Microcapsules and Nanoliposomes Based Strategies to Improve the Stability of Blueberry Anthocyanins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Factor Experiment and Response Surface Optimization of the Microcapsule Preparations
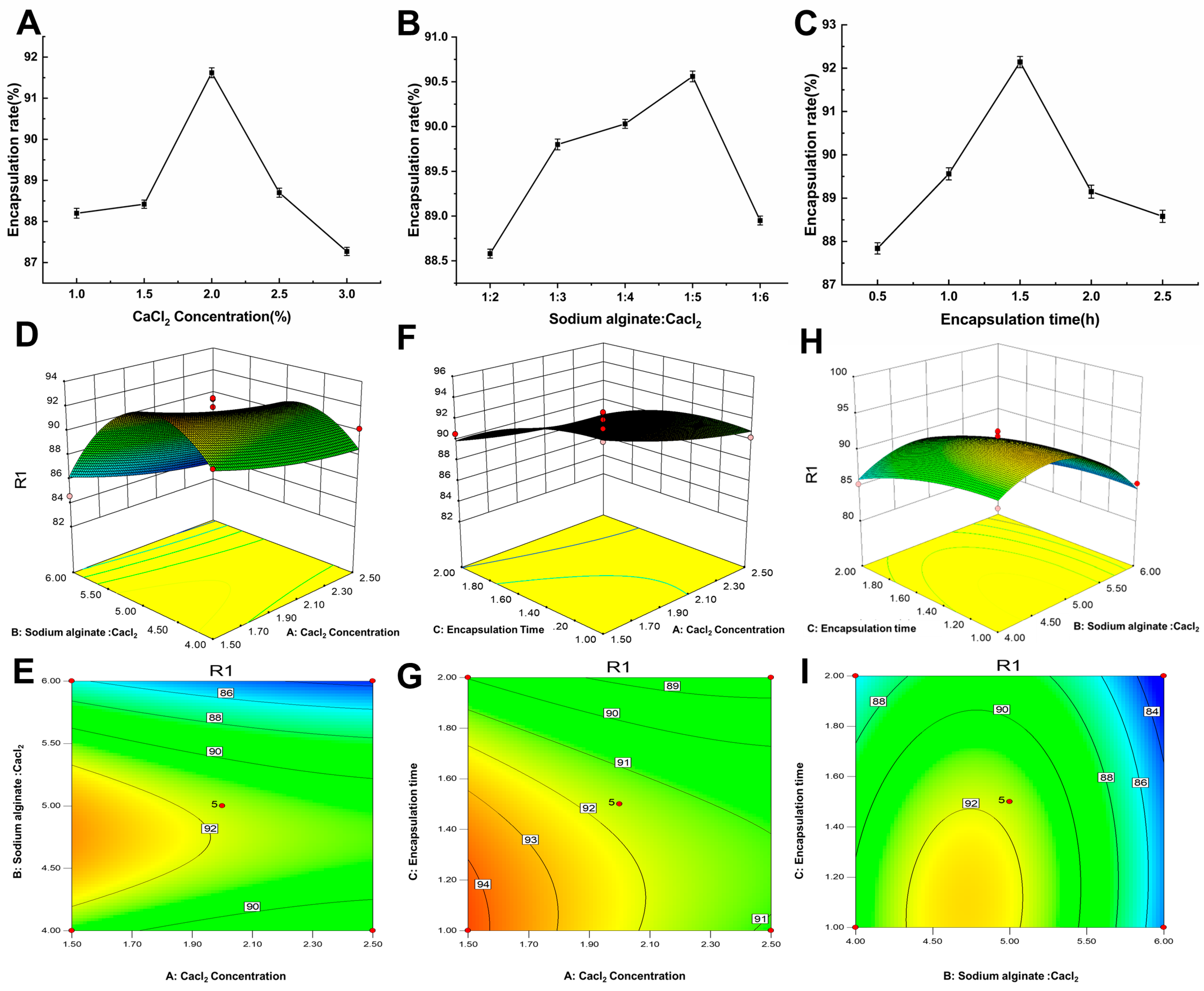
2.1.1. Influence of CaCl2 Concentration on the BAM Encapsulation Efficiency
2.1.2. Effect of the Ratio of Sodium Alginate to CaCl2 on the BAM Encapsulation Efficiency
2.1.3. Effect of Encapsulation Time on the Rate of BAM Encapsulation
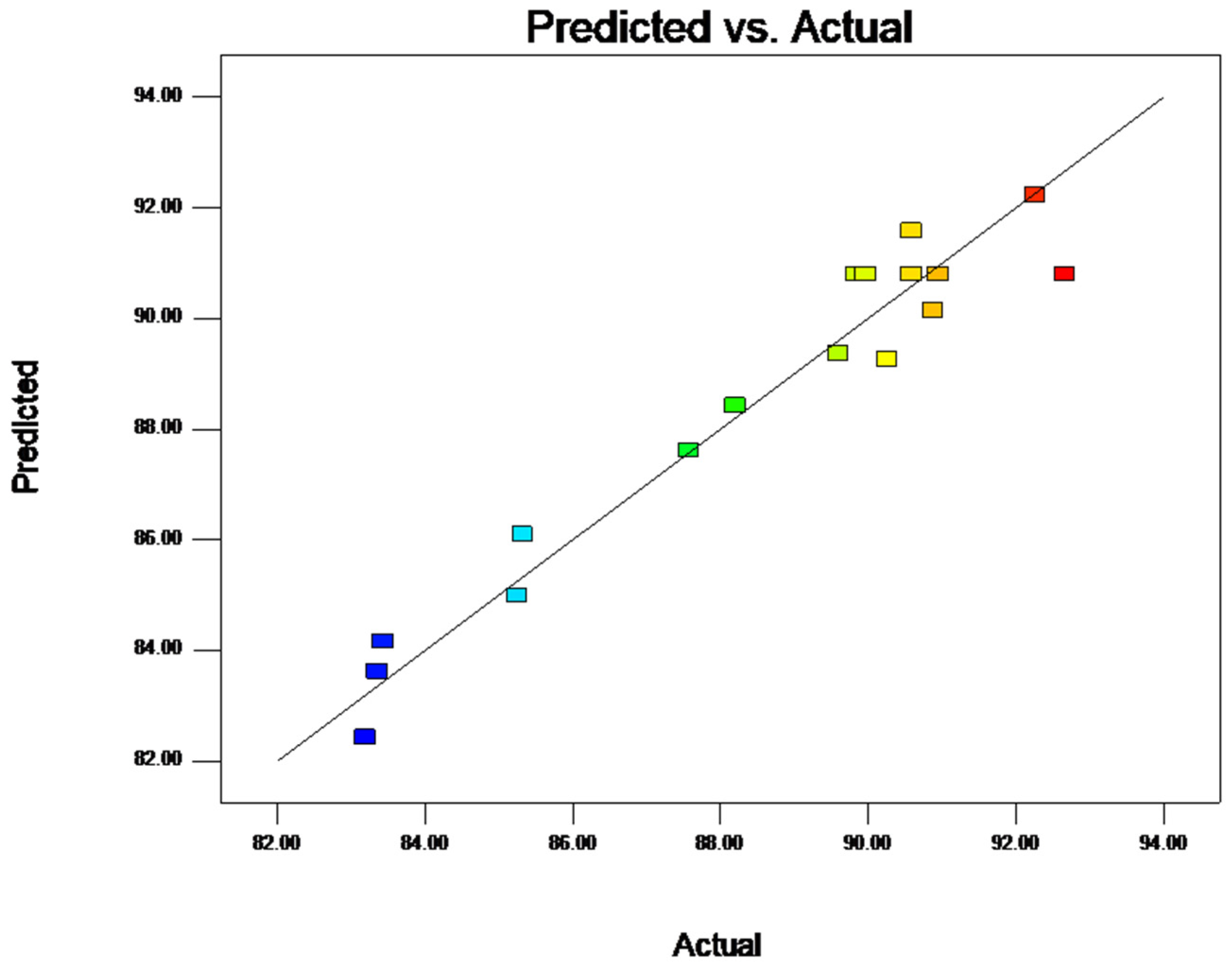
2.1.4. Response Surface Model and Significance Test
2.1.5. Response Surface Analysis of the Encapsulation Efficiency of Microcapsules
2.1.6. Parameter Optimization and Verification
2.2. Single Factor Experiment and Response Surface Optimization of the BAL Preparation
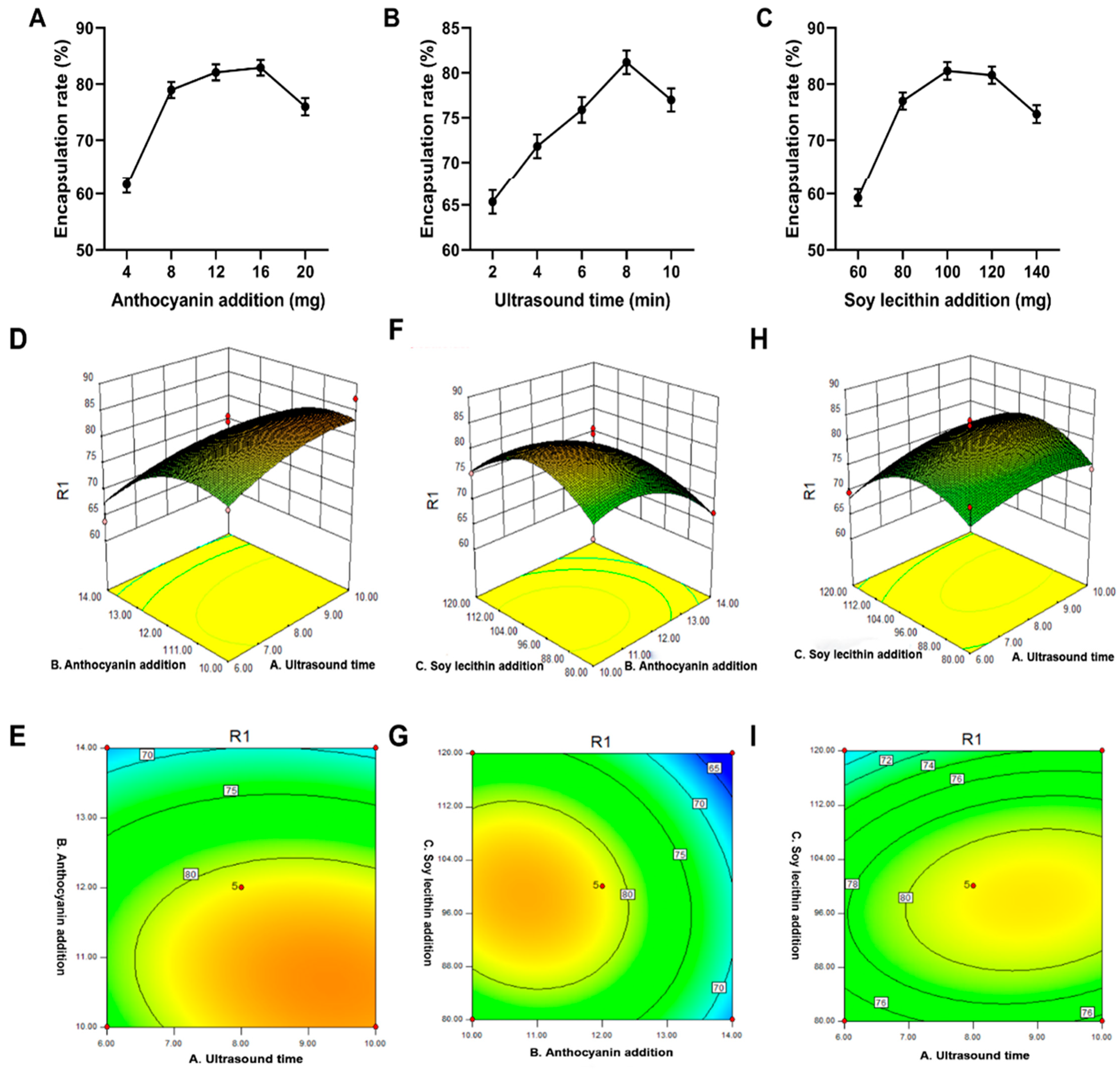
2.2.1. Effect of the Amount of Anthocyanins Added on Encapsulation Efficiency
2.2.2. Effect of Ultrasonic Time on the Encapsulation Rate
2.2.3. Effect of Soy Lecithin on Encapsulation Efficiency
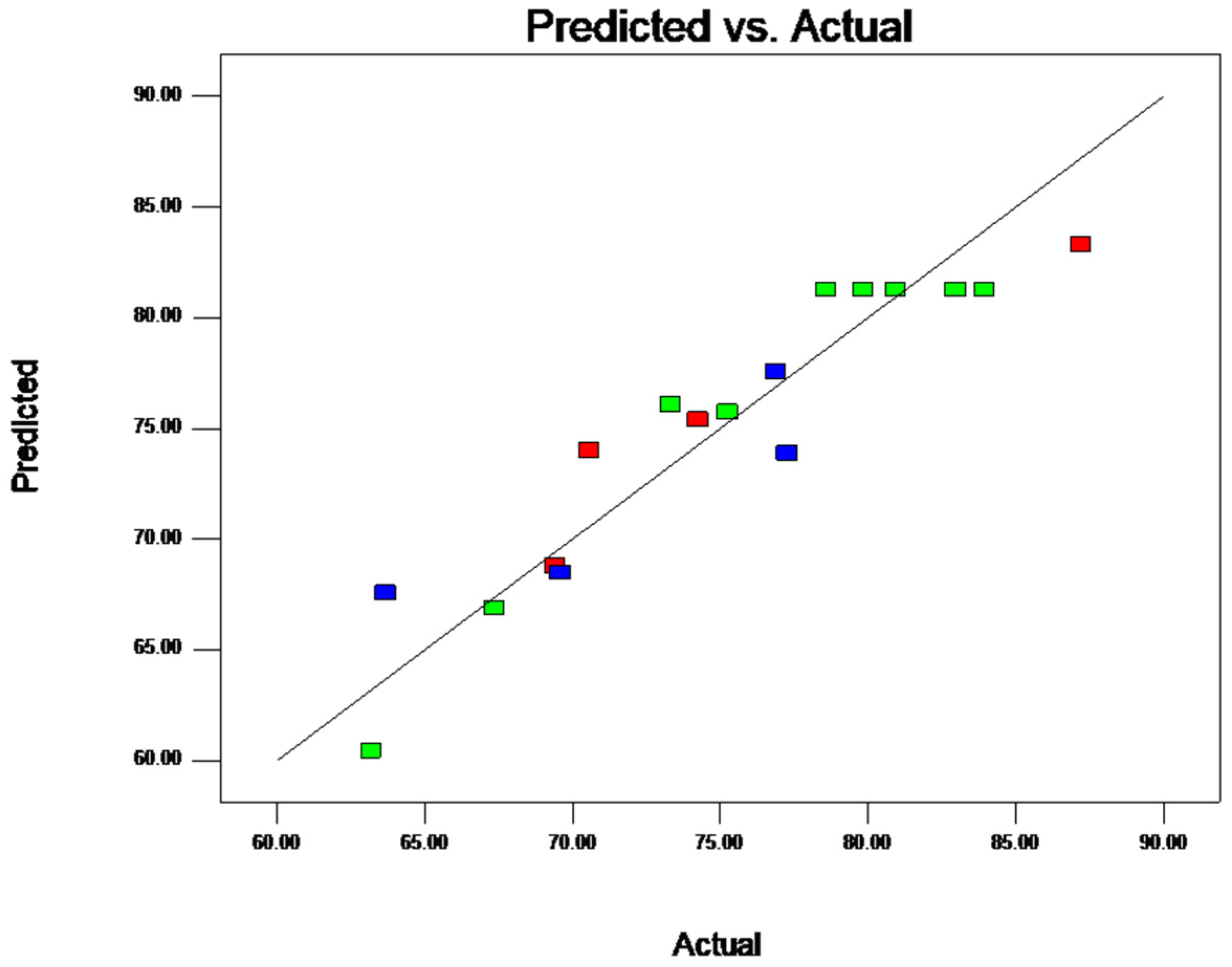
2.2.4. Response Surface Model and Significance Test
2.2.5. Response Surface Analysis of Encapsulation Efficiency
2.2.6. Parameter Optimization and Verification
2.3. Quality Evaluation of BAM and BAL
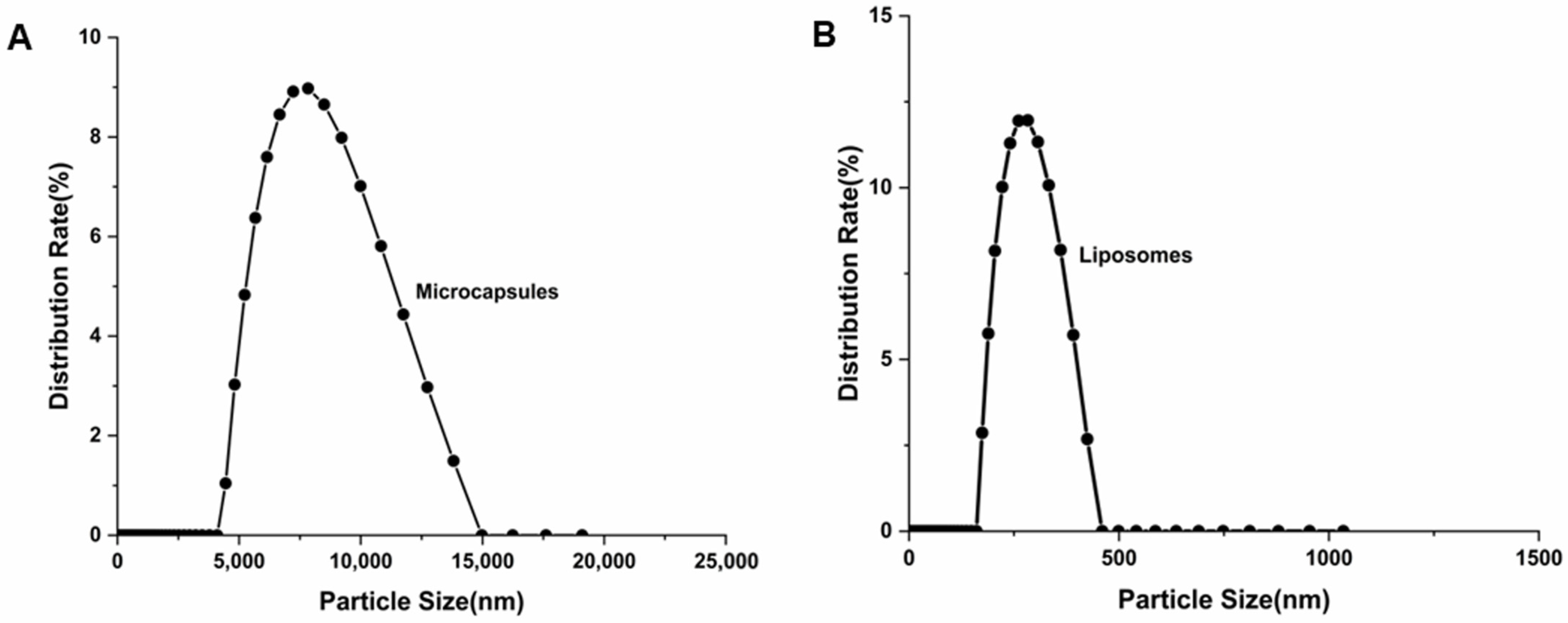
2.3.1. Particle Size and Zeta Potential
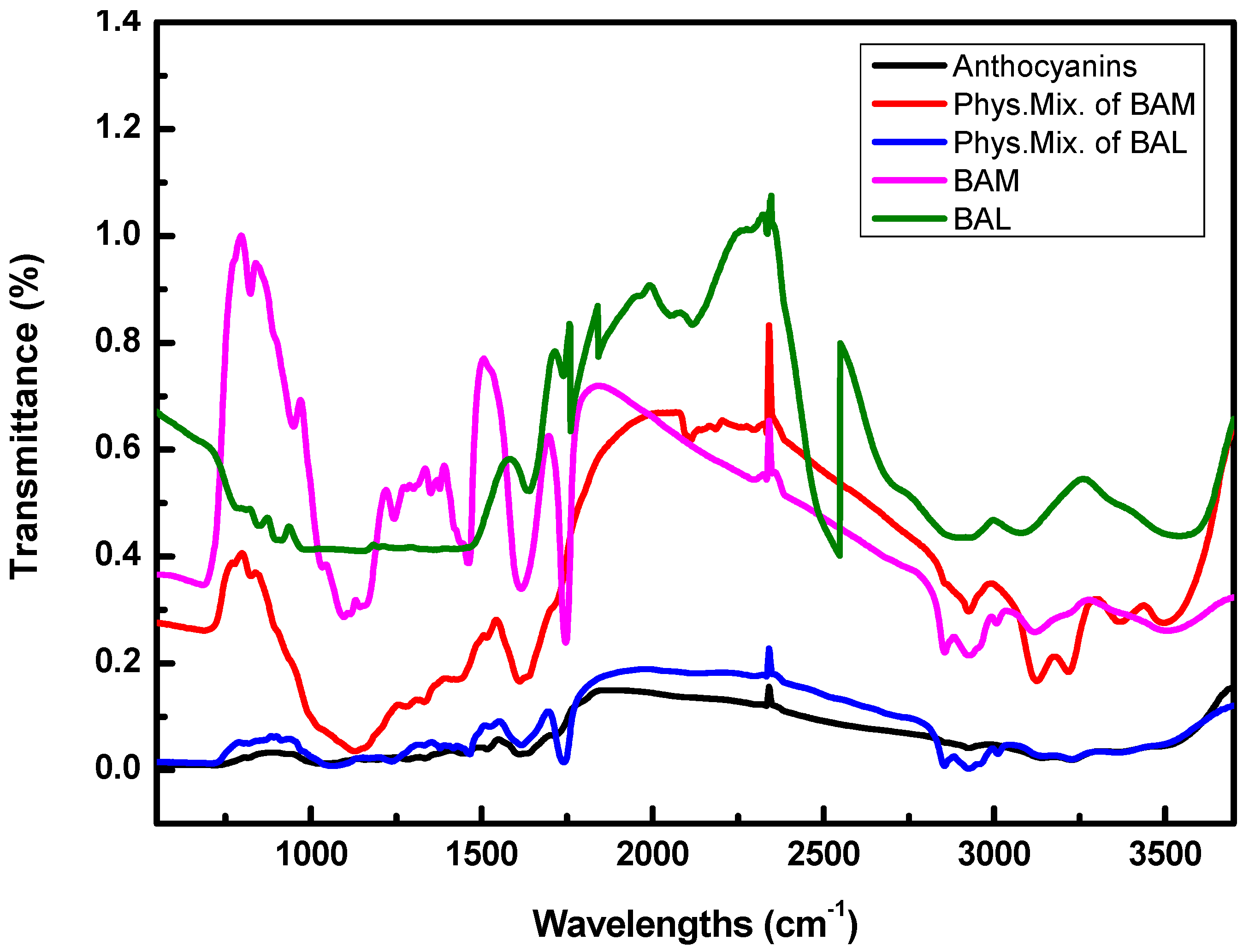
2.3.2. FT-IR Analysis
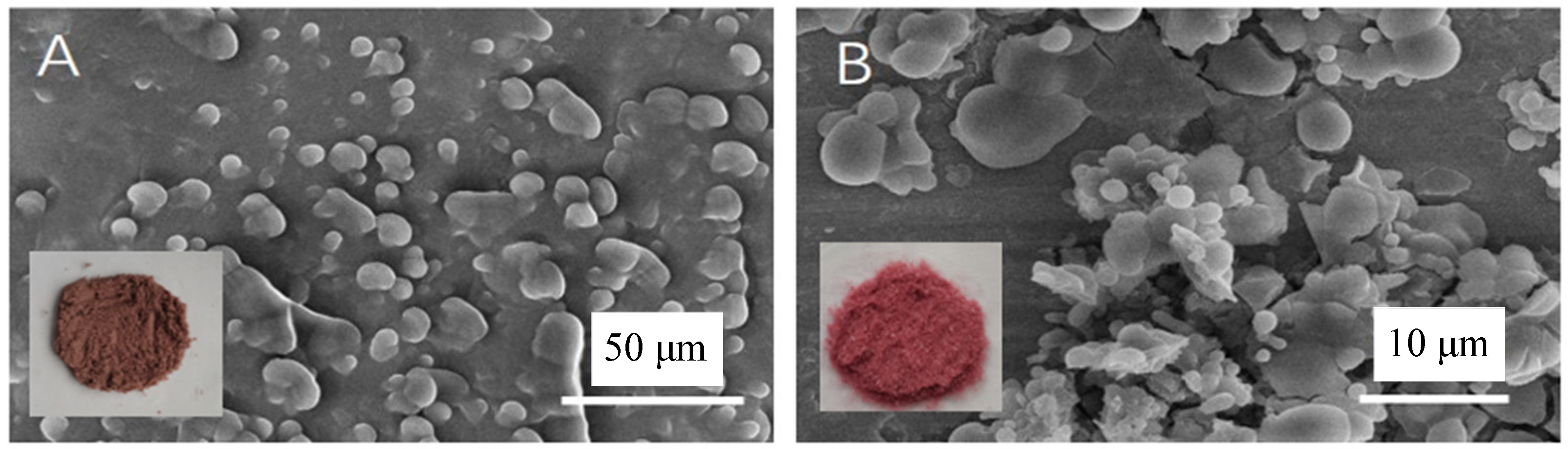
2.3.3. SEM Characterization
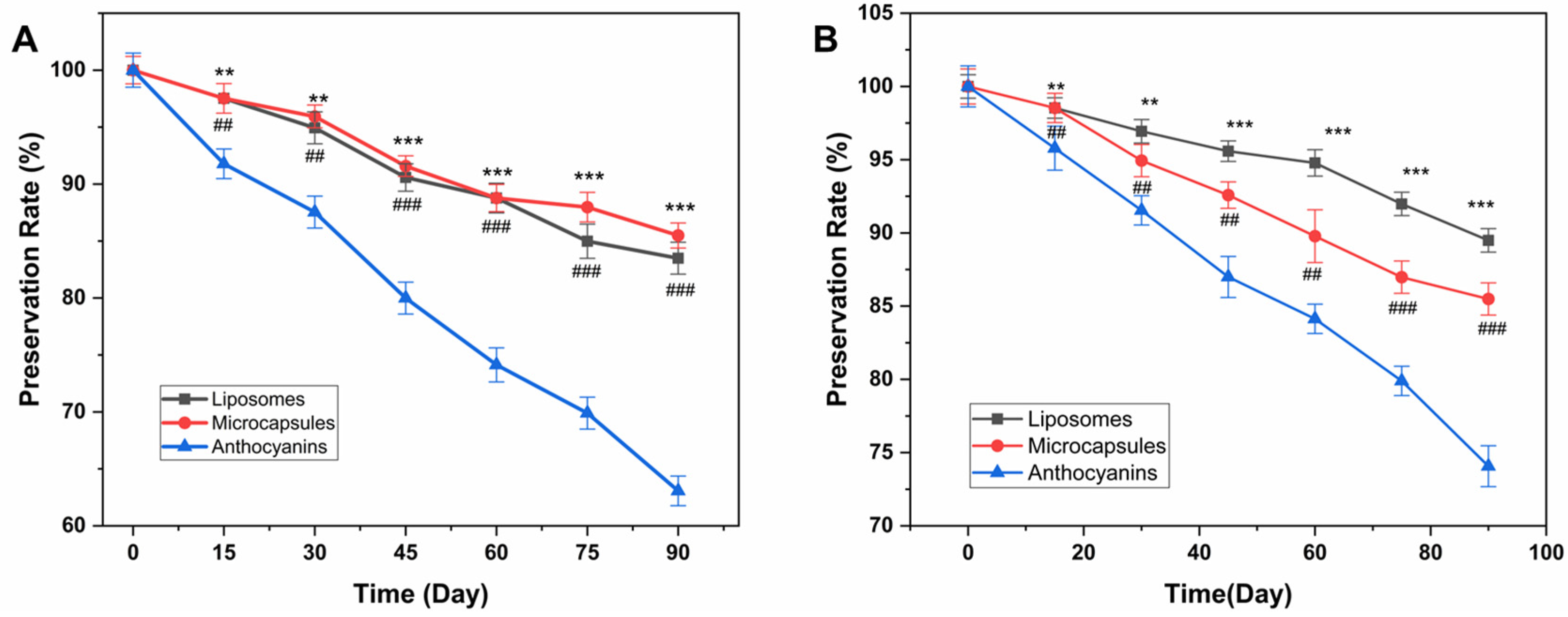
2.3.4. Light Stability
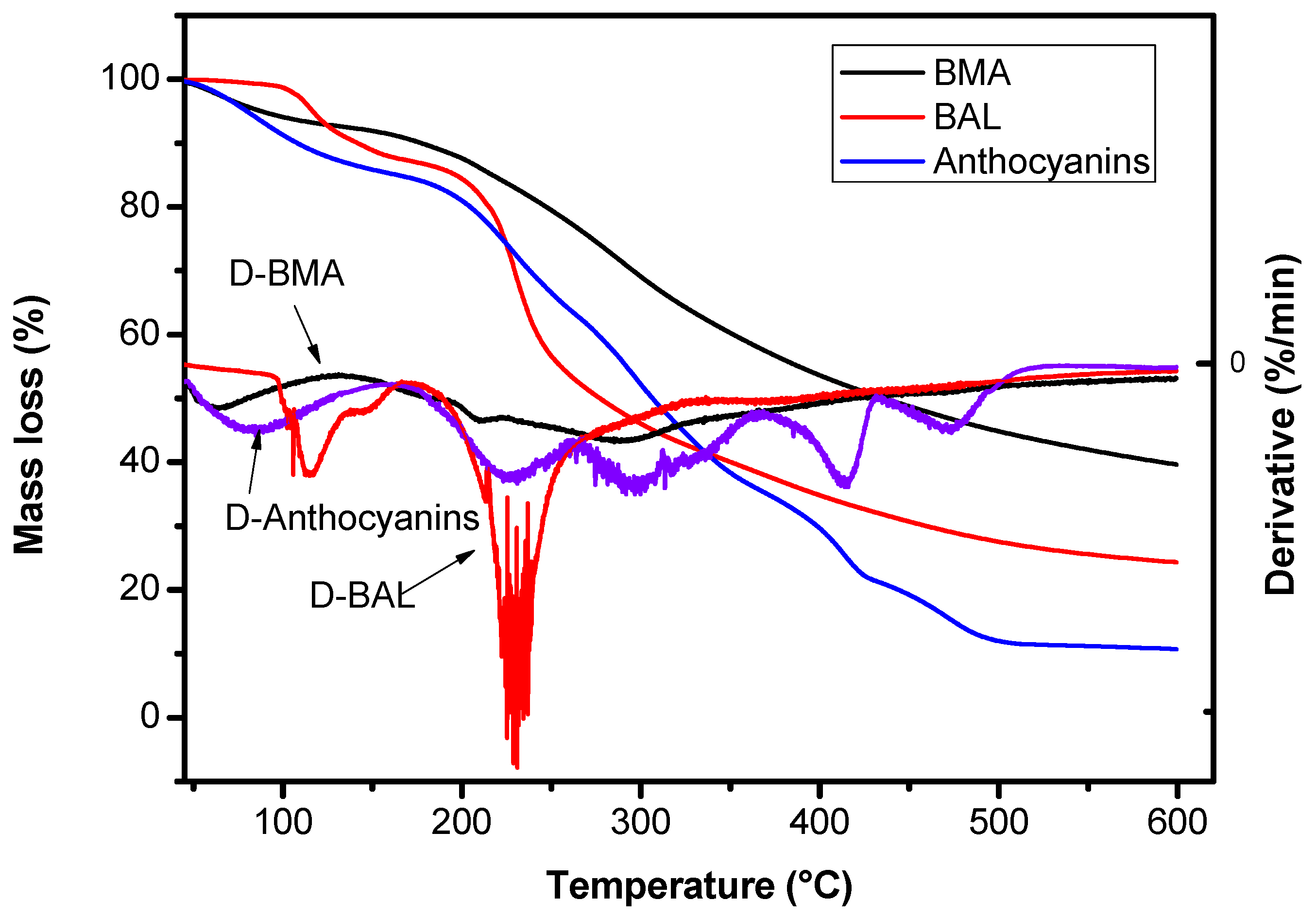
2.3.5. Thermal Stability
3. Materials and Methods
3.1. Materials
3.2. Preparation of BAM and Single-Factor Experiments
3.3. Preparation of BAL and Single Factor Experiments
3.4. Determination of Blueberry Anthocyanin Contents in BAM and BAL
3.5. Determination of the Encapsulation Efficiency
3.5.1. Determination of the BAM Encapsulation Efficiency
3.5.2. Determination of the BAL Encapsulation Efficiency
3.6. Characterization and Evaluation of Stability
3.6.1. Characterization
3.6.2. Light and Thermal Stability
3.7. Statistical Analysi
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ma, Y.H.; Li, Y.H.; Zhang, H.Z.; Wang, Y.; Wu, C.E.; Huang, W.Y. Malvidin induces hepatic stellate cell apoptosis via the endoplasmic reticulum stress pathway and mitochondrial pathway. Food Sci. Nutri. 2020, 8, 5095–5106. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W.Y. Blueberry anthocyanins: An updated review on approaches to enhancing their bioavailability. Trends Food Sci. Technol. 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Yang, W.J.; Guo, Y.X.; Liu, M.; Chen, X.F.; Xiao, X.Y.; Wang, S.N.; Gong, P.; Ma, Y.M.; Chen, F.X. Structure and function of blueberry anthocyanins: A review of recent advances. J. Functional. Foods 2022, 88, 104–864. [Google Scholar] [CrossRef]
- Nazaruddin, N.; Afifah, N.; Bahi, M.; Susilawati, S.; Sani, N.D.M.; Esmaeili, C.; Iqhrammullah, M.; Murniana, M.; Hasanah, U.; Safitri, E. A simple optical pH sensor based on pectin and Ruellia tuberosa L-derived anthocyanin for fish freshness monitoring. F1000 Res. 2021, 10, 422. [Google Scholar] [CrossRef]
- Ge, J.; Yue, P.X.; Chi, J.P.; Liang, J.; Gao, X.L. Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocoll. 2018, 74, 23–31. [Google Scholar] [CrossRef]
- He, W.J.; Mu, H.B.; Liu, Z.M.; Lu, M.; Hang, F.; Chen, J.; Zeng, M.M.; Qin, F.; He, Z.Y. Effect of preheat treatment of milk proteins on their interactions with cyanidin-3-O-glucoside. Food Res. Int. 2018, 107, 394–405. [Google Scholar] [CrossRef]
- Xie, C.J.; Wang, Q.; Ying, R.F.; Wang, Y.S.; Wang, Z.J.; Huang, M.G. Binding a chondroitin sulfate-based nanocomplex with kappa-carrageenan to enhance the stability of anthocyanins. Food Hydrocoll. 2020, 100, 48–104. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, Y.J.; Luo, L.P.; Nie, F.; Gao, G.L. Acylation and Stability Analysis of Blueberry Anthocyanins. Destech Trans. Eng. Technol. Res. 2018. [Google Scholar] [CrossRef]
- de Araujo Santiago, M.C.P.; Nogueira, R.I.; Paim, D.R.S.F.; Gouvêa, A.C.M.S.; de Oliveira Godoy, R.L.; Peixoto, F.M.; Pacheco, S.; Freitas, S.P. Effects of encapsulating agents on anthocyanin retention in pomegranate powder obtained by the spray drying process. LWT Food Sci. Technol. 2016, 73, 551–556. [Google Scholar] [CrossRef]
- Shi, Y.G.; Wang, W.; Zhu, X.Q.; Wang, B.; Hao, Y.; Wang, L.Q.; Yu, D.Y.; Walid, E. Preparation and physicochemical stability of hemp seed oil liposomes. Ind. Crops Prod. 2021, 162, 113–283. [Google Scholar] [CrossRef]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef]
- Cai, X.R.; Du, X.F.; Cui, D.M.; Wang, X.N.; Yang, Z.K.; Zhu, G.L. Improvement of stability of blueberry anthocyanins by carboxymethyl starch/xanthan gum combinations microencapsulation. Food Hydrocoll. 2019, 91, 238–245. [Google Scholar] [CrossRef]
- da Rosa, J.R.; Nunes, G.L.; Motta, M.H.; Fortes, J.P.; Weis, G.C.C.; Hecktheuer, L.H.R.; Muller, E.I.; de Menezes, C.R.; da Rosa, C.S. Microencapsulation of anthocyanin compounds extracted from blueberry (Vaccinium spp.) by spray drying: Characterization, stability and simulated gastrointestinal conditions. Food Hydrocoll. 2019, 89, 742–748. [Google Scholar] [CrossRef]
- Liao, M.J.; Ma, L.J.; Miao, S.; Hu, X.S.; Liao, X.J.; Chen, F.; Ji, J.F. The in-vitro digestion behaviors of milk proteins acting as wall materials in spray-dried microparticles: Effects on the release of loaded blueberry anthocyanins. Food Hydrocoll. 2021, 115, 106–620. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Pegg, R.B.; Kong, F.B. Total phenolics content and antioxidant capacities of microencapsulated blueberry anthocyanins during in vitro digestion. Food Chem. 2014, 153, 272–278. [Google Scholar] [CrossRef]
- He, H.S.; Lu, Y.; Qi, J.P.; Zhu, Q.G.; Chen, Z.J.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Li, M.Y.; Du, C.Y.; Guo, N.; Teng, Y.O.; Meng, X.; Sun, H.; Li, S.S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Chi, J.P.; Ge, J.; Yue, X.Y.; Liang, J.; Sun, Y.; Gao, X.L.; Yue, P.X. Preparation of nanoliposomal carriers to improve the stability of anthocyanins. LWT-Food Sci. Technol. 2019, 109, 101–107. [Google Scholar] [CrossRef]
- Zhao, L.S.; Temelli, F.; Chen, L.Y. Encapsulation of anthocyanin in liposomes using supercritical carbon dioxide: Effects of anthocyanin and sterol concentrations. J. Funct. Foods. 2017, 34, 159–167. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Rahman, M.R.T.; Vuong, Q.V. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Song, J.H.; Yu, Y.; Chen, M.H.; Ren, Z.Y.; Chen, L.; Fu, C.L.; Ma, Z.F.; Li, Z.M. Advancement of Protein- and Polysaccharide-Based Biopolymers for Anthocyanin Encapsulation. Front. Nutri. 2022, 9, 829–938. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Pereira, B.C.S.; Lopes, G.K.; Andrade, C.T. Encapsulation and antioxidant activity of assai pulp oil (Euterpe oleracea) in chitosan/alginate polyelectrolyte complexes. Food Hydrocoll. 2020, 109, 97–106. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49–50, 175–220. [Google Scholar] [CrossRef]
- Hosseini, S.; Varidi, M. Optimization of Microbial Rennet Encapsulation in Alginate-Chitosan Nanoparticles. Food Chem. 2021, 352, 32–129. [Google Scholar] [CrossRef]
- Petraityte, S.; Sipailiene, A. Enhancing encapsulation efficiency of alginate capsules containing lactic acid bacteria by using different divalent cross-linkers sources. LWT-Food Sci. Technol. 2019, 110, 307–315. [Google Scholar] [CrossRef]
- Sridharan, S.; Meinders, M.B.J.; Bitter, J.H.; Nikiforidis, C.V. Pea flour as stabilizer of oil-in-water emulsions: Protein purification unnecessary. Food Hydrocoll. 2020, 101, 105–533. [Google Scholar] [CrossRef]
- Tang, B.; He, Y.; Liu, J.; Zhang, J.; Li, J.L.; Zhou, J.; Ye, Y.; Wang, J.F.; Wang, X.G. Kinetic investigation into pH-dependent color of anthocyanin and its sensing performance. Dye. Pigment. 2019, 170, 107–643. [Google Scholar] [CrossRef]
- Wang, H.J.; Sun, S.; Zhou, Z.; Qiu, Z.K.; Cui, X. Rapid analysis of anthocyanin and its structural modifications in fresh tomato fruit. Food Chem. 2020, 333, 127–439. [Google Scholar] [CrossRef]
- Koh, J.; Xu, Z.M.; Wicker, L. Blueberry pectin and increased anthocyanins stability under in vitro digestion. Food Chem. 2019, 302, 125–343. [Google Scholar] [CrossRef]
- Dong, T.T.; Han, R.P.; Yu, J.W.; Zhu, M.K.; Zhang, Y.; Gong, Y.; Li, Z.Y. Anthocyanins accumulation and molecular analysis of correlated genes by metabolome and transcriptome in green and purple asparaguses (Asparagus officinalis L.). Food Chem. 2019, 271, 18–28. [Google Scholar] [CrossRef]
- Guldiken, B.; Gibis, M.; Boyacioglu, D.; Capanoglu, E.; Weiss, J. Physical and chemical stability of anthocyanin-rich black carrot extract loaded liposomes during storage. Food Res. Int. 2018, 108, 491–497. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.L.; Wang, X.; Lu, B.J.; Zhang, J. Preparation of BAL and changes of vesicle properties, physicochemical properties, in vitro release, and antioxidant activity before and after chitosan modification. Food Sci. Nutr. 2022, 10, 75–87. [Google Scholar] [CrossRef]
- Li, D.N.; Li, B.; Ma, Y.; Sun, X.Y.; Lin, Y.; Meng, X.J. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Dias, S.; Castanheira, E.M.S.; Fortes, A.G.; Pereira, D.M.; Goncalves, M.S.T. Natural Pigments of Anthocyanin and Betalain for Coloring Soy-Based Yogurt Alternative. Foods 2020, 9, 771. [Google Scholar] [CrossRef]
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef]
- Gallez, A.; Palazzo, C.; Blacher, S.; Tskitishvili, E.; Noel, A.; Foidart, J.M.; Evrard, B.; Pequeux, C.; Piel, G. Liposomes and drug-in-cyclodextrin-in-liposomes formulations encapsulating 17 beta-estradiol: An innovative drug delivery system that prevents the activation of the membrane-initiated steroid signaling (MISS) of estrogen receptor alpha. Int. J. Pharm. 2020, 573, 118–861. [Google Scholar] [CrossRef] [PubMed]
- Guldiken, B.; Linke, A.; Capanoglu, E.; Boyacioglu, D.; Kohlus, R.; Weiss, J.; Gibis, M. Formation and characterization of spray dried coated and uncoated liposomes with encapsulated black carrot extract. J. Food Eng. 2019, 246, 42–50. [Google Scholar] [CrossRef]
- Savic, S.; Lukic, M.; Jaksic, I.; Reichl, S.; Tamburic, S.; Muller-Goymann, C. An alkyl polyglucoside-mixed emulsifier as stabilizer of emulsion systems: The influence of colloidal structure on emulsions skin hydration potential. J. Colloid Interface Sci. 2011, 358, 182–191. [Google Scholar] [CrossRef]
- Pereira Souza, A.C.; Gurak, P.D.; Marczak, L.D.F. Maltodextrin, pectin and soy protein isolate as carrier agents in the encapsulation of anthocyanins-rich extract from jaboticaba pomace. Food Bioprod. Process. 2017, 102, 186–194. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, G.; Kong, X.; Wang, J.; Mu, W.; Wang, Y. Encapsulation and characterisation of grape seed proanthocyanidin extract using sodium alginate and diferent cellulose derivatives. Int. J. Food Sci. Technol. 2021, 56, 6420–6430. [Google Scholar] [CrossRef]
- Lima, Á.S.; Soares, C.M.F.; Paltram, R.; Halbwirth, H.; Bica, K. Extraction and consecutive purification of anthocyanins from grape pomaceusing ionic liquid solutions. Fluid Phase Equilibria 2017, 451, 68–78. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hofsommer, H.; Koswig, S.; Krueger, A.D.; Kupina, S.; Martin, S.K.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2023, 5, 1269–1278. [Google Scholar] [CrossRef]
| No. | Independent Variables | Response Value | ||
|---|---|---|---|---|
| CaCl2 Concentration (%) | Sodium Alginate: CaCl2 | Encapsulation Time (h) | Encapsulation Efficiency (%) | |
| 1 | 2 | 1:4 | 2 | 85.25 |
| 2 | 2 | 1:4 | 1 | 89.32 |
| 3 | 2 | 1:5 | 1.5 | 92.66 |
| 4 | 2 | 1:5 | 1.5 | 90.97 |
| 5 | 1.5 | 1:5 | 2 | 90.59 |
| 6 | 2 | 1:5 | 1.5 | 91.95 |
| 7 | 1.5 | 1:4 | 1.5 | 90.88 |
| 8 | 2.5 | 1:4 | 1.5 | 90.2 |
| 9 | 2 | 1:6 | 1 | 85.32 |
| 10 | 2.5 | 1:5 | 2 | 87.57 |
| 11 | 2 | 1:5 | 1.5 | 89.84 |
| 12 | 1.5 | 1:6 | 1.5 | 84.6 |
| 13 | 2.5 | 1:6 | 1.5 | 83.43 |
| 14 | 2 | 1:5 | 1.5 | 92.59 |
| 15 | 2.5 | 1:5 | 1 | 90.86 |
| 16 | 1.5 | 1:5 | 1 | 95.27 |
| 17 | 2 | 1:6 | 2 | 83.19 |
| Source of Variation | Sum of Mean Square | Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Models | 184.78 | 9 | 20.53 | 8.55 | 0.0049 |
| X1 | 12.20 | 1 | 12.20 | 5.08 | 0.0489 |
| X2 | 45.51 | 1 | 45.51 | 18.94 | 0.0033 |
| X3 | 23.12 | 1 | 23.12 | 9.62 | 0.0173 |
| X1X2 | 0.060 | 1 | 0.060 | 0.025 | 0.8789 |
| X1X3 | 0.99 | 1 | 0.99 | 0.41 | 0.5414 |
| X2X3 | 0.91 | 1 | 0.91 | 0.38 | 0.5573 |
| X12 | 0.71 | 1 | 0.71 | 0.29 | 0.6039 |
| X22 | 94.39 | 1 | 94.39 | 39.29 | 0.0004 |
| X32 | 5 | 1 | 5 | 2.08 | 0.1923 |
| Residuals | 16.62 | 7 | 2.40 | ||
| Misfit term | 11.10 | 3 | 3.70 | 2.59 | 0.1905 |
| Error | 5.72 | 4 | 1.43 | ||
| Total | 201.60 | 16 | |||
| R2 | 0.9166 | ||||
| Adj.R2 | 0.8093 |
| No. | Independent Variables | Response Value | ||
|---|---|---|---|---|
| Ultrasonic Time (min) | The Amount of Anthocyanins Added (mg) | Lecithin Addition (mg) | Encapsulation Efficiency (%) | |
| 1 | 8 | 10 | 120 | 75.25 |
| 2 | 8 | 10 | 80 | 73.32 |
| 3 | 8 | 12 | 100 | 82.99 |
| 4 | 8 | 12 | 100 | 83.97 |
| 5 | 6 | 12 | 120 | 69.59 |
| 6 | 8 | 12 | 100 | 80.59 |
| 7 | 6 | 10 | 100 | 76.88 |
| 8 | 10 | 10 | 100 | 87.20 |
| 9 | 8 | 14 | 80 | 67.35 |
| 10 | 10 | 1:5 | 120 | 70.70 |
| 11 | 8 | 1:5 | 100 | 79.78 |
| 12 | 6 | 1:6 | 100 | 63.67 |
| 13 | 10 | 1:6 | 100 | 69.23 |
| 14 | 8 | 1:5 | 100 | 78.58 |
| 15 | 10 | 1:5 | 80 | 74.26 |
| 16 | 6 | 1:5 | 80 | 77.21 |
| 17 | 6 | 14 | 120 | 63.16 |
| Source of Variation | Sum of Mean Square | Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Models | 691.26 | 9 | 76.81 | 5.85 | 0.0148 |
| X1 | 24.68 | 1 | 24.68 | 1.88 | 0.0129 |
| X2 | 300.25 | 1 | 300.25 | 22.85 | 0.0020 |
| X3 | 23.22 | 1 | 23.22 | 1.76 | 0.0263 |
| X1X2 | 5.20 | 1 | 5.20 | 0.40 | 0.5493 |
| X1X3 | 3.98 | 1 | 3.98 | 0.30 | 0.5992 |
| X2X3 | 9.27 | 1 | 9.27 | 0.71 | 0.4287 |
| X12 | 15.42 | 1 | 15.42 | 1.17 | 0.3145 |
| X22 | 107.17 | 1 | 107.17 | 8.20 | 0.0232 |
| X32 | 174.17 | 1 | 174.17 | 13.25 | 0.0003 |
| Residuals | 91.98 | 7 | 13.14 | - | - |
| Misfit term | 72.40 | 3 | 24.13 | 4.93 | 0.0787 |
| Error | 19.56 | 4 | 1.43 | ||
| Total | 763.24 | 16 | |||
| R2 | 0.9026 | ||||
| Adj.R2 | 0.7993 |
| Levels | Factors | ||
|---|---|---|---|
| CaCl2 Concentration (%) | Sodium Alginate: CaCl2 | Encapsulation Time (h) | |
| −1 | 1.5 | 1:4 | 1 |
| 0 | 2 | 1:5 | 1.5 |
| 1 | 2.5 | 1:6 | 2 |
| Levels | Factors | ||
|---|---|---|---|
| Anthocyanins Concentration (mg) | Ultrasonic Time (min) | Soy Lecithin Concentration (mg) | |
| −1 | 6 | 8 | 80 |
| 0 | 8 | 12 | 100 |
| 1 | 10 | 16 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Fang, W.; Liu, W.; Liu, J.; Gong, P. Microcapsules and Nanoliposomes Based Strategies to Improve the Stability of Blueberry Anthocyanins. Molecules 2023, 28, 7344. https://doi.org/10.3390/molecules28217344
Chen J, Fang W, Liu W, Liu J, Gong P. Microcapsules and Nanoliposomes Based Strategies to Improve the Stability of Blueberry Anthocyanins. Molecules. 2023; 28(21):7344. https://doi.org/10.3390/molecules28217344
Chicago/Turabian StyleChen, Jian, Wenjing Fang, Wei Liu, Jianghua Liu, and Pin Gong. 2023. "Microcapsules and Nanoliposomes Based Strategies to Improve the Stability of Blueberry Anthocyanins" Molecules 28, no. 21: 7344. https://doi.org/10.3390/molecules28217344
APA StyleChen, J., Fang, W., Liu, W., Liu, J., & Gong, P. (2023). Microcapsules and Nanoliposomes Based Strategies to Improve the Stability of Blueberry Anthocyanins. Molecules, 28(21), 7344. https://doi.org/10.3390/molecules28217344




