Abstract
The 5,8-disubstituted indolizidines are the largest family of indolizidines isolated from the skin of amphibians. These compounds exhibit interesting biological activities such as noncompetitive blockers of nicotinic receptors. In this paper, we present a short, simple, and general synthesis of these alkaloids based on the hetero Diels–Alder reaction between suitable monoactivated dienes and Δ1-pyrroline as the dienophile. The selectivity of the process is explained based on computational studies. Concise synthesis of the indolizidine alkaloid 181B from a hetero Diels–Alder reaction was accomplished in four steps.
1. Introduction
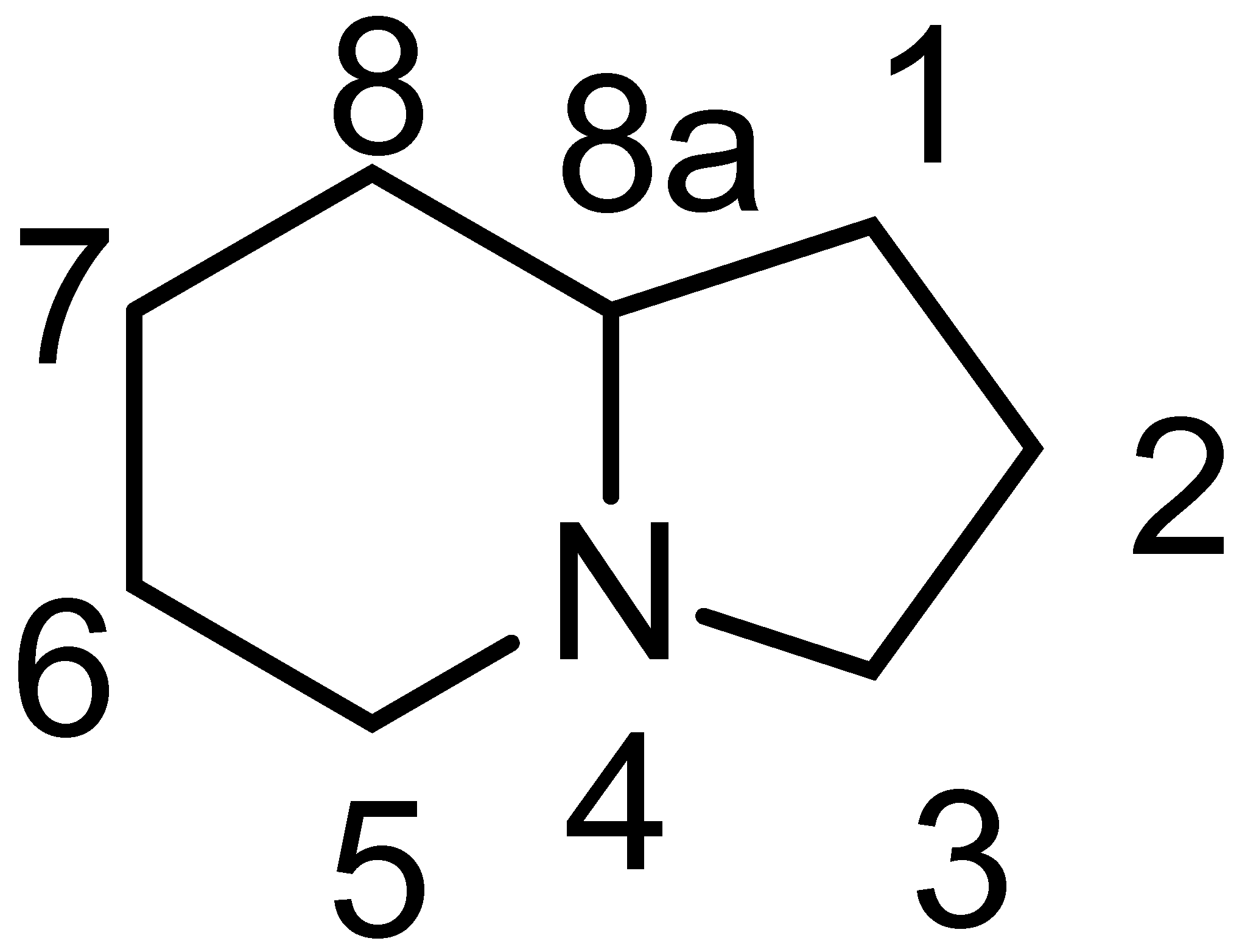
Indolizidine alkaloids constitute a large family of heterocyclic compounds isolated from numerous sources, both terrestrial and marine [1,2,3]. The basic structure of indolizidines is that of a five-membered ring fused with a six-membered ring, with a nitrogen atom at one of the fusion points (Figure 1).

Figure 1.
Structure and numbering of the indolizidine skeleton.
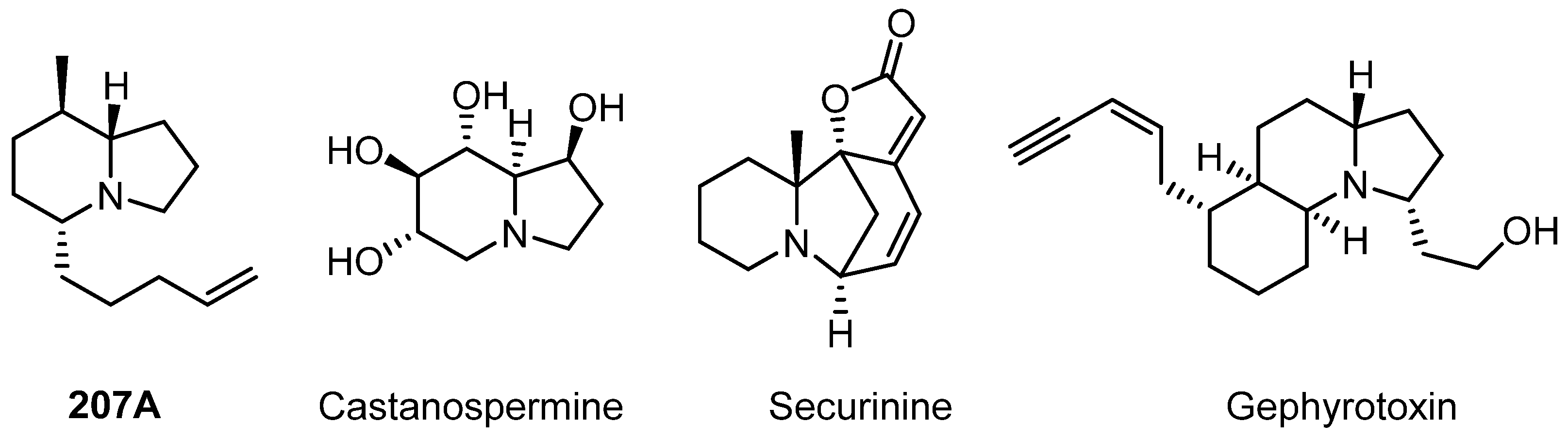
Indolizidines exist with different substitution patterns—from the simple basic skeleton with some hydroxyl groups to more complex structures (Figure 2).

Figure 2.
Examples of naturally occurring indolizidines.
Some of these alkaloids show interesting biological activities, such as antitumor, antiviral, antileukemic, and others [4,5].
A large number of indolizidines have been isolated from or detected on the skins of amphibians, and these are usually substituted with alkyl and hydroxyl groups, composing several families of alkaloids [6]. The largest family is that of the 5,8-disubstituted indolizidines, with more than 80 examples reported. These indolizidines come mainly from dietary sources since many have been isolated from mites that coexist with the amphibian [7,8]. Many of these have tentative structures due to the difficulty of isolation because of the small amount present in samples obtained from natural sources.
Although some species of amphibians that contain these alkaloids are known to be toxic, the toxicity is not produced by the 5,8-disubstituted indolizidines. Their reported biological activity is as noncompetitive blockers of nicotinic receptors [9,10].
Numerous synthetic schemes aimed at the preparation of the indolizidine skeleton, both racemic and enantioselective, have been published. The most usual methodology is the cyclization of a suitable monocyclic intermediate, giving the bicyclic skeleton of the alkaloids [11].
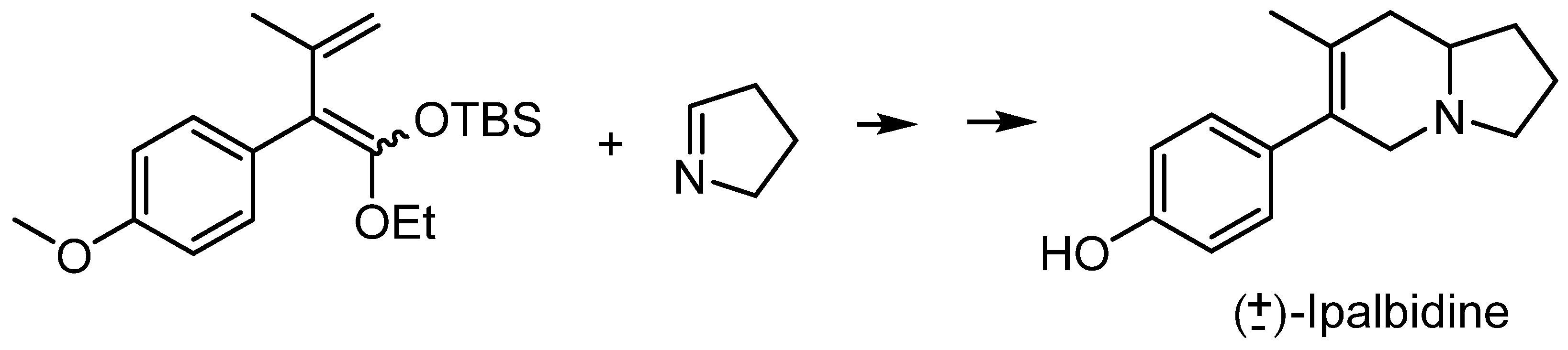
A special mention must be made to Danishefsky and Vogel’s synthesis of (±)-Ipalbidine [12], in which a hetero Diels–Alder reaction between an activated diene and Δ1-pyrroline was used as the key step to prepare the target compound (Figure 3).

Figure 3.
Danishefsky and Vogel’s synthesis of Ipalbidine.
This approach has been used in a few examples, with Δ1-pyrroline derivatives and 1,3-diactivated dienes giving hydroxyl-substituted indolizidines [13,14,15,16], or Δ1-pyrroline and allenyltrimethylsilylthioketenes acting as dienes [17,18].
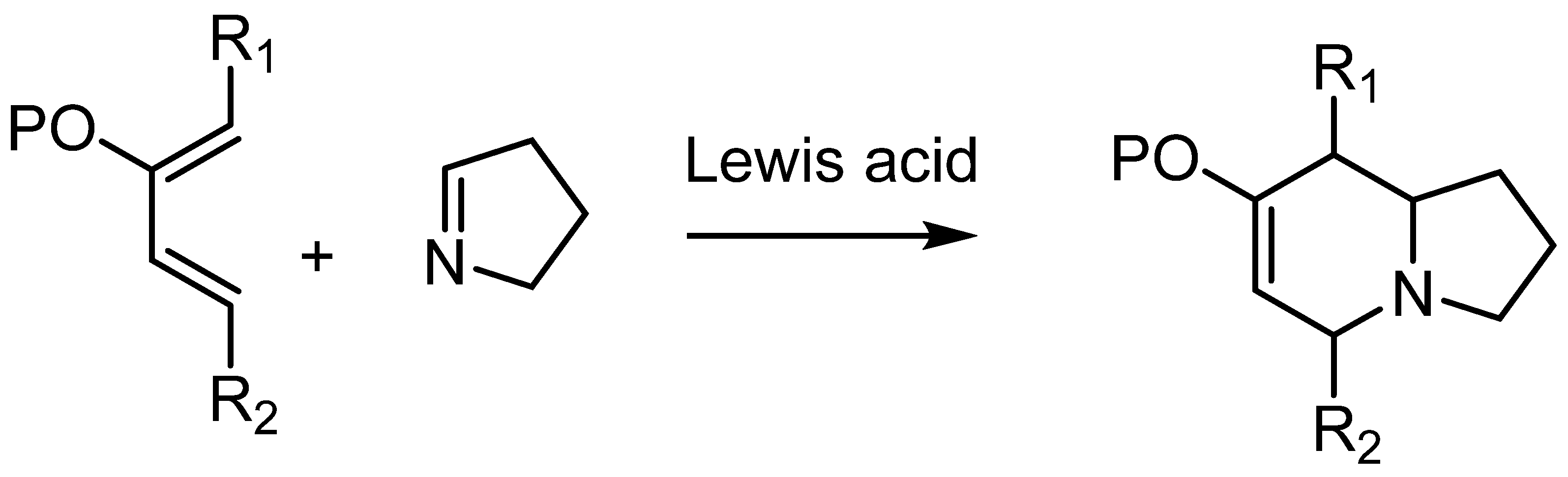
In our group, the hetero Diels–Alder reactions of monoactivated dienes have been used for the synthesis of oxygen-containing compounds via reaction with aldehydes [19]. We found that monoactivated dienes work well in those reactions in the presence of Lewis acids. Thus, we envisioned that the reaction between a suitable monoactivated diene and a cyclic imine (Δ1-pyrroline) could give quick access to the 5,8-alkyl-disubstituted indolizidine skeleton (Scheme 1), providing a simple method to prepare a variety of heterocyclic compounds in a few steps. The relative stereochemistry of the cycloadduct depends on the endo/exo selectivity of the cycloaddition, which in turn depends on the nature of the substituents on the diene. This aspect must be taken into account to match the relative stereochemistry of the natural compounds.

Scheme 1.
Proposed route to the 5,8-disubstituted indolizidine skeleton.
In this article, we present our results in that direction, confirming the idea that the indolizidine skeleton can be accessed from easily prepared dienes and Δ1-pyrroline in a few steps. First, the conditions for the reactions between simple monoactivated dienes and Δ1 pyrroline were studied. Then stereochemical issues were addressed, and finally, the racemic synthesis of the 5,8-disubstituted indolizidine 181B from the hetero Diels–Alder reaction was achieved in only four steps.
2. Results and Discussion
To simplify the synthesis of the indolizidine skeleton, we decided to use monoactivated dienes with a silyloxy group, which works well with hetero dienophiles such as aldehydes under Lewis acid catalysis [19].
2.1. Synthesis of the First Model: Mono-Substituted Indolizidine Skeleton
To test this idea, we decided to start with a simple mono-substituted alkyl indolizidine skeleton following the reactions shown in Scheme 2. Mono-substituted indolizidines with the substituent at position five were proposed as natural compounds; however, it was later determined that their structures were incorrect [6]. This type of 5-substituted indolizidine has been the target of several synthesis efforts [20,21,22,23,24,25].

Scheme 2.
Synthesis of mono-substituted alkyl indolizidine skeleton.
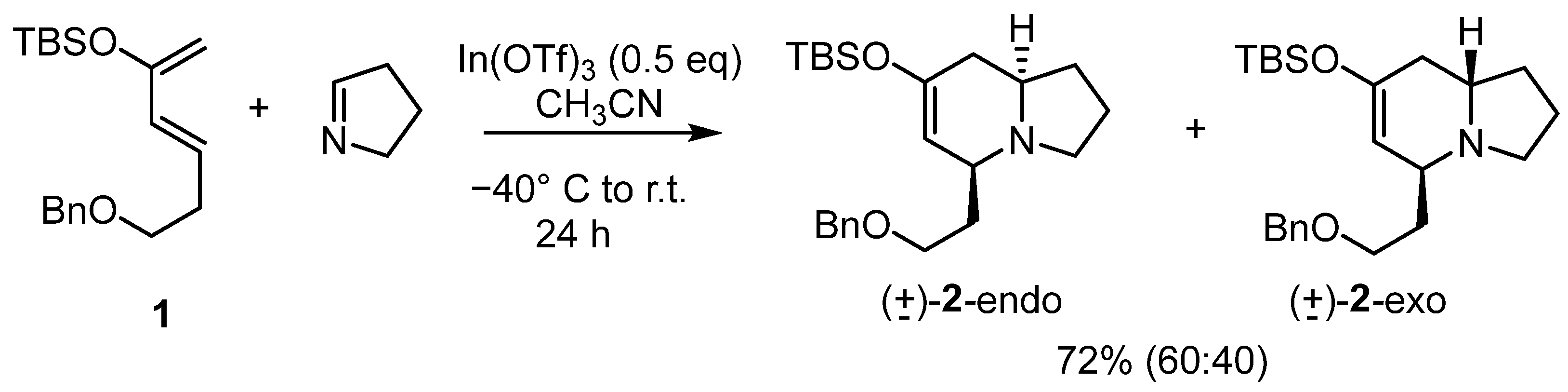
The simple monoactivated diene 1 was chosen to test the reaction. The alkyl chain on 1 contains a benzyloxy group since that group can serve as a reactivity point to make further transformations on the indolizidine skeleton [26,27]. Additionally, these dienes tend to be volatile and somewhat difficult to work with. The presence of the benzyloxy group was expected to reduce the volatility.
Although the hetero Diels–Alder reaction was expected to proceed with high regioselectivity, the endo/exo ratio depends on the structure and degree of substitution on the diene, thus a complete study of the selectivity of the cycloaddition reaction was planned.
The diene was prepared following previous reports [28]. The dienophile Δ1-pyrroline was prepared from the diethylacetal of 4-aminobutyraldehyde as described in the literature [29] and was freshly distilled before each use.
We tested BF3·OEt2, and In(OTf)3 as Lewis acids since in our experience these two acids work well in hetero Diels–Alder reactions. The boron derivative gave the expected cycloadducts (±)-2 in a 40% yield and a 60:40 ratio of endo:exo isomers. The low yield was due to its reactivity with the diene, resulting in recovered unsaturated ketones. With the indium compound, the yield increased to 72% with the same endo:exo ratio. It must be noted that all compounds were slightly volatile even with the benzyloxy group present. The amount of BF3·OEt2 needed for the reaction to proceed to completion was one equivalent, as is usual for this reagent, since the complex it forms with the dienophile is quite strong. As a comparison, three equivalents were needed for the Danishefsky and Vogel hetero Diels–Alder reaction [12]. For the In(OTf)3, it was found that 0.5 equivalents were needed for the reaction to be complete.
The reaction was also studied computationally using the DFT level [30]. These studies concluded that the mechanism is a cycloaddition reaction but with some asynchronicity. The low endo:exo selectivity correlated with the small difference in the energy of the corresponding transition states probably due to the low steric hindrance shown by the diene (for a discussion see the supporting information).
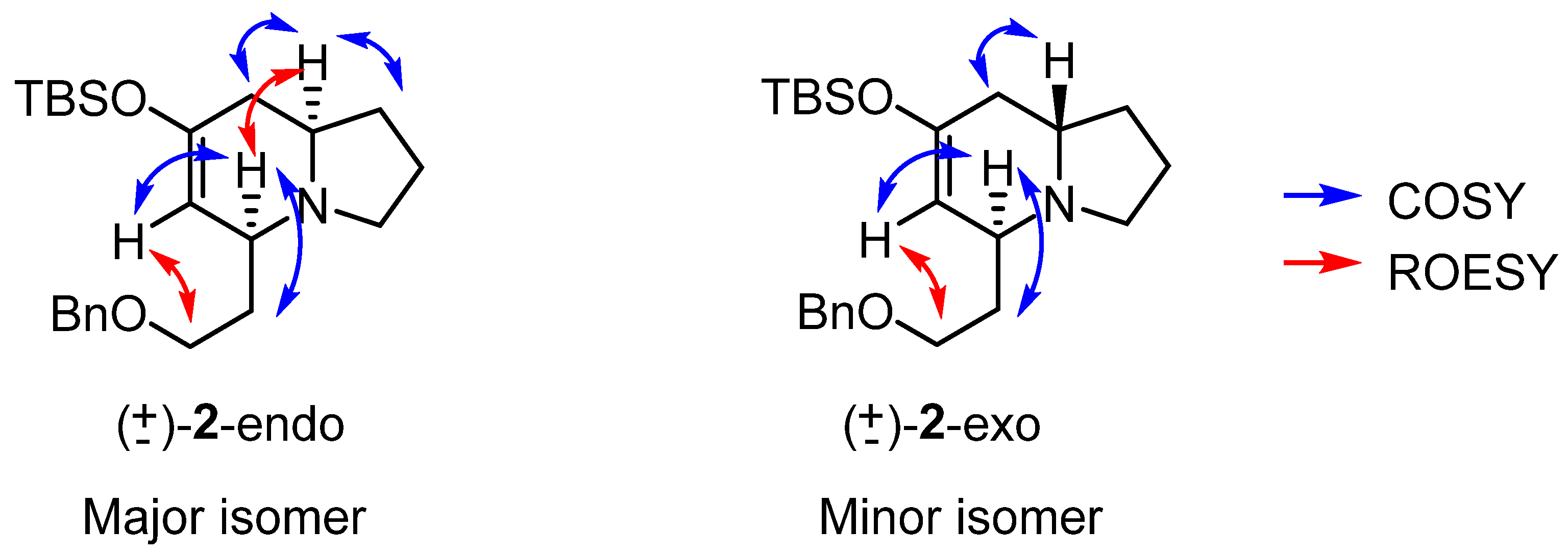
After extraction and purification, the structure and relative stereochemistry of the cycloadducts were studied using standard NMR experiments such as COSY, HMQC, HMBC, and ROESY.
Figure 4 shows the results of these experiments, which allowed us to ascertain that the major compound obtained in the cycloaddition was the endo isomer. This isomer has the same relative stereochemistry as most of the known mono-substituted indolizidines.

Figure 4.
COSY and ROESY correlations for (±)-2-endo and (±)-2-exo.
2.2. Synthesis of 8-Methyl-5-Alkyl Indolizidine Skeleton
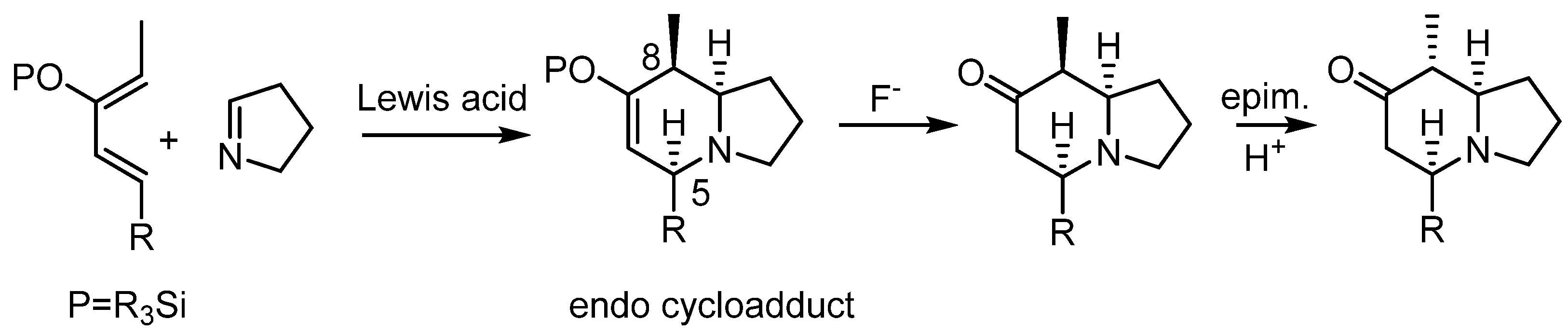
Once we found a set of conditions to carry out the hetero Diels–Alder reaction with Δ1-pyrroline as the dienophile, we moved on to a system closely related to the most abundant indolizidine alkaloids from amphibious sources, the 5,8-disubstituted indolizidines [6]. Several procedures for the syntheses of this family of indolizidines have been published [31,32,33]. The most common among these are the indolizidines with a methyl group at the eighth position, and thus, we decided to prepare an example of a 5-alkyl-8-methyl indolizidine. In these compounds, the stereochemistry of the stereogenic centers is usually cis for the hydrogens at the positions α to the nitrogen (C5 y C8a) and trans between these and the hydrogen at C8. This relative stereochemistry is expected from the endo adduct of a cycloaddition reaction in which the group at the eighth position has undergone epimerization. Since this position is adjacent to the silyloxy group in the expected cycloadducts, we envisioned that after cleaving the silyl group, the resulting carbonyl group could help to epimerize this position (Scheme 3). A computational study indicated that the epimerized ketone should be more stable than the ketone one coming from the cycloaddition reaction. For a discussion see the supporting information.

Scheme 3.
Proposed synthesis of the 8-methyl-5-alkyl indolizidine skeleton with the most common relative stereochemistry found in natural compounds.
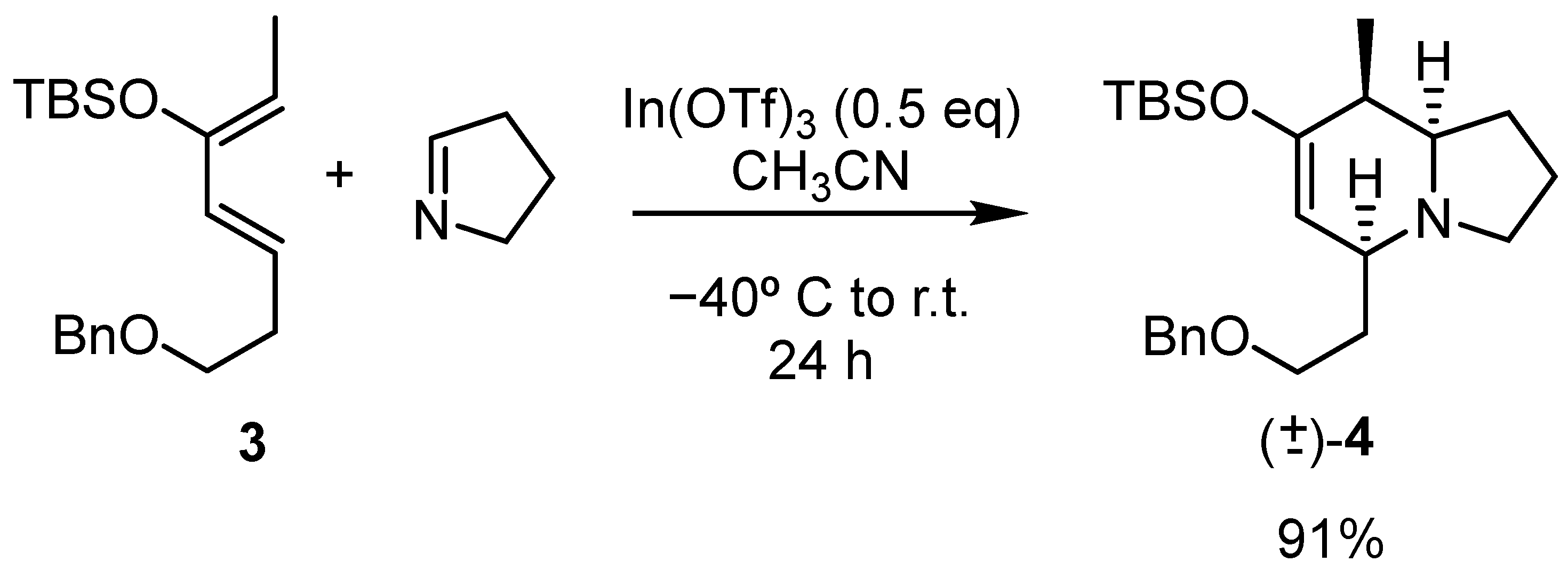
For this study, we decided to maintain the benzyloxy group in the alkyl chain to increase its polarity and facilitate its handling (Scheme 4).

Scheme 4.
Synthesis of cycloadduct (±)-4-endo.
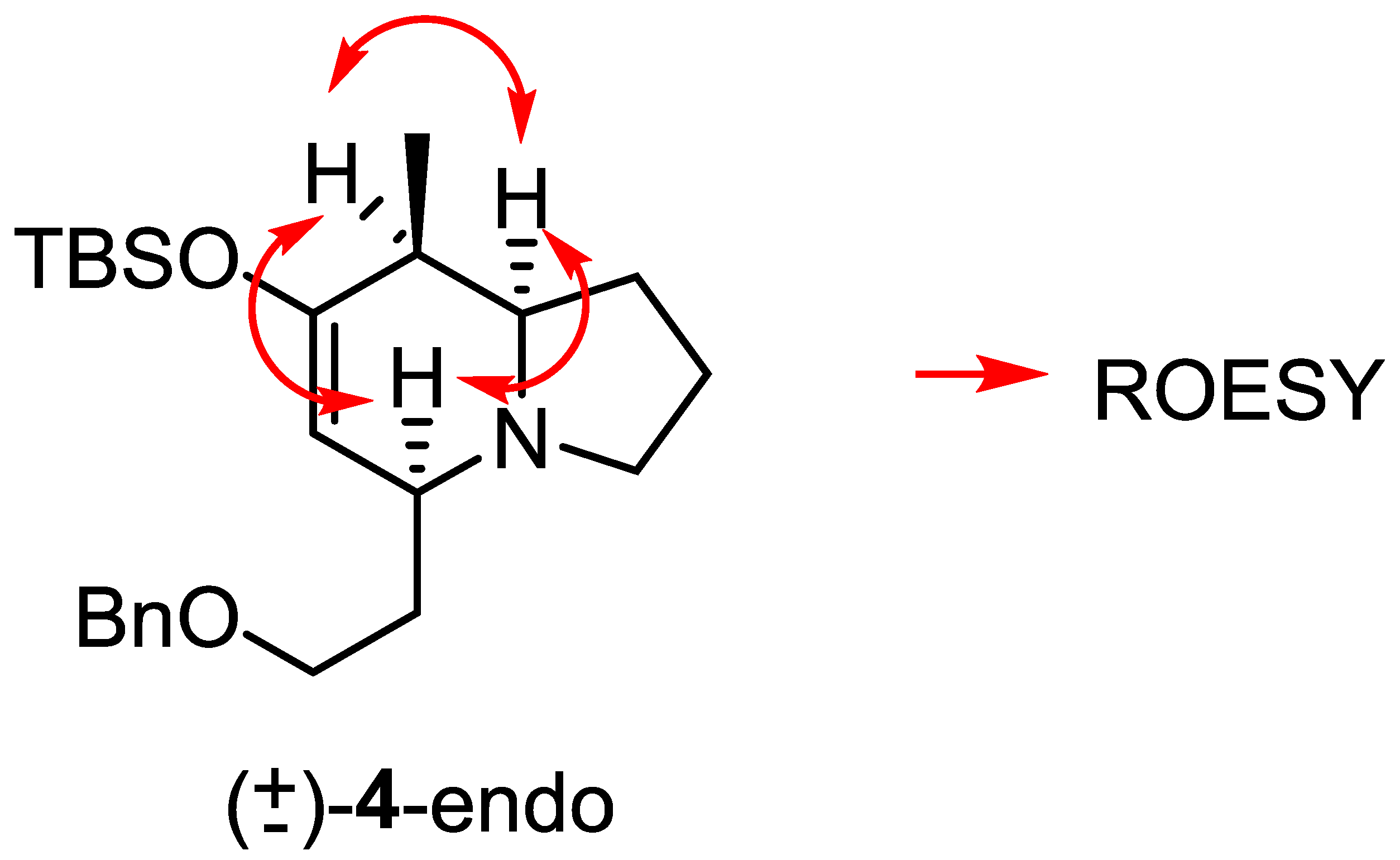
The diene needed for this synthesis was prepared according to the literature [28]. After reacting with Δ1-pyrroline under In(OTf)3 catalysis, one cycloadduct (±)-4 was observed as the major compound (>90%) in an almost quantitative yield. NMR studies of this major cycloadduct (Figure 5) indicated that this compound corresponds to the endo adduct and thus, the three hydrogen atoms in the stereocenters of the molecule are in an all-cis relationship. The computational study of this reaction indicates a larger difference in energy between the respective transition states, favoring the endo one (see the supporting information).

Figure 5.
ROESY correlations with the cycloadduct (±)-4-endo.
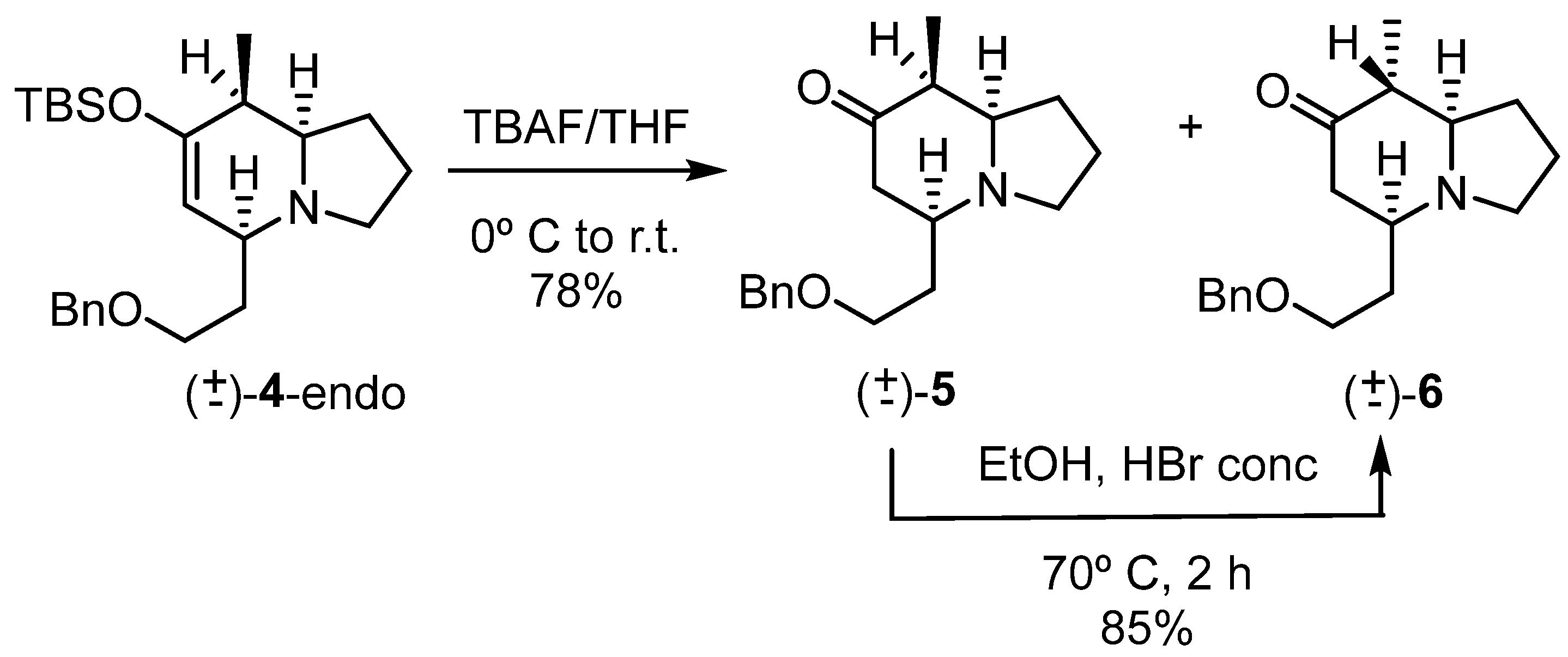
The subsequent step was the cleavage of the enol-silyl group to give the corresponding ketone, which was used to attempt the epimerization of the methyl group on carbon 8 (Scheme 5).

Scheme 5.
Desilylation and epimerization reactions.
Thus, compound (±)-4-endo was treated with TBAF, yielding a mixture of compounds that were identified as two ketones, (±)-5 and (±)-6, epimeric at carbon 8. This result indicated the ease of the epimerization step.
The mixture was treated with HBr [34] to give the completely isomerized ketone (±)-6. The yield from these two steps, desilylation–epimerization, was 66%.
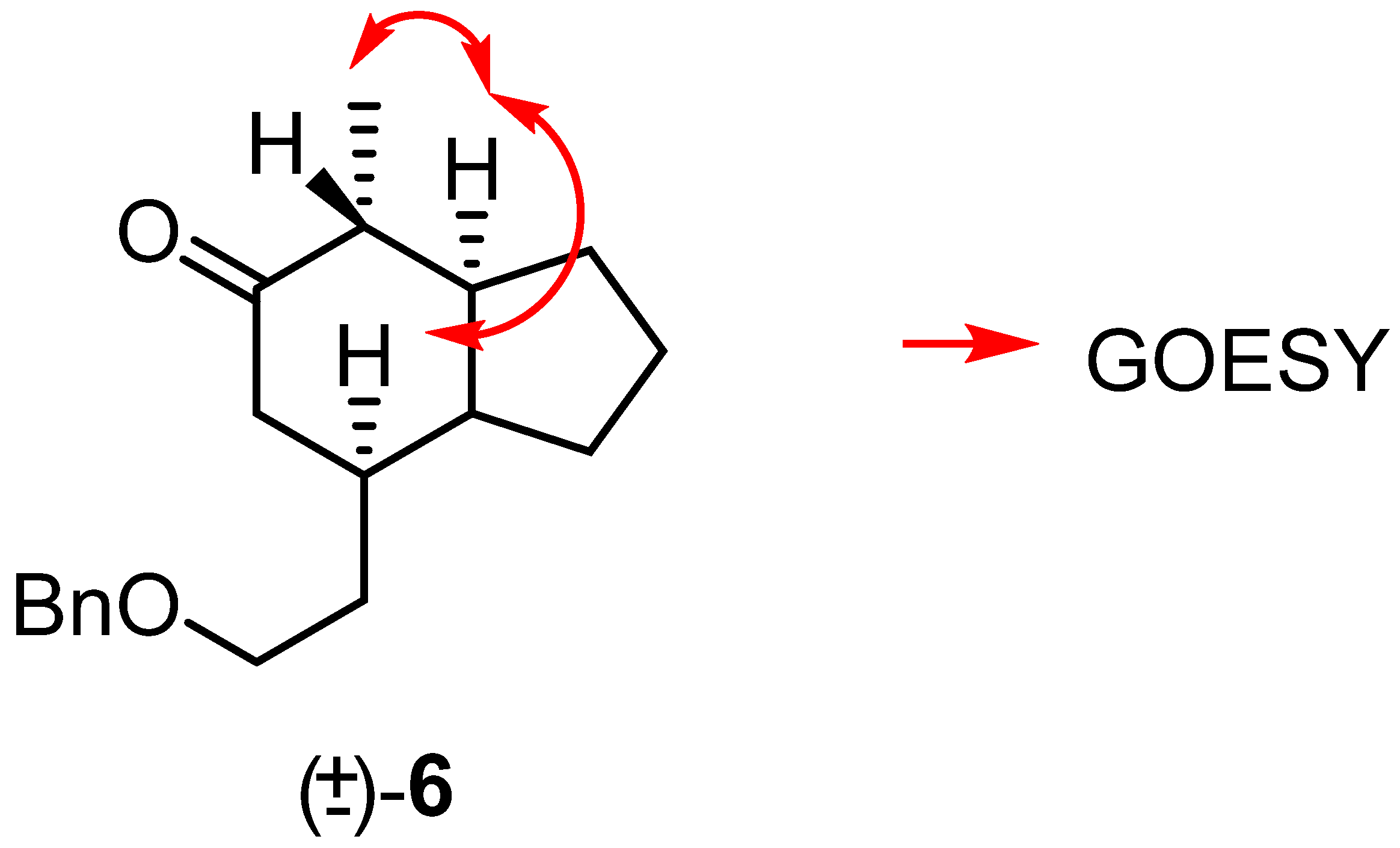
NMR spectroscopic analysis indicated that the new keto-indolizidine (±)-6 had the same relative stereochemistry as most of the naturally occurring 5,8-disubstituted indolizidines (Figure 6).

Figure 6.
Spatial correlations found for (±)-6 from GOESY experiments.
2.3. Synthesis of Indolizidine Rac-181B
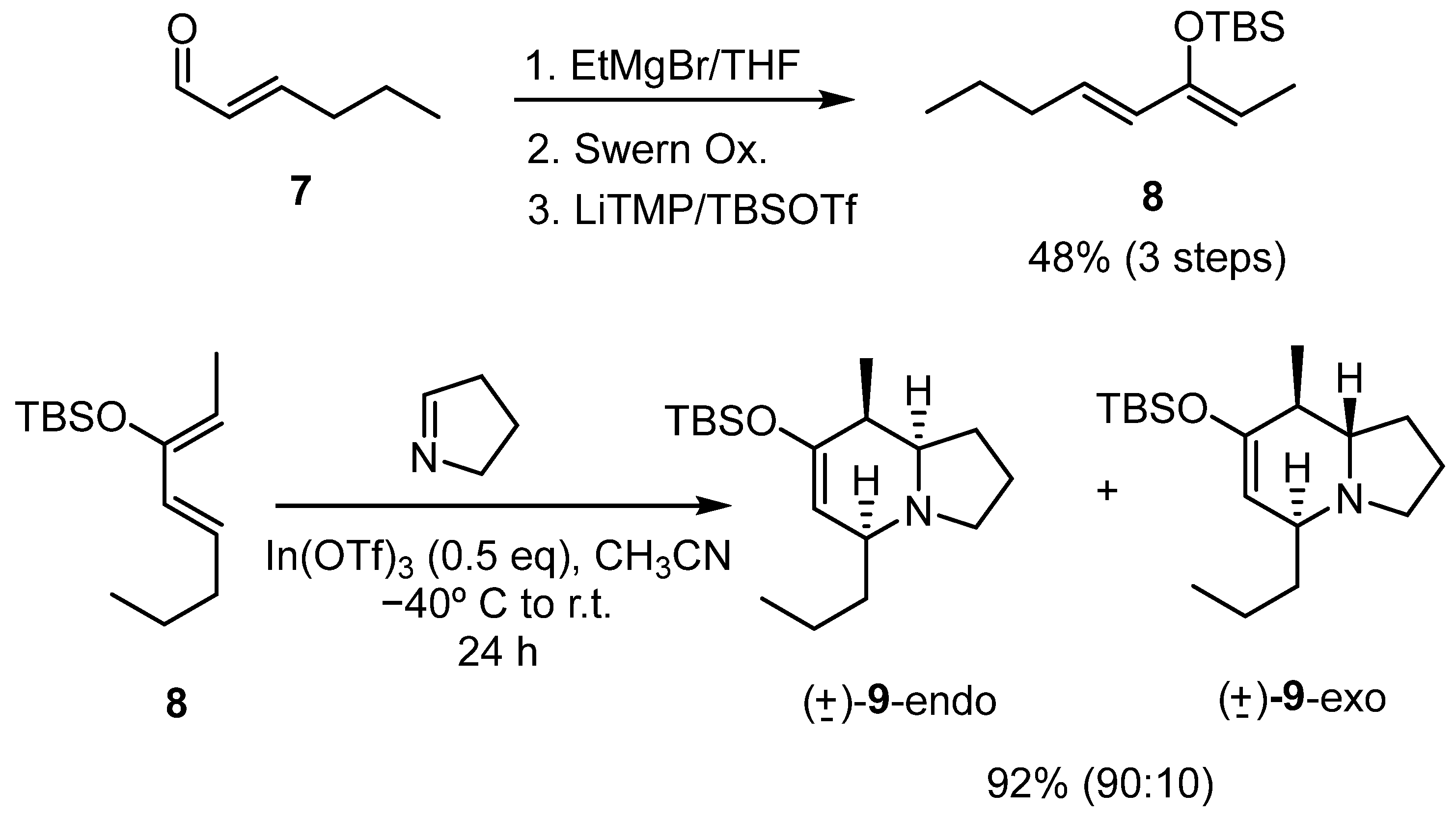
Finally, once the validity of the basic strategy was confirmed, we carried out total synthesis of one natural indolizidine in racemic form. The synthesis could have been performed from the previously obtained keto-indolizidine (±)-6, eliminating the ketone group and transforming the benzyloxy group into a leaving group to introduce the required alkyl chain. However, we felt that it would be interesting to prepare the target molecule in as few steps as possible to check the validity of this fast approach to the 5, 8-disubstituted indolizidine family. Thus, a diene with the required substituents in place was needed. Diene 8 was prepared as shown in Scheme 6 as a 9:1 mixture of Z:E isomers. The major isomer, easily separated by column chromatography using hexanes with 1% of Et3N as eluent, was reacted with Δ1-pyrroline in the presence of In(OTf)3 to give the expected cycloadducts (±)-9 in a 90:10 endo:exo ratio. This ratio is expected from the presence of the methyl group at the eighth position, which increases the selectivity favoring the endo approach as found from the calculation on compound 4. The yield was almost quantitative as judged by the 1H-NMR spectra of the crude reaction mixture, although the isolated yield was 92%, probably due to some decomposition of compounds (±)-9 in contact with the silica gel.

Scheme 6.
Synthesis of cycloadducts (±)-9.
The structures and relative stereochemistry of both cycloadducts were studied using NMR experiments, which confirmed that the major compound was the endo cycloadduct.
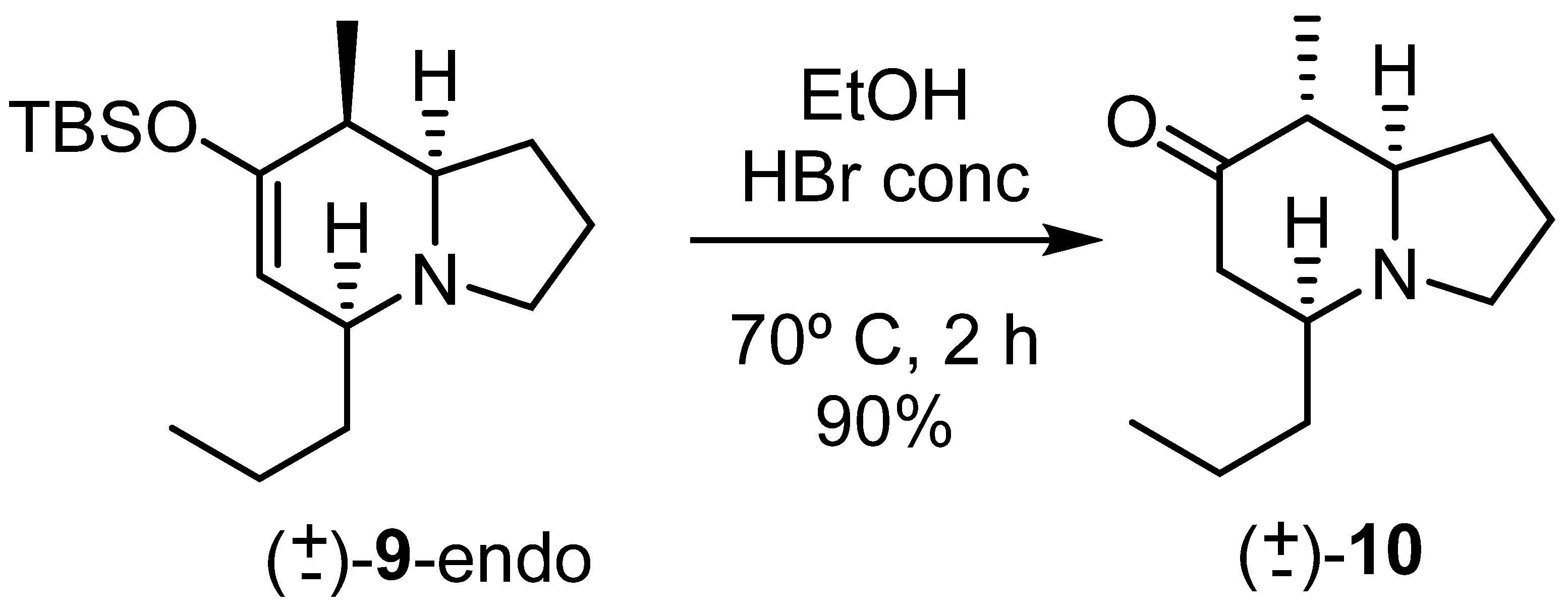
Based on the experience acquired, we decided to treat the endo cycloadduct directly with HBr to obtain the epimerized ketone in one step.
When the crude reaction mixture from the cycloaddition reaction was treated directly with HBr under the same conditions as before, ketone (±)-10 was obtained in a 90% isolated yield (Scheme 7).

Scheme 7.
Synthesis of keto-indolizidine (±)-10.
The structure was confirmed using NMR analysis. The relative stereochemistry was more difficult to prove since all three hydrogen atoms on the tertiary carbons present similar chemical shifts. Thus the assignment was tentatively established as shown in Scheme 7 based on similarity with compound 6 and some correlations observed in the ROESY spectrum.
The next step was the removal of the carbonyl group. In the literature, there are several examples of this transformation in similar compounds, and we tried some of them. The transformation of the ketone into a dithioketal and reduction with Ni-Raney/H2 [35], or treatment with TCDI and radical reduction of the thioester formed [36] gave complex mixtures.
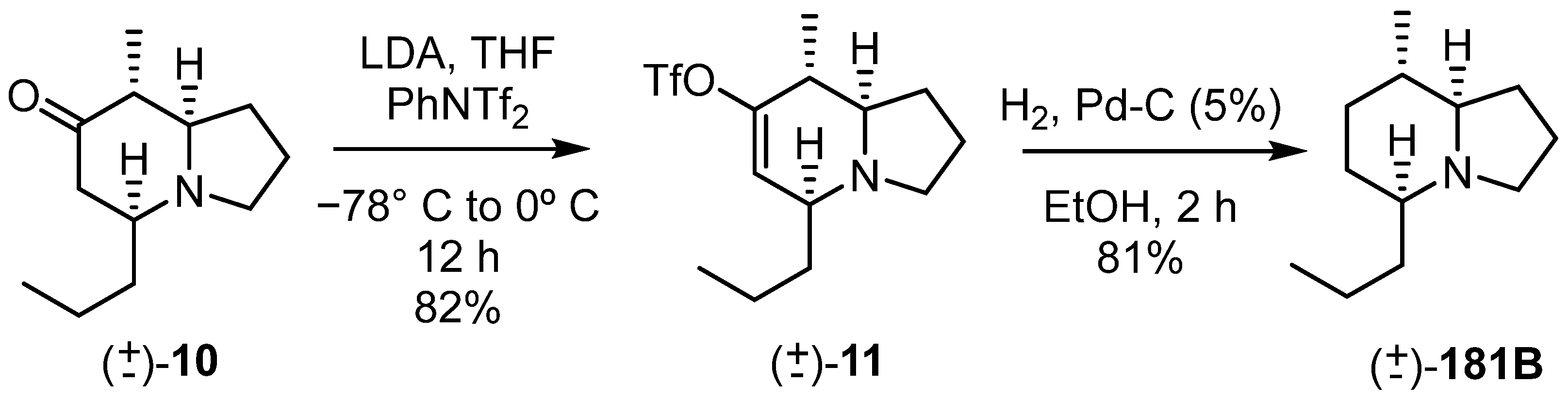
We then resorted to the transformation of the ketone into an enol-triflate and the reduction of this moiety via catalytic hydrogenation (Scheme 8) [37].

Scheme 8.
Synthesis of indolizidine (±)-181B.
The enol-triflate was prepared using LDA as the base and N-phenyl bis-trifluoromethanesulfonimide (PhNTf2), giving (±)-11 in an 82% yield. The hydrogenation step, using Pd/C as the catalyst, gave the reduced compound (±)-181B in an 81% yield. The 1H NMR spectrum of this compound, after forming its salt with trifluoroacetic acid, was compared to that published by Schneider et al. [38]; the two were found to be identical. Thus, the relative stereochemistry assumed previously was correct and it was demonstrated that a 5,8-indolizidine such as 181B can be prepared in a very short synthetic sequence, namely, a hetero Diels–Alder reaction, desilylation–isomerization, formation of the enol-triflate, and catalytic hydrogenation in an overall yield of 49.5%.
The different substitutions in the indolizidine skeleton can be introduced by selecting the adequate diene.
This approach can also be used to confirm the proposed structures of the alkaloids isolated or detected in very small amounts from natural sources.
3. Materials and Methods
3.1. General Experimental Procedures
All moisture-sensitive reactions were carried out under an argon or nitrogen atmosphere with dry solvents under anhydrous conditions. All solvents and reagents were purified using standard techniques or used as supplied from commercial sources. Reactions under standard conditions were monitored by thin-layer chromatography (TLC) on silica gel 60 F254 plates. Visualization was accomplished with UV light, stained with an ethanolic solution of phosphomolybdic acid or ninhydrin, and developed by heating. Silica gel (200–300 mesh) was used for column chromatography. NMR and spectra were recorded in CDCl3, C6D6, or CD3OD at 500 MHz for 1H NMR and 125 MHz for 13C NMR on a Bruker Avance instrument. Chemical shifts are given in (δ) parts per million and coupling constants (J) in Hz. 1H- and 13C-spectra were referenced using the solvent signal as an internal standard. The data are reported as (s = singlet, d = doublet, t = triplet, q = quartet, quintet = quintet, m = multiplet, dd = doublet of doublets, dt = doublet of triplets, and bs = broad singlet). High-resolution mass spectral analysis (HRMS) data were obtained using a VG AutoSpec spectrometer via electrospray ionization (ESI) or electron impact (EI). The 1H NMR and 13C NMR spectra and two-dimensional NMR spectroscopy of new compounds are provided in the Supplementary Materials.
3.2. Compound Synthesis
General Procedure for the Hetero Diels–Alder reaction with In(OTf)3
To a solution of In(OTf)3 (0.50 mmol) in dry acetonitrile was added a solution of Δ1-pyrroline (1.00 mmol) in acetonitrile at 0 °C under argon. After 10 min, the mixture was cooled at −40 °C and a solution of diene (1.00 mmol) in acetonitrile was added. The reaction mixture was allowed to slowly warm to room temperature and stirred for 24 h, quenched with NaHCO3 aqueous solution, extracted with ethyl acetate, washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by flash chromatography to give the corresponding products.
Hetero Diels–Alder reaction between diene 1 and Δ1-pyrroline
According to the general procedure, a 60:40 mixture of isomers 2-endo:2-exo was obtained (72%) and separated by flash chromatography (hexane/ethyl acetate 60:40 to 30:70 + TEA 0.2%)
rac-(5S,8aR)-5-(2-(benzyloxy)ethyl)-7-((tert-butyldimethylsilyl)oxy)-1,2,3,5,8,8a-hexahydroindolizine (2-endo)
Colorless oil. 1H NMR (CDCl3, 500 MHz) δ 0.11 (s, 6H), 0.91 (s, 9H), 1.48 (m, 1H), 1.70–2.18 (m, 8H), 2.35 (m, 1H), 2.89 (bs, 1H), 3.32 (t, J = 9 Hz, 1H), 3.58 (m, 2H), 4.52 (q, J = 12 Hz, 2H), 4.80 (s, 1H), 7.25–7.37 (m, 5H); 13C NMR (CDCl3, 125 MHz) δ −4.5, −4.2, 18.1, 21.7, 25.7, 30.7, 34.6, 37.1, 52.0, 59.0, 61.4, 67.5, 73.1, 106.6, 127.5, 127.6, 128.3, 138.6, 150.0; HRMS (ESI): Calcd for C23H38NO2Si [M + H] 388.2672, found 388.2671.
rac-(5S,8aS)-5-(2-(benzyloxy)ethyl)-7-((tert-butyldimethylsilyl)oxy)-1,2,3,5,8,8a-hexahydroindolizine (2-exo)
Colorless oil. 1H NMR (CDCl3, 500 MHz) δ 0.13 (s, 6H), 0.91 (s, 9H), 1.59 (m, 1H), 1.69 (m, 1H), 1.91 (m, 1H), 2.05 (m, 1H), 2.11 (m, 2H), 2.87 (m, 1H), 3.00 (m, 1H), 3.26 (m, 1H), 3.54 (m, 2H), 3.62 (m, 2H), 3.67 (m, 1H), 4.51 (q, J = 12.5 Hz, 2H), 4.87 (d, J = 4.3 Hz, 1H), 7.27–7.38 (m, 5H); 13C NMR (CDCl3, 125 MHz) δ −4.5, −4.4, 18.0, 21.2, 25.7, 30.3, 32.9, 33.2, 49.4, 53.0, 67.5, 73.1, 127.6, 127.7, 128.4, 138.4, 148.4; HRMS (ESI): Calcd for C23H38NO2Si [M + H] 388.2672, found 388.2666.
Hetero Diels–Alder reaction between diene 3 and Δ1-pyrroline
According to the general procedure, compound 4 was isolated (91%) as a major isomer using hexane/ethyl acetate 70:30 + TEA 0.2%
rac-(5S,8S,8aR)5-(2-(benzyloxy)ethyl)-7-((tert-butyldimethylsilyl)oxy)-8-methyl-1,2,3,5,8,8a-hexahydroindolizine (4)
Colorless oil. 1H NMR (CDCl3, 500 MHz) δ 0.00 (s, 3H), 0.02 (s, 3H), 0.81 (s, 9H), 0.89 (d, J = 6.7 Hz, 3H), 1.51–1.57 (m, 2H), 1.59–1.76 (m, 4H), 1.90–1.95 (m, 2H), 2.36 (td, J = 2.3, 8.5 Hz, 1H), 2.74 (bs, 1H), 3.15 (td, J = 8.4, 11.5 Hz, 1H), 3.40–3.45 (m, 1H), 3.47–3.53 (m, 1H), 4.38 (m, 2H), 4.53 (d, J = 1.8, 1H), 7.19 (m, 5H); 13C NMR (CDCl3, 125 MHz) δ −0.46, −0.41, 12.5, 18.0, 22.2, 25.9, 26.0, 34.5, 37.4, 52.3, 59.3, 64.0, 67.4, 73.0, 105.0, 127.4, 127.6, 128.3, 138.5, 155.0; HRMS (EI): Calcd for C24H39NO2Si [M+] 401.2750, found 401.2745.
Synthesis of Keto-indolizidine 6
To a solution of 4 (0.250 g, 0.62 mmol) in THF (5 mL) at 0 °C was added tetrabutylammonium fluoride trihydrate (0.196 g, 0.62 mmol). The mixture was allowed to reach room temperature and after completion, the solvent was removed by vacuum, affording a 1:2 mixture of C-8 methyl-epimers 5 and 6 (0.139 g, 78%). To a solution of this mixture in EtOH (3 mL) was added HBr (conc.) (1.0 mL). The reaction mixture was heated at 70 °C for 2 h. After cooling to room temperature, the solvent was removed by vacuum, and DCM was added. Then, an aqueous solution of NaHCO3 was added, extracted with DCM, washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by flash chromatography hexane/ethyl acetate 35:65, affording 6 as the only compound (0.118 g, 85%)
rac-(5S,8R,8aR)-5-(2-(benzyloxy)ethyl)-8-methylhexahydroindolizin-7(1H)-one (6)
Yellow oil. 1H NMR (CDCl3, 500 MHz): 1.00 (d, J = 6.7, 3H), 1.60 (m, 1H), 1.71 (m, 1H), 1.79 (m, 1H), 1.90 (m, 1H), 2.00 (m, 2H), 2.12 (m, 2H), 2.32 (m, 2H), 2.43 (m, 1H), 2.52 (m, 1H), 3.27 (t, J = 9.0 Hz, 1H), 3.51 (m, 2H), 4.48 (s, 2H), 7.25–7.37 (m, 5H) 13C NMR (δ, CDCl3): 10.5, 21.4, 30.0, 34.8, 46.0, 50.2, 50.8, 59.2, 66.6, 70.4, 73.1, 127.6, 128.0, 138.4, 210.5; HRMS (EI): Calcd for C18H25NO2 287.1885, found 287.1878.
Synthesis of tert-butyldimethyl(((2Z,4E)-octa-2,4-dien-3-yl)oxy)silane (8)
To an ethylmagnesium bromide solution (1.0 M in THF, 76 mmol, 1.5 equiv) at 0 °C was slowly added trans-2-hexenal (5.0 g, 50.9 mmol, 1.0 equiv) in THF (25 mL). The reaction was allowed to warm to room temperature and stirred for 1 h. The reaction mixture was carefully quenched with saturated aqueous NH4Cl, extracted with diethyl ether, washed with brine, dried over MgSO4, and carefully concentrated in vacuo. The residue was purified by distillation through a vigreux column, bp. 112 °C (760 Torr), yielding the corresponding alcohol (4.6 g, 71%)
(E)-oct-4-en-3-ol (8): Pale yellow oil. 1H NMR (CDCl3, 500 MHz) 0.80 (t, J = 7.3 Hz, 6H), 1.25–1.52 (m, 5H), 1.91 (m, 2H), 3.87 (q, J = 6.7 Hz, 1H), 5.35 (dt, J = 15.2, 7.0 Hz, 1H), 5.53 (dt, J = 15.2, 6.9 Hz, 1H).
A round-bottom flask equipped with a magnetic stirrer and containing DMSO (1.06 mL, 15 mmol) in DCM (100 mL) at −78 °C was charged dropwise with oxalyl chloride 2.0 M in DCM (6 mL, 12 mmol,) and stirred for 30 min. To the resulting mixture, at −60 °C, a solution of the previously prepared alcohol (1.28 g, 10 mmol) in DCM (8 mL) was added dropwise and the reaction mixture was stirred for 1 h at −60 °C, then TEA (6.9 mL, 50 mmol) was added dropwise and stirred for 15 min. The reaction mixture was allowed to warm to room temperature, diluted with DCM, quenched by the addition of water, extracted with DCM, and the combined organic layers washed sequentially with 1% aqueous HCl solution (30 mL), followed by 5% aqueous NaHCO3 solution (30 mL), and brine (30 mL). The organic layers were dried (MgSO4) and filtered, and the solvent was carefully removed by vacuum. The crude product was purified by flash chromatography hexane/ethyl acetate 85:15, affording (E)-oct-4-en-3-one (1.07 g, 85%).
(E)-oct-4-en-3-one: Colorless oil. 1H NMR (CDCl3, 500 MHz) 0.94 (t, J = 7.7 Hz, 3H), 1.09 (t, J = 7.4 Hz, 3H), 1.49 (m, 2H), 2.19 (m, 2H), 2.56 (q, J = 7.4 Hz, 2H), 6.09 (dt, J = 15.7, 1.5 Hz, 1H), 6.85 (dt, J = 15.7, 7.0 Hz, 1H).
To a solution of (E)-oct-4-en-3-one (0.75 g, 5.95 mmol) in THF (20 mL) cooled at −78 °C, tert-butyldimethylsilyl trifluoromethane sulfonate (1.6 mL, 7.14 mmol) was added. Then, 2,2,6,6-tetramethylpiperidine lithium salt (5.95 mmol, 0.3 M in THF) was added. After 15 min, an aqueous solution of NH4Cl was added and the mixture was extracted with diethyl ether, washed with brine, dried over Na2SO4, filtered, and carefully concentrated in vacuo. The combined organic layers were dried over anhydrous Na2SO4 and the solvent was removed by vacuum. The crude product was purified by flash chromatography hexane + 1% TEA, affording 8 (1.14 g, 80%)
tert-Butyldimethyl(((2Z,4E)-octa-2,4-dien-3-yl)oxy)silane (8): Colorless oil. 1H NMR (C6D6, 500 MHz) δ 0.15 (s, 6H), 0.87 (t, J = 7.3 Hz, 3H), 1.07 (s, 9H), 1.35 (quintet, J = 7.3 Hz, 2H), 1.65 (d, J = 6.8 Hz, 3H), 1.99 (q, J = 6.9 Hz, 2H), 4.73 (q, J = 7.0 Hz, 1H), 5.86–5.97 (m, 2H); 13C NMR (C6D6, 125 MHz) δ −3.4, 11.9, 13.9, 18.7, 23.1, 26.2, 34.8, 128.7, 129.6, 149.7; HRMS (EI) calc for C14H28OSi 240.1909, found 240.1904.
Hetero Diels–Alder reaction between diene 8 and Δ1-pyrroline
According to the general procedure, a mixture of compounds 9-endo:9-exo was isolated (92%) as 90:10 isomers, hexane/ethyl acetate 70:30 to 50:50 + TEA 0.2%
rac-(5S,8S,8aR)-7-((tert-butyldimethylsilyl)oxy)-8-methyl-5-propyl-1,2,3,5,8,8a-hexahydroindolizine (9-endo)
Colorless oil. 1H NMR (C6D6, 500 MHz) δ 0.18 (s, 3H), 0.19 (s, 3H), 0.94 (t, J = 7.3 Hz, 3H), 1.00 (s, 9H), 1.24 (d, J = 6.8 Hz, 3H), 1.27–1.40 (m, 1H), 1.41–1.51 (m, 2H), 1.52–1.75 (m, 5H), 1.96 (q, J = 8.6 Hz, 1H), 2.13 (m, 1H), 2.50 (m, 1H), 2.84 (bs, 1H), 3.17 (t, J = 7.4 Hz, 1H), 4.77 (d, J = 1.7 Hz, 1H); 1H NMR (CDCl3, 500 MHz): 0.14 (s, 3H), 0.15 (s, 3H), 0.90 (t, J = 7.0 Hz, 3H), 0.93 (s, 9H), 1.02 (d, J = 6.8 Hz, 3H), 1.27 (m, 1H), 1.40 (m, 2H), 1.49 (m, 2H), 1.66 (m, 1H), 1.75 (m, 2H), 1.99 (m, 1H), 2,05 (m, 1H), 2.46 (m, 1H), 2.66 (m, 1H), 3.26 (td, J = 8.8, 2.1 Hz, 1H), 4.67 (d, J = 1.7 Hz, 1H); 13C NMR (C6D6, 125 MHz) δ −4.5, −4.0, 12.9, 14.9, 17.8, 18.3, 22.8, 26.0, 26.3, 37.5, 38.1, 52.3, 61.7, 64.2, 105.7, 155.5; E.M. m/z (relative intensity): 309 (M+) (2.5), 266 (100), 75 (12), 73 (23); HRMS: Calcd for C18H35NOSi [M+] 309.2488, found 309.2480.
rac-(5S,8S,8aS)-7-((tert-butyldimethylsilyl)oxy)-8-methyl-5-propyl-1,2,3,5,8,8a-hexahydroindolizine (9-exo)
Colorless oil. 1H NMR (C6D6, 500 MHz) δ 0.20 (s, 3H), 0.21 (s, 3H), 0.96 (t, J = 7.0 Hz, 3H), 1.03 (s, 9H), 1.10 (d, J = 6.8 Hz, 3H), 1.44 (m, 2H), 1.58 (m, 1H), 1.69 (q, J = 7.3 Hz, 1H), 1.91 (m, 1H), 2.03 (q, J = 7.0 Hz, 1H), 2.71 (bs, 1H), 2.78 (t, J = 7.0 Hz, 2H), 3.37 (m, 1H), 5.04 (d, J = 3.7 Hz, 1H); 13C NMR (C6D6, 125 MHz) δ −4.5, −4.4, 14.8, 15.6, 18.5, 20.5, 22.3, 26.0, 30.3, 36.1, 37.4, 50.1, 55.2, 60.0, 106.1, 152.2.
Synthesis of Keto-indolizidine (10)
To a solution of 9-endo (225 mg, 1.02 mmol) in EtOH (4 mL) was added HBr (conc.) (1.3 mL). The reaction mixture was heated at 70 °C for 2 h. After cooling to room temperature, the solvent was removed by vacuum, and DCM was added. Then, an aqueous solution of NaHCO3 was added, extracted with DCM, washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by flash chromatography hexane/ethyl acetate 70:30 to give compound 10 (128 mg, 90%)
rac-(5S,8R,8aR)-8-methyl-5-propylhexahydroindolizidin-7(1H)-one (10)
1H NMR (C6D6, 500 MHz) δ 0.78 (t, J = 7.1 Hz, 3H), 1.00 (d, J = 6.5 Hz, 3H), 1.04 (m, 1H), 1.24 (m, 2H), 1.37 (m, 2H), 1.60 (m, 1H), 1.68 (m, 1H), 1.74 (q, J = 8.6 Hz, 1H), 2.09 (m, 2H), 2.31 (d, J = 10.5 Hz, 1H), 2.96 (t, J = 8.5 Hz, 1H); 13C NMR (C6D6, 125 MHz) δ 10.9, 14.5, 17.9, 21.8, 30.4, 37.3, 45.8, 50.3, 50.6, 61.5, 70.6, 208.6; HRMS (ESI): Calcd for C12H22NO [M + H] 196.1701, found 196.1696.
Synthesis of Enol-triflate (11)
To a solution of LDA (1.58 mmol, 0.5 M in THF) at −78 °C was added, dropwise, a solution of keto-indolizidine 10 (0.103 g, 0.53 mmol) in THF (2 mL). The reaction mixture was stirred and allowed to slowly warm to 0 °C for 2 h, then a solution of N-Phenyl-bis(trifluoromethanesulfonimide (0.284 g, 0.8 mmol) in THF (2 mL) was added, and then stirred at 0 °C for 12 h. The reaction mixture was diluted with diethyl ether, quenched by the addition of water, extracted with diethyl ether, washed with brine, dried over MgSO4, and concentrated in vacuo. The residue was purified by flash chromatography column hexane/ethyl acetate 90:10, affording 11 (0.142 g, 82%)
rac-(5S,8S,8aR)-8-methyl-5-propyl-1,2,3,5,8,8a-hexahydroindolizin-7-yl trifluoromethanesulfonate (11)
Colorless oil. 1H NMR (CDCl3, 500 MHz) δ 0.94 (t, J = 7.2 Hz, 3H), 1.11 (d, J = 7.0 Hz, 3H), 1.30 (m, 2H), 1.47 (m, 1H), 1.61 (m, 2H), 1.82 (m, 1H), 1.94 (m, 1H), 2.10 (m, 1H), 2.18 (m, 2H), 2.55 (m, 1H), 2.93 (m, 1H), 3.35 (m, 1H), 5.67 (s, 1H).
Synthesis of 181B
A stirred solution of 11 (0.140 g, 0.43 mmol) in EtOH (2 mL) was degassed and purged with vacuum–argon cycles. To this solution was added 28 mg (20% weight) of 5 % Pd-C. The mixture was stirred under a hydrogen atmosphere at balloon pressure for 2 h, and then argon was bubbled through the solution for a few minutes. The reaction mixture was filtered through Celite and then one drop of trifluoroacetic acid was added. The solvent was removed by vacuum to give 103 mg of compound 181B as its salt (81% yield).
rac-(5S,8S,8aR)-8-methyl-5-propyloctahydroindolizine (181B)
1H NMR (CD3OD, 500 MHz) δ 0.99 (t, J = 7.0 Hz, 3H), 1.03 (d, J = 6.6 Hz, 3H), 1.24–1.57 (m, 5H), 1.67 (m, 2H), 1.90 (m, 2H), 2.00 (m, 1H), 2.13 (m, 2H), 2.37 (m, 1H), 2.86 (m, 1H), 3.07 (m, 2H), 3.73 (m, 1H); 13C NMR (CD3OD, 125 MHz) δ 14.1, 18.3, 19.4, 20.0, 28.3, 30.1, 32.5, 35.2, 36.0, 52.1, 65.2, 73.6; HRMS (ESI): Calcd for C12H24N [M + H] 182.1909, found 182.1905.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28217316/s1: 1H-NMR, 13C-NMR, and 2D spectra of compounds 2-endo, 2-exo, 4-endo, 6, 11-endo, 11-exo, 12, and 181B; 1H-NMR and 13C-NMR of compound 10 and 1H-NMR of compound 13; Discussion of the calculations of the reaction profiles of dienes 1 and 3 with Δ1-pyrroline, epimerization of compounds 5 to 6, and the cartesian coordinates of the stationary points of computational calculations. Refs. [39,40] are cited in the Supplementary Materials.
Author Contributions
M.M.A. and J.A.P. conceived and designed the experiments; J.F.R.-C. and M.M.A. performed the experiments; J.A.P. performed the computational studies. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Michael, J.P. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 2008, 25, 139–165. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.P. Chapter One-Simple Indolizidine and Quinolizidine Alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 75, pp. 1–498. [Google Scholar] [CrossRef]
- De Rop, A.-S.; Rombaut, J.; Willems, T.; De Graeve, M.; Vanhaecke, L.; Hulpiau, P.; De Maeseneire, S.L.; De Mol, M.L.; Soetaert, W.K. Novel Alkaloids from Marine Actinobacteria: Discovery and Characterization. Mar. Drugs 2022, 20, 6. [Google Scholar] [CrossRef]
- Zhang, J.; Morris-Natschke, S.L.; Ma, D.; Shang, X.-F.; Yang, C.-J.; Liu, Y.-Q.; Lee, K.-H. Biologically active indolizidine alkaloids. Med. Res. Rev. 2021, 41, 928–960. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kamal, R.; Kumar, D.; Kumar, V. Indolizidine Alkaloids: Prospective Lead Molecules in Medicinal Chemistry. Curr. Tradit. Med. 2021, 7, 45–56. [Google Scholar] [CrossRef]
- Daly, J.W.; Spande, T.F.; Garraffo, H.M. Alkaloids from Amphibian Skin: A Tabulation of Over Eight-Hundred Compounds. J. Nat. Prod. 2005, 68, 1556–1575. [Google Scholar] [CrossRef] [PubMed]
- Saporito, R.A.; Donnelly, M.A.; Roy, A.; Norton, R.A.; Garraffo, H.M.; Spande, T.F.; Daly, J.W. Oribatid mites as a major dietary source for alkaloids in poison frogs. Proc. Natl. Acad. Sci. USA 2007, 104, 8885–8890. [Google Scholar] [CrossRef]
- Hantak, M.M.; Grant, T.; Reinsch, S.; Mcginnity, D.; Loring, M.; Toyooka, N.; Saporito, R.A. Dietary Alkaloid Sequestration in a Poison Frog: An Experimental Test of Alkaloid Uptake in Melanophryniscus stelzneri (Bufonidae). J. Chem. Ecol. 2013, 39, 1400–1406. [Google Scholar] [CrossRef]
- Toyooka, N.; Tsuneki, H.; Kobayashi, S.; Dejun, Z.; Kawasaki, M.; Kimura, I.; Sasaoka, T.; Nemoto, H. Synthesis of Poison-Frog Alkaloids and Their Pharmacological Effects at Neuronal Nicotinic Acetylcholine Receptors. Curr. Chem. Biol. 2007, 1, 97–114. [Google Scholar] [CrossRef]
- Daly, J.W.; Nishizawa, Y.; Padgett, W.L.; Tokuyama, T.; Smith, A.L.; Holmes, A.B.; Kibayashi, C.; Aronstam, R.S. 5,8-Disubstituted indolizidines: A new class of noncompetitive blockers for nicotinic receptor-channels. Neurochem. Res. 1991, 16, 1213–1218. [Google Scholar] [CrossRef]
- Ratmanovaa, N.K.; Andreev, I.A.; Leontiev, A.V.; Momotova, D.; Novoselov, A.M.; Ivanova, O.A.; Trushkov, I.V. Strategic approaches to the synthesis of pyrrolizidine and indolizidine alkaloids. Tetrahedron 2020, 76, 131031. [Google Scholar] [CrossRef]
- Danishefsky, S.J.; Vogel, C. Concise Total Synthesis of (±)-Ipalbidine by Application of the Aldimine-Diene Cyclocondensation Reaction. J. Org. Chem. 1986, 51, 3915–3916. [Google Scholar] [CrossRef]
- Shao, J.; Yang, J.-S. A Diastereoselective Cyclic Imine Cycloaddition Strategy To Access Polyhydroxylated Indolizidine Skeleton: Concise Syntheses of (+)-/(−)-Lentiginosines and (−)-2-epi-Steviamine. J. Org. Chem. 2012, 77, 7891–7900. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Leoni, S.; Abbiati, G.; Pirovano, V.; Rossi, E. Formal Aza-Diels-Alder Reactions of Spiroindolenines with Electronrich Dienes. Eur. J. Org. Chem. 2021, 2021, 2440–2447. [Google Scholar] [CrossRef]
- Del Fiandra, C.; Moccia, M.; Cerullia, V.; Adamo, M.F.A. Catalytic asymmetric conjugate addition of isocyanoacetate to (Z)-3-substituted-2-(4-pyridyl)-acrylonitrile, a reactive class of Michael acceptor. Chem. Commun. 2016, 52, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, P.; Stecko, S.; Maziarz, E.; Staszewska-Krajewska, O.; Furman, B. Synthesis of Polyhydroxylated Quinolizidine and Indolizidine Scaffolds from Sugar-Derived Lactams via a One-Pot Reduction/Mannich/Michael Sequence. J. Org. Chem. 2014, 79, 10487–10503. [Google Scholar] [CrossRef]
- Aoyagi, S.; Hakoishi, M.; Suzuki, M.; Nakanoya, Y.; Shimada, K.; Takikawa, Y. Synthesis of δ-thiolactams by the aza-Diels–Alder reaction of in situ generated allenyltrimethylsilylthioketenes with imines. Tetrahedron Lett. 2006, 47, 7763–7766. [Google Scholar] [CrossRef]
- Aoyagi, S.; Kikuchi, K.; Shimada, K.; Takikawa, Y. Convenient Synthesis of 4-Methylenecyclobutenones and Their Synthetic Utility as Allenylketene Precursors. Synlett 2007, 16, 2553–2556. [Google Scholar] [CrossRef]
- Martín, M.; Afonso, M.M.; Galindo, A.; Palenzuela, J.A. Enantioselective Synthesis of a Tetrasubstituted Oxocane via a Double Diastereoselective Hetero Diels-Alder Reaction. Synlett 2001, 2001, 117–119. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, S.; Ding, Z.; Ma, D. Enantioselective Addition of Activated Terminal Alkynes to 1-Acylpyridinium Salts Catalyzed by Cu-Bis(oxazoline) Complexes. J. Am. Chem. Soc. 2007, 129, 9300–9301. [Google Scholar] [CrossRef] [PubMed]
- Kapat, A.; Nyfeler, E.; Giuffredi, G.T.; Renaud, P. Intramolecular Schmidt Reaction Involving Primary Azidoalcohols under Nonacidic Conditions: Synthesis of Indolizidine (−)-167B. J. Am. Chem. Soc. 2009, 131, 17746–17747. [Google Scholar] [CrossRef]
- Yang, X.; Gu, X.; Bin, H.; Xie, J.; Zhou, Q. Asymmetric Synthesis of (−)-Indolizidine 167B and (+)-Coniine. Chin. J. Org. Chem. 2020, 40, 3963–3968. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Li, Z.; Cui, L.; Li, C. Silver-Catalyzed Decarboxylative Radical Azidation of Aliphatic Carboxylic Acids in Aqueous Solution. J. Am. Chem. Soc. 2015, 137, 9820–9823. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.-H.; Chen, H.-Y. Synthesis of Dendrobatid Alkaloid (+)-167B and (+)-209D and the Investigation of Diastereoselectivity using DFT Calculations. RSC Adv. 2017, 7, 684–687. [Google Scholar] [CrossRef]
- Miao, P.; Li, R.; Lin, X.; Rao, L.; Sun, Z. Visible-light Induced Metal-free Cascade Wittig/Hydroalkylation Reactions. Green. Chem. 2021, 23, 1638–1641. [Google Scholar] [CrossRef]
- Lee, E.; Li, K.S.; Lim, J. Radical Cyclization of β-Aminoacrylates: Stereoselective Synthesis of Indolizidines 167B and 209D. Tetrahedron Lett. 1996, 37, 1448. [Google Scholar] [CrossRef]
- Ponpandian, T.; Muthusubramanian, S. Sequential deprotection–Cyclisation reaction: Stereoselective synthesis of azabicyclic β-enamino ester derivatives and (−) indolizidine 209D. Tetrahedron 2013, 69, 527–536. [Google Scholar] [CrossRef]
- Mujica, M.T.; Afonso, M.M.; Galindo, A.; Palenzuela, J.A. Hetero diels-alder vs Mukaiyama aldol pathways in the reaction of monoactivated dienes and aldehydes. A Lewis acid study. Tetrahedron 1996, 52, 2167–2176. [Google Scholar] [CrossRef]
- Struve, C.; Christophersen, C. Structural Equilibrium and Ring-Chain Tautomerism of Aqueous Solutions of 4-Aminobutyraldehyde. Heterocycles 2003, 60, 1907–1914. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Michel, P.; Rassat, A.; Daly, J.W.; Spande, T.F. A Stereospecific Synthesis of (±)-5,8-Disubstituted Indolizidines and (±)-1,4-Disubstituted Quinolizidines Found in Poison Frog Skins. J. Org. Chem. 2000, 65, 8908–8918. [Google Scholar] [CrossRef]
- Reddy, C.R.; Ramesh, P.; Latha, B. Formal Syntheses of 5,8-Disubstituted Indolizidine Alkaloids (–)-205A, (–)-207A, and (–)-235B. Synlett 2017, 28, 481–484. [Google Scholar] [CrossRef]
- Zhou, D.-J.; Wang, Z.-H.; Zhang, Y.-R.; Cui, Z.-G. Flexible Syntheses of 5,8-Disubstituted Indolizidine Poisonous-Frog Alkaloids via a Michael-Type Conjugate Addition. J. Chem. Res. 2017, 41, 98–105. [Google Scholar] [CrossRef]
- Liou, B.-S.; Jhang, R.-F.; Chou, S.-S.P. Formal synthesis of (±)-indolizidine 209B. Tetrahedron 2014, 70, 7458–7463. [Google Scholar] [CrossRef]
- Michael, J.P.; Gravestock, D. An Expeditious Synthesis of the Dendrobatid Indolizidine Alkaloid 167B. Eur. J. Org. Chem. 1998, 1998, 865–870. [Google Scholar] [CrossRef]
- Takashima, K.; Okada, T.; Kato, A.; Yamasaki, Y.; Sugouchi, T.; Akanuma, S.-I.; Kubo, Y.; Hosoya, K.-I.; Morita, H.; Ito, T.; et al. Divergent Synthesis of Decahydroquinoline-Type Poison-Frog Alkaloids. ChemistrySelect 2022, 7, e202104533. [Google Scholar] [CrossRef]
- Stoye, A.; Quandt, G.; Brunnhöfer, B.; Kapatsina, E.; Baron, J.; Fischer, A.; Weymann, M.; Kunz, H. Stereoselective Synthesis of Enantiomerically Pure Nupharamine Alkaloids from Castoreum. Angew. Chem. Int. Ed. 2009, 48, 2228–2230. [Google Scholar] [CrossRef]
- Abels, F.; Lindemann, C.; Schneider, C. A General Strategy for the Catalytic, Highly Enantio- and Diastereoselective Synthesis of Indolizidine-Based Alkaloid. Chem. Eur. J. 2014, 20, 1964–1979. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeerschd, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLview20; Université de Sherbrooke: Sherbrooke, QC, USA, 2020; Available online: http://www.cylview.org (accessed on 6 October 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).