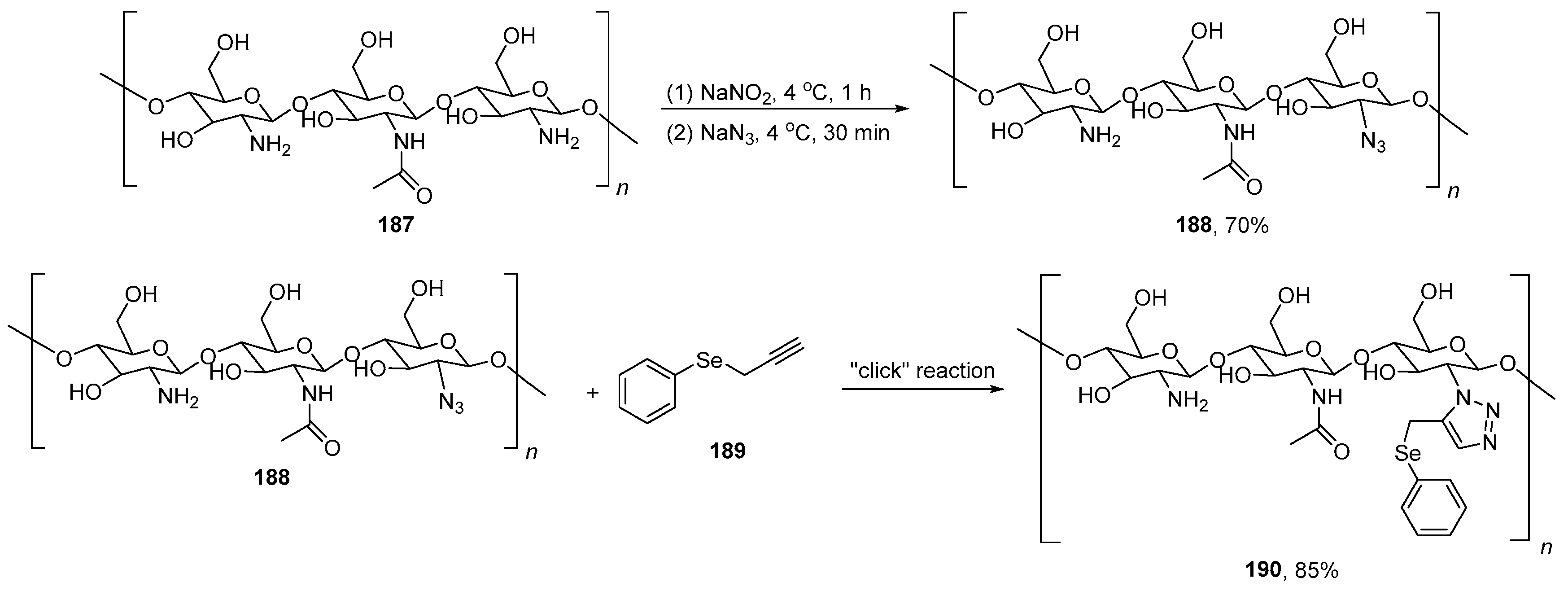
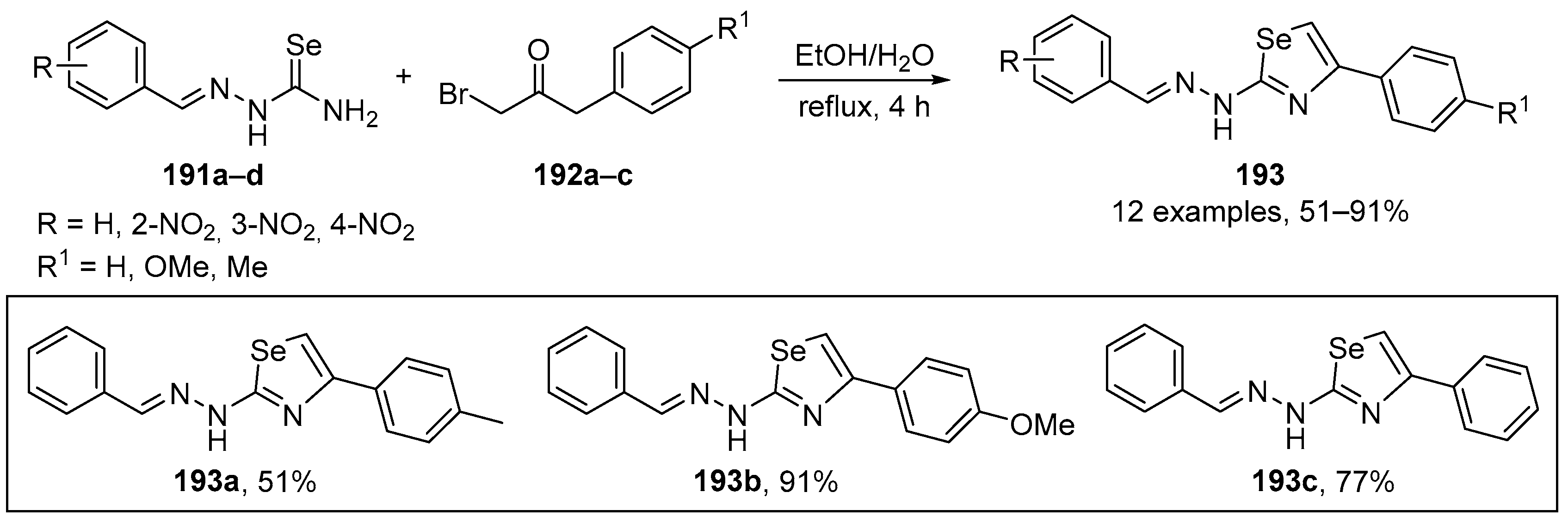
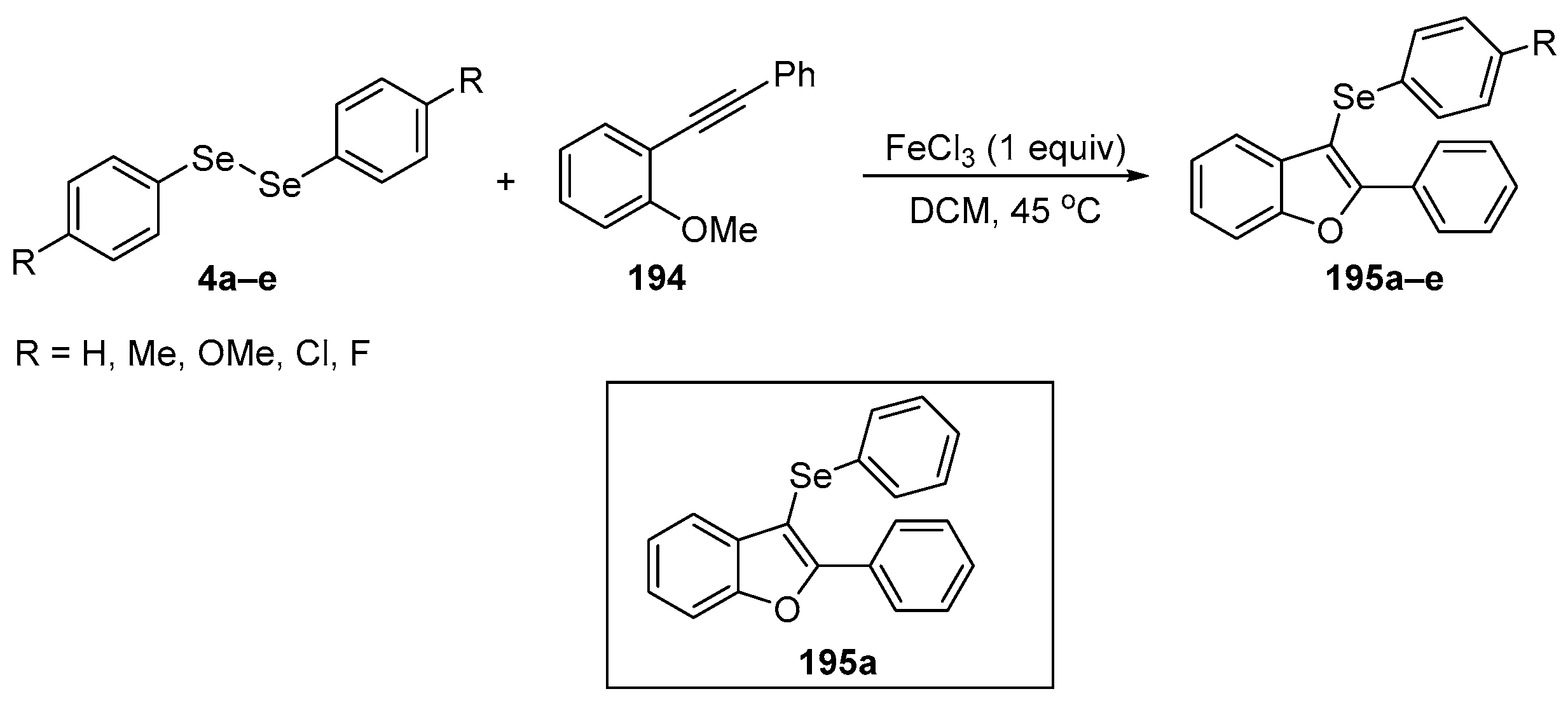
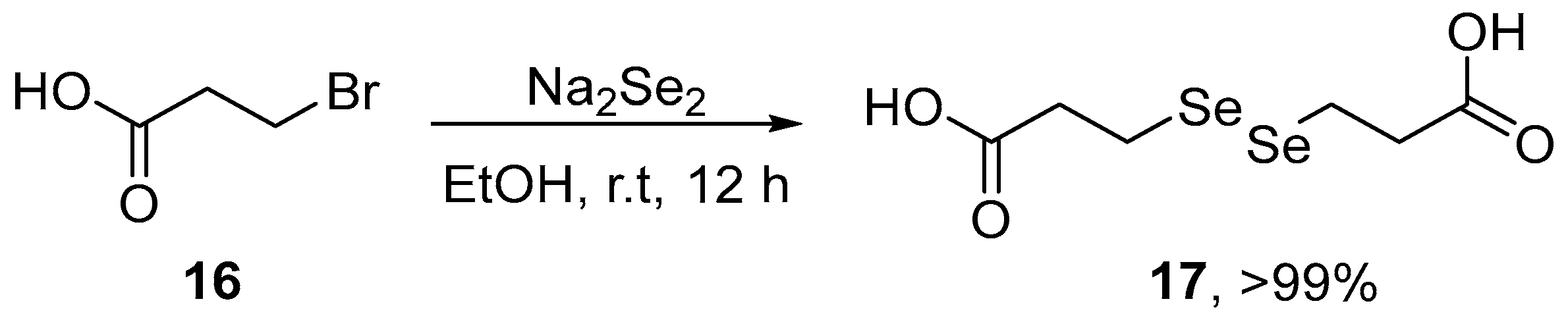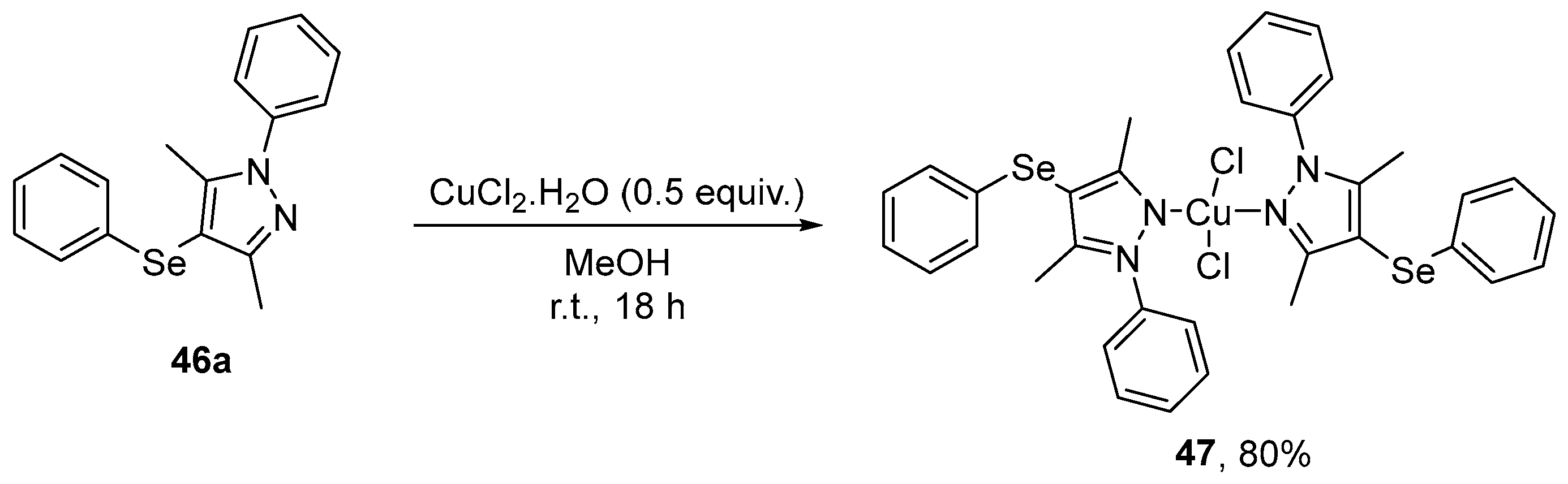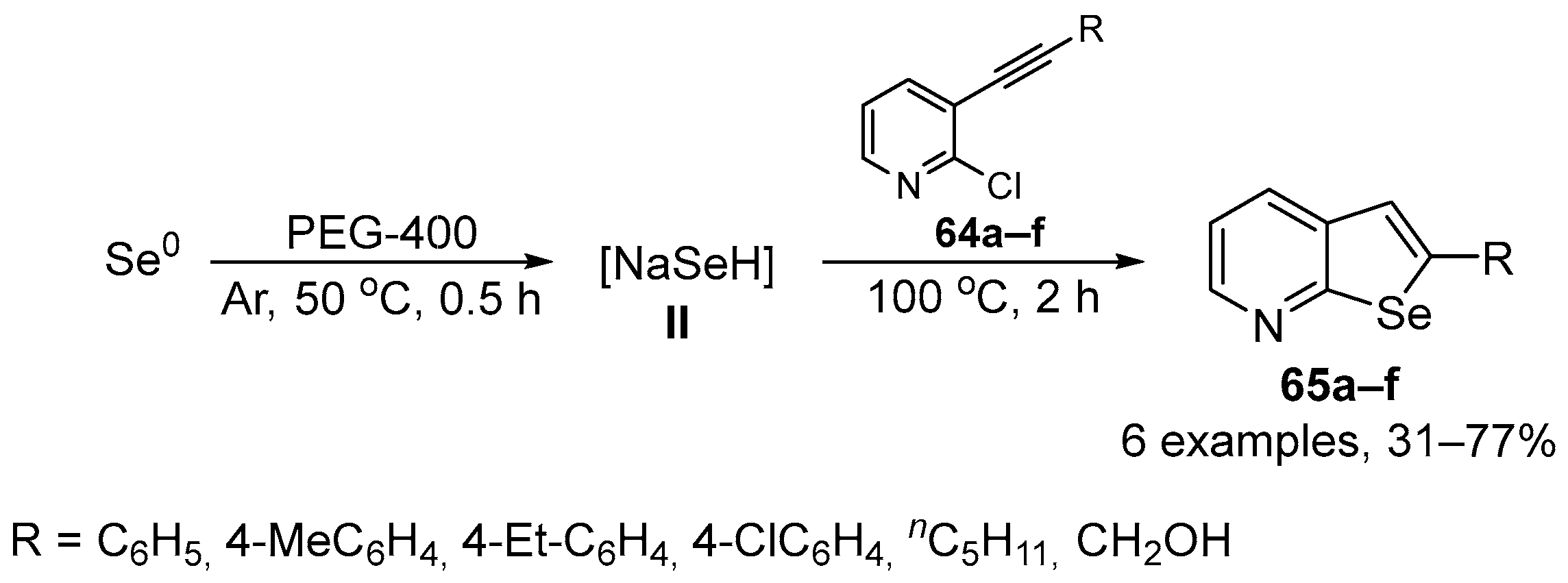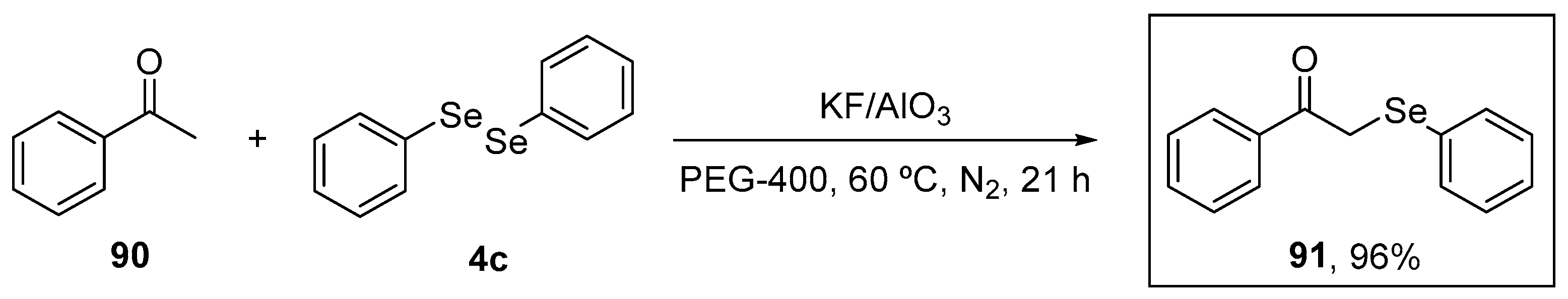Abstract
Selenium is an essential trace element in living organisms, and is present in selenoenzymes with antioxidant activity, like glutathione peroxidase (GPx) and thioredoxin reductase (TrxR). The search for small selenium-containing molecules that mimic selenoenzymes is a strong field of research in organic and medicinal chemistry. In this review, we review the synthesis and bioassays of new and known organoselenium compounds with antioxidant activity, covering the last five years. A detailed description of the synthetic procedures and the performed in vitro and in vivo bioassays is presented, highlighting the most active compounds in each series.
Keywords:
antioxidant; organoselenium; GPx-like; DPPH; ABTS; FRAP; catalase; SOD; lipid peroxidation 1. Introduction
Selenium is an essential trace element in living organisms, being incorporated in the 21st amino acid selenocysteine (Sec), which is present in the selenoenzymes with antioxidant activity including multiple isoforms of glutathione peroxidase (GPx), thioredoxin reductase (TrxR), and other selenoproteins [1,2]. The incorporation of selenium into different molecules permits the synthesis of many Se-containing compounds to explore their biochemistry and potential antioxidant properties [3,4,5]. The protective properties of many different classes of organoselenium compounds, including diselenides, N- or Se-heterocycles, selenides, and Ebselen derivatives have been described [6,7,8,9,10]. This review highlights new research on the synthesis and antioxidant properties of low-molecular mass organoselenium compounds over the period 2018 to early 2023. To prepare this systematic review, we have examined articles, reviews, and book chapters using the databases SciFinder, Web of Science, Scopus, PubMed, and Google Scholar. The search terms used were “antioxidant”, “organoselenium”, “GPx-like”, “selenides”, “glutathione peroxidase”, and “selenium”.
Multiple different methods have been used to investigate and quantify the antioxidant properties of organoselenium compounds that include in vitro assays involving hydrogen atom or electron transfer. These include approaches that measure the extent of damage to biologically important targets such as lipids, proteins, and DNA via the quantification of their oxidation products. This can be achieved via assays that measure loss of the parent species or formation of generic or specific oxidation products. Examples of the former include total hydroperoxides and alcohols, and lipid or protein carbonyls. Examples of specific oxidation products include isoprostanes and regio- and stereoisomeric lipid and cholesterol hydroperoxides and alcohols, oxidized DNA bases, and species formed on individual amino acid sidechains (carbonyls, alcohols, oxyacids, sulfoxides, chlorinated, and nitrated species) [11,12,13]. The overall extent of oxidation in complex systems can also be examined by measuring oxygen consumption via assays such as the total oxygen radical absorbance capacity (ORAC). Other in vitro methods are based on electron transfer, for example, ferric iron-reducing antioxidant power (FRAP), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2′-azinobis [3-ethylbenzothiazoline-6-sulfonic acid] (ABTS) [14,15,16], though these assays cannot be readily applied to complex systems and the data obtained from such assays often show poor correlation with biological assays. Thus, techniques that can be applied to cells or animal models are the most important and reproducible with regard to assessing biological activity. A larger number of studies have reported changes in the activity or protein levels of enzymes such as superoxide dismutase (SOD), catalase (CAT), and GPxs, and changes in gene expression [15]. However, it needs to recognize that enzyme levels and activities can be altered by many pathways; therefore, such data are not direct measures of antioxidant activity (Figure 1). Direct measurement of oxidants, and particularly reactive radicals, is notoriously difficult in complex systems due to the low steady state concentration of such species. Valuable data have been obtained using highly specific techniques such as electron paramagnetic (spin) resonance (EPR or ESR); however, even with this method, trapping agents (spin traps) or spin probes need to be employed for highly reactive species, and these agents have considerable drawbacks and caveats [17]. Fluorescent probes (e.g., dichloro-dihydrofluorescein diacetate, DCFH-DA; dihydroethidium, DHE) have also been widely employed to detect oxidant generation, but many of these probes suffer from considerable problems and artefacts; therefore, large numbers of control experiments are required, and the resulting data need to be treated with great care [12,18].
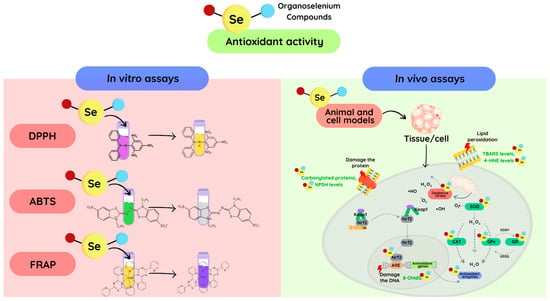
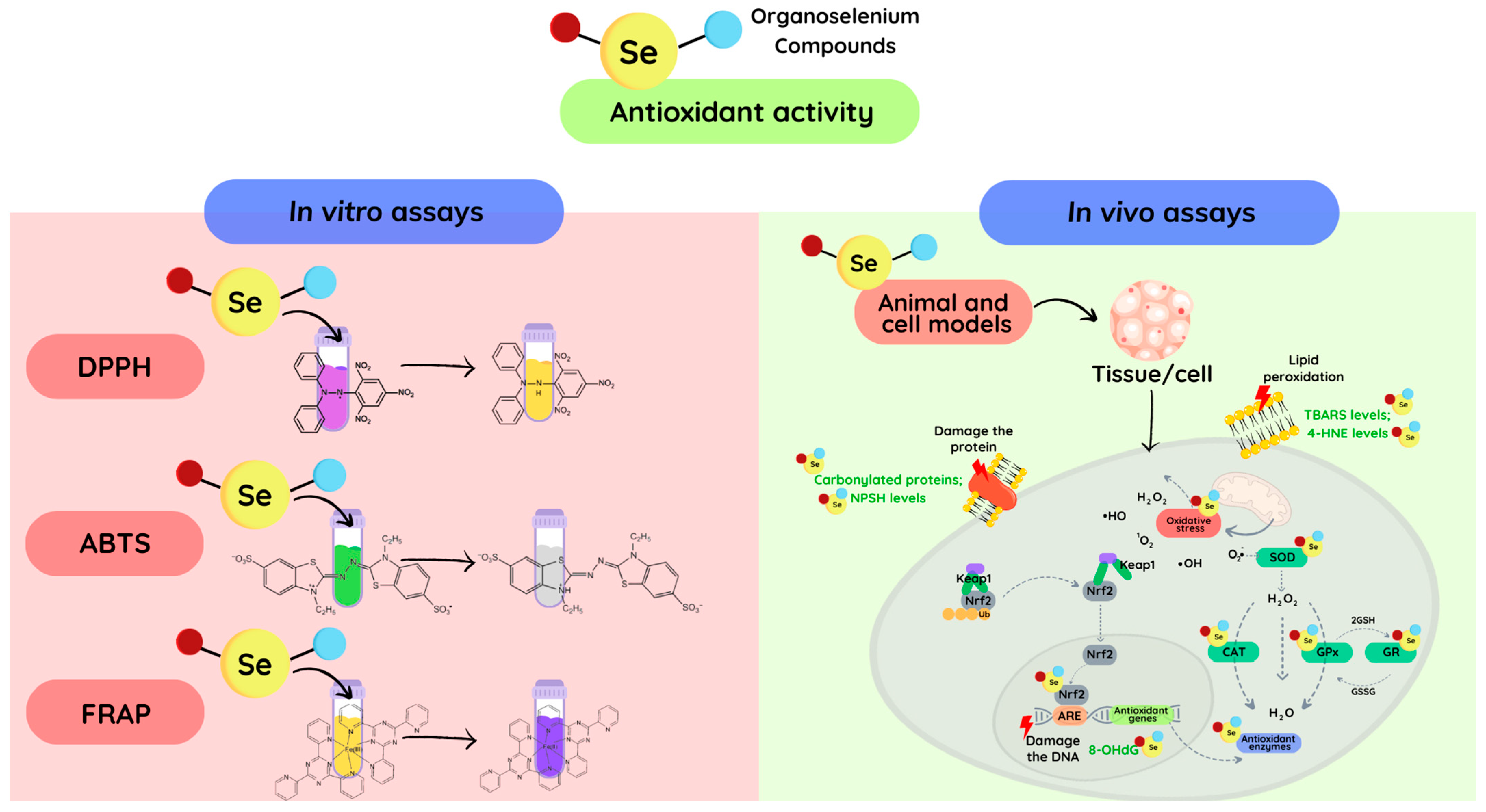
Figure 1.
The routes of the mainly antioxidant assays for exploring the antioxidant properties of novel organoselenium compounds. The antioxidant properties can be studied in a cell through the reduction of the oxidative damage caused to DNA, proteins, and cell membranes, or by mimicking antioxidant enzymes (SOD, CAT, GPx, etc.). Additionally, the redox activity can be observed in vitro through the reduction of oxidant concentrations or synthetic radicals, such as DPPH and ABTS. Finally, oxidants can be directly measured using specific techniques, such as electron paramagnetic (spin) resonance and fluorescent probes. In the DPPH assay, after the reduction of the DPPH radical the color changes from purple to pale yellow. In the ABTS assay, upon reduction of the ABTS radical, the green color discoloration occurs. In the FRAP test, the reduction of Fe3+ yields a violet-blue color.
This review is divided in two main parts: firstly, a short introduction to oxidative stress is provided; secondly, the synthesis of organoselenium compounds and their potential protective activities is discussed at length. This second part is organized according to the class of organoselenium compounds: diselenides, Ebselen derivatives, Se-functionalized heterocycles, Se-heterocycles, selenides, and miscellaneous.
2. Oxidative Stress
Reactive oxidants/species (henceforth, RS), including reactive oxygen species (often termed ‘ROS’, though this is a general term for a large group of species with very different reactivities and effects) are produced both deliberately and accidently during oxygen metabolism. These species can have beneficial effects by mediating cell signaling and harming or killing invading pathogens when they are generated inappropriately. Major ‘ROS’ species include hydrogen peroxide (H2O2), other peroxides (ROOH), the superoxide radical anion (O2•−), hydroxyl radical (HO•), singlet oxygen (1O2), peroxyl radicals (ROO•), and alkoxyl radicals (RO•). The intracellular concentration of oxidants is carefully regulated through a delicate interplay between their production and removal mechanisms, which involves a complex and diverse mixture of antioxidant defenses. Under normal physiological conditions, oxidants play a crucial role in regulating signaling pathways that control numerous cellular processes (‘oxidative eustress’). However, excessive oxidant levels can lead to cell and tissue damage (‘oxidative distress’) [19,20].
Antioxidants mitigate potential detrimental effects of RS, including ROS and related reactive nitrogen, carbon, sulfur, and chlorine species. If oxidant production overwhelms the capacity of antioxidants to eliminate them, or if antioxidant production is compromised, oxidative stress (‘distress’) occurs. Enzymes play a critical role in protecting cells, the extracellular environment and tissues/organs against damage caused by RS [21,22]. These enzymes, which both prevent and repair damage, are supplemented by a battery of low-molecular mass species that can directly scavenge RS, though these are often less important than enzymatic systems.
The superoxide dismutase (SOD) family is a key protective system which catalyzes the conversion of O2•− into H2O2 and molecular oxygen (O2). The resulting H2O2 is then neutralized by multiple enzyme systems including multiple peroxiredoxin (Prxs) and glutathione peroxidase (GPx) isoforms, as well as catalase (CAT). Catalase converts H2O2 into water (H2O) and oxygen (O2) without the use of co-factors, whereas the activities of Prxs and GPxs reduce H2O2 (and lipid hydroperoxides in the case of GPx4) to H2O (or lipid alcohols for GPx4) at the expense of thiol-dependent co-factors. The activity of Prxs is maintained by the thioredoxin (Trx)/thioredoxin reductase (TrxR)/NAPDH system, whereas GPxs use glutathione (GSH) as a cofactor. The glutathione disulfide (GSSG) generated from GSH oxidation is reduced by glutathione reductase using NADPH as the reducing co-factor [23,24]. The glutathione S-transferase (GST) and glutaredoxin families are also involved in protection against oxidative stress. The GST isoforms catalyze the adduction of GSH to a variety of toxic compounds and drugs, facilitating their elimination from the body [23,24,25]. Both the Prx and GPx systems are ultimately dependent on selenium, as both TxrRs and GPxs contain Sec residues in their active sites.
Collectively, these antioxidant enzymes act as an efficient defense system, controlling oxidant levels within and external to cells, and safeguard biological systems against oxidative damage [25]. However, imbalances between oxidant production and their removal can lead to significant detrimental effects to various cellular components, and hence health problems, including neurodegenerative, cardiovascular, lung, skin, kidney and liver diseases, and psychiatric disorders [26,27,28]. Adequate selenium levels are therefore critical to the maintenance of cellular antioxidant levels, and supplementation with biologically-available Se species may be beneficial, particularly in the case of deficiency [29,30,31,32].
The Nrf2 (nuclear factor erythroid 2-related factor 2) pathway is an important cellular transcription factor involved in cellular antioxidant responses [33], as it regulates the expression of genes responsible for synthesizing antioxidant enzymes [33,34]. In the basal state, the Nrf2 is present in the cytoplasm and is associated with the Keap1 protein. This protein targets Nrf2 for rapid proteasomal degradation and thereby prevents its accumulation and translocation to the nucleus. However, Keap1 contains a number of highly reactive cysteine residues and modification of these can result in decreased Keap1 activity and an accumulation of newly synthesized Nrf2 in both the cytosol and nucleus (Figure 2) [33,34]. Nuclear Nrf2 forms a heterodimer with Maf transcription factor proteins and binds to antioxidant response elements (AREs) located in the promoters of target genes, thereby promoting their transcription. These genes include those coding for SOD, CAT, and GPx [33]. In addition, Nrf2 also regulates the expression of genes involved in the synthesis and regeneration of GSH [33,34,35]. GSH therefore plays a central role in protecting against oxidative damage by acting as a reducing enzyme cofactor, as a direct scavenger of oxidants, and also as an agent adducted to exogenous chemicals to enhance their excretion.
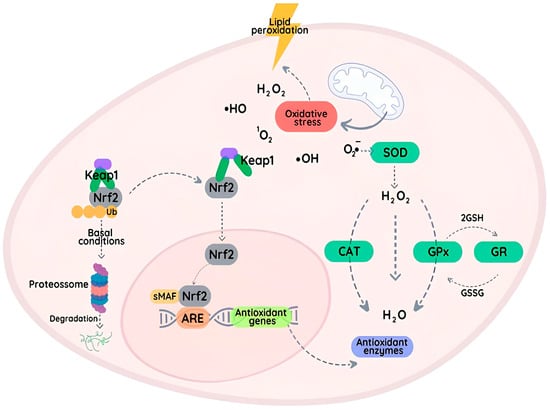
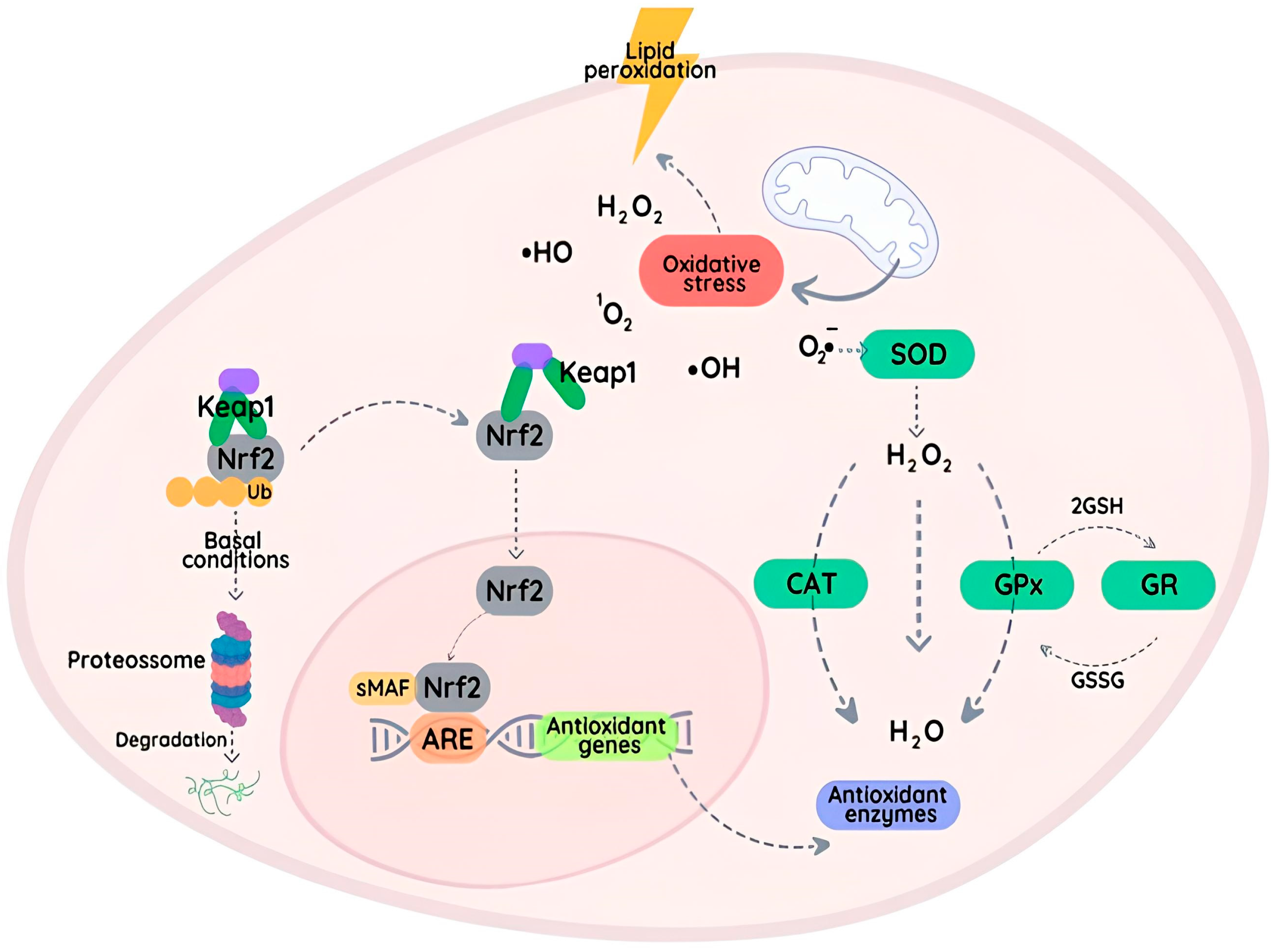
Figure 2.
The Nrf2 pathway is regulated by interactions between Nrf2 and the Keap1 protein in the cytoplasm. Under basal conditions, Nrf2 expression is maintained at low levels through proteasome activity. When the level of reactive species is elevated (oxidative stress), Keap1 is modified, resulting in the accumulation and translocation of Nrf2 to the nucleus, where it binds to AREs and activates the transcription of antioxidant genes, including those coding for SOD, CAT, and GPxs. This adaptive response plays a critical role in protecting cells against damage.
Oxidants also regulate a number of other stress response pathways involving nuclear factor-κ light chain-enhancer of activated B cells (NF-κB), hypoxia-inducible factor (HIF), oestrogen-related receptor (ERR), forkhead box O transcription factor (FOXOs) peroxisome proliferator-activated receptor-γ co-activator 1α (PPAR), peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1a), cellular tumour antigen p53 (p53), 5-adenosine monophosphate-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR), glyceraldehyde-3-phosphate dehydrogenase, (GAPDH), sirtuin protein family (SIRT), and uncoupling protein 1 (UCP) [18,36].
These data indicate a key role for both thiols and Se species in maintaining the correct redox state within cells and tissues and has given rise to widespread interest in the maintenance or enhancement of these defense systems, particularly in pathologies or cases of genetic or nutritional deficiency, where these systems may be compromised. This has resulted in considerable efforts to design and test low molecular mass Se species that can mimic the activity of these key selenium and sulfur dependent systems.
3. Synthesis and Antioxidant Evaluation of Organoselenium Compounds
3.1. Diselenides
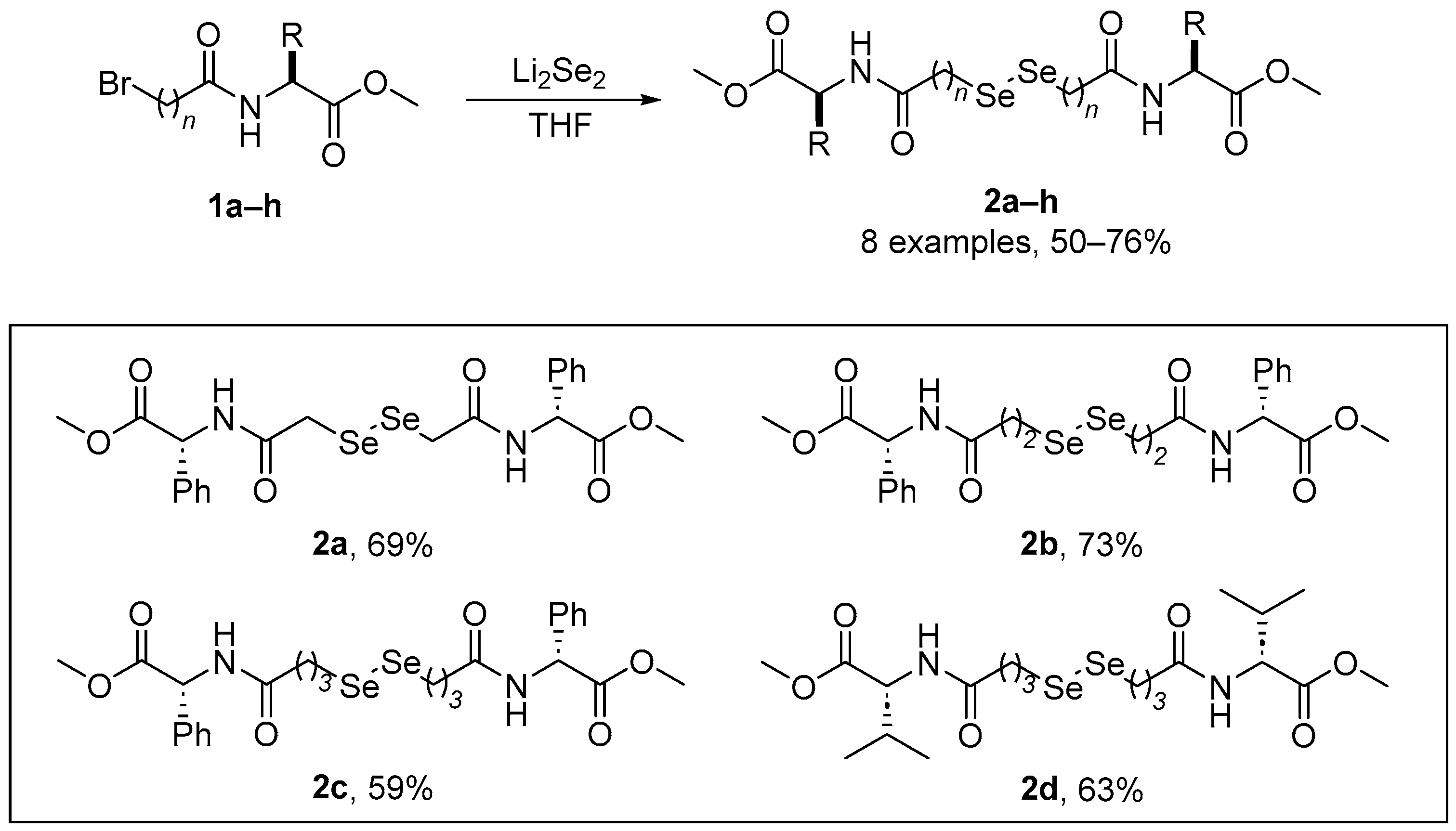
As part of the growing interest in chalcogen-containing amino acid derivatives as chiral building blocks in organic synthesis or for biological protection, Braga and co-workers [37] have reported a facile and inexpensive route for the preparation of a series of Se-substituted amino acids. A major advantage of this strategy is its modular construction, where modifications in the product structure can be easily introduced. The synthesis of the diselenides 2a–h (Scheme 1) involves the reaction of bromo amides 1a–h with Li2Se2 in THF, affording good yields of the expected products (50–76%). The yields of the diselenides derived from L-phenylalanine were not affected by the increase in the carbon chain length. These diselenoamino acid derivatives has been evaluated for GPx- and TxrR-like activity, thiol oxidase, and thiobarbituric acid reactive substances (TBARS, a commonly used, but rather indiscriminate assay for reactive carbonyls including malondialdehyde, MDA). Diselenide 2d, derived from L-valine, showed the best GPx-like activity, followed by 2c and 2b. Compound 2a was the least effective catalyst in the thiol-mediated reduction of H2O2. Compound 2d presented the highest thiol oxidase activity, followed by compounds 2c and 2b. Compound 2a was the less efficient oxidant of GSH (monothiol) and DTT (dithiol). The reactivity of diselenoamino acid derivatives with rat hepatic TrxR was tested:the order of reactivity with 5 μM of the diselenoamino acid derivatives was 2d > 2c > 2b > 2a. At 15 μM, compounds 2d and 2c increased the oxidation of NADPH from 7.5% to 11%, and from 6% to 9%, respectively, but compounds 2a and 2b oxidized less NADPH than observed at 5 μM, indicating that at 15 μM, they were probably inhibiting TrxR activity. In the TBARS assay, compounds 2d and 2c exhibited only a weak antioxidant activity against iron-induced lipid peroxidation, and compounds 2a and 2b were ineffective.
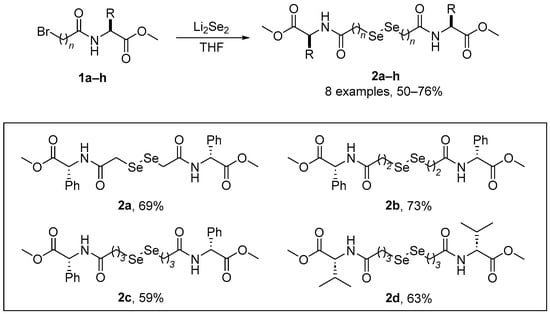
Scheme 1.
Synthesis of diselenoamino acid derivatives 2.
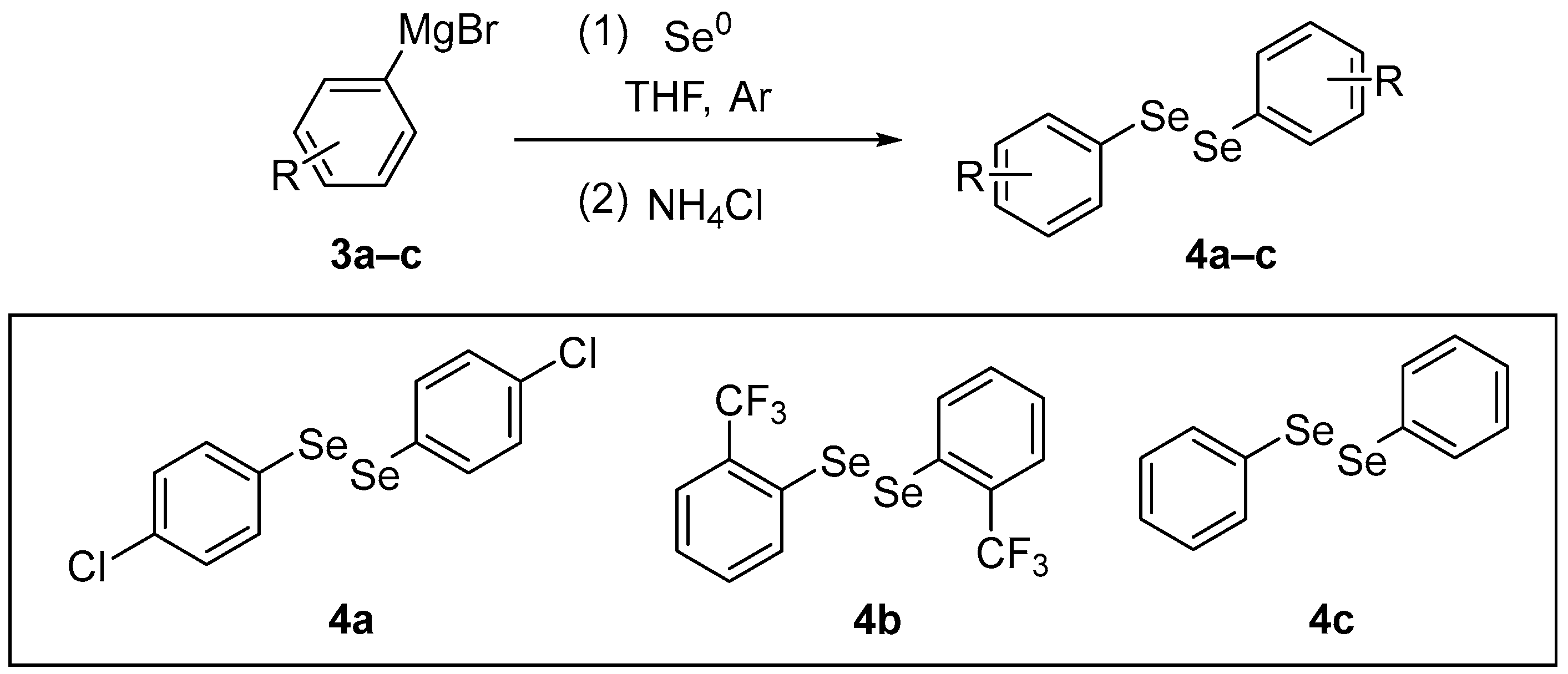
4,4′-Dichlorodiphenyl diselenide (p-ClPhSe)2 4a is an organoselenium compound reported to have antioxidant, antidepressant-like, and neuroprotective actions. Nogueira and co-workers [38] have investigated whether the antioxidant activity and modulation of the glutamatergic system contribute to the antidepressant-like effect of (p-ClPhSe)2 in mice sub-chronically exposed to dexamethasone (DEX). 4,4′-Dichlorodiphenyl diselenide 4a was synthesized according to the literature [39] through the reaction between the Grignard of p-chlorobromobenzene 3a and elemental selenium in THF. After the formation of the selenolate intermediate, the reaction was quenched with saturated NH4Cl, and the resulting selenol was oxidized with atmospheric O2 to give the diselenide 4a (Scheme 2). It was shown that (p-ClPhSe)2 has antioxidant actions in the prefrontal cortex of mice exposed to DEX. The animals received DEX (2 mg/kg, intraperitoneal [i.p.]) for 21 days. Then, the animals were treated with (p-ClPhSe)2 (1, 5, or 10 mg/kg, intragastrically [i.g.]) for 7 days. After the last treatment, the prefrontal cortex was removed to determine the levels of oxidation and CAT and SOD activities. At the tested doses, (p-ClPhSe)2 reduced oxidant levels and increased CAT activity, while at the highest dose (10 mg), it was effective against the decrease in SOD activity induced by DEX exposure.
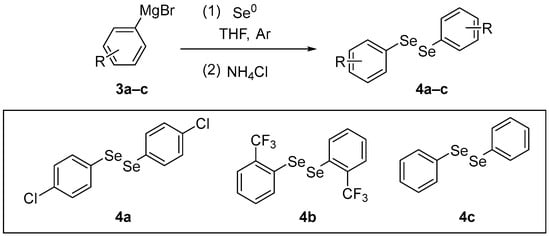
Scheme 2.
Synthesis of diselenides 4.
Brüning et al. [40] have studied the effects of bis(m-(trifluoromethyl)phenyl) diselenide 4b in a reserpine-induced pain-depression dyad model. Diselenide 4b was prepared, as described in Scheme 2, using the Grignard Reagent obtained from 1-bromo-3-(trifluoromethyl)benzene. Mice were injected with reserpine 0.5 mg/kg, i.p. for three consecutive days, once a day, and then they received 4b (10 mg/kg) for the next two days, once a day. Thirty minutes after the last administration of 4b, the behavioral tests were carried out. Diselenide 4b reversed the reserpine-increased thermal hyperalgesia and depressive-like behavior of mice. These effects appear to be related to modulation of oxidative stress, since 4b normalized TBARS and 4-hydroxynonenal- (4-HNE) modified protein levels, markers of lipid oxidation increased by reserpine.
Dos Santos et al. [41] have reported a transcriptional approach to the redox and insulin-signaling pathway using zebrafish (Danio rerio) as a model organism. The authors investigated biomarkers underlying the protective effects of diphenyl diselenide 4c against hyperglycemia. Zebrafish were fed a diet containing diphenyl diselenide (3 mg/kg) for 74 days. During the last 14 days of the study, they were exposed to a 111 mM glucose solution to induce a hyperglycemic state. Following this, the fish were euthanized. The brain tissue was analyzed for levels of TBARS and carbonylated proteins, non-protein sulfhydryl (NPSH) levels, as well as the activities of CAT, SOD, GPx, and GST and mRNA levels of FoxO3A, FoxO3B, Nrf2, GPx3A, SOD1, and SOD2. Compound 4c counteracted the effects of hyperglycemia toward oxidation of lipids and proteins and elevated per se the brain levels of NPSH. In addition, 4c attenuated the effect of hyperglycemia on SOD and increased the activity of GPx and GST enzymes. This compound also normalized the transcriptional levels of FoxO3A, FoxO3B, Nrf2, GPx3A, SOD1, and SOD2, suggesting that the antioxidant effect of 4c in this model is associated with the regulation of oxidative stress.
In order to further examine the effects of Se compounds on hyperglycemia and its consequences, Shen and co-workers [42] have evaluated the preventive and therapeutic effects of 4c against diabetic peripheral neuropathy (DPN), a common microvascular complication of both type 1 and type 2 diabetes mellitus. In vitro, the RSC96 cells were exposed to a very high glucose concentration (100 mM, much higher than detected in poorly controlled diabetes) and then treated with different concentrations of 4c at 1, 10, 25, and 50 μM. These treatments decreased oxidant and malondialdehyde (MDA, a generic product of lipid and sugar oxidation) levels. This compound also activated Nrf2 signaling and inhibited Keap1 expression. In vivo, male rats received a single i.p. injection of streptozotocin (60 mg/kg). After five weeks, the animals were treated with 4c (5 and 15 mg/kg, i.g); 12 weeks later, the rats were sacrificed, and the sciatic nerves removed for evaluation. Treatment with 4c decreased MDA levels and increased GSH, SOD, CAT, and GPx levels in serum and sciatic nerves, reduced the level of Keap1, and stimulated Nrf2 signaling. Taken together, these data indicate that 4c ameliorates DPN in rats by both ameliorating oxidant levels and by activating the Nrf2/Keap1 signaling pathway.
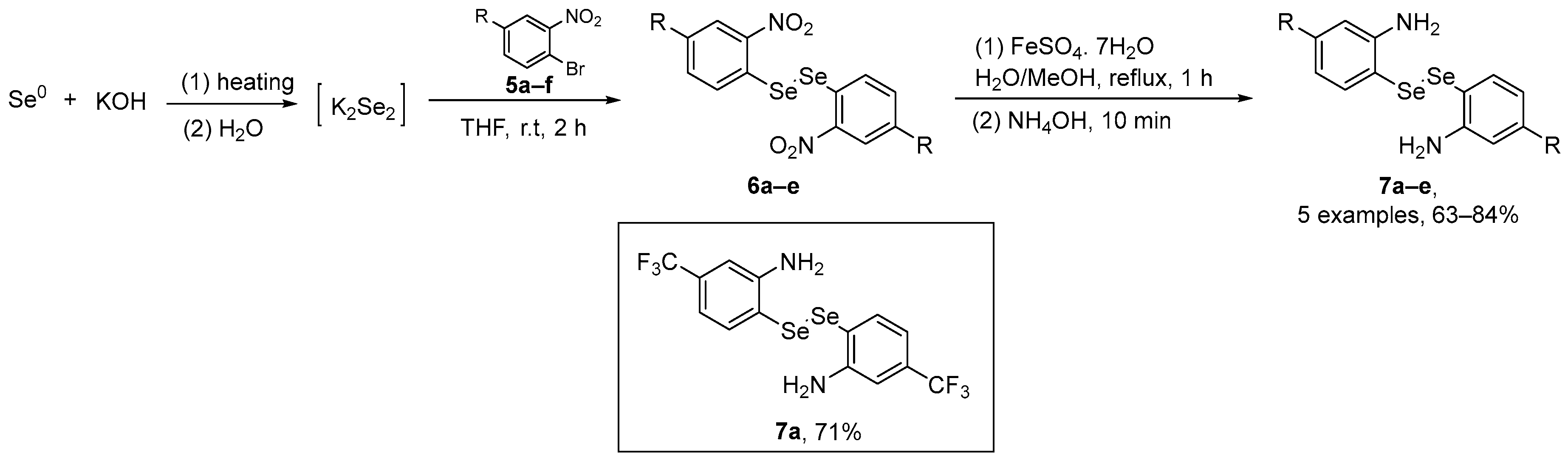
Braga and co-workers [43] have described a simple and efficient route to access aniline-derived diselenides and evaluated their antioxidant/GPx-mimetic properties. The synthesis starts with the aromatic nucleophilic substitution of o-bromonitrobenzene 5a with K2Se2 (generated in situ), affording bis(o-nitrobenzene) diselenide 6. The reduction of bis(o-nitrobenzene) diselenides 6 by a well-established procedure using low-cost iron sulfate heptahydrate (FeSO4.7H2O) afforded the aniline-derivatives 7 (Scheme 3). The aniline-derived diselenides were evaluated GPx-like activity and in kinetic assays. Diselenide 7a, substituted with the CF3 group, showed the best results, being five and two times more effective as a GPx mimetic than Ebselen and 4c, respectively.
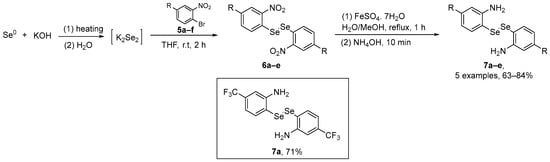
Scheme 3.
Synthesis of aniline-derived diselenides 7.
Studies have also been carried out to assess the effects of Se compounds against hyperglycemia-induced kidney damage (diabetic nephropathy). Thus, Zhou and co-workers [44] have examined the effects of 4c supplementation on diabetic nephropathy in streptozotocin-treated rats. Male rats were subjected to i.p. injection of streptozotocin (60 mg/kg) following fasting for 10 h. After four weeks, they received 4c once daily at 5 or 15 mg/kg for 12 weeks. The animals were then sacrificed, and blood and kidneys were collected for analysis. Administration of 4c modulated GSH levels, the activities of GPx and SOD, and reduced MDA levels in serum and the kidneys. In addition, the compound regulated renal gene expression of Nrf2 and Nrf2-targeted antioxidant enzymes. These data indicate that 4c has a renoprotective effect, which may be attributed to its ability to reduce oxidative stress caused by hyperglycemia. This effect may arise through the activation of the Nrf2/Keap1 system.
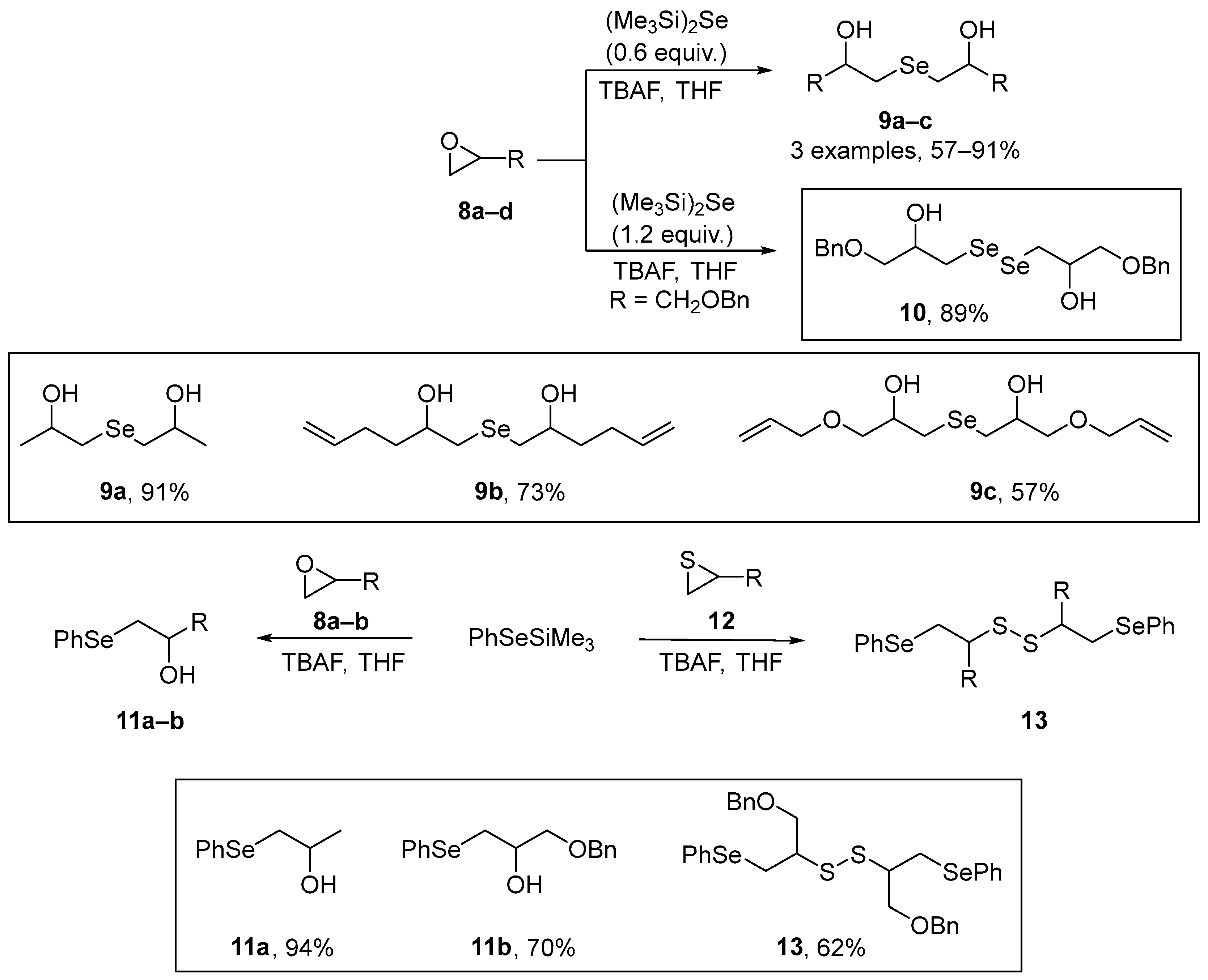
A number of substituted β-hydroxy- and β-amino dialkyl and alkyl-aryl chalcogenides have been synthesized by Capperucci et al. [45] through the ring-opening of epoxides and aziridines by selenium- or tellurium-centered nucleophiles. Synthesis of the symmetrical β-hydroxy selenides 9 and diselenide 10 was achieved through TBAF-induced ring-opening of the epoxides with (Me3Si)2Se [bis(trimethylsilyl)selenide or HMDSS]. By altering the reaction stoichiometry selective formation of 9 or 10 could be achieved (Scheme 4). In a related reaction, a fluoride ion-induced silicon-mediated procedure was used to generate the β-phenylseleno- alcohols 11 and disulfides 13 from (phenylseleno)trimethylsilane and the relevant epoxides or thiiranes. The antioxidant properties of the compounds were investigated in human dermal fibroblasts using the spectrofluorimetric DCFH assay. The cells were exposed to the compounds at 5–50 nM, and the buildup of fluorescence monitored. The results obtained revealed a significant antioxidant potential of these compounds. Thiol-peroxidase assays, using dithiothreitol (DTT), demonstrated that the compounds were effective catalysts of DTT oxidation.
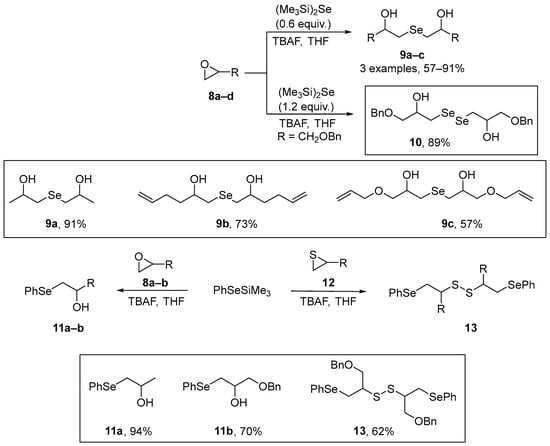
Scheme 4.
Synthesis of differently substituted β-hydroxy dialkyl- and alkyl-aryl selenides.
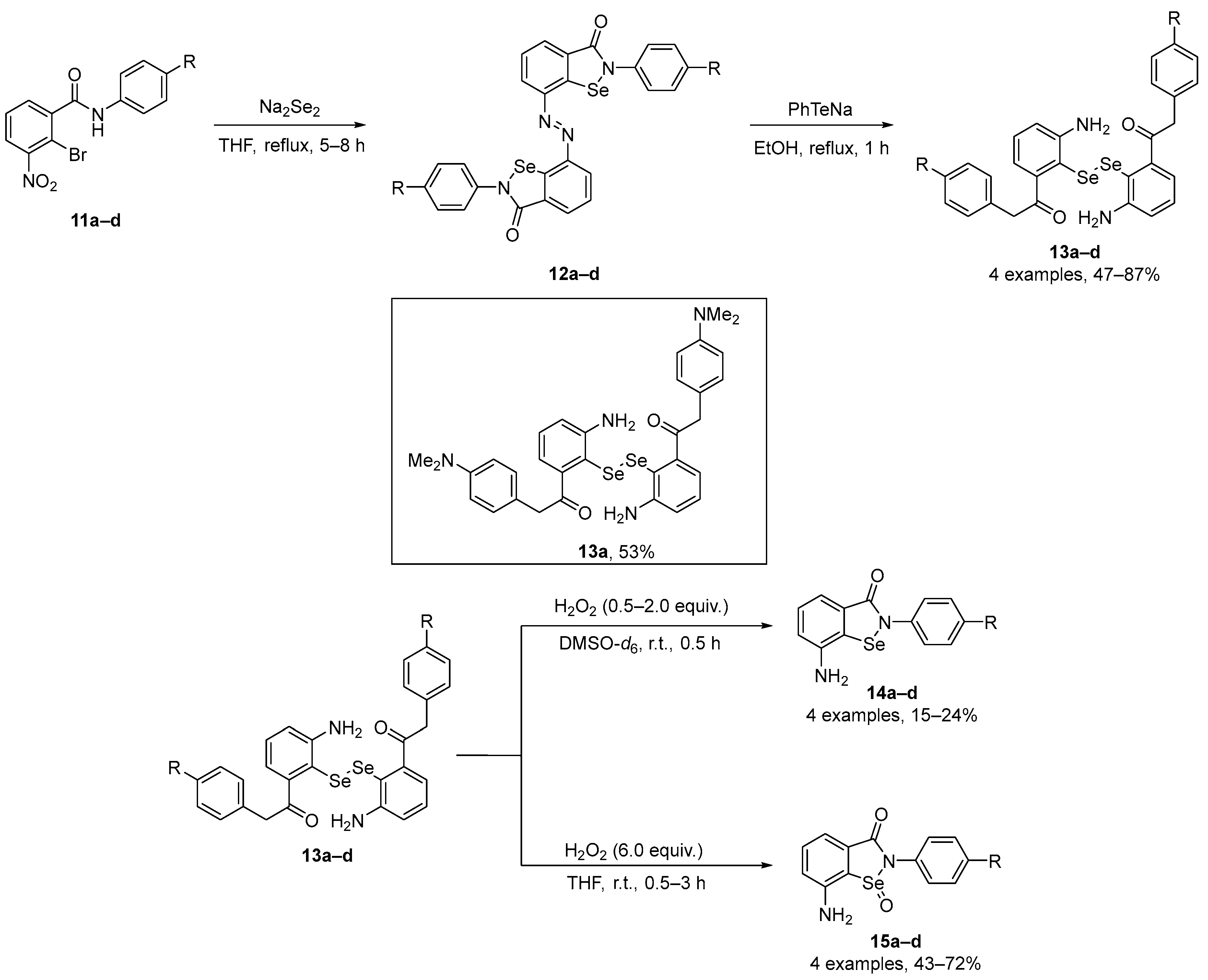
Singh and co-workers [46] have described the synthesis of novel regenerable and multifunctional selenazolonamines 14a–d incorporating free -NH2 group near to the Se atom with remarkable protective and radical-trapping properties. The cyclic selenazolonamines 14a–d were obtained by oxidation of the corresponding diselenides 13a–d with H2O2 under controlled reaction conditions. Azo-bis-ebselen precursors 12a–d were prepared by treating 11a–d with Na2Se2 in dry THF under reflux, according to previously described by the authors [47]. In the sequence, diselenides 13a–d were further utilized for synthesizing selenazolonamines 14a–d. The reaction of 13a–d with H2O2 and DMSO-d6 gave the products 14a–d in 15–24% yield. To generate the corresponding selenoxides, the diselenides 13a–d were treated with H2O2 in THF, giving 15a–d in good yields (43–72%) (Scheme 5). Inhibition rates of conjugated diene (a generic lipid oxidation product) formation (Rinh) and inhibition times (Tinh) with and without ascorbate (AscOH) were evaluated for 14a–d using linoleic acid as the oxidizable lipid. All the compounds at 40 μM diminished the extent of lipid peroxidation. GPx mimetic activity was also evaluated for the same compounds and diselenide 13a, and the resulting data demonstrate that 14a–d (20 μM), 15a–d (20 μM), and 13a (20 μM) have greater GPx-like activity than Ebselen. These new compounds therefore appear to be excellent mimics of GPxs and lipid-soluble antioxidants, protecting lipid membranes and mitochondrial DNA against oxidative stress caused by reactive species in cells.
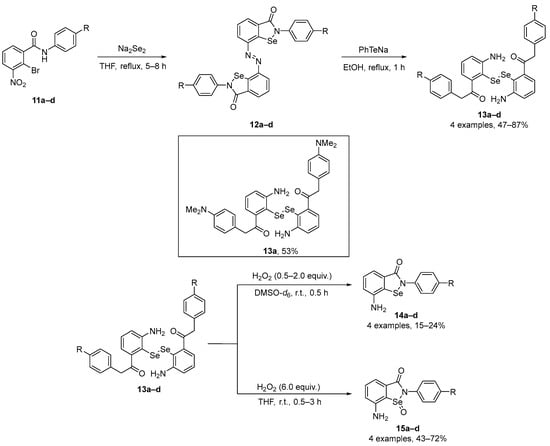
Scheme 5.
Synthesis of novel regenerable and multifunctional selenazolonamines 14 and 15.
Deshmukh et al. [7] have reported data on the interaction of 3,3′-diselenodipropionic acid (DSePA) 17, a well-known pharmacologically active diselenide, with HgCl2 and its ability to prevent HgCl2-induced toxicity in experimental cellular and mice models. DSePA was synthesized starting from the reaction of Na2Se2, generated in situ by the reaction of an ethanolic suspension of Se0 in the presence of NaBH4, with 3-bromopropanoic acid 16. The resulting mixture was stirred for 12 h at room temperature to afford the expected diselenide in almost quantitative yield (Scheme 6). Pre-treatment with DSePA prevented HgCl2-induced oxidative stress in Chinese Hamster Ovary (CHO) cells, and significantly increased the activities of GPx and TrxR and the ratio GSH/GSSG. In vivo studies indicate that the administration of DSePA (2 mg/kg i.p.) prevented HgCl2-induced oxidative stress in mice. Pre-administration of DSePA significantly increased the levels of GPx, TrxR, and GSH/GSSH ratio and decreased TBARS levels in the liver and kidney of mice. Taken together, these results demonstrated that DSePA affords protection against Hg-induced oxidative stress in cell and animal models.
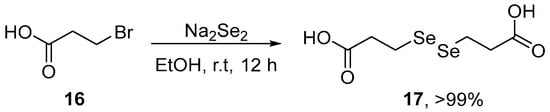
Scheme 6.
Synthesis of 3,3′-diselenodipropionic acid 17 (DSePA).
The kinetics of reaction of DSePA and a range of related species, with hypochlorous acid (HOCl), singlet oxygen (1O2), and a range of peroxides have been reported [48]. These data were compared with the corresponding sulfur-containing species and shown to be significantly higher. The data indicate considerable variations in the rates of reaction of selenols, selenides and diselenides with different oxidants, with the reactivity decreasing along the order selenols > selenides > diselenides. These data complement earlier kinetic and product data reported for sugar-derived selenides (see also below) with different oxidants [49,50,51].
Shen et al. [52] have investigated the capacity of diphenyl diselenide 4c to alleviate tert-butyl hydrogen peroxide (TBHP)-induced oxidative stress, and in lipopolysaccharide (LPS) -induced inflammation in rat glomerular mesangial (HBZY-1) cells, and its potential as a candidate for the prevention and treatment of diabetic nephropathy. The antioxidant activity in HBZY-1 cells was determined by the production of intracellular oxidants and the levels of MDA, GSH, and SOD activity. The cells were exposed to TBHP (400 μM) with 4c at different concentrations (10, 25, or 50 μM) for 24 h. After treatment, the cells were exposed to DCFH-DA and the fluorescence intensity was determined. Oxidation levels in TBHP-stimulated HBZY-1 cells were significantly decreased after treatment with 4c at all concentrations. To evaluate the levels of MDA, GSH, and SOD activity, the HBZY-1 cells were exposed to TBHP (400 μM) and 4c (10, 25, or 50 μM) for 24 h. Such treatment decreased MDA levels and increased intracellular GSH content and SOD activity in the TBHP-stimulated HBZY-1 cells. To address the antioxidant mechanism of 4c, its effects on the protein expression of Nrf2 and downstream antioxidant enzymes were investigated by immunoblotting. Treatment with 4c increased expression of Nrf2, NQO1, HO-1, and GCLC protein, and reduced expression of Keap1 in TBHP-treated HZBY-1 cells. Thus, activation of the Nrf2/Keap1 signaling pathway may be involved in the antioxidant mechanism of 4c. It should be noted that the measurement of intracellular oxidants in these studies may have been compromised by the use of TBHP as the inducer of oxidative stress, as this is itself a powerful cell-penetrating oxidant.
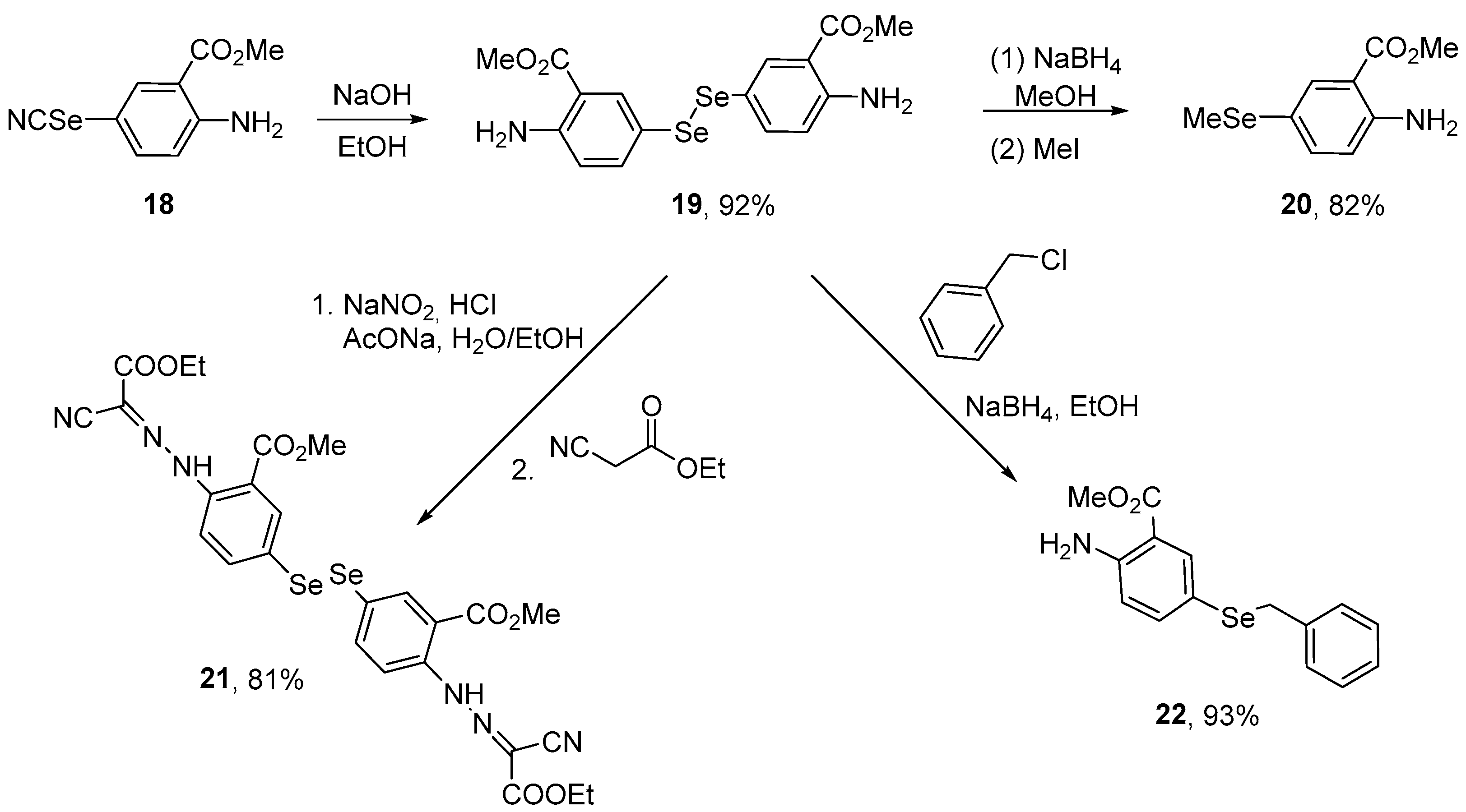
Incorporation of the diselenide functionality into the backbone of anthranilic acid has provided novel compounds designed to interfere with biotargets. Al Abdallah et al. [53] synthesized methyl anthranilate-based hybrid diselenides and evaluated their antioxidant activity using in vitro ABTS and DPPH assays. The selenocyanate 18 was submitted to hydrolysis using NaOH in EtOH to afford the diselenide 19 in 92% yield. Diselenide 19 was converted to selenide 20 (82% yield) through a one-pot alkaline reduction employing a 1:1 mixture of NaBH4 and NaOH in MeOH, followed by the reaction with methyl iodide (Scheme 7). The tested compounds exhibited antioxidant activity in both tests. Compounds 20, 19, 22, 21, and 18 exhibited 96%, 92%, 89%, 85%, and 81% of scavenging activities in the ABTS assay, respectively. In the DPPH assay, compounds 20, 22, 19, 18, and 21 exhibited 91%, 88%, 86%, 73%, and 69% scavenging activities, respectively. Whether such activity also occurs in more complex systems remains to be established.
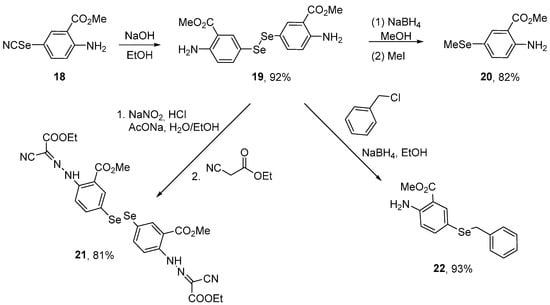
Scheme 7.
Synthesis of methyl anthranilate-based organodiselenide hybrids.
3.2. Ebselen Derivatives
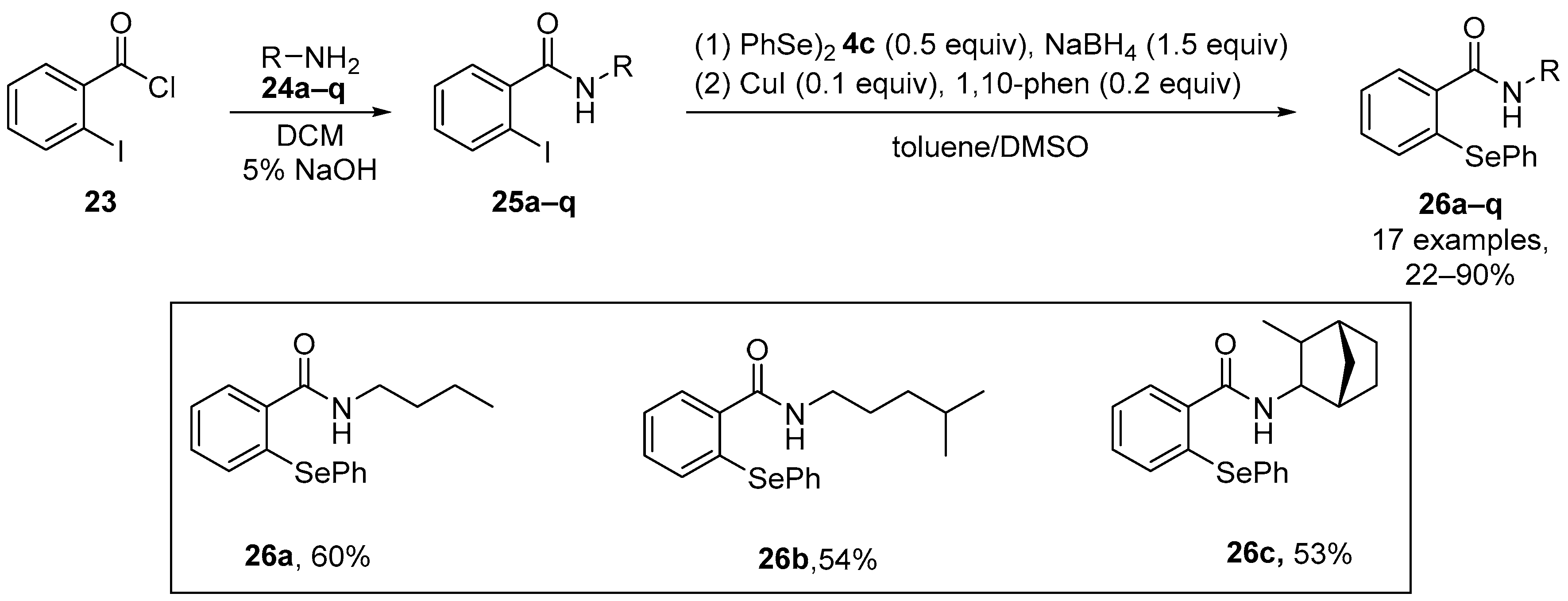
Ebselen and analogues have been widely studied as redox modulators, and consequently Scianowski et al. [54] have synthesized novel Se-based species related to the ebselen skeleton. N-substituted unsymmetrical phenyl selenides containing an o-amido function were generated via copper-catalyzed nucleophilic substitution by PhSe- formed in situ from reduction of diphenyl diselenide by NaBH4. The rate of H2O2 removal by these compounds was evaluated indirectly via an indirect antioxidant assay using dithiothreitol (DTT) as a reducing thiol cofactor. The most potent antioxidants within this new series were the N-butyl-(26a), N-3-methylbutyl-(26b) and phenyl selenides (26c) (Scheme 8).
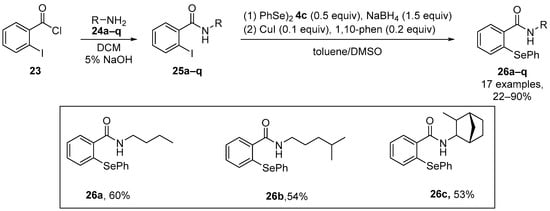
Scheme 8.
Synthesis of N-substituted unsymmetrical phenylselenides 26.
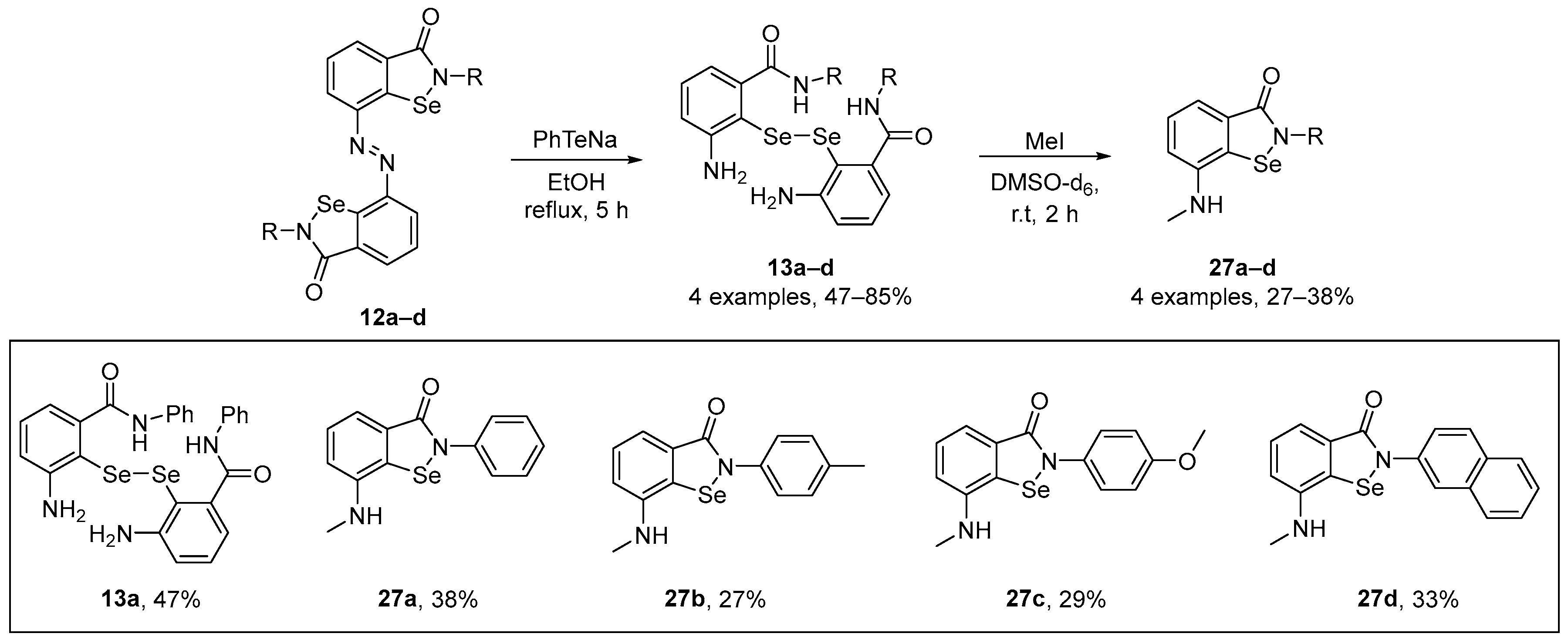
Kumar et al. [55] have described a straightforward synthesis of novel N-methylated ebselenamines and the capacity of these compounds to quench peroxyl radicals. These species showed higher activity than α-tocopherol and were regenerable in the presence of ascorbic acid. Such data contribute to a better understanding of ebselenamine antioxidants as potential drugs against oxidative damage. Diselenides 13a–d were readily prepared from the diazo compounds 12a–d and were used for the construction of novel N-methylated ebselenamines 27a–d. Reduction of 12a–d with PhTeNa prepared in situ produced diselenides 13a–d. Furthermore, the reactions of diselenides 13a–d with four equivalents of MeI in DMSO-d6 at room temperature afforded the N-methylated ebselenamines 27a–d in moderate yields (Scheme 9). The antioxidant activity of the compounds was evaluated by HPLC-based lipid peroxidation assay, GPx-like activity, and oxidant assays. The N-methylated ebselenamine inhibited the peroxidation of linolenic acid, decreased oxidant levels in astroglial cell lines treated with H2O2, and mimicked the functions of GPx.
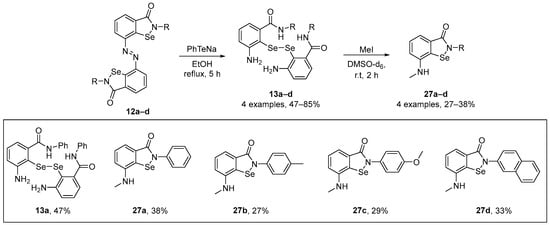
Scheme 9.
Synthesis of novel N-methylated ebselenamine derivatives 27.
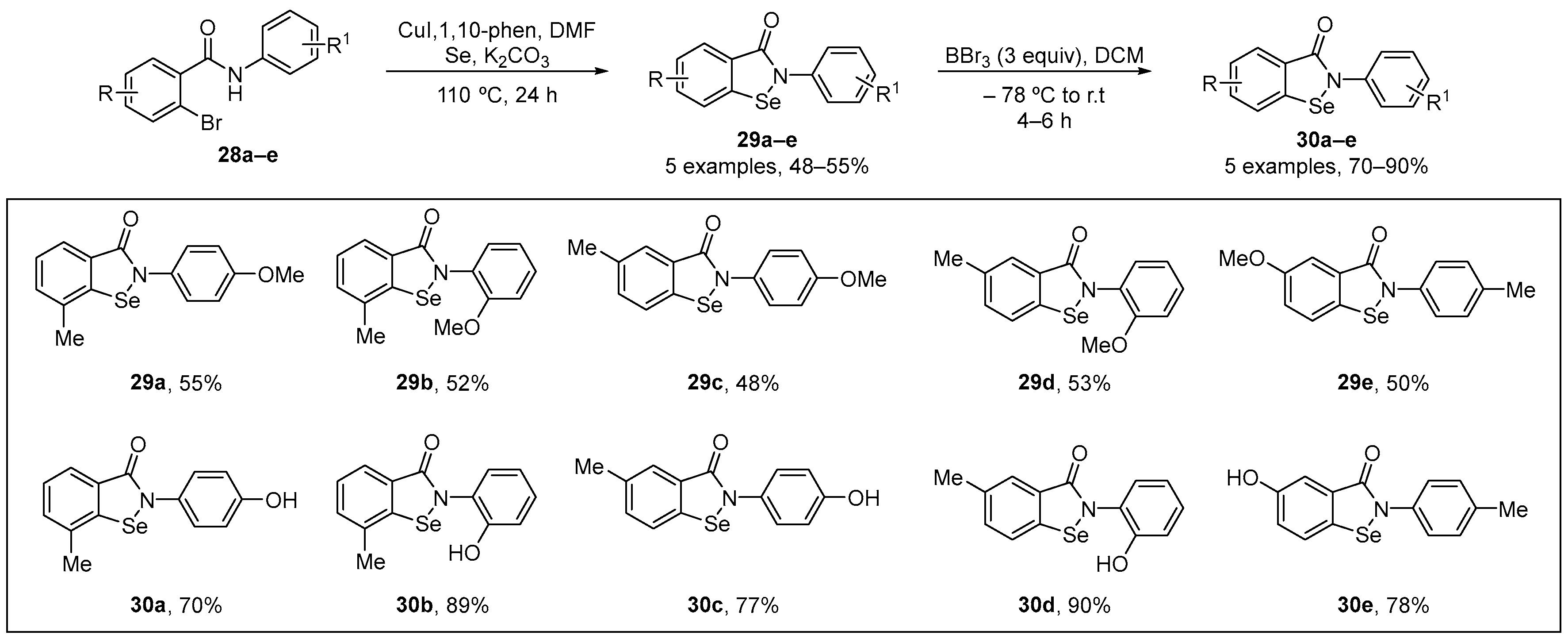
Singh and co-workers [6] have published a copper-catalyzed direct selenation of substituted 2-bromo-N-phenylbenzamide substrates using elemental selenium powder as the Se source to prepare methyl-protected N-phenyl substituted isoselenazolones 29 via simultaneous C-Se and Se-N bond formation. In this process, the substituted phenolic isoselenzolones 30 were generated via a deprotection reaction. After synthesis of the benzamides 28a–e, a copper-catalyzed reaction using CuI and elemental Se was performed using K2CO3 as base and 1,10-phen as a ligand, with this generating the desired isoselenazolones 29a–e. O-demethylation of 29a–e, using stoichiometric amount of BBr3 in DCM at −78 °C, gave the phenolic substituted isoselenazolones 30a–e in yields of 70−90% (Scheme 10). The antioxidant activity of the 30a–e was assessed using the FRAP assay. The compounds at concentrations of 10–150 μM showed activity in the FRAP assay, with the activity following the order 30b > 30a > 30d > 30a > 30e. GPx mimetic activity of was evaluated in both thiophenol and coupled-reductase assays. All compounds exhibited GPx-like activity, indicating that these phenolic isoselenazolones have promising protective properties.
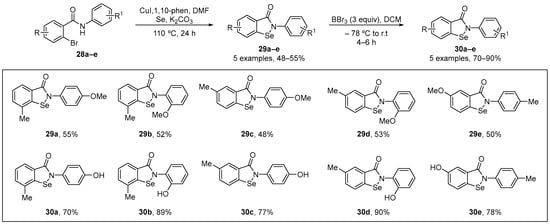
Scheme 10.
Synthesis of methoxy-substituted isoselenazolones via C-Se and Se-N bond formation.
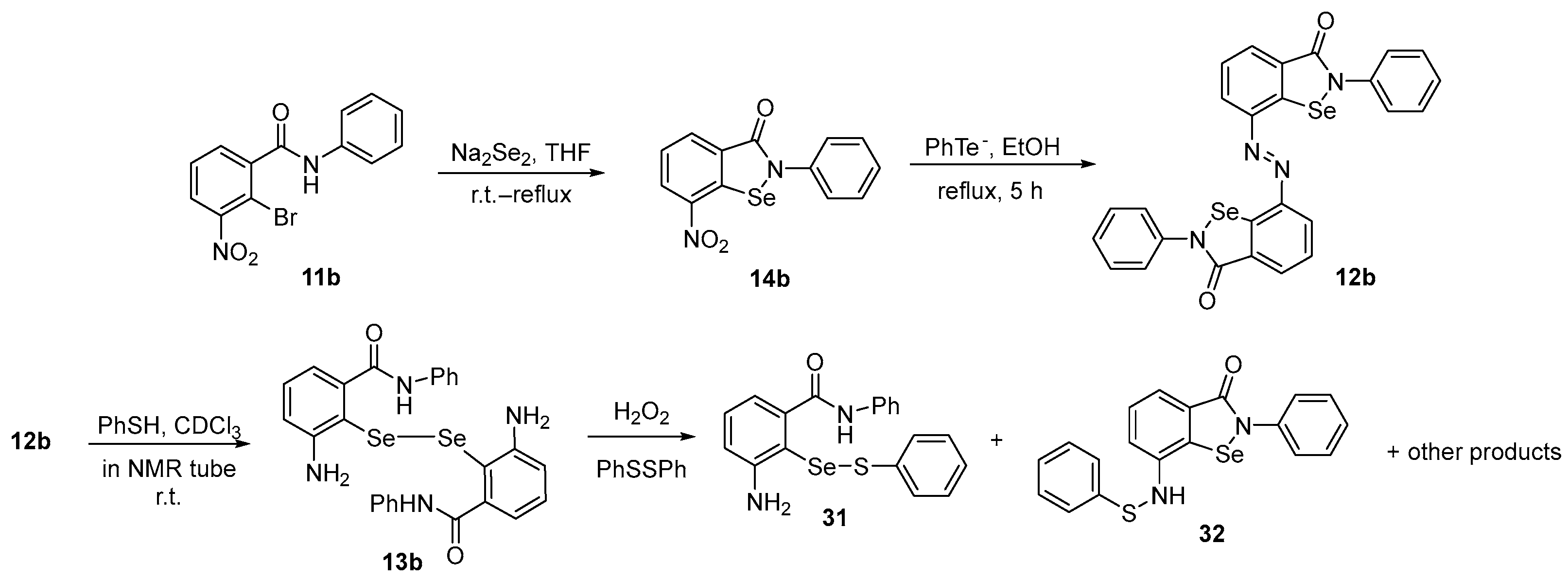
To obtain mechanistic insights into intermediates involved in the GPx-like activity of Ebselen derivatives, the azo-bis-ebselen 12b was prepared by Kumar and Singh [56] using a previously published procedure [47]. Firstly, 12b was reacted with PhSH in an NMR tube at room temperature in CDCl3, being totally reduced to diselenide 13b. Once formed, 13b reacts with H2O2 in the presence of disulfide to form selenenyl sulfide 31, ebselenamine 14b, and N-thiophenyl-ebselenamine 32 (Scheme 11). It was observed that N-thiophenyl ebselenamine 32 and its corresponding selenenyl sulfide 31 were more efficient in catalyzing H2O2 reduction than Ebselen and mimic the action of GPx.
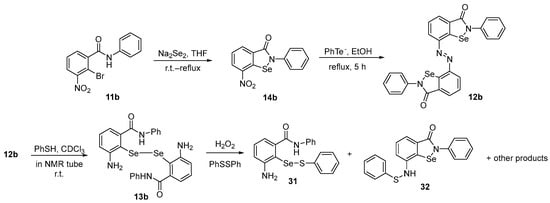
Scheme 11.
Studies on the oxidation of diselenide 13b.
3.3. Se-Functionalized N-Heterocycles
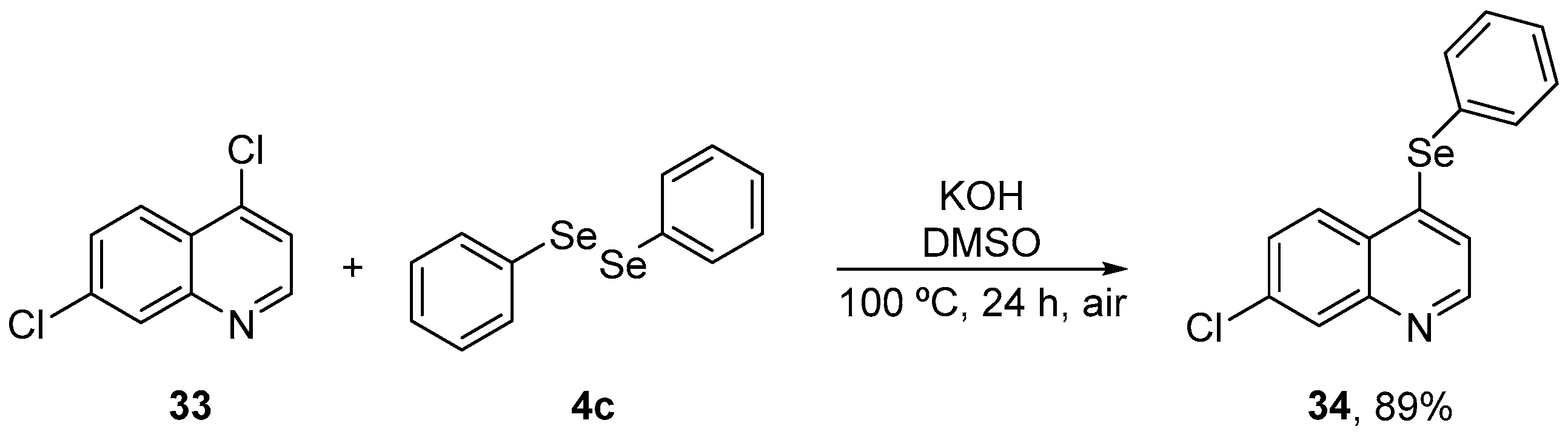
7-Chloroquinoline derivatives are biologically active units and display a broad range of pharmacological activities. Due to their importance in a variety of synthetic and natural products, considerable efforts have been directed to the development of new structures based on this scaffold. Vogt et al. [57] have prepared 7-chloroquinoline containing a selenium moiety (34) to verify the role of the phenylselanyl group in the antioxidant effect. Compound 34 was synthesized in 89% yield by the reaction of 4,7-dichloroquinoline 33 with diphenyl diselenide 4c, using KOH as base and DMSO as solvent at 100 °C (Scheme 12). The activity of 34 was evaluated against oxidative stress induced by sodium nitroprusside (SNP) in the brain of mice and compared to the parent compound 33. Male mice were treated with 34 (50 mg/kg, i.g.) and after 30 min, SNP (0.335 μmol/site, 2 μL, intracerebroventricular (i.c.v.)) was administrated. After 1 h, animals were sacrificed, and the brains were removed for the determination of TBARS, protein carbonyl and NPSH levels, CAT, and GST activities. Compound 34 decreased TBARS and protein carbonyl levels and increased CAT and GST activities, and non-enzymatic NPSH levels. In contrast, 33 did not protect against SNP induced alterations. Therefore, the phenylselenyl group present in the quinoline structure appears to be critical for the protective activities of 34.
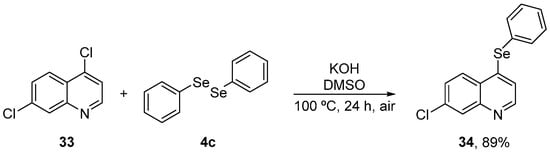
Scheme 12.
Synthesis of 7-chloro-4-(phenylselanyl)quinoline 34 in basic medium.
Prigol and co-workers have further investigated the effect of 34 as a multi-target molecule in flies (Drosophila melanogaster) [58]. Compound 34 may act on the dopaminergic system through multiple different mechanisms including reducing oxidative damage and improving antioxidant defenses factors, thereby protecting dopaminergic neurons, preventing dopamine depletion, and consequently reversing the behavioral motor deficits induced by rotenone. Adult flies were exposed to a diet containing rotenone (500 μM) and/or 34 (25 μM) for 7 days. The flies were then euthanized, and the heads were removed to determine oxidant and TBARS levels, and SOD, CAT, and NPSH activities. Compound 34 reduced oxidant and TBARS levels and restored the activities of SOD and CAT.
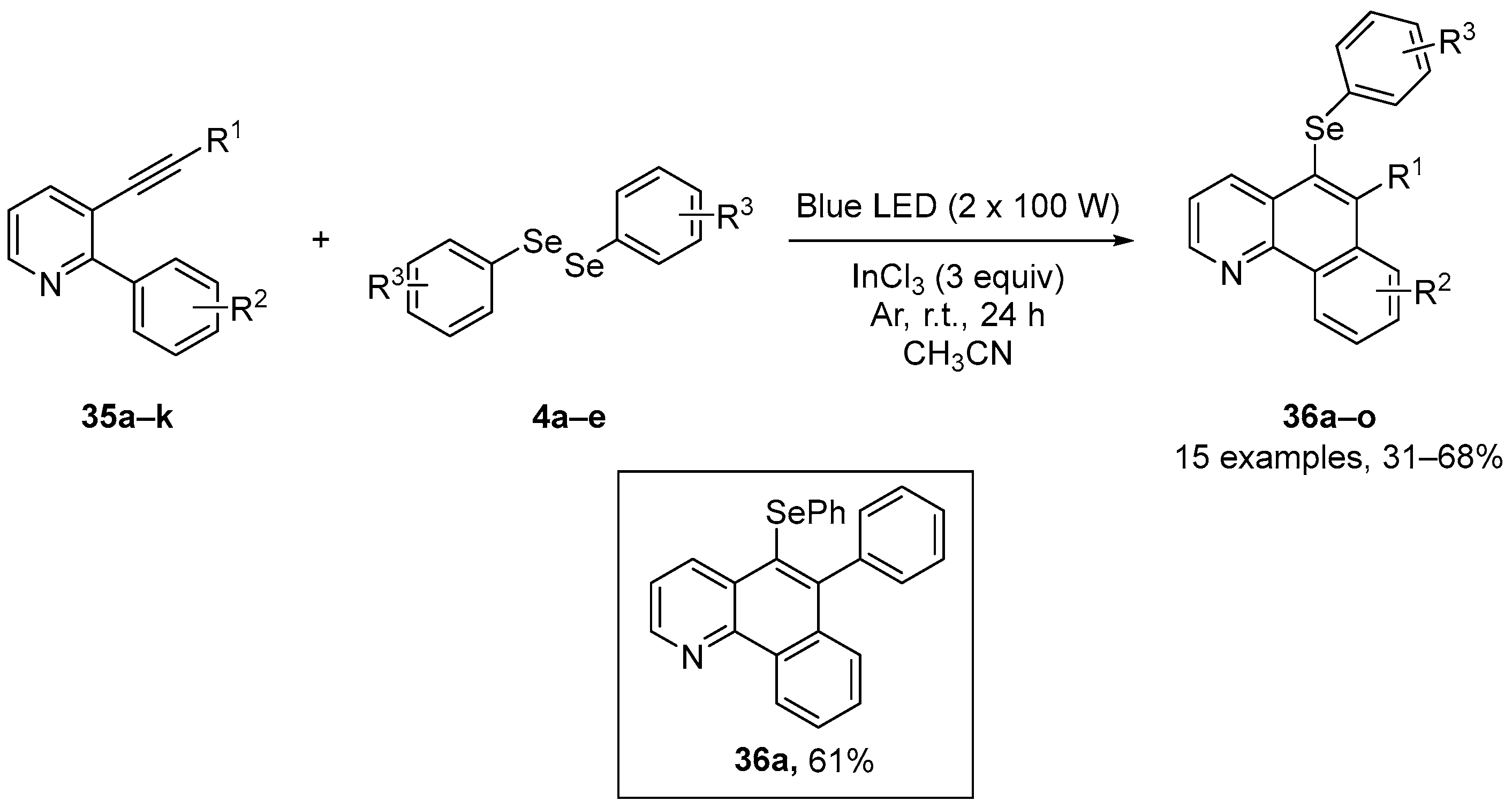
The biological activities of selenium-substituted quinolones were further examined by Schneider and co-workers [9], who reported a simple and efficient method to access the 6-organyl-5-(arylchalcogenyl)benzo[h]quinolines 36. These were prepared in 40–68% yields through the visible light-promoted 6-endo-dig selenocyclization of 2-aryl-3-(organylethynyl)pyridines 35, using diaryl diselenides 4 and indium(III) chloride in CH3CN under Ar atmosphere for 24 h (Scheme 13). The effects of compound 36a in vitro was analyzed using the ABTS, DPPH, and TBARS assays. This compound did not show significant activity when compared to the negative control in the ABTS and DPPH assays, but reduced lipid peroxidation induced by sodium nitroprusside (SNP) in the brain at a concentration of 10 µM, and at concentrations over the range 1–200 µM in the liver of mice. These contrasting data illustrate the difficulties in using simple in vitro assays, such as the ABTS and DPPH assays, to predict biological activity, and emphasize the need for in vivo testing.
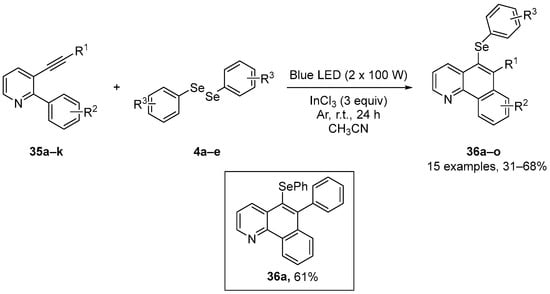
Scheme 13.
Synthesis of 6-organyl-5-(arylselanyl)benzo[h]quinolines 36.
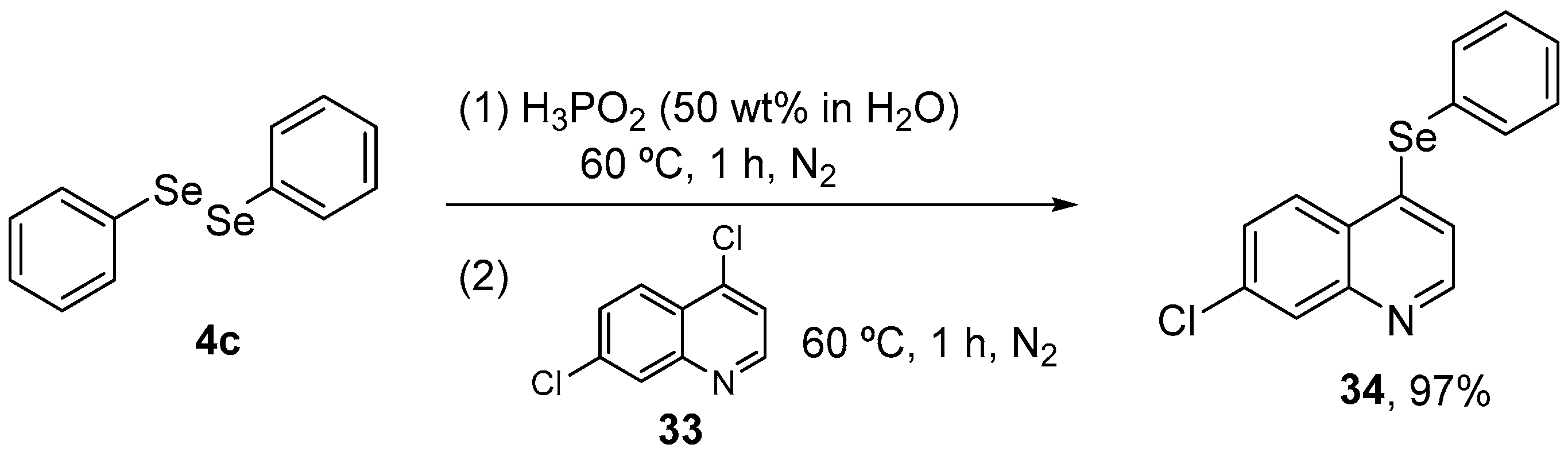
In view of these promising results, Wilhelm and co-workers [59] investigated possible modulation of oxidative stress by 34 in a neuropathic pain animal model. The target molecule was synthesized in 97% yield through the reaction of 4,7-dichloroquinoline 33 with organylselenols, generated in situ by the reaction of diphenyl diselenide 4c with aqueous H3PO2 at 60 °C under N2 atmosphere (Scheme 14). Adult male mice received streptozotocin (200 mg/kg, i.p.) and after 21 days the animals received 34 (5 mg/kg, i.g.) for 15 days. 24 h after the last treatment, the animals were euthanized and the cerebral cortex, hippocampus, and spinal cord samples were collected for analysis. Compound 34 decreased oxidant levels and SOD, NPSH, GPx, and GR activities in the cerebral cortex and hippocampus, while decreasing oxidation levels and NPSH activity in the spinal cord.
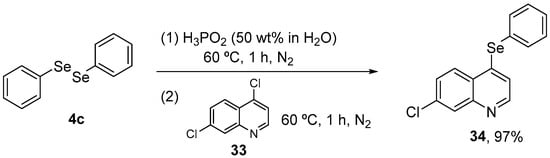
Scheme 14.
Synthesis of 7-chloro-4-(phenylselanyl)quinoline 34 in acidic medium.
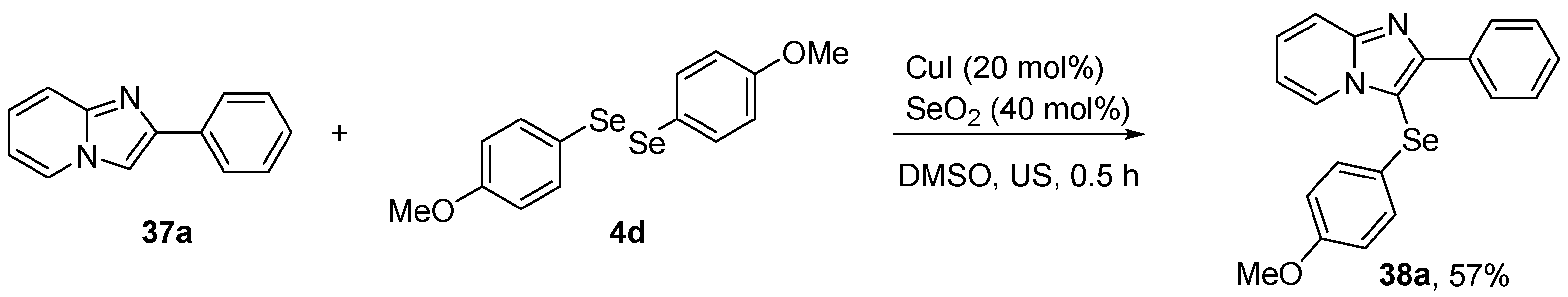
Recent studies have demonstrated the neuroprotective, antidepressant, and antioxidant effects of another organoselenium compound, 3-((4-methoxyphenyl)selanyl)-2-phenylimidazo[1,2-a]pyridine (MPI, 38a). Savegnago et al. [60] have reported the antioxidant activity of 38a in mouse model of LPS-induced depression. 38a was synthesized using 2-phenylimidazo[1,2-a]pyridine 37a, (4-OMePhSe)2 4d and CuI (20 mol%) as copper catalyst in the presence of SeO2 (40 mol%) in DMSO under sonication (US) for 30 min (Scheme 15). Male adult mice were treated with compound 38a (20 and 50 mg/kg, i.g.) 30 min prior the LPS challenge (0.83 mg/kg, i.p.). After 24 h, the animals were sacrificed, followed by brain removal and isolation of prefrontal cortex and hippocampus, for analysis. Pretreatment with 38a prevented an increase in reactive species and lipid peroxidation induced by LPS in the prefrontal cortex and hippocampus. In related studies, Savegnago and co-workers [61] explored the ability of 38a to reverse oxidative stress in a mouse model of inflammation, and stress-induced depressive-like behavior. For the inflammatory model, mice received an injection of TNF-α (0.001 fg/site, i.c.v.). After 30 min, they were treated with 38a (10 mg/kg, i.g.). For the stress model, mice were submitted to physical stress for 240 min. The treatments with 38a (10 mg/kg, i.g.) were given 10 min after the restraint stress. After 30 min, mice were euthanized for isolation of the prefrontal cortex and total hippocampus for determination of the reactive species, nitrate/nitrite (NOx), and TBARS levels. This treatment with 38a abolished the oxidative and nitrosative stress in the prefrontal cortex and hippocampus.
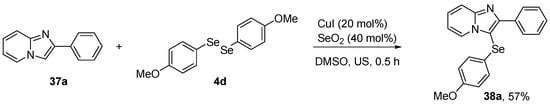
Scheme 15.
Synthesis of 3-[(4-methoxyphenyl)selanyl]-2-phenylimidazo[1,2-a]pyridine (38a).
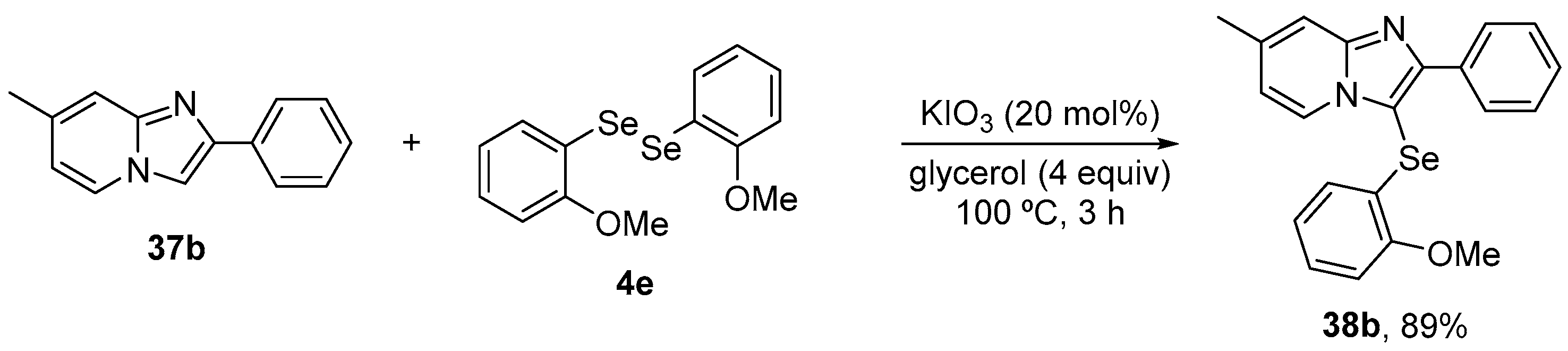
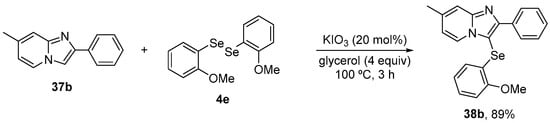
More recently, Ourique and co-workers [62] reported the antioxidant activity and pharmacokinetic characteristics of a related compound, 3-((2-methoxyphenyl)selanyl)-7-methyl-2-phenylimidazo[1,2-a]pyridine (38b), in a glioblastoma cell line. 38b was synthesized by a green protocol, via the direct C(sp2)−H bond chalcogenation of imidazo[1,2-a]pyridine 37b with 0.5 equivalent of (4-OMePhSe)2 4e, using KIO3 as a catalyst and a stoichiometric amount of glycerol, giving the target product in 86% yield (Scheme 16). The cells were exposed at low concentration to 38b (1 μM) for 6 h. After the treatment time, the supernatants were used for TrxR activity, GSH levels, and Nrf2. 38b modulated the inhibition of TrxR activity and the levels of GSH and Nrf2 proteins.
Scheme 16.
Synthesis of 3-((2-methoxyphenyl)selanyl)-7-methyl-2-phenylimidazo[1,2-a]pyridine (38b).
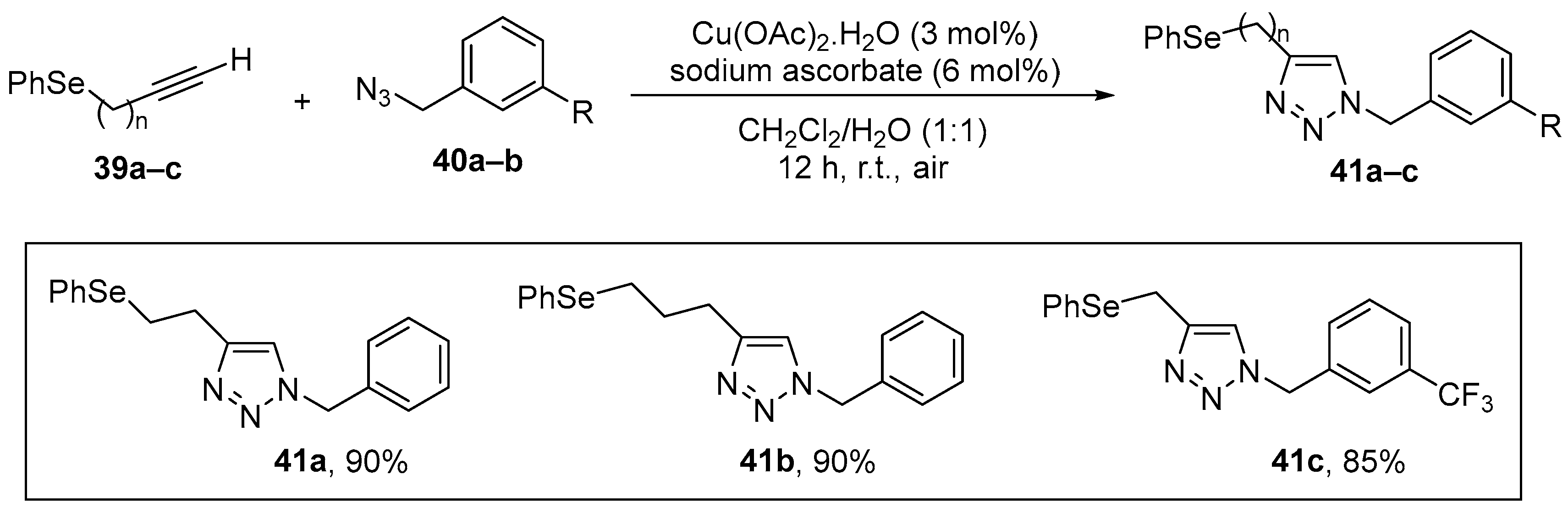
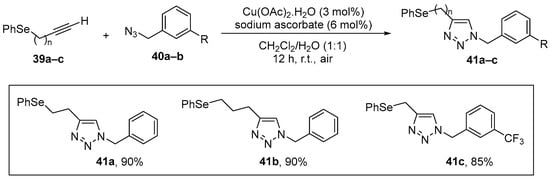
1,2,3-Triazoles are an important class of nitrogen heterocycles, which display a broad spectrum of chemical applications and biological activities. Ávila and co-workers [10] demonstrated that some variations on this skeleton have the potential to repair oxidative damage by different mitochondrial stress inducers, particularly in a genetic mitochondrial dysfunction model (mev-1 mutation). The selected 4-phenyl-1-(phenylselanylmethyl)-1,2,3-triazole (Se-TZ, 41) and their derivatives were synthesized in high yield by click chemistry, the copper catalyzed (Cu(OAc)2.H2O/sodium ascorbate) 1,3-dipolar cycloaddition of azidomethyl arylselenides 39 with alkynes 40 (CuAAC) [63] (Scheme 17). Potential antioxidant effects of 41 were examined in worms (C. elegans), which were exposed to the compounds at 10 μM for 30 min at the first larval stage (L1) and then allowed to develop to the L4 stage. Protective effects were examined by the determination of oxidant and NPSH levels and SOD and CAT activities. The compounds reduced the levels of oxidation and CAT activity. In the mev-1 mutants, which have a reduced life span resulting from enhanced mitochondrial oxidant production, treatment with 41 increased the longevity of the worms, possibly through a radical scavenging activity.
Scheme 17.
Copper-catalyzed 1,3-dipolar cycloaddition of azidomethyl arylselenides with alkynes.
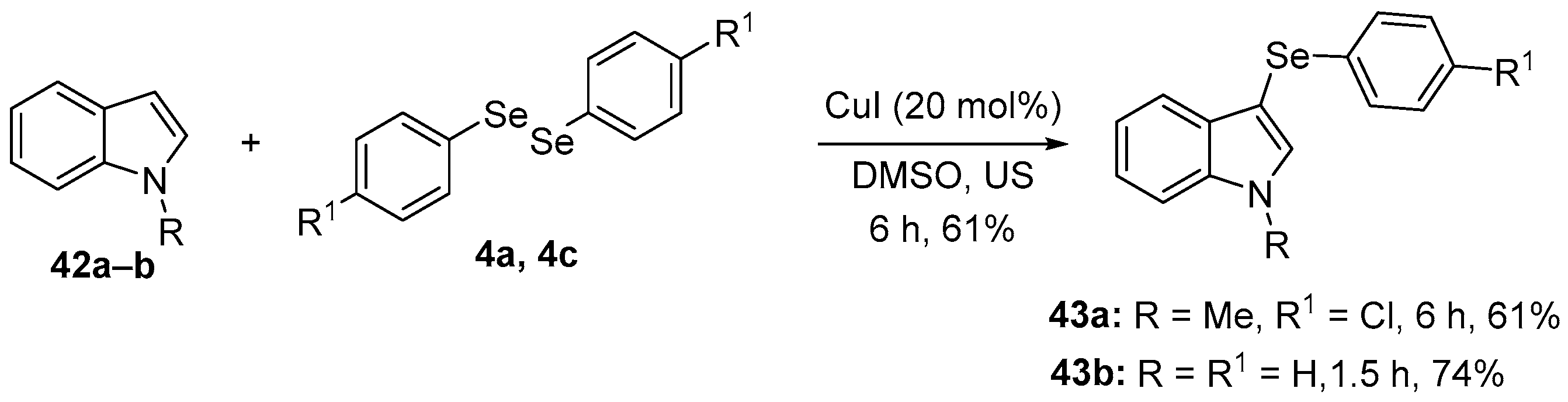
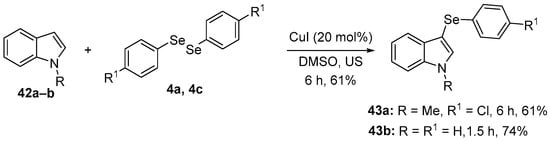
Several studies have highlighted promising protective activities, in different animal models, of N-methylindole combined with an organoselenium moiety, and specifically 3-(4-chlorophenylselanyl)-1-methyl-1H-indole (43a). 43a was prepared in 61% yield through the direct selenation of N-methylindole 42a with 1,2-bis(4-chlorophenyl)diselenide 4a, using CuI as catalyst in DMSO under ultrasonic irradiation [64] (Scheme 18). The pharmacological properties of 43a were investigated by Savegnago and co-workers [65], including in an animal model of neuropathic pain. Male adult mice were submitted to partial sciatic nerve ligation. Four weeks after surgery, the animals were treated with 43a (10 mg/kg, i.g.) and after 30 min, the mice were euthanized for collection of cortex and hippocampus. Treatment with 43a decreased oxidant levels and lipid peroxidation (measured using TBARS) in the cortex and hippocampus of the mice. Later studies [66] examined whether 43a could reverse an oxidative imbalance induced by acute restraint stress in mice. Ten mins after the animals were subjected to 240 min of restraint, they received 43a (1 or 10 mg/kg, i.g.) with possible protective effects evaluated 30 min later. 43a reversed oxidative stress in the cortex and hippocampus of stressed mice by reducing oxidant and TBARS levels, and through the modulation of SOD and CAT activities.
Scheme 18.
Direct selenation of N-methylindole to prepare 43a and 43b.
Compound 43a was also examined for its capacity to attenuate neurochemical alterations in a mammary (4T1) tumor model [67]. Female BALB/c mice were subcutaneously inoculated with 4T1 cancer cells (1 × 105 cells/mice). From days 14 to 20, mice received daily gavage with 43a. Oxidative stress in the prefrontal cortices of tumor-bearing mice was examined by the expression of the NO•-producing enzyme iNOS and the transcription factor Nrf2. 43a decreased the expression of iNOS, increased the expression of Nrf2, and decreased the levels of oxidants, NO•, and lipid peroxidation in the prefrontal cortex.
The antioxidant effect of 43a, and its ability to ameliorate long-term behavioral and biochemical alterations in male mice with sepsis have also been investigated [68]. On day 0, lipopolysaccharide (LPS) (5 mg/kg, i.p.) was administrated to the animals, with treatment with 43a from days 24 to 30 at 1 mg/kg i.g. After sacrifice on day 30, the prefrontal cortex and hippocampus were removed for analysis. 43a decreased the effects of sepsis by reducing the levels of oxidants, NO•, and lipid peroxidation. This work was subsequently extended [69] to examine antidepressant and anxiolytic effect in animals that received corticosterone (20 mg/kg, i.g.) by 14 days, and on the 15th day, 43a (1 mg/kg, i.g.). After 30 min, the mice were anesthetized, and blood was collected by cardiac puncture. Treatment with 43a decreased oxidant levels and lipid peroxidation in the mouse plasma.
Savegnago et al. [70] have also evaluated 43a against H2O2-induced oxidative stress in human dopaminergic neuroblastoma (SH-SY5Y) cells. Cells were pretreated with 43a (4 µM) for 4 h, followed by treatment with H2O2 (343 µM) for 24 h. The levels of NO• metabolites, oxidants, and GSH were then determined. A decrease in oxidants induced by 43a treatment was associated with changes in GSH levels, suggesting that the antidepressant and anxiolytic effect of 43a are related to its antioxidant activity.
1-Methyl-3-(phenylselanyl)-1H-indole (43b) is another indole-based organoselenium compound that has demonstrated promising antioxidant activity in several animal models of depression and anxiety. 43b was synthesized in a 74% yield using the same approach used to prepare 43a (Scheme 18). Male mice received an i.c.v. injection of streptozotocin (0.2 mg/4 μL per mouse). After 24 h, the mice were treated with 43b (10 mg/kg, i.g.) once daily for seven days; 30 min after the final administration of 43b, the animals were euthanized, and the cerebral cortex and hippocampus collected for analysis. Treatment with 43b decreased oxidant levels, metabolites of NO•, and lipid peroxidation [71]. Later studies [72] demonstrated that a single administration of 43b at 10 mg/kg, i.g. was able to induce similar reductions in oxidative stress.
Savegnago and co-workers [73] have also demonstrated that 43b reduces oxidative stress in cerebral structures, liver, and kidney of streptozotocin-induced diabetic mice. In this study, mice were rendered diabetic by a single injection of streptozotocin (200 mg/kg, i.p.). After 7 days of streptozotocin administration, 43b (10 mg/kg, i.g.) was administered once a day for 14 days. 24 h after the last dose of 43b, the animals were euthanized and the hippocampus, prefrontal cortex, liver, and kidney were excised for analysis. Treatment with 43b inhibited lipid peroxidation in the prefrontal cortex, hippocampus, liver, and kidney of the streptozotocin-treated mice. Together, these studies suggest that 43b has antidepressant and anxiolytic effects and can also ameliorate the central and peripheral complications caused by diabetes in mice, potentially via its antioxidant activities.
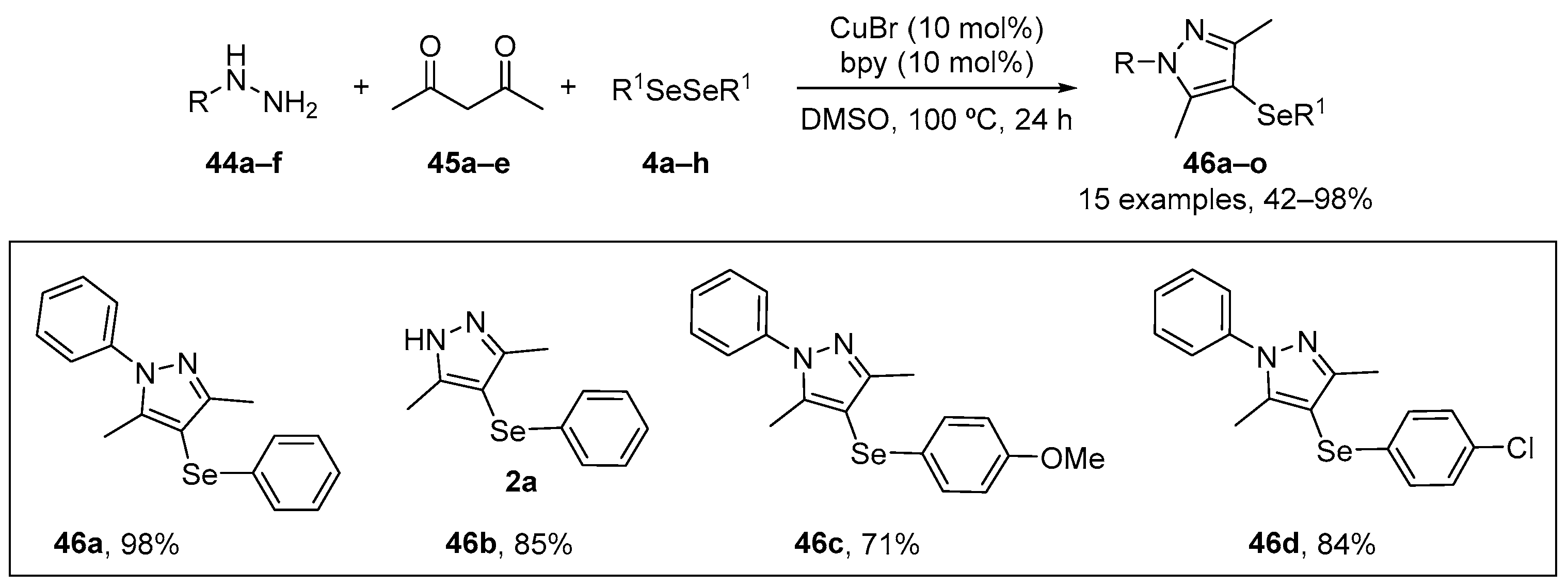
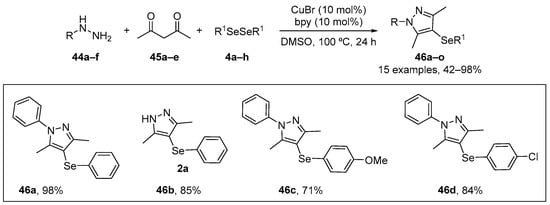
Oliveira et al. [74] have investigated the antioxidant potential of selanyl-substituted pyrazoles through in vitro and in vivo assays. The compounds were synthesized by a previously described procedure involving a cyclo-condensation and copper-catalyzed direct C-H bond selenation reaction [75]. The one-pot multicomponent reaction of different hydrazines 44, 1,3-diketones 45 and diorganyl diselenides 4, using catalytic amounts of copper bromide and bypyridine as ligand afforded a total of fifteen 4-organylselanylpyrazoles 46 in 42–98% yield (Scheme 19). In vitro, the compounds 46a (50–100 µM), 46c (100 µM), and 46d (100 µM) presented FRAP activity, the compound 46a (10 µM) presented DPPH-scavenging activity, the compounds 46a (50–500 µM), 46b (50–500 µM), 46c (50–500 µM) and 46d (100–500 µM) inhibited to NO• scavenging, and the compounds 46a (10–500 µM), 46c (50–500 µM), and 46d (50–500 µM) reduced lipid peroxidation and oxidant levels in mouse brain. In vivo, all compounds at concentrations over the range 10–500 µM reduced lipid peroxidation in the liver and brains of mice, consistent with an antioxidant effect of these pyrazoles.
Scheme 19.
Synthesis of selanyl-substituted pyrazoles 46.
3,5-Dimethyl-1-phenyl-4-(phenylselanyl)-1H-pyrazole (46a, Scheme 19) was examined by Savegnago et al. in a depression-pain syndrome mouse model induced by streptozotocin [76]. The capability of 46a to reverse the depression-pain syndrome in mice was examined by evaluating the levels of oxidants, NO•, lipid peroxidation, and SOD and CAT activities in the prefrontal cortices and hippocampi of mice. Adult male mice received streptozotocin (0.2 mg/4 μL per mouse, i.c.v.), and after 24 h were treated with 46a (1 or 10 mg/kg, i.g.). After 30 min, the animals were euthanized and the prefrontal cortex and hippocampus were removed for examination; 46a treatment decrease oxidant levels, NO• metabolites, and lipid peroxidation in both brain structures. Later studies [77] investigated the anxiolytic-like, antiallodynic, and anti-hyperalgesia effects of 46a in mice subjected to acute restraint stress (ARS). Adult male mice were restrained for 2 h followed by treatment with 46a (1 or 10 mg/kg, i.g.). After 30 min, the animals were euthanized, and the prefrontal cortex and hippocampus were removed for analysis. 46a reversed ARS-induced increased oxidant levels and lipid peroxidation and modulated CAT and SOD activities. Taken together, these studies indicated that the antidepressant-like, antiallodynic, and anti-hyperalgesic effects of 46a may be related to modulation of oxidative and NO• pathways.
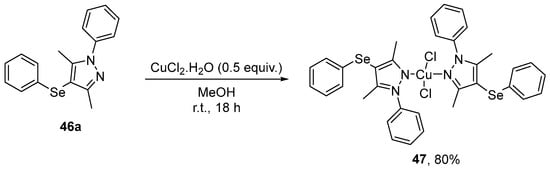
Pinheiro et al. [78] have reported the synthesis, characterization, antioxidant potential, and cytotoxicity of a new Cu(II) complex 47, derived from 46a. The synthesis involved the slow addition of a solution of 46a (0.92 mmol) in MeOH to a solution of CuCl2⋅2H2O (0.46 mmol) in MeOH for 18 h at room temperature, obtaining the desired product in 80% yield (Scheme 20). The antioxidant activity of 47 was evaluated, with concentrations of 10–50 µM shown to inhibit sodium azide-induced formation of reactive species in the hippocampus and cortex of mice. 47 was also effective in scavenging DPPH at different concentrations (1–50 µM), and ABTS•+ at 10 µM.
Scheme 20.
Synthesis of Cu(II) complex 47 derived from 46a.
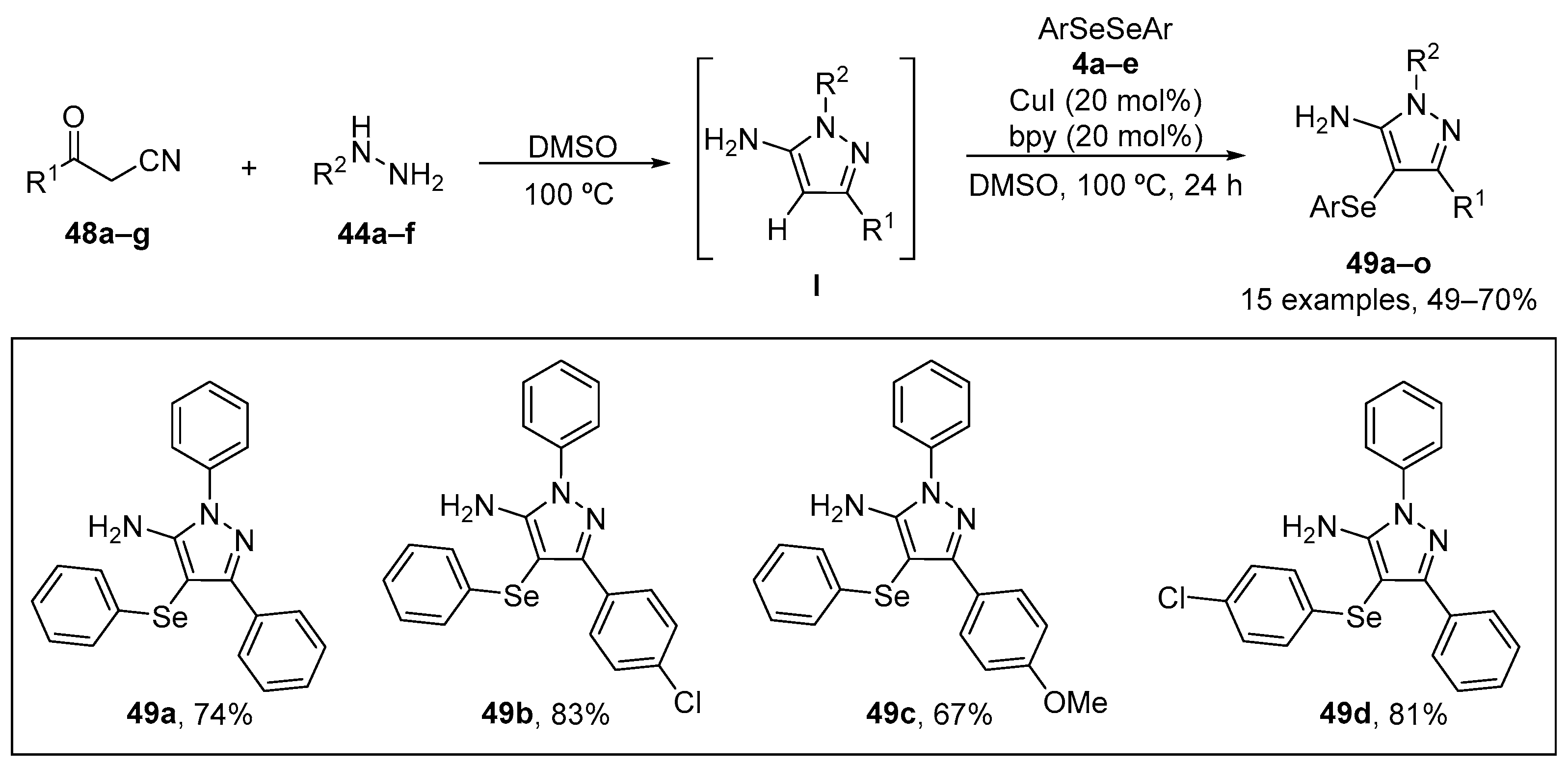
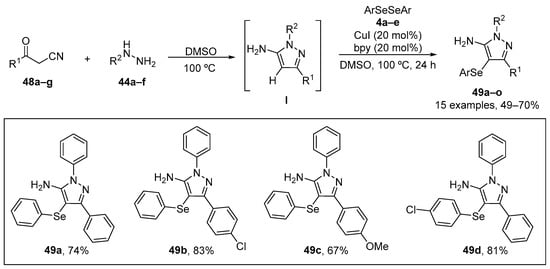
Jacob et al. [79] have reported a sequential one-pot synthesis of 5-amino-4-(arylselanyl)-1H-pyrazoles 49 catalyzed by CuI/bpy under mild conditions. The reaction of easily available benzoylacetonitriles 48, substituted hydrazines 44, and diaryl diselenides 4 in DMSO at 100 °C for 24 h, afforded a range of 5-amino-4-(arylselanyl)-1H-pyrazoles 49 in 49–90% yield (Scheme 21). The antioxidant effect of the 5-amino-4-(arylselanyl)-1H-pyrazoles 49 was evaluated in in vitro studies. Compounds 49a, 49b, 49c, and 49d (50–500 µM) presented reducing power in FRAP assay and the ability to inhibit linoleic acid peroxidation. Compound 49b was more effective in inhibiting linoleic acid peroxidation than the other compounds, with an IC50 value of ~80 µM. Compound 49a (10–500 µM), 49b (1–500 µM), 49c (5–500 µM), and 49d (10–500 µM) demonstrated ABTS•+ radical scavenging activity, with 49a being the most effective (IC50 ~32.5 µM). These 5-amino-4-(arylselanyl)-1H-pyrazoles may therefore be worthy of further study. Whether these relative high drug concentrations can be achieved in vivo remains to be established.
Scheme 21.
Synthesis of 5-amino-4-(arylselanyl)-1H-pyrazoles 49.
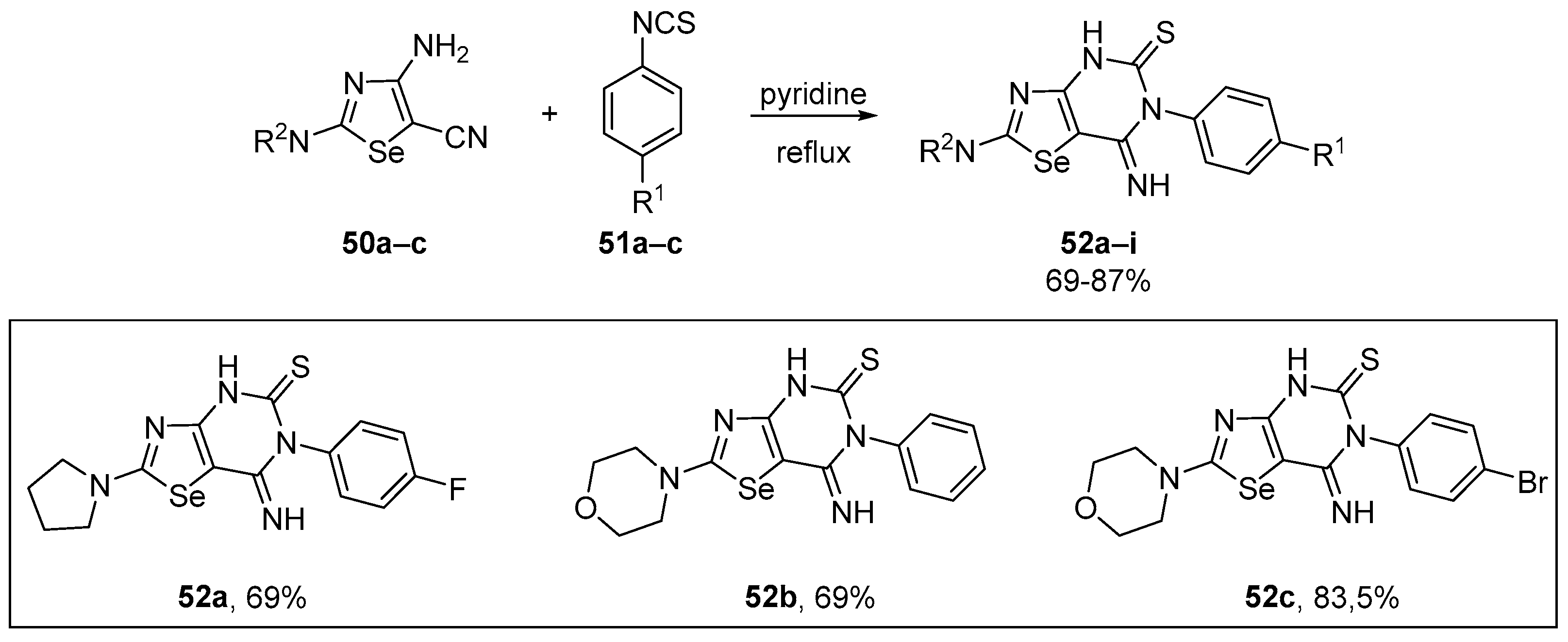
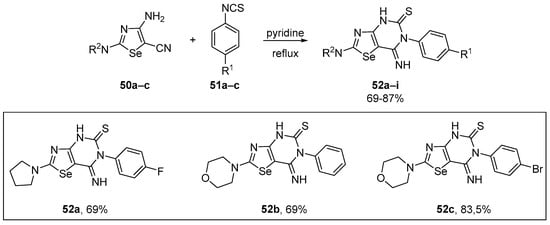
A straightforward strategy for the efficient synthesis of multi-functionalized 7-imino[1,3]selenazolo[4,5-d]pyrimidine-5(4H)-thiones 52 was described by Yarmohammadi and co-workers [80]. The target compounds were obtained by the incorporation of the N-phenylmethanethioamide fragment through the heteroannulation of several 2,4,5-trisubstituted 1,3-selenazoles 50. The reaction was conducted using readily accessible phenyl isothiocyanates in pyridine under reflux, and the novel 7-imino[1,3]-selenazolo[4,5-d]pyrimidine-5(4H)-thione derivatives 52a–i were obtained in 69–87% yield (Scheme 22). The inhibitory activity of the selenium-containing heterocycles was assessed via the DPPH assay, with 52a, 52b, and 52c showing significant activity, with IC50 values in the range of 10–63 μM. 4-Fluorophenyl-substituted compounds bearing 2-morpholine (IC50 14 μM), and 2-piperidine (IC50 19 μM) residuals were ranked in the second and third place of antioxidant efficacy, respectively.
Scheme 22.
Synthesis of 7-imino[1,3]selenazolo[4,5-d]pyrimidine-5(4H)-thiones 52.
3.4. Se-Heterocycles
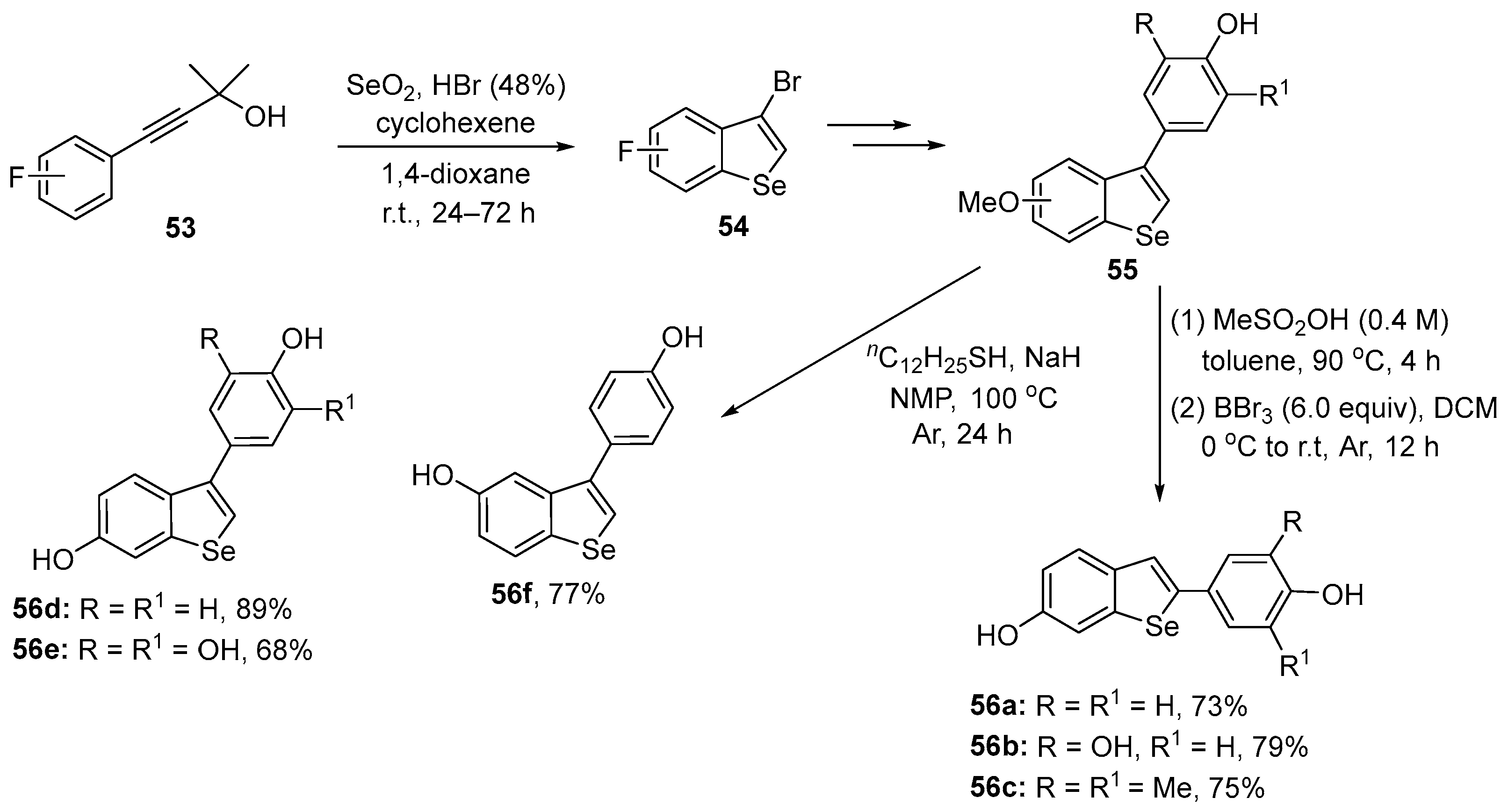
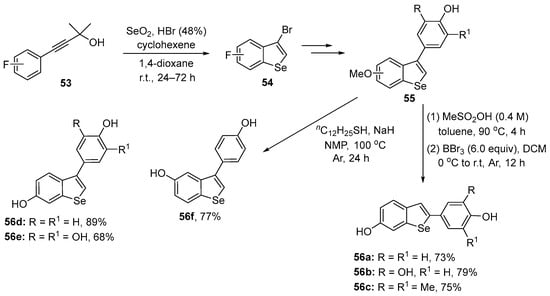
Arsenyan, Chovanec, and co-workers have reported data for a series of poly-hydroxy 2-aryl- and 3-arylbenzo[b]selenophenes 56 and tested their activity in yeast [81]. The compounds were prepared following a procedure described by Arsenyan [82], involving the electrophilic cyclization of arylalkynes 53 with HBr in the presence of SeO2, affording the 3-bromo-benzoselenophene 55, which were converted to the respective 3-aryl derivatives through a Suzuki coupling of 54 with the appropriate arylboronic acid. In the sequence, compounds 2-aryl-substituted 56a–c were obtained by an acid-induced 3,2-aryl shift and deprotection, while 56d–f were prepared after deprotection of the hydroxy groups (Scheme 23).
Scheme 23.
Synthesis of resveratrol-derivative benzoselenophenes 56.
These resveratrol-inspired benzo[b]selenophenes were tested for their capacity to modulate oxidant levels. Compound 56e displayed the most potent antioxidant activity (12.3% of oxidant levels at the high concentration of 5 mM). The 3-aryl derivative, 56d, was more effective than the 2-aryl species 56a in reducing intracellular oxidant levels at equimolar concentrations. Compound 56b exhibited higher antioxidant activity than 56a, probably due to the presence of the additional 3’-OH group. Compound 56c was less effective, and compound 56f increased oxidant levels when compared to the controls; the reasons for this are unclear.
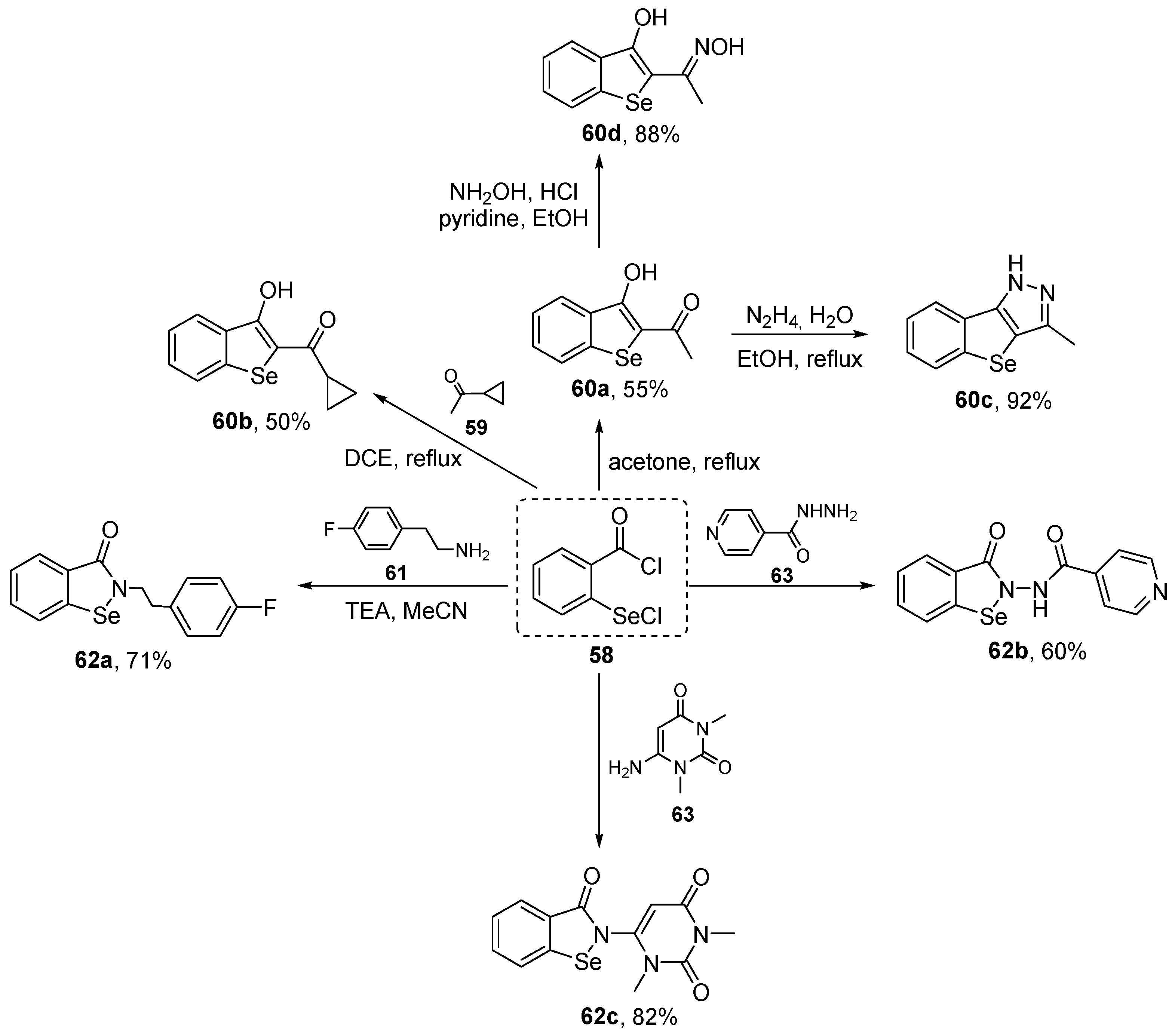
Hamama and co-workers have prepared a series of benzo[b]selenophene 60 and Ebselen analogues 62 starting from the versatile intermediate 2-(chloroseleno)benzoyl chloride 58 [83], and evaluated their activity in vitro using ABTS. A total of four benzo[b]selenophenes 60 and three Ebselen derivatives 62 were prepared in modest to very good yields by the reaction of 58 with ketones and amines, respectively (Scheme 24). The results obtained from the ABTS showed that 60d exhibited the greatest activity, with 89% inhibition at a concentration of 2 mM. Compounds 60d, 60b, and 62b at the same concentration showed antioxidant activities similar to that of ascorbic acid, used as a positive control.
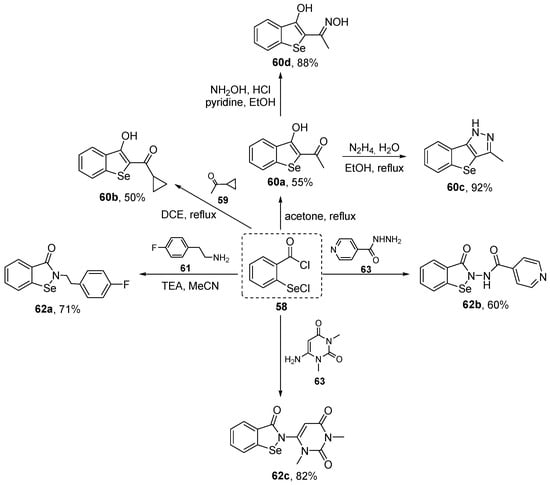
Scheme 24.
Synthesis of benzo[b]selenophenes 60 and Ebselen analogues 62.
Perin and co-workers have developed a new general method to prepare 2-organylselenopheno[2,3-b]pyridines 65 by the insertion of nucleophilic selenium species 64 into 2-chloro-3-(organylethynyl)pyridines 64 [84]. The selenide anion NaHSe was generated in situ from elemental Se and NaBH4/PEG-400 as reducing system, and the nucleophilic attack was followed by an intramolecular cyclization to give the expected selenophene 65. A total of six selenophene-fused to pyridine ring were prepared in 31–77% yield after 2.5 h of reaction at 50–100 °C (Scheme 25). The antioxidant activity of compounds 65 was evaluated in different in vitro assays of thiols, DPPH and ABTS, and SOD-like activity. Selenophene 65 could reduce hepatic production of oxidants induced by sodium azide in mice at the high concentration of 200 μM. However, 65 did not exhibit significant scavenging activity against DPPH and ABTS•+ radicals at any of the tested concentrations, nor demonstrated any SOD-like activity. These results suggest that 60 may have in vivo effects that cannot be modeled by in vitro assays and may not be due to traditional radical scavenging effects.
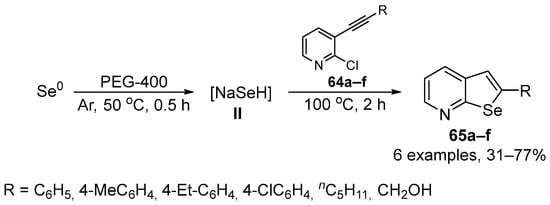
Scheme 25.
Synthesis of 2-organylselenopheno[2,3-b]pyridines 65.
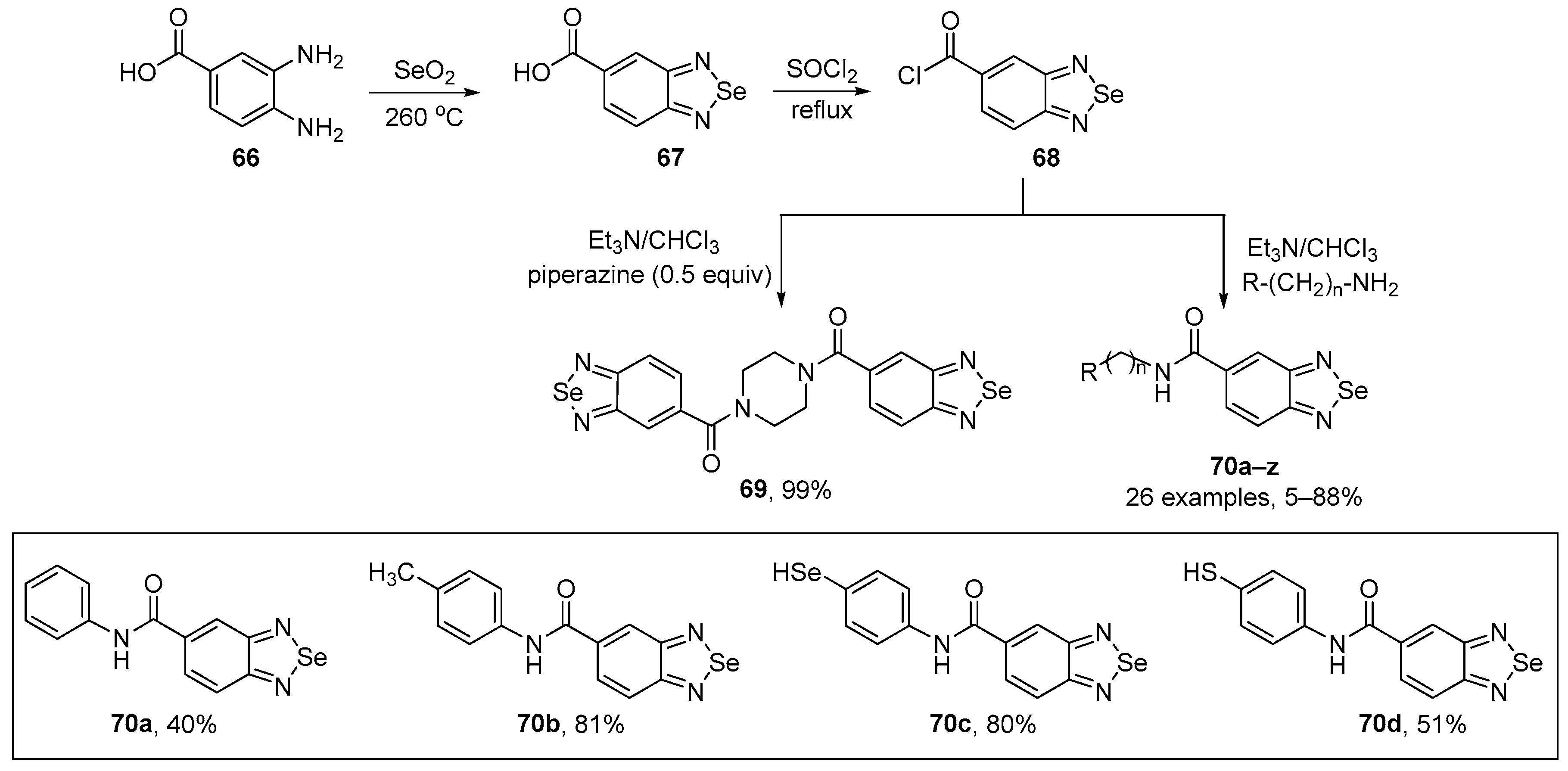
Sanmmartín and co-workers have developed a general method to prepare the benzo[c][1,2,5]selenadiazole-5-amide derivatives 69 and 70, starting from benzo[c][1,2,5]selenadiazole-5-carboxylic acid 67 (BSCA) [85]. The key intermediate 67 was prepared from 3,4-diaminobenzoic acid 66 and selenium dioxide under heating, as previously described by the authors [86]. A total of twenty-seven compounds were prepared from the reaction of acyl chloride 68 and different amines in 5–99% yield (Scheme 26). The new selenadiazoles 70a–d were tested for their radical scavenging activity using DPPH. Compound 70d was the most effective with values exceeding 40% at a concentration of 750 µM. The scavenging capacity was concentration-dependent (0.082 to 828 µM) but not time-dependent over the time frame 30–180 min. Compounds 70a–c also demonstrated potent DPPH radical scavenging activity, with compound 70c displaying the highest activity, followed by 70b and 70a.
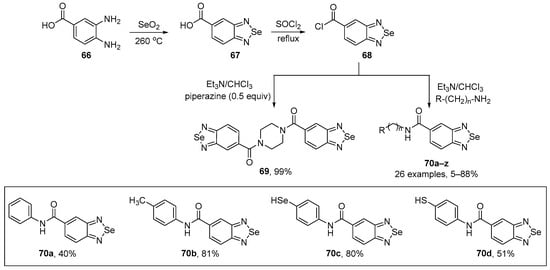
Scheme 26.
Synthesis of benzo[c][1,2,5]selenadiazole derivatives 69 and 70.
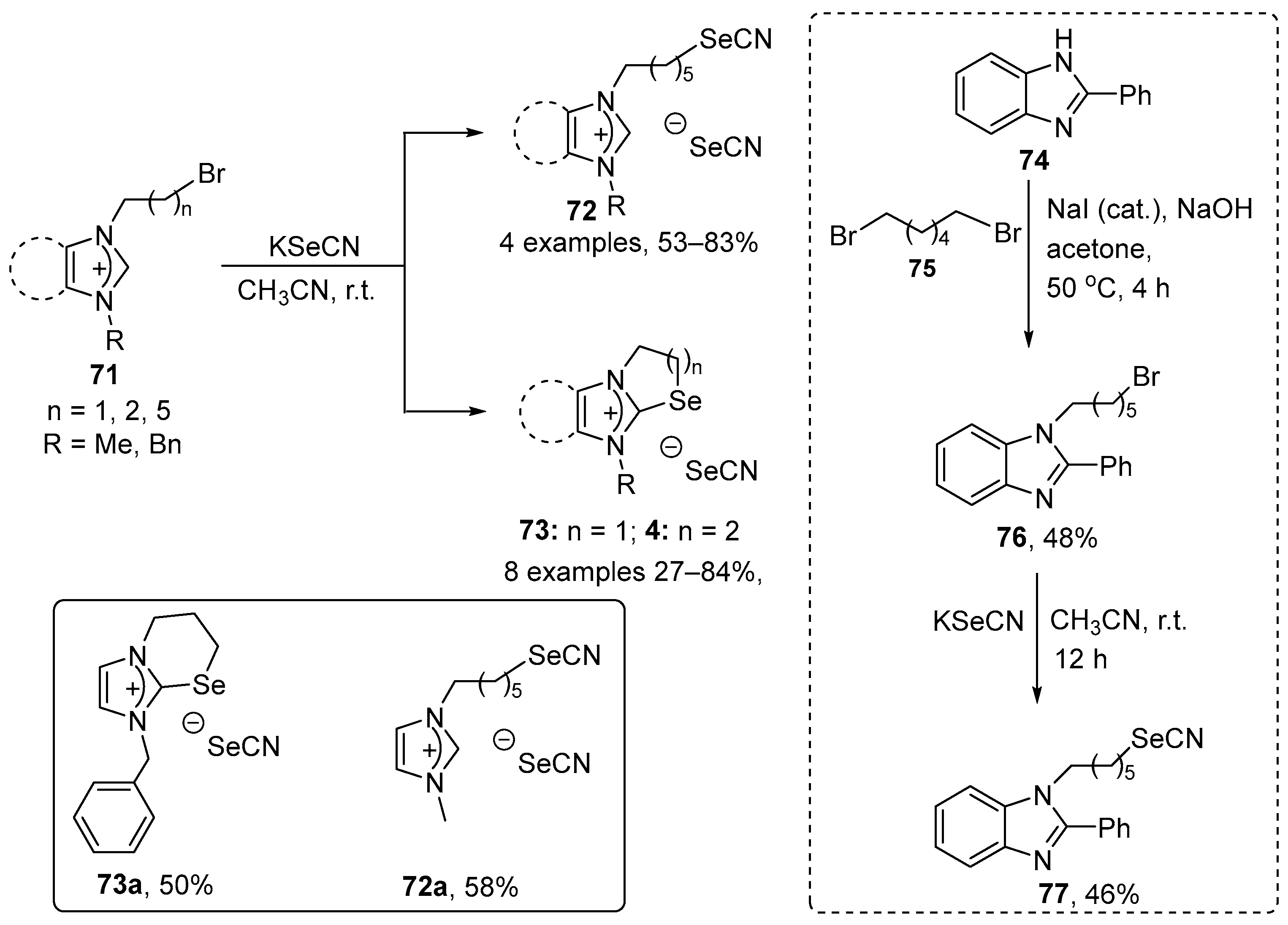
Bhabak and co-workers have described the synthesis of new benzimidazole- and imidazole-fused selenazolium and selenazinium selenocyanates (72, 73, and 77) [87]. Selenazolium and selenazinium selenocyanates 73 were prepared in 27–84% yield through the cyclization of N,N′-disubstituted benzimidazolium and imidazolium bromides 71 containing N-(CH2)2-Br and N-(CH2)3-Br groups in the presence of KSeCN. In contrast, N,N′-disubstituted benzimidazolium and imidazolium bromides 71 substituted with N-(CH2)6-Br afforded the open-chain selenocyanates 72 in 53–83% yield. When bromide 76, derived from 2-phenyl-1H-benzo[d]imidazole 76, was used as stating heterocycle, selenocyanate 77 was obtained in 46% yield after reaction with KSeCN (Scheme 27). The activity of these compounds was evaluated using a GSH–GSSG coupled assay (GPx-like) and a related PhSH–PhSSPh assay. The two compounds were also tested for their capacity to modulate the intracellular level of reactive species in the macrophage-like cell line, RAW 264.7. Most of the compounds displayed significant activity in GPx-like assays. In the GSH–GSSH coupled assay, the reduction of H2O2, cumene hydroperoxide (Cum-OOH), and tert-butyl hydroperoxide (TBHP) were examined. All the compounds reduced H2O2 with higher efficacy than Cum-OOH and TBHP, especially compound 73d. In the PhSH–PhSSPh assay, all the ionic organoselenium compounds exhibited significantly higher activity than the control, with compound 73a being the best in class. The cyclic selenazolium and selenazinium compounds displayed superior activities to their acyclic analogues. The observed activities of the ionic selenocyanates were determined to be the result of the combined effect of both selenium centers, with a higher contribution from the selenocyanate counter anion. Compounds 73d, which exhibited the best activity in the GSH–GSSH assay, and 72e, were also examined for their capacity to remove endogenous H2O2 in the RAW 264.7 cells. The data obtained indicate that the antioxidant activity of compound 73d is dose-dependent and higher than that of 72e.
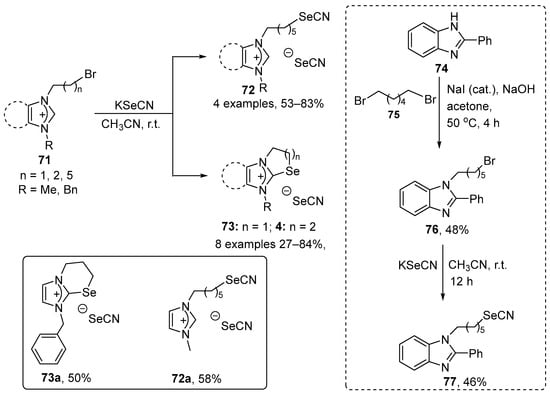
Scheme 27.
Synthesis of imidazole-fused selenazolium and selenazinium selenocyanates.
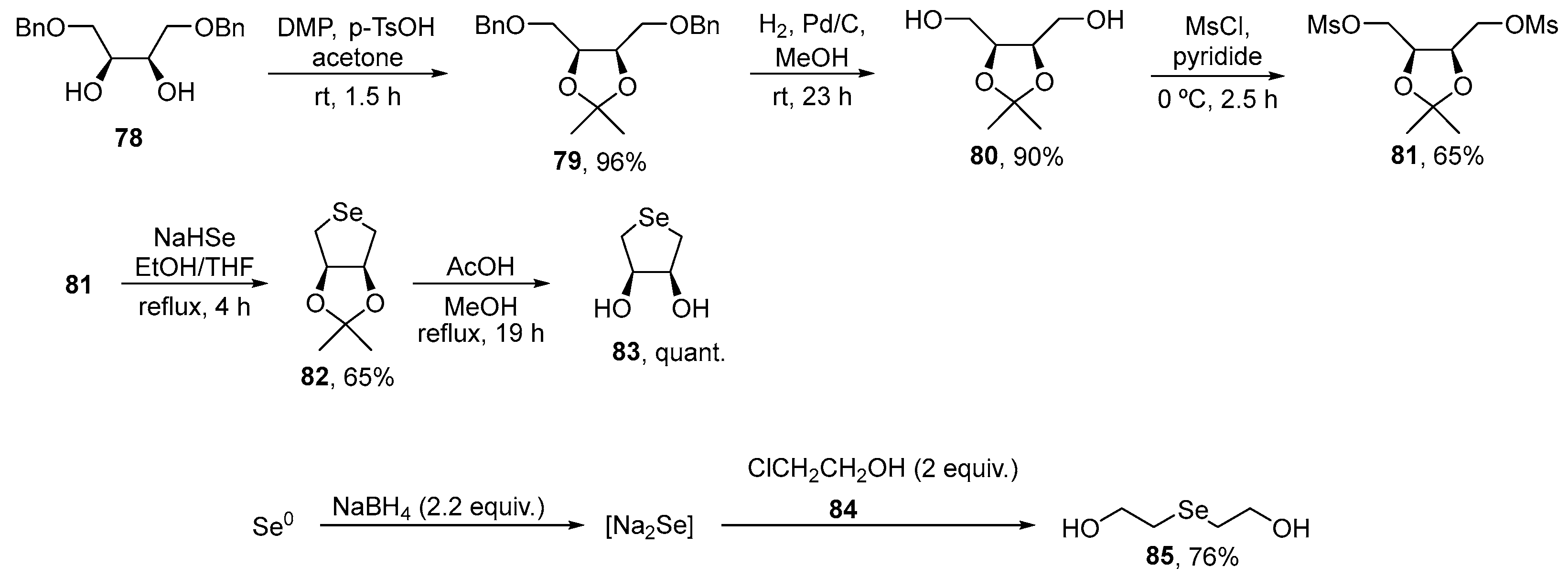
To examine the role of structural changes in the antioxidant activity of organoselenium compounds, Singh, Priyadarsini, and co-workers prepared cyclic DL-trans-3,4-dihydroxy-1-selenide 83 and bis(ethan-2-ol)selenide 85 [88]. Both compounds were prepared via previously described procedures. Synthesis of selenolane 83 was achieved starting from dibenzyl ether 78, derived from cis-2-butene-1,4-diol, that was converted to the ketal 81. After reaction with NaHSe, generated in situ, and deprotection, the cyclic selenide 83 was obtained in 65% yield [89]. The open-chain diol 85, in turn, was prepared in 76% yield by the reaction of chloroethanol 84 with Na2Se, which was obtained in situ from elemental selenium and NaBH4 [90] (Scheme 28). The compounds were assayed for their ability to scavenge peroxynitrite (ONOO−/ONOOH), hydroxyl (HO●), nitrogen dioxide (NO2●), and carbonate (CO3●−) radicals.
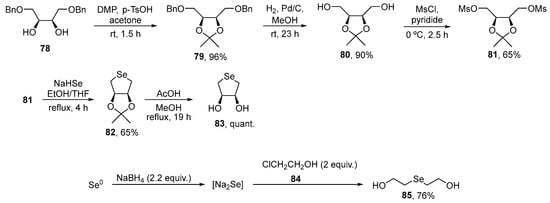
Scheme 28.
Synthesis of di-hydroxy selenides 83 and 85.
Compound 83 showed a higher rate constant for reaction with peroxynitrite as compared to the open-chained compound 85. With CO3●−, the kinetic studies demonstrated a rate constant of 1.2 ± 0.2 × 109 M−1s−1 for compound 83 and 6.5 ± 0.3 × 108 M−1s−1 for 85. No reaction was observed for 83 and 85 with NO2●, while the rate constants for reaction with HO● were comparable. The results indicated that cyclic 83 exhibits higher oxidant reactivity than the linear isomer 85, particularly with peroxynitrite, with a rate constant that was twice as high. These data are consistent with other kinetic data for a range of sulfur and selenium compounds [48] and for enhanced reactivity of 5-membered cyclic species (when compared to 6-membered and acyclic / linear species) [91,92]. The enhanced rates of reaction for the 5-membered ring structures may arise from stabilization of the reaction intermediate formed at the electron-deficient sulfur or selenium center by suitably placed lone pairs of electrons.
These cyclic selenides are related to a family of seleno-substituted sugars that have been previously reported and subsequently tested for biological activity [93]. A range of 5-, 6-, and 7- membered sugar rings have been synthesized with the 5-membered rings containing a Se atom, being kinetically the most favorable synthetic product. All of these species show high reactivity with a wide range of oxidants [49,51,94,95] including H2O2 alkyl peroxides, hypochlorous acid (HOCl), peroxynitrite, and 1O2. These reactions generate the corresponding selenoxide in stoichiometric reactions, with no evidence for ring opening or other products. The selenoxides have been shown to be readily re-reduced by both enzymes (e.g., glutathione reductase at the expense of NADPH), and reductants such as GSH, resulting in regeneration of the selenosugar [96]. As a consequence of this (rapid and efficient) recycling, only low concentrations of these species may be required for efficient oxidant removal in vivo. Testing of these compounds in a range of biological assays including cells, isolated aortic rings, and animal models of inflammation have yielded positive data, and these species are currently under development as skin healing agents [93,97,98,99,100] (e.g., against atopic dermatitis) amongst other pathologies.
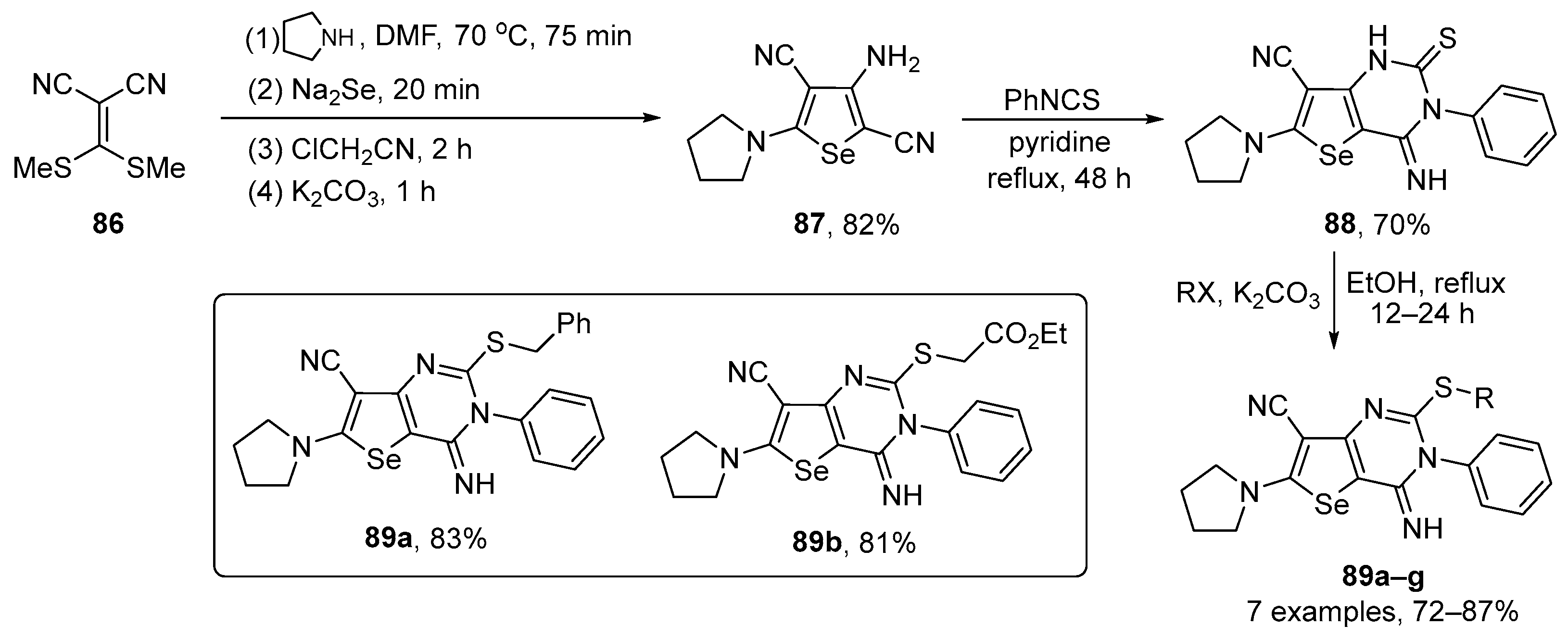
Shiri and co-workers have prepared several pyrimidine-fused dihydroselenophenes 89 and tested their activities in the DPPH assay [101]. The key intermediate in the synthesis is the pyrrolidine-substituted selenophene 87, which was prepared in 82% yield from 2-(bis(ethylthio)methylene)malononitrile 86 after four sequential reactions. Once prepared, 87 was reacted with phenyl isothiocyanate in the presence of pyridine to give the pyrimidine-fused selenophene 88 in 70% yield. After reaction with several alkyl halides, seven S-functionalized products 89 were obtained in 72–87% yield (Scheme 29). Among the tested compounds, the highest antioxidant activity was observed for compound 87 (IC50 ~12 µM), which was much more effective than ascorbic acid, the positive control (IC50 ~22 µM). Activity was also detected for the S-substituted selenpheno[3,2-d]pyrimidines 89a–g, with IC50 values in the range 37–54 µM. The order of radical stability is 87 > 89a–g > 88 and this order is probably due to the presence of –NH2, –SCH2–, and –NHC=S groups acting as hydrogen atom donors, respectively. In the case of 89, the antioxidant activity is more significant in selenopheno[3,2-d] pyrimidines 89a–b (IC50 ~37 and ~39 µM), probably due to the presence of adjacent radical stabilizing groups, including Ph and CO2Et.
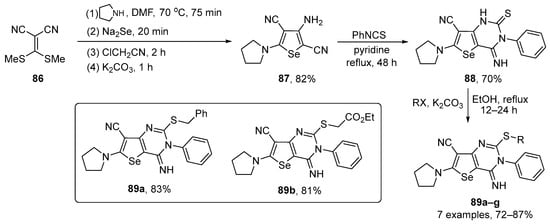
Scheme 29.
Synthesis of pyrimidine-fused selenophenes 89.
3.5. Selenides
α-(Phenylselanyl)acetophenone (91) was evaluated by Sousa et al. [102] for its capacity to modulate oxidative stress induced by acute stress restriction (ARS) in mice. The target molecule was synthesized in 96% yield, as described previously [103], starting from acetophenone 90 and diphenyl diselenide 4c in the presence of the catalytic system KF/Al2O3 and PEG-400 as solvent under N2 atmosphere for 21 h (Scheme 30). Animals were treated with compound 91 at a dose of 10 mg/kg for 10 min after the ARS. Then, after 4.5 h, they were evaluated for the levels of lipid peroxidation in the cortex and hippocampus using the TBARS assay. Treatment with 91 decreased MDA levels, as well reactive species production, and the nitrite and nitrate (NOx) levels induced by ARS.
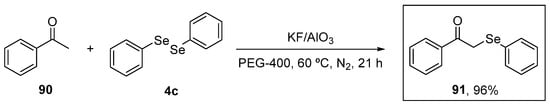
Scheme 30.
Synthetic route to the synthesis of α-(phenylselanyl)acetophenone 91.
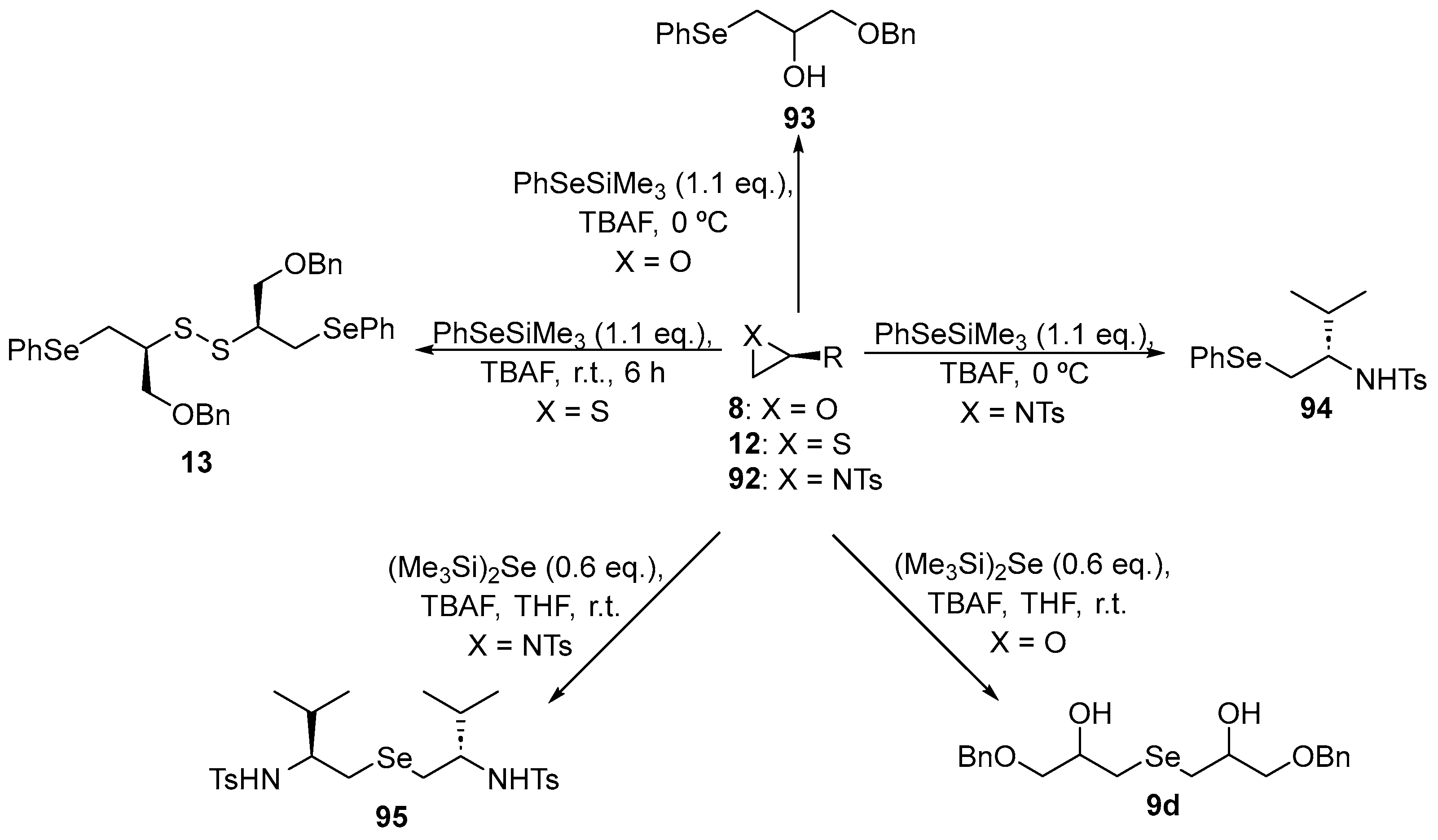
In order to evaluate the thiol peroxidase-like properties of β-functionalized symmetric and non-symmetric organochalcogenides, Capperucci and co-workers [104] prepared a series of new Se-containing compounds. The β-functionalized selenium compounds were synthesized through the ring-opening of oxiranes, aziridines or thiiranes using trimethylsilyl selenides as nucleophiles (Scheme 31). Catalytic activity was examined in the dithiothreitol (DTT) oxidation model. All the compounds exhibited an equal thiol-peroxidase-like activity of 10.0 mol%. Compound 93 gave the shortest time to reduce the initial thiol concentration by 50% (T50 value) after the addition of H2O2, with a value of ~328 s. The β-hydroxy substituted derivatives 9d and 93 exhibited superior catalytic properties compared to the β-amino substituted analogs 94 and 95. Therefore, the substituent at the C-2 position appears to play a crucial role in determining the catalytic properties.
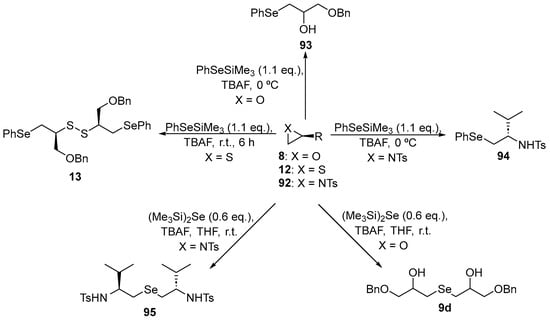
Scheme 31.
Synthesis of β-functionalized symmetric and non-symmetric selenides.
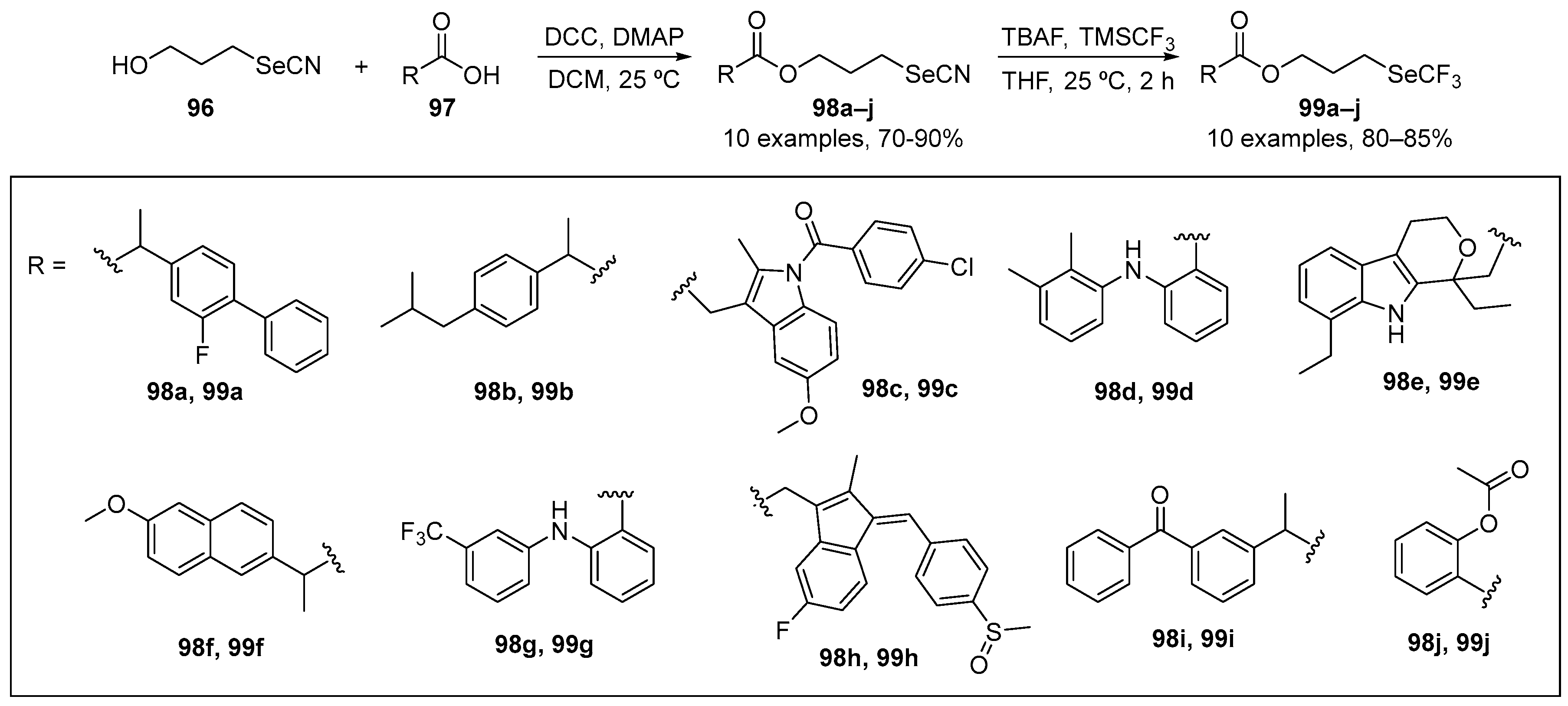
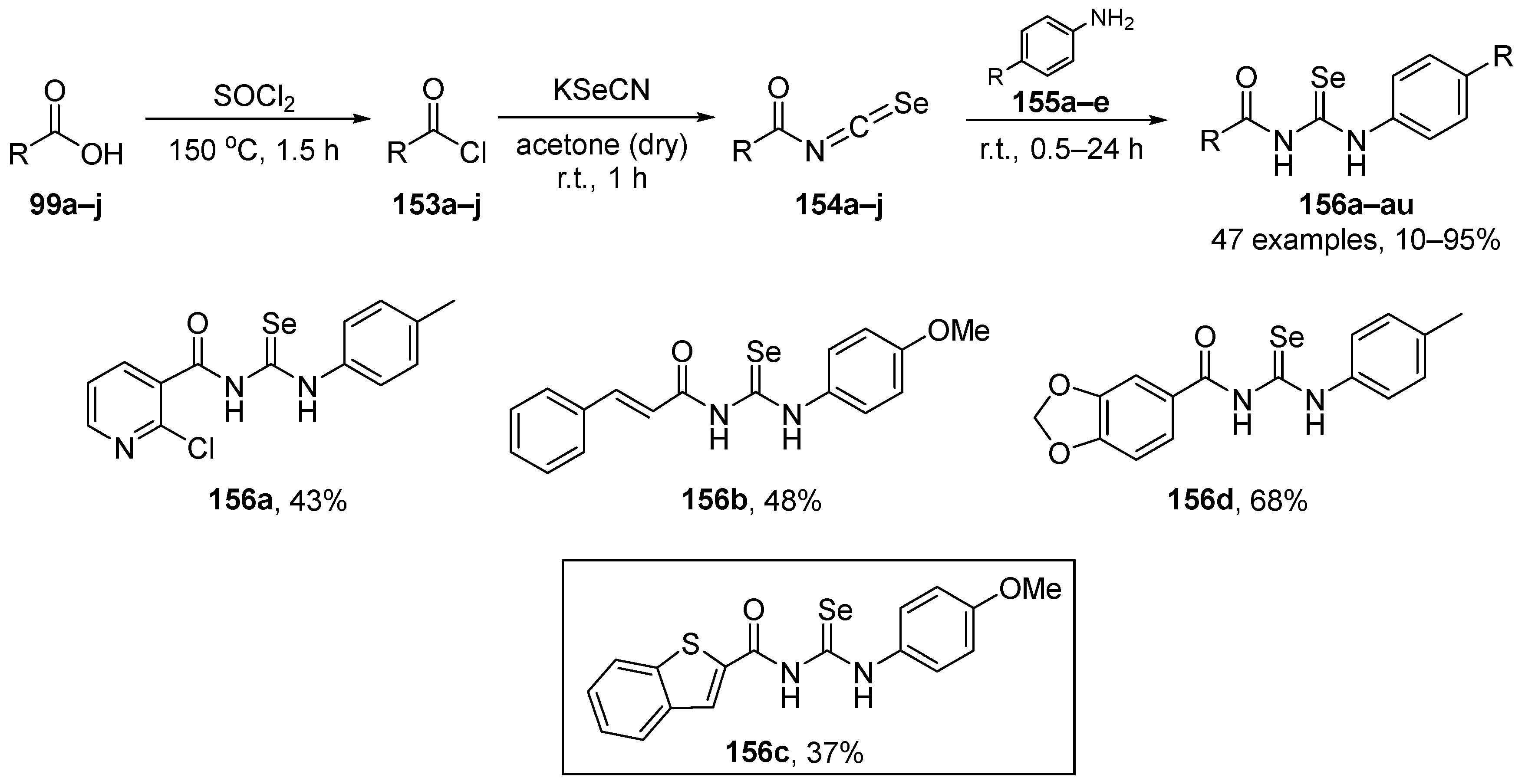
Zhang and co-workers [105] have reported the synthesis of compounds 98a–j and 99a–j based on the hybridization of nonsteroidal anti-inflammatory drugs (NSAIDs) skeleton and an organoselenium motif (-SeCN and -SeCF3). The selenocyanate derivatives 99a–j were obtained by reacting 3-selenocyanatopropan-1-ol with commercially available NSAIDs in the presence of N,N′-dicyclohexylcarbodiimide and 4-dimethylaminopyridine as condensation agents. The trifluoromethyl selenide derivatives were obtained by reacting the corresponding selenocyanate derivatives with trimethyl(trifluoromethyl)silane (TMSCF3) in the presence of TBAF as catalyst to afford 99a–j in yields up to 80% (Scheme 32). The DPPH and GPx-mimetic assays were used to examine the activity of these species. In the DPPH test, compounds 99h and 99i (NSAIDs-SeCF3 derivatives) were the most active, demonstrating ~45 and ~66% inhibition, respectively. The family of NSAIDs-SeCF3 derivatives 99 were more effective than the corresponding NSAIDs-SeCN derivatives 98 (cf. ~17% inhibition for 98a, versus ~73% for 99a). In the GPx-like activity assays, compounds 98h, 98i, 99b, 99e, 99h, and 99i were more active than the other derivatives, with 99h being the most active mimetic.
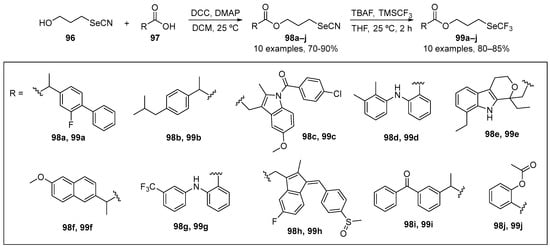
Scheme 32.
Synthesis of SeCN and SeCF3 derivatives.
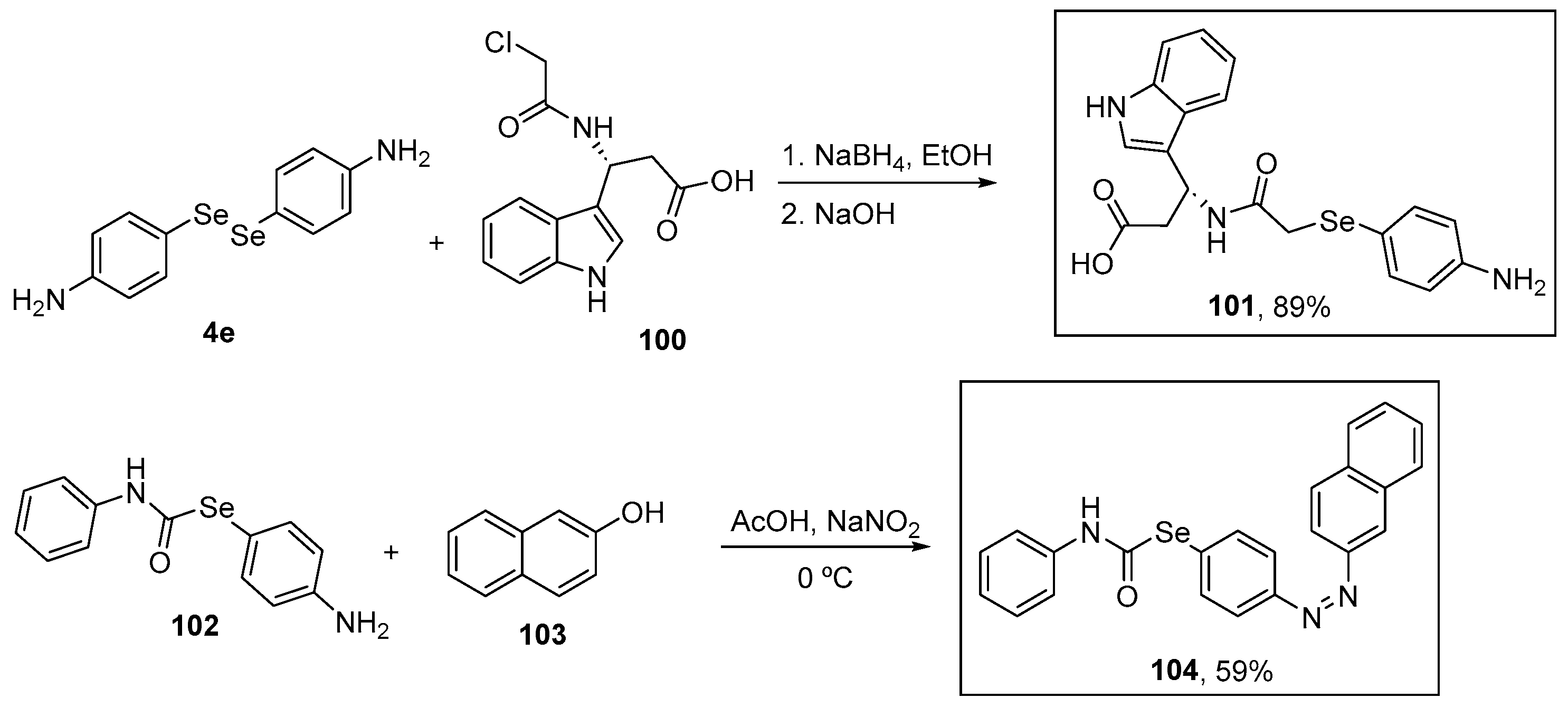
Shaaban and co-workers [106] synthesized several organoselenium compounds functionalized with amide groups in yields of up to 91%. Compound 101 was prepared in 89% yield from the corresponding 4-amino-substituted diselenide 4e after reduction under basic conditions (NaBH4/NaOH) of the Se–Se bond. The reaction of the intermediate selenolate with alkyl chloride 100 proceeded smoothly at room temperature in only 30 min. A sequential diazotization of 2-((4-aminophenyl)selanyl)-N-phenylacetamide 102 and the subsequent coupling with β-naphthol 103 afforded the carbamoselenoate 104 in 59% yield (Scheme 33). Among these OSe compounds, 101 and 104 showed the greatest activity in the DPPH and ABTS assays at (a very high concentration of) 1 mM. In the DPPH assay, compounds 104 and 101 exhibited 93% and 91% inhibition and similar results were found in the ABTS assay with 88% and 85% inhibition, respectively. Compounds 101 and 104 gave IC50 values in the DPPH (~24 and ~20 μM, respectively) and ABTS (~32 and ~29 μM, respectively) assay similar to that of ascorbic acid the positive control (DPPH: 19 μM; ABTS: 29 μM).
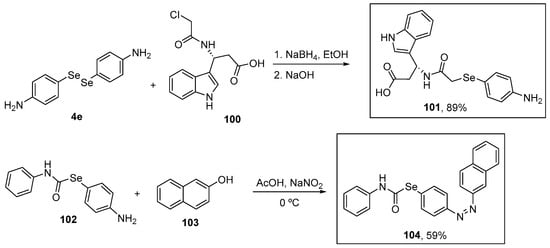
Scheme 33.
Synthesis of selenide-based azo compounds 101 and 104.
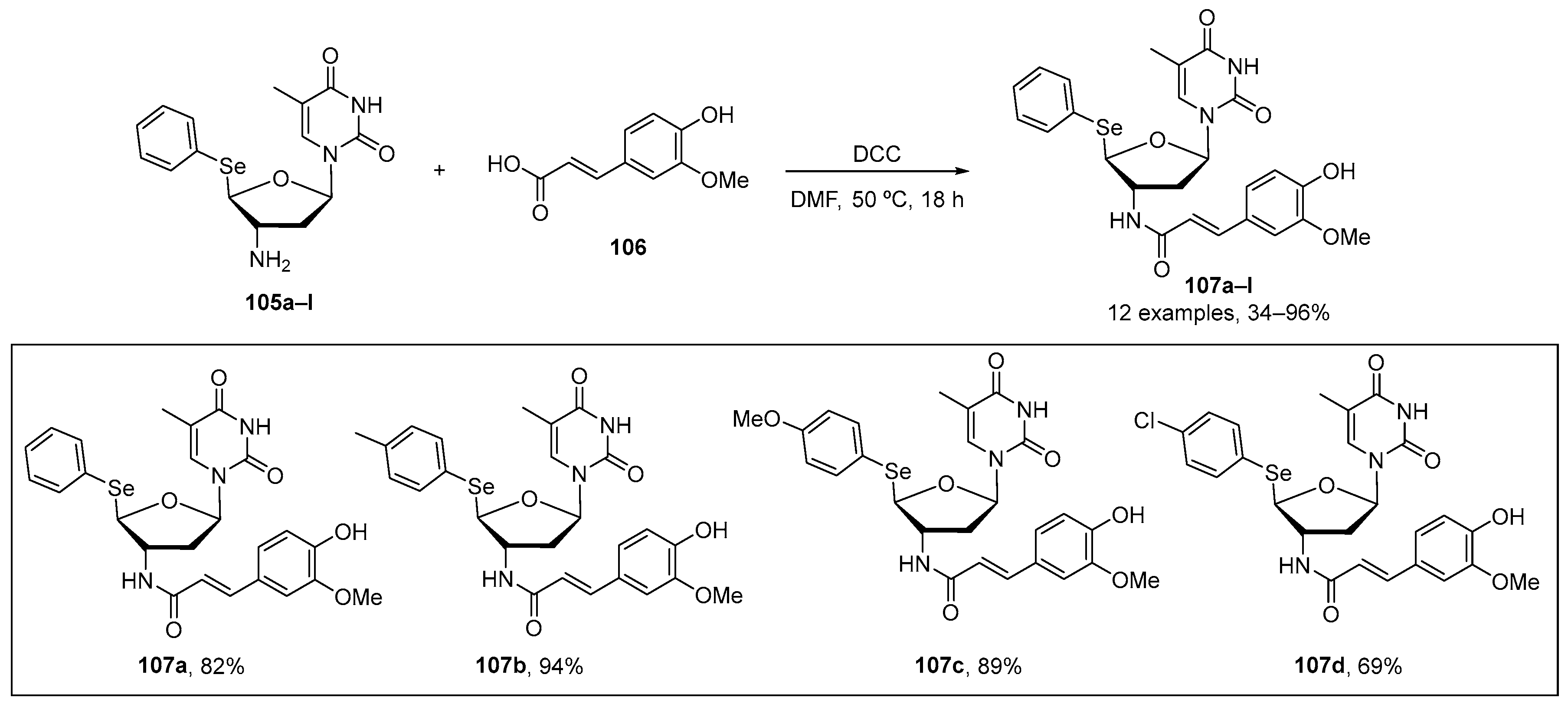
Leal et al. [107] have recently reported the synthesis, antioxidant, and antitumoral activity of new 5′-arylchalcogenyl-3′-N-(E)-feruloyl-3′,5′-dideoxy-amino-thymidine (AFAT) derivatives. 107a–l were prepared in from good to excellent yields through the reaction of 5′-arylchalcogeno-3′-amino-3′-deoxythymidine (ACAT) 105 with cinnamic acid 106 in the presence of N,N′-dicyclohexylcarbodiimide (DCC) as coupling agent in dimethylformamide for 18 h at 50 °C (Scheme 34). DPPH and lipid peroxidation (TBARS) assays were used to evaluate antioxidant activity. In the DPPH test, the AFAT derivatives were evaluated at five different concentrations (0.01–1 mM), and all compounds were able to scavenger this radical at the highest concentration. Compounds 107a–d exhibited higher antioxidant activity than their synthetic precursors (105a–d), particularly compound 107a and its respective precursor. In the TBARS test, compounds 107a, 107b, and 107d showed significant inhibition, with 107a having a six-fold higher effect than 105a. Therefore, fusion of the nucleoside moiety from azidothymidine (AZT) with ferulic acid appears to enhance the antioxidant effects of compounds 105a–d in the TBARS assay.
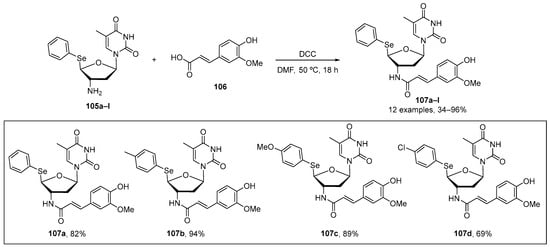
Scheme 34.
Synthesis of selenium-containing amino-thymide derivatives 107.
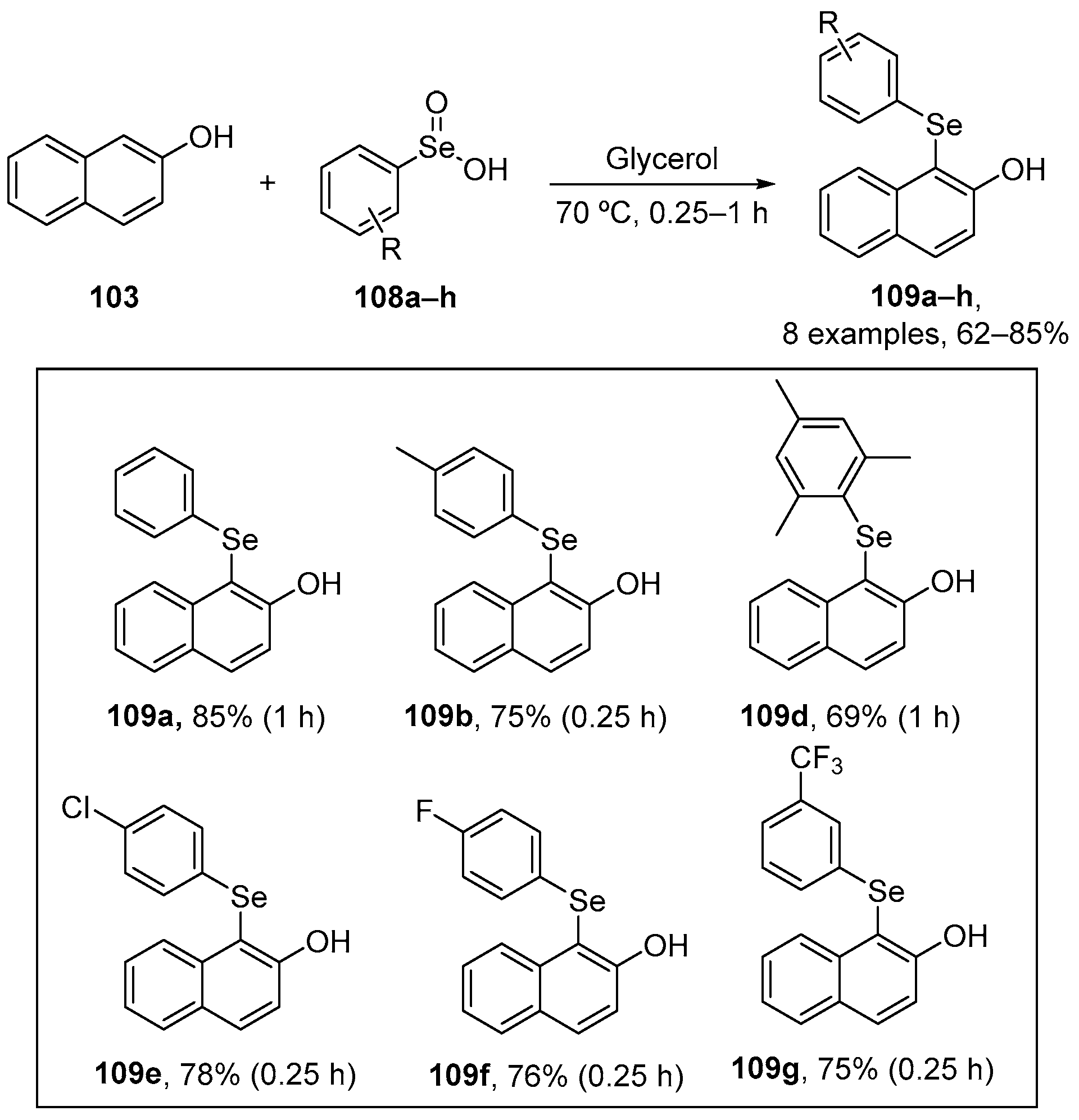
In order to develop greener approaches to organoselenium derivatives, Perin and co-workers designed a new protocol to prepare 1-organoselanyl-naphthalen-2-ol 109 through the functionalization of 2-naphthol 103 with arylseleninic acids 108 using glycerol as solvent. The products were obtained in from moderate to good yields (62–85%) [108] (Scheme 35). Antioxidant assays were performed, such as reactive species levels which were measured using DCFH-DA, and DPPH and ABTS to evaluate radical scavenging activity.
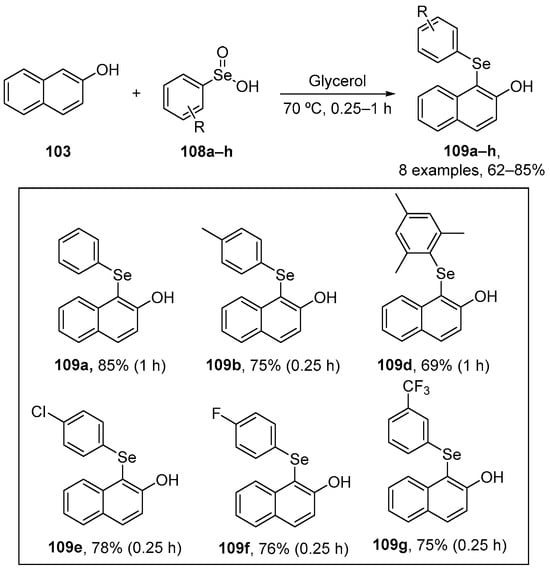
Scheme 35.
Benzeneseleninic acid in the selenofunctionalization of 2-naphthol derivatives.
Compounds 109a, 109d, 109e, and 109g were effective in reducing the reactive species production in the liver of mice. Of these, 109d, 109e, and 109g exhibited the best activity, which can be attributed to the mesitylselanyl, p-chlorophenylselanyl, and [3-(trifluoromethyl)phenyl]selanyl substituents, respectively. Compound 109d was effective in the DPPH assay even at 10 μM. Moreover, this compound displayed the highest Imax value (~98) and the lowest IC50 value (~44 μM), indicating strong antioxidant activity. Compounds 109a, 109b, 109e, and 109f also scavenged the DPPH radical over the concentration range 100–500 μM. In contrast, compounds 109a, 109b, 109d, 109e, 109f, and 109g demonstrated significant activity against ABTS•+ at ≥1 μM. All compounds exhibited high Imax values (~95), comparable to the positive control (99.5), and their IC50 values were < 5 μM, indicating potent activity. These IC50 values were lower than that of ascorbic acid (9.7 μM).
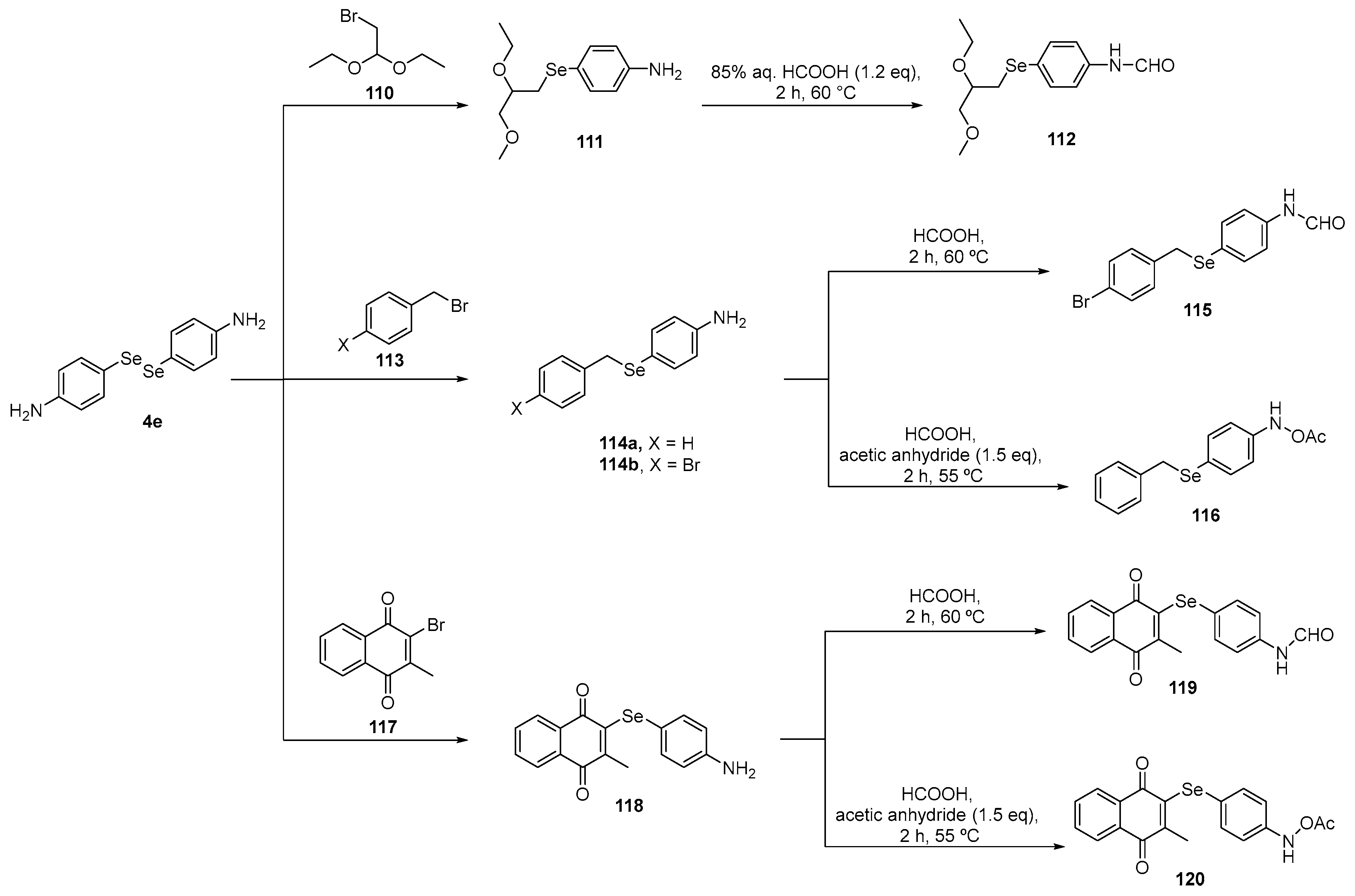
In a study conducted by Shaaban and colleagues [109], the activity of functionalized benzylselenides and naphthalene 1,4-dione derivatives 114–116, 119, and 120 was investigated using DPPH, ABTS, reactive species, and GPx-like assays in cultured oligodendrocytes (158N) cells. These compounds were prepared using a starting material 4-amino-substituted diselenide 4c after a reduction followed by capture of the selenolate intermediate with the respective bromide and reaction with formic acid (Scheme 36). In the same work, the benzylselenides 111 and 114a were reacted with furan-2,5-dione or dihydrofuran-2,5-dione to prepare, after two steps, the N-substituted diselenides 124, 125, 128, and 129 (Scheme 37). In the 158N cell line, most of the compounds reduced reactive species levels, with the rank order in terms of ascending effectiveness at a concentration of 10 µM being 120, 125a, 123b, 127b, 115, 114b, 114a, 123a, and 112.
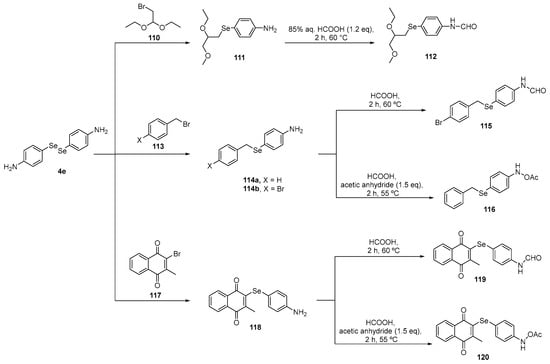
Scheme 36.
Synthesis of functionalized selenides 114, 115, 116, 119, and 120.
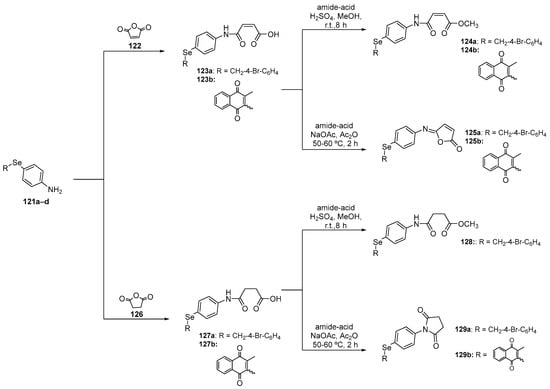
Scheme 37.
Synthesis of functionalized selenides 124, 125, 128, and 129.
In contrast to the data obtained for compounds 114, 115, 116, 119, and 120 (at 10 and 20 μM), selenides 124a, 128, and 129a, as well as the quinone-based organoselenium compounds 124b, 125b, and 129b, caused an increase in intracellular reactive species at all the tested concentrations (10, 20, and 50 μM). Compounds 123b and 124b, which are quinoid-based N-substituted maleanilic acid and its corresponding methyl esters, exhibited significant GPx-like activities at a concentration of ~40 μM. These compounds were 1.5-fold more active than Ebselen. The other compounds showed moderate GPx-like activity, especially 119d, 117a, 128, and 125b. Most of the compounds showed good to moderate activity in the DPPH and ABTS assays, except 119d, 120a, 120b, 123b, 127b, 124b, 125b, and 129b, which demonstrated pro-oxidant effects.
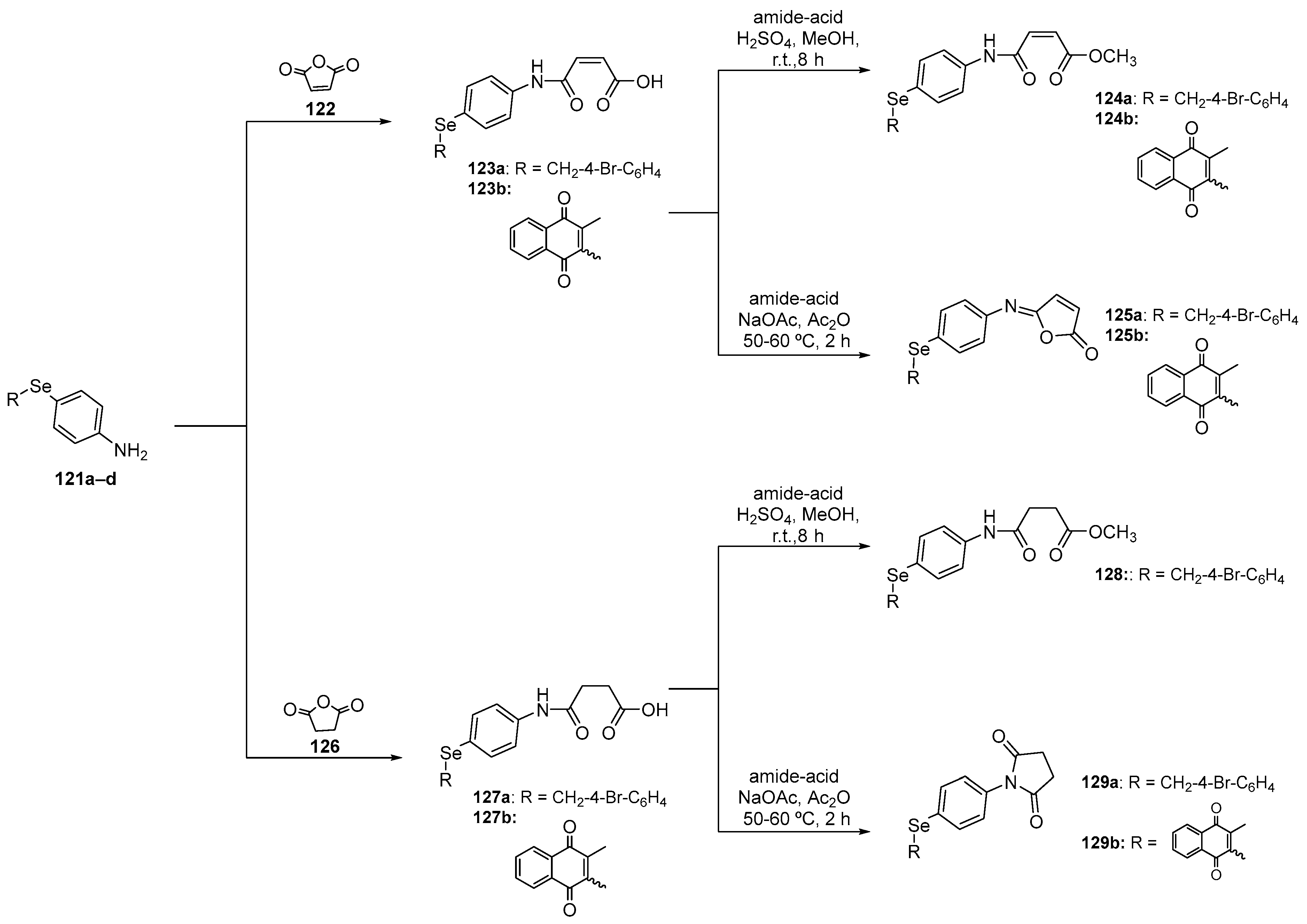
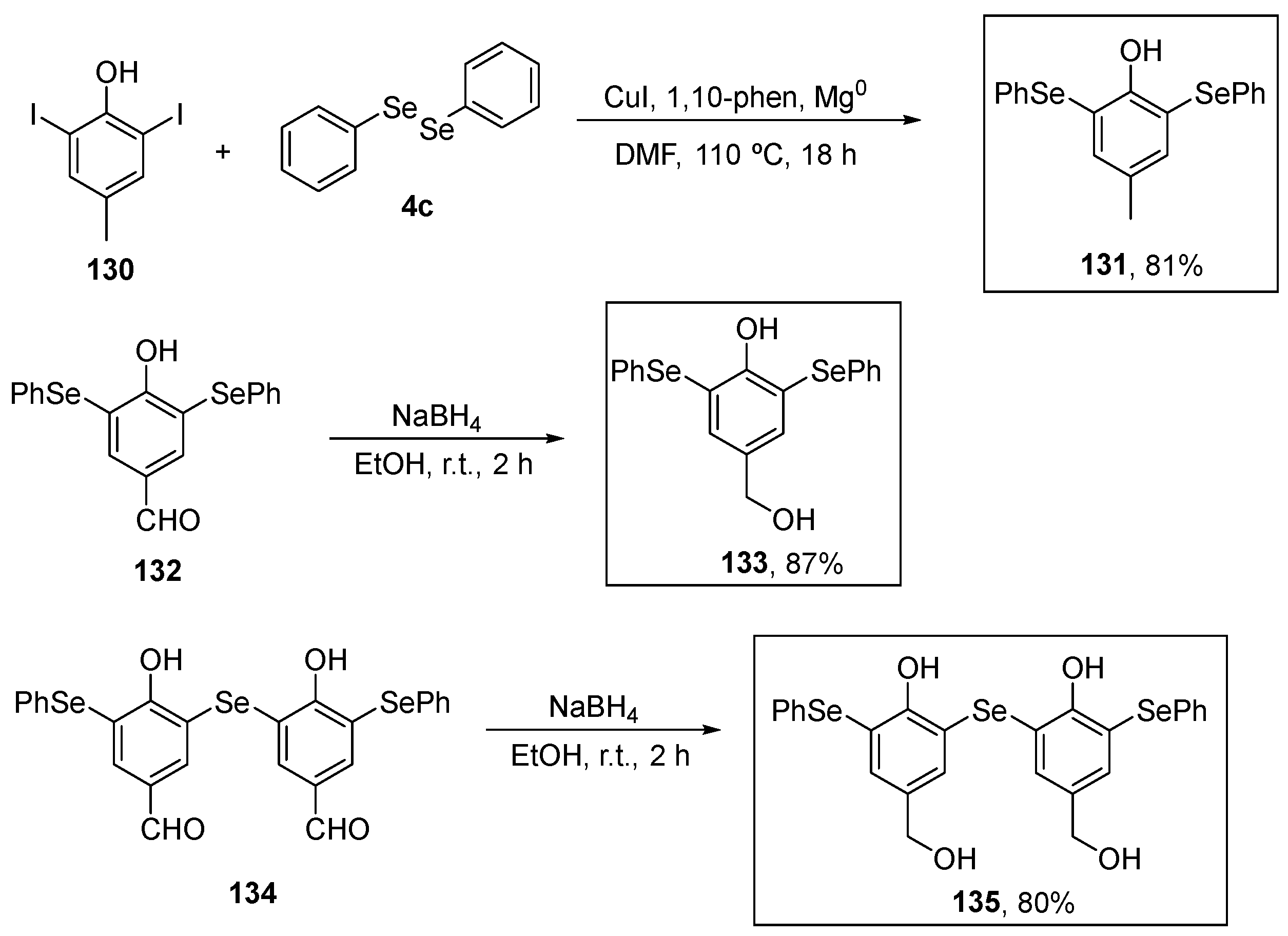
Upadhyay and co-workers (2021) [8] investigated the antioxidant activity of bis- and tris-selenol-bisphenols using the DPPH assay. The target compound 131 was prepared in 81% yield by a new copper(I)-catalyzed reaction of 2,6-diiodophenol in the presence of Mg0 in DMF with diphenyl diselenide. Compounds 133 and 135, in turn, were prepared in 87% and 80% yield by the reduction of the respective aldehydes 132 and 134 (Scheme 38). In the DPPH test, selenophenols 131, 133, and 135 exhibited significant antioxidant activity at a concentration of 6.4 μM, surpassing that of the positive control (vitamin E; 6.4 μM). These compounds displayed maximum rate constants of 0.71 ± 0.269 min−1, 0.78 ± 0.08 min−1, 1.3 ± 0.06 min−1, and 1.2 ± 0.13 min−1, respectively. In the thiol peroxidase assay, the biselenophenols and tris-selenol-bisphenols 133 and 135 demonstrated reduction rates for H2O2 of 4.6 ± 0.62 and 13.7 ± 1.17 μM min−1, respectively. Interestingly, although 133 and 135 exhibited a lower rate of H2O2 decomposition, they were able to decompose H2O2 over significantly longer incubation periods. Lastly, compounds 133 and 135, along with vitamin E, were assessed for their 1O2 quenching activity. The tris- selenophenol 135 exhibited a modest reduction in 1O2 levels, similar to that observed for vitamin E. Therefore, the presence of intramolecular Se….O interactions involving the phenolic OH group appears to enhance the oxidant removal activity of these compounds (Scheme 38).
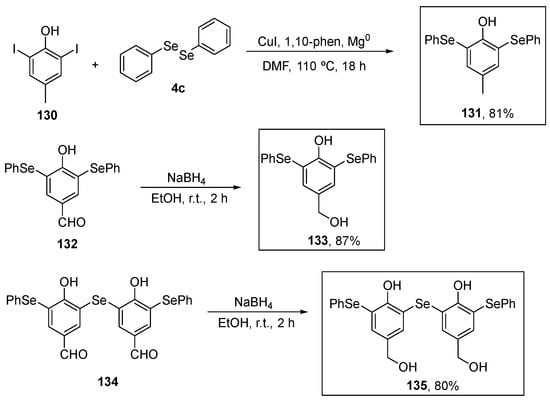
Scheme 38.
Synthesis of phenol-substituted selenides 131, 133, and 135.
3.6. Miscellaneous
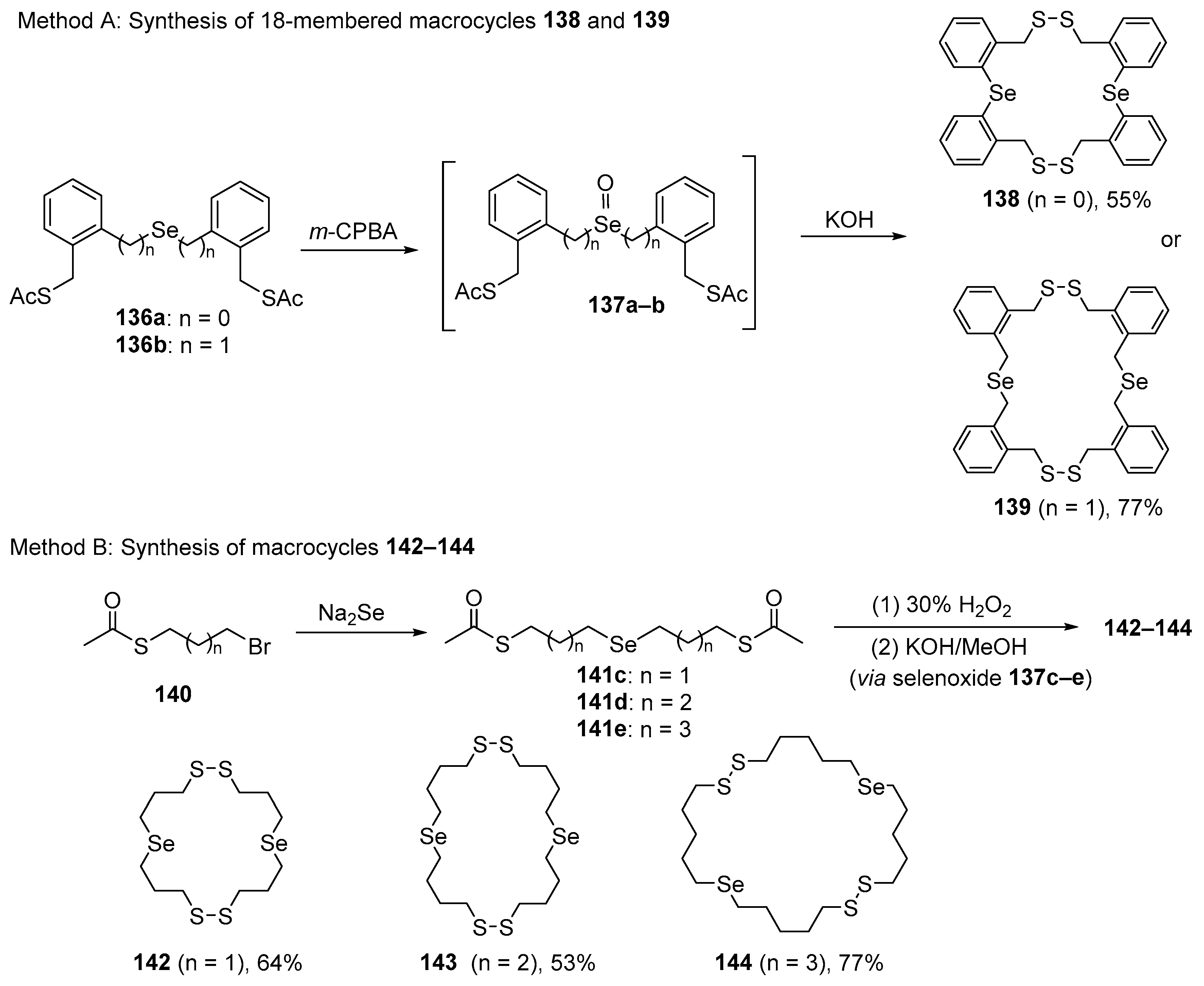
A new class of dimeric macrocycles of 18–26 members with potent GPx-like activity in vitro has been reported by Back and co-workers, while they were trying to prepare spirodithiaselenuranes [110]. The unexpected macrocycles 138–144 were obtained through the base-promoted oxidative cyclization of selenoxide thioacetates 136a–e, which were generated in situ from the respective selenides 136 (Scheme 39). The novel organochalcogen-embedded macrocycles containing two selenide and two disulfide moieties were able to catalyze H2O2 reduction at the expense of a thiol. The dimeric macrocycles 139 and 142–144 showed the best activity in the GPx-mimetic assay. Kinetic plots obtained for the aliphatic macrocycles 139 and 142–144 (10 mol%) revealed that the reactions are remarkably rapid, reaching 50% completion in 2.4 and 4.8 min. Another feature is the considerable induction period for compounds 139 and 142–144, with very rapid consumption of thiol within 7 min.
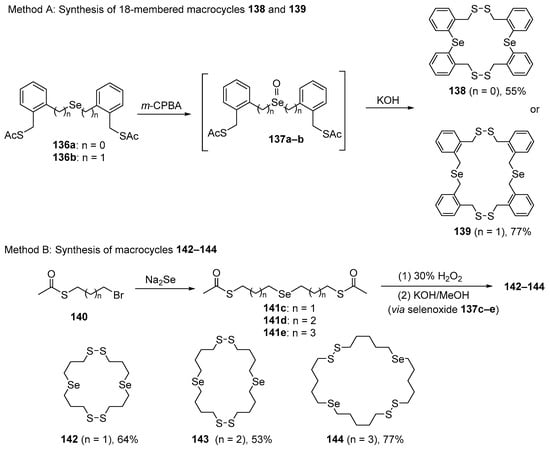
Scheme 39.
Synthesis of macrocycles 138, 139, and 142–144.
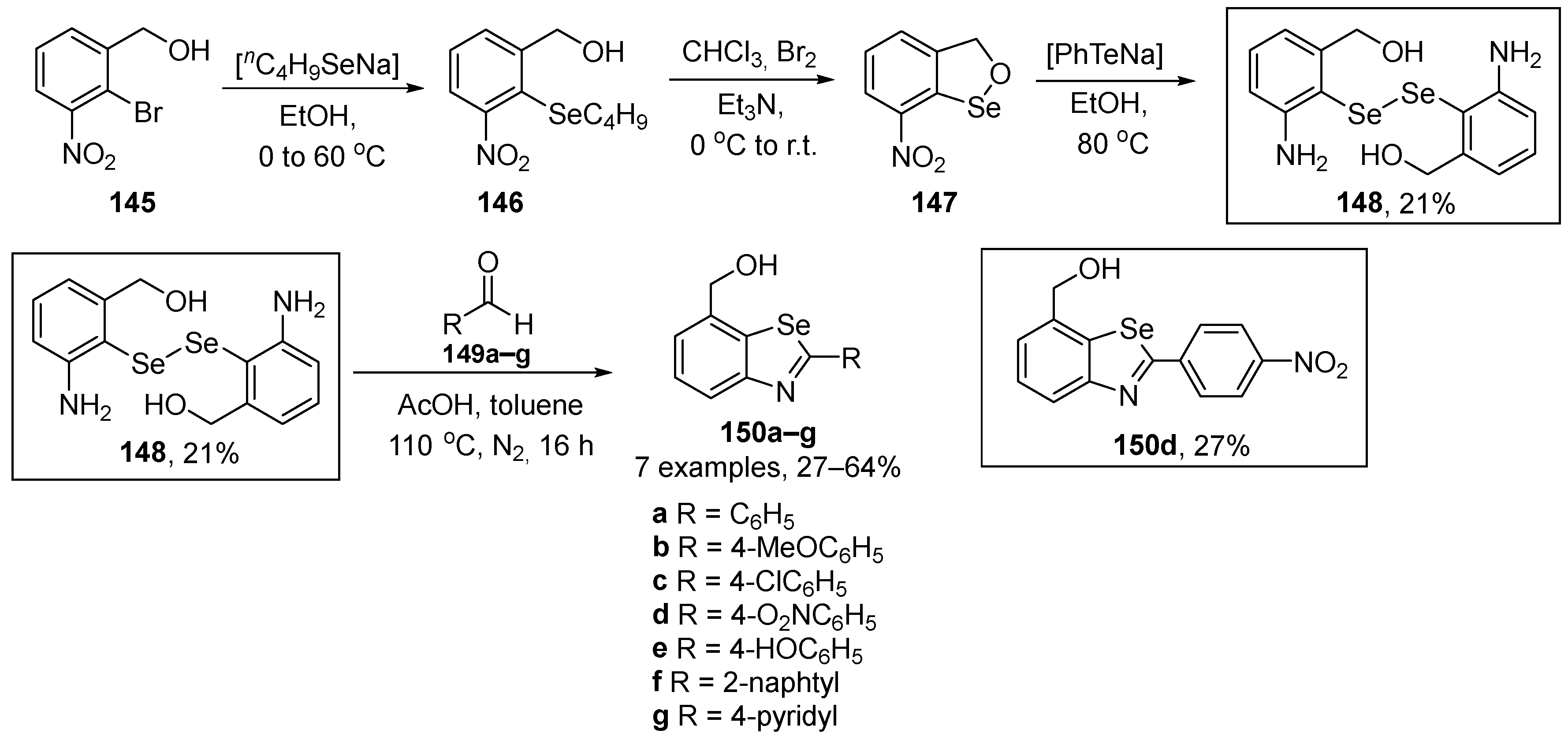
Singh and co-workers have described the synthesis, reactivity, and the GPx-like activity of bis(3-amino-1-hydroxybenzyl)-diselenide 148 and its benzoselenazole derivatives 150 [111]. The key intermediate is the diselenide 148, with two ortho groups at the benzene ring (NH2 and CH2OH). This diselenide was prepared by the reaction of 7-nitro-3H-2,1-benzoxaselenole with PhTeNa, generated in situ in 21% yield after three steps. Once obtained, it was easily converted to the respective benzoselenazoles 150 through the one-pot acetic acid-catalyzed reaction with aldehydes (Scheme 40). All the prepared compounds (148 and 150a–g) presented superior GPx-like activity when compared to diphenyl diselenide and Ebselen, with compounds 148 and 150a being the most active.
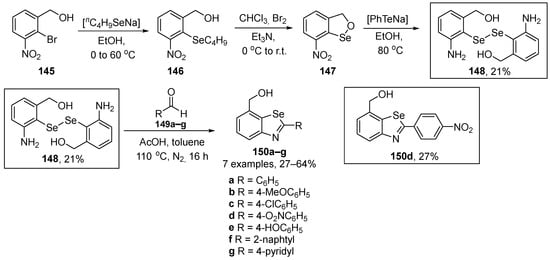
Scheme 40.
Synthesis of diselenide 148 and benzoselenazole 150.
In the GPx-like assay using a thiophenol for the reduction of H2O2 in the presence of PhSH, the compound 148 (11.5 ± 0.4 μM min−1) showed better catalytic activity than Ph2Se2 and Ebselen, probably due to the presence of the two ortho groups to the Se atom. Moreover, the presence of weak secondary Se…H interactions and the various intermediates (selenol, selenenic acid, selenylsulfide) formed during the catalytic cycle from compound 148 are likely to give rise to its high GPx-like activity. Compounds 150a–g demonstrated reduction rates higher than those of Ebselen (1.3 ± 0.1 μM min−1). There was a trend of increasing reduction rates along the order 150e < 150g < 150f < 150c < 150a < 150b < 150d. However, the catalytic activity of compounds 150a–g was low, as only one intermediate (selenoxide) was formed during its catalytic cycle.
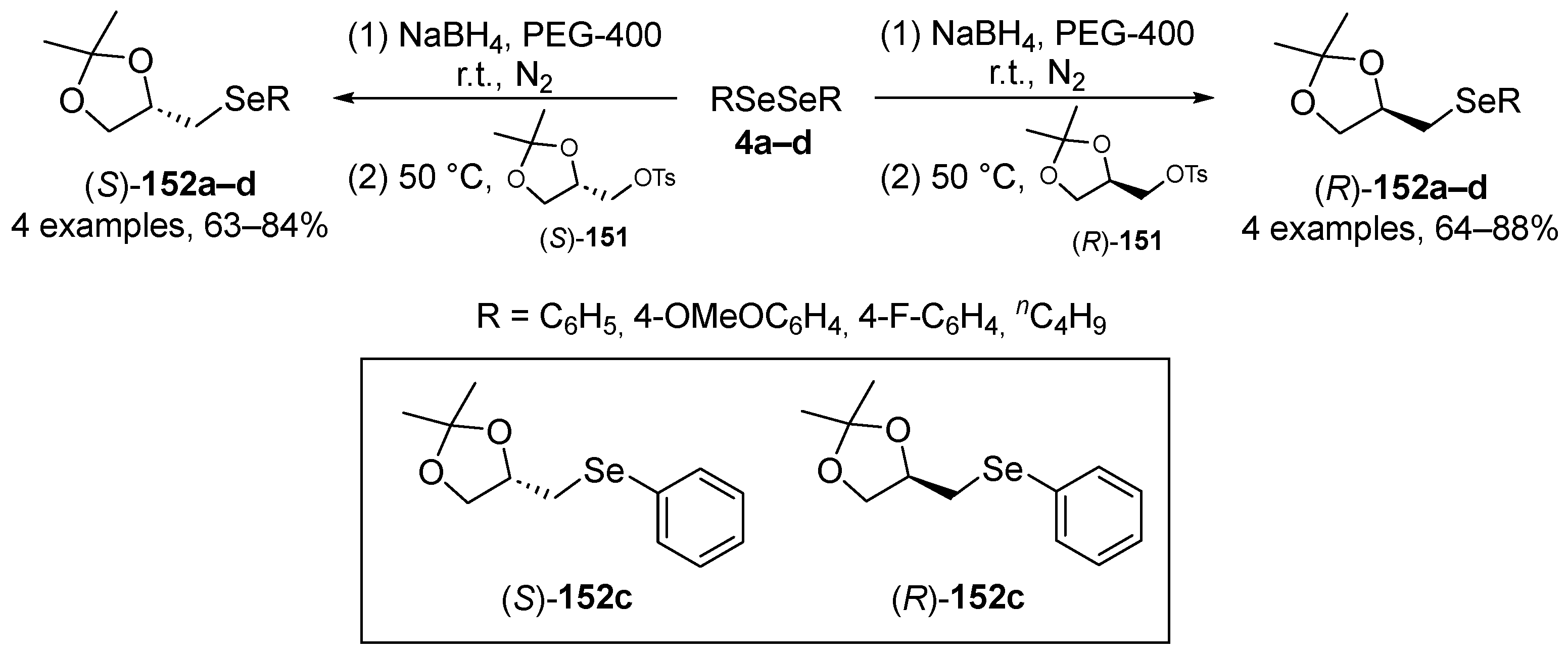
Perin and co-workers have described the synthesis of enantiomerically pure organoselenium compounds (R)-152 and (S)-151, derived from glycerol, which were tested for their in vitro and in vivo (C. elegans) antioxidant activities [112]. The seleno derivatives were prepared through the reaction of chiral solketal tosylates (R)-151 and (S)-151 with selenolate anions, which were generated in situ by reaction of the respective diselenide with NaBH4 (Scheme 41). The most active compounds were the selenide (R)-152c and (S)-152c, derived from diphenyl diselenide 4c. The methodology was successfully extended to sulfide and telluride analogues.
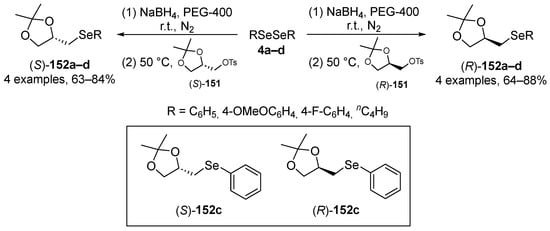
Scheme 41.
Synthesis of chiral selenides (R)-152 and (S)-152.
The authors have also tested these compounds in multiple in vitro assays, including linoleic acid peroxidation assay, ABTS•+, and DPPH scavenging activity, FRAP, chelating potential assay, quantification of reactive species assay, and SOD-like activity. The (R)-152c and (S)-152c enantiomeric compounds inhibited the formation of MDA from linoleic acid. Furthermore, in the sodium azide-induced reactive species test, the compounds decreased reactive species in the cortex of mice (5 μM—(R)-152c; 10 μM—(S)-152c). However, they did not show activity against DPPH and ABTS•+ radicals, the FRAP assay, ion chelation potential, or SOD-like activity. In the in vivo assay, the authors tested (R)-152c and (S)-152c in C. elegans against added H2O2. C. elegans pretreated with sublethal doses of (R)-152c and (S)-152c (1 μM and 50 μM) were completely protected against H2O2-induced mortality. This protection against H2O2 toxicity may be due, in part, to an increase in CAT enzyme levels observed in animals treated with 1 μM (R)-152c. Together, these data reinforce the conclusion that in vivo assays are of great value and confirm that data from in vitro assays do not always predict in vivo activity.
Sanmartín and co-workers have prepared new N,N’-disubstituted acylselenoureas, which were assayed for in vitro DPPH and ABTS•+ assay activity, and for protective effects against H2O2-induced oxidative stress in HT-29 cells [113]. A total of forty-seven selenoureas 156 were prepared and tested in vitro; the four most active ones (156a-d) were evaluated in cell experiments. The best in class was the compound 156c. The N,N’-disubstituted acylselenoureas 156 were prepared in 10–95% yield from the respective carboxylic acids, after a sequential halogenation-isoselenocyanation-amidation (using aniline) (Scheme 42).
Scheme 42.
Synthesis of acyl-selenoureas 156.
The acylselenourea derivatives show excellent activity in the ABTS•+ and DPPH assays (0.77 to 85 µM), with a fast kinetic behavior. In general, phenyl and furyl derivatives are globally the most active with mean DPPH radical inhibition of ~30% at low concentrations. However, ABTS•+ radical scavenging activities of acylselenoureas containing pyridyl (156a, 8.5 µM), cinnamyl (156b, 8.4 µM), benzothienyl (156c, 7.7 µM), or benzodioxyl (156d, 8.3 µM) showed greater antioxidant capacity at low concentration (~0.0007 µM). Furthermore, the acylselenourea 156c (benzothienyl) at 77 µM protected HT-29 cells against H2O2-mediated damage, increasing cell survival by up to 3.6-fold.
The same group have synthesized 30 related N,N′-disubstituted selenoureas 160 containing a para-substituted phenyl ring with different electron-withdrawing and electron-donating groups, and different aliphatic and aromatic nuclei [114]. The synthetic strategy to prepare 160 starts with the preparation of formamide 158 through the Zn/HCl-catalyzed reaction between para-substituted anilines 157 and formic acid. Once prepared, 158 was converted to the key isoselenocyanate intermediate 159, by sequential reaction with triphosgene, OC(OCCl3)2, in Et3N, and selenium powder (Scheme 43). All the prepared compounds showed positive effects in the DPPH and ABTS assays, with 160a, 160b, and 160c showing greater activity than the reference substance (ascorbic acid) around 0.00092 µM.
Scheme 43.
Synthesis of selenoureas 160.
Hussain et al. have prepared a new class of selenoureas 166 having ferrocene and substituted benzoyl functionalities with antioxidant activity in the DPPH test [115]. A total of 17 compounds were prepared by a convergent synthesis starting from p-nitrotoluidine 161 and KSeCN. In the end of each reaction pathway, 3-methyl-4-ferrocenylphenylaniline 163 reacts with the acyl-isoselenocyanante 165 in acetone to afford the desired selenoureas 166 in 49–78% yield (Scheme 44). Among the prepared compounds, in the DPPH test ortho-substituted derivatives (i.e., 2-fluorophenyl derivative) presented higher scavenging activities than meta- and para-fluorophenyl (i.e., 3-fluorophenyl and 4-fluorophenyl) and substituted derivatives (i.e., methylphenyl derivatives). Compounds 166a–d, which showed >50% scavenging activity, have IC50 values of ~152, ~385, ~166, and ~84 μM for 166a, 166d, 166b, and 166c, respectively, compared to ~55 μM for ascorbic acid.
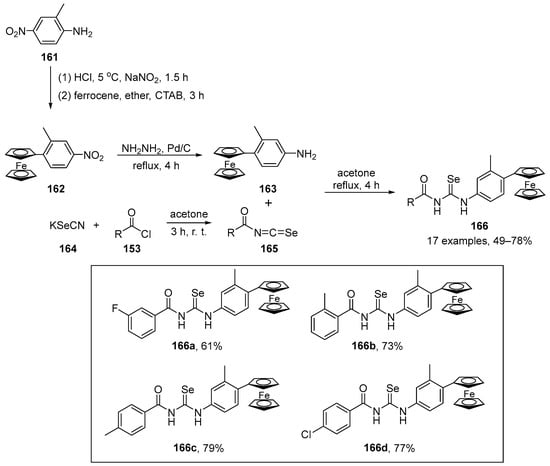
Scheme 44.
Synthesis of ferrocene-functionalized selenoureas 166.
Looking for new GPx-mimic small molecules, Tanini et al. have developed a new approach to prepare selenylsulfides 169 [116]. Fifteen compounds were prepared in 61–86% yield through the reaction of alkyl or arylselenols 167 with N-(organylthio)phthalimides 168 in DMF. In some examples, CsCO3 and tetrabutylammonium iodide (TBAI) were used as additives and the respective diselenides 4 were obtained as co-products in almost all reactions (Scheme 45). The authors experienced difficulties in isolating the prepared compounds, which quickly disproportionated to diselenide and disulfide. In this context, the selenylsufides (S-Se bond) resemble the key intermediates involved in the GPx catalytic cycle, especially compounds 169a and 169b. In the GPx-like assay, the presence of a methoxy group in the arylselanyl moiety renders selenylsulfide 169a more efficient than 169a (10 mol%). Furthermore, diselenides 4a and 4c promote a faster oxidation than selenenylsulfides 169 (ratio of DTTred with 10 mol% of the compounds).
Scheme 45.
Synthesis of selenylsulfides 169.
Silva and co-workers have described a new approach to organoselanyl α-amino phosphonates 173 through the Kabachnik–Fields multicomponent reaction (MCR) [117]. In this MCR, aldehyde 170, arylselanyl-2-propylamine 171, and diorganyl phosphite 172 were reacted in the presence of niobium oxide at 100 °C for 6 h, affording a total of twelve differently substituted organoselanyl α-amino-phosphonates 173 in 48–90% yield (Scheme 46). Six of the prepared compounds were examined for antioxidant assessment in vitro, with compounds 173a, 173c, 173d, and 173e (500 μM) reported to decrease lipid peroxidation induced by sodium nitroprusside. Compounds 173a (100 μM) and 173b (100 μM) showed significant ABTS•+ radical-scavenging activity. However, the compounds did not react with DPPH. The activity of these α-amino phosphonates compounds therefore appears to be dependent on their chemical structure.
Scheme 46.
Synthesis of organoselanyl α-amino phosphonates 173.
The group have described the synthesis of new organophosphate compounds, the phosphoroselenoates 175 [118]. Eleven compounds were prepared in 82–98% yield, by the reaction between diorganyl diselenide 4 and diorganyl H-phosphonate 174 in DMSO at 50 °C for 1–4 h (Scheme 47). Five of the species (175a, 175c, 175d, 175f, 175g) were screened by in vitro (TBARS, reactive species in the brain, FRAP, DPPH, and ABTS•+) and ex vivo (lipid peroxidation, SOD and GPx activities in the brain) assays for activity. The compounds showed in vitro antioxidant activity (DPPH scavenging: 175a, and 175g) and ABTS•+ scavenging: 175a, 175f, and 175g), as well as FRAP activity (175c, 175d, 175f, and 175g). The mechanism of action by which phosphoroselenoates present antioxidant activity is related to the reduction of lipid peroxidation (175c, 175d, 175f, and 175g) and reactive species (175a, 175c, 175d, and 175g). It was demonstrated that, in ex vivo assays, at a dose of 10 mg/kg, compounds 175f and 175g reduced lipid peroxidation levels in the brain of mice, while compounds 175a, 175c, and 175g decreased GPx activity, and 175f–g decreased SOD activity.
Scheme 47.
Synthesis of phosphoroselenoates 175.
An alternative strategy to deliver the reactive selenium species was developed by Pluth and co-workers [119]. The authors have prepared three cyclic P–Se compounds 178 and evaluated their ability to release H2Se in the presence of water. The P–Se compounds were prepared through the reaction between the Woollins’ Reagent 176 and an ortho-substituted phenol 177 (catechol, 2-aminophenol, and N-methyl-2-aminophenol). The reaction was conducted under an atmosphere of N2 in refluxing toluene for 4–10 h, affording the expected products 178a, 178b, and 178c in 13%, 17%, and 49% yield, respectively (Scheme 48). The authors demonstrated H2Se release in the presence of water, which acts as an antioxidant, as demonstrated in liver cells (HeLa) treated with H2O2. In general, 178b demonstrated antioxidant activity toward exogenous (concentration of 25 μM) and endogenous (>5 μM) oxidants, which suggests that this compound exerts antioxidant effects on cells.
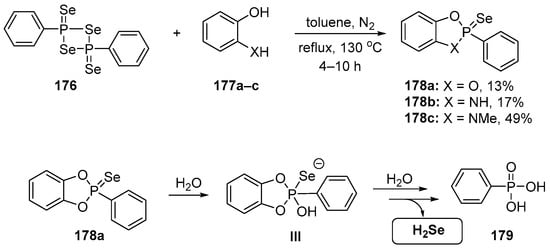
Scheme 48.
Synthesis of cyclic P–Se compounds 178 and the releasing of H2Se from 178a.
Selenocyanates are an interesting class of organoselenium compounds which contain the SeCN unit. Rafique and co-workers have prepared a new class of selenocyanates derived from α,β-unsaturated esters [120]. The most active antioxidant compounds were those having para-substituents in the phenyl ring (4-Br, 4-Cl, and 4-NO2). The synthesis of six methyl (Z)-3-aryl-2-(selenocyanatomethyl)acrylates 182 was achieved in two steps from the Morita–Baylis–Hillman adducts α-methylene-β-hydroxy esters 180. Firstly, 180 was converted to the respective allyl bromide 181, followed by the reaction with NaSeCN in a 4:1 mixture of acetone:H2O at room temperature for 1 h, to give 182 in 86–96% yield (Scheme 49).
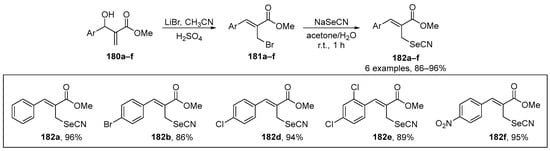
Scheme 49.
Synthesis of allyl selenocyanates 182.
The authors evaluated the antioxidant activity of the compounds using different assays, including TBARS, 4-HNE, and 8-isoprostane (8-ISO) tests for the assessment of lipid peroxidation parameters. They also used the 8-hydroxy-2’-deoxyguanosine (8-OHdG) test to assess DNA damage, and the carbonyl test for the assessment of protein oxidation in cultured mouse neurons exposed to H2O2 and treated with 182. CAT and SOD activities were also evaluated. The cells exposed to H2O2 in culture containing the compounds 182b, 182d, and 182f (10 μM) exhibited the most pronounced pattern of antioxidant activity in TBARS, 4-HNE, 8-ISO, 8-OHdG, and carbonyl levels. These results were similar to those of diphenyl diselenide. Compounds 182a–f showed significant attenuation of CAT activity. Moreover, compounds 182a–d upregulated SOD activity in the neurons exposed to H2O2.
More recently, Shaaban and co-workers described the synthesis and in vitro antioxidant activity (DPPH and ABTS•+) of new organoselenocyanates 185 and 186, derived from anthranilic acid 183 [121]. The key intermediates for the synthesis were 2-amino-5-selenocyanatobenzoic acid 184a and its methyl ester 184b. After several reactions at the amino group, the derivatives 185a–b and 186a–i were prepared in 51–83% yield (Scheme 50). The organoselenocyanate compounds (1 mM) demonstrated scavenging activities of both ABTS•+ and DPPH radicals similar to ascorbic acid (positive control). Among the tested compounds in the DPPH assay, 184b exhibited the highest scavenging activity, followed by 186c, 186b, 186f, 185a, and 186g. In the ABTS test, the most reactive species was 186c followed by 186f, 184b, 185b, 186g, and 185a.
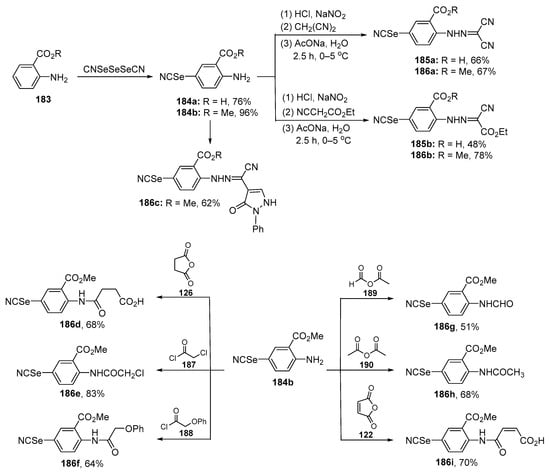
Scheme 50.
Synthesis of selenocyanates 184, 185, and 186 derived of anthranilic acid.
Chitosan (CS) 187, a natural polysaccharide, was used by Fajardo and co-workers as a starting material to prepare the organoselenium-chitosan derivative 190 [122]. The key intermediate in the reaction was the chitosan-azide 188, which was the substrate in the “click” reaction with 3-(phenylseleno)prop-1-yne 189, affording the expected triazole 190 in 85% yield (Scheme 51). Compound 190 demonstrated activity against ABTS•+ radicals in a concentration-dependent manner (≥3.0 g L−1). The maximum inhibition (Imax) achieved by compound 190 was 33.5%. Additionally, compound 190 exhibited significant DPPH scavenging activity at concentrations ≥1.0g L−1, with an Imax of 40.0%.
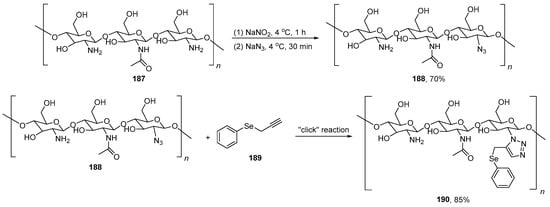
Scheme 51.
Synthesis of selenium-containing chitosan 190.
Filipovic and co-workers have prepared selenazolyl-hydrazones 193, which were tested in in vitro assays (DPPH, TRP, TAOC, ORAC) [123]. Twelve compounds were prepared through the reaction between selenosemicarbazones 191 and α-bromoketones 192 in a 1:1 mixture of EtOH/H2O as solvent (Scheme 52). All the compounds showed antioxidant activity in the DPPH and ORAC assays, with these being superior to ascorbic acid. Compounds without nitro substituents exhibited the highest antioxidant activity amongst those tested, with 193a having the most potent. Compounds with nitro groups at the ortho- and para-positions displayed similar activities to the non-substituted and Me-derived ones in the DPPH test. However, in the ORAC test, compounds containing an ortho-nitro group were less active. Ultimately, the order of activities observed was 193a > 193b > 193c. In the TAOC and TRP assays, the selenazolyl-hydrazones 193 did not yield significant results.
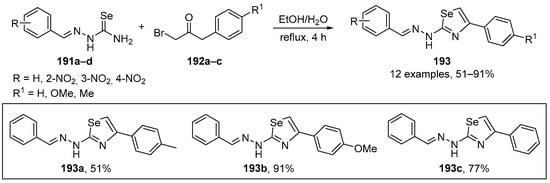
Scheme 52.
Synthesis of selenazolyl-hydrazones 193.
The antioxidant mechanisms involved in the antidepressant-like action of 2-phenyl-3-(arylselanyl)benzofuran 195a were evaluated in vitro and in vivo by Bortolatto et al. [124]. Five compounds were prepared following the procedure of Zeni and co-workers [125] (Scheme 53). The intramolecular cyclization of ortho-alkynyl anisoles 194 with diaryl diselenides 4 in the presence of equivalent amount of FeCl3 afforded the expected benzofurans 195 selectively. Prior to in vivo tests, screening of the compounds 195a–e was carried out to assess their ability to reduce lipid peroxidation (determined by TBARS) in brain tissue. Compound 195, when present at ≥10 μM, demonstrated protective effects against reactive species generation, with 195a giving the lowest IC50 and Imax values (IC50: 9.7 μM; Imax: ~76%). In the TBARS test, 195a displayed the highest effectiveness (IC50: 122 μM; Imax: ~47%). Subsequently, the effects of treatment with 195a at a dose of 50 mg/kg were evaluated in the hippocampus of male mice exposed to p-CPA (a serotonin depletion agent). Parameters evaluated included lipid peroxidation levels, total thiol content, CAT activity, and Na+/K+-ATPase activity. p-CPA exposure increased lipid peroxidation and CAT activity and reduced total thiol content and Na+/K+-ATPase activity, whereas treatment with 195a reversed the decline in total thiols and inhibited lipid peroxidation in the brain tissue, consistent with potential neuroprotective effects.
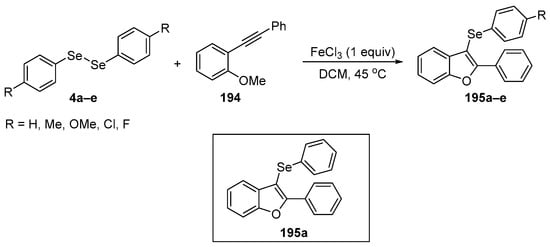
Scheme 53.
Synthesis of 2-phenyl-3-(arylselanyl)benzofurans 195.
4. Conclusions and Perspectives
The synthesis and testing of new organoselenium molecules for biological activities is a field of rapid development. In recent years, many new compounds have been prepared, with many of these being molecular hybrids of known bioactive compounds and organoselenium groups, or unprecedented molecules. In the search for the “Holy Grail” of organoselenium compounds, i.e., compounds that can mimic GPx, a diversity of synthetic approaches has been explored, and multiple bioassays have been used to assess bioactivity. For many years, Ebselen was the most likely candidate to be marketed. It has been tested in several clinical trials and is currently in a Phase 3 study to treat acute noise induced hearing loss, on the basis of its GPx-like activity and anti-inflammatory properties. As discussed above, dozens of studies have reported the synthesis of organoselenium compounds, with the biological activity evaluated mainly in vitro. This is not ideal, and an important limitation, as it is clear from the data described above that (in multiple cases) the in vivo activities differ from those observed in vitro. The in vitro data tend to be over-optimistic with regard to activity, as alternative reactions often occur in vivo, and therefore it is critical that in vivo testing is undertaken. Furthermore, a number of the assays commonly used for in vitro testing are artefact prone and non-physiological (e.g., the DDPH, ABTS, FRAP assays). Use of more complex reaction systems (e.g., tests on cultured or primary cells, insects, and worms, which are less costly than animals) are recommended before animal testing, as are the use of more precise and accurate assays of biological oxidation—particularly those that do not involve added agents, such as fluorescent dyes or probes that may be artefact prone or perturb the systems under study [12,18,126]. Animal studies also have limitations, and consideration needs to be given to dosing methods, pharmacokinetics, metabolism, and multiple other factors, including the possibility of long-term toxicity arising from bioaccumulation of either the parent compound or metabolites. In this respect, highly water-soluble compounds (cf. selenosugars that are rapidly excreted) and those that are resistant to metabolic biotransformation are probably going to be beneficial.
It is likely to be only a matter of time before positive compounds are identified, as new attractive and efficient protocols are emerging to make complex in vitro assays more accessible and reliable. In particular, a number of organic selenium compounds have shown promising protective effects through different mechanisms, including mimetic activities of antioxidant enzymes and modulation of antioxidant genes and redox signaling, which might be considered in the development of new therapeutic approaches for oxidative stress-related diseases.
Regarding the synthesis of organoselenium compounds, the future development in this field will be based on the green chemistry principles, including but not limited to using alternative energy sources, like ultrasound, light, and mechanochemistry, flow chemistry, green solvents, and atom-economic approaches. As demonstrated in this review, this has already been a trend, but there is still room to evolve in this area.
Author Contributions
Conceptualization, C.A.B., L.S. and E.J.L.; methodology, C.A.B., L.S. and E.J.L.; classification and analysis of the collected manuscripts and writing—original draft preparation, J.M.A., P.T.B., M.J.d.R. and C.S.G.; writing—review and editing, P.T.B., C.A.B., M.J.D., L.S. and E.J.L.; supervision, C.A.B., L.S. and E.J.L.; project administration, E.J.L.; funding acquisition, C.A.B., L.S. and E.J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—DOI:10.13039/501100002322—Grant Number 001, and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brasil (CNPq)—DOI: 10.13039/501100003593. FAPERGS and FINEP are also acknowledged for financial support. CNPq is also acknowledged for Fellowship to CAB, LS, and EJL. The Novo Nordisk Foundation (grant: NNF20SA0064214) is acknowledged for the support of MJD.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
M.J.D. is a Director and major shareholder in Seleno Therapeutics plc, which holds patents on selenosugar compounds. The other authors declare no conflict of interest.
References
- Steinbrenner, H.; Speckmann, B.; Klotz, L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Barbosa, N.V.; Rocha, J.B.T. Toxicology and pharmacology of synthetic organoselenium compounds: An update. Arch. Toxicol. 2021, 95, 1179–1226. [Google Scholar] [CrossRef]
- Chuai, H.; Zhang, S.-Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small molecule selenium-containing compounds: Recent development and therapeutic applications. Eur. J. Med. Chem. 2021, 223, 113621. [Google Scholar] [CrossRef]
- Mamgain, R.; Kostic, M.; Singh, F.V. Synthesis and Antioxidant Properties of Organoselenium Compounds. Curr. Med. Chem. 2023, 30, 2421–2448. [Google Scholar] [CrossRef] [PubMed]
- Obieziurska-Fabisiak, M.; Pacuła-Miszewska, A.J.; Laskowska, A.; Ścianowski, J. Organoselenium compounds as antioxidants. Arkivoc 2022, 5, 69–92. [Google Scholar] [CrossRef]
- Kumar, M.; Chhillar, B.; Verma, D.; Nain, S.; Singh, V.P. Introduction of Methyl Group in Substituted Isoselenazolones: Catalytic and Mechanistic Study. J. Org. Chem. 2023, 88, 4273–4285. [Google Scholar] [CrossRef]
- Deshmukh, Y.; Gandhi, V.V.; Singh, B.G.; Kumbhare, L.B.; Debnath, A.K.; Kunwar, A. 3,3′-Diselenodipropionic acid (DSePA) forms 1:1 complex with Hg(II) and prevents oxidative stress in cultured cells and mice model. J. Inorg. Biochem. 2022, 226, 111638. [Google Scholar] [CrossRef]
- Upadhyay, A.; Bhakuni, B.S.; Meena, R.; Kumar, S. Radical Chain Breaking Bis(ortho-organoselenium) Substituted Phenolic Antioxidants. Chem. Asian J. 2021, 16, 966–973. [Google Scholar] [CrossRef]
- Peglow, T.J.; Martins, C.C.; da Motta, K.P.; Luchese, C.; Wilhelm, E.A.; Stieler, R.; Schneider, P.H. Synthesis and biological evaluation of 5-chalcogenyl-benzo[h]quinolines via photocyclization of arylethynylpyridine derivatives. New J. Chem. 2022, 46, 23030–23038. [Google Scholar] [CrossRef]
- Soares, A.T.G.; Junior, L.B.L.R.; Salgueiro, W.G.; Forno, A.H.C.D.; Rodrigues, C.F.; Sacramento, M.; Franco, J.; Alves, D.; Oliveira, R.P.; Pinton, S.; et al. Organoselenotriazoles attenuate oxidative damage induced by mitochondrial dysfunction in mev-1 Caenorhabditis elegans mutants. J. Trace Elem. Med. Biol. 2019, 53, 34–40. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Moore, K.; Roberts, L.J. Measurement of Lipid Peroxidation. Free Radic. 1998, 28, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods 2016, 106, 21–30. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Roy, Z.; Bansal, R.; Siddiqui, L.; Chaudhary, N. Understanding the Role of Free Radicals and Antioxidant Enzymes in Human Diseases. Curr. Pharm. Biotechnol. 2023, 24, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Mohideen, K.; Jeddy, N.; Krithika, C.; Faizee, S.H.; Dhungel, S.; Ghosh, S. Assessment of glutathione peroxidase enzyme response and total antioxidant status in oral cancer—Systematic review and meta-analysis. Cancer Rep. 2023, 6, e1842. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Barchielli, J.; Caperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Selenium and redox signaling. Arch. Biochem. Biophys. 2017, 617, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sudati, J.H.; Nogara, P.A.; Saraiva, R.A.; Wagner, C.; Alberto, E.E.; Braga, A.L.; Fachinetto, R.; Piquini, P.C.; Batista, J.; Rocha, T. Diselenoamino acid derivatives as GPx mimics and as substrates of TrxR: In vitro and in silico studies. Org. Biomol. Chem. 2018, 16, 3777–3787. [Google Scholar] [CrossRef]
- Hecka, S.O.; Zborowskia, V.A.; Quinesb, C.B.; Nogueira, C.W. 4,4′-Dichlorodiphenyl diselenide reverses a depressive-like phenotype, modulates prefrontal cortical oxidative stress and dysregulated glutamatergic neurotransmission induced by subchronic dexamethasone exposure to mice. J. Psychiatr. Res. 2019, 116, 61–68. [Google Scholar] [CrossRef]
- Paulmier, C. Selenium Reagents and Intermediates in Organic Synthesis, 1st ed.; Pergamon Press: Oxford, UK, 1986; pp. 25–51. [Google Scholar]
- Garcia, C.S.; Garcia, P.R.; Espíndola, C.N.S.; Nunes, G.A.; Jardim, N.S.; Müller, S.G.; Bortolatto, C.F.; Brüning, C.A. Effect of m-Trifluoromethyl-diphenyl diselenide on the Pain–Depression Dyad Induced by Reserpine: Insights on Oxidative Stress, Apoptotic, and Glucocorticoid Receptor Modulation. Mol. Neurobiol. 2021, 58, 5078–5089. [Google Scholar] [CrossRef]
- Dos Santos, M.M.; de Macedo, G.T.; Prestes, A.S.; Eckera, A.; Müller, T.E.; Leitempergera, J.; Fontana, B.D.; Daniel, M.P.; Araújo, A.; Rosemberga, D.B.; et al. Modulation of redox and insulin signaling underlie the anti-hyperglycemic and antioxidant effects of diphenyl diselenide in zebrafish. Free Radic. Biol. Med. 2020, 158, 20–31. [Google Scholar] [CrossRef]
- Wanga, X.; Huanb, Y.; Lib, C.; Caob, H.; Sunb, S.; Leib, L.; Liub, Q.; Liub, S.; Jib, W.; Liua, H.; et al. Diphenyl diselenide alleviates diabetic peripheral neuropathy in rats with streptozotocin-induced diabetes by modulating oxidative stress. Biochem. Pharmacol. 2020, 182, 114221. [Google Scholar] [CrossRef]
- Botteselle, G.V.; Elias, W.C.; Bettanin, L.; Canto, R.F.S.; Salin, D.N.O.; Barbosa, F.A.R.; Saba, S.; Gallardo, H.; Ciancaleoni, G.; Domingos, J.B.; et al. Catalytic Antioxidant Activity of Bis-Aniline-Derived Diselenides as GPx Mimics. Molecules 2021, 26, 4446. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Huan, Y.; Cao, H.; Sun, S.; Lei, L.; Liu, Q.; Liu, S.; Ji, W.; Huang, K.; et al. Diphenyl diselenide ameliorates diabetic nephropathy in streptozotocin-induced diabetic rats via suppressing oxidative stress and inflammation. Chem. Biol. Interact. 2021, 338, 109427. [Google Scholar] [CrossRef] [PubMed]
- Capperucci, A.; Coronnello, M.; Salvini, F.; Tanini, D.; Dei, S.; Teodori, E.; Giovannelli, L. Synthesis of functionalised organochalcogenides and in vitro evaluation of their antioxidant activity. Bioorg. Chem. 2021, 110, 104812. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Yadav, M.; Chhillar, B.; Singh, V.P. Regenerable Radical-Trapping and Preventive Selenazolonamine Antioxidants. Asian J. Org. Chem. 2021, 10, 1492–1499. [Google Scholar] [CrossRef]
- Singh, V.P.; Poon, J.; Yan, J.; Lu, X.; Ott, M.K.; Butcher, R.J.; Gates, P.J.; Engman, L. Nitro-, Azo-, and Amino Derivatives of Ebselen: Synthesis, Structure, and Cytoprotective Effects. J. Org. Chem. 2017, 82, 313–321. [Google Scholar] [CrossRef]
- Carroll, L.; Gardiner, K.; Ignasiak, M.; Holmehave, J.; Shimodaira, S.; Breitenbach, T.; Iwaoka, M.; Ogilby, P.R.; Pattison, D.I.; Davies, M.J. Interaction kinetics of selenium-containing compounds with oxidants. Free Radic. Biol. Med. 2020, 155, 58–68. [Google Scholar] [CrossRef]
- Carroll, L.; Pattison, D.I.; Fu, S.; Schiesser, C.H.; Davies, M.J.; Hawkins, C.L. Reactivity of selenium-containing compounds with myeloperoxidase-derived chlorinating oxidants: Second-order rate constants and implications for biological damage. Free Radic. Biol. Med. 2015, 84, 279–288. [Google Scholar] [CrossRef]
- Carroll, L.; Davies, M.J.; Pattison, D.I. Reaction of low-molecular-mass organoselenium compounds (and their sulphur analogues) with inflammation-associated oxidants. Free Radic. Res. 2015, 49, 750–767. [Google Scholar] [CrossRef]
- Storkey, C.; Pattison, D.I.; Ignasiak, M.T.; Schiesser, C.H.; Davies, M.J. Kinetics of reaction of peroxynitrite with selenium- and sulfur-containing compounds: Absolute rate constants and assessment of biological significance. Free Radic. Biol. Med. 2015, 89, 1049–1056. [Google Scholar] [CrossRef]
- Wang, X.; Huan, Y.; Liu, S.; Li, C.; Cao, H.; Lei, L.; Liu, Q.; Ji, W.; Sun, S.; Huang, K.; et al. Diphenyl Diselenide Alleviates Tert-Butyl Hydrogen Peroxide-Induced Oxidative Stress and Lipopolysaccharide-Induced Inflammation in Rat Glomerular Mesangial Cells. Int. J. Mol. Sci. 2022, 23, 11215. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdallah, B.; Al-Faiyz, Y.S.; Shaaban, S. Anticancer, Antimicrobial, and Antioxidant Activities of Organodiselenide-Tethered Methyl Anthranilates. Biomolecules 2022, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Obieziurska-Fabisiak, M.; Pacuła, A.J.; Capoccia, L.; Drogosz-Stachowicz, J.; Janecka, A.; Santi, C.; Scianowski, J. Phenylselanyl Group Incorporation for “Glutathione Peroxidase-like” Activity Modulation. Molecules 2020, 25, 3354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chhillar, B.; Yadav, M.; Sagar, P.; Singhal, N.K.; Gates, P.J.; Butcher, R.J.; Singh, V.P. Catalytic and highly regenerable aminic organoselenium antioxidants with cytoprotective effects. Org. Biomol. Chem. 2021, 19, 2015–2022. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, V.P. Synthesis and antioxidant activities of N-thiophenyl ebselenamines: A 77Se{1H} NMR mechanistic study. New J. Chem. 2022, 46, 12010–12022. [Google Scholar] [CrossRef]
- Vogt, A.G.; Voss, G.T.; de Oliveira, R.L.; Paltian, J.J.; Duarte, L.F.B.; Alves, D.; Jessec, C.R.; Roman, S.S.; Roehrs, J.A.; Wilhelm, E.A.; et al. Organoselenium group is critical for antioxidant activity of 7-chloro-4-phenylselenyl-quinoline. Chem. Biol. Interact. 2018, 282, 7–12. [Google Scholar] [CrossRef]
- Couto, S.F.; Araujo, S.M.; Bortolotto, V.C.; Poetinia, M.R.; Pinheiro, F.C.; Musachio, E.A.S.; Meichtrya, L.B.; do Sacramento, M.; Alves, D.; Novo, D.R.; et al. 7-chloro-4-(phenylselanyl) quinoline prevents dopamine depletion in a Drosophila melanogaster model of Parkinson’s-like disease. J. Trace Elem. Med. Biol. 2019, 54, 232–243. [Google Scholar] [CrossRef]
- Voss, G.T.; de Oliveira, R.L.; do Sacramento, M.; Roehrs, J.A.; Alves, D.; Luchese, C.; Wilhelm, E.A. Contribution of antioxidant action of 7-chloro-4-(phenylselanyl)quinoline to treat streptozotocin-induced diabetic neuropathy in mice. New J. Chem. 2022, 46, 19773–19784. [Google Scholar] [CrossRef]
- Domingues, M.; Casaril, A.M.; Birmann, P.T.; Lourenço, D.A.; Vieira, B.; Begnini, K.; Lenardão, E.J.; Collares, T.; Seixas, F.K.; Savegnago, L. Selanylimidazopyridine Prevents Lipopolysaccharide-Induced Depressive-Like Behavior in Mice by Targeting Neurotrophins and Inflammatory/Oxidative Mediators. Front. Neurosci. 2018, 12, 486. [Google Scholar] [CrossRef]
- Domingues, M.; Casaril, A.M.; Smaniotto, T.A.; Birmann, P.T.; Lourenço, D.A.; Bampi, S.R.; Vieira, B.; Lenardão, E.J.; Savegnago, L. Selanylimidazopyridine abolishes inflammation- and stress-induced depressive-like behaviors by modulating the oxido-nitrosative system. Eur. J. Pharmacol. 2022, 914, 174570. [Google Scholar] [CrossRef]
- Dos Santos, D.C.; Rafique, J.; Saba, S.; Grinevicius, V.M.A.S.; Filho, D.W.; Zamoner, A.; Braga, A.L.; Pedrosa, R.C.; Ourique, F. IP-Se-06, a Selenylated Imidazo[1,2-a]pyridine, Modulates Intracellular Redox State and Causes Akt/mTOR/HIF-1α and MAPK Signaling Inhibition, Promoting Antiproliferative Effect and Apoptosis in Glioblastoma Cells. Oxid. Med. Cell. Longev. 2022, 2022, 3710449. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.T.; Seus, N.; de Souza, D.; Rodrigues, O.E.D.; Paixão, M.W.; Jacob, R.G.; Lenardão, E.J.; Perin, G.; Alves, D. Synthesis of [(Arylselanyl)alkyl]-1,2,3-triazoles by Copper-Catalyzed 1,3-Dipolar Cycloaddition of (Arylselanyl)alkynes with Benzyl Azides. Synthesis 2012, 44, 1997–2004. [Google Scholar] [CrossRef]
- Vieira, B.M.; Thurow, S.; Brito, J.S.; Perin, G.; Alves, D.; Jacob, R.G.; Santi, C.; Lenardão, E.J. Sonochemistry: An efficient alternative to the synthesis of 3-selanylindoles using CuI as catalyst. Ultrason. Sonochem. 2015, 27, 192–199. [Google Scholar] [CrossRef]
- Birmann, P.T.; Sousa, F.S.S.; Domingues, M.; Brüning, C.A.; Vieira, B.M.; Lenardão, E.J.; Savegnago, L. 3-(4-Chlorophenylselanyl)-1-methyl-1H-indole promotes recovery of neuropathic pain and depressive-like behavior induced by partial constriction of the sciatic nerve in mice. J. Trace Elem. Med. Biol. 2019, 54, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Domingues, M.; Bampi, S.R.; Lourenço, D.A.; Padilha, N.B.; Lenardão, E.J.; Sonego, M.; Seixas, F.K.; Collares, T.; Nogueira, C.W.; et al. The selenium-containing compound 3-((4-chlorophenyl)selanyl)-1-methyl-1H-indole reverses depressive-like behavior induced by acute restraint stress in mice: Modulation of oxido-nitrosative stress and inflammatory pathway. Psychopharmacology 2019, 236, 2867–2880. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Domingues, M.; Bampi, S.R.; Lourenço, D.A.; Smaniotto, T.Â.; Segatto, N.; Vieira, B.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. The antioxidant and immunomodulatory compound 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole attenuates depression-like behavior and cognitive impairment developed in a mouse model of breast tumor. Brain Behav. Immun. 2020, 84, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Domingues, M.; Lourenço, D.A.; Vieira, B.; Begnini, K.; Corcini, C.D.; França, R.T.; Junior, A.S.V.; Seixas, F.K.; Collares, T.; et al. 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole ameliorates long-lasting depression- and anxiogenic-like behaviors and cognitive impairment in post-septic mice: Involvement of neuroimmune and oxidative hallmarks. Chem. Biol. Interact. 2020, 331, 109278. [Google Scholar] [CrossRef]
- Casaril, A.M.; Lourenço, D.A.; Domingues, M.; Smaniotto, T.A.; Birmann, P.T.; Vieira, B.; Sonego, M.S.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. Anhedonic- and anxiogenic-like behaviors and neurochemical alterations are abolished by a single administration of a selenium-containing compound in chronically stressed mice. Compr. Psychoneuroendocrinol. 2021, 6, 100054. [Google Scholar] [CrossRef]
- Casaril, A.M.; Segatto, N.; Simões, L.; Paschoal, J.; Domingues, M.; Vieira, B.; Sousa, F.S.S.; Lenardão, E.J.; Seixas, F.K.; Collares, T.; et al. Neuroprotective Effect of 3-[(4-Chlorophenyl)selanyl]-1-methyl-1H-indole on Hydrogen Peroxide-Induced Oxidative Stress in SH-SY5Y Cells. Compr. Psychoneuroendocrinol. 2021, 46, 535–549. [Google Scholar] [CrossRef]
- Bampi, S.R.; Casaril, A.M.; Sousa, F.S.S.; Pesarico, A.P.; Vieira, B.; Lenardão, E.J.; Savegnago, L. Repeated administration of a selenium-containing indolyl compound attenuates behavioural alterations by streptozotocin through modulation of oxidative stress in mice. Pharmacol. Biochem. Behav. 2019, 183, 46–55. [Google Scholar] [CrossRef]
- Bampi, S.R.; Casaril, A.M.; Fronza, M.G.; Domingues, M.; Vieira, B.; Begninic, K.R.; Seixas, F.K.; Collares, T.C.; Lenardão, E.J.; Savegnago, L. The selenocompound 1-methyl-3-(phenylselanyl)-1H-indole attenuates depression-like behavior, oxidative stress, and neuroinflammation in streptozotocin-treated mice. Brain Res. Bull. 2020, 161, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Bampi, S.R.; Casaril, A.M.; Domingues, M.; Lourenço, D.A.; Pesarico, A.P.; Vieira, B.; Begnini, K.R.; Seixas, F.K.; Collares, T.V.; Lenardão, E.J.; et al. Depression-like behavior, hyperglycemia, oxidative stress, and neuroinflammation presented in diabetic mice are reversed by the administration of 1-methyl-3-(phenylselanyl)-1H-indole. J. Psychiatr. Res. 2020, 120, 91–102. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.H.; Sousa, F.S.S.; Birmann, P.T.; Pesarico, A.P.; Alves, D.; Jacob, R.G.; Savegnago, L. Evaluation of antioxidant activity and toxicity of sulfur- or selenium-containing 4-(arylchalcogenyl)-1H-pyrazoles. Can. J. Physiol. Pharmacol. 2020, 98, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.H.; Aquino, T.B.; Nascimento, J.E.R.; Perin, G.; Jacob, R.G.; Alves, D. Direct Synthesis of 4-Organylselanylpyrazoles by Copper-Catalyzed One-Pot Cyclocondensation and C-H Bond Selenylation Reactions. Adv. Synth. Catal. 2015, 357, 4041–4049. [Google Scholar] [CrossRef]
- Birmann, P.T.; Casaril, A.M.; Hartwig, D.; Jacob, R.G.; Seixas, F.K.; Collares, T.; Savegnago, L. A novel pyrazole-containing selenium compound modulates the oxidative and nitrergic pathways to reverse the depression-pain syndrome in mice. Brain Res. 2020, 1741, 146880. [Google Scholar] [CrossRef]
- Birmann, P.T.; Domingues, M.; Casaril, A.M.; Smaniotto, T.A.; Hartwig, D.; Jacob, R.G.; Savegnago, L. A pyrazole-containing selenium compound modulates neuroendocrine, oxidative stress, and behavioral responses to acute restraint stress in mice. Behav. Brain Res. 2021, 396, 112874. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Busatto, F.F.; Schaefer, B.T.; Tomasini, P.P.; Nunes, I.J.; Machado, T.S.; Cargnelutti, R.; de Aquino, T.F.B.; Ferreira, K.Q.; Casaril, A.M.; et al. Synthesis, characterization, antioxidant potential, and cytotoxicity screening of new Cu(II) complexes with 4-(arylchalcogenyl)-1H-pyrazoles ligands. J. Inorg. Biochem. 2022, 237, 112013. [Google Scholar] [CrossRef]
- Jacob, R.G.; Hartwig, D.; Nascimento, J.E.R.; Abib, P.B.; Ebersol, C.P.; Nunes, P.P.P.; Birmann, P.T.; Casaril, A.M.; Savegnago, L.; Schumacher, R.F. Sequential one-pot synthesis and antioxidant evaluation of 5-amino-4-(arylselanyl)-1H-pyrazoles. Tetrahedron Lett. 2022, 103, 153992. [Google Scholar] [CrossRef]
- Sheikhi-Mohammareh, S.; Shiri, A.; Beyzaei, H.; Yarmohammadi, E. New efficient design and synthesis of novel antioxidant and antifungal 7-imino[1,3]selenazolo[4,5-d]pyrimidine-5(4H)-thiones utilizing a base-promoted cascade addition/cyclization sequence. Monatsh. Chem. 2020, 151, 963–969. [Google Scholar] [CrossRef]
- Mániková, D.; Šestáková, Z.; Rendeková, J.; Vlasáková, D.; Lukácová, P.; Paegle, E.; Arsenyan, P.; Chovanec, M. Resveratrol-Inspired Benzo[b]selenophenes Act as Anti-Oxidants in Yeast. Molecules 2018, 23, 507. [Google Scholar] [CrossRef]
- Paegle, E.; Domracheva, I.; Turovska, B.; Petrova, M.; Kanepe-Lapsa, I.; Gulbe, A.; Liepinsh, E.; Arsenyan, P. Natural-Antioxidant-Inspired Benzo[b]selenophenes: Synthesis, Redox Properties, and Antiproliferative Activity. Chem. Asian J. 2016, 11, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, M.; Hamama, W.S.; Zooroba, H.H. An Easy Synthetic Approach to Construct Some Ebselen Analogues and Benzo[b]selenophene Derivatives: Their Antioxidant and Cytotoxic Assessment. J. Heterocycl. Chem. 2018, 55, 1645–1650. [Google Scholar] [CrossRef]
- Peglow, T.J.; Bartz, R.H.; Martins, C.C.; Belladona, A.L.; Luchese, C.; Wilhelm, E.A.; Schumacher, R.F.; Perin, G. Synthesis of 2-Organylchalcogenopheno[2,3-b]pyridines from Elemental Chalcogen and NaBH4/PEG-400 as a Reducing System: Antioxidant and Antinociceptive Properties. ChemMedChem 2020, 15, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Ruberte, A.C.; Plano, D.; Encío, I.; Aydillo, C.; Sharma, A.K.; Carmen Sanmartín, C. Novel selenadiazole derivatives as selective antitumor and radical scavenging agents. Eur. J. Med. Chem. 2018, 157, 14–27. [Google Scholar] [CrossRef]
- Plano, D.; Moreno, E.; Font, M.; Encio, I.; Palop, J.A.; Sanmartin, C. Synthesis and in vitro Anticancer Activities of some Selenadiazole Derivatives. Arch. Pharm. Chem. Life Sci. 2010, 343, 680–691. [Google Scholar] [CrossRef]
- Banerjee, K.; Bhattacherjee, D.; Mahato, S.K.; Sufian, A.; Bhabak, K.P. Benzimidazole- and Imidazole-Fused Selenazolium and Selenazinium Selenocyanates: Ionic Organoselenium Compounds with Efficient Peroxide Scavenging Activities. Inorg. Chem. 2021, 60, 12984–12999. [Google Scholar] [CrossRef]
- Singh, B.G.; Kumar, P.; Phadnis, P.; Iwaoka, M.; Priyadarsini, K.I. Free radical induced selenoxide formation in isomeric organoselenium compounds: The effect of chemical structures on antioxidant activity. New J. Chem. 2019, 43, 13357–13362. [Google Scholar] [CrossRef]
- Arai, K.; Kumakura, F.; Takahira, M.; Sekiyama, N.; Kuroda, N.; Suzuki, T.; Iwaoka, M. Effects of Ring Size and Polar Functional Groups on the Glutathione Peroxidase-Like Antioxidant Activity of Water-Soluble Cyclic Selenides. J. Org. Chem. 2015, 80, 5633–5642. [Google Scholar] [CrossRef]
- Milton, M.D.; Khan, S.; Singh, J.D.; Mishra, V.; Khandelwal, B.L. A facile access to chalcogen and dichalcogen bearing dialkylamines and diols. Tetrahedron Lett. 2005, 46, 755–758. [Google Scholar] [CrossRef]
- Karimi, M.; Ignasiak, M.T.; Chan, B.; Croft, A.K.; Radom, L.; Schiesser, C.H.; Pattison, D.I.; Davies, M.J. Reactivity of disulfide bonds is markedly affected by structure and environment: Implications for protein modification and stability. Sci. Rep. 2016, 6, 38572. [Google Scholar] [CrossRef]
- Gao, Q.; Grzyb, K.; Gamon, L.F.; Ogilby, P.R.; Pędziński, T.; Davies, M.J. The structure of model and peptide disulfides markedly affects their reactivity and products formed with singlet oxygen. Free Radic. Biol. Med. 2023, 207, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Schiesser, C.H. 1,4-Anhydro-4-seleno-d-talitol (SeTal): A remarkable selenium-containing therapeutic molecule. New J. Chem. 2019, 43, 9759–9765. [Google Scholar] [CrossRef]
- Storkey, C.; Davies, M.J.; White, J.M.; Schiesser, C.H. Synthesis and antioxidant capacity of 5-selenopyranose derivatives. Chem. Commun. 2011, 47, 9693–9695. [Google Scholar] [CrossRef] [PubMed]
- Storkey, C.; Pattison, D.I.; White, J.M.; Schiesser, C.H.; Davies, M.J. Preventing Protein Oxidation with Sugars: Scavenging of Hypohalous Acids by 5-Selenopyranose and 4-Selenofuranose Derivatives. Chem. Res. Toxicol. 2012, 25, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.; Pattison, D.I.; Fu, S.; Schiesser, C.H.; Davies, M.J.; Hawkins, C.L. Catalytic oxidant scavenging by selenium-containing compounds: Reduction of selenoxides and N-chloramines by thiols and redox enzymes. Redox Biol. 2017, 12, 872–882. [Google Scholar] [CrossRef]
- Zacharias, T.; Flouda, K.; Jepps, T.A.; Gammelgaard, B.; Schiesser, C.H.; Davies, M.J. Effects of a novel selenium substituted-sugar (1,4-anhydro-4-seleno-d-talitol, SeTal) on human coronary artery cell lines and mouse aortic rings. Biochem. Pharmacol. 2020, 173, 113631. [Google Scholar] [CrossRef]
- Voss, G.T.; Davies, M.J.; Schiesser, C.H.; de Oliveira, R.L.; Nornberg, A.B.; Soares, V.R.; Barcellos, A.M.; Luchese, C.; Fajardo, A.R.; Wilhelm, E.A. Treating atopic-dermatitis-like skin lesions in mice with gelatin-alginate films containing 1,4-anhydro-4-seleno-d-talitol (SeTal). Int. J. Pharm. 2023, 642, 123174. [Google Scholar] [CrossRef]
- Voss, G.T.; de Oliveira, R.L.; Davies, M.J.; Domingues, W.B.; Campos, V.F.; Soares, M.P.; Luchese, C.; Schiesser, C.H.; Wilhelm, E.A. Suppressive effect of 1,4-anhydro-4-seleno-D-talitol (SeTal) on atopic dermatitis-like skin lesions in mice through regulation of inflammatory mediators. J. Trace Elem. Med. Biol. 2021, 67, 126795. [Google Scholar] [CrossRef]
- Ng, H.H.; Leo, C.H.; O’Sullivan, K.; Alexander, S.-A.; Davies, M.J.; Schiesser, C.H.; Parry, L.J. 1,4-Anhydro-4-seleno-d-talitol (SeTal) protects endothelial function in the mouse aorta by scavenging superoxide radicals under conditions of acute oxidative stress. Biochem. Pharmacol. 2017, 128, 34–45. [Google Scholar] [CrossRef]
- Farzamnezhad, I.; Sheikhi-Mohammareh, S.; Beyzaei, H.; Yarmohammadi, E.; Shiri, A. Synthesis of Novel DPPH-Free Radical Scavenger Se-Containing Fused Chalcogenophenes: 2-Alkyl-7-Cyano-4-Imino-3-Phenyl-6-(pyrrolidin-1-yl)-3,4-Dihydroselenopheno[3,2-d]Pyrimidines. Polycycl. Aromat. Compd. 2023, 1–11. [Google Scholar] [CrossRef]
- Sousa, F.S.S.; Birmann, P.T.; Balaguez, R.; Alves, D.; Brüning, C.A.; Savegnago, L. α-(phenylselanyl) acetophenone abolishes acute restraint stress induced-comorbid pain, depression and anxiety-related behaviors in mice. Neurochem. Int. 2018, 120, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Victoria, F.N.; Radatz, C.S.; Sachini, M.; Jacob, R.G.; Perin, G.; da Silva, W.P.; Lenardão, E.J. KF/Al2O3 and PEG-400 as a recyclable medium for the selective α-selenation of aldehydes and ketones. Preparation of potential antimicrobial agents. Tetrahedron Lett. 2009, 50, 6761–6763. [Google Scholar] [CrossRef]
- Tanini, D.; Lupori, B.; Nostro, P.; Capperucci, A. Synthesis and catalytic antioxidant activity of functionalized chalcogen-containing GPx mimics. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 746–749. [Google Scholar] [CrossRef]
- He, X.; Nie, Y.; Zhong, M.; Li, S.; Li, X.; Guo, Y.; Liu, Z.; Gao, Y.; Ding, F.; Wen, D.; et al. New organoselenides (NSAIDs-Se derivatives) as potential anticancer agents: Synthesis, biological evaluation and in silico calculations. Eur. J. Med. Chem. 2021, 218, 113384. [Google Scholar] [CrossRef] [PubMed]
- Marwa Sak, M.; Al-Faiyz, Y.S.; Elsawy, H.; Shaaban, S. Novel Organoselenium Redox Modulators with Potential Anticancer, Antimicrobial, and Antioxidant Activities. Antioxidants 2022, 11, 1231. [Google Scholar] [CrossRef]
- Leal, J.G.; Piccoli, B.C.; Oliveira, C.S.; da Silva, F.A.; Omage, F.B.; da Rocha, J.B.T.; Sonego, M.S.; Segatto, N.V.; Seixas, F.K.; Collares, T.V.; et al. Synthesis, antioxidant and antitumoral activity of new 50-arylchalcogenyl-30-N-(E)-feruloyl-30,50-dideoxy-amino-thymidine (AFAT) derivatives. New J. Chem. 2022, 46, 22306–22313. [Google Scholar] [CrossRef]
- Mailahn, D.H.; Araujo, D.R.; Nobre, P.C.; Fonseca, C.A.R.; Penteado, F.; Lenardão, E.J.; Luchese, C.; Wilhelm, E.A.; Perin, G. Benzeneseleninic Acid Promoting the Selenofunctionalization of 2-naphthol Derivatives: Synthesis and Antioxidant Activity of 1-organoselanyl-naphthalen-2-ols. Curr. Chem. Biol. 2023, 17, 56–66. [Google Scholar] [CrossRef]
- Shaaban, S.; Vervandier-Fasseur, D.; Andreoletti, P.; Zarrouk, A.; Richard, P.; Negm, A.; Jacob, G.M.C.; Cherkaoui-Malki, M. Cytoprotective and antioxidant properties of organic selenides for the myelin-forming cells, oligodendrocytes. Bioorg. Chem. 2018, 80, 43–56. [Google Scholar] [CrossRef]
- McMillan, J.D.R.; Sands, K.N.; Cooney, G.S.; Gelfand, B.S.; Back, T.G. Unexpected Formation and Potent Antioxidant Activity of Macrocyclic Dimers Containing Disulfide and Selenide Groups. Angew. Chem. Int. Ed. 2022, 61, e202213744. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, M.; Chahal, A.; Sodhi, N.; Chhillar, B.; Alajangi, H.K.; Barnwal, R.P.; Singh, V.P. Synthesis, Reactions, and Antioxidant Properties of Bis(3-amino-1-hydroxybenzyl)diselenide. J. Org. Chem. 2023, 88, 3509–3522. [Google Scholar] [CrossRef]
- Nobre, P.C.; Vargas, H.A.; Jacoby, C.G.; Schneider, P.H.; Casaril, A.M.; Savegnago, L.; Schumacher, R.F.; Lenardão, E.J.; Ávila, D.S.; Junior, L.B.L.R.; et al. Synthesis of enantiomerically pure glycerol derivatives containing an organochalcogen unit: In vitro and in vivo antioxidant activity. Arab. J. Chem. 2020, 13, 883–899. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Ramos-Inza, S.; Aydillo, C.; Talavera, I.; Encío, I.; Plano, D.; Sanmartín, C. Novel N,N′-Disubstituted Acylselenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants 2020, 9, 55. [Google Scholar] [CrossRef]
- Calvo-Martín, G.; Plano, D.; Encío, I.; Sanmartín, C. Novel N,N′-Disubstituted Selenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants 2021, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.A.; Badshah, A.; Ahmed, N.; Pezzuto, J.M.; Kondratyuk, T.P.; Park, E.-J.; Hussain, I. Synthesis, characterization and biological applications of selenoureas having ferrocene and substituted benzoyl functionalities. Polyhedron 2019, 170, 12–24. [Google Scholar] [CrossRef]
- Tanini, D.; Bonardi, C.; Viglianisi, C.; Capperucci, A.; Menichetti, S. Towards New Catalytic Antioxidants: A Simple and Mild Synthesis of Selenenylsulfides. Catalysts 2019, 9, 333. [Google Scholar] [CrossRef]
- Vogt, A.G.; Perin, G.; Luchese, C.; da Silva, P.C.; Wilhelm, E.A.; Silva, M.S. Organylselanyl α-Amino Phosphonates: Synthesis, NMR Spectroscopic Study, and Antioxidant and Antinociceptive Activities. Eur. J. Org. Chem. 2018, 2018, 627–639. [Google Scholar] [CrossRef]
- Mailahn, D.H.; Iarocz, L.E.B.; Nobre, P.C.; Perin, G.; Sinott, A.; Pesarico, A.P.; Birmann, P.T.; Savegnago, L.; Silva, M.S. A greener protocol for the synthesis of phosphorochalcogenoates: Antioxidant and free radical scavenging activities. Eur. J. Med. Chem. 2021, 213, 113052. [Google Scholar] [CrossRef]
- Newton, T.D.; Bolton, S.G.; Garcia, A.C.; Chouinard, J.E.; Golledge, S.L.; Zakharov, L.N.; Pluth, M.D. Hydrolysis-Based Small-Molecule Hydrogen Selenide (H2Se) Donors for Intracellular H2Se Delivery. J. Am. Chem. Soc. 2021, 143, 19542–19550. [Google Scholar] [CrossRef]
- Frizon, T.E.A.; Cararo, J.H.; Saba, S.; Dal-Pont, G.C.; Michels, M.; Braga, H.C.; Pimentel, T.; Dal-Pizzol, F.; Valvassori, S.S.; Rafique, J. Synthesis of Novel Selenocyanates and Evaluation of Their Effect in Cultured Mouse Neurons Submitted to Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 5417024. [Google Scholar] [CrossRef]
- Al-Abdallah, B.; Al-Faiyz, Y.S.; Shaaban, S. Organoselenocyanates Tethered Methyl Anthranilate Hybrids with Promising Anticancer, Antimicrobial, and Antioxidant Activities. Inorganics 2022, 10, 246. [Google Scholar] [CrossRef]
- Nornberg, A.B.; de Aquino, T.F.B.; Martins, C.C.; Luchese, C.; Wilhelm, E.A.; Jacob, R.G.; Hartwig, D.; Fajardo, A.R. Organoselenium-chitosan derivative: Synthesis via “click” reaction, characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 191, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Elshaflu, H.; Todorovic, T.R.; Nikolic, M.; Lolic, A.; Višnjevac, A.; Hagenow, S.; Padrón, J.M.; García-Sosa, A.T.; Djordjevic, I.S.; Grubišic, S.; et al. Selenazolyl-hydrazones as Novel Selective MAO Inhibitors with Antiproliferative and Antioxidant Activities: Experimental and In-silico Studies. Front. Chem. 2018, 6, 247. [Google Scholar] [CrossRef]
- Gall, J.I.; Alves, A.G.; Júnior, L.R.C.; Rech, T.S.T.; Neto, J.S.S.; Alves, D.; Soares, M.S.P.; Spohr, L.; Spanevello, R.M.; Brüning, C.A.; et al. Insights into serotonergic and antioxidant mechanisms involved in antidepressant-like action of 2-phenyl-3-(phenylselanyl)benzofuran in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109956. [Google Scholar] [CrossRef] [PubMed]
- Gay, R.M.; Manarin, F.; Schneider, C.C.; Barancelli, D.A.; Costa, M.D.; Zeni, G. FeCl3-Diorganyl Dichalcogenides Promoted Cyclization of 2-Alkynylanisoles to 3-Chalcogen Benzo[b]furans. J. Org. Chem. 2010, 75, 5701–5706. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).