Physicochemical Characterization of Dextran HE29 Produced by the Leuconostoc citreum HE29 Isolated from Traditional Fermented Pickle
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening EPS Producer Strains, Their Isolation and Genotypic Identification
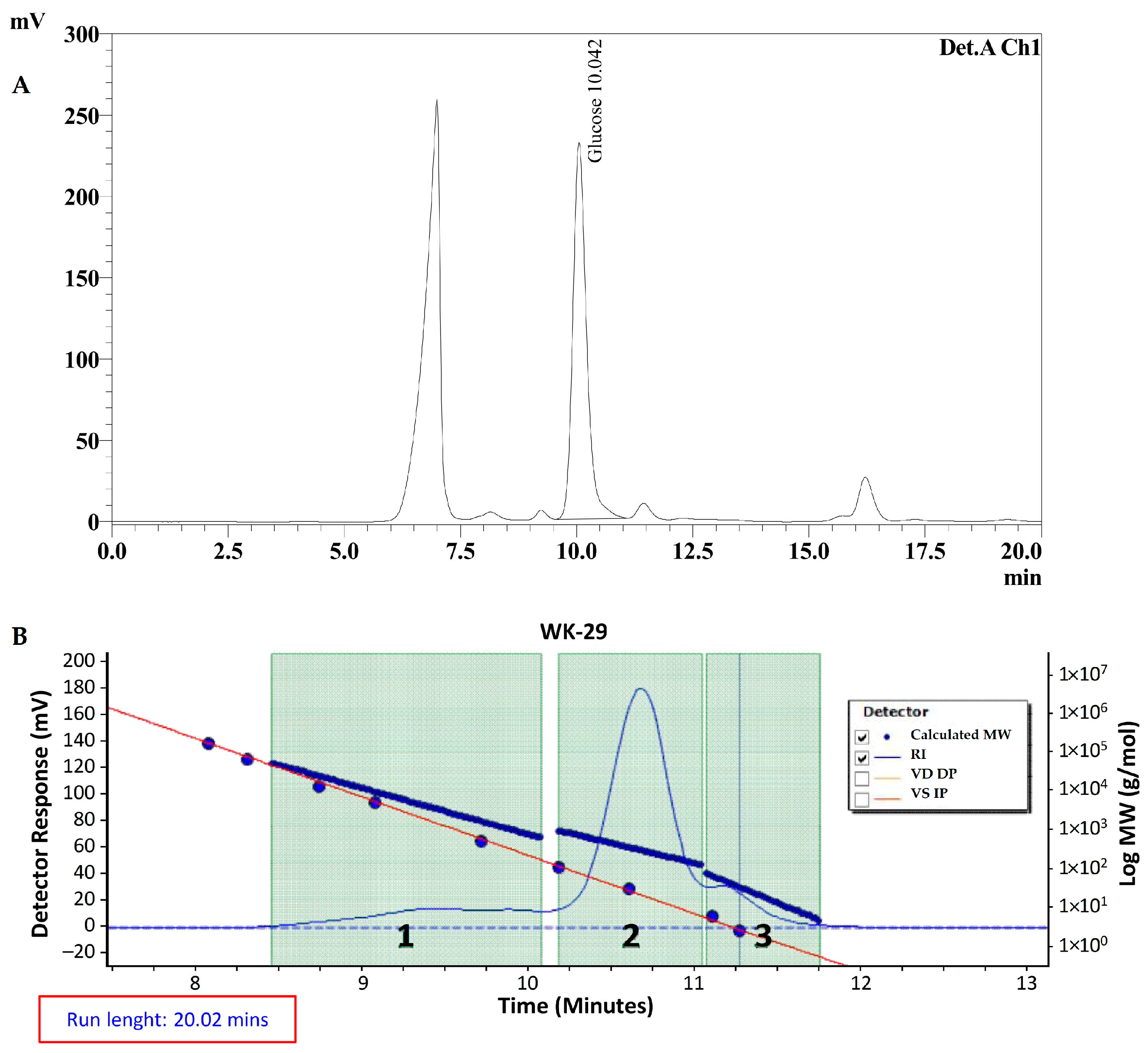
2.2. Monomer Profile and Molecular Weight of EPS HE29
2.3. Structural Characterization of Glucan HE29 through NMR Analysis
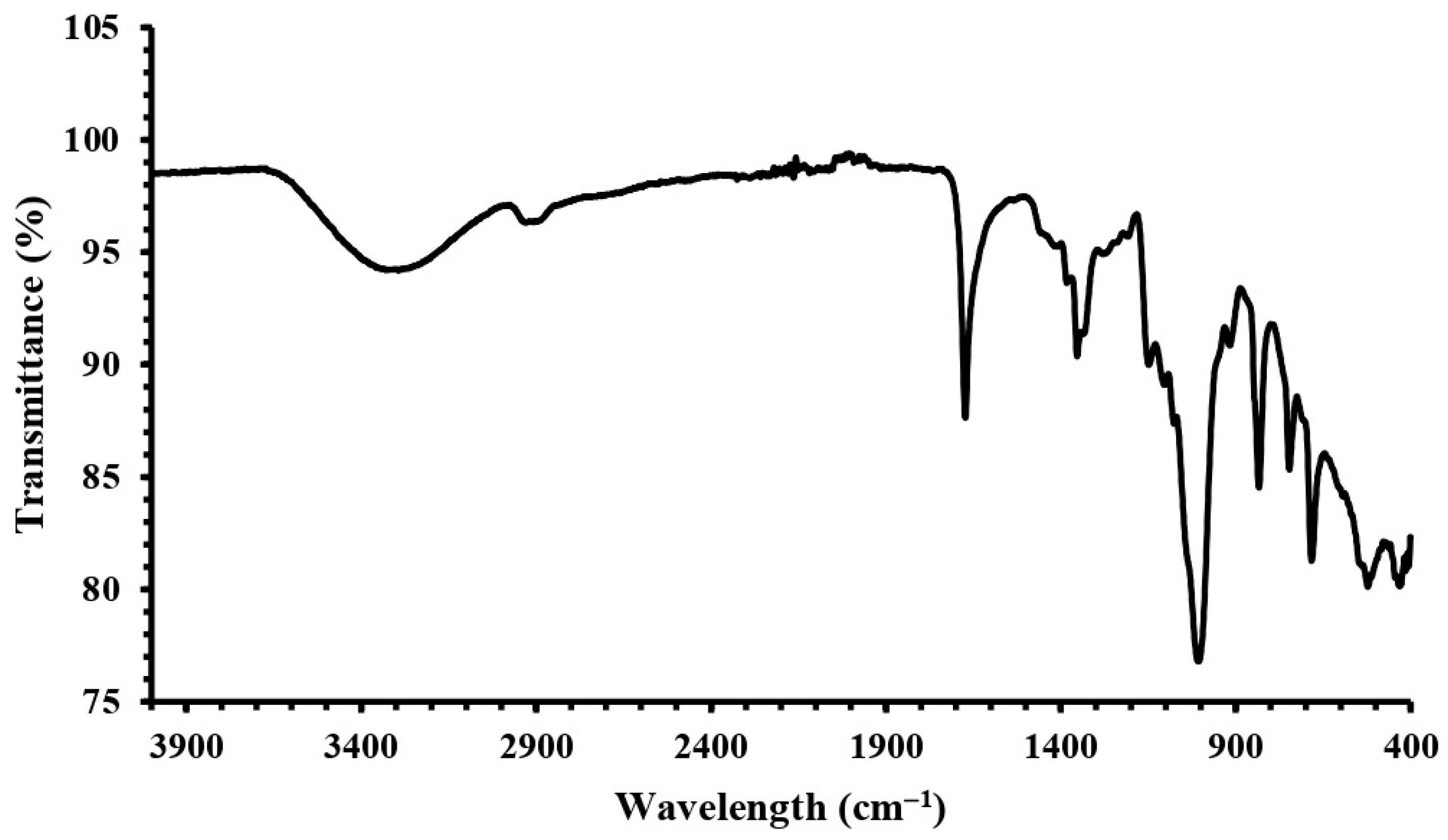
2.4. FT-IR Analysis of Dextran HE29
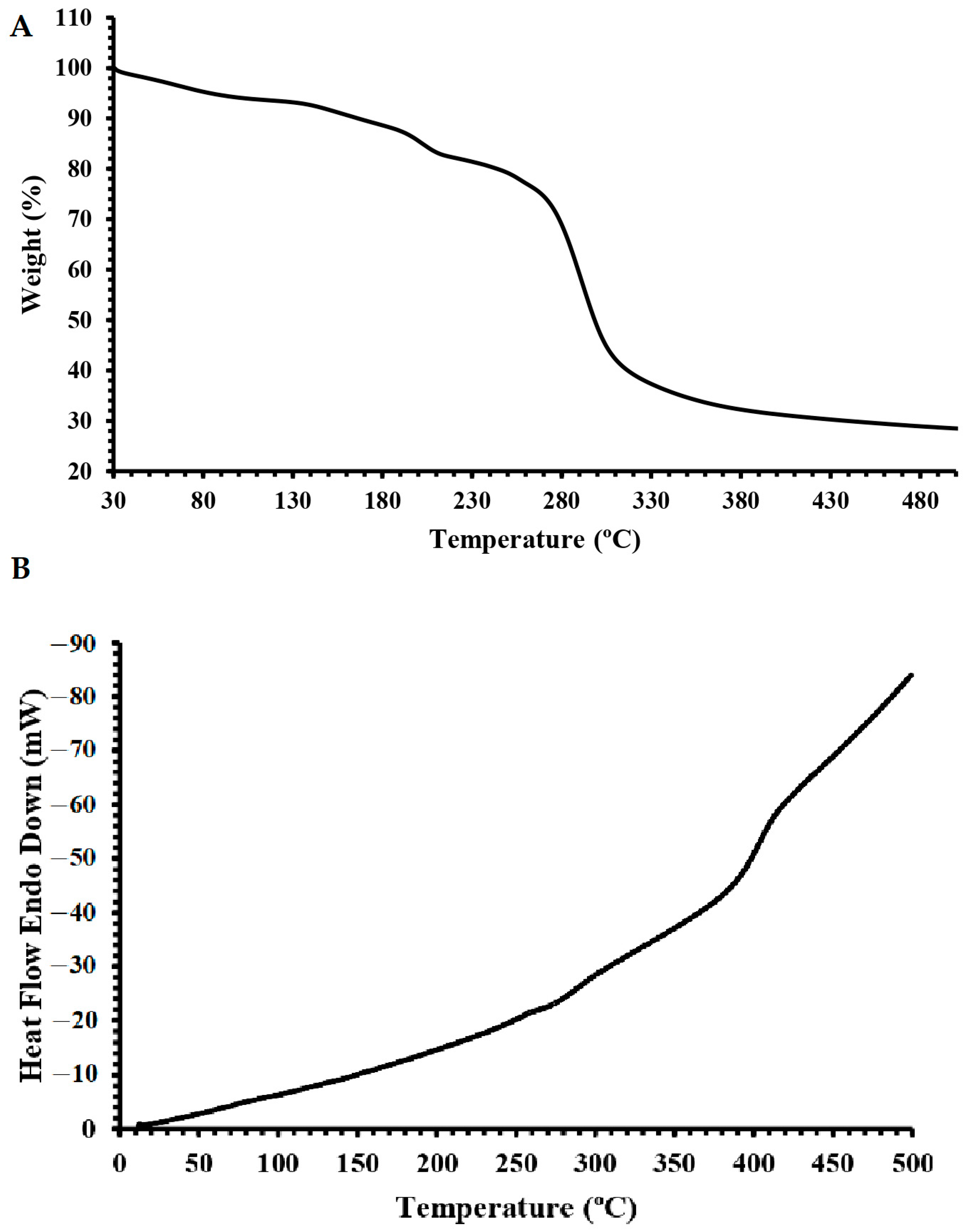
2.5. Thermal Properties of Dextran HE29
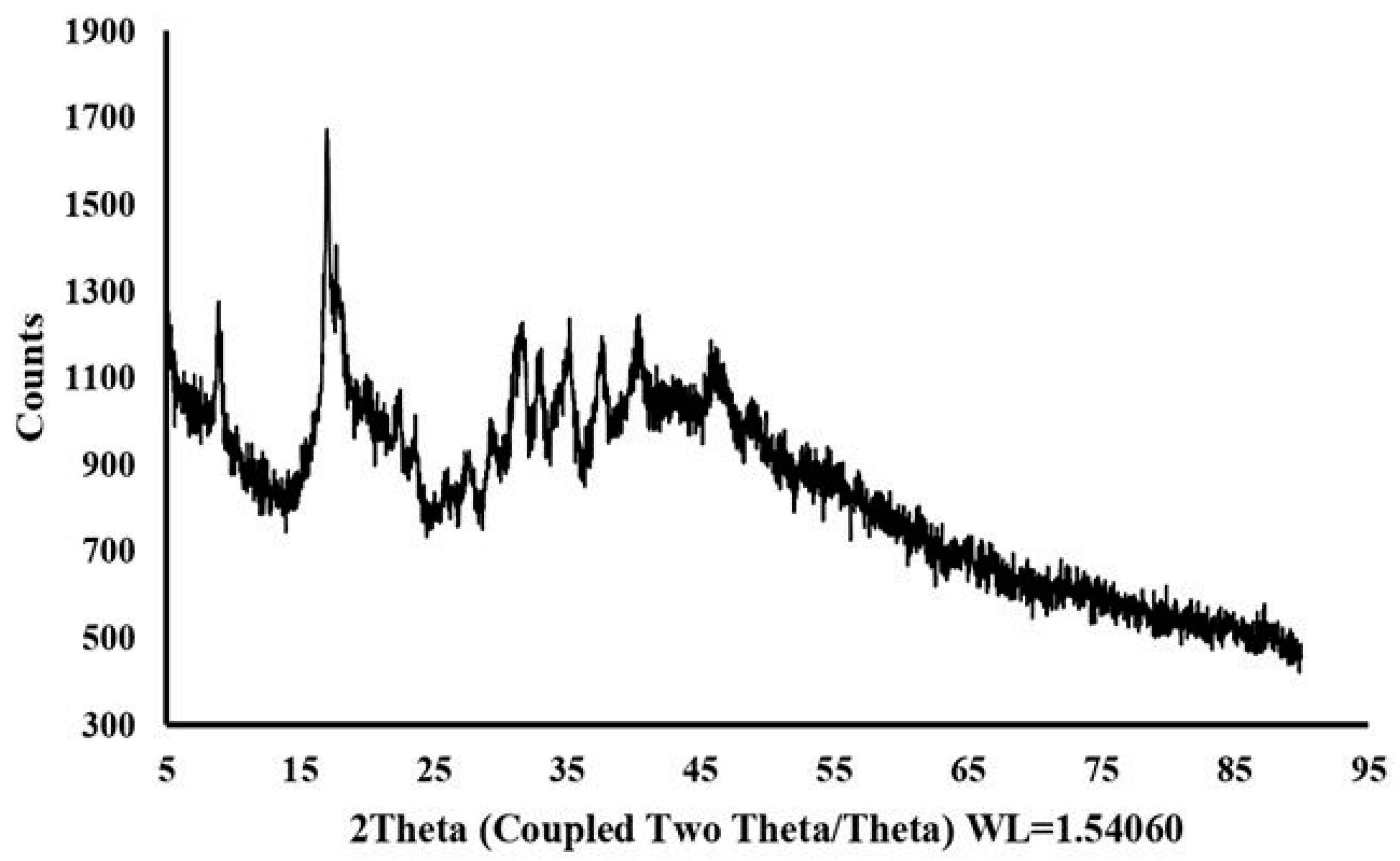
2.6. Crystallographic Properties of Dextran HE29
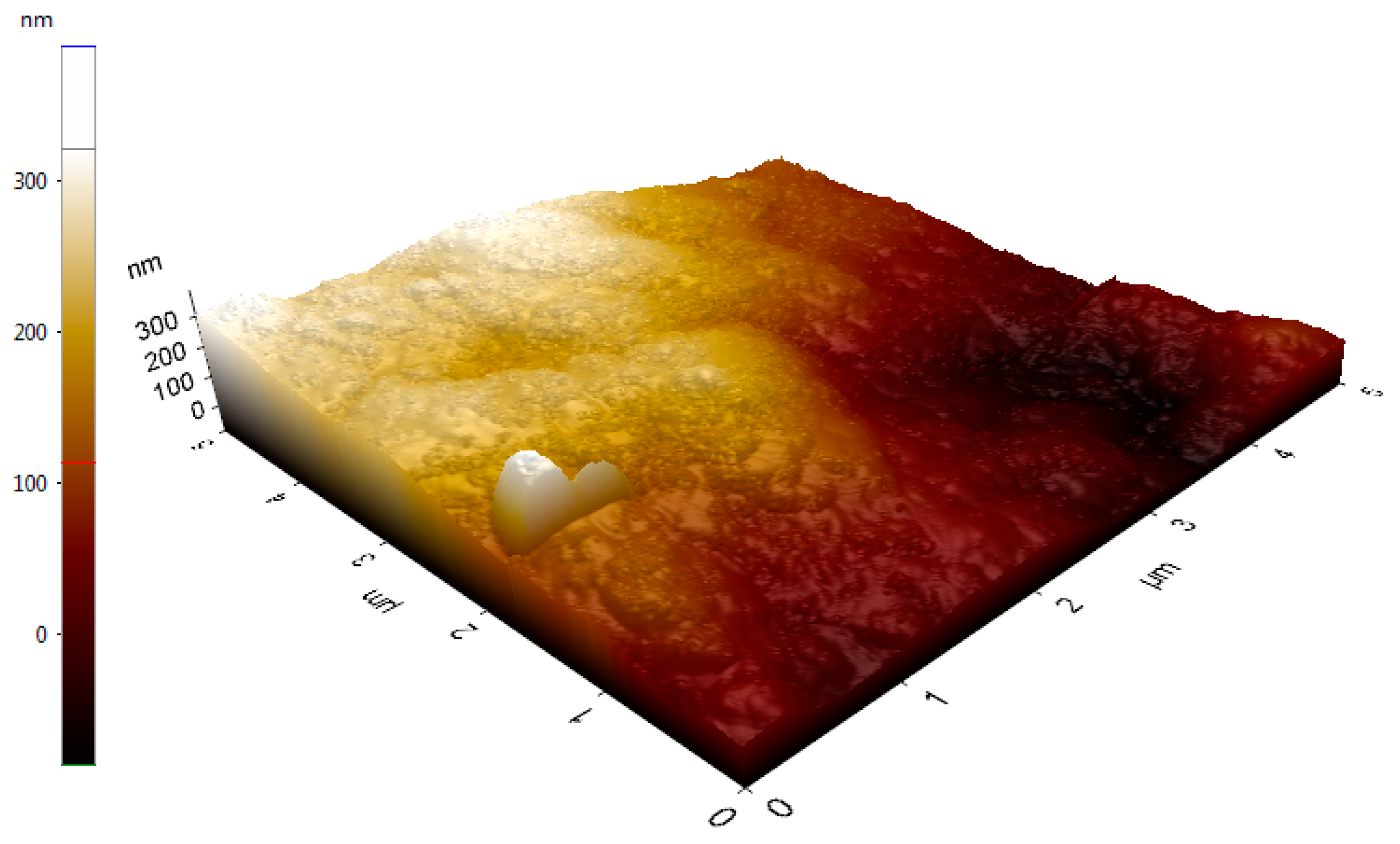
2.7. Atomic Force Microscopy Analysis of Dextran HE29
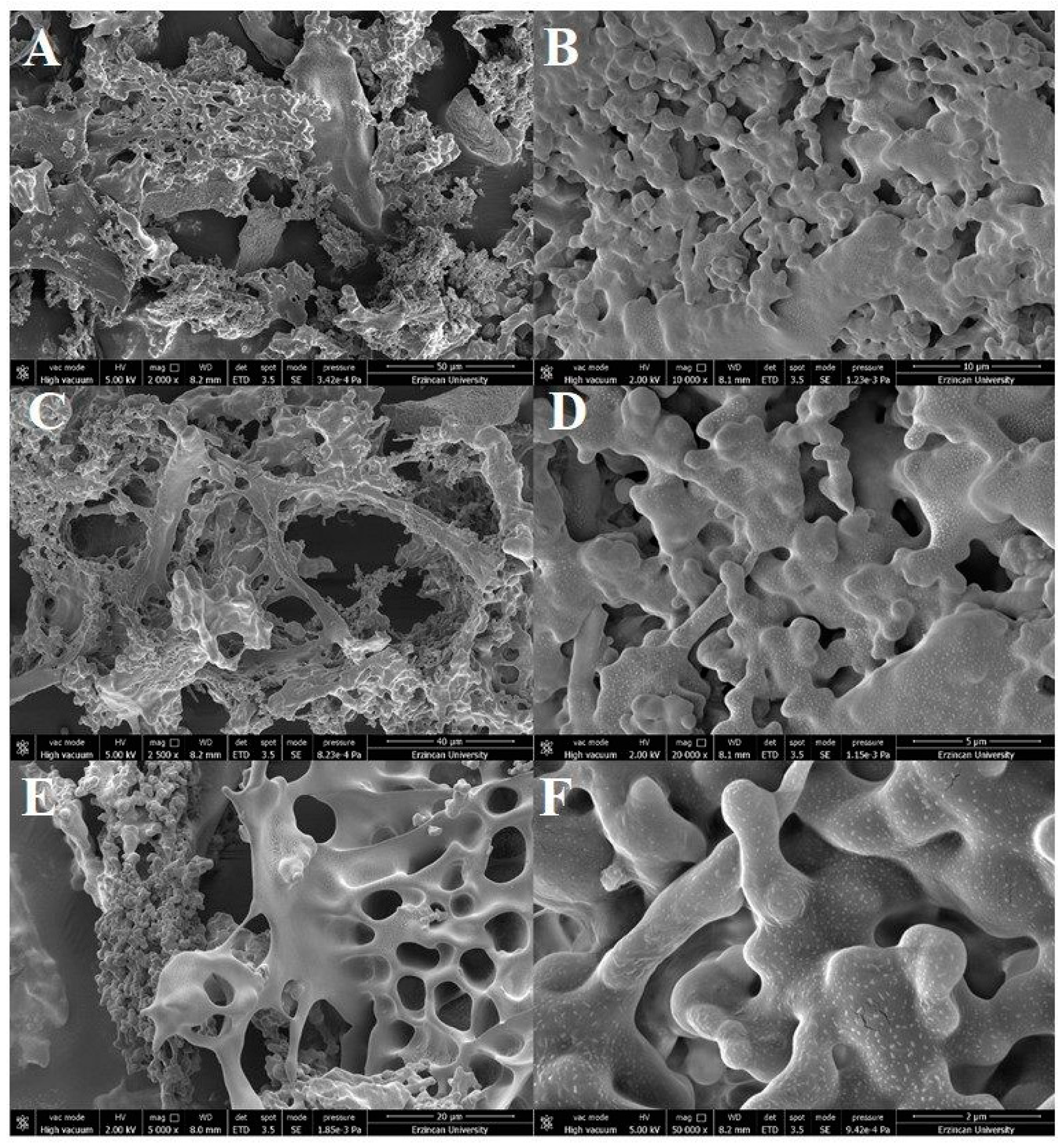
2.8. Scanning Electron Microscopy Analysis of Dextran HE29
3. Materials and Methods
3.1. Collection of Pickle Samples and Isolation of LABs
3.2. Genotypic Characterization of Isolates Using Rep-PCR and Bacterial Identification of EPS Producers
3.3. EPS Extraction from Leuconostoc citreum HE29, Monomer Profile and Molecular Weight Determination
3.4. Structural Determination of EPS HE29 through NMR Analysis
3.5. Determination of the Function Groups within EPS HE29 Structure by FT-IR Spectroscopy Analysis
3.6. Thermal Characterization of EPS HE29 through TGA and DSC Analysis
3.7. Crystallographic Analysis of EPS HE29 through XRD Analysis
3.8. Morphological Characterization of EPS HE29 through SEM and AFM Analysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bounaix, M.-S.; Gabriel, V.; Morel, S.; Robert, H.; Rabier, P.; Remaud-Simeon, M.; Gabriel, B.; Fontagne-Faucher, C. Biodiversity of exopolysaccharides produced from sucrose by sourdough lactic acid bacteria. J. Agric. Food Chem. 2009, 57, 10889–10897. [Google Scholar] [CrossRef]
- Myriam, A.; Arango, L.F.G.; Gabriel, V.; Robert, H.; Morel, S.; Moulis, C.; Gabriel, B.; Remaud-Siméon, M.; Fontagné-Faucher, C. Characterization of a novel dextransucrase from Weissella confusa isolated from sourdough. Appl. Microbiol. Biotechnol. 2013, 97, 5413–5422. [Google Scholar]
- Kralj, S.; van Geel-Schutten, G.; Dondorff, M.; Kirsanovs, S.; Van Der Maarel, M.; Dijkhuizen, L. Glucan synthesis in the genus Lactobacillus: Isolation and characterization of glucansucrase genes, enzymes and glucan products from six different strains. Microbiology 2004, 150, 3681–3690. [Google Scholar] [CrossRef]
- İspirli, H.; Yüzer, M.O.; Skory, C.; Colquhoun, I.J.; Sağdıç, O.; Dertli, E. Characterization of a glucansucrase from Lactobacillus reuteri E81 and production of malto-oligosaccharides. Biocatal. Biotransformation 2019, 37, 421–430. [Google Scholar] [CrossRef]
- Ni, D.; Zhu, Y.; Xu, W.; Bai, Y.; Zhang, T.; Mu, W. Biosynthesis of inulin from sucrose using inulosucrase from Lactobacillus gasseri DSM 20604. Int. J. Biol. Macromol. 2018, 109, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.B.; Kavitake, D.; Shetty, P.H. Physico-chemical characterization of galactan exopolysaccharide produced by Weissella confusa KR780676. Int. J. Biol. Macromol. 2016, 93, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Kavitake, D.; Devi, P.B.; Singh, S.P.; Shetty, P.H. Characterization of a novel galactan produced by Weissella confusa KR780676 from an acidic fermented food. Int. J. Biol. Macromol. 2016, 86, 681–689. [Google Scholar] [CrossRef]
- Castilla-Marroquín, J.; Hernández-Martínez, R.; de la Vequia, H.D.; Ríos-Corripio, M.; Hernández-Rosas, J.; López, M.R.; Hernández-Rosas, F. Dextran synthesis by native sugarcane microorganisms. Rev. Mex. Ing. Química 2020, 19, 177–185. [Google Scholar] [CrossRef]
- Hernández-Rosas, F.; Castilla-Marroquín, J.; Loeza-Corte, J.; Lizardi-Jiménez, M.; Martínez, R.H. The importance of carbon and nitrogen sources on exopolysaccharide synthesis by lactic acid bacteria and their industrial importance. Rev. Mex. Ing. Química 2021, 20, Bio2429. [Google Scholar]
- Tieking, M.; Gänzle, M.G. Exopolysaccharides from cereal-associated lactobacilli. Trends Food Sci. Technol. 2005, 16, 79–84. [Google Scholar] [CrossRef]
- Miao, M.; Bai, A.; Jiang, B.; Song, Y.; Cui, S.W.; Zhang, T. Characterisation of a novel water-soluble polysaccharide from Leuconostoc citreum SK24.002. Food Hydrocoll. 2014, 36, 265–272. [Google Scholar] [CrossRef]
- Ye, G.; Chen, Y.; Wang, C.; Yang, R.; Bin, X. Purification and characterization of exopolysaccharide produced by Weissella cibaria YB-1 from pickle Chinese cabbage. Int. J. Biol. Macromol. 2018, 120, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, K.; Yin, S.; Liu, S.; Zhu, Y.; Yang, Y.; Wang, C. Purification and characterization of an exopolysaccharide produced by Lactobacillus plantarum HY isolated from home-made Sichuan Pickle. Int. J. Biol. Macromol. 2019, 134, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peng, Q.; Guo, Y.; Han, Y.; Xiao, H.; Zhou, Z. Isolation and characterization of dextran produced by Leuconostoc citreum NM105 from manchurian sauerkraut. Carbohydr. Polym. 2015, 133, 365–372. [Google Scholar] [CrossRef]
- Feng, F.; Zhou, Q.; Yang, Y.; Zhao, F.; Du, R.; Han, Y.; Xiao, H.; Zhou, Z. Characterization of highly branched dextran produced by Leuconostoc citreum B-2 from pineapple fermented product. Int. J. Biol. Macromol. 2018, 113, 45–50. [Google Scholar] [CrossRef]
- Maina, N.H.; Tenkanen, M.; Maaheimo, H.; Juvonen, R.; Virkki, L. NMR spectroscopic analysis of exopolysaccharides produced by Leuconostoc citreum and Weissella confusa. Carbohydr. Res. 2008, 343, 1446–1455. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, L.; Tian, J.; Wang, X.; Zhang, X.; Fang, Y.; Li, W. Structural characterization, rheological properties and protection of oxidative damage of an exopolysaccharide from Leuconostoc citreum 1.2461 fermented in soybean whey. Foods 2022, 11, 2283. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Blecker, C.; Gharsallaoui, A.; Corsaro, M.M.; Besbes, S.; Attia, H. Rheological and emulsifying properties of an exopolysaccharide produced by potential probiotic Leuconostoc citreum-BMS strain. Carbohydr. Polym. 2021, 256, 117523. [Google Scholar] [CrossRef]
- Yalmanci, D.; Dertli, E.; Tekin-Cakmak, Z.H.; Karasu, S. Utilization of exopolysaccharide produced by Leuconostoc lactis GW-6 as an emulsifier for low-fat mayonnaise production. Int. J. Biol. Macromol. 2023, 226, 772–779. [Google Scholar] [CrossRef]
- Miao, M.; Huang, C.; Jia, X.; Cui, S.W.; Jiang, B.; Zhang, T. Physicochemical characteristics of a high molecular weight bioengineered α-D-glucan from Leuconostoc citreum SK24.002. Food Hydrocoll. 2015, 50, 37–43. [Google Scholar] [CrossRef]
- Wang, B.; Song, Q.; Zhao, F.; Zhang, L.; Han, Y.; Zhou, Z. Isolation and characterization of dextran produced by Lactobacillus sakei L3 from Hubei sausage. Carbohydr. Polym. 2019, 223, 115111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Feng, F.; Yang, Y.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. Int. J. Biol. Macromol. 2018, 107, 2234–2241. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; İspirli, H.; Alidrisi, H.; Taylan, O.; Dertli, E. Characterisation of dextran AP-27 produced by bee pollen isolate Lactobacillus kunkeei AP-27. Process Biochem. 2023, 129, 22–29. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; İspirli, H.; Taylan, O.; Bilgrami, A.L.; Dertli, E. Structural and bioactive characteristics of a dextran produced by Lactobacillus kunkeei AK1. Int. J. Biol. Macromol. 2022, 200, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Netsopa, S.; Niamsanit, S.; Sakloetsakun, D.; Milintawisamai, N. Characterization and rheological behavior of dextran from Weissella confusa R003. Int. J. Polym. Sci. 2018, 2018, 5790526. [Google Scholar] [CrossRef]
- Aman, A.; Siddiqui, N.N.; Qader, S.A.U. Characterization and potential applications of high molecular weight dextran produced by Leuconostoc mesenteroides AA1. Carbohydr. Polym. 2012, 87, 910–915. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef]
- Yalmanci, D.; İspirli, H.; Dertli, E. Identification of Lactic Acid Bacteria (LAB) from pre-fermented liquids of selected cereals and legumes and characterization of their exopolysaccharides (EPS). Food Biosci. 2022, 50, 102014. [Google Scholar] [CrossRef]
- İspirli, H.; Sagdic, O.; Yılmaz, M.T.; Dertli, E. Physicochemical characterisation of an α-glucan from Lactobacillus reuteri E81 as a potential exopolysaccharide suitable for food applications. Process Biochem. 2019, 79, 91–96. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, B.-G.; Park, H.J.; Yim, J.H. Cryoprotective properties and preliminary characterization of exopolysaccharide (P-Arcpo 15) produced by the Arctic bacterium Pseudoalteromonas elyakovii Arcpo 15. Prep. Biochem. Biotechnol. 2016, 46, 261–266. [Google Scholar] [CrossRef]
- Ljubic, V.; Milosevic, M.; Cvetkovic, S.; Stojanovic, M.; Novovic, K.; Dinic, M.; Popovic, M. The new exopolysaccharide produced by the probiotic strain L. reuteri B2: Extraction, biological properties, and possible application for Ni2+ ion removal from the contaminated water. Biomass Convers. Biorefinery 2022, 1–16. [Google Scholar] [CrossRef]
- Wang, X.; Su, Y.; Su, J.; Xue, J.; Zhang, R.; Li, X.; Li, Y.; Ding, Y.; Chu, X. Optimization of Enzyme—Assisted Aqueous Extraction of Polysaccharide from Acanthopanax senticosus and Comparison of Physicochemical Properties and Bioactivities of Polysaccharides with Different Molecular Weights. Molecules 2023, 28, 6585. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Ahmed, R.Z.; Siddiqui, K.; Arman, M.; Ahmed, N. Characterization of high molecular weight dextran produced by Weissella cibaria CMGDEX3. Carbohydr. Polym. 2012, 90, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Liu, P.; Ahmed, Z.; Xiao, P.; Bai, X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydr. Polym. 2010, 82, 895–903. [Google Scholar] [CrossRef]
- Parikh, A.; Madamwar, D. Partial characterization of extracellular polysaccharides from cyanobacteria. Bioresour. Technol. 2006, 97, 1822–1827. [Google Scholar] [CrossRef]
- Lakra, A.K.; Domdi, L.; Tilwani, Y.M.; Arul, V. Physicochemical and functional characterization of mannan exopolysaccharide from Weissella confusa MD1 with bioactivities. Int. J. Biol. Macromol. 2020, 143, 797–805. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Tian, Z.; Yang, Y.; Yang, Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet Kefir. Carbohydr. Polym. 2015, 125, 16–25. [Google Scholar] [CrossRef]
- Taylan, O.; Yilmaz, M.T.; Dertli, E. Partial characterization of a levan type exopolysaccharide (EPS) produced by Leuconostoc mesenteroides showing immunostimulatory and antioxidant activities. Int. J. Biol. Macromol. 2019, 136, 436–444. [Google Scholar] [CrossRef]
- Yilmaz, M.; Dertli, E.; Toker, O.; Tatlisu, N.; Sagdic, O.; Arici, M. Effect of in situ exopolysaccharide production on physicochemical, rheological, sensory, and microstructural properties of the yogurt drink ayran: An optimization study based on fermentation kinetics. J. Dairy Sci. 2015, 98, 1604–1624. [Google Scholar] [CrossRef]
- Dertli, E.; Mercan, E.; Arıcı, M.; Yılmaz, M.T.; Sağdıç, O. Characterisation of lactic acid bacteria from Turkish sourdough and determination of their exopolysaccharide (EPS) production characteristics. LWT Food Sci. Technol. 2016, 71, 116–124. [Google Scholar] [CrossRef]
- Baker, G.; Smith, J.J.; Cowan, D.A. Review and re-analysis of domain-specific 16S primers. J. Microbiol. Methods 2003, 55, 541–555. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
İspirli, H. Physicochemical Characterization of Dextran HE29 Produced by the Leuconostoc citreum HE29 Isolated from Traditional Fermented Pickle. Molecules 2023, 28, 7149. https://doi.org/10.3390/molecules28207149
İspirli H. Physicochemical Characterization of Dextran HE29 Produced by the Leuconostoc citreum HE29 Isolated from Traditional Fermented Pickle. Molecules. 2023; 28(20):7149. https://doi.org/10.3390/molecules28207149
Chicago/Turabian Styleİspirli, Hümeyra. 2023. "Physicochemical Characterization of Dextran HE29 Produced by the Leuconostoc citreum HE29 Isolated from Traditional Fermented Pickle" Molecules 28, no. 20: 7149. https://doi.org/10.3390/molecules28207149
APA Styleİspirli, H. (2023). Physicochemical Characterization of Dextran HE29 Produced by the Leuconostoc citreum HE29 Isolated from Traditional Fermented Pickle. Molecules, 28(20), 7149. https://doi.org/10.3390/molecules28207149





