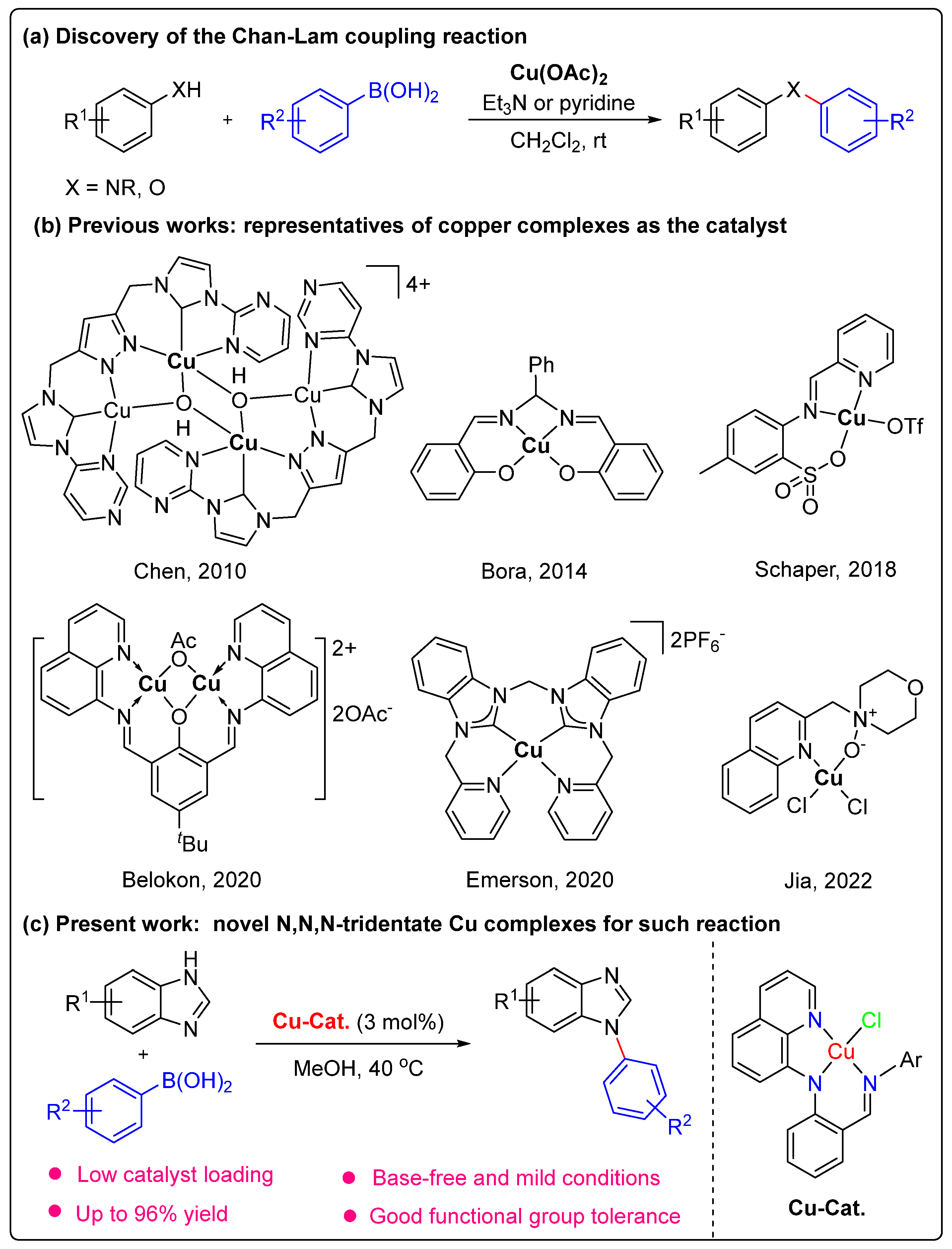
Copper Complexes with N,N,N-Tridentate Quinolinyl Anilido-Imine Ligands: Synthesis and Their Catalytic Application in Chan−Lam Reactions
Abstract
:1. Introduction
2. Results and Discussion
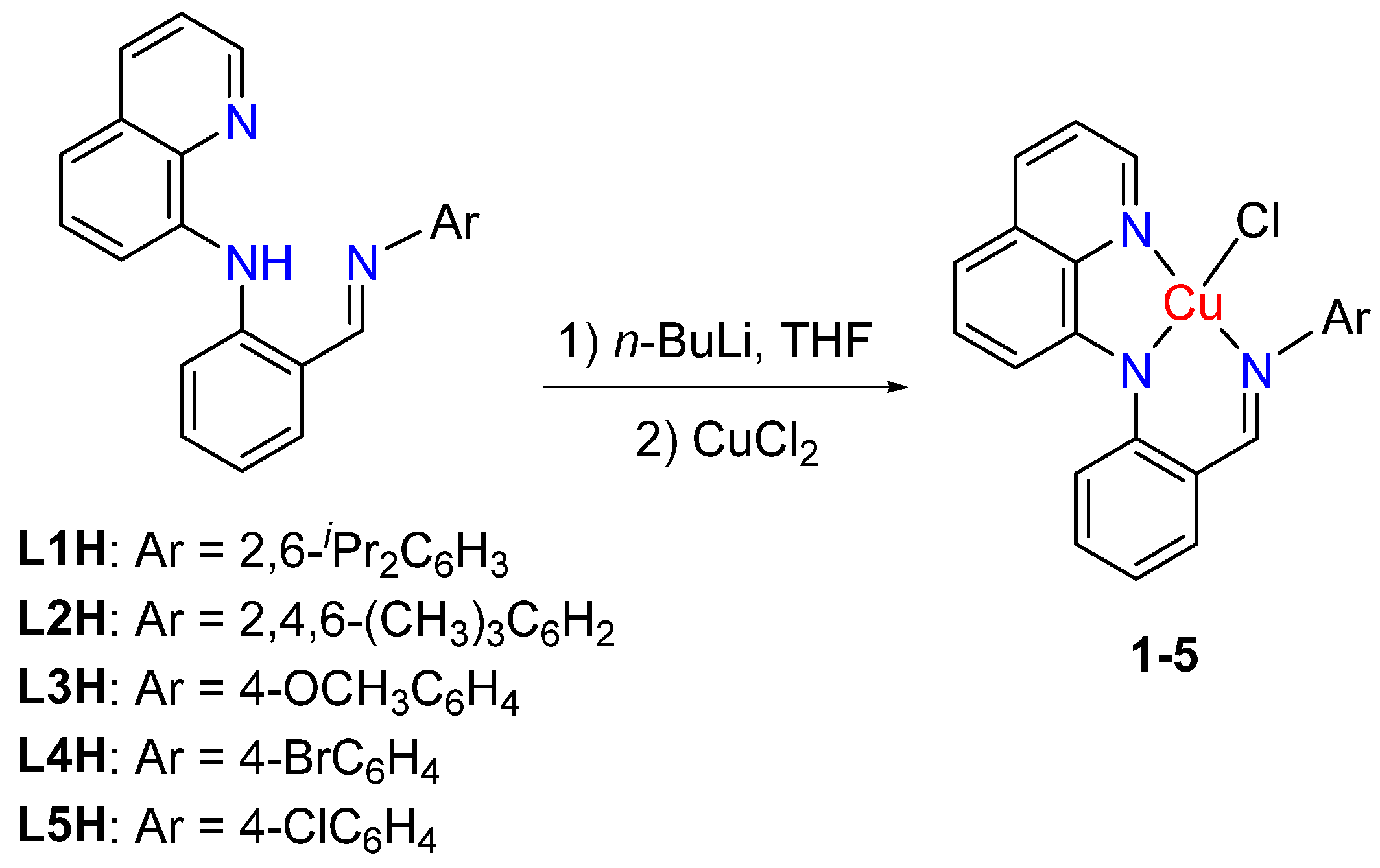
2.1. Synthesis and Characterization of Ligands and Cu(II) Complexes
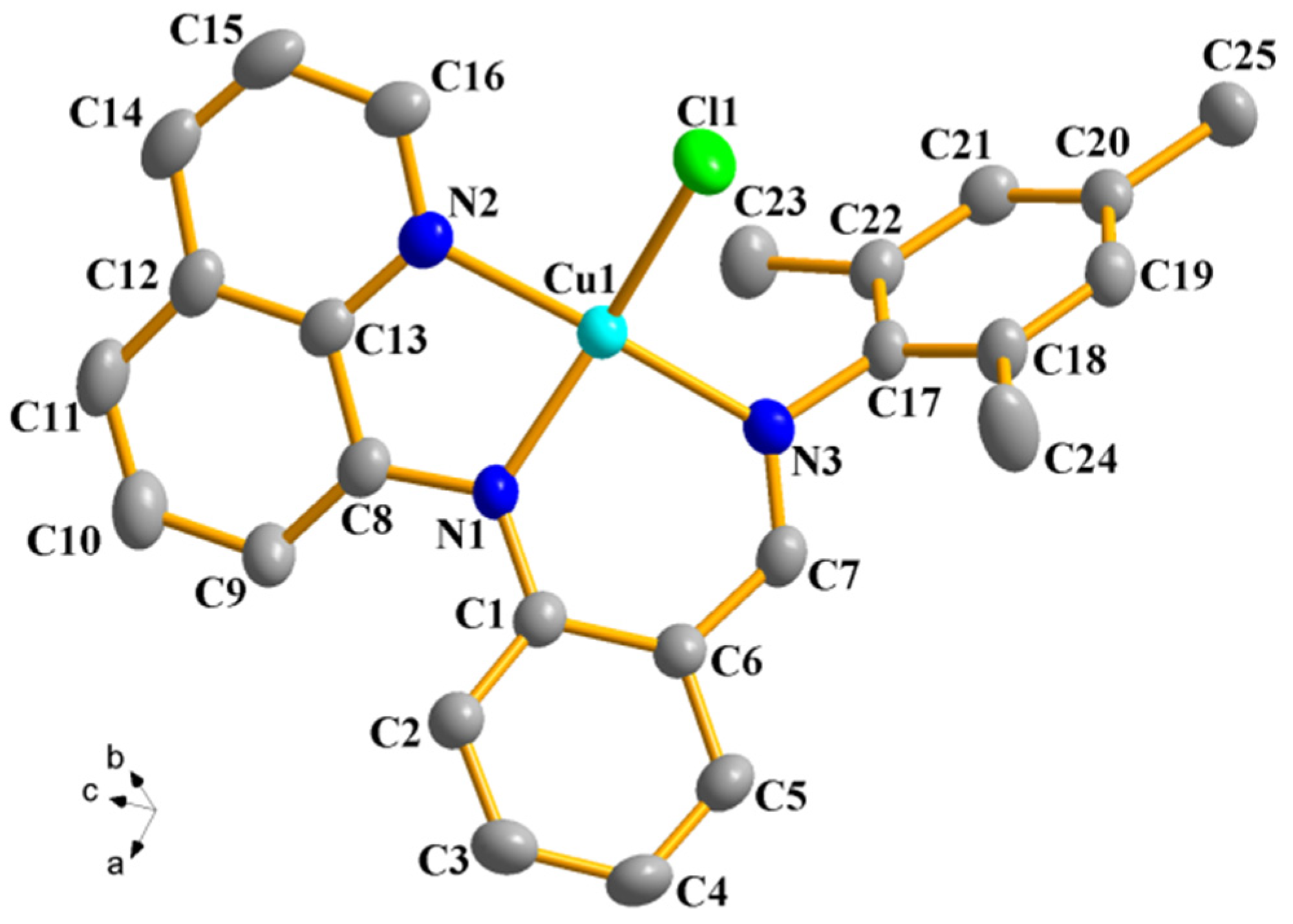
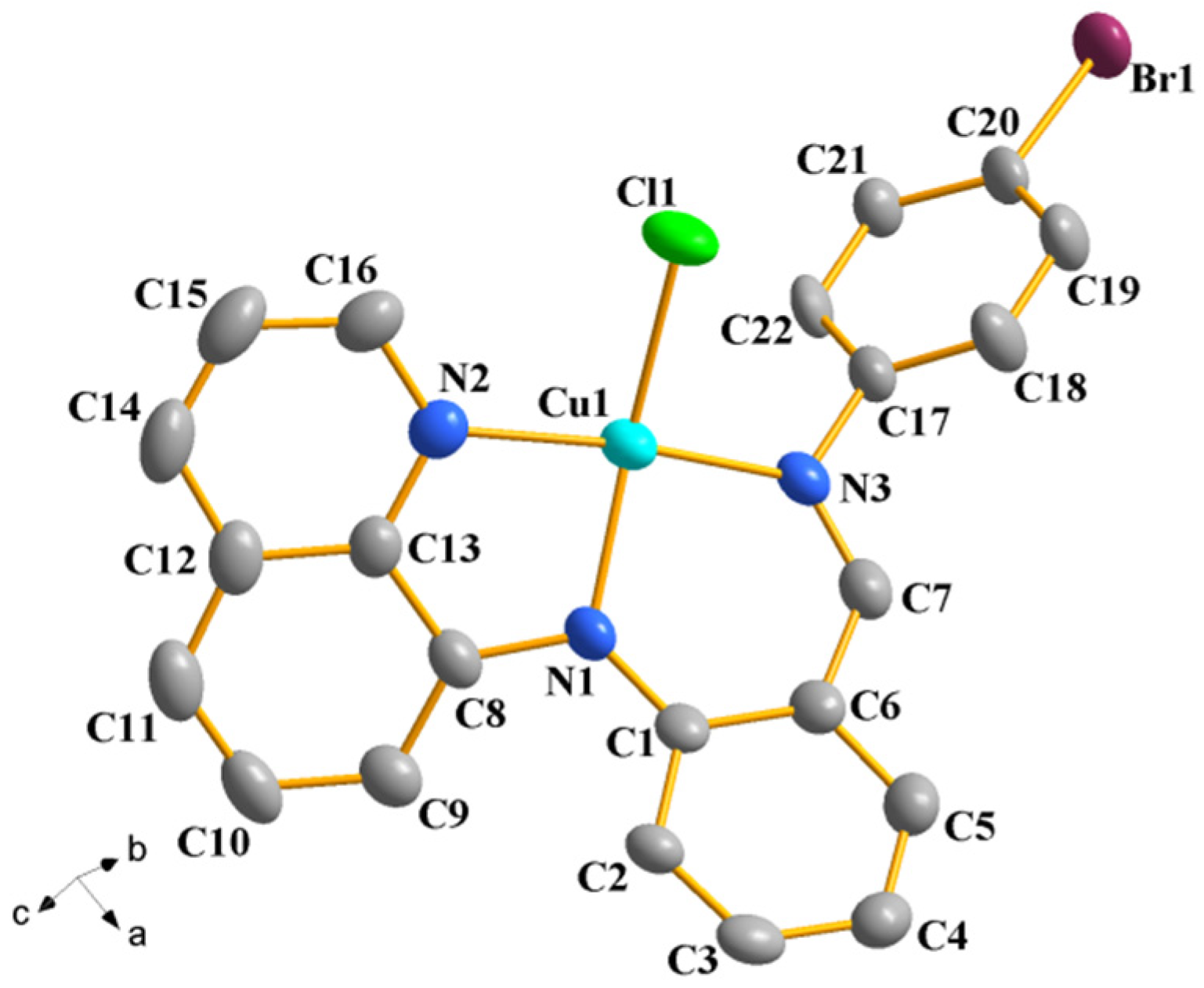
2.2. Description of the Crystal Structures of Complexes 2 and 4
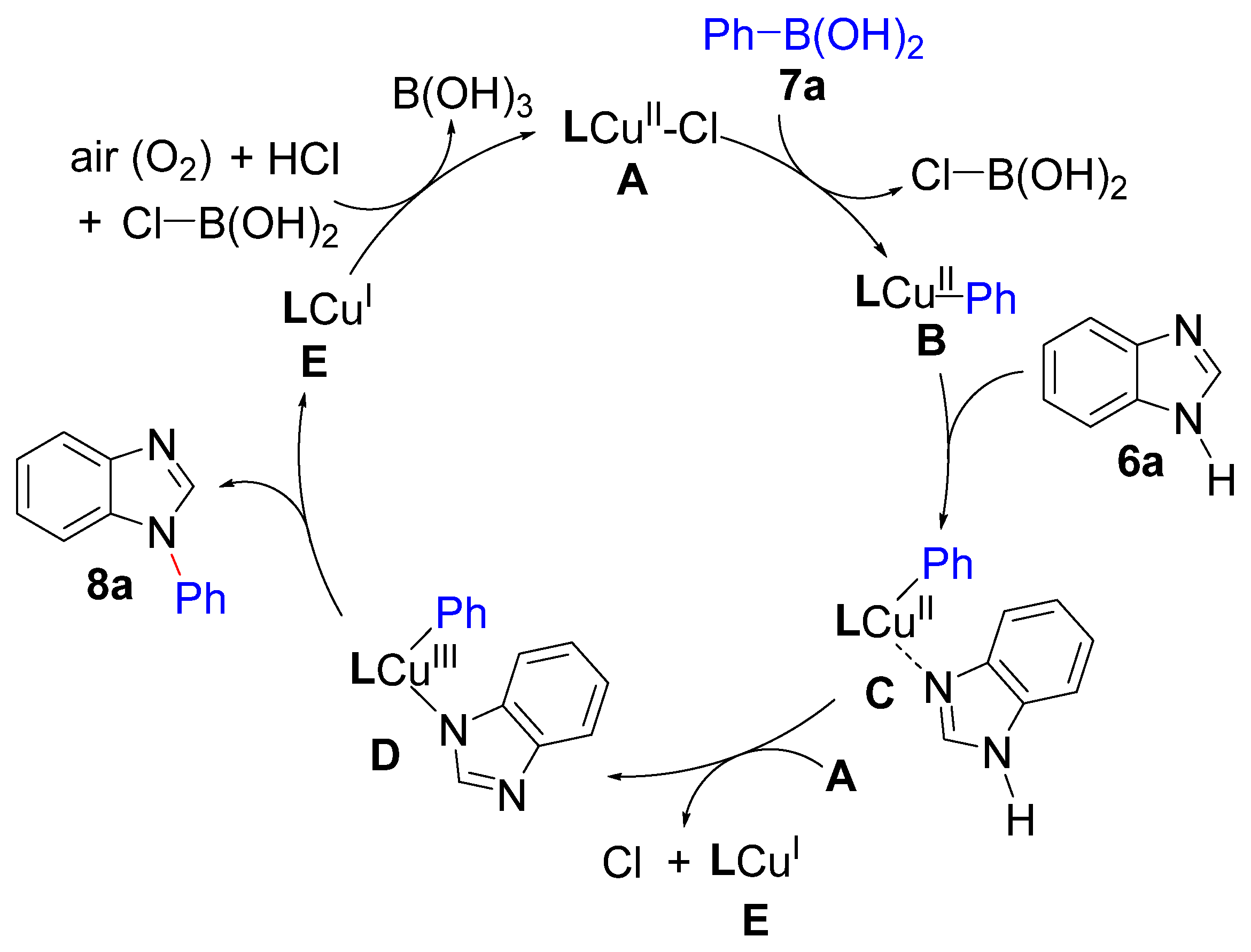
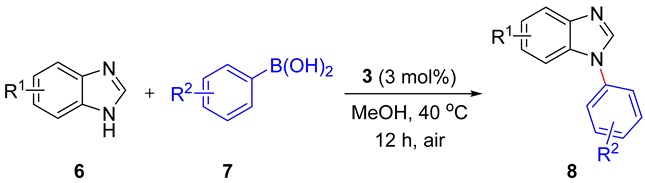
2.3. Catalytic Activity
3. Materials and Methods
3.1. General Considerations
3.2. X-ray Crystallographic Studies
3.3. Synthesis of Ligand L3H
3.4. Synthesis of Complex 2
3.5. Synthesis of Complex 3
3.6. Synthesis of Complex 4
3.7. Synthesis of Complex 5
3.8. General Procedure for the Cu-Catalyzed Chan-Lam Coupling Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, H.; Gilliam, A.M.; Qu, F.; Shaughnessy, K.H. Enolizable ketones as activators of palladium(II) precatalysts in amine arylation reactions. ACS Catal. 2020, 10, 4127–4135. [Google Scholar] [CrossRef]
- Lu, C.-J.; Xu, Q.; Feng, J.; Liu, R.-R. The asymmetric Buchwald-Hartwig amination reaction. Angew. Chem. Int. Ed. 2023, 62, e202216863. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Li, J.-H.; Dong, Z.-B. A review on the latest progress of Chan-Lam coupling reaction. Adv. Synth. Catal. 2020, 362, 3311–3331. [Google Scholar] [CrossRef]
- Jia, X.; Tong, X. Recent progress on Chan-Lam coupling reactions catalyzed by copper(II) complexes. Chin. J. Org. Chem. 2022, 42, 2640–2658. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, Y.; Zhang, L.; Guo, Y.; Ma, D. Oxalic diamides and tert-butoxide: Two types of ligands enabling practical access to alkyl aryl ethers via Cu-catalyzed coupling reaction. J. Am. Chem. Soc. 2019, 141, 3541–3549. [Google Scholar] [CrossRef]
- Roh, J.; Vavrová, K.; Hrábálek, A. Synthesis and functionalization of 5-substituted tetrazoles. Eur. J. Org. Chem. 2012, 2012, 6101–6118. [Google Scholar] [CrossRef]
- Chan, D.M.T.; Monaco, K.L.; Wang, R.-P.; Winters, M.P. New N- and O-arylations with phenylboronic acids and cupric acetate. Tetrahedron Lett. 1998, 39, 2933–2936. [Google Scholar] [CrossRef]
- Evans, D.A.; Katz, J.L.; West, T.R. Synthesis of diaryl ethers through the copper-promoted arylation of phenols with arylboronic acids. An expedient synthesis of thyroxine. Tetrahedron Lett. 1998, 39, 2937–2940. [Google Scholar] [CrossRef]
- Lam, P.Y.S.; Clarkt, C.G.; Saubernt, S.; Adamst, J.; Winters, M.P.; Chan, D.M.T.; Combs, A. New aryl/heteroaryl C-N bond cross-coupling reactions via arylboronic acid/cupric acetate arylation. Tetrahedron Lett. 1998, 39, 2941–2944. [Google Scholar] [CrossRef]
- Fu, T.; Qiao, H.; Peng, Z.; Hu, G.; Wu, X.; Gao, Y.; Zhao, Y. Palladium-catalyzed air-based oxidative coupling of arylboronic acids with H-phosphine oxides leading to aryl phosphine oxides. Org. Biomol. Chem. 2014, 12, 2895–2902. [Google Scholar] [CrossRef]
- Kumar, K.A.; Kannaboina, P.; Rao, D.N.; Das, P. Nickel-catalyzed Chan-Lam cross-coupling: Chemoselective N-arylation of 2-aminobenzimidazoles. Org. Biomol. Chem. 2016, 14, 8989–8997. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Kumar, S.; Singh, K.N.; Drew, M.G.B.; Singh, N. Synthesis and characterization of new square planar heteroleptic cationic complexes [Ni(II) β-oxodithioester-dppe]+; their use as a catalyst for Chan-Lam coupling. New J. Chem. 2020, 44, 12143–12153. [Google Scholar] [CrossRef]
- Shi, W.; Shi, Y.; Xü, M.; Zou, G.; Wu, X.-Y. Chemoselective Chan-Lam coupling by directly using copper powders via mechanochemical metal activation for catalysis. Mol. Catal. 2022, 528, 112472. [Google Scholar] [CrossRef]
- Collman, J.P.; Zhong, M. An efficient diamine copper complex-catalyzed coupling of arylboronic acids with imidazoles. Org. Lett. 2000, 2, 1233–1236. [Google Scholar] [CrossRef]
- Liu, B.; Liu, B.; Zhou, Y.; Chen, W. Copper(II) hydroxide complexes of N-heterocyclic carbenes and catalytic oxidative amination of arylboronic acids. Organometallics 2010, 29, 1457–1464. [Google Scholar] [CrossRef]
- Gogoi, A.; Sarmah, G.; Dewan, A.; Bora, U. Unique copper-salen complex: An efficient catalyst for N-arylations of anilines and imidazoles at room temperature. Tetrahedron Lett. 2014, 55, 31–35. [Google Scholar] [CrossRef]
- Duparc, V.H.; Schaper, F. Sulfonato-diketimine copper(II) complexes: Synthesis and application as catalysts in Chan−Evans−Lam couplings. Organometallics 2017, 36, 3053–3060. [Google Scholar] [CrossRef]
- Duparc, V.H.; Bano, G.L.; Schaper, F. Chan−Evans−Lam couplings with copper iminoarylsulfonate complexes: Scope and mechanism. ACS Catal. 2018, 8, 7308–7325. [Google Scholar] [CrossRef]
- Akatyev, N.; Il’in, M., Jr.; Peregudova, S.; Peregudov, A.; Buyanovskaya, A.; Kudryavtsev, K.; Dubovik, A.; Grinberg, V.; Orlov, V.; Pavlov, A.; et al. Chan-Evans-Lam C-N coupling promoted by a dinuclear positively charged Cu(II) complex. catalytic performance and some evidence for the mechanism of CEL reaction obviating Cu(III)/Cu(I) catalytic cycle. ChemCatChem 2020, 12, 3010–3021. [Google Scholar] [CrossRef]
- Cope, J.D.; Sheridan, P.E.; Galloway, C.J.; Awoyemi, R.F.; Stokes, S.L.; Emerson, J.P. Synthesis and characterization of a tetradentate, N-heterocyclic carbene copper(II) complex and its use as a Chan-Evans-Lam coupling catalyst. Organometallics 2020, 39, 4457–4464. [Google Scholar] [CrossRef]
- Jia, X.; He, J. Three copper(II) complexes derived from 2-methylquinoline and cyclic secondary amines: Synthesis and catalytic application in C-N bond forming reactions. Appl. Organomet. Chem. 2022, 36, e6743. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Z.; Shi, S.; Li, Q.; Zhu, X. AGET ATRP of methyl methacrylate catalyzed by FeCl3/iminodiacetic acid in the presence of air. Polymer 2008, 49, 3054–3059. [Google Scholar] [CrossRef]
- Hao, Z.; Yang, N.; Gao, W.; Xin, L.; Luo, X.; Mu, Y. Nickel complexes bearing N,N,N-tridentate quinolinyl anilido-imine ligands: Synthesis, characterization and catalysis on norbornene addition polymerization. J. Organomet. Chem. 2014, 749, 350–355. [Google Scholar] [CrossRef]
- Hao, Z.; Han, Y.; Gao, W.; Xin, L.; Mu, Y. Iron(II) complexes bearing anilido-imine ligands: Synthesis and catalysis on ATRP of methyl methacrylate. Polyhedron 2014, 83, 236–241. [Google Scholar] [CrossRef]
- Hao, Z.; Xu, B.; Gao, W.; Han, Y.; Zeng, G.; Zhang, J.; Li, G.; Mu, Y. Chromium complexes with N,N,N-tridentate quinolinyl anilidoimine ligand: Synthesis, characterization, and catalysis in ethylene polymerization. Organometallics 2015, 34, 2783–2790. [Google Scholar] [CrossRef]
- Hao, Z.; Ma, A.; Xu, B.; Gao, W.; Mu, Y. Cu(II) complexes with anilido-imine ligands: Synthesis, characterization and catalysis on reverse atom transfer radical polymerization of styrene. Polyhedron 2017, 126, 276–281. [Google Scholar] [CrossRef]
- Gao, W.; Cui, D.; Liu, X.; Zhang, Y.; Mu, Y. Rare-earth metal bis(alkyl)s supported by a quinolinyl anilido-imine ligand: Synthesis and catalysis on living polymerization of ε-caprolactone. Organometallics 2008, 27, 5889–5893. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, H.; Hao, Z.; Han, Z.; Lu, G.-L.; Lin, J. Nickel complexes bearing N,N,N-tridentate quinolinyl anilido-imine ligands: Synthesis, characterization and catalysis in Suzuki-Miyaura cross-coupling reaction. Polyhedron 2023, 246, 116676. [Google Scholar] [CrossRef]
- Kongkaew, M.; Sitthisuwannakul, K.; Nakarajouyphon, V.; Pornsuwan, S.; Kongsaeree, P.; Sangtrirutnugul, P. Benzimidazole–triazole ligands with pendent triazole functionality: Unexpected formation and effects on copper-catalyzed aerobic alcohol oxidation. Dalton Trans. 2016, 45, 16810–16819. [Google Scholar] [CrossRef]
- Gao, T.; Meng, L.; Zeng, G.; Hao, Z.; Han, Z.; Feng, Q.; Lin, J. Copper(II) complexes supported by 8-hydroxyquinoline-imine ligands: Synthesis, characterization and catalysis in aerobic alcohols oxidation. Polyhedron 2022, 224, 115984. [Google Scholar] [CrossRef]
- Fliedel, C.; Rosa, V.; Santos, C.I.M.; Gonzalez, P.J.; Almeida, R.M.; Gomes, C.S.B.; Gomes, P.T.; Lemos, M.A.N.D.A.; Aullón, G.; Weltere, R.; et al. Copper(II) complexes of bis(aryl-imino)-acenaphthene ligands: Synthesis, structure, DFT studies and evaluation in reverse ATRP of styrene. Dalton Trans. 2014, 43, 13041–13054. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, K.; Ma, H.; Wu, S.; Huang, Y.; Duan, Y.; Luo, Y.; Yan, J.; Yang, G. An optimized Ni-catalyzed Chan-Lam type coupling: Enantioretentive access to chiral N-aryl sulfinamides. Chem. Eur. J. 2022, 28, e202202190. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Ryland, B.L.; Brunold, T.C.; Stahl, S.S. Kinetic and spectroscopic studies of aerobic copper(II)-catalyzed methoxylation of arylboronic esters and insights into aryl transmetalation to copper(II). Organometallics 2012, 31, 7948–7957. [Google Scholar] [CrossRef] [PubMed]
- Vantourout, J.C.; Li, L.; Bendito-Moll, E.; Chabbra, S.; Arrington, K.; Bode, B.E.; Isidro-Llobet, A.; Kowalski, J.A.; Nilson, M.G.; Wheelhouse, K.M.P.; et al. Mechanistic insight enables practical, scalable, room temperature Chan-Lam N-arylation of N-aryl sulfonamides. ACS Catal. 2018, 8, 9560–9566. [Google Scholar] [CrossRef]
- Phillips, A.J.; Uto, Y.; Wipf, P.; Reno, M.J.; Williams, D.R. Synthesis of functionalized oxazolines and oxazoles with DAST and Deoxo-Fluor. Org. Lett. 2000, 2, 1165–1168. [Google Scholar] [CrossRef]
- King, A.E.; Brunold, T.C.; Stahl, S.S. Mechanistic study of copper-catalyzed aerobic oxidative coupling of arylboronic esters and methanol: Insights into an organometallic oxidase reaction. J. Am. Chem. Soc. 2009, 131, 5044–5045. [Google Scholar] [CrossRef]
- Vantourout, J.C.; Miras, H.N.; Isidro-Llobet, A.; Sproules, S.; Watson, A.J.B. Spectroscopic studies of the Chan-Lam amination: A mechanism-inspired solution to boronic ester reactivity. J. Am. Chem. Soc. 2017, 139, 4769–4779. [Google Scholar] [CrossRef]
- West, M.J.; Fyfe, J.W.B.; Vantourout, J.C.; Watson, A.J.B. Mechanistic development and recent applications of the Chan-Lam amination. Chem. Rev. 2019, 119, 12491–12523. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
| Complex | 2 | 4 |
|---|---|---|
| Formula | C25H22ClCuN3 | C22H15BrClCuN3 |
| Fw | 463.44 | 500.27 |
| T (K) | 199.99 (10) | 199.99 (10) |
| Crystal system | monoclinic | monoclinic |
| Space group | Cc | P21/c |
| a (Å) | 17.2718 (8) | 15.5617 (4) |
| b (Å) | 15.2065 (6) | 9.4245 (2) |
| c (Å) | 8.1558 (3) | 27.3576 (7) |
| α (°) | 90 | 90 |
| β (°) | 98.532 (4) | 96.439 (2) |
| γ (°) | 90 | 90 |
| Volume (Å3) | 2118.36 (15) | 3986.99 (17) |
| Z | 4 | 8 |
| Dcalc (g/cm3) | 1.453 | 1.667 |
| μ (mm−1) | 2.736 | 5.220 |
| F (000) | 956.0 | 1992.0 |
| Crystal size (mm) | 0.14 × 0.12 × 0.1 | 0.14 × 0.12 × 0.1 |
| 2Θ range for data collection (°) | 7.784 to 146.896 | 5.716 to 147.15 |
| Reflections collected | 4242 | 14,755 |
| Data/restraints/parameters | 2723/2/274 | 7793/0/505 |
| Goodness-of-fit on F2 | 1.074 | 1.008 |
| R1, wR2 [I > 2σ(I)] | 0.0327, 0.0888 | 0.0533, 0.1325 |
| R1, wR2 (all data) | 0.0339, 0.0899 | 0.0758, 0.1496 |
| Max. peak (e·Å−3) | 0.49 | 0.58 |
| Mini. peak (e·Å−3) | −0.71 | −0.71 |
| CCDC | 2248465 | 2248467 |
| Entry | Cat. (mol%) | Solvent | T (°C) | Time (h) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | 1 (5) | MeOH | 50 | 20 | 90 |
| 2 | 1 (5) | iPrOH | 50 | 20 | 6 |
| 3 | 1 (5) | 1,4-Dioxane | 50 | 20 | 40 |
| 4 | 1 (5) | DMF | 50 | 20 | trace |
| 5 | 1 (5) | CH3CN | 50 | 20 | trace |
| 6 | 1 (5) | THF | 50 | 20 | 46 |
| 7 | 1 (5) | Toluene | 50 | 20 | 25 |
| 8 | 1 (5) | MeOH | 50 | 16 | 92 |
| 9 | 1 (5) | MeOH | 50 | 12 | 90 |
| 10 | 1 (5) | MeOH | 50 | 8 | 83 |
| 11 | 1 (5) | MeOH | 40 | 12 | 92 |
| 12 | 1 (5) | MeOH | rt | 12 | 81 |
| 13 | 1 (5) | MeOH | 60 | 12 | 88 |
| 14 | 1 (3) | MeOH | 40 | 12 | 93 |
| 15 | 1 (2) | MeOH | 40 | 12 | 84 |
| 16 | 1 (1) | MeOH | 40 | 12 | 71 |
| 17 | 2 (3) | MeOH | 40 | 12 | 91 |
| 18 | 3 (3) | MeOH | 40 | 12 | 96 |
| 19 | 4 (3) | MeOH | 40 | 12 | 94 |
| 20 | 5 (3) | MeOH | 40 | 12 | 90 |
| 21 | CuCl2 (3) | MeOH | 40 | 12 | 48 |
| 22 | - | MeOH | 40 | 12 | - |
| 23 c | 3 (3) | MeOH | 40 | 12 | - |
 | |||
 | 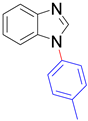 | 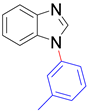 | 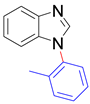 |
| 8a, 96% | 8b, 96% | 8c, 95% | 8d, 91% |
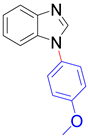 | 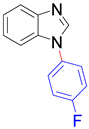 | 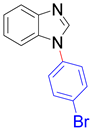 | 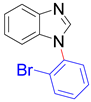 |
| 8e, 89% | 8f, 94% | 8g, 93% | 8h, 85% |
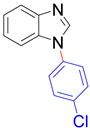 | 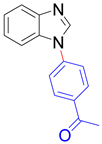 | 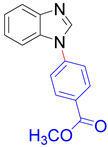 | 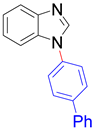 |
| 8i, 96% | 8j, 88% | 8k, 91% | 8l, 83% |
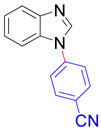 | 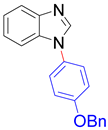 | 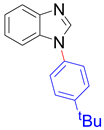 | 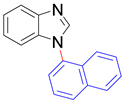 |
| 8m, 75% | 8n, 77% | 8o, 84% | 8p, 87% |
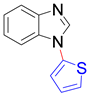 | 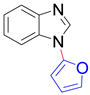 | 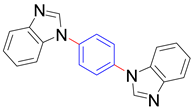 | |
| 8q, trace | 8r, trace | 8s b, 76% | |
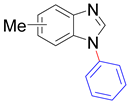 | 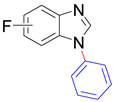 | 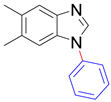 | 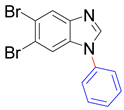 |
| 8t/8t’ (5/6-Me), 95% | 8u/8u’ (5/6-F), 79% | 8v, 93% | 8w, 58% |
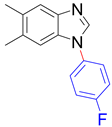 | 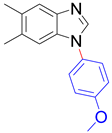 | ||
| 8x, 88% | 8y, 90% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Yang, J.; Hao, Z.; Han, Z.; Lin, J.; Lu, G.-L. Copper Complexes with N,N,N-Tridentate Quinolinyl Anilido-Imine Ligands: Synthesis and Their Catalytic Application in Chan−Lam Reactions. Molecules 2023, 28, 7406. https://doi.org/10.3390/molecules28217406
Zhou X, Yang J, Hao Z, Han Z, Lin J, Lu G-L. Copper Complexes with N,N,N-Tridentate Quinolinyl Anilido-Imine Ligands: Synthesis and Their Catalytic Application in Chan−Lam Reactions. Molecules. 2023; 28(21):7406. https://doi.org/10.3390/molecules28217406
Chicago/Turabian StyleZhou, Xiaoyu, Jiaxin Yang, Zhiqiang Hao, Zhangang Han, Jin Lin, and Guo-Liang Lu. 2023. "Copper Complexes with N,N,N-Tridentate Quinolinyl Anilido-Imine Ligands: Synthesis and Their Catalytic Application in Chan−Lam Reactions" Molecules 28, no. 21: 7406. https://doi.org/10.3390/molecules28217406
APA StyleZhou, X., Yang, J., Hao, Z., Han, Z., Lin, J., & Lu, G.-L. (2023). Copper Complexes with N,N,N-Tridentate Quinolinyl Anilido-Imine Ligands: Synthesis and Their Catalytic Application in Chan−Lam Reactions. Molecules, 28(21), 7406. https://doi.org/10.3390/molecules28217406