Abstract
Echinacea purpurea (L.) Moench is a medicinal plant commonly used for the treatment of upper respiratory tract infections, the common cold, sore throat, migraine, colic, stomach cramps, and toothaches and the promotion of wound healing. Based on the known pharmacological properties of essential oils (EOs), we hypothesized that E. purpurea EOs may contribute to these medicinal properties. In this work, EOs from the flowers of E. purpurea were steam-distilled and analyzed by gas chromatography–mass spectrometry (GC–MS), GC with flame-ionization detection (GC–FID), and chiral GC–MS. The EOs were also evaluated for in vitro antimicrobial and innate immunomodulatory activity. About 87 compounds were identified in five samples of the steam-distilled E. purpurea EO. The major components of the E. purpurea EO were germacrene D (42.0 ± 4.61%), α-phellandrene (10.09 ± 1.59%), β-caryophyllene (5.75 ± 1.72%), γ-curcumene (5.03 ± 1.96%), α-pinene (4.44 ± 1.78%), δ-cadinene (3.31 ± 0.61%), and β-pinene (2.43 ± 0.98%). Eleven chiral compounds were identified in the E. purpurea EO, including α-pinene, sabinene, β-pinene, α-phellandrene, limonene, β-phellandrene, α-copaene, β-elemene, β-caryophyllene, germacrene D, and δ-cadinene. Analysis of E. purpurea EO antimicrobial activity showed that they inhibited the growth of several bacterial species, although the EO did not seem to be effective for Staphylococcus aureus. The E. purpurea EO and its major components induced intracellular calcium mobilization in human neutrophils. Additionally, pretreatment of human neutrophils with the E. purpurea EO or (+)-δ-cadinene suppressed agonist-induced neutrophil calcium mobilization and chemotaxis. Moreover, pharmacophore mapping studies predicted two potential MAPK targets for (+)-δ-cadinene. Our results are consistent with previous reports on the innate immunomodulatory activities of β-caryophyllene, α-phellandrene, and germacrene D. Thus, this study identified δ-cadinene as a novel neutrophil agonist and suggests that δ-cadinene may contribute to the reported immunomodulatory activity of E. purpurea.
1. Introduction
The genus Echinacea L. (Asteraceae) contains ten species generally known as coneflowers. Coneflowers have big, showy heads of composite flowers with spiny central disks that bloom throughout the summer in Europe and North America. Echinacea purpurea (L.) Moench (purple coneflower) is a well-known medicinal-ornamental perennial plant native to damp prairies, meadows, and open forests of the central to southeastern parts of the United States (Ohio, Michigan, Iowa, Louisiana, and Georgia) [1]. Due to its pharmacological importance, Echinacea is cultivated worldwide. It typically grows from 2 to 4’ tall and has coarse, ovate to broad-lanceolate, dark green leaves. Brauneria purpurea (L.) Britton, Echinacea intermedia Lindl. Ex Paxton, Echinacea serotina (Sweet) D. Don ex G. Don f., Echinacea speciosa (Wender.) Paxton, Helichroa purpurea (L.) Raf., and Rudbeckia purpurea L. are known botanical synonyms of E. purpurea [2].
E. purpurea preparations are among the best-selling herbal supplements [3]. Historically, E. purpurea has been used in the treatment of upper respiratory tract infections [4], common colds [5], sore throats, migraines, colic, stomach cramps, and toothaches [6] and to promote wound healing [7]. Likewise, extracts of various parts of E. purpurea have been reported to exhibit antioxidant, anti-inflammatory, anxiolytic, immunoregulatory, antiproliferative, antiviral, antibacterial, and antifungal properties [1,8,9,10,11,12,13,14]. This plant has a reputation for stimulating the immune system to help fight infectious diseases. Standardized preparations of E. purpurea have several applications for respiratory tract infections [5,15,16,17,18,19,20,21,22,23], viral infections [24,25,26,27], tumor suppression [28], acne, and skin diseases [29,30]. In addition, a mixture of E. purpurea and E. angustifolia was reported to protect against acetic acid-induced ulcerative colitis in rats [31]. Attempts to use Echinacea as an immune modulator date back over a century, with early experiments revealing its potential to stimulate the immune system. Echinacea’s immunological properties have been extensively studied since 1923 [32]. Early studies confirmed phagocyte-stimulating, hyaluronidase-inhibiting, and properdin-generating activities. Experiments using all three medicinal species of Echinacea have demonstrated macrophage-activating properties [33], including reports of increased macrophage phagocytic activity accompanied with enhanced cytokine production, in vitro and in vivo [33]. E. purpurea EOs have been shown to exhibit anti-inflammatory effects in mice and rats [12,34], and EOs from various medicinal plants have been reported to exhibit immunomodulatory activity through their ability to modulate neutrophil functional activity. Since neutrophils perform essential host defense functions, they represent an ideal pharmacological target for therapeutic development [35]. Likewise, the antimicrobial properties of EOs have been reported in several studies (reviewed in [36]). Thus, we propose that a combination of antimicrobial activity with innate immunomodulatory activity may represent an ideal approach for protection against pathogens while modulating the ensuing inflammatory response and suggest that such EOs may offer promise as an alternative treatment option.
The efficacy of E. purpurea preparations has been investigated in several clinical trials, but the results varied greatly depending on the plant parts used, the extract type, the variability of active components, and the sample size. Previous studies identified several bioactive compounds from ethanolic and water extracts of E. purpurea, including caffeic acid derivatives (caffeic acid, caftaric acid, cichoric acid, echinacoside, chlorogenic acid, and cynarin), alkylamides, flavonoids (rutin, quercetin, nicotiflorin, and luteolin), polysaccharides, and polyacetylenes [37,38,39,40,41,42,43]. These compounds have been extensively studied and are thought to be responsible for the bioactivity of E. purpurea extracts. Although a few studies investigated the E. purpurea EO, little is known about their biological activities. Based on previous studies demonstrating immunomodulatory activity of EOs, we hypothesized that the E. purpurea EO may contribute to the medicinal properties of extracts from this plant. Thus, the current study aims to explore the volatile composition of EOs extracted from the flowers of E. purpurea from Bulgaria and evaluate their in vitro antibacterial activity and innate immunomodulatory potential in human neutrophils.
2. Results and Discussion
2.1. Chemical Composition
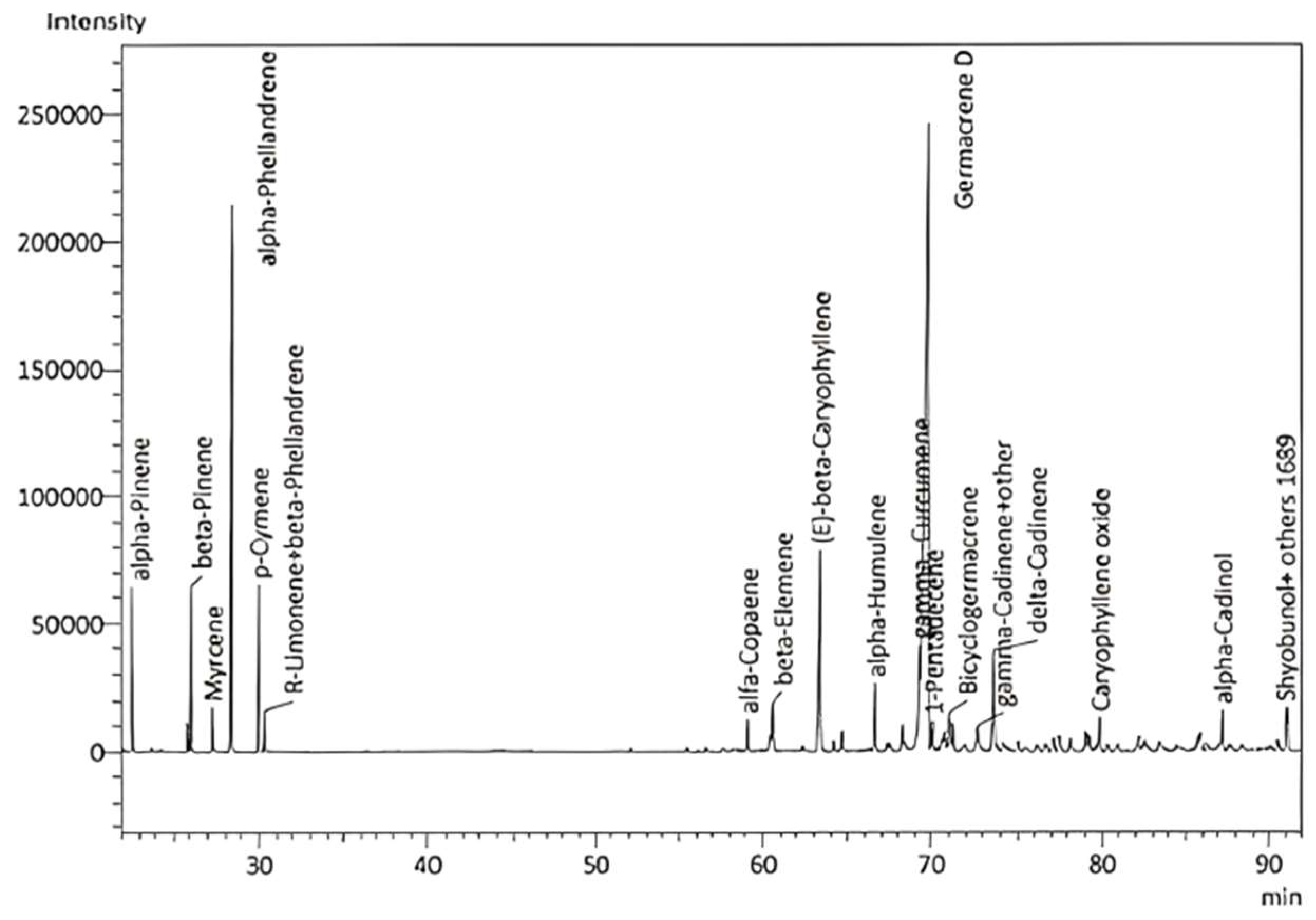
Five E. purpurea samples (E1–5) were steam-distilled for 3–4 h in a Clevenger-type apparatus. The average yield was 0.13 ± 0.06%. The EO had a deeply sweet, herbaceous, floral, slightly grassy, and lightly hay-like scent and was analyzed by GC–MS and GC–FID to identify and quantify the EO component compounds, respectively (Figure 1). A total of 87 compounds were identified, representing 97.65–100% of the total EO composition (Table 1). Note, it has been reported that the volatile components can be detected in the aerial parts and roots of E. purpurea, with variable yields and chemical compositions that can be affected by pathogen attacks [44]. The major components of the E. purpurea EO in this study were germacrene D (42.0 ± 4.61%), α-phellandrene (10.09 ± 1.59%), β-caryophyllene (5.75 ± 1.72%), γ-curcumene (5.03 ± 1.96%), α-pinene (4.44 ± 1.78%), δ-cadinene (3.31 ± 0.61%), and β-pinene (2.43 ± 0.98%) (values indicate % of the EO). The abundance of germacrene D correlates well with previous reports on essential oils from the flowerheads of E. purpurea [45,46,47]. Interestingly, analysis of the flowerheads revealed that α- and β-pinene, β-myrcene, ocimene, limonene, camphene, and terpinene were the main components [48]. The hydrodistilled EO of cultivated E. purpurea flowerheads during ontogenesis comprised mainly β-caryophyllene (25.0%), fatty acids (17.2%), germacrene D (13.8%), α,β-pinene (9.5%), nerolidol (6.6%), and α-phellandrene (4.2%) [49].
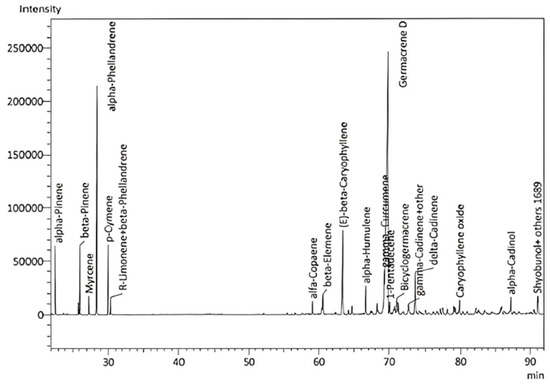
Figure 1.
GC–MS chromatogram of E. purpurea flower EO.

Table 1.
Chemical composition of five samples (E1–5) of the flower essential oil of Echinacea purpurea, expressed as percent (%).
2.2. Enantiomeric Distribution
The enantiomeric distribution of chiral compounds in E. purpurea EO is presented in Table 2. The results revealed a total of 11 chiral compounds: α-pinene, sabinene, β-pinene, α-phellandrene, limonene, β-phellandrene, α-copaene, β-elemene, β-caryophyllene, germacrene D, and δ-cadinene. (−)-α-phellandrene, (+)-α-copaene, and (−)-β-caryophyllene appeared as pure enantiomers. Thus, these chiral constituents and their enantiomeric distributions could be used as reference standards for identifying adulteration in E. purpurea EO.

Table 2.
Enantiomeric distributions of chiral compounds present in essential oils of Echinacea purpurea.
2.3. Antimicrobial Activity of E. purpurea EO
It has been previously published that extracts from E. purpurea had MIC values of 93.8 mg/mL against S. mutans and 375 mg/mL against E. coli [50]. It is likely that much of the antimicrobial activity of the E. purpurea EO is derived from the germacrene D content, as the Echinacea EO has been shown to contain about 42% germacrene D [51]. As shown in Table 3, the E. purpurea EO inhibited the growth of several bacterial species, although the EO did not seem to be effective against S. aureus and had lower activity for E. coli and S. epidermidis. The major constituents contributing to the antimicrobial activity of the E. purpurea EO are likely β-caryophyllene and α-pinene, which have both exhibited potent antimicrobial activities as single compounds in vitro [36,52,53,54,55,56]. The other major constituents in the E. purpurea EO, including germacrene D, α-phellandrene, β-pinene, γ-curcumene, and δ-cadinene, may provide additive or synergistic antimicrobial effects, as these molecules, or EOs derived from other plants that contain high levels of these molecules, have been reported to exhibit moderate antimicrobial activities [56,57,58,59,60,61,62,63,64].

Table 3.
Summary of antimicrobial data of E. purpurea flower EO.
2.4. Innate Immunomodulatory Activity of E. purpurea EO and Its Components
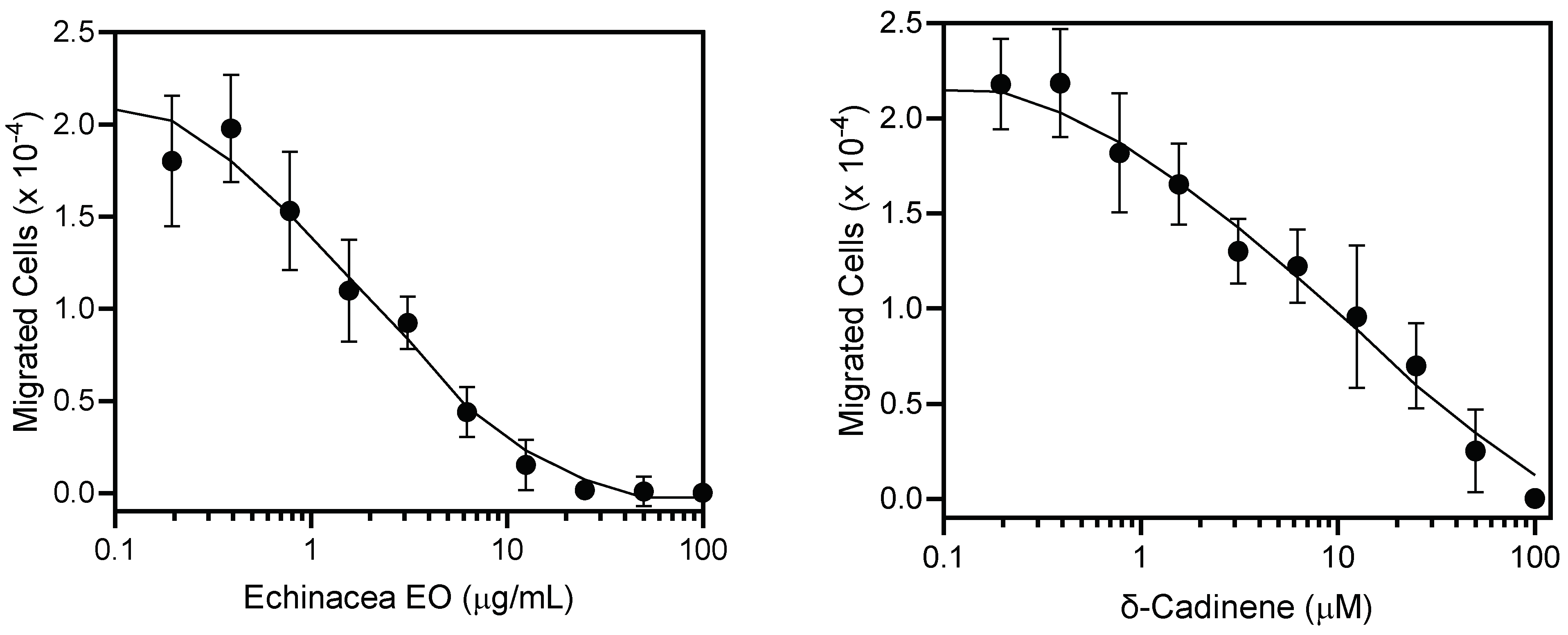
A growing body of research suggests that E. purpurea has immunostimulatory properties. For example, the E. purpurea root extract was reported to have immune-enhancing properties by lowering the frequency and function of regulatory T cells [65]. In another study, oral administration of an E. purpurea extract increased natural killer cell activity in mice by increasing the levels of MHC II, CD4 T cells, and Th1 cytokines [66]. Likewise, an ethanolic extract of the aerial parts was reported to modulate cytokine response in human T-cells [67]. We hypothesized that the E. purpurea EO could have innate immunomodulatory properties. In the present study, human neutrophils were used to evaluate the innate immunomodulatory effects of the E. purpurea EO and their major components. Neutrophils are immune cells that serve as both effectors and regulators in the development of the innate inflammatory response [68]. As shown in Table 4, the treatment of human neutrophils with the E. purpurea EO induced intracellular Ca2+ mobilization (EC50 = 19.9 ± 4.5 μg/mL). This is an important finding, as intracellular Ca2+ mobilization plays an important role in neutrophil activation and function [69,70]. As shown in Table 4, the E. purpurea EO components (+)-δ-cadinene and α-phellandrene also induced comparable changes in intracellular Ca2+ concentrations ([Ca2+]i) in neutrophils compared to that observed previously with germacrene D [71] with EC50 values between 20.8 and 24.6 μM. In contrast, no activity was observed for β-caryophyllene. Previously, we found that EOs and their components could inhibit neutrophil migration [71,72,73]. Thus, we analyzed the effects of the E. purpurea EO and (+)-δ-cadinene on neutrophil chemotaxis and found that pretreatment with the E. purpurea EO (IC50 = 1.8 ± 0.6 μg/mL) or (+)-δ-cadinene (IC50 = 0.48 ± 0.1 μM) for 10 min suppressed fMLF-induced human neutrophil chemotaxis in a dose-dependent manner (Table 4, Figure 2).

Table 4.
Effect of E. purpurea EO and pure major components on [Ca2+]i and chemotaxis in human neutrophils and cytotoxicity in THP-1 monocytic cells.
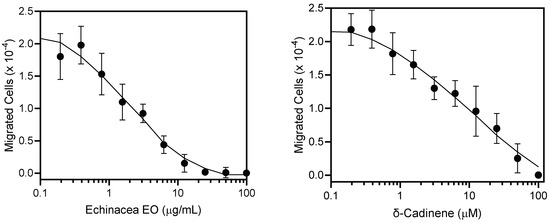
Figure 2.
Inhibition of neutrophil chemotaxis by Echinacea purpurea EO and (+)-δ-cadinene. Neutrophil chemotaxis toward 1 nM fMLF was measured, as described under Section 3. The data are presented as the mean ± SEM and are based on two independent experiments.
Furthermore, we tested the cytotoxicity of the E. purpurea EO and its components at various concentrations in human THP-1 monocytic cells during 90 min and 24 h incubation periods to ensure that the effects on neutrophil functional activity (i.e., inhibition of Ca2+ mobilization and cell migration) were not significantly influenced by potential toxicity. As shown in Table 4, the E. purpurea EO and components had no cytotoxicity after 90 min and very little cytotoxicity after 24 h, confirming that the Ca2+ flux and chemotaxis assays were not influenced by possible cytotoxicity.
Although determining the mechanism is beyond the scope of this study, we sought to explore the potential protein targets of (+)-δ-cadinene. Reverse-pharmacophore mapping using the molecular structure of (+)-δ-cadinene was performed to identify its potential cellular targets. Table 5 shows the top 10 human protein targets for (+)-δ-cadinene out of 300 potential targets ranked by normalized fit scores in descending order. Interestingly, two MAPKs are indicated as possible targets among the top selected targets, namely mitogen-activated protein (MAP) kinase 1 and MAP kinase-activated protein kinase 2. Since MAP kinases play an important role in neutrophil activation and host defense mechanisms (reviewed in [74]), the possibility that (+)-δ-cadinene might interact with these kinases is very interesting and warrants future investigation of this area.

Table 5.
Potential human protein targets of (+)-δ-cadinene identified by PharmMapper.
3. Materials and Methods
3.1. Materials
Dichloromethane, dimethyl sulfoxide (DMSO), N-formyl-Met-Leu-Phe (fMLF), streptomycin, and penicillin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Germacrene D, (+)-δ-cadinene, α-phellandrene, and β-caryophyllene were purchased from Cayman Chemical (Ann Arbor, MI, USA), Fluo-4AM was purchased from Invitrogen (Carlsbad, CA, USA). RPMI 1640 medium was purchased from Mediatech Inc., Herndon, VA, USA. Fetal bovine serum (FBS) was purchased from ATCC (Manassas, VA, USA). Hanks’ balanced salt solution (HBSS; 0.137 M NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 5.56 mM glucose, and 10 mM HEPES, pH 7.4) was purchased from Life Technologies (Grand Island, NY, USA). HBSS without Ca2+ and Mg2+ is referred to as HBSS−; HBSS containing 1.3 mM CaCl2 and 1.0 mM MgSO4 is referred to as HBSS+.
3.2. Essential Oil Extraction
Cultivated E. purpurea was harvested from Dobrich, Bulgaria, in June–July 2020. Fresh flowers were handpicked in the early morning, transferred immediately, and steam-distilled for 3–4 h in a Clevenger-type apparatus. The plant material-to-water ratio was 1:6.
3.3. Gas Chromatography-Mass Spectrometry (GC–MS) Analysis
The E. purpurea EOs were analyzed using a Shimadzu GC–MS-QP2010 Ultra (Shimadzu Scientific Instruments, Columbia, MD, USA) with electron impact (EI) mode with 70 eV, using 40–400 m/z range scans with a scan rate of 3.0 scan/s. The GC column used was a ZB-5ms fused silica capillary column with a (5% phenyl)-polymethylsiloxane stationary phase and a film thickness of 0.25 μm, a length of 30 m, and an internal diameter of 0.25 mm. The column temperature was set at 50 °C for 2 min and then increased by 2 °C/min to the temperature of 260 °C. The carrier gas was helium with a column head pressure of 552 kPa and a constant flow rate of 1.37 mL/min. The injector temperature was kept at 260 °C, and the ion source temperature was 200 °C. For each essential oil sample, a 1:10 v/v solution in dichloromethane (DCM) was prepared, and 0.3 μL was injected using a split ratio of 1:30. The EO components were identified by comparing mass spectral fragmentation patterns (over 80% similarity match) and retention indices (RI) based on a series of homologous C8-C20 n-alkanes with those reported in databases [NIST database, and our in-house library] using the Lab Solutions GC–MS post-run analysis software version 4.45 (Shimadzu Scientific Instruments, Columbia, MD, USA).
3.4. Gas Chromatography–Flame Ionization Detection (GC–FID) Analysis
GC–FID analysis of the E. purpurea EO was performed using a Shimadzu GC 2010 equipped with a flame ionization detector (Shimadzu Scientific Instruments, Columbia, MD, USA), as previously described [75], with a ZB-5 capillary column (Phenomenex, Torrance, CA, USA).
3.5. Enantiomeric Analysis by Chiral Gas Chromatography–Mass Spectrometry (CGC–MS)
A Shimadzu GC–MS-QP2010S with EI mode (70 eV) and B-Dex 325 chiral capillary GC column was used to perform the enantiomeric analysis of E. purpurea EO. Scans were in the 40–400 m/z range at a scan rate of 3.0 scan/s. The column temperature was set to 50 °C and, at first, increased by 1.5 °C/min to 120 °C and then 2 °C/min to 200 °C. The final temperature of the column was 200 °C and was kept constant. The carrier gas was helium, with a constant flow rate of 1.8 mL/min. For each EO sample, 3% w/v solution in DCM was prepared and 0.1 μL was injected using a split ratio of 1:45 [75,76]. The enantiomer percentages were determined from the peak area. A comparison of retention times and mass spectral fragmentation patterns was performed against authentic samples obtained from Sigma-Aldrich (Milwaukee, WI, USA) and was used to identify the enantiomers.
3.6. Isolation of Human Neutrophils
Human neutrophils were obtained using blood collected from healthy donors. Blood collection was approved by the Institutional Review Board at Montana State University (Protocol #2022-168). The isolated neutrophils were resuspended in HBSS+ for all biological assays. Neutrophil preparations were routinely >95% pure and >98% viable, as determined by light microscopy and trypan blue exclusion, respectively.
3.7. Cell Culture
Human THP-1 monocytic cells obtained from ATCC (Manassas, VA, USA) were cultured in RPMI 1640 medium (Mediatech Inc., Herndon, VA, USA) supplemented with 10% (v/v) FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin.
3.8. Ca2+ Mobilization Assay
Changes in the intracellular Ca2+ concentrations ([Ca2+]i) were monitored with a FlexStation 3 (Molecular Devices, Sunnyvale, CA, USA). For these assays, neutrophils were loaded with 1.25 μg/mL Fluo-4AM and incubated in the dark at 37 °C for 30 min. The cells were then washed with HBSS−. The dye-loaded cells were resuspended in HBSS+ and pipetted into the wells of black microtiter plates at 2 × 105 cells/well. To measure the direct effects of samples on [Ca2+]i, the test samples were added to the wells (final concentration of DMSO was 1%), and the fluorescence was monitored (λex = 485 nm, λem = 538 nm). Changes in fluorescence were monitored every 5 s at room temperature for 240 s. To evaluate the inhibitory effects of the test samples, the samples were added to the wells and incubated for 10 min, with the subsequent addition of 5 nM fMLF. Responses were normalized to the response induced by control fMLF (5 nM) alone without pretreatment. These responses were assigned as 100%. To calculate median effective concentrations (EC50 or IC50), we used curve fitting (at least five or six points) and nonlinear regression analysis of the dose–response curves. Curve fitting was performed with Prism 9 (GraphPad Software, Inc., San Diego, CA, USA).
3.9. Chemotaxis Assay
To evaluate the effects of the E. purpurea EO and its components on neutrophil migration, we resuspended the neutrophils in chemotaxis media (HBSS+ containing 2% (v/v) heat-inactivated FBS) at 2 × 106 cells/mL. We analyzed chemotaxis using 96-well ChemoTx chambers (Neuroprobe, Gaithersburg, MD). Neutrophils were first preincubated in Greiner flat-bottom 96-well plates (Millipore Sigma, Burlington, MA, USA) with different concentrations of test samples at room temperature for 30 min. To set up the chemotaxis chambers, a known number of neutrophils were aliquoted into eight wells of the lower chamber to be used for creating the standard curve (linear range of 103–4 × 104 neutrophils in 30 µL of chemotaxis media). Thirty microliters of the chemotaxis media containing the indicated test samples or control 1% DMSO and 1 nM fMLF as the chemoattractant was aliquoted into the remaining wells of the lower chamber, except for 3 lower wells that were reserved for background controls (DMSO treated cells in the upper wells and DMSO without fMLF in the lower wells). The lower chamber plate was then covered with the upper filter plate. The pretreated cells were then transferred from the 96-well Greiner plate into the upper wells of the chemotaxis chamber (4 × 104 cells/well in 20 µL), except for the 3 background control wells indicated above and the 8 upper wells corresponding to the lower wells containing neutrophils used for the standard curve. The cells were then allowed to migrate from the upper wells through the polycarbonate membrane filter for 60 min at 37 °C/5% CO2. Any remaining unmigrated neutrophils were wiped from the upper membrane in each well using filter paper, and 20 µL of 2.5 mM EDTA was added to each well to detach any cells that migrated through the filter membrane but were still attached to the lower membrane surface (10 min incubation at 4 °C). The number of migrated cells was then determined by measuring the ATP in lysates of transmigrated cells and comparing this to the standard curve obtained using known neutrophil numbers, as described above. Calculation of median effective concentrations (IC50) was performed by nonlinear regression analysis of the dose-response curves.
3.10. Cytotoxicity Assays
We analyzed monocyte cytotoxicity using THP-1 monocytic cells using a CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI, USA). Briefly, the cells were incubated (104 cells/well) with the indicated concentrations of the essential oil or compound for 90 min or 24 h at 37 °C/5% CO2. After incubation, we added the substrate. The samples were analyzed using a Fluoroscan Ascent FL microplate reader.
3.11. Antimicrobial Activity
The standard broth dilution method was used to determine the minimum inhibitory concentration (MIC). The following strains were selected for the testing and were obtained through BEI Resources, NIAID, NIH: Escherichia coli K-12 (Strain DC10B, NR-49804), Shigella sonnei (Strain WRAIR I Virulent, NR-519), Shigella flexneri (Strain 24570, NR-517), Salmonella enterica subsp. enterica (Strain MDCH01 (Serovar Tennessee), NR-20742), Pseudomonas aeruginosa (Strain Shr42, NR-48982), Staphylococcus epidermidis (Strain M0881, NR-41888), Staphylococcus aureus (Strain Sa1912, NR-51347), Streptococcus pyogenes (Strain MGAS9882, NR-15272), Streptococcus pneumoniae (Strain NP112, NR-19213), and Bacillus cereus (Strain Tor 16585, NR-12151) and through BEI Resources, NIAID, NIH as part of the Human Microbiome Project: Klebsiella pneumoniae subsp. Pneumoniae (Strain WGLW3, HM-748), Citrobacter freundii (Strain GED7749C, HM-1280), and Enterococcus faecalis (Strain TX2137, HM-432). The assay was performed with a 1% EO stock solution in DMSO (~2500 µg/mL). The EO stock solution was serially diluted in a 96-well plate with TSB broth that had been inoculated with bacterial species. Plates were sealed with sterile gas exchange film and incubated at 37 °C overnight. Optical density was measured at 600 nm using a Thermo Scientific Spectronic 200 spectrophotometer. Assays were performed in quadruplicate.
3.12. Molecular Modeling
The PharmMapper Server was utilized for exploring the potential protein targets for (+)-δ-cadinene [77]. The 3D structure of (+)-δ-cadinene was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/441005; accessed on 7 September 2022). The pharmacophore mapping was performed with the “Human Protein Targets Only” database containing 2241 targets. The top 300 potential human protein targets were retrieved and sorted by the normalized fit score.
3.13. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 9 for Windows (GraphPad Software, San Diego, CA, USA). A p value of less than 0.05 was considered statistically significant.
4. Conclusions
The flower EOs of E. purpurea mainly comprised germacrene D (42.0 ± 4.61%), α-phellandrene (10.09 ± 1.59%), β-caryophyllene (5.75 ± 1.72%), γ-curcumene (5.03 ± 1.96%), α-pinene (4.44 ± 1.78%), δ-cadinene (3.31 ± 0.61%), and β-pinene (2.43 ± 0.98%). Analysis of the E. purpurea EO microbicidal activity showed relatively high activity against a variety of bacterial pathogens. Further analysis showed that the E. purpurea EO, germacrene D, α-phellandrene, β-caryophyllene, and δ-cadinene all induced intracellular Ca2+ mobilization in human neutrophils, suggesting that they also exhibited innate immunomodulatory activity, as intracellular Ca2+ mobilization is a key component of neutrophil activation. Indeed, pretreatment of cells with the E. purpurea EO or δ-cadinene inhibited subsequent heterologous agonist-induced Ca2+ mobilization and inhibited human neutrophil chemotaxis toward N-formyl peptide. This study shows that the E. purpurea EO and major components were microbicidal but also had immunomodulatory effects on neutrophil activation, and we suggest that this combination of host defense against pathogens and modulation of the inflammatory response may contribute to the reported beneficial health effects of Echinacea extracts. Furthermore, we identified δ-cadinene as one of the active components in E. purpurea oil, and pharmacophore mapping studies predicted two potential MAPK targets for (+)-δ-cadinene.
Author Contributions
Conceptualization, N.S.D., P.S. and M.T.Q.; methodology, N.S.D., L.N.K., I.A.S., B.L.L. and J.L.B.; pharmacophore analysis, A.I.K.; formal analysis, P.S., L.N.K., I.A.S., A.I.K. and T.L.T.; data curation, N.S.D., I.A.S., M.T.Q. and B.L.G.; writing—original draft preparation, N.S.D. and I.A.S.; writing—review and editing, N.S.D., P.S., I.A.S., M.T.Q., B.L.G. and H.W.; supervision, P.S. and M.T.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Institutes of Health IDeA Program Grants GM115371 and GM103474, the USDA National Institute of Food and Agriculture Hatch project 1009546, the Montana State University Agricultural Experiment Station, and the Tomsk Polytechnic University Development Program (project Priority-2030-NIP/IZ-009-375-2023).
Institutional Review Board Statement
This study was approved by the Institutional Review Board at Montana State University (Protocol #2022-168).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to thank Tim Valentiner and Plamen Nikolov for kindly providing authentic samples of the Echinacea purpurea essential oils. Special thanks to Samantha Lewis and Scott Johnson for the valuable discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shemluck, M. Medicinal and Other Uses of the Compositae by Indians in the United States and Canada. J. Ethnopharmacol. 1982, 5, 303–358. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Sharif, K.O.; Tufekci, E.F.; Ustaoglu, B.; Altunoglu, Y.C.; Zengin, G.; Llorent-Martínez, E.J.; Guney, K.; Baloglu, M.C. Anticancer and Biological Properties of Leaf and Flower Extracts of Echinacea purpurea (L.) Moench. Food Biosci. 2021, 41, 101005. [Google Scholar] [CrossRef]
- WFO Plant List: Echinacea purpurea (L.) Moench. Available online: https://wfoplantlist.org/plant-list/taxon/wfo-0000036347-2022-12?page=1 (accessed on 6 April 2023).
- Moltó, J.; Valle, M.; Miranda, C.; Cedeño, S.; Negredo, E.; Clotet, B. Herb-Drug Interaction between Echinacea purpurea and Etravirine in HIV-Infected Patients. Antimicrob. Agents Chemother. 2012, 56, 5328–5331. [Google Scholar] [CrossRef] [PubMed]
- Ogal, M.; Johnston, S.L.; Klein, P.; Schoop, R. Echinacea Reduces Antibiotic Usage in Children through Respiratory Tract Infection Prevention: A Randomized, Blinded, Controlled Clinical Trial. Eur. J. Med. Res. 2021, 26, 33. [Google Scholar] [CrossRef]
- Linde, K.; Barrett, B.; Wölkart, K.; Bauer, R.; Melchart, D. Echinacea for Preventing and Treating the Common Cold. Cochrane Database Syst. Rev. 2006, 2006, CD000530. [Google Scholar] [CrossRef]
- Temerdashev, Z.; Vinitskaya, E.; Meshcheryakova, E.; Shpigun, O. Chromatographic Analysis of Water and Water-Alcohol Extracts of Echinacea purpurea L. Obtained by Various Methods. Microchem. J. 2022, 179, 107507. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D. Studies on the Antioxidant Activity of Echinacea Root Extract. J. Agric. Food Chem. 2000, 48, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.; Krecsak, L.; Zámbori, J. Double-Blind Placebo Controlled Trial of the Anxiolytic Effects of a Standardized Echinacea Extract. Phytother. Res. 2020, 34, 660–668. [Google Scholar] [CrossRef]
- Bauer, R. New Findings on the Pharmacological Activity and Therapeutical Efficacy of Preparations of the Pressed Juice of Echinacea purpurea. Wien. Med. Wochenschr. 2002, 152, 407–411. [Google Scholar] [CrossRef]
- Ardjomand-Woelkart, K.; Bauer, R. Review and Assessment of Medicinal Safety Data of Orally Used Echinacea Preparations. Planta Med. 2015, 82, 17–31. [Google Scholar] [CrossRef]
- Yu, D.; Yuan, Y.; Jiang, L.; Tai, Y.; Yang, X.; Hu, F.; Xie, Z. Anti-Inflammatory Effects of Essential Oil in Echinacea purpurea L. Pak. J. Pharm. Sci. 2013, 26, 403–408. [Google Scholar] [PubMed]
- Xu, W.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Echinacea purpurea Suppresses the Cell Survival and Metastasis of Hepatocellular Carcinoma through Regulating the PI3K/Akt Pathway. Int. J. Biochem. Cell Biol. 2022, 142, 106115. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C. Echinacea: A Miracle Herb against Aging and Cancer? Evidence in vivo in Mice. Evid.-Based Complement. Altern. Med. 2005, 2, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B.; Brown, R.; Rakel, D.; Mundt, M.; Bone, K.; Barlow, S.; Ewers, T. Echinacea for Treating the Common Cold: A Randomized Controlled Trial. Ann. Intern. Med. 2010, 153, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Karsch-Völk, M.; Kiefer, B.B.; Bauer, R.; Linde, A.-W.K. Echinacea for Preventing and Treating the Common Cold (Review). Cochrane Database Syst. Rev. 2014, 2014, CD000530. [Google Scholar] [CrossRef] [PubMed]
- Nahas, R.; Balla, A. Clinical Review Complementary and Alternative Medicine for Prevention and Treatment of the Common Cold. Can. Fam. Physician 2011, 57, 31–36. [Google Scholar]
- Jawad, M.; Schoop, R.; Suter, A.; Klein, P.; Eccles, R. Safety and Efficacy Profile of Echinacea purpurea to Prevent Common Cold Episodes: A Randomized, Double-Blind, Placebo-Controlled Trial. Evid.-Based Complement. Altern. Med. 2012, 2012, 841315. [Google Scholar] [CrossRef]
- Ross, S.M. Echinacea purpurea: A Proprietary Extract of Echinacea purpurea Is Shown to Be Safe and Effective in the Prevention of the Common Cold. Holist. Nurs. Pract. 2016, 30, 54–57. [Google Scholar] [CrossRef]
- Schapowal, A.; Klein, P.; Johnston, S.L. Echinacea Reduces the Risk of Recurrent Respiratory Tract Infections and Complications: A Meta-Analysis of Randomized Controlled Trials. Adv. Ther. 2015, 32, 187–200. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Schoop, R.; Suter, A.; Hudson, J. Prevention of Influenza Virus Induced Bacterial Superinfection by Standardized Echinacea purpurea, via Regulation of Surface Receptor Expression in Human Bronchial Epithelial Cells. Virus Res. 2017, 233, 51–59. [Google Scholar] [CrossRef]
- Isbaniah, F.; Wiyono, W.H.; Yunus, F.; Setiawati, A.; Totzke, U.; Verbruggen, M.A. Echinacea purpurea along with Zinc, Selenium and Vitamin C to Alleviate Exacerbations of Chronic Obstructive Pulmonary Disease: Results from a Randomized Controlled Trial. J. Clin. Pharm. Ther. 2011, 36, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.; Taylor, J.A.; Vander Stoep, A.; Weiss, N.S.; Standish, L.J.; Calabrese, C. Echinacea purpurea for Prevention of Upper Respiratory Tract Infections in Children. J. Altern. Complement. Med. 2005, 11, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Signer, J.; Jonsdottir, H.R.; Albrich, W.C.; Strasser, M.; Züst, R.; Ryter, S.; Ackermann-Gäumann, R.; Lenz, N.; Siegrist, D.; Suter, A.; et al. In Vitro Virucidal Activity of Echinaforce®, an Echinacea purpurea Preparation, against Coronaviruses, Including Common Cold Coronavirus 229E and SARS-CoV-2. Virol. J. 2020, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Pleschka, S.; Stein, M.; Schoop, R.; Hudson, J.B. Anti-Viral Properties and Mode of Action of Standardized Echinacea purpurea Extract against Highly Pathogenic Avian Influenza Virus (H5N1, H7N7) and Swine-Origin H1N1 (S-OIV). Virol. J. 2009, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Ladenheim, D.; Horn, O.; Werneke, U.; Phillpot, M.; Murungi, A.; Theobald, N.; Orkin, C. Potential Health Risks of Complementary Alternative Medicines in HIV Patients. HIV Med. 2008, 9, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Kolev, E.; Mircheva, L.; Edwards, M.R.; Johnston, S.L.; Kalinov, K.; Stange, R.; Gancitano, G.; Berghe, W.V.; Kreft, S. Echinacea purpurea For the Long-Term Prevention of Viral Respiratory Tract Infections During Covid-19 Pandemic: A Randomized, Open, Controlled, Exploratory Clinical Study. Front. Pharmacol. 2022, 13, 856410. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-T.; Huang, C.-C.; Shieh, X.-H.; Chen, C.-L.; Chen, L.-J.; Yu, B. Flavonoid, Phenol and Polysaccharide Contents of Echinacea purpurea L. and Its Immunostimulant Capacity In Vitro. Int. J. Environ. Sci. Dev. 2010, 1, 5–9. [Google Scholar] [CrossRef]
- Sharma, M.; Schoop, R.; Suter, A.; Hudson, J.B. The Potential Use of Echinacea in Acne: Control of Propionibacterium Acnes Growth and Inflammation. Phytother. Res. 2011, 25, 517–521. [Google Scholar] [CrossRef]
- Oláh, A.; Szabó-Papp, J.; Soeberdt, M.; Knie, U.; Dähnhardt-Pfeiffer, S.; Abels, C.; Bíró, T. Echinacea purpurea-Derived Alkylamides Exhibit Potent Anti-Inflammatory Effects and Alleviate Clinical Symptoms of Atopic Eczema. J. Dermatol. Sci. 2017, 88, 67–77. [Google Scholar] [CrossRef]
- Dogan, Z.; Ergul, B.; Sarikaya, M.; Filik, L.; Gonultaş, A. The Protective Effect of Echinacea spp. (Echinacea angustifolia and Echinacea purpurea) in a Rat Colitis Model Induced by Acetic Acid. Pak. J. Pharm. Sci. 2014, 27, 1827–1835. [Google Scholar]
- Bauer, R. Echinacea: Biological Effects and Active Principles. In Phytomedicines of Europe: Chemistry and Biological Activity; Lawson, L., Bauer, R., Eds.; American Chemical Society: Washington, DC, USA, 1998; pp. 140–157. [Google Scholar]
- Rininger, J.A.; Kickner, S.; Chigurupati, P.; McLean, A.; Franck, Z. Immunopharmacological Activity of Echinacea Preparations Following Simulated Digestion on Murine Macrophages and Human Peripheral Blood Mononuclear Cells. J. Leukoc. Biol. 2000, 68, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Nyalambisa, M.; Oyemitan, I.A.; Matewu, R.; Oyedeji, O.O.; Oluwafemi, O.S.; Songca, S.P.; Nkeh-Chungag, B.N.; Oyedeji, A.O. Volatile Constituents and Biological Activities of the Leaf and Root of Echinacea Species from South Africa. Saudi Pharm. J. 2017, 25, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Oüzek, G.; Schepetkin, I.A.; Utegenova, G.A.; Kirpotina, L.N.; Andrei, S.R.; Oüzek, T.; Baser, K.H.C.; Abidkulova, K.T.; Kushnarenko, S.V.; Khlebnikov, A.I.; et al. Chemical Composition and Phagocyte Immunomodulatory Activity of Ferula iliensis Essential Oils. J. Leukoc. Biol. 2017, 101, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.; Williams, C. Phytochemistry of the Genus Echinacea. In Echinacea: The Genus Echinacea (Medicinal and Aromatic Plants—Industrial Profiles); Miller, S.C., Yu, H., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 55–71. [Google Scholar]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea Species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A Review of Their Chemistry, Pharmacology and Clinical Properties. J. Pharm. Pharmacol. 2010, 57, 929–954. [Google Scholar] [CrossRef]
- Lin, Z.; Neamati, N.; Zhao, H.; Kiryu, Y.; Turpin, J.A.; Aberham, C.; Strebel, K.; Kohn, K.; Witvrouw, M.; Pannecouque, C.; et al. Chicoric Acid Analogues as HIV-1 Integrase Inhibitors. J. Med. Chem. 1999, 42, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Scagel, C.F. Chicoric Acid: Chemistry, Distribution, and Production. Front. Chem. 2013, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.L.; Liu, R.; Smith, M.L.; Harris, C.S. Echinacea Fruit: Phytochemical Localization and Germination in Four Species of Echinacea. Botany 2018, 96, 461–470. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Salehi, B.; Martorell, M.; del Mar Contreras, M.; Soltani-Nejad, A.; et al. Echinacea Plants as Antioxidant and Antibacterial Agents: From Traditional Medicine to Biotechnological Applications. Phytother. Res. 2018, 32, 1653–1663. [Google Scholar] [CrossRef]
- Cozzolino, R.; Malvagna, P.; Spina, E.; Giori, A.; Fuzzati, N.; Anelli, A.; Garozzo, D.; Impallomeni, G. Structural Analysis of the Polysaccharides from Echinacea Angustifolia Radix. Carbohydr. Polym. 2006, 65, 263–272. [Google Scholar] [CrossRef]
- Pellati, F.; Epifano, F.; Contaldo, N.; Orlandini, G.; Cavicchi, L.; Genovese, S.; Bertelli, D.; Benvenuti, S.; Curini, M.; Bertaccini, A.; et al. Chromatographic Methods for Metabolite Profiling of Virus- and Phytoplasma-Infected Plants of Echinacea purpurea. J. Agric. Food Chem. 2011, 59, 10425–10434. [Google Scholar] [CrossRef] [PubMed]
- Hudaib, M.; Bellardi, M.G.; Rubies-Autonell, C.; Fiori, J.; Cavrini, V. Chromatographic (GC-MS, HPLC) and Virological Evaluations of Salvia Sclarea Infected by BBWV-I. Farmaco 2001, 56, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Merdivan, M.; Tashakkori, P.; Erdem, P.; Anderson, J.L. Analysis of Echinacea Flower Volatile Constituents by HS-SPME-GC/MS Using Laboratory-Prepared and Commercial SPME Fibers. J. Essent. Oil Res. 2019, 31, 91–98. [Google Scholar] [CrossRef]
- Mirjalili, M.H.; Salehi, P.; Badi, H.N.; Sonboli, A. Volatile Constituents of the Flowerheads of ThreeEchinacea Species Cultivated in Iran. Flavour Fragr. J. 2006, 21, 355–358. [Google Scholar] [CrossRef]
- Mazza, G.; Cottrell, T. Volatile Components of Roots, Stems, Leaves, and Flowers of Echinacea Species. J. Agric. Food Chem. 1999, 47, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- Vaverková, S.; Mikulásová, M.; Habán, M.; Tekel’, J.; Hollá, M.; Otepka, P. Variability of the Essential Oil from Three Sorts of Echinacea MOENCH Genus during Ontogenesis. Ceska Slov. Farm. 2007, 56, 121–124. [Google Scholar] [PubMed]
- Yazdanian, M.; Rostamzadeh, P.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Moghaddam, M.M.; Kahnamoei, M.B. Evaluation of Antimicrobial and Cytotoxic Effects of Echinacea and Arctium Extracts and Zataria Essential Oil. AMB Express 2022, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Pérez Zamora, C.; Torres, C.; Nuñez, M. Antimicrobial Activity and Chemical Composition of Essential Oils from Verbenaceae Species Growing in South America. Molecules 2018, 23, 544. [Google Scholar] [CrossRef]
- Leite-Sampaio, N.F.; Gondim, C.N.F.L.; Martins, R.A.A.; Siyadatpanah, A.; Norouzi, R.; Kim, B.; Sobral-Souza, C.E.; Gondim, G.E.C.; Ribeiro-Filho, J.; Coutinho, H.D.M. Potentiation of the Activity of Antibiotics against ATCC and MDR Bacterial Strains with (+)-α-Pinene and (-)-Borneol. BioMed Res. Int. 2022, 2022, 8217380. [Google Scholar] [CrossRef]
- Yoo, H.-J.; Jwa, S.-K. Inhibitory Effects of β-Caryophyllene on Streptococcus Mutans Biofilm. Arch. Oral Biol. 2018, 88, 42–46. [Google Scholar] [CrossRef]
- Moo, C.-L.; Yang, S.-K.; Osman, M.-A.; Yuswan, M.H.; Loh, J.-Y.; Lim, W.-M.; Lim, S.-H.-E.; Lai, K.-S. Antibacterial Activity and Mode of Action of β-Caryophyllene on Bacillus cereus. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria Crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.R.; Lopes, P.M.; de Azevedo, M.M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of A-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- de Souza, W.F.C.; de Lucena, F.A.; de Castro, R.J.S.; de Oliveira, C.P.; Quirino, M.R.; Martins, L.P. Exploiting the Chemical Composition of Essential Oils from Psidium Cattleianum and Psidium Guajava and Its Antimicrobial and Antioxidant Properties. J. Food Sci. 2021, 86, 4637–4649. [Google Scholar] [CrossRef] [PubMed]
- Radice, M.; Durofil, A.; Buzzi, R.; Baldini, E.; Martínez, A.P.; Scalvenzi, L.; Manfredini, S. Alpha-Phellandrene and Alpha-Phellandrene-Rich Essential Oils: A Systematic Review of Biological Activities, Pharmaceutical and Food Applications. Life 2022, 12, 1602. [Google Scholar] [CrossRef] [PubMed]
- Adolpho, L.O.; Paz, L.H.A.; Rosa, O.; Morel, A.F.; Dalcol, I.I. Chemical Profile and Antimicrobial Activity of Leonotis nepetifolia (L.) R. Br. Essential Oils. Nat. Prod. Res. 2023, 15, 1–5. [Google Scholar] [CrossRef]
- Cárdenas, J.; Rojas, J.; Rojas-Fermin, L.; Lucena, M.; Buitrago, A. Essential Oil Composition and Antibacterial Activity of Monticalia greenmaniana (Asteraceae). Nat. Prod. Commun. 2012, 7, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Uçüncü, O.; Kahriman, N.; Terzioğlu, S.; Karaoğlue, S.A.; Yayli, N. Composition and Antimicrobial Activity of the Essential Oils from Flowers of Senecio othonnae, S. racemosus, and S. nemorensis. Nat. Prod. Commun. 2010, 5, 831–834. [Google Scholar]
- González, A.M.; Tracanna, M.I.; Amani, S.M.; Schuff, C.; Poch, M.J.; Bach, H.; Catalán, C.A.N. Chemical Composition, Antimicrobial and Antioxidant Properties of the Volatile Oil and Methanol Extract of Xenophyllum poposum. Nat. Prod. Commun. 2012, 7, 1663–1666. [Google Scholar] [CrossRef]
- Hoi, T.M.; Chung, N.T.; Huong, L.T.; Ogunwande, I.A. Studies on Asteraceae: Chemical Compositions of Essential Oils and Antimicrobial Activity of the Leaves of Vernonia patula (Dryand.) Merr. and Grangea maderaspatana (L.) Poir. from Vietnam. J. Essent. Oil Bear. Plants 2021, 24, 500–509. [Google Scholar] [CrossRef]
- Thinh, B.B.; Thin, D.B. Essential Oil Composition, Antimicrobial and Antioxidant Properties of Pluchea eupatorioides Kurz Collected from Vietnam. J. Essent. Oil Bear. Plants 2023, 26, 653–663. [Google Scholar] [CrossRef]
- Kim, H.-R.; Oh, S.-K.; Lim, W.; Lee, H.K.; Moon, B.-I.; Seoh, J.-Y.; Commun, N.P. Immune Enhancing Effects of Echinacea purpurea Root Extract by Reducing Regulatory T Cell Number and Function. Nat. Prod. Commun. 2014, 9, 511–514. [Google Scholar]
- Park, S.J.; Lee, M.; Kim, D.; Oh, D.H.; Prasad, K.S.; Eun, S.; Lee, J. Echinacea purpurea Extract Enhances Natural Killer Cell Activity in Vivo by Upregulating MHC II and Th1-Type CD4+T Cell Responses. J. Med. Food 2021, 24, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.N.; Papanicolaou, G.; Lin, H.; Lau, C.B.S.; Kennelly, E.J.; Cassileth, B.R.; Cunningham-Rundles, S. Echinacea purpurea (L.) Moench Modulates Human T-Cell Cytokine Response. Int. Immunopharmacol. 2014, 19, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Malech, H.L.; DeLeo, F.R.; Quinn, M.T. The Role of Neutrophils in the Immune System: An Overview. Neutrophil Methods Protoc. 2014, 1124, 3–10. [Google Scholar]
- Dixit, N.; Kim, M.-H.; Rossaint, J.; Yamayoshi, I.; Zarbock, A.; Simon, S.I. Leukocyte Function Antigen-1, Kindlin-3, and Calcium Flux Orchestrate Neutrophil Recruitment during Inflammation. J. Immunol. 2012, 189, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Gronski, M.A.; Kinchen, J.M.; Juncadella, I.J.; Franc, N.C.; Ravichandran, K.S. An Essential Role for Calcium Flux in Phagocytes for Apoptotic Cell Engulfment and the Anti-Inflammatory Response. Cell Death Differ. 2009, 16, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.; Özek, G.; Özek, T.; Kirpotina, L.; Khlebnikov, A.; Quinn, M. Chemical Composition and Immunomodulatory Activity of Hypericum Perforatum Essential Oils. Biomolecules 2020, 10, 916. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S.V.; Özek, G.; Kirpotina, L.N.; Sinharoy, P.; Utegenova, G.A.; Abidkulova, K.T.; Özek, T.; Başer, K.H.C.; Kovrizhina, A.R.; et al. Modulation of Human Neutrophil Responses by the Essential Oils from Ferula akitschkensis and Their Constituents. J. Agric. Food Chem. 2016, 64, 7156–7170. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S.V.; Özek, G.; Kirpotina, L.N.; Utegenova, G.A.; Kotukhov, Y.A.; Danilova, A.N.; Özek, T.; Başer, K.H.C.; Quinn, M.T. Inhibition of Human Neutrophil Responses by the Essential Oil of Artemisia kotuchovii and Its Constituents. J. Agric. Food Chem. 2015, 63, 4999–5007. [Google Scholar] [CrossRef]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil Cell Surface Receptors and Their Intracellular Signal Transduction Pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef]
- Decarlo, A.; Johnson, S.; Ouédraogo, A.; Dosoky, N.S.; Setzer, W.N. Chemical Composition of the Oleogum Resin Essential Oils of Boswellia dalzielii from Burkina Faso. Plants 2019, 8, 223. [Google Scholar] [CrossRef]
- Kumar Poudel, D.; Dangol, S.; Rokaya, A.; Maharjan, S.; Kumar Ojha, P.; Rana, J.; Dahal, S.; Timsina, S.; Dosoky, N.S.; Satyal, P.; et al. Quality Assessment of Zingiber officinale Roscoe Essential Oil from Nepal. Nat. Prod. Commun. 2022, 17, 1934578X2210803. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper Server: A Web Server for Potential Drug Target Identification Using Pharmacophore Mapping Approach. Nucleic Acids Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).