Combining Raman Microspectroscopy and X-ray Microcomputed Tomography for the Study of Bone Quality in Apolipoprotein-Deficient Animal Models
Abstract
1. Introduction
1.1. Bone and Its Constituents
1.2. Bone and Raman Spectroscopy
1.3. Bone and Microcomputed Tomography
1.4. Scope of the work
2. Results
2.1. Raman Spectroscopy
2.1.1. Raman Spectra of Bone
2.1.2. Raman Study of Femoral and Tibial Cortical Bones
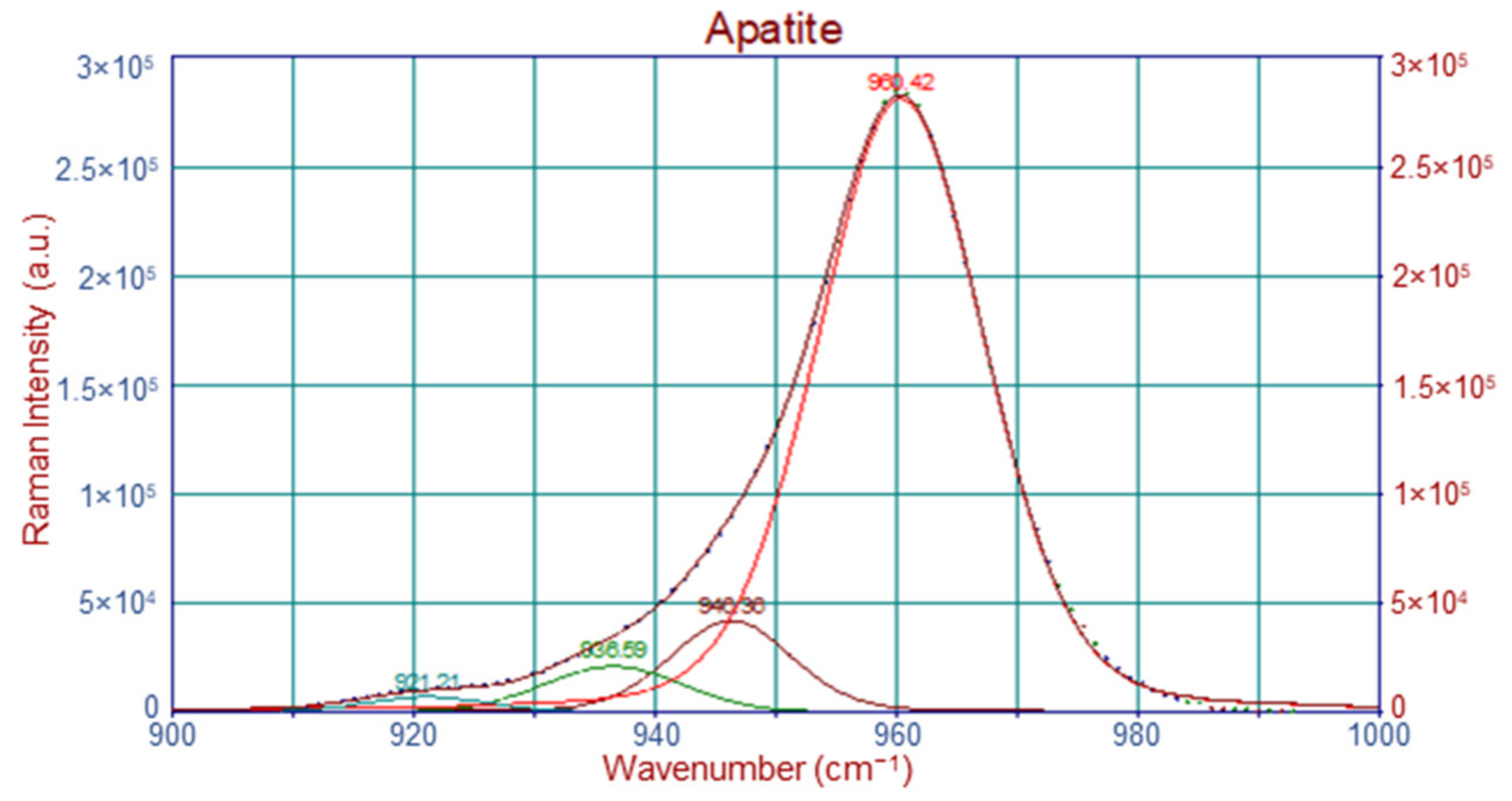
Mineral-to-Matrix Ratio
Carbonate-to-Phosphate Ratio (CPR)
Crystallinity
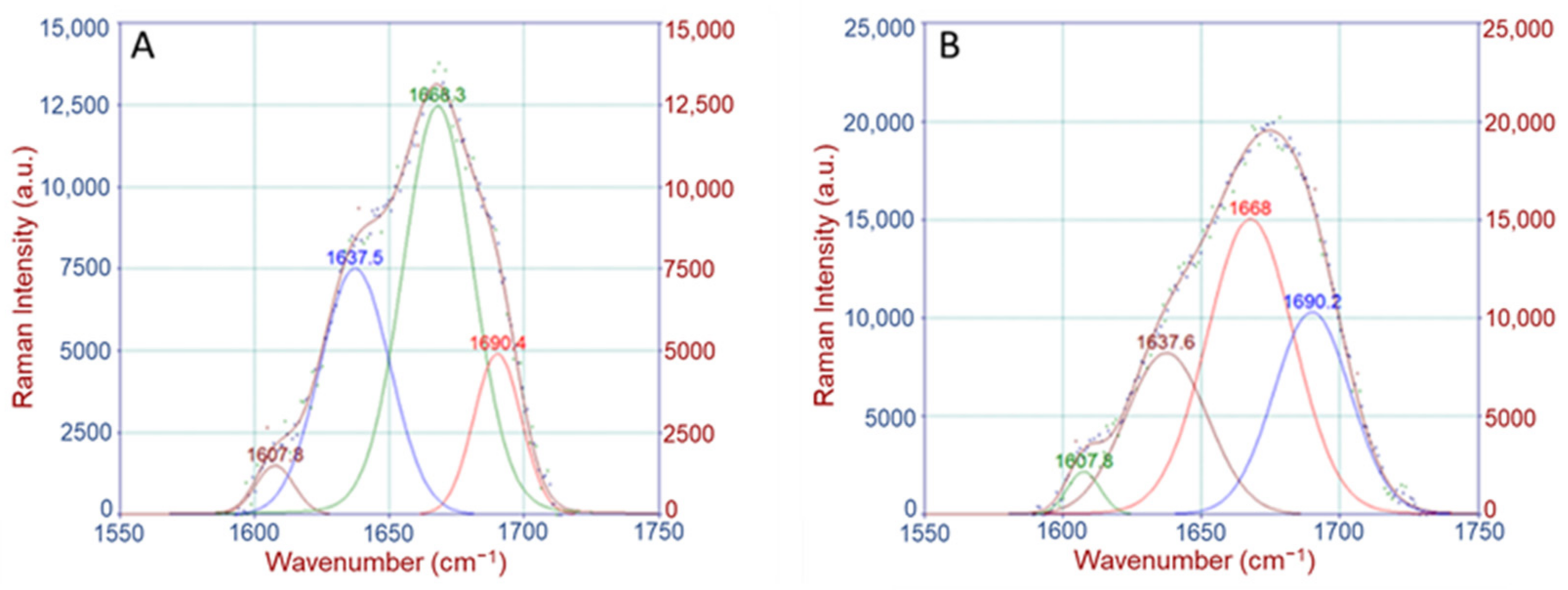
Crosslinking Ratio (CLR)
2.2. X-ray Microcomputed Tomography
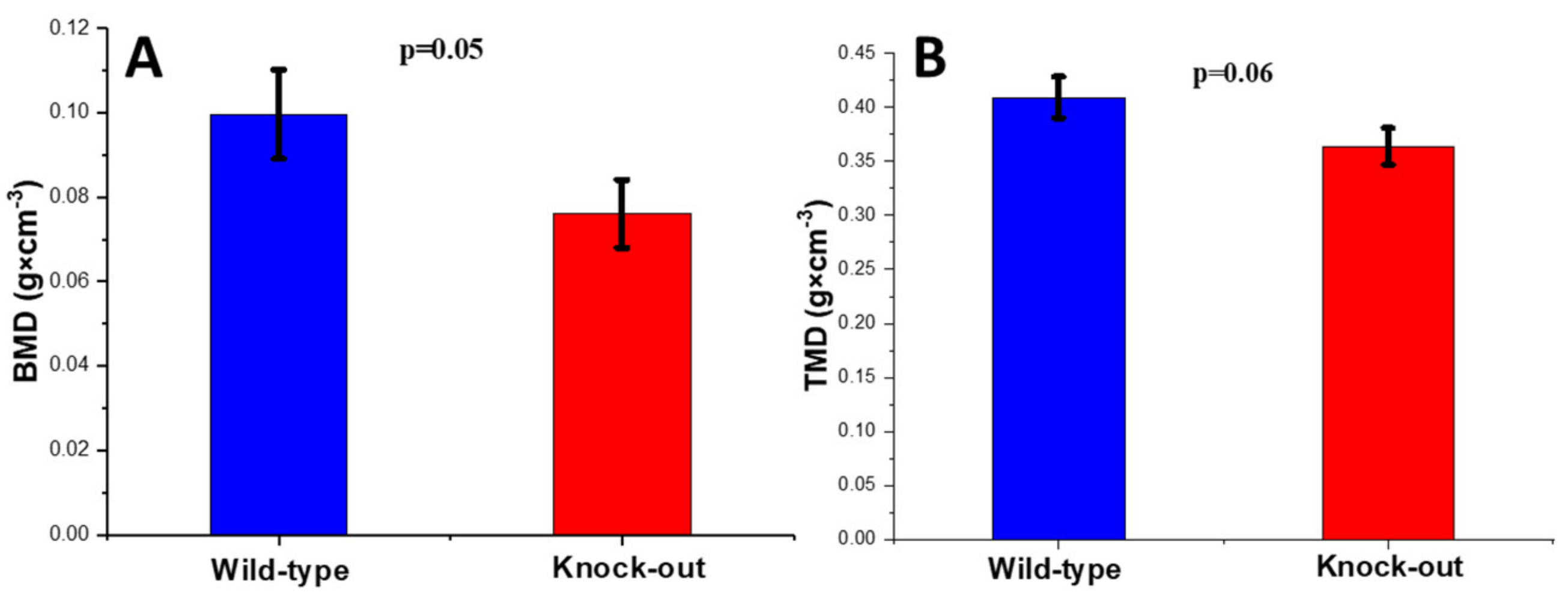
2.2.1. Density and Morphometric Parameters of the Cortical Bones
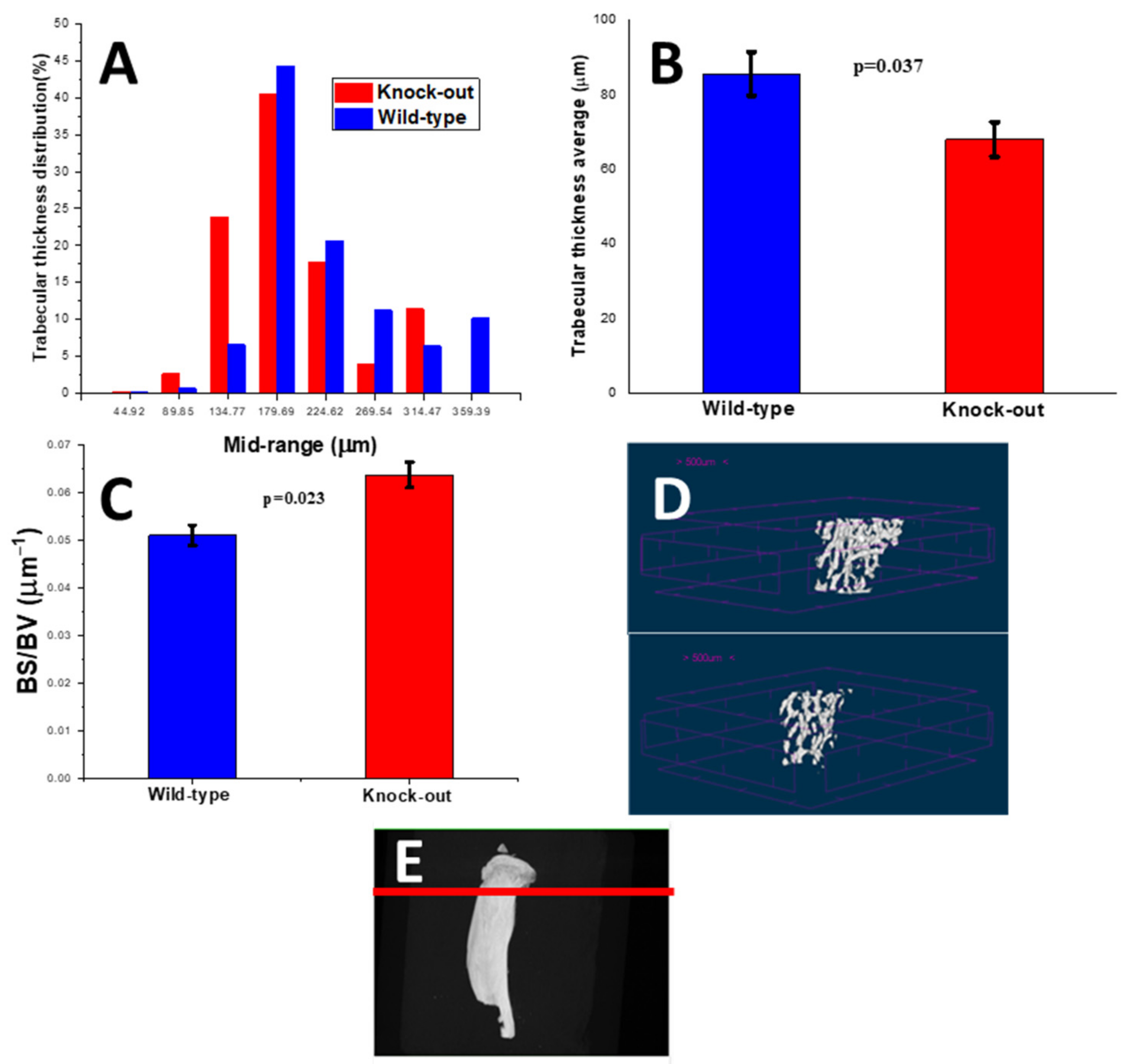
2.2.2. Density and Morphometric Parameters of the Trabecular Bones
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Raman Spectroscopy
4.2.1. Raman Metrics
4.2.2. Raman Band Deconvolution
4.3. Atomic Absorption Spectroscopy
4.4. Thermal Gravimetric Analysis
4.5. Microcomputed Tomography
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Unal, M.; Jung, H.; Akkus, O. Novel Raman Spectroscopic Biomarkers Indicate that Postyield Damage Denatures Bone’s Collagen. J. Bone Miner. Res. 2016, 31, 1015–1025. [Google Scholar] [CrossRef]
- Morris, M.D.; Mandair, G.S. Raman Assessment of Bone Quality. Clin. Orthop. Relat. Res. 2011, 469, 2160–2169. [Google Scholar] [CrossRef]
- Currey, J.D. The effect of porosity and mineral content on the Young’s modulus of elasticity of compact bone. J. Biomech. 1988, 21, 131–139. [Google Scholar] [CrossRef]
- Grynpas, M. Age and disease-related changes in the mineral of bone. Calcif. Tissue Int. 1993, 53, S57–S64. [Google Scholar] [CrossRef]
- Kingsmill, V.J.; Boyde, A. Mineralisation density of human mandibular bone: Quantitative backscattered electron image analysis. J. Anat. 1998, 192, 245–256. [Google Scholar] [CrossRef]
- Boyce, T.M.; Bloebaum, R.D. Cortical aging differences and fracture implications for the human femoral neck. Bone 1993, 14, 769–778. [Google Scholar] [CrossRef]
- Jowsey, J. Age changes in human bone. Clin. Orthop. 1960, 17, 210–218. [Google Scholar]
- Crofts, R.; Boyce, T.; Bloebaum, R. Aging changes in osteon mineralization in the human femoral neck. Bone 1994, 15, 147–152. [Google Scholar] [CrossRef]
- McNerny, E.M.; Gardinier, J.D.; Kohn, D.H. Exercise increases pyridinoline cross-linking and counters the mechanical effects of concurrent lathyrogenic treatment. Bone 2015, 81, 327–337. [Google Scholar] [CrossRef]
- McNerny, E.M.; Gong, B.; Morris, M.D.; Kohn, D.H. Bone Fracture Toughness and Strength Correlate with Collagen Cross-Link Maturity in a Dose-Controlled Lathyrism Mouse Model. J. Bone Miner. Res. 2015, 30, 455–464. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Shane, E.; Lyritis, G.; Skarantavos, G.; Mendelsohn, R.; Boskey, A.L. Bone Fragility and Collagen Cross-Links. J. Bone Miner. Res. 2004, 19, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Knott, L.; Whitehead, C.C.; Fleming, R.H.; Bailey, A.J. Biochemical changes in the collagenous matrix of osteoporotic avian bone. Biochem. J. 1995, 310 Pt 3, 1045–1051. [Google Scholar] [CrossRef]
- Saito, M.; Fujii, K.; Mori, Y.; Marumo, K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos. Int. 2006, 17, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B. Bone material properties and mineral matrix contributions to fracture risk or age in women and men. J. Musculoskelet. Neuronal. Interact. 2002, 2, 201–204. [Google Scholar] [PubMed]
- Mandair, G.S.; Morris, M.D. Contributions of Raman spectroscopy to the understanding of bone strength. Bone Key Rep. 2015, 4, 620. [Google Scholar] [CrossRef]
- Kim, G.; Cole, J.H.; Boskey, A.L.; Baker, S.P.; van der Meulen, M.C.H. Reduced Tissue-Level Stiffness and Mineralization in Osteoporotic Cancellous Bone. Calcif. Tissue Int. 2014, 95, 125–131. [Google Scholar] [CrossRef]
- McCreadie, B.R.; Morris, M.D.; Chen, T.-C.; Rao, D.S.; Finney, W.F.; Widjaja, E.; Goldstein, S.A. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone 2006, 39, 1190–1195. [Google Scholar] [CrossRef]
- Takahata, M.; Maher, J.R.; Juneja, S.C.; Inzana, J.; Xing, L.; Schwarz, E.M.; Berger, A.J.; Awad, H.A. Mechanisms of bone fragility in a mouse model of glucocorticoid-treated rheumatoid arthritis Implications for insufficiency fracture risk. Arthritis Rheum. 2012, 64, 3649–3659. [Google Scholar] [CrossRef]
- Akkus, O.; Adar, F.; Schaffler, M.B. Age-related changes in physicochemical properties of mineral crystals are related to impaired mechanical function of cortical bone. Bone 2004, 34, 443–453. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Fernández-Murga, M.L.; Vinué, Á.; Caeiro, J.R.; Guede, D.; Tarín, J.J.; Andrés, V.; Cano, A. Impact of estrogens on atherosclerosis and bone in the apolipoprotein E–deficient mouse model. Menopause 2015, 22, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, M.; Beil, F.T.; Mueller, B.; Bartelt, A.; Marshall, R.P.; Koehne, T.; Amling, M.; Ruether, W.; Cooper, J.A.; Humphries, S.E.; et al. Human apolipoprotein E isoforms differentially affect bone mass and turnover in vivo. J. Bone Miner. Res. 2013, 28, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Beil, F.T.; Schinke, T.; Roeser, K.; Ruether, W.; Heeren, J.; Niemeier, A. Apolipoprotein E-dependent inverse regulation of vertebral bone and adipose tissue mass in C57Bl/6 mice: Modulation by diet-induced obesity. Bone 2010, 47, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Ackert-Bicknell, C.L. HDL cholesterol and bone mineral density: Is there a genetic link? Bone 2012, 50, 525–533. [Google Scholar] [CrossRef]
- Pirih, F.; Lu, J.; Ye, F.; Bezouglaia, O.; Atti, E.; Ascenzi, M.-G.; Tetradis, S.; Demer, L.; Aghaloo, T.; Tintut, Y. Adverse effects of hyperlipidemia on bone regeneration and strength. J. Bone Miner. Res. 2012, 27, 309–318. [Google Scholar] [CrossRef]
- Tsezou, A.; Iliopoulos, D.; Malizos, K.N.; Simopoulou, T. Impaired expression of genes regulating cholesterol efflux in human osteoarthritic chondrocytes. J. Orthop. Res. 2010, 28, 1033–1039. [Google Scholar] [CrossRef]
- Blair, H.C.; Kalyvioti, E.; I Papachristou, N.; Tourkova, I.L.; A Syggelos, S.; Deligianni, D.; Orkoula, M.G.; Kontoyannis, C.G.; A Karavia, E.; E Kypreos, K.; et al. Apolipoprotein A-1 regulates osteoblast and lipoblast precursor cells in mice. Lab. Investig. 2016, 96, 763–772. [Google Scholar] [CrossRef]
- Liu, L.; Alonso, V.; Guo, L.; Tourkova, I.; Henderson, S.E.; Almarza, A.J.; Friedman, P.A.; Blair, H.C. Na+/H+ Exchanger Regulatory Factor 1 (NHERF1) Directly Regulates Osteogenesis. J. Biol. Chem. 2012, 287, 43312–43321. [Google Scholar] [CrossRef]
- Raghavan, M.; Sahar, N.D.; Kohn, D.H.; Morris, M.D. Age-specific profiles of tissue-level composition and mechanical properties in murinecortical bone. Bone 2012, 50, 942–953. [Google Scholar] [CrossRef]
- Friedman, M.A.; Bailey, A.M.; Rondon, M.J.; McNerny, E.M.; Sahar, N.D.; Kohn, D.H. Calcium- and Phosphorus-Supplemented Diet Increases Bone Mass after Short-Term Exercise and Increases Bone Mass and Structural Strength after Long-Term Exercise in Adult Mice. PLoS ONE 2016, 11, e0151995. [Google Scholar] [CrossRef]
- Hassler, N.; Gamsjaeger, S.; Hofstetter, B.; Brozek, W.; Klaushofer, K.; Paschalis, E.P. Effects of long-term alendronate treatment on postmenopausal osteoporosis bone material properties. Osteoporos. Int. 2015, 26, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Kavukcuoglu, N.B.; Arteaga-Solis, E.; Lee-Arteaga, S.; Ramirez, F.; Mann, A.B. Nanomechanics and Raman spectroscopy of fibrillin 2 knock-out mouse bones. J. Mater. Sci. 2007, 42, 8788–8794. [Google Scholar] [CrossRef]
- Crane, N.J.; Popescu, V.; Morris, M.D.; Steenhuis, P.; Ignelzi, M.A. Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembranous mineralization. Bone 2006, 39, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Kazanci, M.; Fratzl, P.; Klaushofer, K.; Paschalis, E.P. Complementary Information on In Vitro Conversion of Amorphous (Precursor) Calcium Phosphate to Hydroxyapatite from Raman Microspectroscopy and Wide-Angle X-ray Scattering. Calcif. Tissue Int. 2006, 79, 354–359. [Google Scholar] [CrossRef]
- McElderry, J.-D.P.; Zhao, G.; Khmaladze, A.; Wilson, C.G.; Franceschi, R.T.; Morris, M.D. Tracking circadian rhythms of bone mineral deposition in murine calvarial organ cultures. J. Bone Miner. Res. 2013, 28, 1846–1854. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Verdelis, K.; Doty, S.B.; Boskey, A.L.; Mendelsohn, R.; Yamauchi, M. Spectroscopic Characterization of Collagen Cross-Links in Bone. J. Bone Miner. Res. 2001, 16, 1821–1828. [Google Scholar] [CrossRef]
- Carden, A.; Rajachar, R.; Morris, M.D.; Kohn, D.H. Ultrastructural Changes Accompanying the Mechanical Deformation of Bone Tissue: A Raman Imaging Study. Calcif. Tissue Int. 2003, 72, 166–175. [Google Scholar] [CrossRef]
- Molino, G.; Montalbano, G.; Pontremoli, C.; Fiorilli, S.; Vitale-Brovarone, C. Imaging Techniques for the Assessment of the Bone Osteoporosis-Induced Variations with Particular Focus on Micro-CT Potential. Appl. Sci. 2020, 10, 8939. [Google Scholar] [CrossRef]
- Guha, I.; Klintström, B.; Klintström, E.; Zhang, X.; Smedby, Ö.; Moreno, R.; Saha, P.K. A comparative study of trabecular bone micro-structural measurements using different CT modalities. Phys. Med. Biol. 2020, 65, 235029. [Google Scholar] [CrossRef]
- Yerramshetty, J.S.; Akkus, O. The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone 2008, 42, 476–482. [Google Scholar] [CrossRef]
- Bone Mineral Density (BMD) and Tissue Mineral Density (TMD) Calibration and Measurement Using Bruker Microct BMD Phantoms and CT-Analyser Software, Method Note MN 9, Bruker Microct, Bruker (Bruker Belgium SA, Kontich, Belgium). Available online: https://www.med.nagasaki-u.ac.jp/generes/machine/SkyScan%20phantoms.pdf (accessed on 1 March 2013).
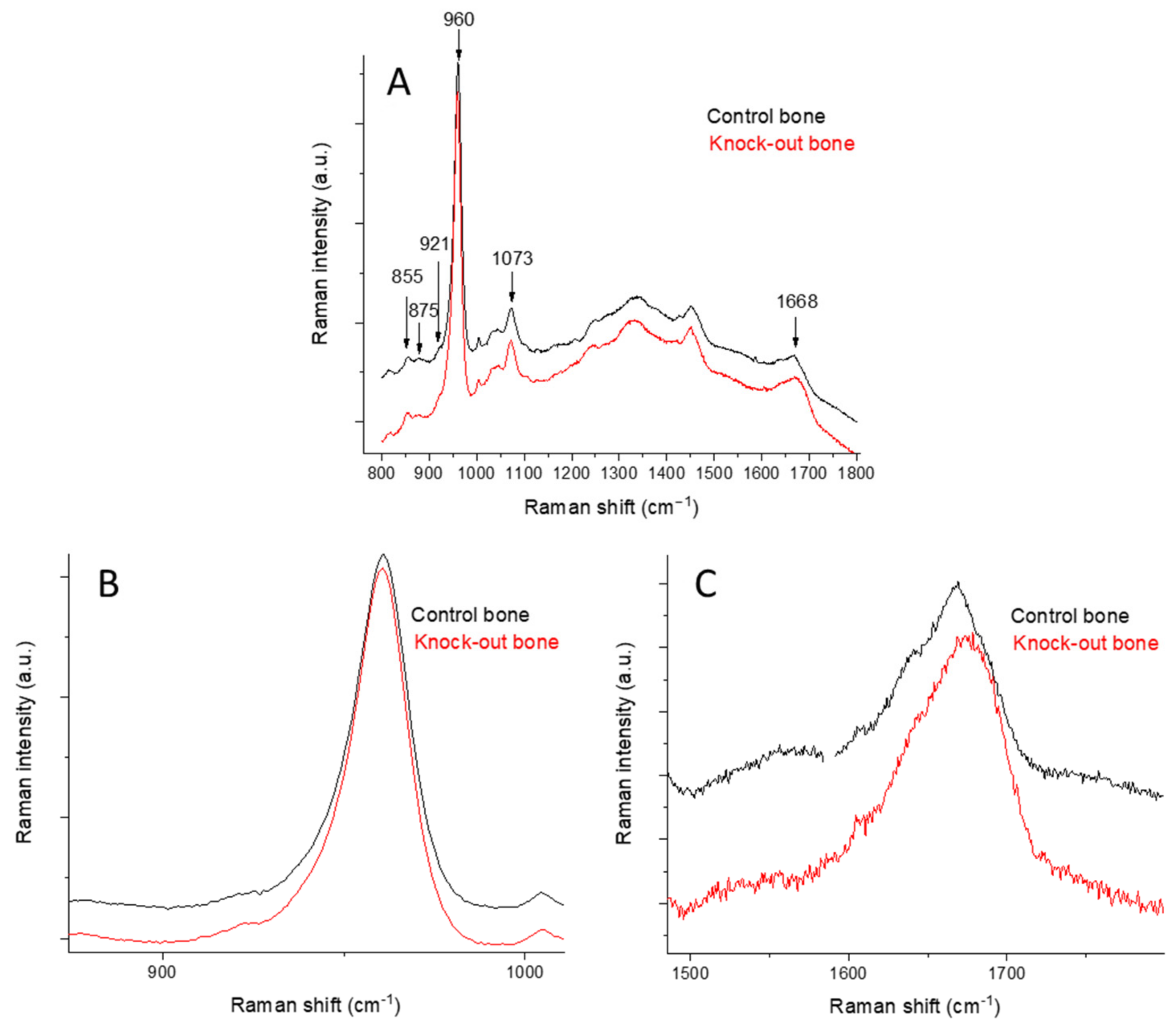
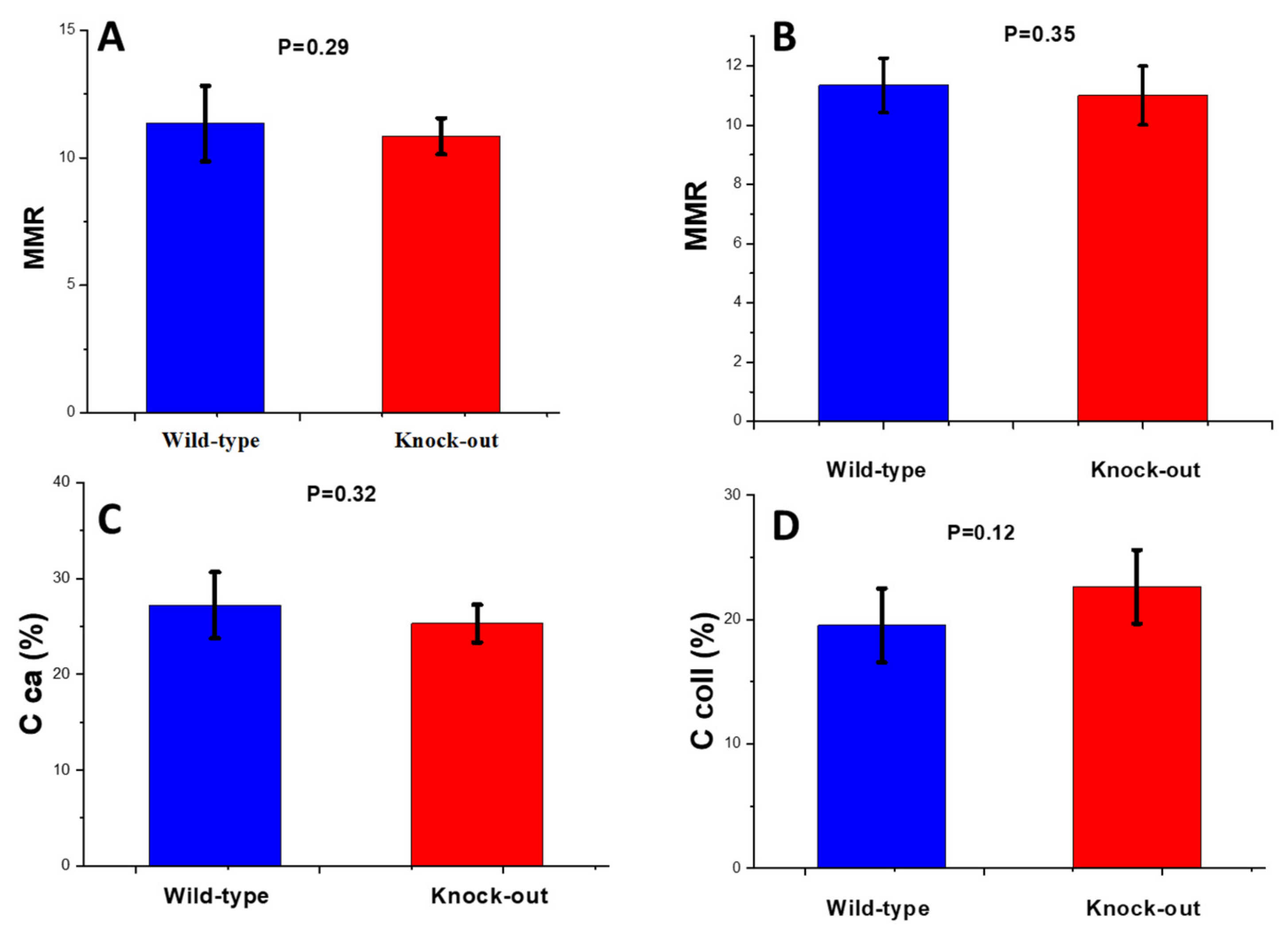
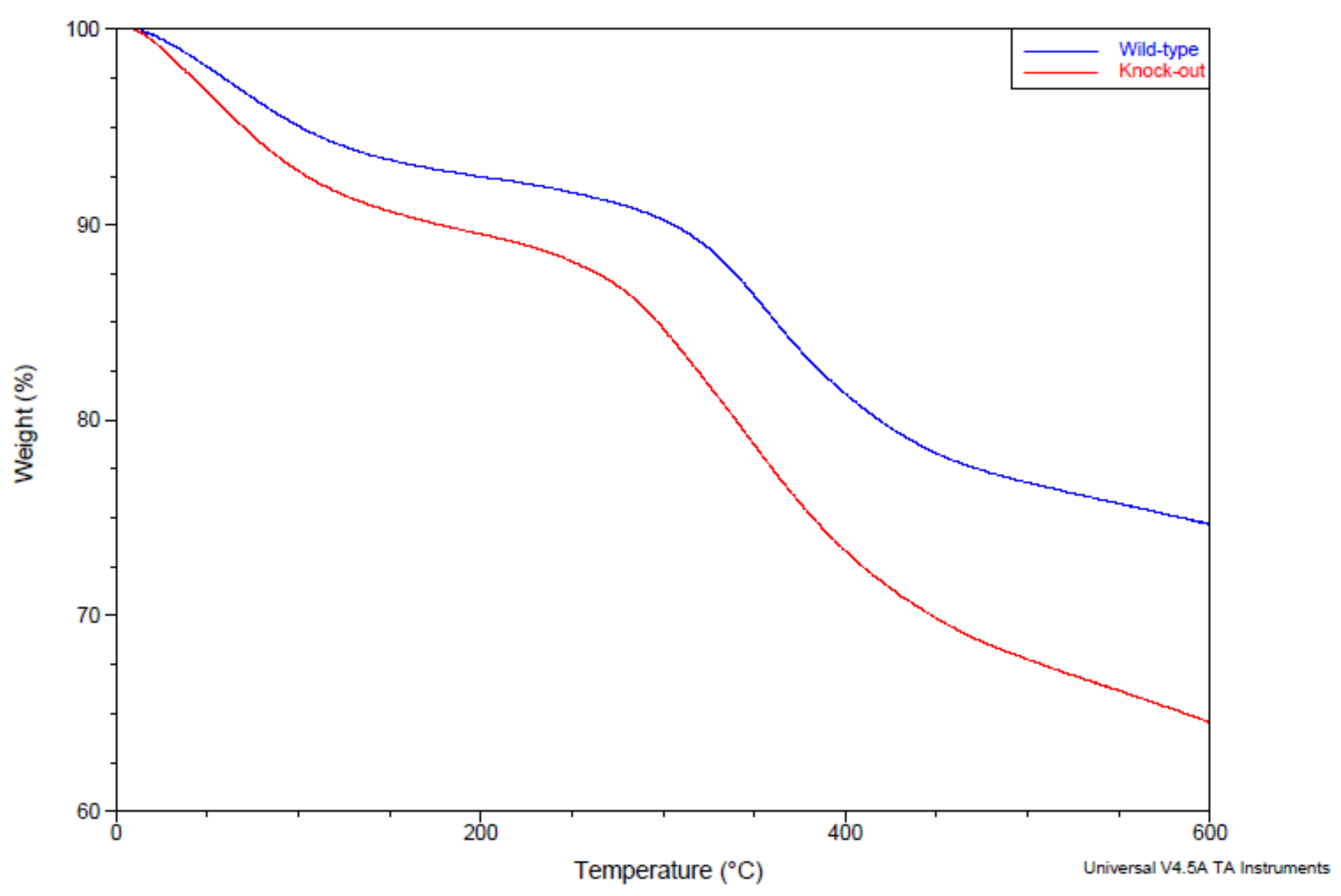

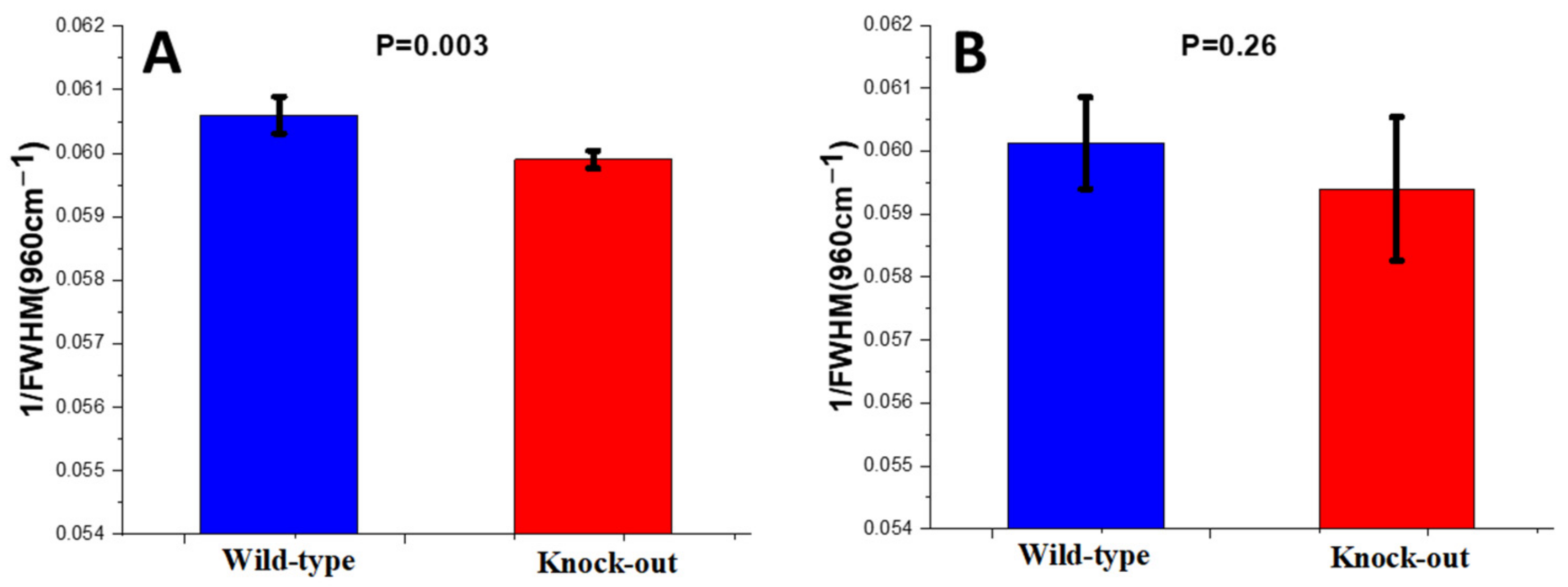


| Raman Shift (cm−1) | Band Assignment |
|---|---|
| 855 | ν(C-C), collagen proline |
| 875 | ν(C-C), collagen hydroxyproline |
| 921 | ν(C-C), collagen proline |
| 937 | ν(C-C), proline and protein backbone |
| 946 | ν1PO43− |
| 960 | ν1PO43−, bone mineral |
| 1003 | ν(C-C), phenylalanine |
| 1035 | ν3PO43− |
| 1050 | ν3PO43− |
| 1060 | proteoglycan, lipids, collagen, ν3PO43− |
| 1073 | ν1CO32− |
| 1087 | ν3PO43−, ν1CO32− |
| 1637 | ν(C=C) |
| 1668 | amide I, ν(C=O) |
| 1690 | amide I, β-sheets, disordered secondary structure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fertaki, S.; Giannoutsou, P.; Orkoula, M.G. Combining Raman Microspectroscopy and X-ray Microcomputed Tomography for the Study of Bone Quality in Apolipoprotein-Deficient Animal Models. Molecules 2023, 28, 7196. https://doi.org/10.3390/molecules28207196
Fertaki S, Giannoutsou P, Orkoula MG. Combining Raman Microspectroscopy and X-ray Microcomputed Tomography for the Study of Bone Quality in Apolipoprotein-Deficient Animal Models. Molecules. 2023; 28(20):7196. https://doi.org/10.3390/molecules28207196
Chicago/Turabian StyleFertaki, Stefani, Panagiota Giannoutsou, and Malvina G. Orkoula. 2023. "Combining Raman Microspectroscopy and X-ray Microcomputed Tomography for the Study of Bone Quality in Apolipoprotein-Deficient Animal Models" Molecules 28, no. 20: 7196. https://doi.org/10.3390/molecules28207196
APA StyleFertaki, S., Giannoutsou, P., & Orkoula, M. G. (2023). Combining Raman Microspectroscopy and X-ray Microcomputed Tomography for the Study of Bone Quality in Apolipoprotein-Deficient Animal Models. Molecules, 28(20), 7196. https://doi.org/10.3390/molecules28207196






