Chemical Composition, Functional and Anticancer Properties of Carrot
Abstract
:1. Introduction
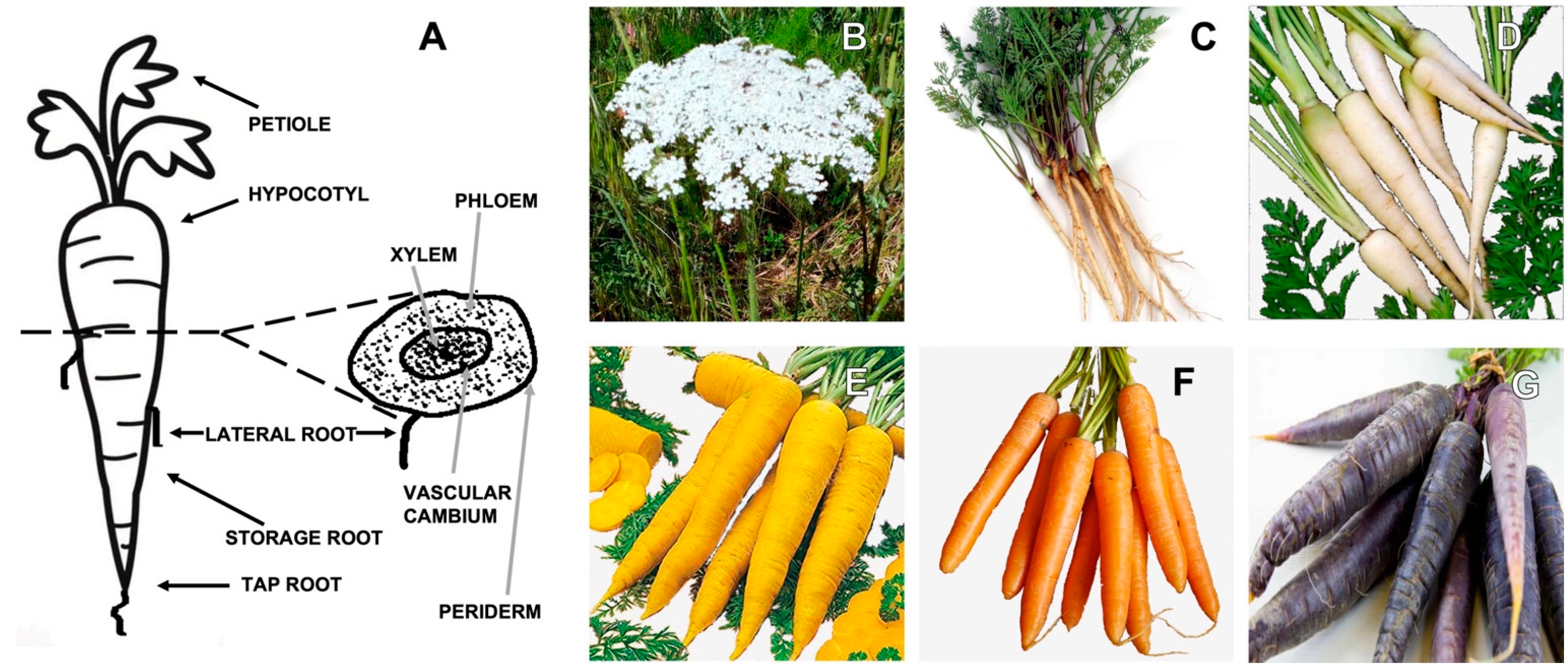
Daucus carota L. Chemical Composition
2. Daucus carota L. Bioactive Components with Health-Promoting Function or Phytochemicals
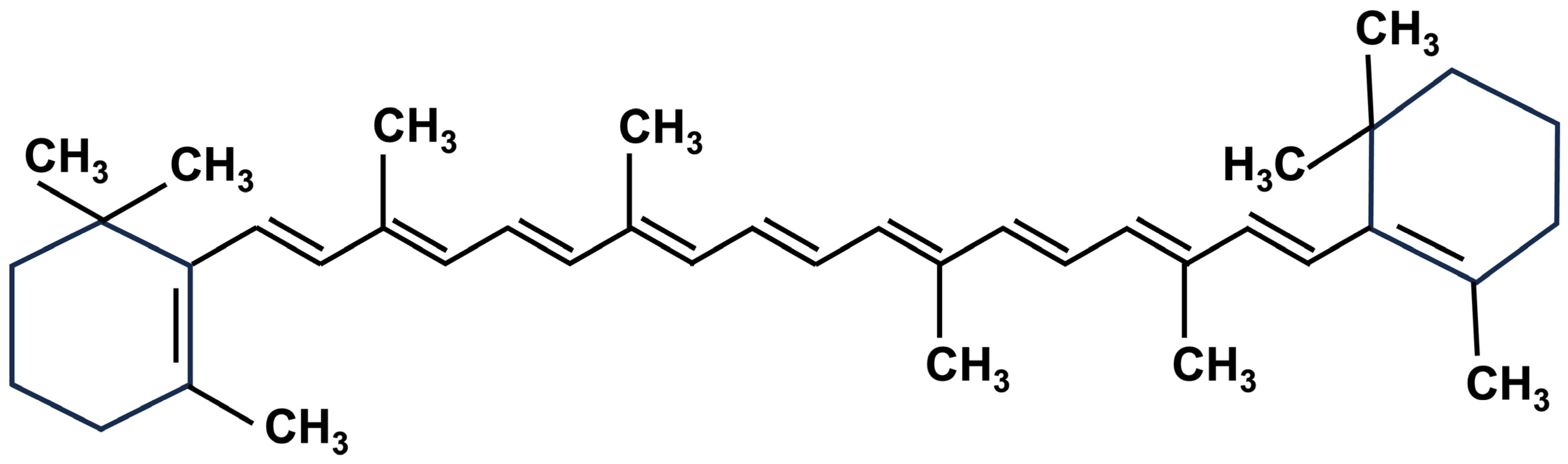
2.1. Carotenoids
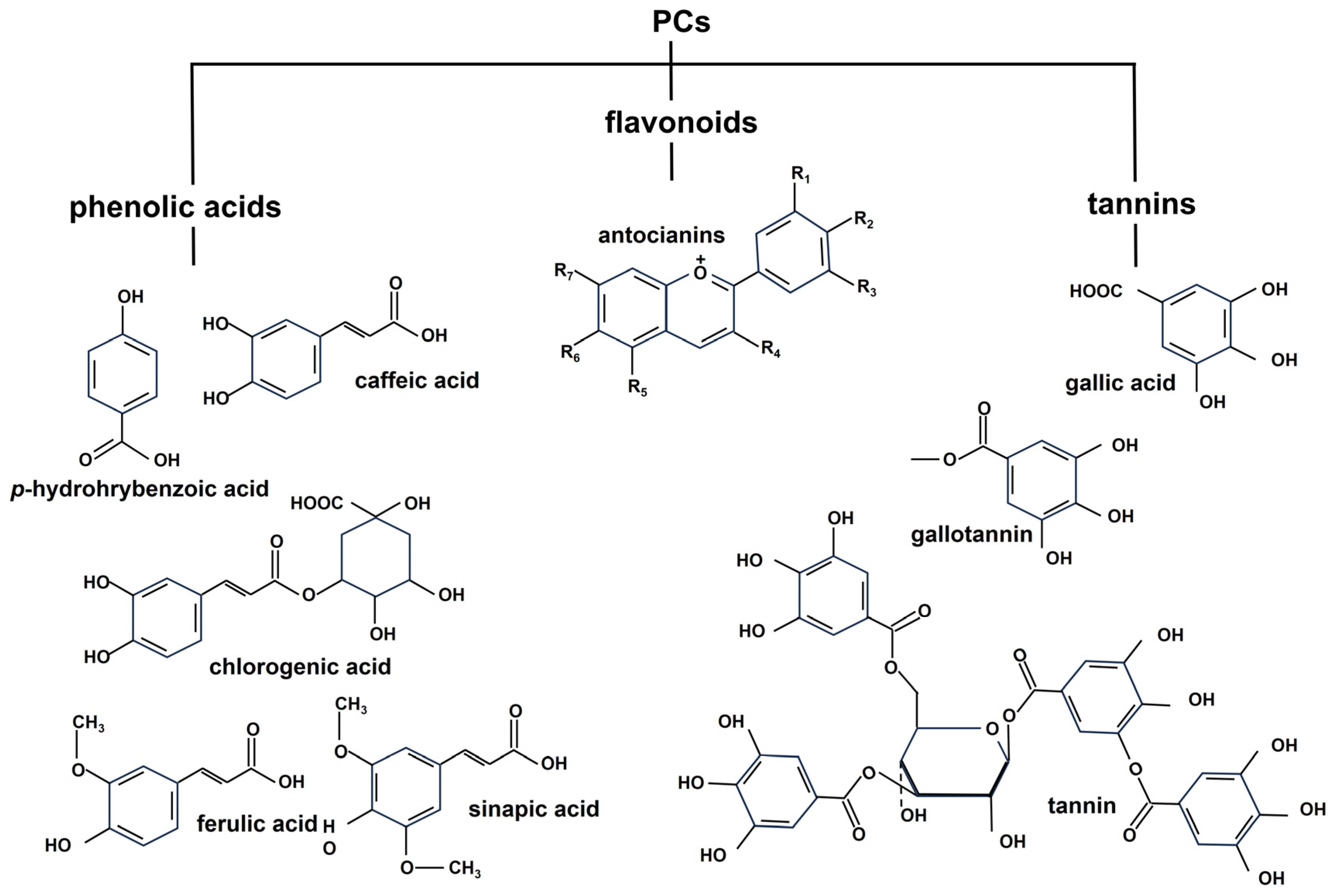
2.2. Phenolics
2.3. Vitamins
2.4. Fatty Acids and Their Derivatives
3. Carrot Compounds and Their Role in Cancer
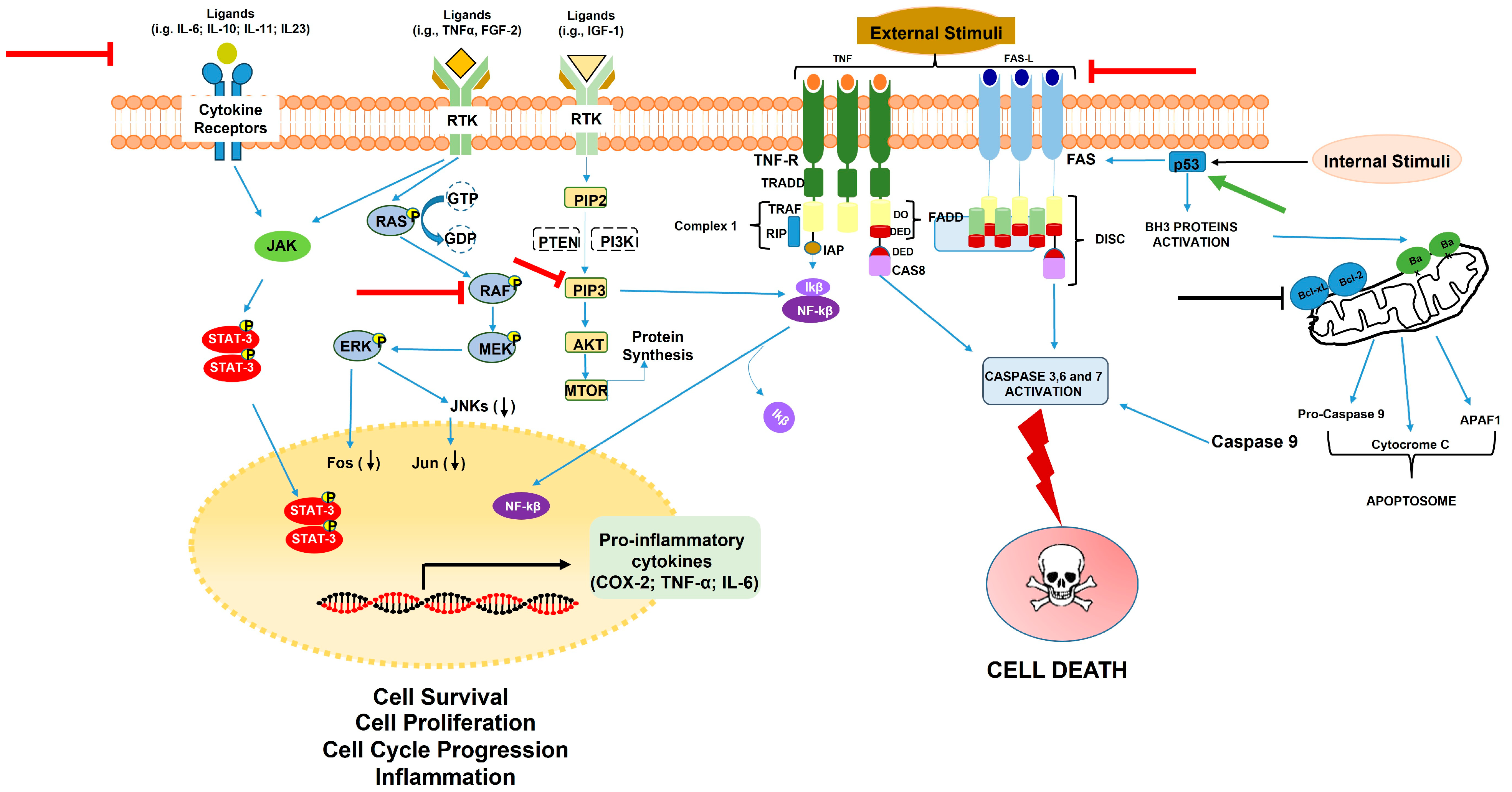
3.1. Carrot Compounds and Their Role in Cell Survival, Proliferation, Apoptosis, and Inflammation
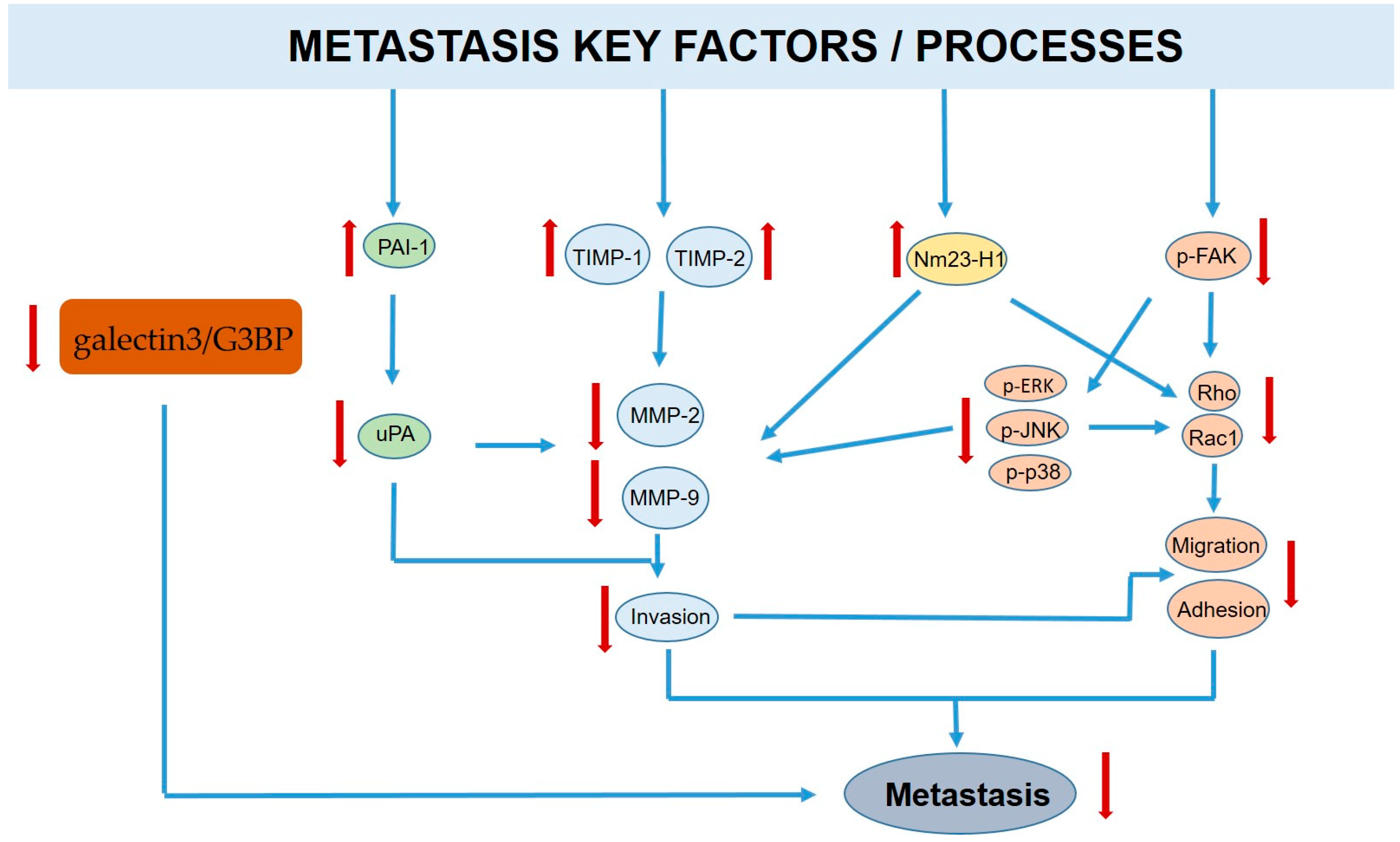
3.2. Carrot Compounds and Their Anti-Metastatic Role
3.3. Carrot Compounds and Their Role in Multidrug Resistance, MDR
3.4. Carrot Compounds and Their Role as Lipid Metabolism Modulators in Cancer
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nguyen, H.H.V. Handbook of Vegetables Preservation Processing, 2nd ed.; Routledge: Abington, UK, 2015. [Google Scholar]
- Heywood, V.H. Relationships and evolution in the Daucus carota complex. Isr. J. Plant Sci. 1983, 32, 51–65. [Google Scholar]
- Stolarczyk, J.; Janick, J. Carrot: History and iconography. Chron. Hortic. 2011, 51, 2. [Google Scholar]
- Que, F.; Hou, X.L.; Wang, G.L.; Xu, Z.-S.; Tan, G.-F.; Li, T.; Wang, Y.-H.; Khadr, A.; Xiong, A.-S. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Sadler, M.J.; Gibson, S.; Whelan, K.; Ha, M.A.; Lovegrove, J.; Higgs, J. Dried fruit and public health—What does the evidence tell us? Int. J. Food Sci. Nutr. 2019, 70, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Surbhi, S.; Verma, R.C.; Deepak, R.; Jain, H.K.; Yadav, K.K. A review: Food, chemical composition and utilization of carrot (Daucus carota L.) pomace. Int. J. Chem. Stud. 2018, 6, 2921–2926. [Google Scholar]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef]
- Yusuf, E.; Tkacz, K.; Turkiewicz, I.P.; Wojdyło, A.; Nowicka, P. Analysis of chemical compounds’ content in different varieties of carrots, including qualification and quantification of sugars, organic acids, minerals, and bioactive compounds by UPLC. Eur. Food Res. Technol. 2021, 247, 3053–3062. [Google Scholar] [CrossRef]
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive C17-Polyacetylenes in Carrots (Daucus carota L.): Current Knowledge and Future Perspectives. J. Agric. Food Chem. 2015, 63, 9211–9222. [Google Scholar] [CrossRef]
- Szczepanek, M.; Wilczewski, E.; Pobereżny, J.; Wszelaczyńska, E.; Keutgen, A.; Ochmian, I. Effect of biostimulants and storage on the content of macroelements in storage roots of carrot. J. Elem. 2015, 20, 1021–1031. [Google Scholar] [CrossRef]
- MedlinePlus. Definitions of Health Terms: Minerals. 2019. Available online: medlineplus.gov/definitions/mineralsdefinitions.Html (accessed on 2 March 2022).
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of volatiles, phenolics, sugars, antioxidant vitamins, and sensory quality of different colored carrot varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef]
- Sistrunk, W.A.; Bradley, G.A.; Smittle, D. Influence of preharvest factors on carbohydrates in carrots. Proc. Am. Soc. Hortic. Sci. 1967, 90, 239–251. [Google Scholar]
- Miedzobrodzka, A.; Ciesllk, E.; Sikora, E. Changes in the content of nitrate and nitrites in carrot roots during storage in the clamp. Rocz. Panstw. Zakl. Hig. 1992, 43, 33–36. [Google Scholar]
- Luby, C.H.; Maeda, H.A.; Goldman, I.L. Genetic and phenological variation of tocochromanol (Vitamin E) content in wild (Daucus carota L. var. carota) and domesticated carrot (D. carota L. var. sativa). Hortic. Res. 2014, 1, 15. [Google Scholar] [CrossRef]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Ergun, M.; Susluoğlu, Z. Evaluating carrot as a functional food. Middle East. J. Sci. 2018, 4, 113–119. [Google Scholar] [CrossRef]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Arino, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and their health benefits—Review article. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Tvrzicka, E.; Kremmyda, L.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. 2011, 155, 117–130. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Varzakas, T. A Review of the Structure, Biosynthesis, Absorption of Carotenoids-Analysis and Properties of their Common Natural Extracts. Curr. Res. Nutr. Food Sci. J. 2016, 4, 25–37. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Zhai, K.; Abotaleb, M.; Ashrafizadeh, M.; Brockmueller, A.; Shakibaei, M.; Biringer, K.; Bugos, O.; et al. Carotenoids in Cancer Metastasis—Status Quo and Outlook. Biomolecules 2020, 10, 1653. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.; Hartmann, L.; Dubois-Laurent, C.; Welsch, R.; Huet, S.; Hamama, L.; Briard, M.; Peltier, D.; Gagné, S.; Geoffriau, E. Carotenoid gene expression explains the difference of carotenoid accumulation in carrot root tissues. Planta 2017, 245, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, C.; Simon, G.; Rock, E.; Amouroux, P.; Remesy, C. Genetic Variability Influences Carotenoid, Vitamin, Phenolic, and Mineral Content in White, Yellow, Purple, Orange, and Dark-Orange Carrot Cultivars. J. Am. Soc. Hortic. Sci. 2004, 129, 523–529. [Google Scholar] [CrossRef]
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The Content of Phenolic Compounds and Radical Scavenging Activity Varies with Carrot Origin and Root Color. Plant Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef]
- Kidmose, U.; Hansen, S.L.; Christensen, L.P.; Edelenbos, M.; Larsen, E.; Nørbaek, R. Effects of Genotype, Root Size, Storage, and Processing on Bioactive Compounds in Organically Grown Carrots (Daucus carota L.). J. Food Sci. 2004, 69, S388–S394. [Google Scholar] [CrossRef]
- Seljasen, R.; Bengtsson, G.B.; Hoftun, H.; Vogt, G. Sensory and chemical changes in five varieties of carrot (Daucus carota L.) in response to mechanical stress at harvest and post-harvest. J. Sci. Food Agric. 2001, 81, 436–447. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S.; Daglia, M.; Rengasamy, K.R. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef]
- Jahns, L.; Conrad, Z.; Johnson, L.K.; Whigham, L.D.; Wu, D.; Claycombe-Larson, K.J. A diet high in carotenoid-rich vegetables and fruits favorably impacts inflammation status by increasing plasma concentrations of IFN-α2 and decreasing MIP-1β and TNF-α in healthy individuals during a controlled feeding trial. Nutr. Res. 2018, 52, 98–104. [Google Scholar] [CrossRef]
- Rowles, J.L., 3rd; Erdman, J.W., Jr. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar] [CrossRef]
- Toydemir, G.; Gultekin Subasi, B.; Hall, R.D.; Beekwilder, J.; Boyacioglu, D.; Capanoglu, E. Effect of Food Processing on Antioxidants, Their Bioavailability and Potential Relevance to Human Health. Food Chem. X 2022, 14, 100334. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, H.; Wang, Y.; Schneider, J.A.; Willett, W.C.; Morris, M.C. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: A community-based cohort of older adults. Am. J. Clin. Nutr. 2021, 113, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.J.; Tomas, M.; et al. Functional implications of bound phenolic compounds and phenolics–food interaction: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, J.; Jin, P.; Li, X.; Wang, L.; Zheng, Y. The Effect of Temperature on Phenolic Content in Wounded Carrots. Food Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Babic, I.; Amiot, M.J.; Nguyen-The, A.C. Accumulation of chlorogenic acids in shredded carrots during storage in oriented polypropylene films. J. Food Sci. 1993, 58, 840–841. [Google Scholar] [CrossRef]
- Lorenc-Kukuła, K.; Jafra, S.; Oszmiański, J.; Szopa, J. Ectopic expression of anthocyanin 5-o-glucosyltransferase in potato tuber causes increased resistance to bacteria. J. Agric. Food Chem. 2005, 53, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition and Antioxidant Potential of Grain Legume Seeds: A Review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Insights into the phenolic compounds present in jambolan (Syzygium cumini) along with their health-promoting effects. Int. J. Food Sci. Technol. 2018, 53, 2431–2447. [Google Scholar] [CrossRef]
- Chawla, J.; Kvarnberg, D. Hydrosoluble Vitamins. In Neurologic Aspects of Systemic Disease Part II; Biller, J., Ferro, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 59; Volume 120, pp. 891–914. [Google Scholar]
- Bhandari, S.R.; Choi, C.S.; Rhee, J.; Shin, Y.K.; Song, J.W.; Kim, S.-H.; Kang, S.; Lee, J.G. Influence of Root Color and Tissue on Phytochemical Contents and Antioxidant Activities in Carrot Genotypes. Foods 2023, 12, 120. [Google Scholar] [CrossRef]
- Bouzari, A.; Holstege, D.M.; Barrett, D.M. Vitamin Retention in Eight Fruits and Vegetables: A Comparison of Refrigerated and Frozen Storage. J. Agric. Food Chem. 2015, 63, 957–962. [Google Scholar] [CrossRef]
- Leong, S.Y.; Oey, I. Effect of endogenous ascorbic acid oxidase activity and stability on vitamin C in carrots (Daucus carota subsp. sativus) during thermal treatment. Food Chem. 2012, 134, 2075–2085. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin e in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of Antioxidants in the Skin: Anti-Aging Effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Christensen, L.P. Aliphatic C17-polyacetylenes of the falcarinol-type as potential health promoting compounds in food plants of the Apiaceae family. Recent Pat. Food Nutr. Agric. 2011, 3, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef]
- Hansen, S.L.; Purup, S.; Christensen, L.P. Bioactivity of falcarinol and the influence of processing and storage on its content in carrots (Daucus carota L.). J. Sci. Food Agric. 2003, 83, 1010–1017. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R.B. The hormesis database: The occurrence of hormetic dose responses in the toxicological literature. Regul. Toxicol. Pharm. 2011, 61, 73–81. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharm. Sci. 2001, 22, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Duthie, S.J.; Milne, L.; Christensen, L.P.; Duthie, G.G.; Bestwick, C.S. Biphasic effect of falcarinol on CaCo-2 cell proliferation, DNA damage, and apoptosis. J. Agric. Food Chem. 2007, 55, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Christensen, L.P.; Theil, P.K.; Oksbjerg, N. The polyacetylenes falcarinol and falcarindiol affect stress responses in myotube cultures in a biphasic manner. Dose-Response 2008, 6, 239–251. [Google Scholar] [CrossRef]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of falcarinol and related aliphatic C17-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar] [CrossRef] [PubMed]
- Zaini, R.G.; Brandt, K.; Clench, M.R.; Le Maitre, C.L. Effects of bioactive compounds from carrots (Daucus carota L.), polyacetylenes, beta-carotene and lutein on human lymphoid leukaemia cells. Anticancer Agents Med. Chem. 2012, 12, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Gu, M.; Zhao, Y.; Zheng, X.; Xing, C. Autophagy contributes to falcarindiol-induced cell death in breast cancer cells with enhanced endoplasmic reticulum stress. PLoS ONE 2017, 12, e0176348. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P. Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef]
- Kundu, J.K.; Surh, Y.-J. Inflammation: Gearing the journey to cancer. Mutat. Res. 2008, 659, 15–30. [Google Scholar] [CrossRef]
- Multho, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2012, 2, 98. [Google Scholar]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Coussens, M.L.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kobaek-Larsen, M.; Baatrup, G.; Notabi, M.K.; El-Houri, R.B.; Pipó-Ollé, E.; Arnspang, E.C.; Christensen, L.P. Dietary polyacetylenic oxylipins falcarinol and falcarindiol prevent inflammation and colorectal neoplastic transformation: A mechanistic and dose-response study in a rat model. Nutrients 2019, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Kobaek-Larsen, M.; El-Houri, R.B.; Christensen, L.P.; Al-Najami, I.; Fretté, X.; Baatrup, G. Dietary polyacetylenes, falcarinol and falcarindiol, isolated from carrots prevents the formation of neoplastic lesions in the colon of azoxymethane-induced rats. Food Funct. 2017, 8, 964–974. [Google Scholar] [CrossRef]
- Deding, U.; Baatrup, G.; Christensen, L.P.; Kobaek-Larsen, M. Carrot Intake and Risk of Colorectal Cancer: A Prospective Cohort Study of 57,053 Danes. Nutrients 2020, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Satoh, M.; Takeuchi, N.; Kirisawa, M. Synthesis and absolute configurations of the cytotoxic polyacetylenes isolated from the callus of Panax ginseng. Chem. Pharm. Bull. 1990, 38, 1447–1450. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Satoh, M. A new cytotoxic chlorine-containing polyacetylene from the callus of Panax ginseng. Chem. Pharm. Bull. 1988, 36, 4206–4208. [Google Scholar] [CrossRef]
- Ahn, B.Z. Beziehung zwischen Struktur und cytotoxischer Aktivität von Panaxydol-Analogen gegen L1210 Zellen. Arch. Pharm. 1988, 321, 61–63. [Google Scholar] [CrossRef]
- Sun, W.; He, Y.S.; Xu, L.H.; Zhang, B.Y.; Qi, L.W.; Yang, J.; Li, P.; Wen, X.D. Pharmacokinetic profiles of falcarindiol and oplopandiol in rats after oral administration of polyynes extract of Oplopanax elatus. Chin. J. Nat. Med. 2016, 14, 714–720. [Google Scholar] [CrossRef]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Christensen, L.P.; Brandt, K. Acetylenes and psoralens. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M.N., Ashihara, H., Eds.; Blackwell: Oxford, UK, 2009; Chapter 5; pp. 147–173. [Google Scholar]
- Brandt, K.; Christensen, L.P.; Hansen-Møller, J.; Hansen, S.L.; Haraldsdóttir, J.; Jespersen, L.; Purup, S.; Kharazmi, A.; Barkholdt, V.; Frøkiær, H.; et al. Health promoting compounds in vegetables and fruits: A systematic approach for identifying plant components with impact on human health. Trends Food Sci. Technol. 2004, 15, 384–393. [Google Scholar] [CrossRef]
- Crosby, D.G.; Aharonson, N. The structure of carotatoxin, a natural toxicant from carrot. Tetrahedron 1967, 23, 465–472. [Google Scholar] [CrossRef]
- Uwai, K.; Ohashi, K.; Takaya, Y.; Ohta, T.; Tadano, T.; Kisara, K.; Shibusawa, K.; Sakakibara, R.; Oshima, Y. Exploring the structural basis of neurotoxicity in C17-polyacetylenes isolated from water hemlock. J. Med. Chem. 2000, 43, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Kobaek-Larsen, M.; Christensen, L.P.; Vach, W.; Ritskes-Hoitinga, J.; Brandt, K. Inhibitory effects of feeding with carrots or (−)-falcarinol on development of azoxymethane-induced preneoplastic lesions in the rat colon. J. Agric. Food Chem. 2005, 53, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- Raju, J. Azoxymethane-induced rat aberrant crypt foci: Relevance in studying chemoprevention of colon cancer. World J. Gastroenterol. 2008, 14, 6632–6635. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995, 93, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Corpet, D.E.; Pierre, F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon cancer chemoprevention in rats, mice and humans. Eur. J. Cancer 2005, 41, 1911–1922. [Google Scholar] [CrossRef]
- Stefanson, A.L.; Bakovic, M. Falcarinol is a potent inducer of heme oxygenase-1 and was more effective than sulforaphane in attenuating intestinal inflammation at diet-achievable doses. Oxid. Med. Cell. Longev. 2018, 2018, 3153527. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhang, Z.; Huang, W.H.; Du, G.J.; Wen, X.D.; Calway, T.; Yu, C.; Nass, R.; Zhao, J.; Du, W.; et al. Identification of potential anticancer compounds from Oplopanax horridus. Phytomedicine 2013, 20, 999–1006. [Google Scholar] [CrossRef]
- Jin, H.R.; Liao, Y.; Li, X.; Zhang, Z.; Zhao, J.; Wang, C.Z.; Huang, W.H.; Li, S.P.; Yuan, C.S.; Du, W. Anticancer compound oplopantriol A kills cancer cells through inducing ER stress and BH3 proteins Bim and Noxa. Cell Death Dis. 2014, 5, e1190. [Google Scholar] [CrossRef]
- Jin, H.R.; Zhao, J.; Zhang, Z.; Liao, Y.; Wang, C.Z.; Huang, W.H.; Li, S.P.; He, T.C.; Yuan, C.S.; Du, W. The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e376. [Google Scholar] [CrossRef]
- Le, H.T.; Nguyen, H.T.; Min, H.Y.; Hyun, S.Y.; Kwon, S.; Lee, Y.; Le, T.H.V.; Lee, J.; Park, J.H.; Lee, H.Y. Panaxynol, a natural Hsp90 inhibitor, effectively targets both lung cancer stem and non-stem cells. Cancer Lett. 2018, 412, 297–307. [Google Scholar] [CrossRef]
- Mandrich, L.; Piccolella, S.; Esposito, A.V.; Costa, S.; Mercadante, V.; Pacifico, S.; Caputo, E. Different Extraction Procedures Revealed the Anti-Proliferation Activity from Vegetable Semi-Purified Sources on Breast Cancer Cell Lines. Antioxidants 2023, 12, 1242. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef]
- Murakoshi, M.; Takayasu, J.; Kimura, O.; Kohmura, E.; Nishino, H.; Iwashima, A.; Okuzumi, J.; Sakai, T.; Sugimoto, T.; Imanishi, J.; et al. Inhibitory effects of α-carotene on proliferation of the human neuroblastoma cell line GOTO. J. Natl. Cancer Inst. 1989, 81, 1649–1652. [Google Scholar] [CrossRef] [PubMed]
- Dulińska, J.; Gil, D.; Zagajewski, J.; Hartwich, J.; Bodzioch, M.; Dembińska-Kieć, A.; Langmann, T.; Schmitz, G.; Laidler, P. Different Effect of Beta-Carotene on Proliferation of Prostate Cancer Cells. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Sowmya Shree, K.G.; Yogendra Prasad, H.S.; Arpitha, U.R.; Deepika, K.; Nawneet Kumar, P.; Mondal, P.; Ganesan, P. β-carotene at physiologically attainable concentration induces apoptosis and down-regulates cell survival and antioxidant markers in human breast cancer (MCF-7) cells. Mol. Cell. Biochem. 2017, 436, 1–12. [Google Scholar] [CrossRef]
- Antunes, A.; Carmo, F.; Pinto, S.; Andrade, N.; Martel, F. The Anti-Proliferative Effect of β-Carotene against a Triple-Negative Breast Cancer Cell Line Is Cancer Cell-Specific and JNK-Dependent. PharmaNutrition 2022, 22, 100320. [Google Scholar] [CrossRef]
- Hu, C.; Huang, Y.; Luo, P.; Yang, Y. Effect of antioxidants coenzyme Q10 and β-carotene on the cytotoxicity of vemurafenib against human malignant melanoma. Oncol. Lett. 2021, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Sevimli-Gur, C.; Cetin, B.; Akay, S.; Gulce-Iz, S.; Yesil-Celiktas, O. Extracts from Black Carrot Tissue Culture as Potent Anticancer Agents. Plant Foods Hum. Nutr. 2013, 68, 293–298. [Google Scholar] [CrossRef]
- Liu, R.; Choi, H.S.; Kim, S.L.; Kim, J.H.; Yun, B.S.; Lee, D.S. 6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling. Molecules 2020, 25, 4374. [Google Scholar] [CrossRef]
- Elasbali, A.M.; Al-Soud, W.A.; Elfaki, E.M.; Alanazi, H.H.; Alharbi, B.; Alharethi, S.H.; Anwer, K.; Mohammad, T.; Hassan, M.I. Identification of novel c-Kit inhibitors from natural sources using virtual screening and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2023, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Yueh, T.-C.; Chen, Y.-C.; Huang, C.-H.; Yang, C.-M.; Hu, M.-L. Antimetastatic Effects of α-Carotene and Possible Mechanisms of Action in Human Hepatocarcinoma SK-Hep-1 Cells. J. Agric. Food Chem. 2013, 61, 10368–10376. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Lee, H.-A.; Lim, J.Y.; Kim, Y.; Jung, C.-H.; Yoo, S.-H.; Kim, Y. β-Carotene inhibits neuroblastoma cell invasion and metastasis in vitro and in vivo by decreasing level of hypoxia-inducible factor-1α. J. Nutr. Biochem. 2014, 25, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Kwon, H.S.; Kang, M.; Leem, H.H.; Lee, K.H.; Kim, D.Y. The antitumor natural compound falcarindiol disrupts neural stem cell homeostasis by suppressing Notch pathway. Int. J. Mol. Sci. 2018, 19, 3432. [Google Scholar] [CrossRef]
- Venkatesh, V.; Nataraj, R.; Thangaraj, G.S.; Karthikeyan, M.; Gnanasekaran, A.; Kaginelli, S.B.; Kuppanna, G.; Kallappa, C.G.; Basalingappa, K.M. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig. 2018, 5, 5. [Google Scholar] [CrossRef]
- Natarajamurthy, S.H.; Sistla, S.; Dharmesh, S.M. Disruption of galectin-3 and galectin-3 binding protein (G3BP) interaction by dietary pectic polysaccharides (DPP)—Arrest of metastasis, inhibition of proliferation and induction of apoptosis. Int. J. Biol. Macromol. 2019, 139, 486–499. [Google Scholar] [CrossRef]
- Zgheib, P.; Daher, C.F.; Mroueh, M.; Nasrallah, A.; Taleb, R.I.; El-Sibai, M. Daucus carota Pentane/Diethyl Ether Fraction Inhibits Motility and Reduces Invasion of Cancer Cells. Chemotherapy 2014, 60, 302–309. [Google Scholar] [CrossRef]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef]
- Teng, Y.-N.; Sheu, M.-J.; Hsieh, Y.-W.; Wang, R.-Y.; Chiang, Y.-C.; Hung, C.-C. β-carotene reverses multidrug resistant cancer cells by selectively modulating human P-glycoprotein function. Phytomedicine 2016, 23, 316–323. [Google Scholar] [CrossRef]
- Tan, K.W.; Killeen, D.P.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Dietary polyacetylenes of the falcarinol type are inhibitors of breast cancer resistance protein (BCRP/ABCG2). Eur. J. Pharmacol. 2014, 723, 346–352. [Google Scholar] [CrossRef]
- Liao, J.; Sun, A.; Xie, Y.; Isse, T.; Kawamoto, T.; Zou, Y.; Ge, J. Aldehyde Dehydrogenase-2 Deficiency Aggravates Cardiac Dysfunction Elicited by Endoplasmic Reticulum Stress Induction. Mol. Med. 2012, 18, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef] [PubMed]
- Tomokazu, O.; Takao, K.; Shinji, N.; Takahito, N.; Kenichiro, O.; Akira, H. Induction of antioxidant and phase 2 drug metabolizing enzymes by falcarindiol isolated from Notopterygium incisum extract, which activates the Nrf2/ARE pathway, leads to cytoprotection against oxidative and electrophilic stress. Arch. Biochem. Biophys. 2009, 488, 34–41. [Google Scholar]
- Tomokazu, O.; Shinji, N.; Eisaburo, A.; Takahito, N.; Kenichiro, O.; Akira, H. Activation of the Nrf2/ARE pathway via S-alkylation of cysteine 151 in the chemopreventive agent-sensor Keap1 protein by falcarindiol, a conjugated diacetylene compound. Toxicol. Appl. Pharmacol. 2010, 244, 27–36. [Google Scholar]
- Tirinato, L.; Pagliari, F.; Limongi, T.; Marini, M.; Falqui, A.; Seco, J.; Candeloro, P.; Liberale, C.; Di Fabrizio, E. An Overview of Lipid Droplets in Cancer and Cancer Stem Cells. Stem Cells Int. 2017, 2017, 1656053. [Google Scholar] [CrossRef]
- Cruz, A.L.S.; Barreto, E.d.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef]
- Huang, B.; Song, B.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef]
- Smith, B.; Hartmut, L. Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep. 2012, 2, 580–590. [Google Scholar] [CrossRef]
- Liang, G.; Sourav, T.S.; Jodie, T.; Mandeep, K. Targeting cellular cholesterol for anticancer therapy. FEBS J. 2019, 286, 4192–4208. [Google Scholar]
- Furlan, V.; Bren, U. Helichrysum italicum: From Extraction, Distillation, and Encapsulation Techniques to Beneficial Health Effects. Foods 2023, 12, 802. [Google Scholar] [CrossRef]
- Poljsak, B.; Milisav, I. The role of antioxidants in cancer, friends or foes? Curr. Pharm. Des. 2018, 24, 5234–5244. [Google Scholar] [CrossRef]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015, 7, 308re8. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; De Berardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Pantiora, P.; Furlan, V.; Matiadis, D.; Mavroidi, B.; Perperopoulou, F.; Papageorgiou, A.C.; Sagnou, M.; Bren, U.; Pelecanou, M.; Labrou, N.E. Monocarbonyl Curcumin Analogues as Potent Inhibitors against Human Glutathione Transferase P1-1. Antioxidants 2023, 12, 63. [Google Scholar] [CrossRef] [PubMed]



| Chemical Constituents | Observed Amount | References |
|---|---|---|
| Moisture | 86–89 mg/100 g FW | [7] |
| Ca | 34–80 mg/100 g FW | [7] |
| P | 25–53 mg/100 g FW | [7] |
| K | 240 mg/100 g FW | [7] |
| Mg | 9 mg/100 g FW | [7] |
| Mn | 0.2–0.8 mg/kg FW | [8] |
| Fe | 0.4–2.2 mg/100 g | [7] |
| Na | 40 mg/100 g | [7] |
| Total sugars | 2.73–11.24 g/100 g FW | [8] |
| Total organic acids | 1.07–2.79 g/100 g FW | [8] |
| Vitamin C (ascorbic acid) | 1.0–5.3 mg/100 g * FW | [8] |
| Total phenolics | 7.3–224 mg/100 g FW | [8] |
| Tetraterpenoids (carotenoids, chlorophylls) | 0.2–4.1 mg/100 g FW | [8] |
| Falcarinol § | 16–84 mg/kg FW | [9] |
| (C17-polyacetilens) falcarindol § | 8–27 mg/kg FW | [9] |
| Falcarindol-3acetate § | 8–40 mg/kg FW | [9] |
| Compounds | Biological Effects in Cancer | References |
|---|---|---|
| α-Carotene (AC) | Anti-proliferative, anti-metastatic | [88,96] |
| β-Carotene (BC) | Anti-proliferative, anti-inflammatory, anti-apoptotic, anti-metastatic, ABC efflux transporter modulator | [89,90,91,92,97] |
| Antocianins | Cytotoxic | [93] |
| 6-Methoximellein | Anti-inflammatory, anti-metastatic | [94] |
| Falcarinol (FaOH) | Cytotoxic, ABC efflux transporter modulator, anti-inflammatory | [55,57,58,65,66,77,81] |
| Falcarindiol (FaDOH) | Anti-inflammatory, cytotoxic, ABC efflux transporter modulator, lipid modulator, | [59,65,66,84,98,99,108] |
| Glycerophosphocoline derivatives (GPC) | Anti-proliferative | [86] |
| Monogalactosyl-monoacylglycerol (MGMG) | Anti-proliferative | [86] |
| Carrot pectic polysaccharide (CRPP) | Anti-metastatic | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandrich, L.; Esposito, A.V.; Costa, S.; Caputo, E. Chemical Composition, Functional and Anticancer Properties of Carrot. Molecules 2023, 28, 7161. https://doi.org/10.3390/molecules28207161
Mandrich L, Esposito AV, Costa S, Caputo E. Chemical Composition, Functional and Anticancer Properties of Carrot. Molecules. 2023; 28(20):7161. https://doi.org/10.3390/molecules28207161
Chicago/Turabian StyleMandrich, Luigi, Antonia Valeria Esposito, Silvio Costa, and Emilia Caputo. 2023. "Chemical Composition, Functional and Anticancer Properties of Carrot" Molecules 28, no. 20: 7161. https://doi.org/10.3390/molecules28207161
APA StyleMandrich, L., Esposito, A. V., Costa, S., & Caputo, E. (2023). Chemical Composition, Functional and Anticancer Properties of Carrot. Molecules, 28(20), 7161. https://doi.org/10.3390/molecules28207161






