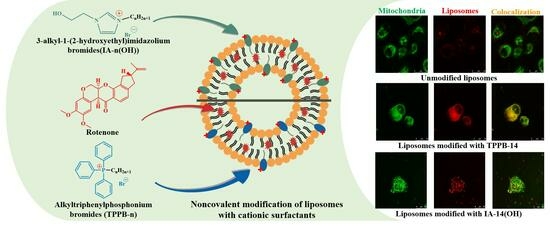
Mitochondria-Targeted Lipid Nanoparticles Loaded with Rotenone as a New Approach for the Treatment of Oncological Diseases
Abstract
:1. Introduction
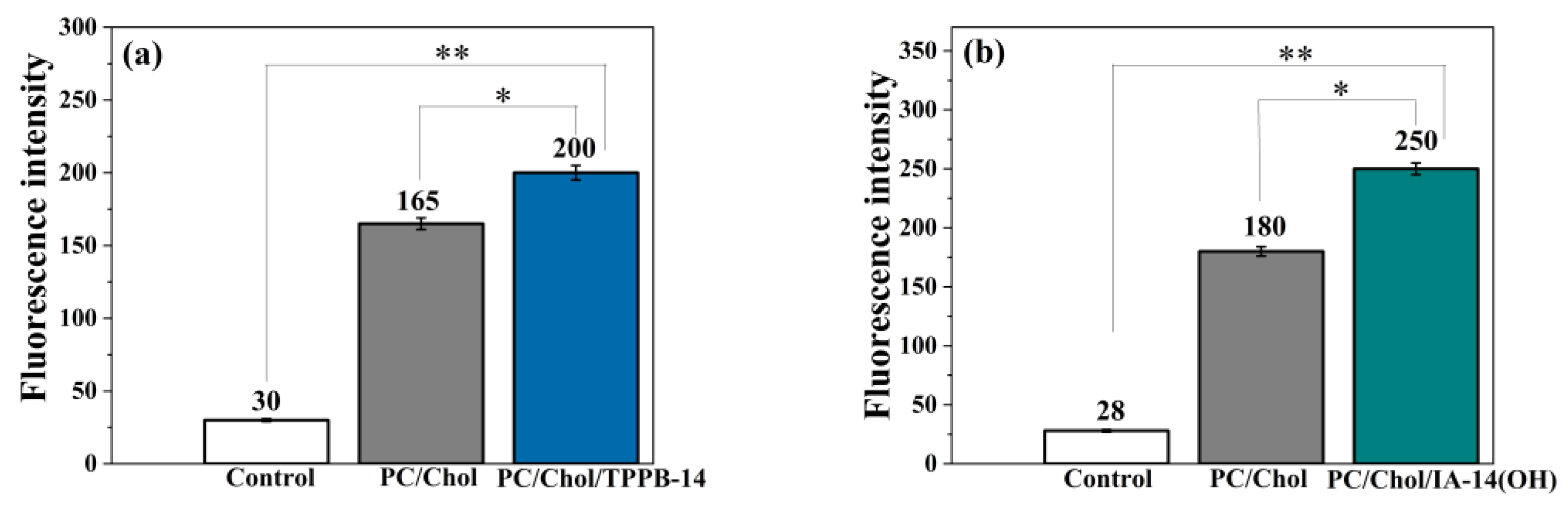
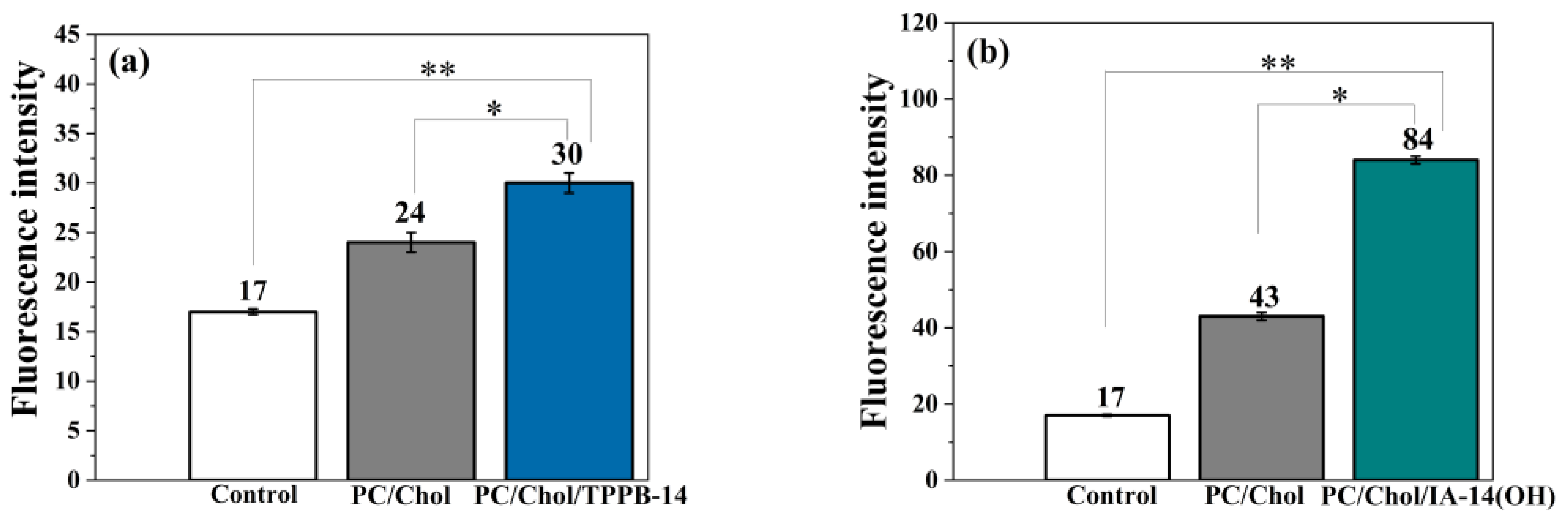
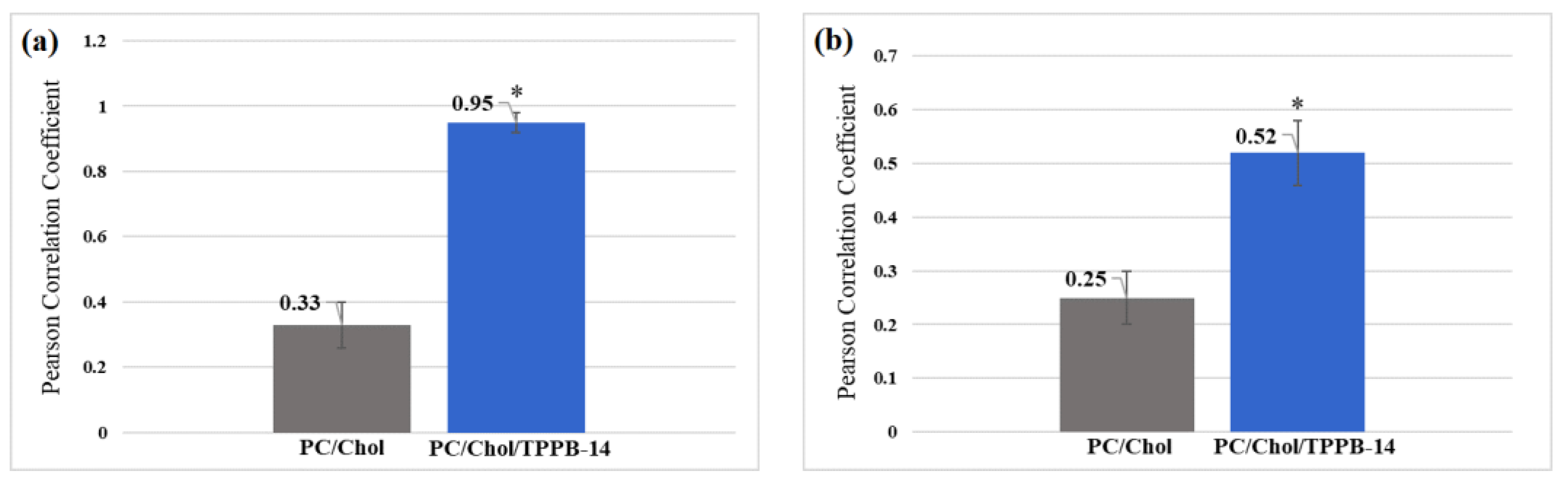
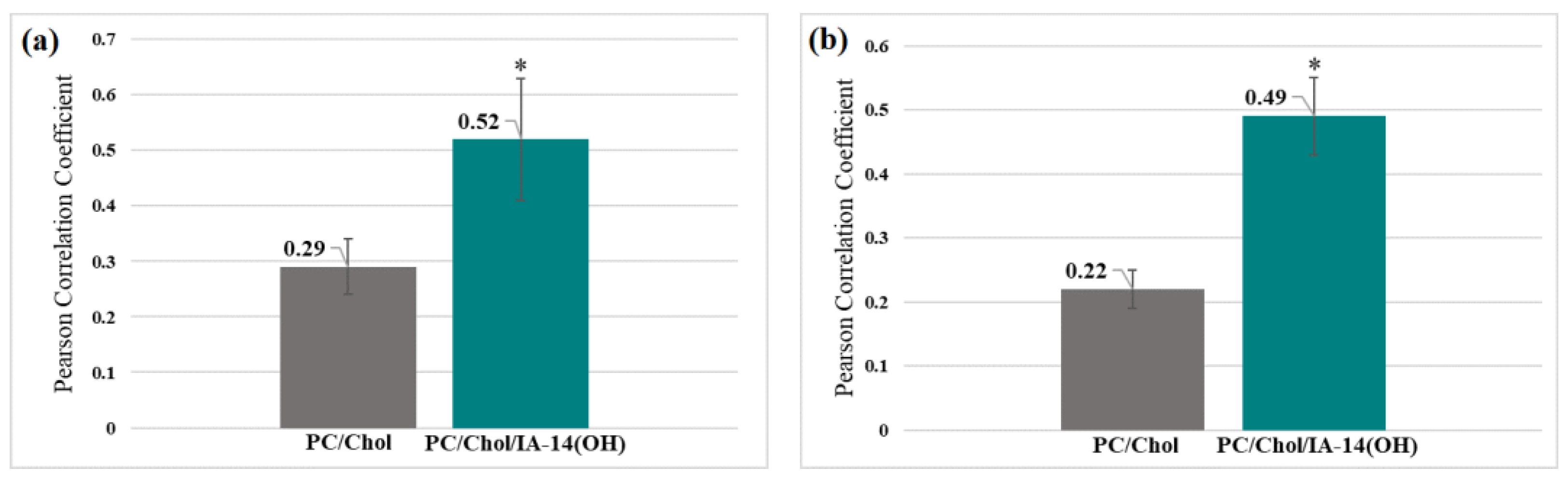
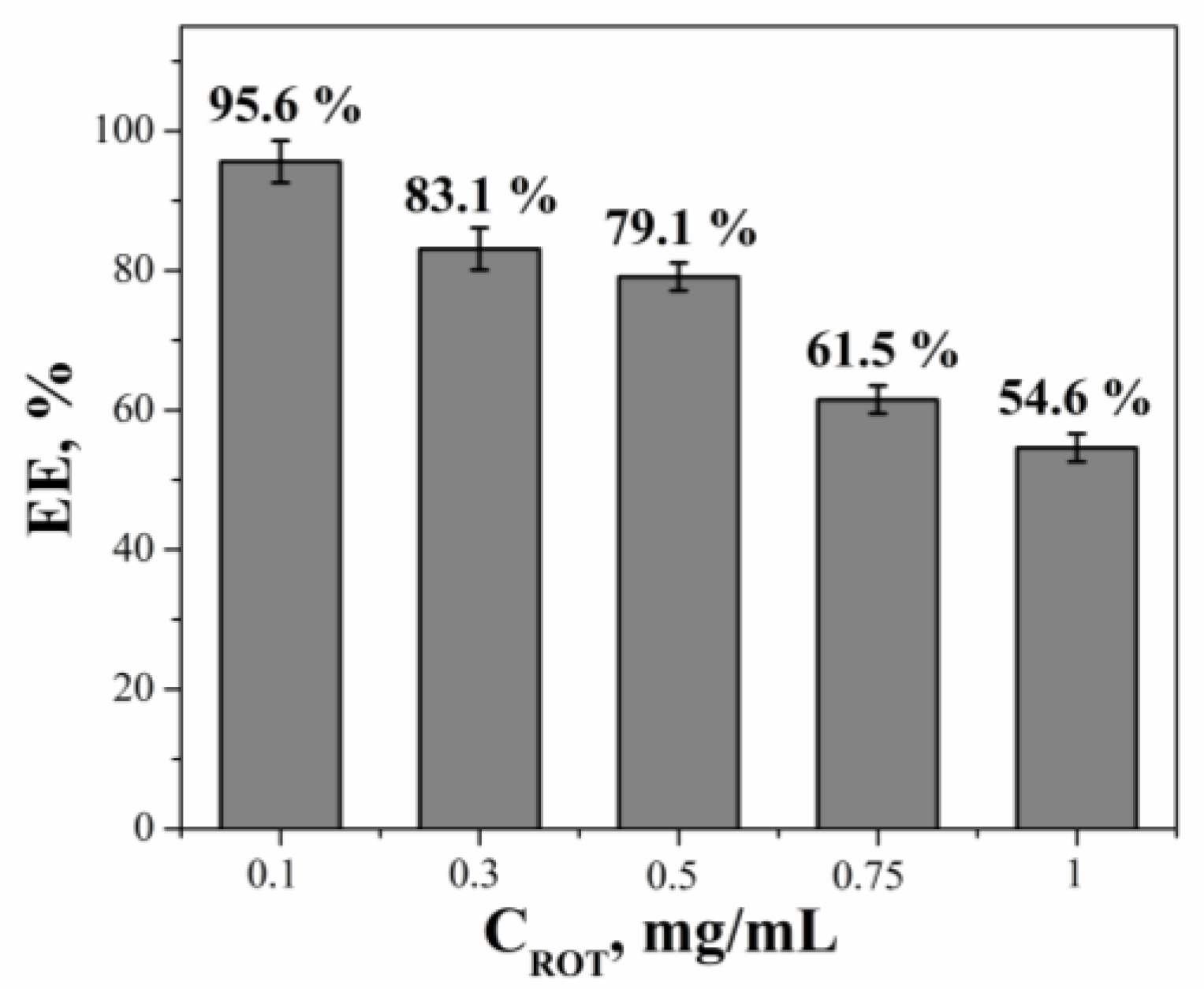
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Liposome Preparation
3.3. Dynamic and Electrophoretic Light Scattering (DLS/ELS)
3.4. Transmission Electron Microscopy (TEM)
3.5. Encapsulation Efficiency (EE) and Release Rate of Substrate
3.6. Cell Culture
3.7. Cellular Uptake
3.8. Cytotoxicity
3.9. Colocalization Assay
3.10. Mitochondrial Membrane Potential
3.11. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milane, L.; Dolare, S.; Jahan, T.; Amiji, M. Mitochondrial Nanomedicine: Subcellular Organelle-Specific Delivery of Molecular Medicines. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102422. [Google Scholar] [CrossRef]
- Du, H.; Yan, S.S. Mitochondrial Medicine for Neurodegenerative Diseases. Int. J. Biochem. Cell Biol. 2010, 42, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The Cell Biology of Mitochondrial Membrane Dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Milane, L.; Trivedi, M.; Singh, A.; Talekar, M.; Amiji, M. Mitochondrial Biology, Targets, and Drug Delivery. J. Control. Release 2015, 207, 40–58. [Google Scholar] [CrossRef]
- Zorov, D.B.; Isaev, N.K.; Plotnikov, E.Y.; Silachev, D.N.; Zorova, L.D.; Pevzner, I.B.; Morosanova, M.A.; Jankauskas, S.S.; Zorov, S.D.; Babenko, V.A. Perspectives of Mitochondrial Medicine. Biochemistry 2013, 78, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Rahman, J.; Rahman, S. Mitochondrial Medicine in the Omics Era. Lancet 2018, 391, 2560–2574. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, M.; Morra, F.; Di Meo, I.; Tiranti, V. Mitochondrial Transplantation in Mitochondrial Medicine: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 1969. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Vásquez-Trincado, C.; García-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial Dynamics, Mitophagy and Cardiovascular Disease: Mitochondria and Cardiovascular Disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The Warburg Effect 97 Years after Its Discovery. Cancers 2020, 12, 2819. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg Effect: Historical Dogma versus Current Understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Neuzil, J. Targeting Mitochondria as an Anticancer Strategy. Cancer Commun. 2019, 39, 63. [Google Scholar] [CrossRef]
- Battogtokh, G.; Cho, Y.-Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondrial-Targeting Anticancer Agent Conjugates and Nanocarrier Systems for Cancer Treatment. Front. Pharmacol. 2018, 9, 922. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Vasilieva, E.A.; Mirgorodskaya, A.B.; Zakharov, S.V.; Pavlov, R.V.; Kashapova, N.E.; Gaynanova, G.A. Hydrotropes: Solubilization of Nonpolar Compounds and Modification of Surfactant Solutions. J. Mol. Liq. 2023, 370, 120923. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Mirgorodskaya, A.B.; Kuznetsov, D.M.; Razuvaeva, Y.S.; Zakharova, L.Y. Nanosized Supramolecular Systems: From Colloidal Surfactants to Amphiphilic Macrocycles and Superamphiphiles. Colloid J. 2022, 84, 502–517. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.; Kushnazarova, R.; Pavlov, R.; Valeeva, F.; Lenina, O.; Bushmeleva, K.; Kuryashov, D.; Vyshtakalyuk, A.; Gaynanova, G.; Petrov, K.; et al. Supramolecular Tools to Improve Wound Healing and Antioxidant Properties of Abietic Acid: Biocompatible Microemulsions and Emulgels. Molecules 2022, 27, 6447. [Google Scholar] [CrossRef]
- Heise, N.; Becker, S.; Mueller, T.; Bache, M.; Csuk, R.; Güttler, A. Mitochondria-Targeting 1,5-Diazacyclooctane-Spacered Triterpene Rhodamine Conjugates Exhibit Cytotoxicity at Sub-Nanomolar Concentration against Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 10695. [Google Scholar] [CrossRef]
- Rajaputra, P.; Nkepang, G.; Watley, R.; You, Y. Synthesis and in Vitro Biological Evaluation of Lipophilic Cation Conjugated Photosensitizers for Targeting Mitochondria. Bioorganic Med. Chem. 2013, 21, 379–387. [Google Scholar] [CrossRef]
- Muli, D.K.; Rajaputra, P.; You, Y.; McGrath, D.V. Asymmetric ZnPc–Rhodamine B Conjugates for Mitochondrial Targeted Photodynamic Therapy. Bioorganic Med. Chem. Lett. 2014, 24, 4496–4500. [Google Scholar] [CrossRef]
- Han, M.; Vakili, M.R.; Soleymani Abyaneh, H.; Molavi, O.; Lai, R.; Lavasanifar, A. Mitochondrial Delivery of Doxorubicin via Triphenylphosphine Modification for Overcoming Drug Resistance in MDA-MB-435/DOX Cells. Mol. Pharm. 2014, 11, 2640–2649. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Dai, L.; Ji, M.; Wang, H. Mitochondria-Targeted Triphenylphosphonium Conjugated Glycyrrhetinic Acid Derivatives as Potent Anticancer Drugs. Bioorganic Chem. 2019, 85, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhang, T.; Yuan, H.; Li, D.; Lou, H.; Fan, P. Mitochondria-Targeted Lupane Triterpenoid Derivatives and Their Selective Apoptosis-Inducing Anticancer Mechanisms. J. Med. Chem. 2017, 60, 6353–6363. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Li, W.; Li, T.; Xiao, S.; Lu, J.; Xu, J.; Zhao, Y. Rational Design, Synthesis and Biological Evaluation of Triphenylphosphonium-Ginsenoside Conjugates as Mitochondria-Targeting Anti-Cancer Agents. Bioorganic Chem. 2020, 103, 104150. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Dodwadkar, N.S.; Deshpande, P.P.; Torchilin, V.P. Liposomes Loaded with Paclitaxel and Modified with Novel Triphenylphosphonium-PEG-PE Conjugate Possess Low Toxicity, Target Mitochondria and Demonstrate Enhanced Antitumor Effects in Vitro and in Vivo. J. Control. Release 2012, 159, 393–402. [Google Scholar] [CrossRef]
- Khatun, Z.; Choi, Y.S.; Kim, Y.G.; Yoon, K.; Nurunnabi, M.; Li, L.; Lee, E.; Kang, H.C.; Huh, K.M. Bioreducible Poly(Ethylene Glycol)–Triphenylphosphonium Conjugate as a Bioactivable Mitochondria-Targeting Nanocarrier. Biomacromolecules 2017, 18, 1074–1085. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, W.-Y.; Ma, X.; Ju, R.-J.; Li, X.-Y.; Li, N.; Sun, M.-G.; Shi, J.-F.; Zhang, C.-X.; Lu, W.-L. The Anticancer Efficacy of Paclitaxel Liposomes Modified with Mitochondrial Targeting Conjugate in Resistant Lung Cancer. Biomaterials 2013, 34, 3626–3638. [Google Scholar] [CrossRef]
- Gaynanova, G.; Vasileva, L.; Kashapov, R.; Kuznetsova, D.; Kushnazarova, R.; Tyryshkina, A.; Vasilieva, E.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembling Drug Formulations with Tunable Permeability and Biodegradability. Molecules 2021, 26, 6786. [Google Scholar] [CrossRef]
- Benien, P.; Solomon, M.A.; Nguyen, P.; Sheehan, E.M.; Mehanna, A.S.; D’Souza, G.G.M. Hydrophobized Triphenyl Phosphonium Derivatives for the Preparation of Mitochondriotropic Liposomes: Choice of Hydrophobic Anchor Influences Cytotoxicity but Not Mitochondriotropic Effect. J. Liposome Res. 2016, 26, 21–27. [Google Scholar] [CrossRef]
- Lu, J.; Li, R.; Mu, B.; Peng, Y.; Zhao, Y.; Shi, Y.; Guo, L.; Hai, L.; Wu, Y. Multiple Targeted Doxorubicin-Lonidamine Liposomes Modified with p-Hydroxybenzoic Acid and Triphenylphosphonium to Synergistically Treat Glioma. Eur. J. Med. Chem. 2022, 230, 114093. [Google Scholar] [CrossRef]
- Benien, P.; Almuteri, M.; Shah, S.; Böhlke, M.; Mehanna, A.; D’Souza, G.G.M. Synthesis of Triphenylphosphonium Phospholipid Conjugates for the Preparation of Mitochondriotropic Liposomes. In Mitochondrial Medicine; Methods in Molecular Biology; Weissig, V., Edeas, M., Eds.; Springer: New York, NY, USA, 2021; Volume 2275, pp. 119–126. ISBN 978-1-07-161261-3. [Google Scholar]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z. Triphenylphosphonium Decorated Liposomes and Dendritic Polymers: Prospective Second Generation Drug Delivery Systems for Targeting Mitochondria. Mol. Pharm. 2016, 13, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.A.; Vasileva, L.A.; Gaynanova, G.A.; Pavlov, R.V.; Sapunova, A.S.; Voloshina, A.D.; Sibgatullina, G.V.; Samigullin, D.V.; Petrov, K.A.; Zakharova, L.Y.; et al. Comparative Study of Cationic Liposomes Modified with Triphenylphosphonium and Imidazolium Surfactants for Mitochondrial Delivery. J. Mol. Liq. 2021, 330, 115703. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasileva, L.A.; Sibgatullina, G.V.; Samigullin, D.V.; Sapunova, A.S.; Voloshina, A.D.; Galkina, I.V.; Petrov, K.A.; Zakharova, L.Y. Mitochondria-Targeted Cationic Liposomes Modified with Alkyltriphenylphosphonium Bromides Loaded with Hydrophilic Drugs: Preparation, Cytotoxicity and Colocalization Assay. J. Mater. Chem. B 2019, 7, 7351–7362. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.; Gaynanova, G.; Valeeva, F.; Belyaev, G.; Zueva, I.; Bushmeleva, K.; Sibgatullina, G.; Samigullin, D.; Vyshtakalyuk, A.; Petrov, K.; et al. Mitochondria-Targeted Delivery Strategy of Dual-Loaded Liposomes for Alzheimer’s Disease Therapy. Int. J. Mol. Sci. 2023, 24, 10494. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.A.; Shah, A.A.; D’Souza, G.G.M. In Vitro Assessment of the Utility of Stearyl Triphenyl Phosphonium Modified Liposomes in Overcoming the Resistance of Ovarian Carcinoma Ovcar-3 Cells to Paclitaxel. Mitochondrion 2013, 13, 464–472. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Ahtamyanova, L.R.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Novel Hybrid Liposomal Formulations Based on Imidazolium-Containing Amphiphiles for Drug Encapsulation. Colloids Surf. B 2019, 178, 352–357. [Google Scholar] [CrossRef]
- Viale, A.; Pettazzoni, P.; Lyssiotis, C.A.; Ying, H.; Sánchez, N.; Marchesini, M.; Carugo, A.; Green, T.; Seth, S.; Giuliani, V.; et al. Oncogene Ablation-Resistant Pancreatic Cancer Cells Depend on Mitochondrial Function. Nature 2014, 514, 628–632. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters With Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Briuglia, M.-L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of Cholesterol on Liposome Stability and on in Vitro Drug Release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Mutlu-Ağardan, N.B.; Tort, S. Cholesterol Included Self-Assembled Electrospun Proliposomes as a Feasible Approach for Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 86, 104749. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasilieva, E.A.; Pavlov, R.V.; Zueva, I.V.; Babaev, V.M.; Kuznetsov, D.M.; Voloshina, A.D.; Petrov, K.A.; Zakharova, L.Y.; et al. Oxime Therapy for Brain AChE Reactivation and Neuroprotection after Organophosphate Poisoning. Pharmaceutics 2022, 14, 1950. [Google Scholar] [CrossRef] [PubMed]
- Sęk, A.; Perczyk, P.; Szcześ, A.; Machatschek, R.; Wydro, P. Studies on the Interactions of Tiny Amounts of Common Ionic Surfactants with Unsaturated Phosphocholine Lipid Model Membranes. Chem. Phys. Lipids 2022, 248, 105236. [Google Scholar] [CrossRef] [PubMed]
- Pashirova, T.N.; Sapunova, A.S.; Lukashenko, S.S.; Burilova, E.A.; Lubina, A.P.; Shaihutdinova, Z.M.; Gerasimova, T.P.; Kovalenko, V.I.; Voloshina, A.D.; Souto, E.B.; et al. Synthesis, Structure-Activity Relationship and Biological Evaluation of Tetracationic Gemini Dabco-Surfactants for Transdermal Liposomal Formulations. Int. J. Pharm. 2020, 575, 118953. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Chen, M.; Liu, X.; Fahr, A. Skin Penetration and Deposition of Carboxyfluorescein and Temoporfin from Different Lipid Vesicular Systems: In Vitro Study with Finite and Infinite Dosage Application. Int. J. Pharm. 2011, 408, 223–234. [Google Scholar] [CrossRef]
- Granja, A.; Nunes, C.; Sousa, C.T.; Reis, S. Folate Receptor-Mediated Delivery of Mitoxantrone-Loaded Solid Lipid Nanoparticles to Breast Cancer Cells. Biomed. Pharmacother. 2022, 154, 113525. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, W.Y.; Ko, Y.T. The Effect of Surface Charges on the Cellular Uptake of Liposomes Investigated by Live Cell Imaging. Pharm. Res. 2017, 34, 704–717. [Google Scholar] [CrossRef]
- Jarallah, S.J.; Aldossary, A.M.; Tawfik, E.A.; Altamimi, R.M.; Alsharif, W.K.; Alzahrani, N.M.; As Sobeai, H.M.; Qamar, W.; Alfahad, A.J.; Alshabibi, M.A.; et al. GL67 Lipid-Based Liposomal Formulation for Efficient SiRNA Delivery into Human Lung Cancer Cells. Saudi Pharm. J. 2023, 31, 1139–1148. [Google Scholar] [CrossRef]
- Mazumdar, S.; Chitkara, D.; Mittal, A. Exploration and Insights into the Cellular Internalization and Intracellular Fate of Amphiphilic Polymeric Nanocarriers. Acta Pharm. Sin. B 2021, 11, 903–924. [Google Scholar] [CrossRef]
- Wang, C.; Sun, A.; Qiao, Y.; Zhang, P.; Ma, L.; Su, M. Cationic Surface Modification of Gold Nanoparticles for Enhanced Cellular Uptake and X-Ray Radiation Therapy. J. Mater. Chem. B 2015, 3, 7372–7376. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; Du Toit, L.C.; Pillay, V. Parameters and Characteristics Governing Cellular Internalization and Trans-Barrier Trafficking of Nanostructures. Int. J. Nanomedicine. 2015, 10, 2191–2206. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, Y. Mitochondrial Targeted Strategies and Their application for Cancer and Other Diseases Treatment. J. Pharm. Investig. 2020, 50, 271–293. [Google Scholar] [CrossRef]
- Profillidis, V.A.; Botzoris, G.N. Statistical Methods for Transport Demand Modeling. In Modeling of Transport Demand; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–224. ISBN 978-0-12-811513-8. [Google Scholar]
- Pathak, R.K.; Marrache, S.; Harn, D.A.; Dhar, S. Mito-DCA: A Mitochondria Targeted Molecular Scaffold for Efficacious Delivery of Metabolic Modulator Dichloroacetate. ACS Chem. Biol. 2014, 9, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Thuy, L.T.; Lee, S.; Park, J.-I.; Choi, J.S. Liposomes Containing Cholesterol and Mitochondria-Penetrating Peptide (MPP) for Targeted Delivery of Antimycin A to A549 Cells. Colloids Surf. B 2018, 161, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R. Estrogenic Flavonoids and Their Molecular Mechanisms of Action. J. Nutr. Biochem. 2023, 114, 109250. [Google Scholar] [CrossRef]
- Trabjerg, M.S.; Andersen, D.C.; Huntjens, P.; Mørk, K.; Warming, N.; Kullab, U.B.; Skjønnemand, M.-L.N.; Oklinski, M.K.; Oklinski, K.E.; Bolther, L.; et al. Inhibition of Carnitine Palmitoyl-Transferase 1 Is a Potential Target in a Mouse Model of Parkinson’s Disease. npj Park. Dis. 2023, 9, 6. [Google Scholar] [CrossRef]
- Peng, K.; Yang, L.; Wang, J.; Ye, F.; Dan, G.; Zhao, Y.; Cai, Y.; Cui, Z.; Ao, L.; Liu, J.; et al. The Interaction of Mitochondrial Biogenesis and Fission/Fusion Mediated by PGC-1α Regulates Rotenone-Induced Dopaminergic Neurotoxicity. Mol. Neurobiol. 2017, 54, 3783–3797. [Google Scholar] [CrossRef]
- Ibarra-Gutiérrez, M.T.; Serrano-García, N.; Orozco-Ibarra, M. Rotenone-Induced Model of Parkinson’s Disease: Beyond Mitochondrial Complex I Inhibition. Mol. Neurobiol. 2023, 60, 1929–1948. [Google Scholar] [CrossRef]
- Shi, G.; Scott, H.; Azhar, N.I.F.M.; Gialeli, A.; Clennell, B.; Lee, K.S.; Hurcombe, J.; Whitcomb, D.; Coward, R.; Wong, L.-F.; et al. AZD5438 a GSK-3a/b and CDK Inhibitor Is Antiapoptotic Modulates Mitochondrial Activity and Protects Human Neurons from Mitochondrial Toxins. Sci. Rep. 2023, 13, 8334. [Google Scholar] [CrossRef]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial Complex I Inhibitor Rotenone Induces Apoptosis through Enhancing Mitochondrial Reactive Oxygen Species Production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef]
- Zhang, K.; Ji, X.; Song, Z.; Song, W.; Huang, Q.; Yu, T.; Shi, D.; Wang, F.; Xue, X.; Guo, J. Butyrate Inhibits the Mitochondrial Complex Ι to Mediate Mitochondria-Dependent Apoptosis of Cervical Cancer Cells. BMC Complement. Med. Ther. 2023, 23, 212. [Google Scholar] [CrossRef]
- Sauerová, P.; Verdánová, M.; Mravec, F.; Pilgrová, T.; Venerová, T.; Hubálek Kalbáčová, M.; Pekař, M. Hyaluronic Acid as a Modulator of the Cytotoxic Effects of Cationic Surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 155–161. [Google Scholar] [CrossRef]
- Khan, D.R.; Rezler, E.M.; Lauer-Fields, J.; Fields, G.B. Effects of Drug Hydrophobicity on Liposomal Stability: Effects of Drug Hydrophobicity. Chem. Biol. Drug Des. 2007, 71, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Kawano, K.; Furuya, A.; Matsuda, Y.; Kimura, C.; Yamaguchi, K.; Wakabayashi, S.; Taniguchi, K.; Ozaki, K.; Hattori, Y. Preparation of Liposomes Co-Encapsulating Doxorubicin and Mifepristone for Treating Multidrug-Resistant Cancer. J. Drug Deliv. Sci. Technol. 2023, 85, 104605. [Google Scholar] [CrossRef]
- Zhao, L.; Ling, L.; Lu, J.; Jiang, F.; Sun, J.; Zhang, Z.; Huang, Y.; Liu, X.; Zhu, Y.; Fu, X.; et al. Reactive Oxygen Species-responsive Mitochondria-targeted Liposomal Quercetin Attenuates Retinal Ischemia–Reperfusion Injury via Regulating SIRT1/FOXO3A and P38 MAPK Signaling Pathways. Bioeng. Transl. Med. 2023, 8, e10460. [Google Scholar] [CrossRef] [PubMed]
- Vasilieva, E.A.; Kuznetsova, D.A.; Valeeva, F.G.; Kuznetsov, D.M.; Zakharov, A.V.; Amerhanova, S.K.; Voloshina, A.D.; Zueva, I.V.; Petrov, K.A.; Zakharova, L.Y. Therapy of Organophosphate Poisoning via Intranasal Administration of 2-PAM-Loaded Chitosomes. Pharmaceutics 2022, 14, 2846. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Voloshina, A.D.; Sapunova, A.S.; Kulik, N.V.; Nizameev, I.R.; Kadirov, M.K.; Kashapov, R.R.; Zakharova, L.Y. Supramolecular Systems Based on Cationic Imidazole-Containing Amphiphiles Bearing Hydroxyethyl Fragment: Aggregation Properties and Functional Activity. J. Mol. Liq. 2019, 289, 111058. [Google Scholar] [CrossRef]
- Gainanova, G.A.; Vagapova, G.I.; Syakaev, V.V.; Ibragimova, A.R.; Valeeva, F.G.; Tudriy, E.V.; Galkina, I.V.; Kataeva, O.N.; Zakharova, L.Y.; Latypov, S.K.; et al. Self-Assembling Systems Based on Amphiphilic Alkyltriphenylphosphonium Bromides: Elucidation of the Role of Head Group. J. Colloid Interface Sci. 2012, 367, 327–336. [Google Scholar] [CrossRef]
- Amerkhanova, S.K.; Voloshina, A.D.; Mirgorodskaya, A.B.; Lyubina, A.P.; Kuznetsova, D.A.; Kushnazarova, R.A.; Mikhailov, V.A.; Zakharova, L.Y. Antimicrobial Properties and Cytotoxic Effect of Imidazolium Geminis with Tunable Hydrophobicity. Int. J. Mol. Sci. 2021, 22, 13148. [Google Scholar] [CrossRef]
- Vasileva, L.; Gaynanova, G.; Valeeva, F.; Romanova, E.; Pavlov, R.; Kuznetsov, D.; Belyaev, G.; Zueva, I.; Lyubina, A.; Voloshina, A.; et al. Synthesis, Properties, and Biomedical Application of Dicationic Gemini Surfactants with Dodecane Spacer and Carbamate Fragments. Int. J. Mol. Sci. 2023, 24, 12312. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.Y.; Kaupova, G.I.; Gabdrakhmanov, D.R.; Gaynanova, G.A.; Ermakova, E.A.; Mukhitov, A.R.; Galkina, I.V.; Cheresiz, S.V.; Pokrovsky, A.G.; Skvortsova, P.V.; et al. Alkyl Triphenylphosphonium Surfactants as Nucleic Acid Carriers: Complexation Efficacy toward DNA Decamers, Interaction with Lipid Bilayers and Cytotoxicity Studies. Phys. Chem. Chem. Phys. 2019, 21, 16706–16717. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, S.; Hosseini, S.A.; Soraya, H.; Roosta, Y.; Mohammadzadeh, A. Development of Doxorubicin-Encapsulated Magnetic Liposome@PEG for Treatment of Breast Cancer in BALB/c Mice. Drug Deliv. Transl. Res. 2023, 13, 2589–2603. [Google Scholar] [CrossRef] [PubMed]
- Sumathi, R.; Punitha, S.; Tamizharasi, S.; Sivakumar, T.; Jeyaprakash, R. Formulation and Characterization of Soya Lecithin-Based Liposomes for Encapsulating a Weakly Soluble Naringenin. J. Med. Pharm. Allied Sci. 2021, 10, 2018–4023. [Google Scholar] [CrossRef]
- Ndemazie, N.B.; Bulusu, R.; Zhu, X.Y.; Frimpong, E.K.; Inkoom, A.; Okoro, J.; Ebesoh, D.; Rogers, S.; Han, B.; Agyare, E. Evaluation of Anticancer Activity of Zhubech, a New 5-FU Analog Liposomal Formulation, against Pancreatic Cancer. Int. J. Mol. Sci. 2023, 24, 4288. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Helder, M.N.; Naderinezhad, S.; Sheikhha, M.H.; Forouzanfar, T.; Zandieh-doulabi, B. A Comprehensive Mathematical Model of Drug Release Kinetics from Nano-Liposomes, Derived from Optimization Studies of Cationic PEGylated Liposomal Doxorubicin Formulations for Drug-Gene Delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 169–177. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Jain, A.; Jain, S.K. In Vitro Release Kinetics Model Fitting of Liposomes: An Insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef]
- Ang, S.-S.; Thoo, Y.Y.; Siow, L.F. Encapsulation of Hydrophobic Apigenin into Small Unilamellar Liposomes Coated with Chitosan Through Ethanol Injection and Spray Drying. Food Bioprocess Technol. 2023, 1–16. [Google Scholar] [CrossRef]
- Lafi, Z.; Alshaer, W.; Hatmal, M.M.; Zihlif, M.; Alqudah, D.A.; Nsairat, H.; Azzam, H.; Aburjai, T.; Bustanji, Y.; Awidi, A. Aptamer-Functionalized PH-Sensitive Liposomes for a Selective Delivery of Echinomycin into Cancer Cells. RSC Adv. 2021, 11, 29164–29177. [Google Scholar] [CrossRef]
- Huang, X.-F.; Chang, K.-F.; Lin, Y.-L.; Liao, K.-W.; Hsiao, C.-Y.; Sheu, G.-T.; Tsai, N.-M. Enhancement of Cytotoxicity and Induction of Apoptosis by Cationic Nano-Liposome Formulation of n -Butylidenephthalide in Breast Cancer Cells. Int. J. Med. Sci. 2021, 18, 2930–2942. [Google Scholar] [CrossRef] [PubMed]
- Nurcahyanti, A.D.; Wink, M. L-Canavanine Potentiates the Cytotoxicity of Doxorubicin and Cisplatin in Arginine Deprived Human Cancer Cells. PeerJ 2016, 4, e1542. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Júnior, A.D.; Vieira, F.P.; De Melo, V.J.; Lopes, M.T.P.; Silveira, J.N.; Ramaldes, G.A.; Garnier-Suillerot, A.; Pereira-Maia, E.C.; De Oliveira, M.C. Preparation and Cytotoxicity of Cisplatin-Containing Liposomes. Braz. J. Med. Biol. Res. 2007, 40, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zang, G.; Li, N.; Sun, C.; Du, R. Agonist-Induced Piezo1 Activation Promote Mitochondrial-Dependent Apoptosis in Vascular Smooth Muscle Cells. BMC Cardiovasc. Disord. 2022, 22, 287. [Google Scholar] [CrossRef]
- Akintade, D.D.; Chaudhuri, B. Apoptosis, Induced by Human α-Synuclein in Yeast, Can Occur Independent of Functional Mitochondria. Cells 2020, 9, 2203. [Google Scholar] [CrossRef]
- Zoughaib, M.; Pavlov, R.V.; Gaynanova, G.A.; Garifullin, R.; Evtugyn, V.G.; Abdullin, T.I. Amphiphilic RGD and GHK Peptides Synergistically Enhance Liposomal Delivery into Cancer and Endothelial Cells. Mater. Adv. 2021, 2, 7715–7730. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zhukova, N.A.; Lukashenko, S.S.; Valeeva, F.G.; Burilova, E.A.; Sapunova, A.S.; Voloshina, A.D.; Mirgorodskaya, A.B.; Zakharova, L.Y.; Sinyashin, O.G.; et al. Multi-Targeted Approach by 2-Benzimidazolylquinoxalines-Loaded Cationic Arginine Liposomes against Cervical Cancer Cells in Vitro. Colloids Surf. B 2019, 178, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Mirgorodskaya, A.B.; Kuznetsova, D.A.; Kushnazarova, R.A.; Gabdrakhmanov, D.R.; Zhukova, N.A.; Lukashenko, S.S.; Sapunova, A.S.; Voloshina, A.D.; Sinyashin, O.G.; Mamedov, V.A.; et al. Soft Nanocarriers for New Poorly Soluble Conjugate of Pteridine and Benzimidazole: Synthesis and Cytotoxic Activity against Tumor Cells. J. Mol. Liq. 2020, 317, 114007. [Google Scholar] [CrossRef]
- AAT Bioquest. Inc. Quest Graph™ IC50 Calculator. AAT Bioquest. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 10 November 2022).
- Kuznetsova, D.A.; Kuznetsov, D.M.; Vasileva, L.A.; Amerhanova, S.K.; Valeeva, D.N.; Salakhieva, D.V.; Nikolaeva, V.A.; Nizameev, I.R.; Islamov, D.R.; Usachev, K.S.; et al. Complexation of Oligo- and Polynucleotides with Methoxyphenyl-Functionalized Imidazolium Surfactants. Pharmaceutics 2022, 14, 2685. [Google Scholar] [CrossRef]





| Formulation | Dh, nm | PdI | ζ, mV | Dh, nm | PdI | ζ, mV |
|---|---|---|---|---|---|---|
| 1st Day | 2 Months | |||||
| PC | 131 ± 1 | 0.214 ± 0.013 | +1.8 ± 0.9 | 115 ± 1 | 0.105 ± 0.008 | −13 ± 1 |
| PC/Chol | 133 ± 5 | 0.215 ± 0.022 | −7.0 ± 0.2 | 112 ± 1 | 0.118 ± 0.023 | −14 ± 1 |
| 50/1 | ||||||
| PC/Chol/TPPB-10 | 152 ± 1 | 0.239 ± 0.003 | +29 ± 2 | 114 ± 2 | 0.102 ± 0.010 | +31 ± 3 |
| PC/Chol/TPPB-12 | 102 ± 1 | 0.116 ± 0.018 | +30 ± 2 | 100 ± 1 | 0.145 ± 0.008 | +35 ± 2 |
| PC/Chol/TPPB-14 | 120 ± 1 | 0.085 ± 0.012 | +33 ± 2 | 106 ± 1 | 0.086 ± 0.012 | +37.2 ± 0.5 |
| PC/Chol/TPPB-16 | 98 ± 1 | 0.103 ± 0.018 | +38 ± 2 | 120 ± 1 | 0.124 ± 0.010 | +44 ± 1 |
| PC/Chol/IA-10(OH) | 150 ± 1 | 0.200 ± 0.012 | +10.5 ± 0.2 | 96 ± 2 | 0.078 ± 0.013 | +3.3 ± 0.4 |
| PC/Chol/IA-12(OH) | 104 ± 1 | 0.177 ± 0.002 | +26 ± 1 | 116 ± 4 | 0.269 ± 0.037 | +13 ± 1 |
| PC/Chol/IA-14(OH) | 111 ± 1 | 0.083 ± 0.012 | +39 ± 1 | 113 ± 1 | 0.090 ± 0.011 | +27 ± 1 |
| PC/Chol/IA-16(OH) | 109 ± 1 | 0.105 ± 0.011 | +44.2 ± 0.4 | 121 ± 1 | 0.168 ± 0.006 | +25.1 ± 0.5 |
| 35/1 | ||||||
| PC/Chol/TPPB-10 | 110 ± 1 | 0.092 ± 0.023 | +28.8 ± 0.6 | 122 ± 1 | 0.130 ± 0.021 | +33 ± 1 |
| PC/Chol/TPPB-12 | 104 ± 1 | 0.134 ± 0.014 | +31 ± 1 | 103 ± 1 | 0.137 ± 0.022 | +45 ± 3 |
| PC/Chol/TPPB-14 | 109 ± 1 | 0.083 ± 0.012 | +35 ± 1 | 108 ± 2 | 0.138 ± 0.019 | +42 ± 1 |
| PC/Chol/TPPB-16 | 102 ± 1 | 0.119 ± 0.008 | +30.3 ± 0.3 | 122 ± 1 | 0.147 ± 0.012 | +47 ± 4 |
| PC/Chol/IA-10(OH) | 106 ± 2 | 0.204 ± 0.034 | +12.4 ± 0.6 | 99 ± 2 | 0.133 ± 0.028 | +7.5 ± 0.5 |
| PC/Chol/IA-12(OH) | 94 ± 1 | 0.100 ± 0.015 | +31 ± 1 | 113 ± 5 | 0.237 ± 0.015 | +19 ± 2 |
| PC/Chol/IA-14(OH) | 110 ± 1 | 0.094 ± 0.002 | +41 ± 1 | 116 ± 1 | 0.101 ± 0.014 | +26 ± 3 |
| PC/Chol/IA-16(OH) | 107 ± 1 | 0.151 ± 0.004 | +49.2 ± 0.4 | 132 ± 3 | 0.217 ± 0.004 | +34 ± 3 |
| 25/1 | ||||||
| PC/Chol/TPPB-10 | 113 ± 1 | 0.076 ± 0.019 | +39 ± 1 | 114 ± 1 | 0.101 ± 0.004 | +38 ± 1 |
| PC/Chol/TPPB-12 | 120 ± 1 | 0.137 ± 0.032 | +42 ± 1 | 103 ± 1 | 0.114 ± 0.004 | +43 ± 3 |
| PC/Chol/TPPB-14 | 111 ± 1 | 0.133 ± 0.005 | +38.8 ± 0.5 | 122 ± 1 | 0.124 ± 0.012 | +47 ± 2 |
| PC/Chol/TPPB-16 | 123 ± 1 | 0.097 ± 0.009 | +40.3 ± 0.4 | 119 ± 2 | 0.103 ± 0.006 | +53 ± 2 |
| PC/Chol/IA-10(OH) | 108 ± 1 | 0.203 ± 0.006 | +15 ± 1 | 97 ± 2 | 0.120 ± 0.024 | +6 ± 2 |
| PC/Chol/IA-12(OH) | 97 ± 1 | 0.132 ± 0.004 | +35 ± 1 | 134 ± 3 | 0.255 ± 0.004 | +25 ± 0.8 |
| PC/Chol/IA-14(OH) | 111 ± 1 | 0.101 ± 0.023 | +45 ± 1 | 117 ± 1 | 0.103 ± 0.010 | +35 ± 1 |
| PC/Chol/IA-16(OH) | 112 ± 1 | 0.094 ± 0.018 | +51 ± 1 | 131 ± 4 | 0.207 ± 0.107 | +38 ± 1 |
| Formulation | EE, % | Dh, nm | PdI | ζ, mV | Dh, nm | PdI | ζ, mV |
|---|---|---|---|---|---|---|---|
| 1st Day | 2 Months | ||||||
| 50/1 | |||||||
| PC/Chol/TPPB-10 | 91 ± 2 | 110 ± 1 | 0.103 ± 0.005 | 29 ± 1 | 121 ± 1 | 0.099 ± 0.017 | 29 ± 1 |
| PC/Chol/TPPB-12 | 94 ± 1 | 119 ± 2 | 0.115 ± 0.011 | 31 ± 1 | 123 ± 2 | 0.115 ± 0.018 | 31 ± 1 |
| PC/Chol/TPPB-14 | 94 ± 1 | 114 ± 1 | 0.109 ± 0.011 | 33 ± 1 | 115 ± 1 | 0.098 ± 0.027 | 31 ± 1 |
| PC/Chol/TPPB-16 | 95 ± 2 | 116 ± 2 | 0.106 ± 0.005 | 25.5 ± 0.7 | 120 ± 2 | 0.098 ± 0.017 | 34 ± 3 |
| PC/Chol/IA-10(OH) | 97 ± 1 | 109 ± 1 | 0.068 ± 0.011 | 6.4 ± 0.5 | 125 ± 2 | 0.124 ± 0.007 | 3.2 ± 0.4 |
| PC/Chol/IA-12(OH) | 97 ± 1 | 109 ± 1 | 0.083 ± 0.004 | 25.4 ± 0.1 | 118 ± 1 | 0.146 ± 0.003 | 8.5 ± 0.1 |
| PC/Chol/IA-14(OH) | 96 ± 2 | 111 ± 1 | 0.075 ± 0.003 | 36.2 ± 0.5 | 124 ± 1 | 0.169 ± 0.012 | 14.3 ± 0.5 |
| PC/Chol/IA-16(OH) | 98 ± 1 | 110 ± 1 | 0.090 ± 0.003 | 39 ± 1 | 119 ± 1 | 0.160 ± 0.005 | 13.2 ± 0.5 |
| 35/1 | |||||||
| PC/Chol/TPPB-10 | 91 ± 3 | 114 ± 2 | 0.116 ± 0.015 | 31 ± 1 | 110 ± 2 | 0.096 ± 0.014 | 37.2 ± 0.3 |
| PC/Chol/TPPB-12 | 92 ± 2 | 138 ± 3 | 0.250 ± 0.006 | 34 ± 2 | 106 ± 2 | 0.072 ± 0.008 | 44 ± 1 |
| PC/Chol/TPPB-14 | 94 ± 1 | 122 ± 1 | 0.203 ± 0.019 | 35 ± 1 | 114 ± 1 | 0.082 ± 0.020 | 45 ± 3 |
| PC/Chol/TPPB-16 | 93 ± 1 | 117 ± 1 | 0.172 ± 0.001 | 32 ± 1 | 120 ± 1 | 0.171 ± 0.013 | 48 ± 2 |
| PC/Chol/IA-10(OH) | 89 ± 2 | 109 ± 1 | 0.095 ± 0.007 | 9 ± 1 | 151 ± 3 | 0.282 ± 0.016 | 7.2 ± 0.2 |
| PC/Chol/IA-12(OH) | 94 ± 2 | 110 ± 2 | 0.109 ± 0.002 | 30.2 ± 0.2 | 117 ± 1 | 0.107 ± 0.025 | 24 ± 1 |
| PC/Chol/IA-14(OH) | 92 ± 1 | 118 ± 1 | 0.155 ± 0.013 | 40 ± 1 | 113 ± 2 | 0.127 ± 0.004 | 35 ± 1 |
| PC/Chol/IA-16(OH) | 94 ± 2 | 115 ± 2 | 0.133 ± 0.014 | 45.1 ± 0.2 | 115 ± 1 | 0.072 ± 0.008 | 28 ± 1 |
| Formulation | Lipid/Surfactant Molar Ratio | Korsmeyer–Peppas | Higuchi | |||
|---|---|---|---|---|---|---|
| kKP | n | R2 | kH | R2 | ||
| PC/Chol | - | 30.52 ± 0.57 | 0.496 ± 0.014 | 0.9968 | 30.36 ± 0.24 | 0.9971 |
| PC/Chol/IA-14(OH) | 50/1 | 29.29 ± 0.35 | 0.340 ± 0.009 | 0.9973 | 24.26 ± 0.84 | 0.9288 |
| 35/1 | 33.56 ± 0.47 | 0.325 ± 0.011 | 0.9961 | 27.31 ± 1.05 | 0.9098 | |
| 25/1 | 33.92 ± 0.44 | 0.354 ± 0.010 | 0.9971 | 28.51 ± 0.91 | 0.9415 | |
| PC/Chol/TPPB-14 | 50/1 | 23.24 ± 0.90 | 0.623 ± 0.028 | 0.9914 | 27.05 ± 0.73 | 0.9723 |
| 35/1 | 23.18 ± 0.76 | 0.614 ± 0.023 | 0.9940 | 26.30 ± 0.82 | 0.9646 | |
| 25/1 | 23.15 ± 0.70 | 0.591 ± 0.022 | 0.9943 | 25.52 ± 0.70 | 0.9719 | |
| PC/Chol/IA-10(OH) | 50/1 | 25.30 ± 0.35 | 0.344 ± 0.011 | 0.9964 | 21.03 ± 0.71 | 0.9317 |
| PC/Chol/IA-12(OH) | 24.23 ± 0.40 | 0.379 ± 0.013 | 0.9958 | 20.99 ± 0.56 | 0.9611 | |
| PC/Chol/IA-14(OH) | 28.94 ± 0.35 | 0.341 ± 0.009 | 0.9973 | 23.96 ± 0.83 | 0.9288 | |
| PC/Chol/IA-16(OH) | 27.18 ± 0.63 | 0.389 ± 0.018 | 0.9921 | 23.82 ± 0.62 | 0.9641 | |
| PC/Chol/TPPB-10 | 25.28 ± 1.50 | 0.593 ± 0.043 | 0.9782 | 28.32 ± 0.81 | 0.9682 | |
| PC/Chol/TPPB-12 | 27.66 ± 1.22 | 0.547 ± 0.032 | 0.9855 | 29.31 ± 0.58 | 0.9834 | |
| PC/Chol/TPPB-14 | 28.70 ± 1.27 | 0.546 ± 0.032 | 0.9853 | 30.34 ± 0.60 | 0.9834 | |
| PC/Chol/TPPB-16 | 34.38 ± 1.51 | 0.476 ± 0.033 | 0.9809 | 33.39 ± 0.65 | 0.9817 | |
| Formulation | IC50, µM | SIChang Liver/HuTu 80 | |||
|---|---|---|---|---|---|
| Tumor Cell Lines | Normal Cell Lines | ||||
| HuTu 80 | PANC-1 | Chang Liver | WI-38 | ||
| ROT | 2.8 | >1000 | 484 | 1000 | >173 |
| PC/Chol | 5.0 | 13.2 | 125 | 27.4 | 25 |
| PC/Chol/TPPB-14 | 0.07 | 4.9 | 21.5 | 3.2 | 307 |
| PC/Chol/IA-10(OH) | 1.3 | - | >63.5 | - | >49 |
| PC/Chol/IA-12(OH) | 1 | - | 35 | - | 35 |
| PC/Chol/IA-14(OH) | 1 | 7.8 | >63.5 | - | >63.5 |
| PC/Chol/IA-16(OH) | 0.5 | 8.8 | 56.3 | - | 113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileva, L.; Gaynanova, G.; Kuznetsova, D.; Valeeva, F.; Lyubina, A.; Amerhanova, S.; Voloshina, A.; Sibgatullina, G.; Samigullin, D.; Petrov, K.; et al. Mitochondria-Targeted Lipid Nanoparticles Loaded with Rotenone as a New Approach for the Treatment of Oncological Diseases. Molecules 2023, 28, 7229. https://doi.org/10.3390/molecules28207229
Vasileva L, Gaynanova G, Kuznetsova D, Valeeva F, Lyubina A, Amerhanova S, Voloshina A, Sibgatullina G, Samigullin D, Petrov K, et al. Mitochondria-Targeted Lipid Nanoparticles Loaded with Rotenone as a New Approach for the Treatment of Oncological Diseases. Molecules. 2023; 28(20):7229. https://doi.org/10.3390/molecules28207229
Chicago/Turabian StyleVasileva, Leysan, Gulnara Gaynanova, Darya Kuznetsova, Farida Valeeva, Anna Lyubina, Syumbelya Amerhanova, Alexandra Voloshina, Guzel Sibgatullina, Dmitry Samigullin, Konstantin Petrov, and et al. 2023. "Mitochondria-Targeted Lipid Nanoparticles Loaded with Rotenone as a New Approach for the Treatment of Oncological Diseases" Molecules 28, no. 20: 7229. https://doi.org/10.3390/molecules28207229
APA StyleVasileva, L., Gaynanova, G., Kuznetsova, D., Valeeva, F., Lyubina, A., Amerhanova, S., Voloshina, A., Sibgatullina, G., Samigullin, D., Petrov, K., & Zakharova, L. (2023). Mitochondria-Targeted Lipid Nanoparticles Loaded with Rotenone as a New Approach for the Treatment of Oncological Diseases. Molecules, 28(20), 7229. https://doi.org/10.3390/molecules28207229