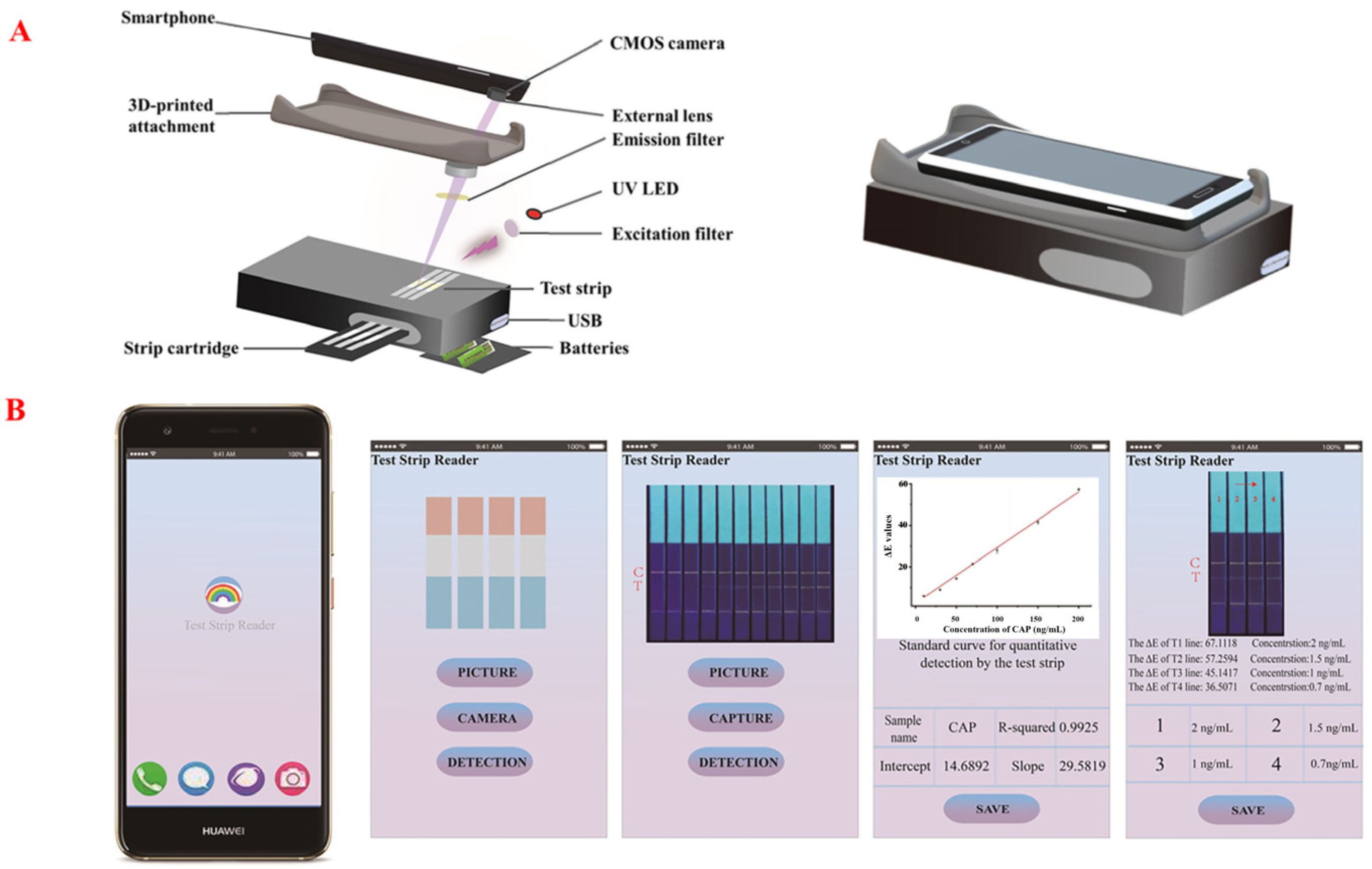
2.8. Conceptual Products for on-Site Detection
3. Materials and Methods
3.1. Materials and Reagents
3.2. Apparatus
3.3. Preparation of QBs-mAb Probes
3.4. Fabrication of QBs-LFIA
3.5. Optimization of QBs-LFIA
3.6. Specificity
3.7. Sample Preparation and QBs-LFIA Detection
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Moghazy, A.Y.; Zhao, C.; Istamboulie, G.; Amaly, N.; Si, Y.; Noguer, T.; Sun, G. Ultrasensitive label-free electrochemical immunosensor based on PVA-co-PE nanofibrous membrane for the detection of chloramphenicol residues in milk. Biosens. Bioelectron. 2018, 117, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, J.; Yao, L.; Xue, F.; Lu, J.; Li, B.; Chen, W. Aptamer-mediated colorimetric method for rapid and sensitive detection of chloramphenicol in food. Food Chem. 2018, 260, 208–212. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.; Yang, L.; Wang, X.; Zheng, Y.; Bao, L. Covalently bonded aptamer-functionalised magnetic mesoporous carbon for high-efficiency chloramphenicol detection. J. Sep. Sci. 2020, 43, 2610–2618. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, B.; Huang, P.; Wu, F. A novel colorimetric aptasensor for detection of chloramphenicol based on lanthanum ion–assisted gold nanoparticle aggregation and smartphone imaging. Anal. Bioanal. Chem. 2019, 411, 7511–7518. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; He, F.; Liu, X.; Zhang, F.; Wang, X.; Peng, Y.; Liu, J. Detection of chloramphenicol with an aptamer-based colorimetric assay: Critical evaluation of specific and unspecific binding of analyte molecules. Microchim. Acta 2020, 187, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Hong, C.; Lin, Z.; Chen, X.; Huang, Z. Aptamer-based fluorometric determination of chloramphenicol by controlling the activity of hemin as a peroxidase mimetic. Anal. Methods 2020, 12, 2391–2397. [Google Scholar] [CrossRef]
- Dong, B.; Li, H.; Sun, J.; Li, Y.; Mari, G.M.; Yu, X.; Yu, W.; Wen, K.; Shen, J.; Wang, Z. Magnetic assisted fluorescence immunoassay for sensitive chloramphenicol detection using carbon dots@CaCO3 nanocomposites. J. Hazard. Mater. 2021, 402, 123942. [Google Scholar] [CrossRef]
- Ding, J.; Li, Q.; Xu, X.; Zhang, X.; Su, Y.; Yue, Q.; Gao, B. A wheat straw cellulose-based hydrogel for Cu (II) removal and preparation copper nanocomposite for reductive degradation of chloramphenicol. Carbohyd. Polym 2018, 190, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Luo, C.; Cheng, W.; Mao, W.; Zhang, D.; Ding, S. A simple and sensitive electrochemical aptasensor for determination of Chloramphenicol in honey based on target-induced strand release. Electroanal. Chem. 2012, 687, 89–94. [Google Scholar] [CrossRef]
- Rajaji, U.; Muthumariappan, A.; Chen, S.M.; Chen, T.W.; Tseng, T.W.; Wang, K.; Qi, D.; Jiang, J. Facile sonochemical synthesis of porous and hierarchical manganese(III) oxide tiny nanostructures for super sensitive electrocatalytic detection of antibiotic (chloramphenicol) in fresh milk. Ultrason. Sonochem 2019, 58, 104648. [Google Scholar] [CrossRef]
- Moudgil, P.; Bedi, J.S.; Aulakh, R.S.; Gill, J.P.S.; Kumar, A. Validation of HPLC Multi-residue Method for Determination of Fluoroquinolones, Tetracycline, Sulphonamides and Chloramphenicol Residues in Bovine Milk. Food Anal. Method. 2019, 12, 338–346. [Google Scholar] [CrossRef]
- Yao, T.; Yao, S. Magnetic ionic liquid aqueous two-phase system coupled with high performance liquid chromatography: A rapid approach for determination of chloramphenicol in water environment. J. Chromatogr. A 2017, 1481, 12–22. [Google Scholar] [CrossRef]
- Imran, M.; Habib, F.E.; Majeed, S.; Tawab, A.; Rauf, W.; Rahman, M.; Umer, M.; Iqbal, M. LC-MS/MS-based determination of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in poultry meat from the Punjab-Pakistan. Food Addit. Contam. A 2018, 35, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Sakai, T.; Teshima, R.; Nemoto, S.; Akiyama, H. Total determination of chloramphenicol residues in foods by liquid chromatography-tandem mass spectrometry. Food Chem. 2017, 230, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xie, J.; Zhao, J.; Song, G.; Hu, Y. Magnetic Chitosan Nanocomposite Used as Cleanup Material to Detect Chloramphenicol in Milk by GC-MS. Food Anal. Method 2014, 7, 814–819. [Google Scholar] [CrossRef]
- Zhao, C.; Si, Y.; Pan, B.; Taha, A.Y.; Pan, T.; Sun, G. Design and fabrication of a highly sensitive and naked-eye distinguishable colorimetric biosensor for chloramphenicol detection by using ELISA on nanofibrous membranes. Talanta 2020, 217, 121054. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, B.; Chen, E.; Yu, X.; Ye, Z.; Sun, C.; Zhang, M. Dual fluorescent immunochromatographic assay for simultaneous quantitative detection of citrinin and zearalenone in corn samples. Food Chem. 2021, 336, 127713. [Google Scholar] [CrossRef]
- Xu, L.; Du, F.; Zhu, J.; Ding, S. Luminous silica colloids with carbon dot incorporation for sensitive immunochromatographic assay of Zika virus. Analyst 2021, 146, 706–713. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Deng, Y.; Yao, L.; Adeloju, S.; Pan, D.; Xue, F.; Wu, Y.; Zheng, L.; Chen, W. Ultrasensitive detection of mercury with a novel one-step signal amplified lateral flow strip based on gold nanoparticle-labeled ssDNA recognition and enhancement probes. Biosens. Bioelectron. 2014, 61, 14–20. [Google Scholar] [CrossRef]
- Chen, E.; Xu, Y.; Ma, B.; Cui, H.; Sun, C.; Zhang, M. Carboxyl-functionalized, europium nanoparticle-based fluorescent immunochromatographic assay for sensitive detection of citrinin in monascus fermented food. Toxins 2019, 11, 605. [Google Scholar] [CrossRef]
- Cai, Y.; Kang, K.; Liu, Y.; Wang, Y.; He, X. Development of a lateral flow immunoassay of C-reactive protein detection based on red fluorescent nanoparticles. Anal. Biochem. 2018, 556, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Deng, J.; Wang, Y.; Chen, H.; Ding, Y.; Hua, X.; Wang, M. Competitive immunoassay for simultaneous detection of imidacloprid and thiacloprid by upconversion nanoparticles and magnetic nanoparticles. Environ. Sci. Pollut. R. 2019, 26, 23471–23479. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; You, H.; Luo, P.; Tao, Z.; Chen, H.; Liu, F.; Wang, M. Upconversion fluorescence immunoassay for imidaclothiz by magnetic nanoparticle separation. Anal. Bioanal. Chem. 2017, 409, 6885–6892. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Luo, K.; Xia, J.; Xu, G.; Wu, C.; Han, J.; Zhang, G.; Liu, M.; Lai, W. Advantages of time-resolved fluorescent nanobeads compared with fluorescent submicrospheres, quantum dots, and colloidal gold as label in lateral flow assays for detection of ractopamine. Biosens. Bioelectron. 2017, 91, 95–103. [Google Scholar] [CrossRef]
- Qie, Z.; Yan, W.; Gao, Z.; Meng, W.; Xiao, R.; Wang, S. An anti-BSA antibody-based immunochromatographic assay for chloramphenicol and aflatoxin M 1 by using carboxy-modified CdSe/ZnS core—Shell nanoparticles as label. Microchim. Acta 2020, 187, 1–8. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Q.; Ding, S.; Xu, J.; Chen, H. Ultrasensitive Detection of Severe Fever with Thrombocytopenia Syndrome Virus Based on Immunofluorescent Carbon Dots/SiO2 Nanosphere-Based Lateral Flow Assay. ACS Omega 2019, 4, 21431–21438. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, J.; Beloglazova, N.; Yang, S.; De Saeger, S.; Mari, G.M.; Zhang, S.; Shen, J.; Wang, Z.; Yu, X. Portable Multiplex Immunochromatographic Assay for Quantitation of Two Typical Algae Toxins Based on Dual-Color Fluorescence Microspheres. J. Agric. Food Chem. 2019, 67, 6041–6047. [Google Scholar] [CrossRef]
- Rong, Z.; Bai, Z.; Li, J.; Tang, H.; Shen, T.; Wang, Q.; Wang, C.; Xiao, R.; Wang, S. Dual-color magnetic-quantum dot nanobeads as versatile fluorescent probes in test strip for simultaneous point-of-care detection of free and complexed prostate-specific antigen. Biosens. Bioelectron. 2019, 145, 111719. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Wen, C.; Tang, M.; Wu, L.; Liu, C.; Zhu, L.; Pang, D. Sensitive and Quantitative Detection of C-Reaction Protein Based on Immunofluorescent Nanospheres Coupled with Lateral Flow Test Strip. Anal. Chem. 2016, 88, 6577–6584. [Google Scholar] [CrossRef]
- Xie, S.; Wen, K.; Wang, S.; Wang, J.; Peng, T.; Mari, G.M.; Li, J.; Wang, Z.; Yu, X.; Jiang, H. Quantitative and rapid detection of amantadine and chloramphenicol based on various quantum dots with the same excitations. Anal. Bioanal. Chem. 2019, 411, 2131–2140. [Google Scholar] [CrossRef]
- Ma, M.; Wen, K.; Beier, R.C.; Eremin, S.A.; Li, C.; Zhang, S.; Shen, J.; Wang, Z. Chemiluminescence Resonance Energy Transfer Competitive Immunoassay Employing Hapten-Functionalized Quantum Dots for the Detection of Sulfamethazine. ACS Appl. Mater. Inter. 2016, 8, 17745–17750. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Meng, C.; Wen, Y.; Fu, W.; He, P. Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B1, zearalenone and deoxynivalenol) using quantum dot microbeads. Microchim. Acta 2019, 186, 748. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Liu, Q.; Yang, Q.; Pang, C.; Xu, H.; Du, X.; Wei, L.; Nie, K.; Guo, Y.; Sun, X. An immediate and antibody protected carboxyl quantum dot immunochromatographic analysis hierarchical signal amplification test strip based on biotin-streptavidin system for the detection of aflatoxin B1 in peanuts. J. Food Compos. Anal. 2024, 125, 105759. [Google Scholar] [CrossRef]
- Hu, M.; Hu, X.; Wang, G.; Cheng, Y.; Yu, X.; Huang, X.; Li, Y. A fluorescent lateral flow immunoassay based on CdSe/CdS/ZnS quantum dots for sensitive detection of olaquindox in feedstuff. Food Chem. 2023, 419, 136025. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, P.; Duan, H.; Liu, B.; Shao, Y.; Li, P.; Zhang, C.; Xiong, Y. Quantum dot bead-based immunochromatographic assay for the quantitative detection of triazophos. Food Agric. Immunol. 2019, 30, 955–967. [Google Scholar] [CrossRef]
- Duan, H.; Li, Y.; Shao, Y.; Huang, X.; Xiong, Y. Multicolor quantum dot nanobeads for simultaneous multiplex immunochromatographic detection of mycotoxins in maize. Sens. Actuators B-Chem. 2019, 291, 411–417. [Google Scholar] [CrossRef]
- Liu, B.; Li, P.; Wang, Y.; Guo, Y.; Zhang, H.; Dong, S.; Xiong, Y.; Zhang, C. Quantum Dot Submicrobead–Based Immunochromatographic Assay for the Determination of Parathion in Agricultural Products. Food Anal. Method. 2020, 13, 1736–1745. [Google Scholar] [CrossRef]
- Jia, B.; Liao, X.; Sun, C.; Fang, L.; Zhou, L.; Kong, W. Development of a quantum dot nanobead-based fluorescent strip immunosensor for on-site detection of aflatoxin B1 in lotus seeds. Food Chem. 2021, 356, 129614. [Google Scholar] [CrossRef]
- Jia, B.; He, X.; Cui, P.; Liu, J.; Wang, J. Detection of chloramphenicol in meat with a chemiluminescence resonance energy transfer platform based on molecularly imprinted graphene. Anal. Chim. Acta 2019, 1063, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Jiang, S.; Li, X.; Jiang, S. Films based on κ-carrageenan incorporated with curcumin for freshness monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Byzova, N.A.; Zvereva, E.A.; Zherdev, A.V.; Eremin, S.A.; Dzantiev, B.B. Rapid Pretreatment-Free Immunochromatographic Assay of Chloramphenicol in Milk. Talanta 2010, 81, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.; Zvereva, E.; Shanin, I.; Zherdev, A.; Dzantiev, B. Development of a Multicomponent Immunochromatographic Test System for the Detection of Fluoroquinolone and Amphenicol Antibiotics in Dairy Products. J. Sci. Food Agric. 2019, 99, 3834–3842. [Google Scholar] [CrossRef]
- Wang, S.; Du, T.; Liu, S.; Li, Y.; Wang, Y.; Zhang, L.; Zhang, D.; Sun, J.; Zhu, M.; Wang, J. Dyestuff Chemistry Auxiliary Instant Immune-Network Label Strategy for Immunochromatographic Detection of Chloramphenicol. Food Chem. 2023, 401, 134140. [Google Scholar] [CrossRef] [PubMed]
- Gantverg, A.; Shishani, I.; Hoffman, M. Determination of Chloramphenicol in Animal Tissues and Urine. Anal. Chim. Acta 2003, 483, 125–135. [Google Scholar] [CrossRef]
- Shen, H.-Y.; Jiang, H.-L. Screening, Determination and Confirmation of Chloramphenicol in Seafood, Meat and Honey Using ELISA, HPLC–UVD, GC–ECD, GC–MS–EI–SIM and GCMS–NCI–SIM Methods. Anal. Chim. Acta 2005, 535, 33–41. [Google Scholar] [CrossRef]
- Batish, S.; Rajput, J.K. Quercetin Capped Silver Nanoparticles as an Electrochemical Sensor for Ultrasensitive Detection of Chloramphenicol in Food and Water Samples. J. Food Compos. Anal. 2023, 122, 105421. [Google Scholar] [CrossRef]



| Sample | Spiked (ng/mL) | Measured (Mean ± SD, ng/mL) | Recovery (%) | CV (%) |
|---|---|---|---|---|
| Red drum | 0.1 | 0.10 ± 0.01 | 104.91 | 9.56 |
| 0.7 | 0.65 ± 0.04 | 92.44 | 6.86 | |
| 1.5 | 1.24 ± 0.07 | 82.82 | 5.83 | |
| Grass carp | 0.1 | 0.1 ± 0.01 | 95.06 | 6.69 |
| 0.7 | 0.67 ± 0.04 | 96.39 | 6.01 | |
| 1.5 | 1.32 ± 0.10 | 87.91 | 7.36 | |
| Freshwater shrimp | 0.1 | 0.09 ± 0.01 | 94.40 | 8.34 |
| 0.7 | 0.66 ± 0.04 | 94.93 | 5.96 | |
| 1.5 | 1.32 ± 0.12 | 88.33 | 9.29 | |
| Scallop | 0.1 | 0.09 ± 0.01 | 89.90 | 6.21 |
| 0.7 | 0.64 ± 0.05 | 91.90 | 7.43 | |
| 1.5 | 1.40 ± 0.10 | 93.10 | 6.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Fan, L.; Liu, X.; Tang, Y.; Wang, P.; Shu, Z.; Zhang, W.; Zhu, L. Lateral Flow Immunoassay Based on Quantum-Dot Nanobeads for Detection of Chloramphenicol in Aquatic Products. Molecules 2023, 28, 7496. https://doi.org/10.3390/molecules28227496
Han Q, Fan L, Liu X, Tang Y, Wang P, Shu Z, Zhang W, Zhu L. Lateral Flow Immunoassay Based on Quantum-Dot Nanobeads for Detection of Chloramphenicol in Aquatic Products. Molecules. 2023; 28(22):7496. https://doi.org/10.3390/molecules28227496
Chicago/Turabian StyleHan, Qian, Ling Fan, Xiuying Liu, Yiwei Tang, Pingping Wang, Zaixi Shu, Wei Zhang, and Lijie Zhu. 2023. "Lateral Flow Immunoassay Based on Quantum-Dot Nanobeads for Detection of Chloramphenicol in Aquatic Products" Molecules 28, no. 22: 7496. https://doi.org/10.3390/molecules28227496
APA StyleHan, Q., Fan, L., Liu, X., Tang, Y., Wang, P., Shu, Z., Zhang, W., & Zhu, L. (2023). Lateral Flow Immunoassay Based on Quantum-Dot Nanobeads for Detection of Chloramphenicol in Aquatic Products. Molecules, 28(22), 7496. https://doi.org/10.3390/molecules28227496








