Abstract
For the first time, a novel dithiomaleimides (DTM) based tetra-antennary GalNAc conjugate was developed, which enable both efficient siRNA delivery and good traceability, without incorporating extra fluorophores. This conjugate can be readily constructed by three click-type reactions, that is, amidations, thiol-dibromomaleimide addition and copper catalyzed azide–alkyne cycloaddition (CuAAC). And it also has comparable siRNA delivery efficiency, with a GalNAc L96 standard to mTTR target. Additionally, due to the internal DTMs, a highly fluorescent emission was observed, which benefited delivery tracking and reduced the cost and side effects of the extra addition of hydrophobic dye molecules. In all, the simple incorporation of DTMs to the GalNAc conjugate structure has potential in gene therapy and tracking applications.
1. Introduction
Synthetic small interfering RNAs (siRNAs) can inhibit the expression of ill genes through gene silencing when mediated by the endogenous RNA interference (RNAi) approach [1,2]. siRNAs have many advantages over small molecular and monoclonal antibodies because of their function of complete Watson–Crick base pairing with mRNA, whereas small molecule and monoclonal antibodies need to recognize the complicated conformation of proteins. Therefore, many diseases that are not treatable by small molecule and monoclonal antibodies since some target molecules cannot be identified. In contrast, any gene can be targeted by siRNA because only the right sequence along the targeting mRNA needs to be selected. As a result, siRNA has a wider therapeutic area than small molecules or antibodies [3,4,5,6,7]. However, efficient and targeted delivery remains the bottleneck, due to the large molecular weight and negative charge [8,9,10]. Recently, N-acetylgalactosamine (GalNAc) conjugates were successfully and widely applied for liver-targeted siRNA delivery due to their excellent interaction with the asialoglycoprotein receptor (ASGPR, also known as Ashwell-Morell receptor) [11,12,13]. By conjugating GalNAc to nucleic acid molecules, drug payloads can be efficiently delivered into hepatocytes and elicit the corresponding biological response. This represents a potent and promising delivery strategy for nucleic acid-based therapeutics. As a trailblazer in this research field, Alnylam Pharmaceuticals has made significant contributions on GalNAc conjugate based delivery system [14,15,16,17,18,19,20,21,22,23].
A common issue for tracking the GalNAc delivery system should mandatorily incorporating fluorophore into the conjugate (Scheme 1A). As a result, it is often ambiguous regarding the location of the binding molecule and its mobility in the micellar host of the binding agent and also the payloads. Another problem arising from the conjugates aggregation could decrease the fluorescent emissions through either fluorophore-– interaction or fluorophore–fluorophore self-quenching events [24]. Furthermore, the incorporation of an emission handle, often a large hydrophobic dye molecule, such as fluorescein- and cyanine-type dyes, can lead to changes in the scaffold size and stability of the GalNAc conjugates [25]. Additionally, inducing extra fluorophore would increase the cost of the siRNA synthesis. Thus, new methods for the facile, economic and nondisruptive fluorophore labeling of GalNAc conjugates are required.
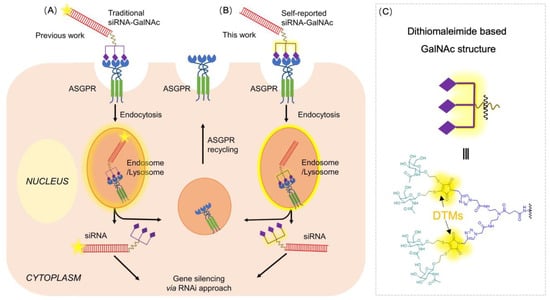
Scheme 1.
(A) General illustration of conventional GalNAc-decorated oligonucleotides (siRNA) delivery mechanism and tracking strategy, (B) The mechanism of our DTM based tetra-antennary GalNAc-siRNA conjugate without incorporating extra fluorophores, (C) The chemical structure of DTM based tetra-antennary GalNAc. For simplicity, only trivalent GalNAc conjugates are indicated.
One of the reasonable solutions to these issues is the addition of a fluorophore, which can be readily and non-ambiguous incorporated into the GalNAc scaffold during the synthesis. Dithiomaleimides (DTMs) as a versatile and high-emission fluorophores, have been applied in protein and polymer labeling [26,27,28,29,30]. It could be prepared by a rapid and efficient reaction of 2,3-dibromomaleimide with two thiol motifs [31,32,33,34]. However, DTMs were also reported to exhibit a strong fluorescence property which can potentially act as a trackable and self-reported functional handle [35,36].
Herein, for the first time, we present a novel DTM-based tetra-antennary GalNAc conjugate, which enables both efficient siRNA delivery and good traceability without incorporating extra fluorophores (Scheme 1B,C). This conjugate can be readily constructed by three click-type reactions, that is, amidations, thiol-dibromomaleimide addition and copper catalyzed azide–alkyne cycloaddition (CuAAC). And, it also has comparable siRNA delivery efficiency with GalNAc L96 standard to mTTR target [15]. Additionally, due to the internal DTMs, a highly fluorescent emission was observed, which benefited the delivery tracking and reduced the cost and side effects of the extra addition of hydrophobic dye molecules. In all, the simple incorporation of DTMs inthe GalNAc conjugate structure could seem trivial, but we propose this new system has potential in gene therapy and tracking applications.
2. Results and Discussion
2.1. DTM GalNAc Ligand Synthesis
The DTM containing intermediate 2Gal-DTM-alkyne was synthesis as Scheme 2. Firstly, GalNAc containing the thiol group (Gal-SH) was first synthesized through the deprotection of acetyl protected Gal-SAc. Gal-SH was then conjugated to N-propargyl-2,3-dibromomaleimide in the presence of NaOAc as the catalyst to yield 2Gal-DTM-alkyne. The overall yield of these two-step reactions was around 80% (Scheme S1 and Figures S1–S5).
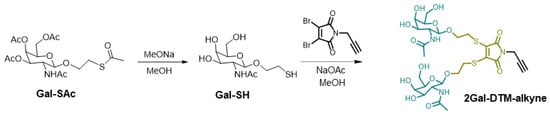
Scheme 2.
Synthesis of DTM-GalNAc ligand (2Gal-DTM-alkyne).
2.2. DTM-GalNAc-siRNA Conjugate Synthesis
As shown in Figure 1, the sense (oligo-NH2) and antisense (AS-oligomer) strands (Table 1) were synthesized, respectively on an ABI Synthesizer using commercially available 5′-O-(4,4′-dimethoxytrityl)-2′-deoxy-2′-fluoro-, 5′-O-(4,4′-dimethoxytrityl)-2′-O-(tert-butyldimethylsilyl)- and 5′-O-(4,4′-dimethoxytrityl)-2′-O-methyl-3′-O-(2-cyanoethyl-N,N-diisopropyl) phosphoramidite monomers of uridine and 4-N-acetylcytidine, 6-N-benzoyladenosine, and 2-N-isobutyrylguanosine using standard solid-phase oligonucleotide synthesis and deprotection protocols. Then, the sense strand (oligo-NH2) was successively coupled with two NHS linkers to equip two azido groups at the end (oligo-2N3) via efficient amidation reactions, in 82% and 87% of the yield, respectively. Oligo-2N3 was easily coupled with two 2Gal-DTM-alkyne via a CuAAc reaction to yield the tetra-antennary DTM-GalNAc-decorated sense strand in 75% of the yield (a detailed experimental methodology is listed in Section 3.2.2 in maintext). Moreover, the molecular structure and the purity of each oligomer was further validated by high performance liquid chromatography (HPLC) and electrospray ionization mass spectrometry (ESI-MS) (Figure 2A–H). Unimodal and symmetrical HPLC traces with high purity (90.9% to 96.0%), as well as single-peak signal, agree with the calculated value of the molecular mass, confirmed by the successful synthesis of the strands. Additionally, equimolar amounts of complementary sense and antisense strands were mixed and annealed by heating to 90 °C and slowly cooled to obtain the desired DTM-GalNAc-siRNA conjugate.
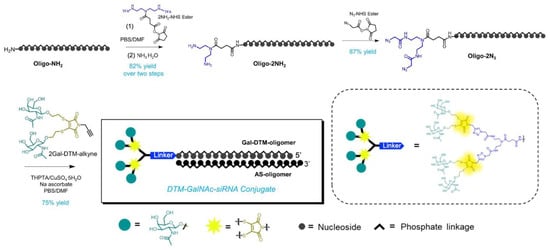
Figure 1.
Synthetic route of the DTM-GalNAc-siRNA conjugate.

Table 1.
siRNA used in this study.
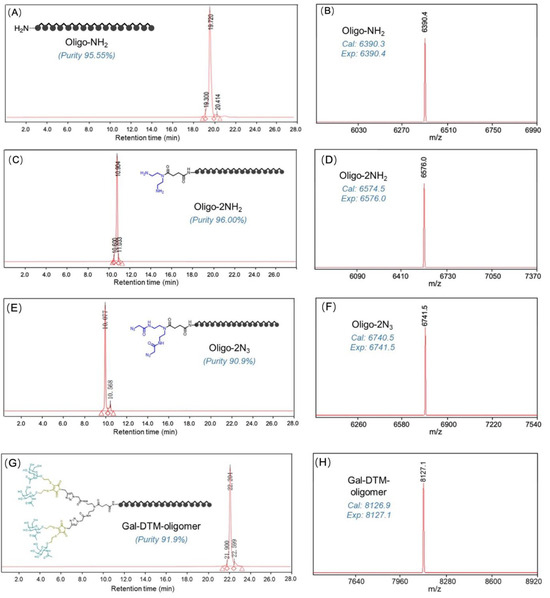
Figure 2.
Structure and purity characterization: HPLC traces (A,E,C,G) and mass spectra (B,D,F,H).
2.3. Cell Uptake of DTM-GalNAc-siRNA Conjugate
The uptake of the siRNA-GalNAc conjugates was evaluated in freshly isolated primary mouse hepatocytes [37]. After cells were incubated with 10 nM siRNA, a comparable robust uptake was observed with standard GalNAc-L96 and our DTM-GalNAc (Figure 3A, part 3 in Supplementary Information). We next evaluated the ability to silence gene expression using GalNAc-L96 and our DTM-GalNAc, designed to target the rodent transthyretin (TTR) gene. The results of the fluorescence quantitative PCR also confirmed the comparable TTR gene silencing ability, especially in high concentrations (Figure 3B). As shown in Figure 3C, the DTM-GalNAc showed an excitation wavelength of 467 nm and an emission wavelength of 515 nm, which is similar to the fluorescence property of GalNAc-L96 (FAM). Moreover, for confocal laser scanning microscopy (CLSM), DTM-GalNAc-treated hepatocytes exhibited stronger green fluorescence in the cytoplasm than that of the FAM-labeled antisense GalNAc-L96-siRNA conjugate-treated group after 2 h incubation, as shown in Figure 3D. Overall, both IC50 detection and CLSM results indicated the efficient cellular uptake behavior of the obtained DTM-GalNAc, implying its potential to be an effective carrier for both good siRNA delivery and excellent traceability. The endosomal escape mechanism of DTM-GalNAc-siRNA conjugate is the same as the GalNAc-L96-siRNA conjugate. DTM-GalNAc-siRNA binds to the ASGPR expressed by liver hepatocytes and is taken up in endosomes, where the DTM-GalNAc-siRNAs dissociate from the receptors. Then, the DTM-GalNAc sugars and branches are very quickly lysed from the siRNAs before the siRNAs escape to the cytoplasm.
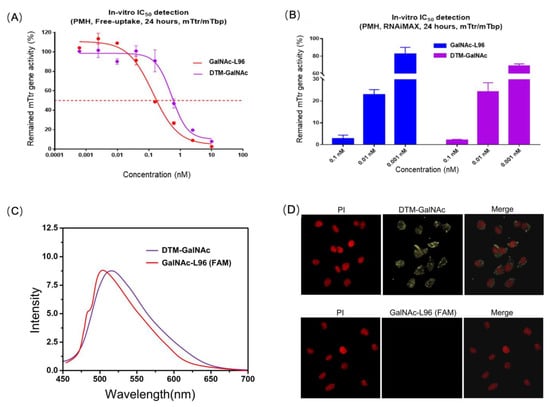
Figure 3.
(A) Uptake of conjugates into primary mouse hepatocytes. Freshly isolated hepatocytes from livers of wild-type mice were incubated with 10 nM siRNA. (B) TTR gene silencing 24 h after transfection, samples were collected, and RNA was extracted with cell lysate added with RNase inhibitor. Kits were then used to remove genomic DNA and reverse transcription. The obtained cDNA was diluted and detected by fluorescence quantitative PCR. (C) Fluorescence spectra of DTM-GalNAc (purple line) and GalNAc-L96 (FAM) (red line). (D) CLSM images of hepatocytes incubated with FAM labeled antisense GalNAc-L96-siRNA conjugate (yellow), and label free DTM-GalNAc-siRNA conjugate (yellow) at an oligonucleotide concentration of 2 μm at 37 °C for 2 h. Cell nuclei (red) were stained with PI. (Scale bar: 20 μm).
3. Materials and Methods
3.1. General Information
Unless stated otherwise, commercially available reagents were purchased from Sigma-Aldrich, Acros Organic, Alfa Aesar, TCI, Energy chemical, Adams and Sinopharm Chem. Dry tetrahydrofuran (THF), dichloromethane (DCM), toluene and N, N-dimethylformamide (DMF) were collected fresh from an Innovative Technology PS-MD-5 solvent purification system. All other dry solvents used were dried over 4 Å molecular sieves and stored under argon. Primary mouse liver cells were purchased from Hunan Fenghui Biotechnology Co., Ltd. (Changsha, China). RNAiMAX was purchased from Invitrogen. Opti-MEM and Paraformaldehyde was purchased from Sigma-Aldrich (Shanghai, China). PBS was purchased from Shanghai Life-iLab Co., Ltd. (Shanghai, China).
All 1H NMR, 13C NMR spectra were collected using a Bruker nuclear magnetic resonance instrument (300 MHz) using tetramethylsilane (TMS) as the internal standard at room temperature. The 1H NMR spectra were referenced to 7.26 ppm in CDCl3. Electrospray Ionization Mass spectrometry (ESI-MS) of chemical was performed by electrospray ionization on a 70-VSE. Ultraviolet-Visible Absorption (UV-Vis) of the samples were measured using a Thermo Electron-EV300 UV-Vis spectrophotometer at room temperature. The slit-width was set at 1 nm with a scan speed of 480 nm/min.
3.2. Synthesis of DTS Containing GalNAc Conjugate
3.2.1. Synthesis of the DTS Containing GalNAc Scaffold
- Synthesis of compound 2: In an oven dried round-bottom flask, compound 1 (197 mg, 0.51 mmol), 2-bromoethanol (0.11 mL, 1.54 mmol) and Sc(OTf)3 (37 mg, 0.075 mmol as catalyst) were disolved in dry DCM (3 mL). After 27 h, TLC indicated the reaction was complete. The reaction mixture concentrated and directly purified by column chromatography (1:2 PE/EtOAc) to give compound 2 (153 mg, 67 %) as white crystals [38]. 1H NMR (300 MHz, CDCl3) δ 5.95 (d, J = 8.6 Hz, 1H), 5.52–5.12 (m, 2H), 4.77 (d, J = 8.4 Hz, 1H), 4.21–4.06 (m, 3H), 4.00–3.89 (m, 2H), 3.86–3.75 (m, 1H), 3.46 (dd, J = 9.1, 3.7 Hz, 2H), 2.12 (s, 3H), 2.02 (s, 3H), 1.97 (s, 3H), 1.96 (s, 3H).
- Synthesis of compound 3: In a 10 mL round-bottom flask with a condenser, compound 2 (454.3 mg, 1.0 mmol) was dissolved in 5.0 mL dry acetone. Then, K2CO3 (196.5 mg 1.72 mmol) was added. The mixture was heated to 45 °C for 24 h. After cooling to 25 °C, the mixture was filtered. The filter cake was washed with 3 × 10 mL acetone and the combined filtrate was concentrated. The residue was re-dissolved in 15 mL CHCl3, and this solution was washed with 3 × 10 mL water. The organic phase was dried with anhydrous Na2SO4, and concentrated under a vacuum to afford the crude product as a deep yellow oil, which was purified by flash column on neutral alumina. Eluting with a mixed solvent of PE/EA (v/v = 4/1 to 1/1) to afford compound 3 (359.6 mg, yield 84%) as a colorless solid [39]. 1H NMR (300 MHz, CDCl3) δ 5.71 (d, J = 8.6 Hz, 1H), 5.47–5.18 (m, 2H), 4.73 (d, J = 8.4 Hz, 1H), 4.22–4.08 (m, 2H), 3.97 (dq, J = 9.2, 6.4 Hz, 3H), 3.65 (dt, J = 10.5, 6.7 Hz, 1H), 3.10 (ddd, J = 20.7, 13.8, 7.4 Hz, 2H), 2.35 (s, 3H), 2.15 (s, 3H), 2.05 (s, 3H), 2.01 (s, 3H), 1.99 (s, 3H).
- Synthesis of compound 4: NaOMe (324.1 mg, 6.0 mmol) was added to a solution of compound 3 (449.5 mg, 1.0 mmol) in CH3OH (10 mL) and stirred at RT for 3 h under a nitrogen atmosphere. The mixture was then neutralized using Dowex resin and filtered through a pad of Celite. The collected filtrate was concentrated under reduced pressure and compound 4 (230.7 mg, yield 82 %) was obtained as a foaming oil, which was used without further purification and stored under −20 °C in a nitrogen atmosphere.
- Synthesis of compound 6: 2,3-dibromomaleimide (5) (254.9 mg, 1.0 mmol) and propargyl bromide (297.4 mg, 2.5 mmol,) were dissolved in acetone (15 mL), followed by the addition of potassium carbonate (552.8 mg, 4.0 mmol). The mixture was stirred at 25 °C for 48 h. The precipitate was filtrated off and the filtrate was concentrated to afford the crude product, which was purified by the silica gel column chromatography using hexane/ethyl acetate (1/1, v/v) as the eluent to obtain the desire product (158.2 mg, yield 54.0%) [34]. 1H NMR (300 MHz, CDCl3) δ 4.38 (d, J = 2.5 Hz, 2H), 2.27 (s, 1H).
- Synthesis of compound 7: In a 25 mL round-bottom flask under argon, compound 6 (290.9 mg, 1.0 mmol) was dissolved in MeOH (4.0 mL). Then, NaOAc (172.3 mg, 2.1 mmol) was added to the mixture. Then, a solution of compound 4 (618.9 mg, 2.2 mmol) in MeOH (2 mL) under argon was added dropwise over 5 min, to give an orange solution. After that, the mixture was stirred at 20 °C for 3 h. Then, quenched with 10 mL H2O and extracted with EtOAc (2 × 20 mL). The combined organic layer was dried (MgSO4), filtered and concentrated under a vacuum to yield a yellow solid which was used without any purification [40].
3.2.2. Synthesis of DTS Containing GalNAc Conjugated and Unconjugated siRNAs
Sense and antisense strands (Table 1) were synthesized on an ABI394 Synthesizer (Applied Biosystems, Foster City, CA, USA) using commercially available 5′-O-(4,4′-dimethoxytrityl)-2′-deoxy-2′-fluoro- and 5′-O-(4,4′-dimethoxytrityl)-2′-O-methyl- 3′-O-(2-cyanoethyl-N,N-diisopropyl) phosphoramidite monomers of uridine, 4-N-acetylcytidine, 6-N-benzoyladenosine, and 2-N-isobutyrylguanosine (Hongene Biotech Corporation, Shanghai, China) using standard solid-phase oligonucleotide synthesis and deprotection protocols. After synthesis, the support was treated with 25–28% aqueous ammonia at 55 °C for 16 h. The suspension was filtered through a 0.22 μm filter to remove solid residues. The oligonucleotides were purified by an anion-exchange high-performance liquid chromatography (IEX-HPLC) with Source 15Q (Cytiva, Shrewsbury, MA, USA), using a linear gradient of 15–65% buffer B over 50 min with a 15 mL/min flow rate. Buffer A was 0.02 M Na2HPO4 in 10% CH3CN (pH 8.5) and buffer B was buffer A plus 1 M NaCl. The pure fractions were combined, concentrated and desalted on a Sartorius ultrafiltration station. The integrities of the purified oligonucleotides were confirmed by LC-MS (Table S1), and by analytical IEX HPLC. Equimolar amounts of complementary sense and antisense strands were mixed and annealed by heating to 90 °C and slowly cooling to obtain the desired siRNAs (Table 1).
According to Figure 1, Oligo-2NH2 was synthesized by oligo-NH2 reacting with 2NH2-NHS Ester (10-fold excess over oligo-NH2) in the mixture of DMSO and phosphate-buffered saline (PBS) (3:2, v/v) at 25 °C overnight. The mixture was filtered through a 0.22 μm filter and purified by RP-HPLC with Waters X-Bridge C18 column (5 μm, 10 mm × 250 mm) using a linear gradient of 5–45% buffer B over 36 min with a 5 mL/min flow rate (Buffer A: 0.1 M TEAA (pH 7.0), Buffer B: ACN). The Oligo-2NH2 was characterized by RP-HPLC and electrospray ionization mass spectrometry (ESI-MS), (Figure 2).
Oligo-2N3 was synthesized by oligo-2NH2 reacting with the N3-NHS Ester (50-fold excess over oligo-NH2) in the mixture of DMSO and phosphate-buffered saline (PBS) (1:3, v/v) at 4 °C overnight. The mixture was filtered through a 0.22 μm filter and purified by RP-HPLC with Waters X-Bridge C18 column (5 μm, 10 mm × 250 mm) using a linear gradient of 5–60% buffer B over 40 min with 5 mL/min flow rate (Buffer A: 0.1 M TEAA (pH 7.0), Buffer B: ACN). Oligo-2N3 was characterized by RP-HPLC and electrospray ionization mass spectrometry (ESI-MS) (Figure 2).
DTM-GalNAc-siRNA was synthesized by Oligo-2N3 reacting with 2Gal-DTM-alkyne (12-fold excess over oligo-2N3) in the presence of an excess of CuSO4·5H2O (12-fold excess over oligo-2N3), THPTA (30-fold excess over oligo-2N3) and sodium ascorbate (50-fold excess over oligo-2N3) in a PBS/DMF (1:1, v/v) solvent mixture at room temperature for 1 h. The mixture was filtered through a 0.22 μm filter and purified by RP-HPLC with Waters X-Bridge C18 column (5 μm, 10 mm × 250 mm) using a linear gradient of 5–60% buffer B over 55 min with 5 mL/min flow rate (Buffer A: 0.1 M TEAA (pH 7.0), Buffer B: ACN). DTM-GalNAc-siRNA was characterized by RP-HPLC and electrospray ionization mass spectrometry (ESI-MS) (Figure 2). Equimolar amounts of complementary sense and antisense strands were mixed and annealed by heating it to 90 °C and slowly cooling it to obtain the desired siRNAs (Table 1).
4. Conclusions
In summary, we have developed a novel DTM-based tetra-antennary GalNAc conjugate, which could be readily fabricated by three efficient click-type reactions, that is, amidations, thiol-dibromomaleimide addition and CuAAC. With this DTM GalNAc scaffold, a siRNA delivery efficiency comparable with the GalNAc L96 standard to mTTR target was achieved. Additionally, due to the internal DTMs, a highly fluorescent emission was observed, which would benefit the delivery tracking and avoid the incorporation of extra hydrophobic fluorophores. The insertion of DTMs into GalNAc conjugate might seem trivial, but this two-birds-with-one-stone strategy has potential applications in gene and drug delivery in medical therapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28207184/s1. Scheme S1: Synthetic route of compound 7 (2Gal-DTM-alkyne); Figure S1: 1H NMR spectrum of compound 2; Figure S2: 1H NMR spectrum of compound 3; Figure S3: 1H NMR spectrum of compound 4; Figure S4: 1H NMR spectrum of compound 6; Figure S5: 1H NMR spectrum of compound 7.
Author Contributions
Conceptualization, S.K., L.Q. and M.X.; methodology, S.K., X.G. and Q.W.; analysis, S.K., X.G. and Q.W.; data curation, S.K. and J.L.; writing, S.K., L.Q. and M.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Major Scientific Research Project of Jiangsu Commission of Health (ZD2022036) and the Science Technology and Development Project of Wuxi (Y20212013).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data are available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Bumcrot, D.; Manoharan, M.; Koteliansky, V.; Sah, D.W.Y. RNAi therapeutics: A potential new class of pharmaceutical drugs. Nat. Chem. Biol. 2006, 2, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Deleavey, G.F.; Damha, M.J. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 2012, 19, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.Y.; Ayyar, V.S.; Mitra, A. Pharmacokinetic and Pharmacodynamic Modeling of siRNA Therapeutics—A Minireview. Pharm. Res. 2022, 39, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhao, J.; Shah, M.; Migliorati, J.M.; Tawfik, S.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X.B. Nedosiran, a Candidate siRNA Drug for the Treatment of Primary Hyperoxaluria: Design, Development, and Clinical Studies. ACS Pharmacol. Transl. Sci. 2022, 5, 1007–1016. [Google Scholar] [CrossRef]
- Narasipura, E.A.; VanKeulen-Miller, R.; Ma, Y.; Fenton, O.S. Ongoing Clinical Trials of Nonviral siRNA Therapeutics. Bioconjugate Chem. 2023, 34, 1177–1197. [Google Scholar] [CrossRef]
- Ahn, I.; Kang, C.S.; Han, J. Where should siRNAs go: Applicable organs for siRNA drugs. Exp. Mol. Med. 2023, 55, 1283–1292. [Google Scholar] [CrossRef]
- Ranjbar, S.; Zhong, X.B.; Manautou, J.; Lu, X. A holistic analysis of the intrinsic and delivery-mediated toxicity of siRNA therapeutics. Adv. Drug. Deliv. Rev. 2023, 201, 115052. [Google Scholar] [CrossRef]
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013, 21, 1570–1578. [Google Scholar] [CrossRef]
- Coelho, T.; Adams, D.; Silva, A.; Lozeron, P.; Hawkins, P.N.; Mant, T.; Perez, J.; Chiesa, J.; Warrington, S.; Tranter, E.; et al. Safety and Efficacy of RNAi Therapy for Transthyretin Amyloidosis. N. Engl. J. Med. 2013, 369, 819–829. [Google Scholar] [CrossRef]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.-H.J.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef]
- Spiess, M. The asialoglycoprotein receptor: A model for endocytic transport receptors. Biochemistry 1990, 29, 10009–10018. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.L.; Rup, D.; Lodish, H.F. Difficulties in the quantification of asialoglycoprotein receptors on the rat hepatocyte. J. Biol. Chem. 1980, 255, 9033–9036. [Google Scholar] [CrossRef] [PubMed]
- Stockert, R.J. The asialoglycoprotein receptor: Relationships between structure, function, and expression. Physiol. Rev. 1995, 75, 591–609. [Google Scholar] [CrossRef] [PubMed]
- Zlatev, I.; Castoreno, A.; Brown, C.R.; Qin, J.; Waldron, S.; Schlegel, M.K.; Degaonkar, R.; Shulga-Morskaya, S.; Xu, H.; Gupta, S.; et al. Reversal of siRNA-mediated gene silencing in vivo. Nat. Biotech. 2018, 36, 509–511. [Google Scholar] [CrossRef]
- Nair, J.K.; Willoughby, J.L.; Chan, A.; Charisse, K.; Alam, M.R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kel’in, A.V.; Milstein, S.; et al. Multivalent N-Acetylgalactosamine-Conjugated siRNA Localizes in Hepatocytes and Elicits Robust RNAi-Mediated Gene Silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. [Google Scholar] [CrossRef]
- Parmar, R.; Willoughby, J.L.; Liu, J.; Foster, D.J.; Brigham, B.; Theile, C.S.; Charisse, K.; Akinc, A.; Guidry, E.; Pei, Y.; et al. 5′-(E)-Vinylphosphonate: A Stable Phosphate Mimic Can Improve the RNAi Activity of siRNA–GalNAc Conjugates. Chembiochem 2016, 17, 985–989. [Google Scholar] [CrossRef]
- Willoughby, J.L.; Chan, A.; Sehgal, A.; Butler, J.S.; Nair, J.K.; Racie, T.; Shulga-Morskaya, S.; Nguyen, T.; Qian, K.; Yucius, K.; et al. Evaluation of GalNAc-siRNA Conjugate Activity in Pre-clinical Animal Models with Reduced Asialoglycoprotein Receptor Expression. Mol. Therapy 2018, 26, 105–114. [Google Scholar] [CrossRef]
- Zimmermann, T.S.; Karsten, V.; Chan, A.; Chiesa, J.; Boyce, M.; Bettencourt, B.R.; Hutabarat, R.; Nochur, S.; Vaishnaw, A.; Gollob, J. Clinical Proof of Concept for a Novel Hepatocyte-Targeting GalNAc-siRNA Conjugate. Mol. Therapy 2017, 25, 71–78. [Google Scholar] [CrossRef]
- Matsuda, S.; Keiser, K.; Nair, J.K.; Charisse, K.; Manoharan, R.M.; Kretschmer, P.; Peng, C.G.; VKel’in, A.; Kandasamy, P.; Willoughby, J.L.; et al. siRNA Conjugates Carrying Sequentially Assembled Trivalent N-Acetylgalactosamine Linked Through Nucleosides Elicit Robust Gene Silencing In Vivo in Hepatocytes. ACS Chem. Bio 2015, 10, 1181–1187. [Google Scholar] [CrossRef]
- Schlegel, M.K.; Foster, D.J.; Kel’in, A.V.; Zlatev, I.; Bisbe, A.; Jayaraman, M.; Lackey, J.G.; Rajeev, K.G.; Charissé, K.; Harp, J.; et al. Chirality Dependent Potency Enhancement and Structural Impact of Glycol Nucleic Acid Modification on siRNA. J. Am. Chem. Soc. 2017, 139, 8537–8546. [Google Scholar] [CrossRef]
- Janas, M.M.; Schlegel, M.K.; Harbison, C.E.; Yilmaz, V.O.; Jiang, Y.; Parmar, R.; Zlatev, I.; Castoreno, A.; Xu, H.; Shulga-Morskaya, S.; et al. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat. Commun. 2018, 9, 723. [Google Scholar] [CrossRef]
- Holland, R.J.; Lam, K.; Ye, X.; Martin, A.D.; Wood, M.C.; Palmer, L.; Fraser, D.; McClintock, K.; Majeski, S.; Jarosz, A.; et al. Ligand conjugate SAR and enhanced delivery in NHP. Mol. Therapy 2021, 29, 2910–2919. [Google Scholar] [CrossRef]
- Kumar, P.; Degaonkar, R.; Guenther, D.C.; Abramov, M.; Schepers, G.; Capobianco, M.; Jiang, Y.; Harp, J.; Kaittanis, C.; Janas, M.M.; et al. Chimeric siRNAs with chemically modified pentofuranose and hexopyranose nucleotides: Altritol-nucleotide (ANA) containing GalNAc–siRNA conjugates: In vitro and in vivo RNAi activity and resistance to 5′-exonuclease. Nucleic Acids Res. 2020, 48, 4028–4040. [Google Scholar] [CrossRef] [PubMed]
- Anthony, O.; Zana, R. Interactions between Water-Soluble Polymers and Surfactants: Effect of the Polymer Hydrophobicity. 1. Hydrophilic Polyelectrolytes. Langmuir 1996, 12, 1967–1975. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Robin, M.P.; Jones, M.W.; Haddleton, D.M.; O’Reilly, R.K. Dibromomaleimide End Functional Polymers by RAFT Polymerization Without the Need of Protecting Groups. ACS Macro Lett. 2012, 1, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, F.F.; Nobles, M.; Ryan, C.P.; Smith, M.E.; Tinker, A.; Caddick, S.; Baker, J.R. In Situ Maleimide Bridging of Disulfides and a New Approach to Protein PEGylation. Bioconjugate Chem. 2011, 22, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.E.; Chudasama, V.; Moody, P.; Smith, M.E.; Caddick, S. A novel synthetic chemistry approach to linkage-specific ubiquitin conjugation. Org. Biomol. Chem. 2015, 13, 4165–4168. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, W.; Liu, X.; Wang, G.; Wang, Y.; Li, D.; Xie, L.; Gao, Y.; Deng, H.; Gao, W. Site-selective in situ growth of fluorescent polymer–antibody conjugates with enhanced antigen detection by signal amplification. Biomaterials 2015, 64, 2–9. [Google Scholar] [CrossRef]
- Li, Z.L.; Sun, L.; Ma, J.; Zeng, Z.; Jiang, H. Synthesis and post-polymerization modification of polynorbornene bearing dibromomaleimide side groups. Polymer 2016, 84, 336–342. [Google Scholar] [CrossRef]
- Robin, M.P.; Wilson, P.; Mabire, A.B.; Kiviaho, J.K.; Raymond, J.E.; Haddleton, D.M.; O’Reilly, R.K. Conjugation-Induced Fluorescent Labeling of Proteins and Polymers Using Dithiomaleimides. J. Am. Chem. Soc. 2013, 135, 2875–2878. [Google Scholar] [CrossRef] [PubMed]
- Robin, M.P.; Mabire, A.B.; Damborsky, J.C.; Thom, E.S.; Winzer-Serhan, U.H.; Raymond, J.E.; O’Reilly, R.K. New Functional Handle for Use as a Self-Reporting Contrast and Delivery Agent in Nanomedicine. J. Am. Chem. Soc. 2013, 135, 9518–9524. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wilson, P.; Kempe, K.; Chen, H.; Haddleton, D.M. Reversible Regulation of Thermoresponsive Property of Dithiomaleimide-Containing Copolymers via Sequential Thiol Exchange Reactions. ACS Macro Lett. 2016, 5, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, M.; Xiong, M.; Cheng, J. Reduction-responsive dithiomaleimide-based nanomedicine with high drug loading and FRET-indicated drug release. Chem. Commun. 2015, 51, 4807–4810. [Google Scholar] [CrossRef] [PubMed]
- Sikder, A.; Xie, Y.; Thomas, M.; Derry, M.J.; O’Reilly, R.K. Precise control over supramolecular nanostructures via manipulation of H-bonding in π-amphiphiles. Nanoscale 2021, 13, 20111–20118. [Google Scholar] [CrossRef]
- Tan, M.; Wang, X.; Xie, T.; Zhang, Z.; Shi, Y.; Li, Y.; Chen, Y. Fluorogenic Mechanophore Based on Dithiomaleimide with Dual Responsiveness. Macromolecules 2022, 55, 6860–6865. [Google Scholar] [CrossRef]
- Ware, B.R.; Brown, G.E.; Soldatow, V.Y.; LeCluyse, E.L.; Khetani, S.R. Long-Term Engineered Cultures of Primary Mouse Hepatocytes for Strain and Species Comparison Studies During Drug Development. J. Liver Res. 2019, 19, 199–214. [Google Scholar] [CrossRef]
- Christensen, H.; Christiansen, M.S.; Petersen, J.; Jensen, H.H. Direct formation of beta-glycosides of N-acetyl glycosamines mediated by rare earth metal triflates. Org. Biomol. Chem. 2008, 6, 3276–3283. [Google Scholar] [CrossRef]
- Gorityala, B.K.; Lu, Z.; Leow, M.L.; Ma, J.; Liu, X.W. Design of a “turn-off/turn-on” biosensor: Understanding carbohydrate-lectin interactions for use in noncovalent drug delivery. J. Am. Chem. Soc. 2012, 134, 15229–15232. [Google Scholar] [CrossRef]
- Schumacher, F.F.; Nunes, J.P.; Maruani, A.; Chudasama, V.; Smith, M.E.; Chester, K.A.; Baker, J.R.; Caddick, S. Next generation maleimides enable the controlled assembly of antibody-drug conjugates via native disulfide bond bridging. Org. Biomol. Chem. 2014, 12, 7261–7269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
