Natural Drugs: A New Direction for the Prevention and Treatment of Diabetes
Abstract
1. Introduction
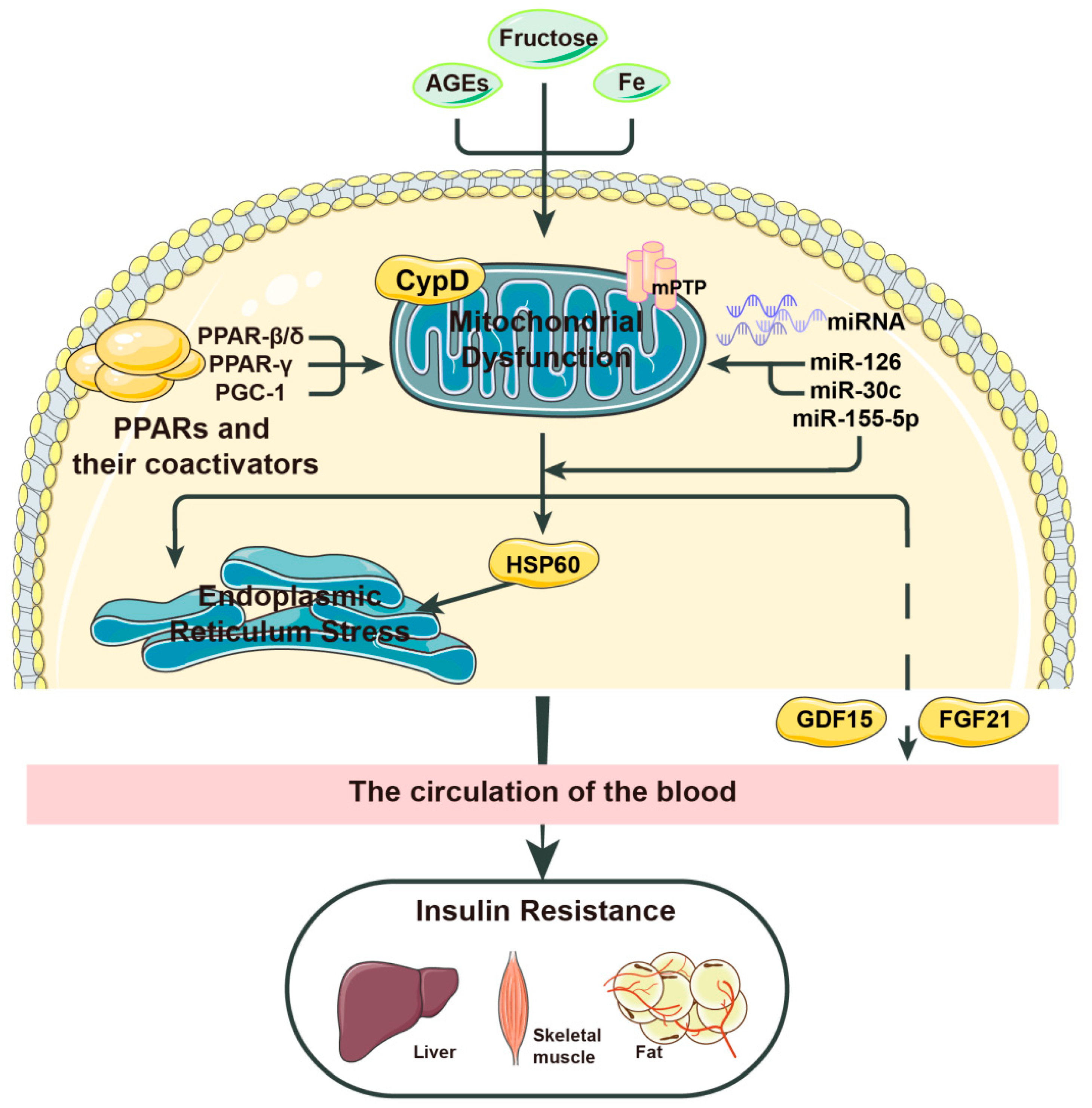
2. Mitochondrial Stress, a Key Inducer of Insulin Resistance
2.1. Major Factors Leading to Mitochondrial Stress
2.2. How Does Mitochondrial Stress Affect Insulin Resistance?
2.2.1. HSP60
2.2.2. FGF21
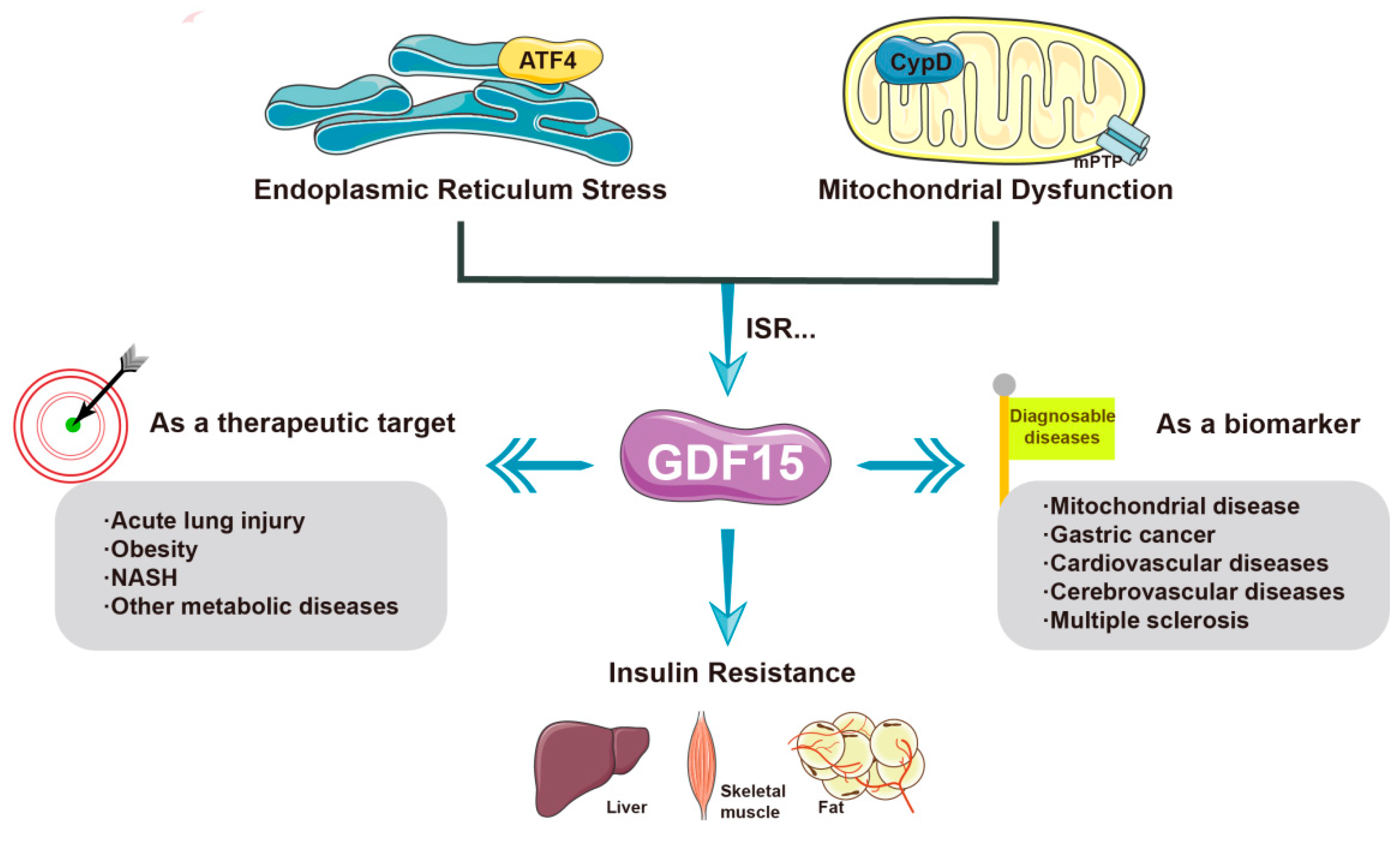
2.2.3. GDF-15
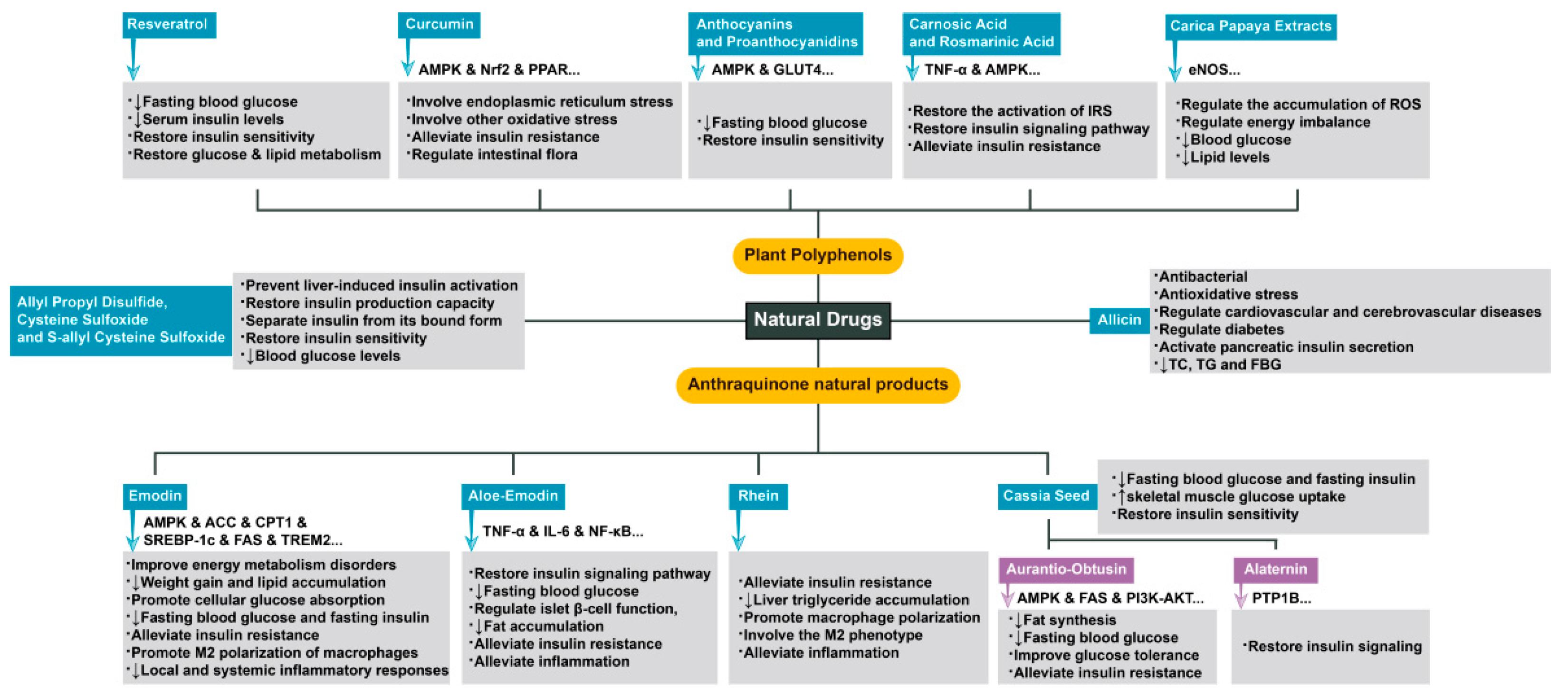
3. Complex Mechanisms of Natural Drugs in the Treatment of Diseases Related to Insulin Resistance
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sundaram, K.; Mu, J.; Dryden, G.W.; Sriwastva, M.K.; Lei, C.; Zhang, L.; Qiu, X.; Xu, F.; Yan, J.; et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat. Commun. 2021, 12, 213–234. [Google Scholar] [CrossRef]
- Broughton, D.L.; Taylor, R. Review: Deterioration of Glucose Tolerance with Age: The Role of Insulin Resistance. Age Ageing 1991, 20, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of beta-Cell Dysfunction and Insulin Resistance to the Pathogenesis of Impaired Glucose Tolerance and Impaired Fasting Glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Perseghin, G.; Price, T.B.; Petersen, K.F.; Roden, M.; Cline, G.W.; Gerow, K.; Rothman, D.L.; Shulman, G.I. Increased Glucose Transport–Phosphorylation and Muscle Glycogen Synthesis after Exercise Training in Insulin-Resistant Subjects. N. Engl. J. Med. 1996, 335, 1357–1362. [Google Scholar] [CrossRef]
- Burkey, B.F.; Li, X.; Bolognese, L.; Balkan, B.; Mone, M.; Russell, M.; Hughes, T.E.; Wang, P.R. Acute and Chronic Effects of the Incretin Enhancer Vildagliptin in Insulin-Resistant Rats. J. Pharmacol. Exp. Ther. 2005, 315, 688–695. [Google Scholar] [CrossRef]
- Klymenko, O.; Brecklinghaus, T.; Dille, M.; Springer, C.; de Wendt, C.; Altenhofen, D.; Binsch, C.; Knebel, B.; Scheller, J.; Hardt, C.; et al. Histone deacetylase 5 regulates interleukin 6 secretion and insulin action in skeletal muscle. Mol. Metab. 2020, 42, 101062. [Google Scholar] [CrossRef]
- Beck, G.R.; Khazai, N.B.; Bouloux, G.F.; Camalier, C.E.; Lin, Y.; Garneys, L.M.; Siqueira, J.; Peng, L.; Pasquel, F.; Umpierrez, D.; et al. The effects of thiazolidinediones on human bone marrow stromal cell differentiation in vitro and in thiazolidinedione-treated patients with type 2 diabetes. Transl. Res. 2012, 161, 145–155. [Google Scholar] [CrossRef]
- Bonnet, F.; Scheen, A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes. Metab. 2017, 19, 473–481. [Google Scholar] [CrossRef]
- Taylor, S.I.; Blau, J.E.; Rother, K.I.; Beitelshees, A.L. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: Balancing benefits and risks. Lancet Diabetes Endocrinol. 2019, 7, 949–958. [Google Scholar] [CrossRef]
- Ko, E.; Um, M.Y.; Choi, M.; Han, T.; Kim, I.-H.; Shin, S. Cassia tora Seed Improves Pancreatic Mitochondrial Function Leading to Recovery of Glucose Metabolism. Am. J. Chin. Med. 2020, 48, 615–629. [Google Scholar] [CrossRef]
- Cheng, F.-R.; Cui, H.-X.; Fang, J.-L.; Yuan, K.; Guo, Y. Ameliorative Effect and Mechanism of the Purified Anthraquinone-Glycoside Preparation from Rheum palmatum L. on Type 2 Diabetes Mellitus. Molecules 2019, 24, 1454. [Google Scholar] [CrossRef]
- Deora, N.; Sunitha, M.; Satyavani, M.; Harishankar, N.; Vijayalakshmi, M.A.; Venkataraman, K.; Venkateshan, V. Alleviation of diabetes mellitus through the restoration of β-cell function and lipid metabolism by Aloe vera (L.) Burm. f. extract in obesogenic WNIN/GR-Ob rats. J. Ethnopharmacol. 2021, 272, 113921. [Google Scholar] [CrossRef]
- Jeong, E.M.; Chung, J.; Liu, H.; Go, Y.; Gladstein, S.; Farzaneh-Far, A.; Lewandowski, E.D.; Dudley, S.C., Jr. Role of Mitochondrial Oxidative Stress in Glucose Tolerance, Insulin Resistance, and Cardiac Diastolic Dysfunction. J. Am. Heart Assoc. 2016, 5, e003046. [Google Scholar] [CrossRef]
- Schrauwen, P.; Schrauwen-Hinderling, V.; Hoeks, J.; Hesselink, M.K. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2010, 1801, 266–271. [Google Scholar] [CrossRef]
- Choi, K.-M.; Ryan, K.K.; Yoon, J.C. Adipose Mitochondrial Complex I Deficiency Modulates Inflammation and Glucose Homeostasis in a Sex-Dependent Manner. Endocrinology 2022, 163, bqac018. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T.; Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Wang, X.; Du, H.; Shao, S.; Bo, T.; Yu, C.; Chen, W.; Zhao, L.; Li, Q.; Wang, L.; Liu, X.; et al. Cyclophilin D deficiency attenuates mitochondrial perturbation and ameliorates hepatic steatosis. Hepatology 2018, 68, 62–77. [Google Scholar] [CrossRef]
- Nassir, F.; Ibdah, J.A. Role of Mitochondria in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 8713–8742. [Google Scholar] [CrossRef]
- Galloway, C.A.; Lee, H.; Brookes, P.; Yoon, Y. Decreasing mitochondrial fission alleviates hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 307, G632–G641. [Google Scholar] [CrossRef]
- Taanman, J.-W. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta BBA-Bioenergies 1999, 1410, 103–123. [Google Scholar] [CrossRef]
- Gustafsson, C.M.; Falkenberg, M.; Larsson, N.-G. Maintenance and Expression of Mammalian Mitochondrial DNA. Annu. Rev. Biochem. 2016, 85, 133–160. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef] [PubMed]
- Supinski, G.S.; Schroder, E.A.; Callahan, L.A. Mitochondria and Critical Illness. Chest 2020, 157, 310–322. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Becker, Y.L.C.; Duvvuri, B.; Fortin, P.R.; Lood, C.; Boilard, E. The role of mitochondria in rheumatic diseases. Nat. Rev. Rheumatol. 2022, 18, 621–640. [Google Scholar] [CrossRef]
- Kleele, T.; Rey, T.; Winter, J.; Zaganelli, S.; Mahecic, D.; Lambert, H.P.; Ruberto, F.P.; Nemir, M.; Wai, T.; Pedrazzini, T.; et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 2021, 593, 435–439. [Google Scholar] [CrossRef]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta (BBA) Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Wu, B.; Chen, Y.; Clarke, R.; Akala, E.; Yang, P.; He, B.; Gao, H. AMPK Signaling Regulates Mitophagy and Mitochondrial ATP Production in Human Trophoblast Cell Line BeWo. Front. Biosci. 2022, 27, 118. [Google Scholar] [CrossRef]
- Paolisso, G.; D’Amore, A.; Volpe, C.; Balbi, V.; Saccomanno, F.; Galzerano, D.; Giugliano, D.; Varricchio, M.; D’Onofrio, F. Evidence for a relationship between oxidative stress and insulin action in non-insulin-dependent (type II) diabetic patients. Metabolism 1994, 43, 1426–1429. [Google Scholar] [CrossRef]
- Silva, A.K.S.; Peixoto, C.A. Role of peroxisome proliferator-activated receptors in non-alcoholic fatty liver disease inflammation. Cell. Mol. Life Sci. 2018, 75, 2951–2961. [Google Scholar] [CrossRef]
- Chen, J.; Montagner, A.; Tan, N.S.; Wahli, W. Insights into the Role of PPARβ/δ in NAFLD. Int. J. Mol. Sci. 2018, 19, 1893. [Google Scholar] [CrossRef]
- Lee, H.J.; Yeon, J.E.; Ko, E.J.; Yoon, E.L.; Suh, S.J.; Kang, K.; Kim, H.R.; Kang, S.H.; Yoo, Y.J.; Je, J.; et al. Peroxisome proliferator-activated receptor-delta agonist ameliorated inflammasome activation in nonalcoholic fatty liver disease. World J. Gastroenterol. 2015, 21, 12787–12799. [Google Scholar] [CrossRef]
- Liu, X.; Du, H.; Chai, Q.; Jia, Q.; Liu, L.; Zhao, M.; Li, J.; Tang, H.; Chen, W.; Zhao, L.; et al. Blocking mitochondrial cyclophilin D ameliorates TSH-impaired defensive barrier of artery. Redox Biol. 2018, 15, 418–434. [Google Scholar] [CrossRef]
- Hou, D.; Hu, F.; Mao, Y.; Yan, L.; Zhang, Y.; Zheng, Z.; Wu, A.; Forouzanfar, T.; Pathak, J.L.; Wu, G. Cationic antimicrobial peptide NRC-03 induces oral squamous cell carcinoma cell apoptosis via CypD-mPTP axis-mediated mitochondrial oxidative stress. Redox Biol. 2022, 54, 102355. [Google Scholar] [CrossRef]
- Castillo, E.C.; Morales, J.A.; Chapoy-Villanueva, H.; Silva-Platas, C.; Treviño-Saldaña, N.; Guerrero-Beltrán, C.E.; Bernal-Ramírez, J.; Torres-Quintanilla, A.; García, N.; Youker, K.; et al. Mitochondrial Hyperacetylation in the Failing Hearts of Obese Patients Mediated Partly by a Reduction in SIRT3: The Involvement of the Mitochondrial Permeability Transition Pore. Cell. Physiol. Biochem. 2019, 53, 465–479. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Lu, C.-H.; Wu, C.-H.; Li, K.-J.; Kuo, Y.-M.; Hsieh, S.-C.; Yu, C.-L. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef]
- Selenscig, D.; Ferreira, M.D.R.; Chicco, A.; Lombardo, Y.B. Dietary fish oil ameliorates adipose tissue dysfunction in insulin-resistant rats fed a sucrose-rich diet improving oxidative stress, peroxisome proliferator-activated receptor γ and uncoupling protein 2. Food Funct. 2018, 9, 2496–2507. [Google Scholar] [CrossRef]
- Sil, R.; Chakraborti, A.S. Oxidative Inactivation of Liver Mitochondria in High Fructose Diet-Induced Metabolic Syndrome in Rats: Effect of Glycyrrhizin Treatment. Phytother. Res. 2016, 30, 1503–1512. [Google Scholar] [CrossRef]
- Rabelo, F.; Stefano, J.T.; Cavaleiro, A.M.; Lima, R.V.C.; de Campos Mazo, D.F.; Carrilho, F.J.; Correa-Giannella, M.L.; Oliveira, C.P. Association between the CYBA and NOX4 genes of NADPH oxidase and its relationship with metabolic syndrome in non-alcoholic fatty liver disease in Brazilian population. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Besse-Patin, A.; Léveillé, M.; Oropeza, D.; Nguyen, B.N.; Prat, A.; Estall, J.L. Estrogen Signals Through Peroxisome Proliferator-Activated Receptor−γ Coactivator 1α to Reduce Oxidative Damage Associated with Diet-Induced Fatty Liver Disease. Gastroenterology 2017, 152, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; Lal, M.; Binukumar, B.K. Crosstalk Between the Unfolded Protein Response, MicroRNAs, and Insulin Signaling Pathways: In Search of Biomarkers for the Diagnosis and Treatment of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.M.F.; Klein, D.; Álvarez-Cubela, S.; Domínguez-Bendala, J.; Pastori, R.L. The Role of MicroRNAs in Diabetes-Related Oxidative Stress. Int. J. Mol. Sci. 2019, 20, 5423. [Google Scholar] [CrossRef] [PubMed]
- Dotta, F.; Ventriglia, G.; Snowhite, I.V.; Pugliese, A. MicroRNAs: Markers of beta-cell stress and autoimmunity. Curr. Opin. Endocrinol. Diabetes 2018, 25, 237–245. [Google Scholar] [CrossRef]
- LaPierre, M.P.; Stoffel, M. MicroRNAs as stress regulators in pancreatic beta cells and diabetes. Mol. Metab. 2017, 6, 1010–1023. [Google Scholar] [CrossRef]
- Zhu, M.; Wei, Y.; Geißler, C.; Abschlag, K.; Campos, J.C.; Hristov, M.; Möllmann, J.; Lehrke, M.; Karshovska, E.; Schober, A. Hyperlipidemia-Induced MicroRNA-155-5p Improves β-Cell Function by Targeting Mafb. Diabetes 2017, 66, 3072–3084. [Google Scholar] [CrossRef]
- Yin, Z.; Zhao, Y.; He, M.; Li, H.; Fan, J.; Nie, X.; Yan, M.; Chen, C.; Wang, D.W. MiR-30c/PGC-1β protects against diabetic cardiomyopathy via PPARα. Cardiovasc. Diabetol. 2019, 18, 7. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Liu, H.; Chen, Y.; Li, P.; Liu, L.; Li, J.; Ren, Y.; Huang, J.; Xiong, E.; et al. Empagliflozin Ameliorates Diabetic Cardiomyopathy via Attenuating Oxidative Stress and Improving Mitochondrial Function. Oxidative Med. Cell. Longev. 2022, 2022, 1122494. [Google Scholar] [CrossRef]
- Xue, R.-Q.; Yu, X.-J.; Zhao, M.; Xu, M.; Wu, Q.; Cui, Y.-L.; Yang, S.; Li, D.-L.; Zang, W.-J. Pyridostigmine alleviates cardiac dysfunction via improving mitochondrial cristae shape in a mouse model of metabolic syndrome. Free. Radic. Biol. Med. 2019, 134, 119–132. [Google Scholar] [CrossRef]
- Wang, X.; Younis, S.; Cen, J.; Wang, Y.; Krizhanovskii, C.; Andersson, L.; Welsh, N. ZBED6 counteracts high-fat diet-induced glucose intolerance by maintaining beta cell area and reducing excess mitochondrial activation. Diabetologia 2021, 64, 2292–2305. [Google Scholar] [CrossRef]
- Nishida, Y.; Nawaz, A.; Kado, T.; Takikawa, A.; Igarashi, Y.; Onogi, Y.; Wada, T.; Sasaoka, T.; Yamamoto, S.; Sasahara, M.; et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J. Cachex-Sarcopenia Muscle 2020, 11, 241–258. [Google Scholar] [CrossRef]
- Xiao, T.; Liang, X.; Liu, H.; Zhang, F.; Meng, W.; Hu, F. Mitochondrial stress protein HSP60 regulates ER stress-induced hepatic lipogenesis. J. Mol. Endocrinol. 2020, 64, 67–75. [Google Scholar] [CrossRef]
- Wen, Z.; Li, J.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C. Hypertrophic Adipocyte–Derived Exosomal miR-802-5p Contributes to Insulin Resistance in Cardiac Myocytes Through Targeting HSP60. Obesity 2020, 28, 1932–1940. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, Y.T.; Oh, H.; Kim, S.H.; Cho, J.M.; Kim, Y.-N.; Kim, S.S.; Kim, D.H.; Hur, K.Y.; Kim, H.K.; et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 2012, 19, 83–92. [Google Scholar] [CrossRef]
- Forsström, S.; Jackson, C.B.; Carroll, C.J.; Kuronen, M.; Pirinen, E.; Pradhan, S.; Marmyleva, A.; Auranen, M.; Kleine, I.-M.; Khan, N.A.; et al. Fibroblast Growth Factor 21 Drives Dynamics of Local and Systemic Stress Responses in Mitochondrial Myopathy with mtDNA Deletions. Cell Metab. 2019, 30, 1040–1054.e1047. [Google Scholar] [CrossRef]
- Lehtonen, J.M.; Auranen, M.; Darin, N.; Sofou, K.; Bindoff, L.; Hikmat, O.; Uusimaa, J.; Vieira, P.; Tulinius, M.; Lönnqvist, T.; et al. Diagnostic value of serum biomarkers FGF21 and GDF15 compared to muscle sample in mitochondrial disease. J. Inherit. Metab. Dis. 2020, 44, 469–480. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Nga, H.T.; Tian, J.; Yi, H.-S. Mitochondrial Metabolic Signatures in Hepatocellular Carcinoma. Cells 2021, 10, 1901. [Google Scholar] [CrossRef]
- Ost, M.; Coleman, V.; Voigt, A.; van Schothorst, E.M.; Keipert, S.; van der Stelt, I.; Ringel, S.; Graja, A.; Ambrosi, T.; Kipp, A.P.; et al. Muscle mitochondrial stress adaptation operates independently of endogenous FGF21 action. Mol. Metab. 2015, 5, 79–90. [Google Scholar] [CrossRef]
- Park, J.; Joe, Y.; Ryter, S.W.; Surh, Y.-J.; Chung, H.T. Similarities and Distinctions in the Effects of Metformin and Carbon Monoxide in Immunometabolism. Mol. Cells 2019, 42, 292–300. [Google Scholar] [CrossRef]
- Moon, J.S.; Goeminne, L.; Kim, J.T.; Tian, J.W.; Kim, S.-H.; Nga, H.T.; Kang, S.G.; Kang, B.E.; Byun, J.; Lee, Y.; et al. Growth differentiation factor 15 protects against the aging-mediated systemic inflammatory response in humans and mice. Aging Cell 2020, 19, e13195. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Jung, S.-B.; Lee, S.E.; Kang, S.G.; Lee, J.H.; Ryu, M.J.; Chung, H.K.; Chang, J.Y.; Kim, Y.K.; Hong, H.J.; et al. An adipocyte-specific defect in oxidative phosphorylation increases systemic energy expenditure and protects against diet-induced obesity in mouse models. Diabetologia 2020, 63, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-S. Implications of Mitochondrial Unfolded Protein Response and Mitokines: A Perspective on Fatty Liver Diseases. Endocrinol. Metab. 2019, 34, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Kurniawan, A. Appetite problem in cancer patients: Pathophysiology, diagnosis, and treatment. Cancer Treat. Res. Commun. 2021, 27, 100336. [Google Scholar] [CrossRef]
- Rosenberg, B.J.; Hirano, M.; Quinzii, C.M.; Colantuoni, E.; Needham, D.M.; Lederer, D.J.; Baldwin, M.R. Growth differentiation factor-15 as a biomarker of strength and recovery in survivors of acute respiratory failure. Thorax 2019, 74, 1099–1101. [Google Scholar] [CrossRef]
- Dominguez-Gonzalez, C.; Badosa, C.; Madruga-Garrido, M.; Martí, I.; Paradas, C.; Ortez, C.; Diaz-Manera, J.; Berardo, A.; Alonso-Pérez, J.; Trifunov, S.; et al. Growth Differentiation Factor 15 is a potential biomarker of therapeutic response for TK2 deficient myopathy. Sci. Rep. 2020, 10, 10111. [Google Scholar] [CrossRef]
- Poulsen, N.S.; Madsen, K.L.; Hornsyld, T.M.; Eisum, A.-S.V.; Fornander, F.; Buch, A.E.; Stemmerik, M.G.; Ruiz-Ruiz, C.; Krag, T.; Vissing, J. Growth and differentiation factor 15 as a biomarker for mitochondrial myopathy. Mitochondrion 2020, 50, 35–41. [Google Scholar] [CrossRef]
- Montero, R.; Yubero, D.; Villarroya, J.; Henares, D.; Jou, C.; Rodríguez, M.A.; Ramos, F.; Nascimento, A.; Ortez, C.I.; Campistol, J.; et al. GDF-15 Is Elevated in Children with Mitochondrial Diseases and Is Induced by Mitochondrial Dysfunction. PLoS ONE 2016, 11, e0148709. [Google Scholar] [CrossRef]
- Straub, I.R.; Weraarpachai, W.; Shoubridge, E.A. Multi-OMICS study of a CHCHD10 variant causing ALS demonstrates metabolic rewiring and activation of endoplasmic reticulum and mitochondrial unfolded protein responses. Hum. Mol. Genet. 2021, 30, 687–705. [Google Scholar] [CrossRef]
- Steffen, J.; Ngo, J.; Wang, S.-P.; Williams, K.; Kramer, H.F.; Ho, G.; Rodriguez, C.; Yekkala, K.; Amuzie, C.; Bialecki, R.; et al. The mitochondrial fission protein Drp1 in liver is required to mitigate NASH and prevents the activation of the mitochondrial ISR. Mol. Metab. 2022, 64, 101566. [Google Scholar] [CrossRef]
- Aguilar-Recarte, D.; Barroso, E.; Zhang, M.; Rada, P.; Pizarro-Delgado, J.; Peña, L.; Palomer, X.; Valverde, Á.M.; Wahli, W.; Vázquez-Carrera, M. A positive feedback loop between AMPK and GDF15 promotes metformin antidiabetic effects. Pharmacol. Res. 2023, 187, 106578. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Bruce, K.; Danaei, Z.; Li, R.J.; Barros, D.R.; Kuah, R.; Lim, Y.-M.; Mariani, L.H.; Cherney, D.Z.; Chiu, J.F.; et al. Metformin triggers a kidney GDF15-dependent area postrema axis to regulate food intake and body weight. Cell Metab. 2023, 35, 875–886.e5. [Google Scholar] [CrossRef]
- Chung, H.K.; Ryu, D.; Kim, K.S.; Chang, J.Y.; Kim, Y.K.; Yi, H.-S.; Kang, S.G.; Choi, M.J.; Lee, S.E.; Jung, S.-B.; et al. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J. Cell Biol. 2017, 216, 149–165. [Google Scholar] [CrossRef]
- Akazawa, S.; Sun, F.; Ito, M.; Kawasaki, E.; Eguchi, K. Efficacy of troglitazone on body fat distribution in type 2 diabetes. Diabetes Care 2000, 23, 1067–1071. [Google Scholar] [CrossRef]
- Ensuring drug safety: Lessons from the thiazolidinediones. Lancet 2007, 370, 1101. [CrossRef]
- Barber, T.M.; Kabisch, S.; Randeva, H.S.; Pfeiffer, A.F.H.; Weickert, M.O. Implications of Resveratrol in Obesity and Insulin Resistance: A State-of-the-Art Review. Nutrients 2022, 14, 2870. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017, 14, 60. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Hekmatdoost, A.; Mirmiran, P. Anti-Hyperglycemic and Insulin Sensitizer Effects of Turmeric and Its Principle Constituent Curcumin. Int. J. Endocrinol. Metab. 2014, 12, e18081. [Google Scholar] [CrossRef]
- Na, L.-X.; Zhang, Y.-L.; Li, Y.; Liu, L.-Y.; Li, R.; Kong, T.; Sun, C.-H. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 526–533. [Google Scholar] [CrossRef]
- Pirola, L.; Balcerczyk, A.; Okabe, J.; El-Osta, A. Epigenetic phenomena linked to diabetic complications. Nat. Rev. Endocrinol. 2010, 6, 665–675. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, Y.; Gao, J.; Zheng, Z.; Zhang, Z.; Yao, L.; Li, D. Curcumin improves insulin sensitivity in high-fat diet-fed mice through gut microbiota. Nutr. Metab. 2022, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Thirone, A.C.; Huang, C.; Klip, A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol. Metab. 2006, 17, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Jayanthy, G.; Devi, V.R.; Ilango, K.; Subramanian, S.P. Rosmarinic Acid Mediates Mitochondrial Biogenesis in Insulin Resistant Skeletal Muscle Through Activation of AMPK. J. Cell. Biochem. 2017, 118, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Hartogh, D.J.D.; Vlavcheski, F.; Tsiani, E. Muscle Cell Insulin Resistance Is Attenuated by Rosmarinic Acid: Elucidating the Mechanisms Involved. Int. J. Mol. Sci. 2023, 24, 5094. [Google Scholar] [CrossRef]
- Mimura, J.; Inose-Maruyama, A.; Taniuchi, S.; Kosaka, K.; Yoshida, H.; Yamazaki, H.; Kasai, S.; Harada, N.; Kaufman, R.J.; Oyadomari, S.; et al. Concomitant Nrf2- and ATF4-Activation by Carnosic Acid Cooperatively Induces Expression of Cytoprotective Genes. Int. J. Mol. Sci. 2019, 20, 1706. [Google Scholar] [CrossRef]
- Santana, L.F.; Inada, A.C.; Santo, B.L.S.D.E.; Filiú, W.F.O.; Pott, A.; Alves, F.M.; Guimarães, R.d.C.A.; Freitas, K.d.C.; Hiane, P.A. Nutraceutical Potential of Carica papaya in Metabolic Syndrome. Nutrients 2019, 11, 1608. [Google Scholar] [CrossRef]
- Elsayed, R.H.; Kamel, E.M.; Mahmoud, A.M.; El-Bassuony, A.A.; Bin-Jumah, M.; Lamsabhi, A.M.; Ahmed, S.A. Rumex dentatus L. phenolics ameliorate hyperglycemia by modulating hepatic key enzymes of carbohydrate metabolism, oxidative stress and PPARγ in diabetic rats. Food Chem. Toxicol. 2020, 138, 111202. [Google Scholar] [CrossRef]
- Song, P.; Kim, J.H.; Ghim, J.; Yoon, J.H.; Lee, A.; Kwon, Y.; Hyun, H.; Moon, H.-Y.; Choi, H.-S.; Berggren, P.-O.; et al. Emodin Regulates Glucose Utilization by Activating AMP-activated Protein Kinase. J. Biol. Chem. 2013, 288, 5732–5742. [Google Scholar] [CrossRef]
- Yu, F.; Yu, N.; Peng, J.; Zhao, Y.; Zhang, L.; Wang, X.; Xu, X.; Zhou, J.; Wang, F. Emodin inhibits lipid accumulation and inflammation in adipose tissue of high-fat diet-fed mice by inducing M2 polarization of adipose tissue macrophages. FASEB J. 2021, 35, e21730. [Google Scholar] [CrossRef]
- Fu, S.; Dang, Y.; Xu, H.; Li, A.; Zhou, X.; Gao, X.; Li, Z. Aloe vera-Fermented Beverage Ameliorates Obesity and Gut Dysbiosis in High-Fat-Diet Mice. Foods 2022, 11, 3728. [Google Scholar] [CrossRef]
- Dou, F.; Liu, M.Y.; Liu, L.; Wang, J.; Sun, M.T.; Mu, M.F.; Guo, Q.; Guo, C.; Jia, N.; Liu, W.; et al. Aloe-Emodin Ameliorates Renal Fibrosis Via Inhibiting PI3K/Akt/mTOR Signaling Pathway In Vivo and In Vitro. Rejuvenation Res. 2019, 22, 218–229. [Google Scholar] [CrossRef]
- Quan, Y.; Gong, L.; He, J.; Zhou, Y.; Liu, M.; Cao, Z.; Li, Y.; Peng, C. Aloe emodin induces hepatotoxicity by activating NF-κB inflammatory pathway and P53 apoptosis pathway in zebrafish. Toxicol. Lett. 2019, 306, 66–79. [Google Scholar] [CrossRef]
- Régnier, M.; Rastelli, M.; Morissette, A.; Suriano, F.; Le Roy, T.; Pilon, G.; Delzenne, N.M.; Marette, A.; Van Hul, M.; Cani, P.D. Rhubarb Supplementation Prevents Diet-Induced Obesity and Diabetes in Association with Increased Akkermansia muciniphila in Mice. Nutrients 2020, 12, 2932. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, C.; Vong, C.T.; Tao, H.; Li, H.; Wang, S.; Wang, Y. Rhein regulates redox-mediated activation of NLRP3 inflammasomes in intestinal inflammation through macrophage-activated crosstalk. Br. J. Pharmacol. 2022, 179, 1978–1997. [Google Scholar] [CrossRef]
- Genua, M.; Rutella, S.; Correale, C.; Danese, S. The triggering receptor expressed on myeloid cells (TREM) in inflammatory bowel disease pathogenesis. J. Transl. Med. 2014, 12, 293. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Tong, A.-H.; Pan, Y.-Y.; Zhang, X.-D.; Ding, W.-Y.; Xiong, W. The effect of cassia seed extract on the regulation of the LKB1–AMPK–GLUT4 signaling pathway in the skeletal muscle of diabetic rats to improve the insulin sensitivity of the skeletal muscle. Diabetol. Metab. Syndr. 2019, 11, 1–10. [Google Scholar] [CrossRef]
- Zhou, F.; Ding, M.; Gu, Y.; Fan, G.; Liu, C.; Li, Y.; Sun, R.; Wu, J.; Li, J.; Xue, X.; et al. Aurantio-Obtusin Attenuates Non-Alcoholic Fatty Liver Disease Through AMPK-Mediated Autophagy and Fatty Acid Oxidation Pathways. Front. Pharmacol. 2021, 12, 826628. [Google Scholar] [CrossRef]
- Guo, C.; Liao, W.; Qiu, R.; Zhou, D.; Ni, W.; Yu, C.; Zeng, Y. Aurantio-obtusin improves obesity and insulin resistance induced by high-fat diet in obese mice. Phytother. Res. 2020, 35, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Ali, M.Y.; Choi, J.S. Promising Inhibitory Effects of Anthraquinones, Naphthopyrone, and Naphthalene Glycosides, from Cassia obtusifolia on α-Glucosidase and Human Protein Tyrosine Phosphatases 1B. Molecules 2017, 22, 28. [Google Scholar] [CrossRef]
- Patel, D.; Prasad, S.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Zhang, C.; Sheng, Y.; Zhao, C.; He, X.; Xu, W.; Huang, K.; Luo, Y. Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci. Rep. 2018, 8, 3527. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.; Al-Sagan, A.A.; El-Hack, A.; Taha, M.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Faroughi, F.; Mohammad-Alizadeh-Charandabi, S.; Javadzadeh, Y.; Mirghafourvand, M. Effects of Garlic Pill on Blood Glucose Level in Borderline Gestational Diabetes Mellitus: A Randomized Controlled Trial. Iran. Red Crescent Med. J. 2018, 20, e60675. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef]
- Zhou, T.; Gai, Z.; Gao, X.; Li, L. The Potential Mechanism of Exercise Combined with Natural Extracts to Prevent and Treat Postmenopausal Osteoporosis. J. Health Eng. 2021, 2021, 2852661. [Google Scholar] [CrossRef]
- Wieërs, G.; Verbelen, V.; Driessche, M.V.D.; Melnik, E.; Vanheule, G.; Marot, J.-C.; Cani, P.D. Do Probiotics During In-Hospital Antibiotic Treatment Prevent Colonization of Gut Microbiota with Multi-Drug-Resistant Bacteria? A Randomized Placebo-Controlled Trial Comparing Saccharomyces to a Mixture of Lactobacillus, Bifidobacterium, and Saccharomyces. Front. Public Health 2021, 8, 578089. [Google Scholar] [CrossRef]
- Leyva-Gómez, G.; González-Torres, M.; Alcalá-Alcalá, S.; Bernal-Chávez, S.A.; Morales-Morfin, J.C.; Carmen, M.G.-D.; Sharifi-Rad, J.; Figueroa-González, G.; Reyes-Hernández, O.D.; Del Prado-Audelo, M.L.; et al. Development of films from natural sources for infections during wound healing. Cell. Mol. Biol. 2021, 67, 96–100. [Google Scholar] [CrossRef]
- Bellik, Y.; Hammoudi, S.M.; Abdellah, F.; Iguer-Ouada, M.; Boukraâ, L. Phytochemicals to prevent inflammation and allergy. Recent Patents Inflamm. Allergy Drug Discov. 2012, 6, 147–158. [Google Scholar] [CrossRef]
| Factor | Mechanism |
|---|---|
| AGEs | Promotes mitochondrial stress by activating NADPH |
| CypD | Promotes mitochondrial stress when the MPTP is open and NEFAs are present |
| PPARs | Inhibit mitochondrial stress by inhibiting IL-6 |
| miR-126 | Inhibits mitochondrial stress by activating SIRT1 and SOD |
| miR-30c | Inhibits mitochondrial stress by activating PGC-1 and PPAR-α |
| HSP60 | Mitochondrial stress causes overexpression of HSP60; HSP60 promotes mTORC1-SREBP1 signal transduction and promotes insulin resistance together with endoplasmic reticulum stress |
| FGF21 | Metformin induces mitochondrial stress through the Perk-eIF2α-ATF4 axis; mitochondrial stress promotes FGF21 expression by activating the comprehensive stress response (ISR); FGF21 improves insulin resistance |
| GDF-15 | GDF-15 improves insulin resistance; CYPD may cause insulin resistance by inhibiting GDF-15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Wang, X. Natural Drugs: A New Direction for the Prevention and Treatment of Diabetes. Molecules 2023, 28, 5525. https://doi.org/10.3390/molecules28145525
Wu P, Wang X. Natural Drugs: A New Direction for the Prevention and Treatment of Diabetes. Molecules. 2023; 28(14):5525. https://doi.org/10.3390/molecules28145525
Chicago/Turabian StyleWu, Peishan, and Xiaolei Wang. 2023. "Natural Drugs: A New Direction for the Prevention and Treatment of Diabetes" Molecules 28, no. 14: 5525. https://doi.org/10.3390/molecules28145525
APA StyleWu, P., & Wang, X. (2023). Natural Drugs: A New Direction for the Prevention and Treatment of Diabetes. Molecules, 28(14), 5525. https://doi.org/10.3390/molecules28145525









