A High-Efficiency Deep Blue Emitter for OLEDs with a New Dual-Core Structure Incorporating ETL Characteristics
Abstract
:1. Introduction
2. Results and Discussion
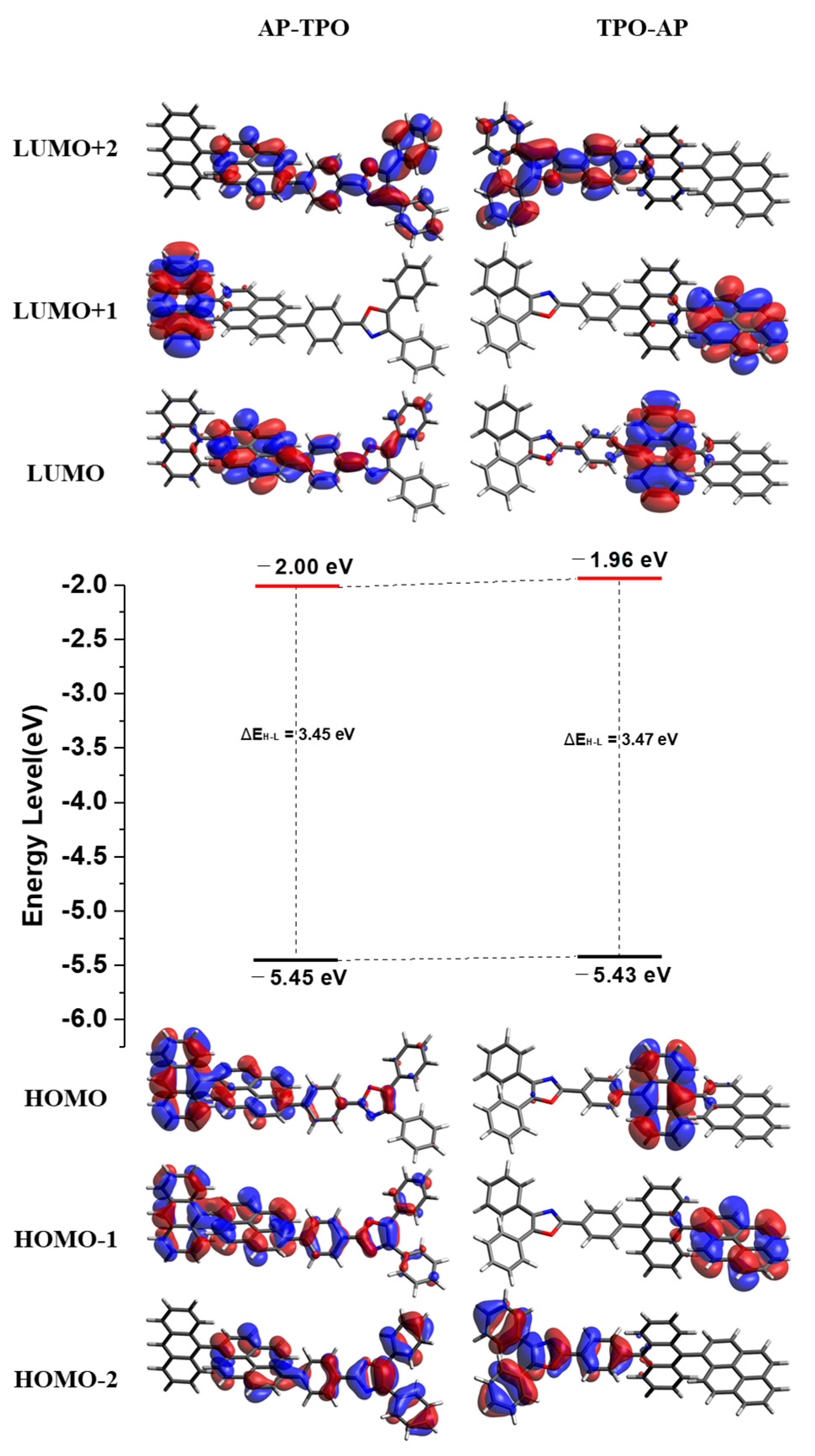
2.1. Photophysical Properties and Theoretical Calculations
2.2. Thermal and Electrical Properties
2.3. Electroluminescence Properties
3. Experimental
3.1. Materials, Measurements, and Device Fabrication
3.2. Synthesis
3.2.1. 2-(4-Bromophenyl)-4,5-diphenyloxazole (1)
3.2.2. 4,5-Diphenyl-2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)oxazole (2)
3.2.3. 1-(10-Bromoanthracen-9-yl)pyrene (3)
3.2.4. 2-(4-(6-(Anthracen-9-yl)pyren-1-yl)phenyl)-4,5-diphenyloxazole (5) (AP-TPO)
3.2.5. 4,5-Diphenyl-2-(4-(10-(pyren-1-yl)anthracen-9-yl)phenyl)oxazole (6) (TPO-AP)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, C.-H.; Chen, C.H.; Hsu, F.M.; Shih, P.I.; Shu, C.-F. Efficient non-doped blue-light-emitting diodes incorporating an anthracene derivative end-capped with fluorene groups. J. Mater. Chem. 2009, 19, 1464. [Google Scholar] [CrossRef]
- Thomas, K.R.J.; Kapoor, N.; Prasad, M.N.K.; Jou, J.-H.; Chen, Y.-L.; Joun, Y.-C. Pyrene-Fluorene Hybrids Containing Acetylene Linkage as Color-Tunable Emitting Materials for Organic Light-Emitting Diodes. J. Org. Chem. 2012, 77, 3921. [Google Scholar] [CrossRef]
- Jeon, S.; Jeon, Y.; Kim, J.; Lee, C.; Gong, M. Blue organic light-emitting diode with improved color purity using 5-naphthyl-spiro[fluorene-7,9′-benzofluorene]. Org. Electron. 2008, 9, 522. [Google Scholar] [CrossRef]
- Hayashi, T.; Mataga, N.; Sakata, Y.; Misumi, S.; Morita, M.; Tanaka, J. Excimer fluorescence and photodimerization of anthracenophanes and 1,2-dianthrylethanes. J. Am. Chem. Soc. 1976, 98, 5910. [Google Scholar] [CrossRef]
- Matsui, A.H. Excitonic processes in aromatic molecular crystals of strong exciton-phonon coupling. Pure Appl. Chem. 1995, 67, 429. [Google Scholar] [CrossRef]
- Feng, X.; Xu, Z.; Hu, Z.; Qi, C.; Luo, D.; Zhao, X.; Mu, Z.; Redshaw, C.; Lam, J.W.Y.; Ma, D.; et al. Pyrene-based blue emitters with aggregation-induced emission features for high-performance organic light-emitting diodes. J. Mater. Chem. C 2019, 7, 2283. [Google Scholar] [CrossRef]
- Kim, B.; Park, Y.; Lee, J.; Yokohama, D.; Lee, J.; Kido, J.; Park, J. Synthesis and electroluminescence properties of highly efficient blue fluorescence emitters using dual core chromophores. J. Mater. Chem. C 2013, 1, 432. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.; Kim, S.; Kim, J.; Lee, J.; Shin, H.; Lee, J.H.; Park, J. Synthesis and electroluminescence properties of highly efficient dual core chromophores with side groups for blue emission. J. Mater. Chem. C 2014, 2, 4737. [Google Scholar] [CrossRef]
- Xing, Z.H.; Zhuang, J.Y.; Xu, X.P.; Ji, S.J.; Su, W.-M.; Cui, Z. Novel oxazole-based emitters for high efficiency fluorescent OLEDs: Synthesis, characterization, and optoelectronic properties. Tetrahedron 2017, 73, 2036. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.; Wu, H.; Liu, Z.; Li, J.; Zhang, G.; Wen, G.; Shuang, S.; Dong, C.; Choi, M.M.F. Synthesis and photophysical studies of oxazole rings containing compounds as electron accepting units. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 256. [Google Scholar] [CrossRef]
- Jones, R.N. The Ultraviolet Absorption Spectra of Anthracene Derivatives. Chem. Rev. 1947, 41, 353. [Google Scholar] [CrossRef]
- Desai, N.K.; Mahajan, P.G.; Mali, S.S.; Kolekar, G.B.; Patil, S.R. Enhanced Exciplex Emission of Pyrene Thin Films Doped by Perylene: Structural, Photophysical and Morphological Investigation. J. Fluoresc. 2018, 28, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xing, J.; Gong, Q.; Chen, L.C.; Liu, G.; Yao, C.; Wang, Z.; Zhang, H.L.; Chen, Z.; Zhang, Q. Reducing aggregation caused quenching effect through co-assembly of PAH chromophores and molecular barriers. Nat. Commun. 2019, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Zhang, D.; Wei, J.; Zhang, Y.; Huang, T.; Liu, Z.; Yin, C.; Hong, X.; Wang, X.; Zeng, X.; et al. Highly efficient and stable deep-blue OLEDs based on narrowband emitters featuring an orthogonal spiro-configured indolo[3,2,1-de]acridine structure. Chem. Sci. 2022, 13, 5622. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Lu, P.; Chen, S.; Lam, J.W.Y.; Wang, Z.; Liu, Y.; Kwok, H.S.; Ma, Y.; Tang, B.Z. Changing the Behavior of Chromophores from Aggregation-Caused Quenching to Aggregation-Induced Emission: Development of Highly Efficient Light Emitters in the Solid State. Adv. Mater. 2010, 22, 2159. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, H.; Jin, J.; Bodedla, G.B.; Tao, P.; Ma, D.; Wong, W.Y. Orthogonal anthracene and pyrene derivatives for efficient pure deep-blue organic light-emitting diodes. J. Mater. Chem. C 2023, 11, 6438–6443. [Google Scholar] [CrossRef]
- Ki, M.S.; Sim, M.; Kwon, O.; Im, K.; Choi, B.; Cha, B.J.; Kim, Y.; Jin, T.Y.; Paeng, K. Improved Thermal Stability and Operational Lifetime of Blue Fluorescent Organic Light-Emitting Diodes by Using a Mixed-Electron Transporting Layer. ACS Mat. Lett. 2022, 4, 1676. [Google Scholar] [CrossRef]
- Zhang, G.; Musgrave., C.B. Comparison of DFT Methods for Molecular Orbital Eigenvalue Calculations. J. Phys. Chem. A 2007, 111, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.; Sancho-García, J.C.; Pershin, A.; Beljonne, D.; Zysman-Colman, E.; Olivier, Y. Benchmarking DFT Functionals for Excited-State Calculations of Donor–Acceptor TADF Emitters: Insights on the Key Parameters Determining Reverse Inter-System Crossing. J. Phys. Chem. A 2023, 127, 4743–4757. [Google Scholar] [CrossRef]
- Sasaki, T.; Hasegawa, M.; Inagaki, K.; Ito, H.; Suzuki, K.; Onno, T.; Morii, K.; Shimizu, T.; Fukagawa, H. Unravelling the electron injection/transport mechanism in organic light-emitting diodes. Nat. Commun. 2021, 12, 2706. [Google Scholar] [CrossRef]
- Sasaki, T.; Onno, T.; Shimizu, T.; Fukagawa, H. Effects of Energy-Level Alignment on Operating Voltages of Blue Organic Light-Emitting Diodes. Adv. Mater. Interfaces 2023, 10, 220192. [Google Scholar] [CrossRef]
- Shan, T.; Gao, Z.; Tang, X.; He, X.; Gao, Y.; Li, J.; Sun, X.; Liu, Y.; Liu, H.; Yang, B.; et al. Highly efficient and stable pure blue nondoped organic light-emitting diodes at high luminance based on phenanthroimidazole-pyrene derivative enabled by triplei-triplet annihilation. Dyes. Pigm. 2017, 142, 189–197. [Google Scholar] [CrossRef]
- Lim, H.; Woo, S.J.; Ha, Y.H.; Kim, Y.H.; Kim, J.J. Breaking the Efficiency Limit of Deep-Blue Fluorescent OLEDs Based on Anthracene Derivatives. Adv. Mater. 2022, 34, 2100161. [Google Scholar] [CrossRef]
- Kondakov, D.Y.; Pawlik, T.D.; Hatwar, T.K.; Spindler, J.P. Triplet annihilation exceeding spin statistical limit in highly efficient fluorescent organic light-emitting diodes. J. Appl. Phys. 2009, 106, 124510. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, Y.; Huang, T.; Liu, Z.; Duan, L.; Zhang, D. Highly efficient and nearly roll-off–free electrofluorescent devices via multiple sensitizations. Sci. Adv. 2022, 8, eabp9203. [Google Scholar] [CrossRef]
- Lee, S.; Koo, H.; Kwon, O.; Park, Y.J.; Choi, H.; Lee, K.; Ahn, B.; Park, Y.M. The Role of Charge Balance and Excited State Levels on Device Performance of Exciplex-based Phosphorescent Organic Light Emitting Diodes. Sci. Rep. 2017, 7, 11995. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.H.; Maeng, J.H.; Lee, H.; Yoo, H.; Lampande, R.; Lee, J.Y.; Kwon, J.H. Rigid Oxygen-Bridged Boron-Based Blue Thermally Activated Delayed Fluorescence Emitter for Organic Light-Emitting Diode: Approach towards Satisfying High Efficiency and Long Lifetime Together. Adv. Optical Mater. 2020, 8, 2000102. [Google Scholar] [CrossRef]






| Solution a | Film b | PLQY c (%) | |||||
|---|---|---|---|---|---|---|---|
| UVmax (nm) | PLmax (nm) | FWHM (nm) | UVmax (nm) | PLmax (nm) | FWHM (nm) | ||
| AP-TPO | 371, 390 | 433 | 54 | 377, 396 | 464 | 80 | 82/20 |
| TPO-AP | 328, 341 | 443 | 58 | 332, 345 | 462 | 72 | 88/24 |
| Compound | Absorption Wavelength (nm) | Oscillator Strength | Characteristic of Transition | Contribution a (%) |
|---|---|---|---|---|
| AP-TPO | 412.9 | 0.011 | HOMO−1→ LUMO HOMO → LUMO | 23.5 70.0 |
| 403.2 | 0.014 | HOMO−1→ LUMO+1 HOMO → LUMO+1 | 52.7 34.7 | |
| 386.1 | 1.321 | HOMO−1→ LUMO HOMO → LUMO+1 HOMO → LUMO | 54.5 12.8 21.6 | |
| 363.9 | 0.010 | HOMO−1→ LUMO+1 HOMO → LUMO+1 | 25.1 51.0 | |
| TPO-AP | 399.4 | 0.005 | HOMO → LUMO | 93.4 |
| 398.7 | 0.003 | HOMO−1→ LUMO+1 | 94.0 | |
| 382.8 | 0.383 | HOMO−2→ LUMO+2 | 83.9 | |
| 372.7 | 0.164 | HOMO−1→ LUMO HOMO → LUMO+1 | 19.1 78.7 |
| Td (°C) | Tg (°C) | Tm (°C) | HOMO (eV) | LUMO (eV) | Band Gap (eV) | |
|---|---|---|---|---|---|---|
| AP-TPO | 448 | 151 | 283 | −5.80 | −2.88 | 2.92 |
| TPO-AP | 395 | 162 | 307 | −5.84 | −2.96 | 2.88 |
| Volt (V) | CE (Cd/A) | PE (lm/W) | EQE (%) | CIE (x, y) | ELmax (nm) | |
|---|---|---|---|---|---|---|
| AP-TPO | 5.44 | 4.33 | 2.52 | 3.73 | (0.156, 0.134) | 447 |
| TPO-AP | 5.86 | 5.49 | 2.94 | 4.26 | (0.168, 0.154) | 453 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, Y.; Kwon, H.; Park, S.; Dae, S.; Lee, H.; Lee, K.; Park, J. A High-Efficiency Deep Blue Emitter for OLEDs with a New Dual-Core Structure Incorporating ETL Characteristics. Molecules 2023, 28, 7485. https://doi.org/10.3390/molecules28227485
Heo Y, Kwon H, Park S, Dae S, Lee H, Lee K, Park J. A High-Efficiency Deep Blue Emitter for OLEDs with a New Dual-Core Structure Incorporating ETL Characteristics. Molecules. 2023; 28(22):7485. https://doi.org/10.3390/molecules28227485
Chicago/Turabian StyleHeo, Yeongjae, Hyukmin Kwon, Sangwook Park, Sunwoo Dae, Hayoon Lee, Kiho Lee, and Jongwook Park. 2023. "A High-Efficiency Deep Blue Emitter for OLEDs with a New Dual-Core Structure Incorporating ETL Characteristics" Molecules 28, no. 22: 7485. https://doi.org/10.3390/molecules28227485
APA StyleHeo, Y., Kwon, H., Park, S., Dae, S., Lee, H., Lee, K., & Park, J. (2023). A High-Efficiency Deep Blue Emitter for OLEDs with a New Dual-Core Structure Incorporating ETL Characteristics. Molecules, 28(22), 7485. https://doi.org/10.3390/molecules28227485









