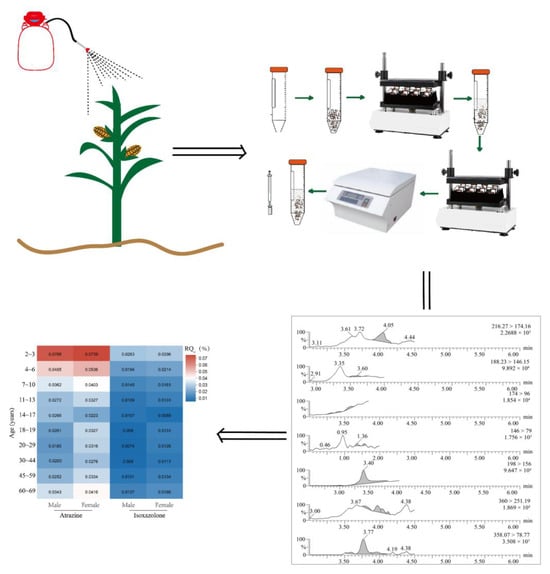
Terminal Residue and Dietary Risk Assessment of Atrazine and Isoxaflutole in Corn Using High-Performance Liquid Chromatography–Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. Optimization of UPLC–MS/MS Analysis
2.2. QuEChERS Pre-Treatment
2.3. Method Validation
2.4. Terminal Residue
2.5. Chronic Dietary Risk Assessment
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Solvent Standard and Matrix Matching Standard Solution
3.3. Field Experiment Design and Sampling
3.4. Residue Determination
3.4.1. Extraction and Purification Procedures
3.4.2. Instrumental Parameters
3.4.3. Method Validation
3.5. Terminal Residue and Dietary Risk Assessment
3.5.1. Residue Definition
3.5.2. Dietary Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nuss, E.T.; Tanumihardjo, S.A. Maize: A Paramount Staple Crop in the Context of Global Nutrition. Compr. Rev. Food Sci. Food Saf. 2010, 9, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, K.; Yousefi, A.R.; Oveisi, M. Effect of cowpea (Vigna unguiculata) intercropping on weed biomass and maize (Zea mays) yield. N. Z. J. Crop Hort. Sci. 2013, 41, 180–188. [Google Scholar] [CrossRef]
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Wu, Y.; An, Q.; Hao, X.; Li, D.; Zhou, C.; Zhang, J.; Wei, X.; Pan, C. Dissipative behavior, residual pattern, and risk assessment of four pesticides and their metabolites during tea cultivation, processing and infusion. Pest Manag. Sci. 2022, 78, 3019–3029. [Google Scholar] [CrossRef]
- Gao, J.; Wang, F.; Jiang, W.; Miao, J.; Wang, P.; Zhou, Z.; Liu, D. A full evaluation of chiral phenylpyrazole pesticide flufiprole and the metabolites to non-target organism in paddy field. Environ. Pollut. 2020, 264, 114808. [Google Scholar] [CrossRef]
- Pallett, K.E.; Cramp, S.M.; Little, J.P.; Veerasekaran, P.; Crudace, A.J.; Slater, A.E. Isoxaflutole: The background to its discovery and the basis of its herbicidal properties. Pest Manag. Sci. 2001, 57, 133–142. [Google Scholar] [CrossRef]
- Beltran, E.; Fenet, H.; Cooper, J.-F.; Coste, C.-M. Fate of isoxaflutole in soil under controlled conditions. J. Agric. Food Chem. 2003, 51, 146–151. [Google Scholar] [CrossRef]
- Papiernik, S.K.; Yates, S.R.; Koskinen, W.C.; Barber, B. Processes affecting the dissipation of the herbicide isoxaflutole and its diketonitrile metabolite in agricultural soils under field conditions. J. Agric. Food Chem. 2007, 55, 8630–8639. [Google Scholar] [CrossRef]
- Muller, G.; LeBaron, H.; McFarland, J.; Burnside, O. History of the discovery and development of triazine herbicides. Triazine Herbic. 2008, 50, 13–29. [Google Scholar]
- Jin, Y.; Zhang, X.; Shu, L.; Chen, L.; Sun, L.; Qian, H.; Liu, W.; Fu, Z. Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 2010, 78, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, L.S.; Wang, J.; Wang, J.H.; Liu, W.; Xie, H. DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil. Biol. Biochem. 2009, 41, 905–909. [Google Scholar] [CrossRef]
- Stoker, T.E.; Laws, S.C.; Guidici, D.L.; Cooper, R.L. The effect of atrazine on puberty in male wistar rats: An evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol. Sci. 2000, 58, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Gammon, D.W.; Aldous, C.N.; Carr, W.C., Jr.; Sanborn, J.R.; Pfeifer, K.F. A risk assessment of atrazine use in California: Human health and ecological aspects. Pest Manag. Sci. 2005, 61, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Hao, B.; Zhang, N.; Lv, H.; Zhao, B.; Tian, Y. Rapid determination of thiram and atrazine pesticide residues in fruit and aqueous system based on surface-enhanced Raman scattering. Spectrochim. Acta Part A 2023, 285, 121873. [Google Scholar] [CrossRef]
- Sun, W.; Huang, G.; Sai, N.; Xu, B. Carboxylated hapten coated directly coated ELISA for the detection of atrazine residue in water. J. Hyg. Res. 2017, 46, 109–119. [Google Scholar]
- Yuan, L.; Chai, Y.; Li, C.; Liu, R.; Chen, Z.; Li, L.; Li, W.; He, Y. Dissipation, residue, dietary, and ecological risk assessment of atrazine in apples, grapes, tea, and their soil. Environ. Sci. Pollut. Res. 2021, 28, 35064–35072. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Z.; Zhang, T.; Du, M.; Lv, B.; Yang, S.; Chen, R. Simultaneous detection of topramezon, atrazine, and atrazine metabolite residues in environmental water by large-volume SPE-UPLC-MS/MS. Int. J. Environ. Anal. Chem. 2022. [Google Scholar] [CrossRef]
- Tandon, S.; Singh, A. Field dissipation kinetics of atrazine in soil and post-harvest residues in winter maize crop under subtropical conditions. Chem. Ecol. 2015, 31, 273–284. [Google Scholar] [CrossRef]
- Lin, C.H.; Lerch, R.N.; Garrett, H.E.; Li, Y.X.; George, M.F. Improved HPLC-MS/MS method for determination of isoxaflutole (balance) and its metabolites in soils and forage plants. J. Agric. Food Chem. 2007, 55, 3805–3815. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Lerch, R.N.; Thurman, E.M.; Garrett, H.E.; George, M.F. Determination of isoxaflutole (balance) and its metabolites in water using solid phase extraction followed by high-performance liquid chromatography with ultraviolet or mass spectrometry. J. Agric. Food Chem. 2002, 50, 5816–5824. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Li, Q.; Sun, L.; Yu, W.; Jiang, W.; Wang, Z.; Liu, C. Simultaneous determination of isoxaflutole and its two metabolites in corn under field conditions by LC-MS/MS. J. Sci. Food Agric. 2022, 102, 3480–3486. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS-Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Wilkowska, A.; Biziuk, M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011, 125, 803–812. [Google Scholar] [CrossRef]
- Lehotay, S.J. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: Collaborative study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef]
- Cui, K.; Wu, X.; Zhu, L.; Zhang, Y.; Dai, G.; Cao, J.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Development and establishment of a QuEChERS-based extraction method for determining tembotrione and its metabolite AE 1417268 in corn, corn oil and certain animal-origin foods by HPLC-MS/MS. Food Addit. Contam. Part A 2020, 37, 1678–1686. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, T.; Hu, J. Dissipation kinetics and residues of triazolopyrimidine herbicides flumetsulam and florasulam in corn ecosystem. Environ. Monit. Assess. 2015, 187, 390. [Google Scholar] [CrossRef] [PubMed]
- Pihlström, T.; Fernández-Alba, A.R.; Gamón, M.; Poulsen, M.E.; Lippold, R.; Anastassiades, M. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. Sante 2017, 11813, 21–22. [Google Scholar]
- Su, Y.; Wang, W.; Hu, J.; Liu, X. Dissipation behavior, residues distribution and dietary risk assessment of tembotrione and its metabolite in maize via QuEChERS using HPLC-MS/MS technique. Ecotoxicol. Environ. Saf. 2020, 191, 110187. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, L.; Zhang, Y.; Shao, H.; Li, H.; Li, N.; Zou, P.; Lu, N.; Guo, Y. Residue behavior and dietary risk assessment of spinetoram (XDE-175-J/L) and its two metabolites in cauliflower using QuEChERS method coupled with UPLC-MS/MS. Ecotoxicol. Environ. Saf. 2020, 202, 110942. [Google Scholar] [CrossRef] [PubMed]
- Makinde, J.; Ogunbodede, B. Evaluation of atrazine plus isoxaflutole (Atoll®) mixture for weed control in maize. Ghana J. Agric. Sci. 2008, 40, 193–198. [Google Scholar] [CrossRef]
- Willemse, C.; Soltani, N.; David, C.H.; Jhala, A.J.; Robinson, D.E.; Sikkema, P.H. Interaction of 4-hydroxyphenylpyruvate dioxygenase (HPPD) and atrazine alternative photosystem II (PS II) inhibitors for control of multiple herbicide-resistant waterhemp (Amaranthus tuberculatus) in corn. Weed Sci. 2021, 69, 492–503. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, J. Total residue levels and risk assessment of flufenacet and its four metabolites in corn. J. Food Compos. Anal. 2022, 106, 104268. [Google Scholar] [CrossRef]
- Wang, K.; Jiao, B.; Gao, H.; Pan, X.; Wu, X.; Xu, J.; Dong, F.; Zheng, Y. Residue and dietary risk assessment of glyphosate, glufosinate-ammonium, and their metabolites in maize and soybean. J. Food Compos. Anal. 2023, 120, 105298. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. National Food Safety Standard-Maximum Residue Limits for Pesticides in Food; China Agricultural Press: Beiing, China, 2021. [Google Scholar]
- Chang, C.-H.; MacIntosh, D.; Lemos, B.; Zhang, Q.; Lu, C. Characterization of Daily Dietary Intake and the Health Risk of Neonicotinoid Insecticides for the U.S. Population. J. Agric. Food Chem. 2018, 66, 10097–10105. [Google Scholar] [CrossRef]
- Bian, Y.; Feng, Y.; Zhang, A.; Qi, X.; Pan, J.; Han, J.; Ma, X.; Liang, L. Residue distribution and risk assessment of bifenazate and its metabolite in garlic plant. Food Chem. 2022, 379, 132013. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Y.; Hu, J. Dissipation, residues and risk assessment of pyraclostrobin and picoxystrobin in cucumber under field conditions. J. Sci. Food Agric. 2020, 100, 5145–5151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Dong, Z.; Bian, C.; Wang, L.; Wu, T.; Zhou, W.; Li, Y.; Li, B. Residue analysis, dissipation behavior, storage stability and dietary risk assessment of florpyrauxifen-benzyl in natural paddy field environment using UPLC-QTOF-MS/MS. J. Food Compos. Anal. 2022, 114, 104781. [Google Scholar] [CrossRef]
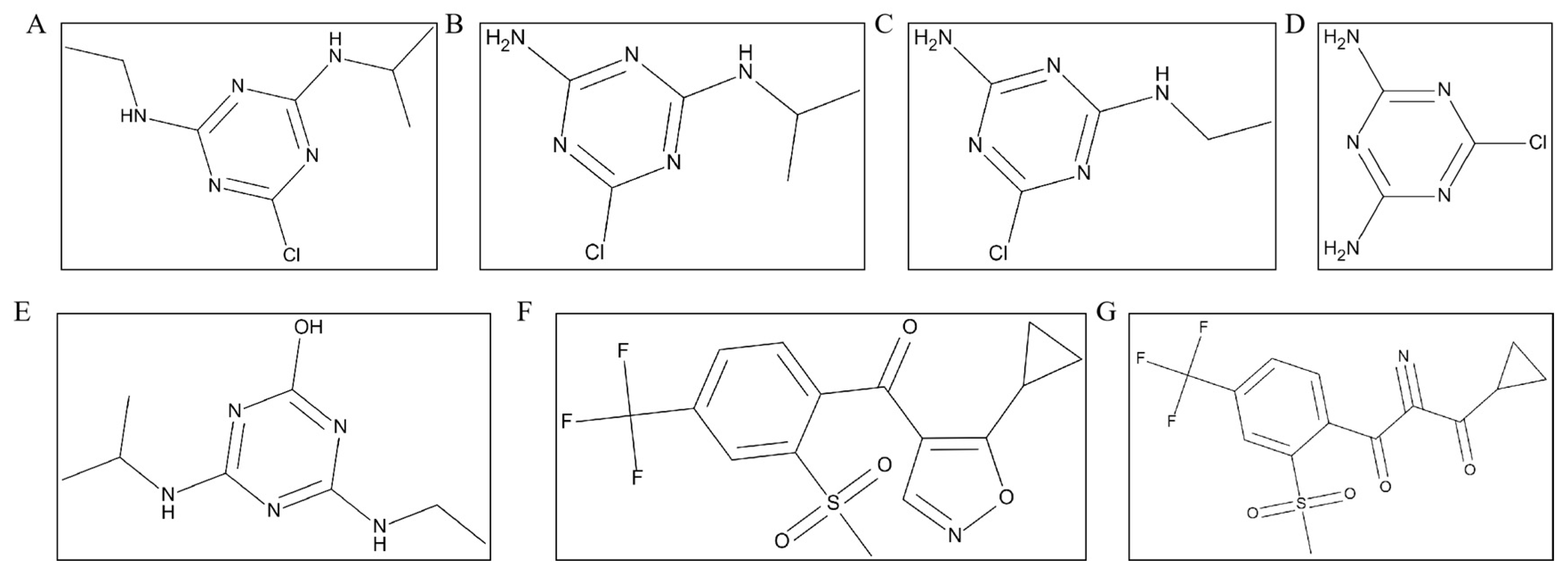
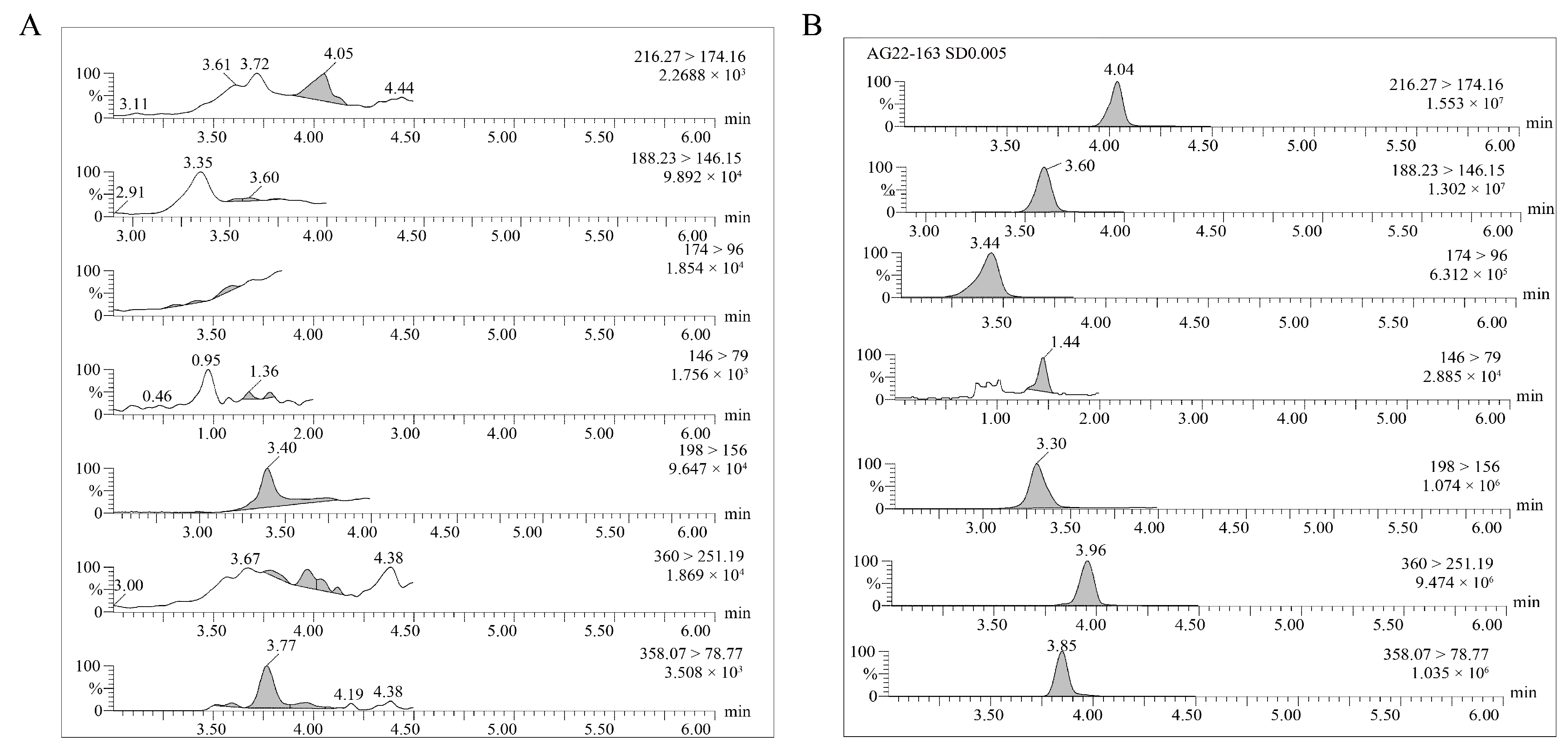

| Compounds | Retention Time (min) | Precursor Ion (m z−1) | Product Ion (m z−1) | Cone Voltage (CV, V) | Collision Energy (CE, eV) |
|---|---|---|---|---|---|
| ATR | 4.04 | 216.27 | 174.16 * | 20 | 28 |
| 96.14 | 21 | ||||
| DEA | 3.60 | 188.23 | 78.9 | 30 | 30 |
| 146.15 * | 19 | ||||
| DIA | 3.44 | 174.0 | 96.0 * | 65 | 20 |
| 131.9 | 15 | ||||
| DACT | 1.44 | 146.0 | 79.0 * | 70 | 19 |
| 103.99 | 17 | ||||
| HA | 3.30 | 198.0 | 156.0 * | 30 | 17 |
| 113.9 | 21 | ||||
| IFT | 3.96 | 360.0 | 251.19 * | 30 | 18 |
| 219.9 | 40 | ||||
| IFT-DKN | 3.85 | 358.07 | 78.77 * | −20 | −17 |
| 278.0 | −14 |
| Compound | Matrix | Equation | Determination Coefficient (R2) | ME (%) |
|---|---|---|---|---|
| ATR | Acetonitrile | y = 1.92473 × 108x + 171,768 | 0.9961 | - |
| Fresh corn | y = 1.78484 × 108x + 151,436 | 0.9972 | −7.3 | |
| Corn kernels | y = 1.72312 × 108x − 36,266.5 | 0.9990 | −10.5 | |
| Corn straw | y = 1.45725 × 108x + 64,352.9 | 0.9991 | −24.3 | |
| DEA | Acetonitrile | y = 1.69395 × 108x + 222,823 | 0.9912 | - |
| Fresh corn | y = 1.53907 × 108x + 183,749 | 0.9945 | −9.1 | |
| Corn kernels | y = 1.48905 × 108x − 28,122.8 | 0.9963 | −12.1 | |
| Corn straw | y = 8.71969 × 107x + 8952.86 | 0.9996 | −48.5 | |
| DIA | Acetonitrile | y = 1.22314 × 107x + 12,671.5 | 0.9965 | - |
| Fresh corn | y = 1.17502 × 107x + 7327.07 | 0.9981 | −3.9 | |
| Corn kernels | y = 1.54207 × 107x + 565.797 | 0.9986 | 26.1 | |
| Corn straw | y = 8.11043 × 106x − 1082.61 | 0.9999 | −33.7 | |
| DACT | Acetonitrile | y = 92,470.9x + 15.5547 | 0.9994 | - |
| Fresh corn | y = 98,205.3x + 21.2445 | 0.9977 | 6.2 | |
| Corn kernels | y = 150,640x + 103.791 | 0.9915 | 62.9 | |
| Corn straw | y = 99,527x − 4.04451 | 0.9991 | 7.6 | |
| HA | Acetonitrile | y = 2.51197 × 107x − 13,594.8 | 0.9995 | - |
| Fresh corn | y = 2.8312 × 107x + 40,610.5 | 0.9933 | 12.7 | |
| Corn kernels | y = 2.74648 × 107x + 43,150.3 | 0.9912 | 9.3 | |
| Corn straw | y = 3.17866 × 107x + 49,750.2 | 0.9906 | 26.5 | |
| IFT | Acetonitrile | y = 1.31221 × 108x + 97,738.5 | 0.9940 | - |
| Fresh corn | y = 1.16984 × 108x + 144,660 | 0.9944 | −10.8 | |
| Corn kernels | y = 1.02943 × 108x − 71,139.6 | 0.9917 | −21.5 | |
| Corn straw | y = 7.02378 × 107x − 3031.07 | 0.9960 | −46.5 | |
| IFT-DKN | Acetonitrile | y = 1.96818 × 107x − 18,509.5 | 0.9962 | - |
| Fresh corn | y = 2.69316 × 107x − 5800.49 | 0.9994 | 36.8 | |
| Corn kernels | y = 1.97881 × 107x − 13,759.9 | 0.9967 | 0.5 | |
| Corn straw | y = 3.48496 × 107x + 26,353 | 0.9971 | 77.1 |
| Location | Total ATR Residue (mg kg−1) * | Total IFT residue (mg kg−1) * | ||||
|---|---|---|---|---|---|---|
| Fresh Corn | Corn Kernels | Corn Straw | Fresh Corn | Corn Kernels | Corn Straw | |
| Liaoning | <0.05 | <0.05 | 0.067 ± 0.0014 | <0.02 | <0.02 | <0.02 |
| Heilongjiang | <0.05 | <0.05 | <0.05 | <0.02 | <0.02 | <0.02 |
| Neimenggu | <0.05 | <0.05 | 0.11 ± 0.085 | <0.02 | <0.02 | <0.02 |
| Shanxi | <0.05 | <0.05 | 0.071 ± 0.0014 | <0.02 | <0.02 | <0.02 |
| Beijing | <0.05 | <0.05 | 0.135 ± 0.0071 | <0.02 | <0.02 | <0.02 |
| Yunnan | <0.05 | <0.05 | <0.05 | <0.02 | <0.02 | <0.02 |
| Gender | Age (Years) | Average bw (kg) | Fi (kg) | ATR | IFT | ||||
|---|---|---|---|---|---|---|---|---|---|
| STMR (mg kg−1) | NEDI (mg kg−1 bw Day−1) | RQc (%) | STMR (mg kg−1) | NEDI (mg kg−1 bw Day−1) | RQc (%) | ||||
| Male | 2–3 | 16.6 | 0.0047 | 0.05 | 1.42 × 10−5 | 0.0708 | 0.02 | 5.66 × 10−6 | 0.0283 |
| 4–6 | 20.6 | 0.004 | 9.71 × 10−6 | 0.0485 | 3.88 × 10−6 | 0.0194 | |||
| 7–10 | 31.8 | 0.0046 | 7.23 × 10−6 | 0.0362 | 2.89 × 10−6 | 0.0145 | |||
| 11–13 | 46.8 | 0.0051 | 5.45 × 10−6 | 0.0272 | 2.18 × 10−6 | 0.0109 | |||
| 14–17 | 59.1 | 0.0063 | 5.33 × 10−6 | 0.0266 | 2.13 × 10−6 | 0.0107 | |||
| 18–19 | 63.4 | 0.0051 | 4.02 × 10−6 | 0.0201 | 1.61 × 10−6 | 0.0080 | |||
| 20–29 | 68.8 | 0.0051 | 3.71 × 10−6 | 0.0185 | 1.48 × 10−6 | 0.0074 | |||
| 30–44 | 71.4 | 0.0057 | 3.99 × 10−6 | 0.0200 | 1.60 × 10−6 | 0.0080 | |||
| 45–59 | 70.3 | 0.0071 | 5.05 × 10−6 | 0.0252 | 2.02 × 10−6 | 0.0101 | |||
| 60–69 | 67.1 | 0.0092 | 6.86 × 10−6 | 0.0343 | 2.74 × 10−6 | 0.0137 | |||
| Female | 2–3 | 15.9 | 0.0047 | 1.48 × 10−5 | 0.0739 | 5.91 × 10−6 | 0.0296 | ||
| 4–6 | 19.6 | 0.0042 | 1.07 × 10−5 | 0.0536 | 4.29 × 10−6 | 0.0214 | |||
| 7–10 | 29.8 | 0.0048 | 8.05 × 10−6 | 0.0403 | 3.22 × 10−6 | 0.0161 | |||
| 11–13 | 44.4 | 0.0058 | 6.53 × 10−6 | 0.0327 | 2.61 × 10−6 | 0.0131 | |||
| 14–17 | 51.6 | 0.0046 | 4.46 × 10−6 | 0.0223 | 1.78 × 10−6 | 0.0089 | |||
| 18–19 | 52.7 | 0.0069 | 6.55 × 10−6 | 0.0327 | 2.62 × 10−6 | 0.0131 | |||
| 20–29 | 54.6 | 0.0069 | 6.32 × 10−6 | 0.0316 | 2.53 × 10−6 | 0.0126 | |||
| 30–44 | 57.9 | 0.0064 | 5.53 × 10−6 | 0.0276 | 2.21 × 10−6 | 0.0111 | |||
| 45–59 | 59.9 | 0.008 | 6.68 × 10−6 | 0.0334 | 2.67 × 10−6 | 0.0134 | |||
| 60–69 | 59.5 | 0.0099 | 8.32 × 10−6 | 0.0416 | 3.33 × 10−6 | 0.0166 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Pei, T.; Wang, Y.; Qin, S.; Qi, Y.; Ren, P.; Li, J. Terminal Residue and Dietary Risk Assessment of Atrazine and Isoxaflutole in Corn Using High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2023, 28, 7225. https://doi.org/10.3390/molecules28207225
Cao J, Pei T, Wang Y, Qin S, Qi Y, Ren P, Li J. Terminal Residue and Dietary Risk Assessment of Atrazine and Isoxaflutole in Corn Using High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules. 2023; 28(20):7225. https://doi.org/10.3390/molecules28207225
Chicago/Turabian StyleCao, Junli, Tao Pei, Yonghui Wang, Shu Qin, Yanli Qi, Pengcheng Ren, and Jindong Li. 2023. "Terminal Residue and Dietary Risk Assessment of Atrazine and Isoxaflutole in Corn Using High-Performance Liquid Chromatography–Tandem Mass Spectrometry" Molecules 28, no. 20: 7225. https://doi.org/10.3390/molecules28207225
APA StyleCao, J., Pei, T., Wang, Y., Qin, S., Qi, Y., Ren, P., & Li, J. (2023). Terminal Residue and Dietary Risk Assessment of Atrazine and Isoxaflutole in Corn Using High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules, 28(20), 7225. https://doi.org/10.3390/molecules28207225