Preparation and Characterization of a Novel Morphosis of Dextran and Its Derivatization with Polyethyleneimine
Abstract
:1. Introduction
2. Results and Discussion
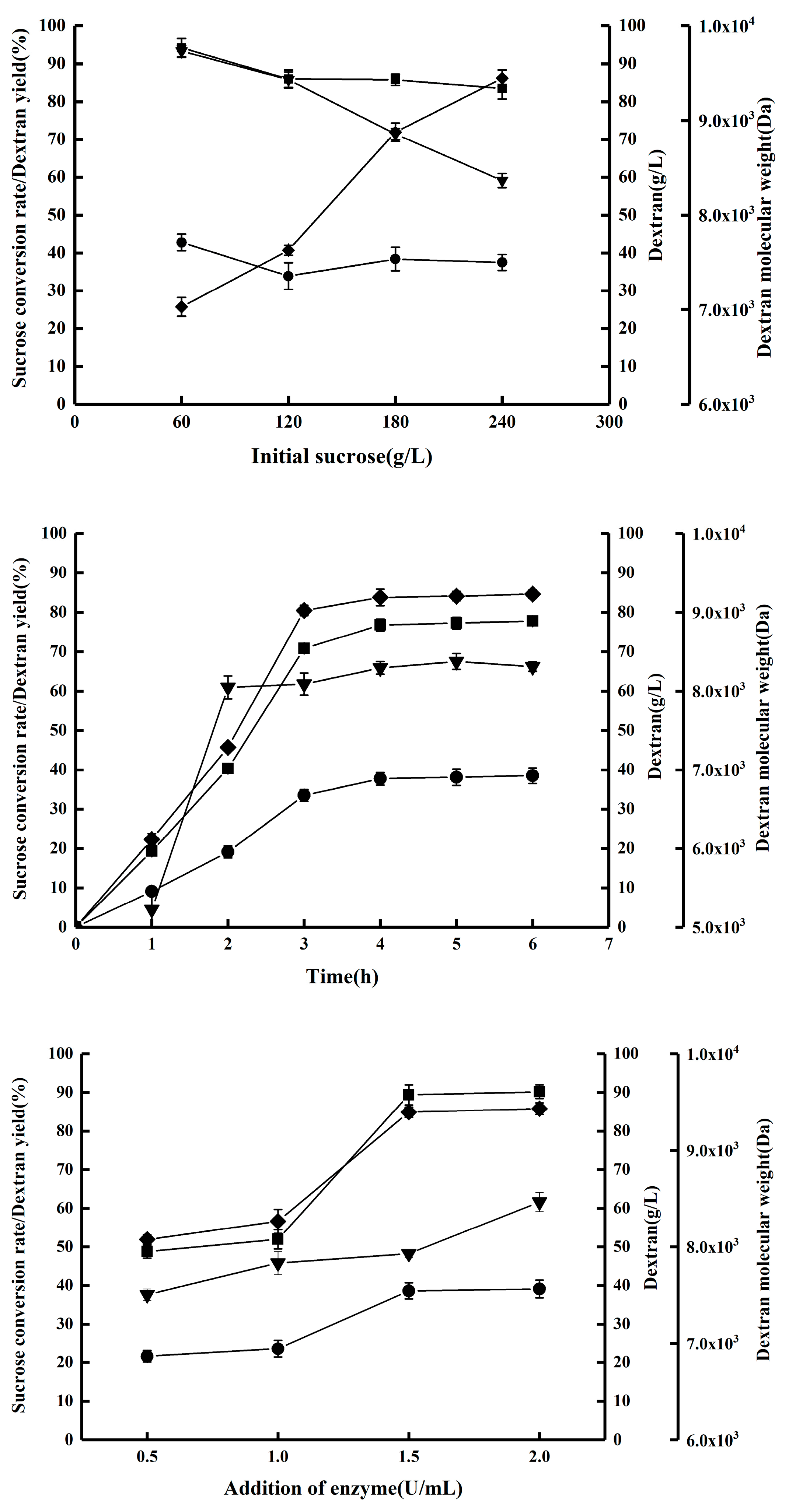
2.1. Synthesis of Dextran by DSR-MΔ2-K654A
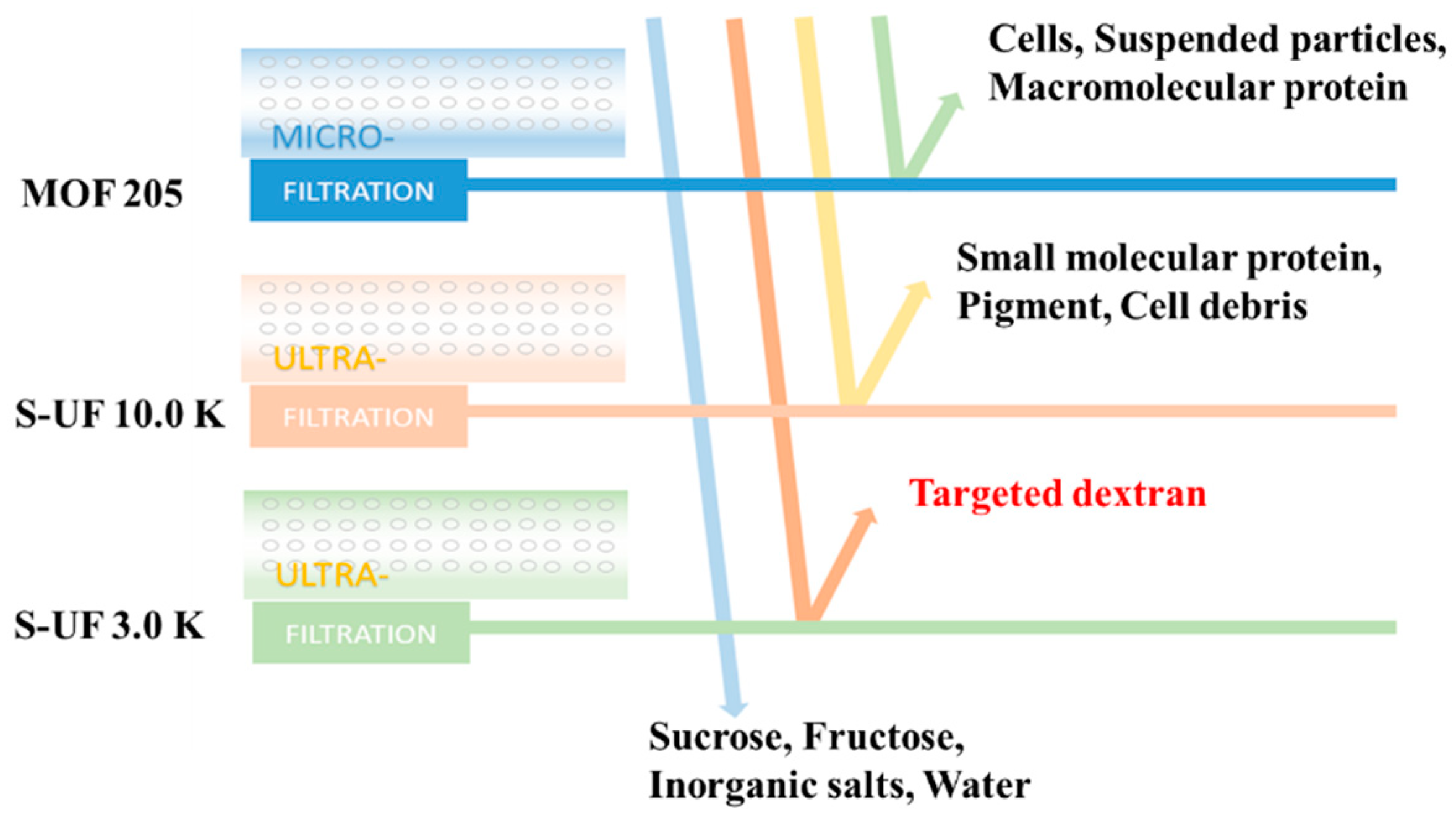
2.2. Preparation and Storage of Purified Dextran Solution
2.3. Effects of Drying Rate on the Formation of Dextran Precipitates
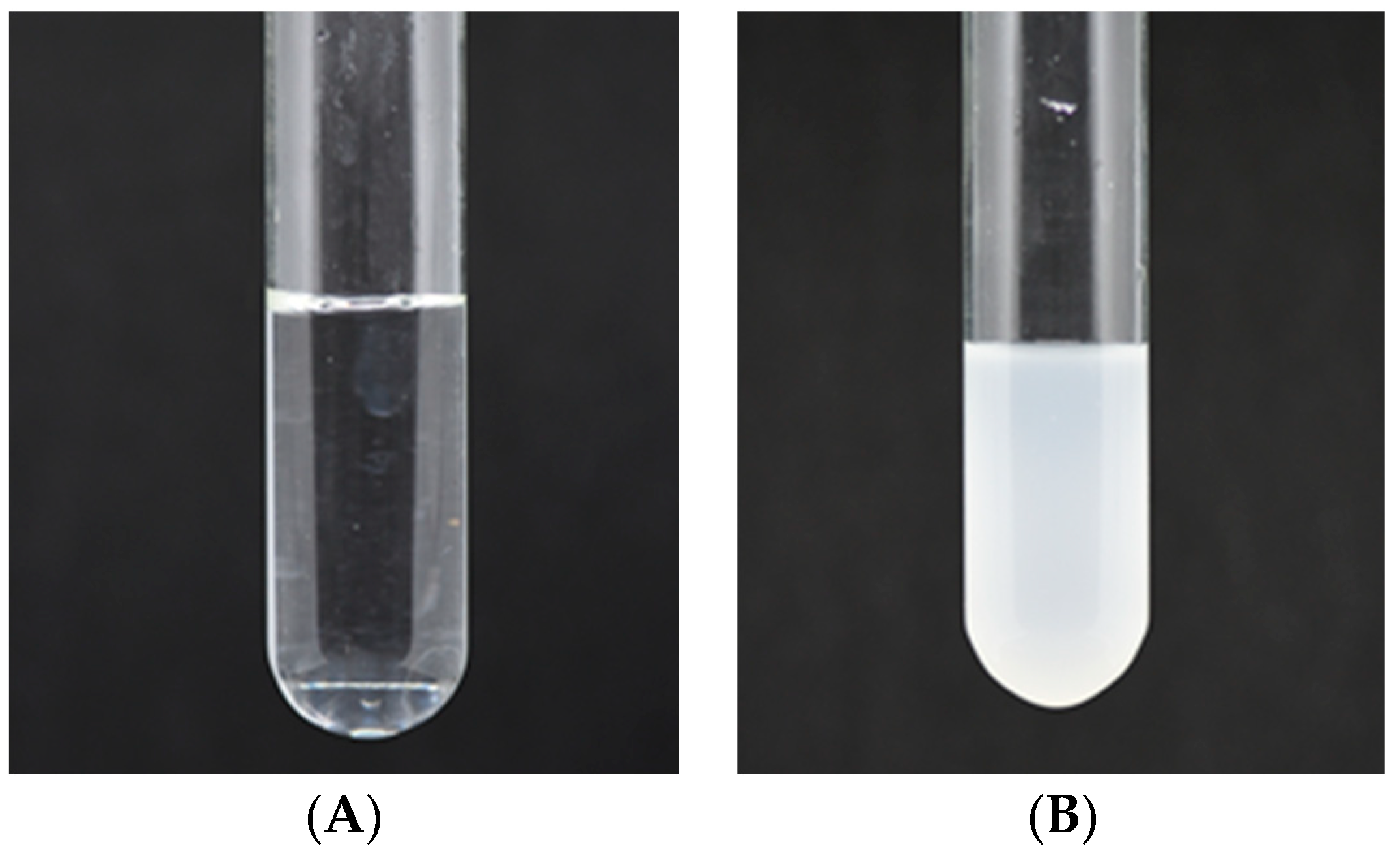
2.4. Characterization of Insoluble Dextran Precipitates
2.4.1. Solubility of IS-Dextran in Different Solvents
2.4.2. Molecular Weights Analysis
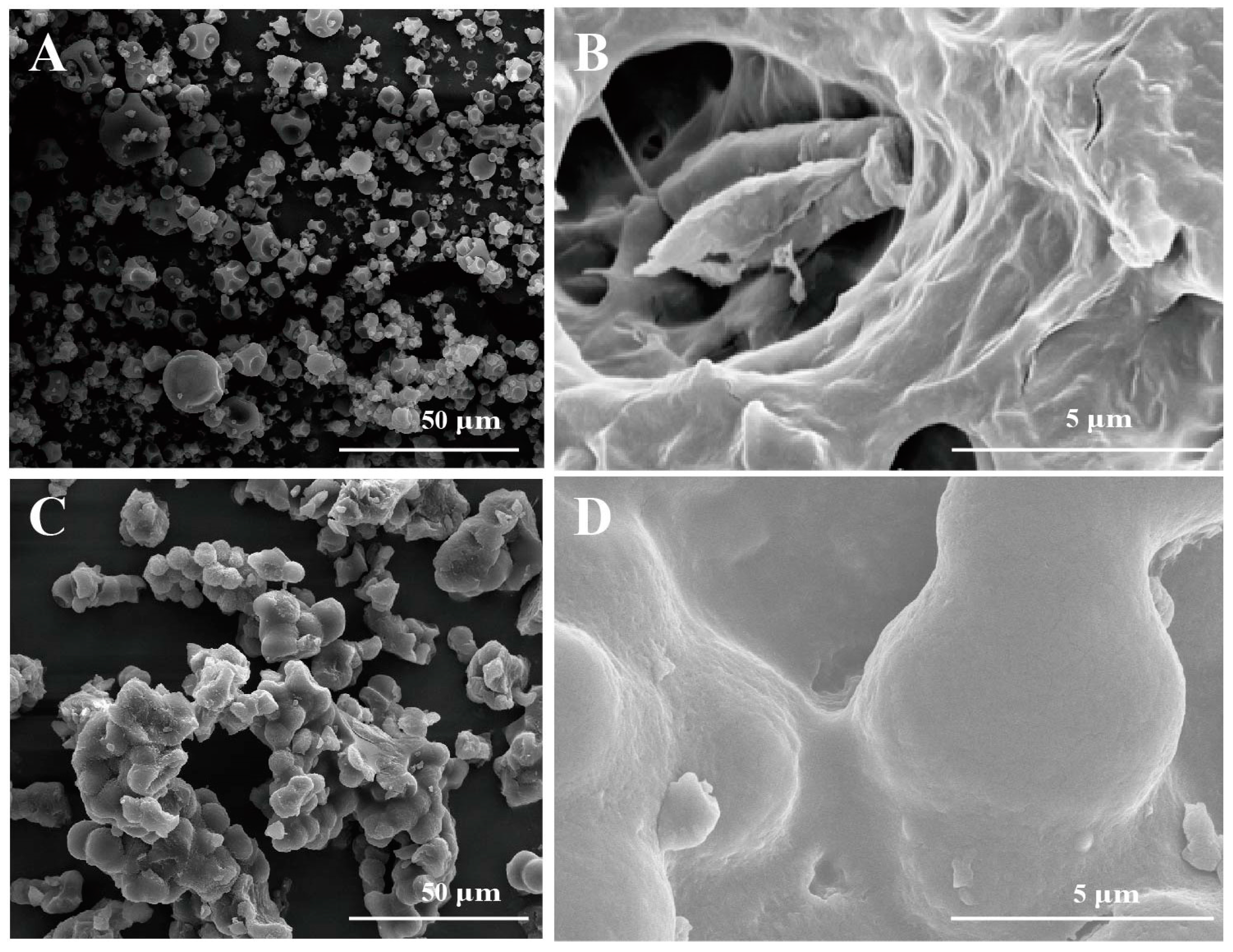
2.4.3. SEM Analysis
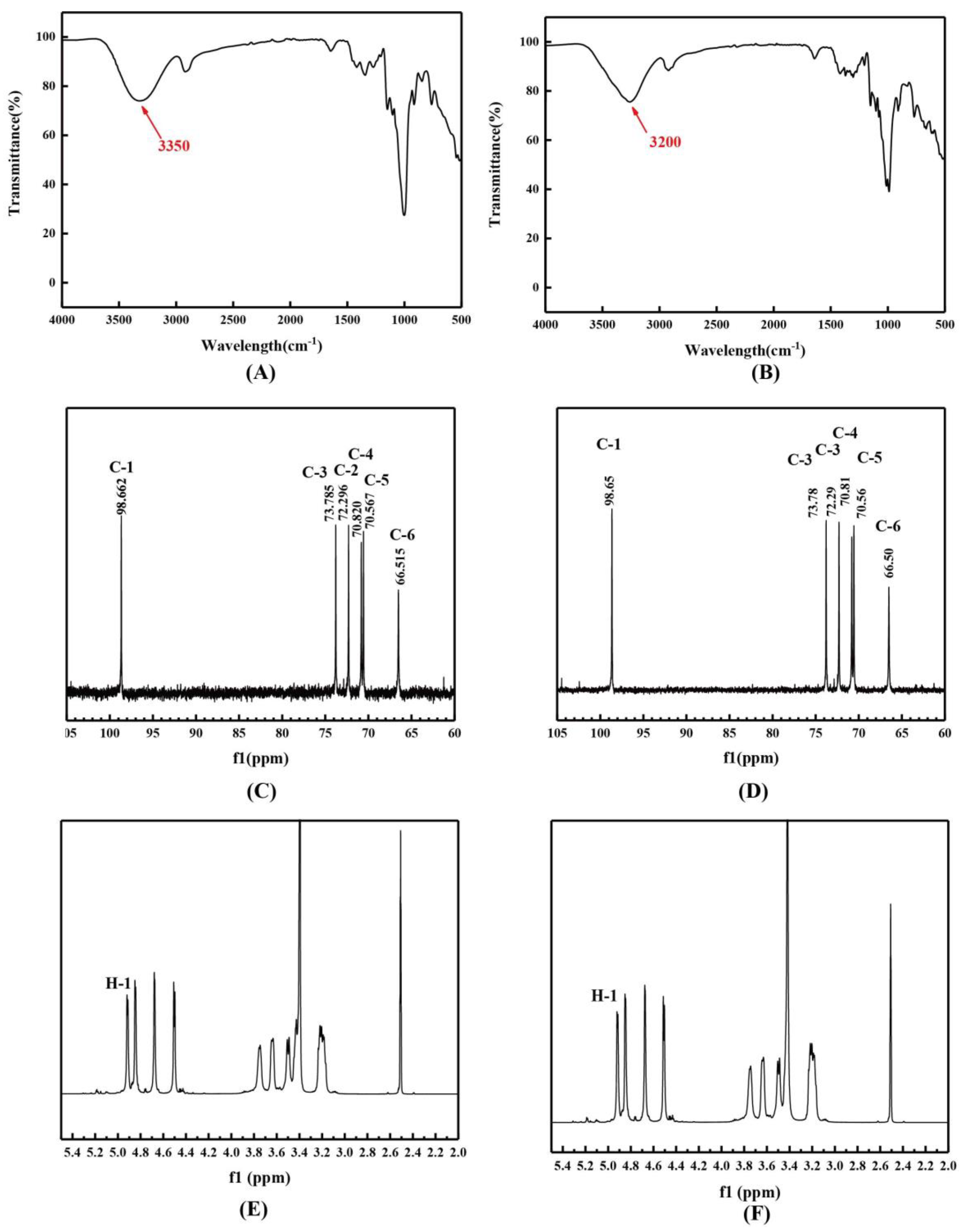
2.4.4. Spectroscopic Analysis
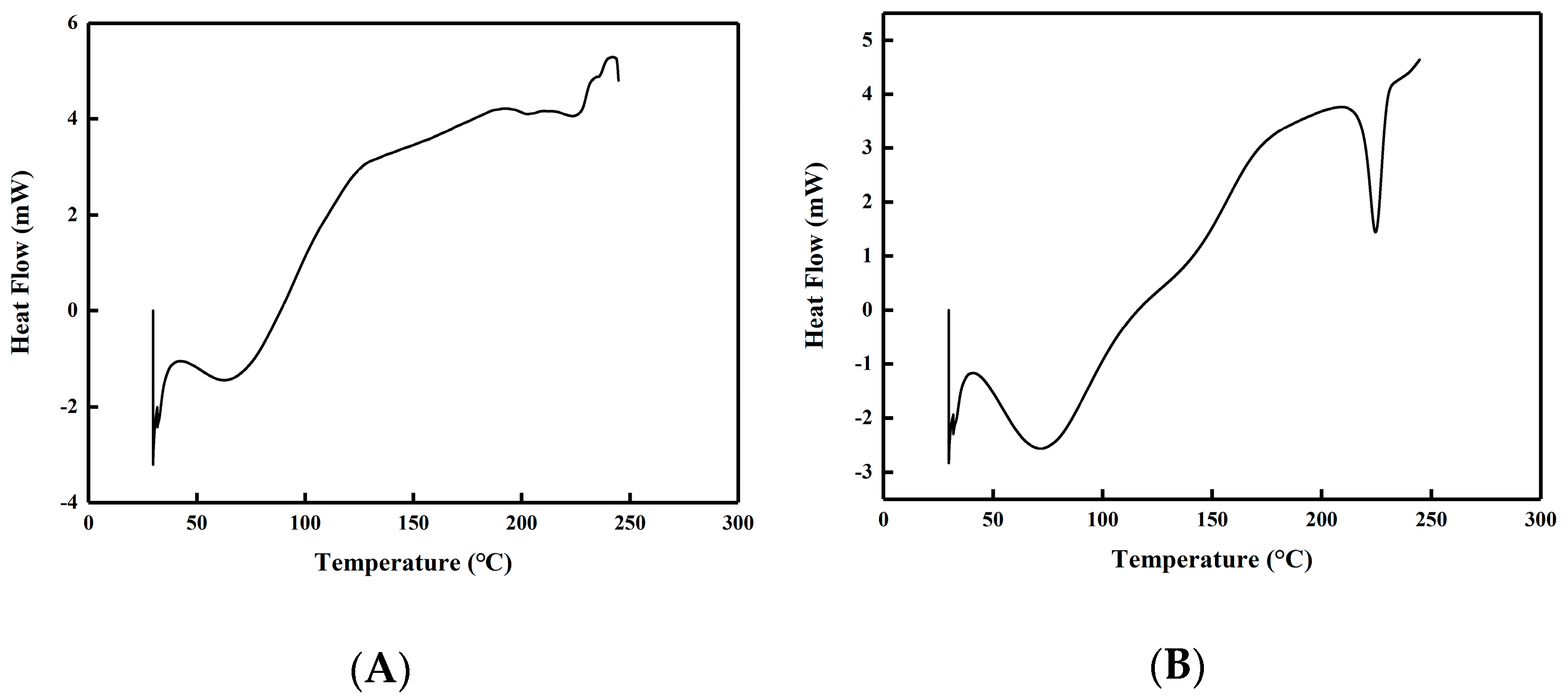
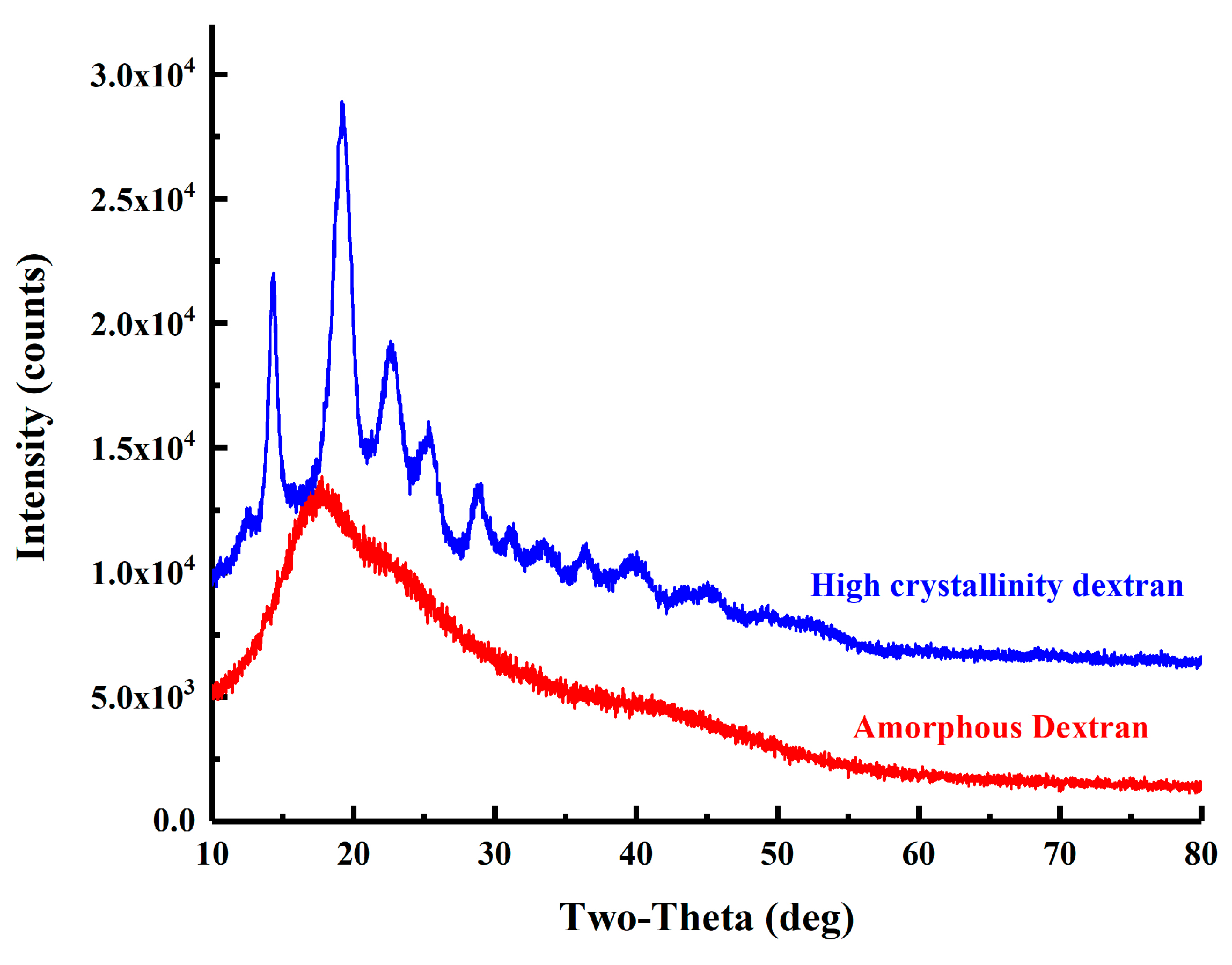
2.4.5. DSC and XRD Analysis
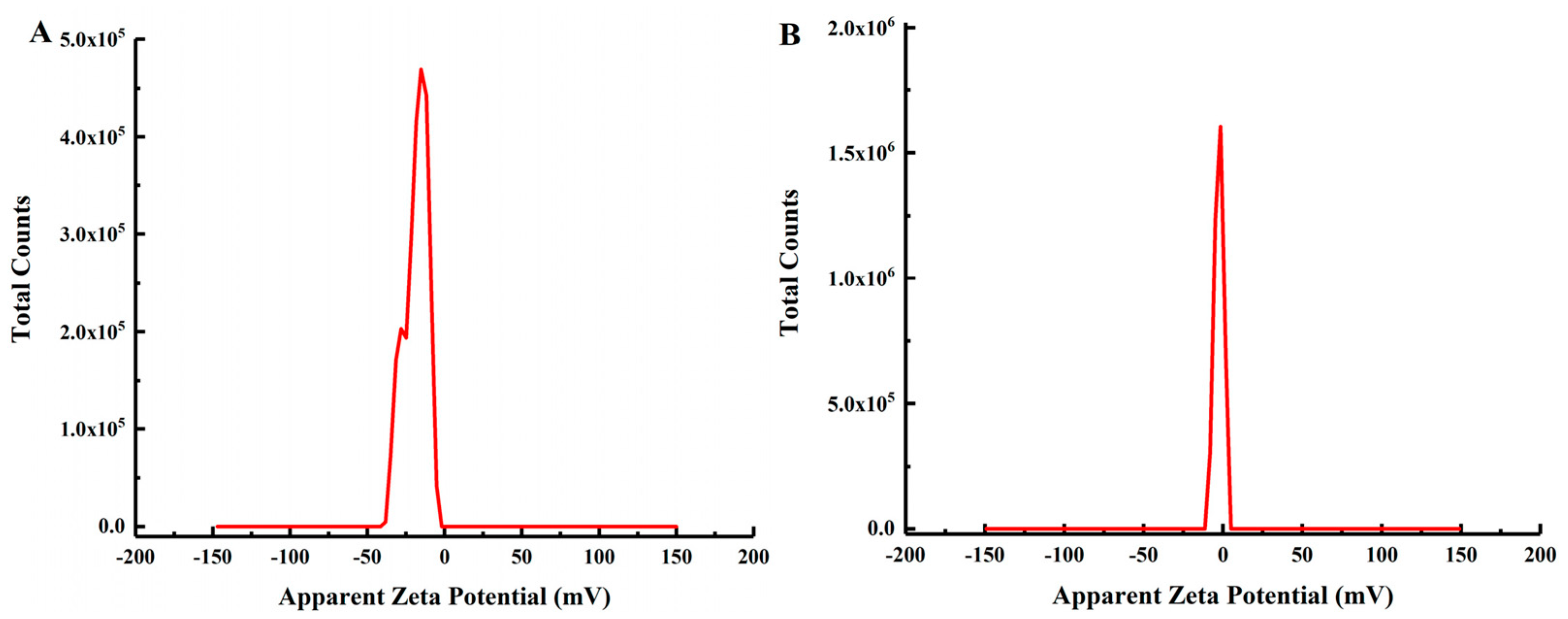
2.4.6. Zeta Potential Analysis
2.5. Preparation of Positively Charged IS-Dextran with PEI
3. Materials and Methods
3.1. Strains, Plasmids, Media, and Conditions
3.2. Dextransucrase Activity Assay and Reaction Condition
3.3. Preparation of Purified Dextran Samples
3.4. Solubility of SI-Dextran in Different Solvents
3.5. Gel Chromatography Technology
3.6. Infrared Spectrum
3.7. SEM, Scanning Electron Microscope
3.8. Zeta Potential
3.9. Differential Scanning Calorimetry
3.10. X-ray Diffraction
3.11. Coating IS-Dextran with PEI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mohanrao, R.; Hema, K.; Sureshan, K.M. Topochemical synthesis of different polymorphs of polymers as a paradigm for tuning properties of polymers. Nat. Commun. 2020, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- Losev, E.A.; Mikhailenko, M.A.; Achkasov, A.F.; Boldyreva, E.V. The effect of carboxylic acids on glycine polymorphism, salt and co-crystal formation. A comparison of different crystallisation techniques. New J. Chem. 2013, 37, 1973–1981. [Google Scholar] [CrossRef]
- Maidur, S.R.; Patil, P.S.; Katturi, N.K.; Soma, V.R.; Wong, Q.A.; Quah, C.K. Ultrafast nonlinear optical and structure–property relationship studies of pyridine-based anthracene chalcones using z-scan, degenerate four-wave mixing, and computational approaches. J. Phys. Chem. B 2021, 125, 3883–3898. [Google Scholar] [CrossRef] [PubMed]
- Hetmanczyk, Ł.; Szklarz, P.; Kwocz, A.; Wierzejewska, M.; Pagacz-Kostrzewa, M.; Melnikov, M.Y.; Tolstoy, P.M.; Filarowski, A. Polymorphism and conformational equilibrium of nitro-acetophenone in solid state and under matrix conditions. Molecules 2021, 26, 3109. [Google Scholar] [CrossRef]
- Robyt, J.F.; Yoon, S.-H.; Mukerjea, R. Dextransucrase and the mechanism for dextran biosynthesis. Carbohyd. Res. 2008, 343, 3039–3048. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Kim, J.M.; Han, K.-I.; Jung, E.-G.; Kim, Y.H.; Patnaik, B.B.; Yoon, M.S.; Chung, S.K.; Kim, W.J.; Han, M.-D. Mutan: A mixed linkage α-[(1,3)- and (1,6)]-d-glucan from Streptococcus mutans, that induces osteoclast differentiation and promotes alveolar bone loss. Carbohyd. Polym. 2016, 137, 561–569. [Google Scholar] [CrossRef]
- Hirata, Y.; Sano, Y.; Aoki, M.; Shoji, H.; Kato, S.; Yamamoto, H. Small-angle X-ray scattering studies on nucleation formation of dextran precipitation in the presence of boron. J. Colloid Interface Sci. 2000, 223, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Sano, Y.; Aoki, M.; Kobatake, H.; Kato, S.; Yamamoto, H. Structural change in dextran: Mechanism of insolubilization by adsorption on the air–liquid Interface. J. Colloid Interface Sci. 1999, 212, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Stenekes, R.J.H.; Talsma, H.; Hennink, W.E. Formation of dextran hydrogels by crystallization. Biomaterials 2001, 22, 1891–1898. [Google Scholar] [CrossRef]
- Durand, A.; Marie, E.; Rotureau, E.; Leonard, M.; Dellacherie, E. Amphiphilic polysaccharides: Useful tools for the preparation of nanoparticles with controlled surface characteristics. Langmuir 2004, 20, 6956–6963. [Google Scholar] [CrossRef]
- Mellors, R.; Benzeval, I.; Eisenthal, R.; Hubble, J. Preparation of self-assembled microspheres and their potential for drug delivery. Pharm. Dev. Technol. 2010, 15, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Sun, H.X.; Tang, S.H.; Feng, H.M.; Zhang, H.B.; Chen, K.; Li, P.; Niu, Q.J. Nanofiltration membranes with enhanced performance by constructing an interlayer integrated with dextran nanoparticles and polyethyleneimine coating. J. Membr. Sci. 2022, 654, 120537. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Bartolome-Cabrero, R.; Rodriguez, M.D.; Dos Santos, C.S.; Rueda, N.; Fernandez-Lafuente, R. Stabilizing effects of cations on lipases depend on the immobilization protocol. RSC Adv. 2015, 5, 83868–83875. [Google Scholar] [CrossRef]
- Sharma, A.; Thatai, K.S.; Kuthiala, T.; Singh, G.; Arya, S.K. Employment of polysaccharides in enzyme immobilization. React. Funct. Polym. 2021, 167, 105005. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar] [PubMed]
- Martino, A.; Durante, M.; Pifferi, P.G.; Spagna, G.; Bianchi, G. Immobilization of β-glucosidase from a commercial preparation. Part 1. A comparative study of natural supports. Process Biochem. 1996, 31, 281–285. [Google Scholar] [CrossRef]
- Wang, T.T.; Jiang, Z.M.; Wang, Y.Y.; Wu, H.; Fang, Y.; Dong, W.L.; Wu, B.; Ma, J.F.; Jiang, M. Low Molar Mass Dextran: One-Step Synthesis With Dextransucrase by Site-Directed Mutagenesis and its Potential of Iron-Loading. Front. Bioeng. Biotechnol. 2021, 9, 747602. [Google Scholar] [CrossRef]
- Liu, T.; Liu, T.T.; Liu, H.C.; Fan, H.X.; Chen, B.Y.; Wang, D.W.; Zhang, Y.R.; Sun, F.J. Preparation and Characterization of a Novel Polysaccharide-Iron(III) Complex in Auricularia auricula Potentially Used as an Iron Supplement. BioMed Res. Int. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Hoy, R.S.; O’Hern, C.S. Glassy dynamics of crystallite formation: The role of covalent bonds. Soft Matter 2012, 8, 1215–1225. [Google Scholar] [CrossRef]
- Shingel, K.I. Determination of structural peculiarities of dextran, pullulan and γ-irradiated pullulan by Fourier-transform IR spectroscopy. Carbohydr. Res. 2002, 337, 1445–1451. [Google Scholar] [CrossRef]
- Miao, M.; Bai, A.J.; Jiang, B.; Song, Y.; Cui, S.W.; Zhang, T. Characterisation of a novel water-soluble polysaccharide from Leuconostoc citreum SK24.002. Food Hydrocoll. 2014, 36, 265–272. [Google Scholar] [CrossRef]
- Majumder, A.; Singh, A.; Goyal, A. Application of response surface methodology for glucan production from Leuconostoc dextranicum and its structural characterization. Carbohyd. Polym. 2009, 75, 150–156. [Google Scholar] [CrossRef]
- Alemán-Domínguez, M.E.; Giusto, E.; Ortega, Z.; Tamaddon, M.; Benítez, A.N.; Liu, C. Three-dimensional printed polycaprolactone-microcrystalline cellulose scaffolds. J. Biomed. Mater. Res. B 2019, 107, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Abu-Thabit, N.Y.; Judeh, A.A.; Hakeem, A.S.; UI-Hamid, A.; Umar, Y.; Ahmad, A. Isolation and characterization of microcrystalline cellulose from date seeds (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2020, 155, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Du, H.S.; Liu, W.; Zhang, M.M.; Si, C.L.; Zhang, X.Y.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohyd. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Kosmulski, M.; Mączka, E.; Ruchomski, L. Two types of electrokinetic behavior of solid particles in the presence of anionic surfactants. J. Colloid Interface Sci. 2019, 533, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Wang, S.C.; Xing, Z.; Niu, Y.M.; Liao, Z.C.; Lu, Y.; Qiu, J.N.; Zhang, J.F.; Wang, C.M.; Dong, L. Destructing biofilms by cationic dextran through phase transition. Carbohyd. Polym. 2022, 279, 118778. [Google Scholar] [CrossRef]
- Caroline Diana Sherly, M.; Rekha, M.R. Efficacy of vinyl imidazole grafted cationized pullulan and dextran as gene delivery vectors: A comparative study. Int. J. Biol. Macromol. 2017, 105, 947–955. [Google Scholar] [CrossRef]
- Jiang, D.H.; Salem, A.K. Optimized dextran–polyethylenimine conjugates are efficient non-viral vectors with reduced cytotoxicity when used in serum containing environments. Int. J. Pharmaceut. 2012, 427, 71–79. [Google Scholar] [CrossRef]
- Zaitsev, S.Y.; Savina, A.A.; Zaitsev, I.S. Biochemical aspects of lipase immobilization at polysaccharides for biotechnology. Adv. Colloid Interfac. 2019, 272, 102016. [Google Scholar] [CrossRef]
- Jin, W.P.; Wang, Z.F.; Peng, D.F.; Shen, W.Y.; Zhu, Z.Z.; Cheng, S.Y.; Li, B.; Huang, Q.R. Effect of linear charge density of polysaccharides on interactions with α-amylase: Self-Assembling behavior and application in enzyme immobilization. Food Chem. 2020, 331, 127320. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.W.; Zhang, H.B.; Zhang, Y.Q.; Hu, X.Q. Biosynthesis of oligodextrans with different Mw by synergistic catalysis of dextransucrase and dextranase. Carbohyd. Polym. 2014, 112, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Bejar, W.; Gabriel, V.; Amari, M.; Morel, S.; Mezghani, M.; Maguin, E.; Fontagné-Faucher, C.; Bejar, S.; Chouayekh, H. Characterization of glucansucrase and dextran from Weissella sp. TN610 with potential as safe food additives. Int. J. Biol. Macromol. 2013, 52, 125–132. [Google Scholar] [CrossRef] [PubMed]
| Initial Concentration of Dextran Solution (g/L) | Yield of IS-Dextran (%) |
|---|---|
| 90 | 0.51 ± 0.05 |
| 120 | 1.30 ± 0.11 |
| 240 | 28.71 ± 5.38 |
| Initial Concentration of Dextran (g/L) | Drying Method | Yield of IS-Dextran (%) |
|---|---|---|
| 90 | Spray drying | N.D. |
| 90 | Oven drying | 59.1 ± 5.3 |
| 120 | Spray drying | N.D. |
| 120 | Oven drying | 65.8 ± 4.9 |
| 240 | Spray drying | N.D. |
| 240 | Oven drying | 67.4 ± 4.1 |
| Solvent | Condition | Solubility |
|---|---|---|
| Distilled water | 80 °C,15 min | Insoluble |
| 0.5 M NaOH | 80 °C, 15 min | Insoluble |
| 0.5 M NaOH + 1 M Urea | 80 °C, 15 min | Insoluble |
| DMSO | 80 °C, 15 min | Dissolved completely |
| Ethanol | 40 °C, 15 min | Insoluble |
| Mw (Da) | PDI | |
|---|---|---|
| S-dextran | 6702 ± 274 | 1.64 ± 0.09 |
| IS-dextran | 6555 ± 155 | 1.98 ± 0.06 |
| Condition | PDI | |
|---|---|---|
| IS-dextran-PEI600 | PEI600 | 20.9 ± 2.1 |
| IS-dextran-PEI1800 | PEI1800 | 34.4 ± 3.9 |
| IS-dextran-PEI10,000 | PEI10,000 | 37.7 ± 2.4 |
| IS-dextran-PEI600 | PEI600 + Epichlorohydrin | 34.7 ± 3.5 |
| IS-dextran-PEI1800 | PEI1800 + Epichlorohydrin | 39.6 ± 3.1 |
| IS-dextran-PEI10,000 | PEI10,000 + Epichlorohydrin | 35.3 ± 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Sun, K.; Wu, H.; Dong, W.; Ma, J.; Jiang, M. Preparation and Characterization of a Novel Morphosis of Dextran and Its Derivatization with Polyethyleneimine. Molecules 2023, 28, 7210. https://doi.org/10.3390/molecules28207210
Jiang Z, Sun K, Wu H, Dong W, Ma J, Jiang M. Preparation and Characterization of a Novel Morphosis of Dextran and Its Derivatization with Polyethyleneimine. Molecules. 2023; 28(20):7210. https://doi.org/10.3390/molecules28207210
Chicago/Turabian StyleJiang, Zhiming, Kaifeng Sun, Hao Wu, Weiliang Dong, Jiangfeng Ma, and Min Jiang. 2023. "Preparation and Characterization of a Novel Morphosis of Dextran and Its Derivatization with Polyethyleneimine" Molecules 28, no. 20: 7210. https://doi.org/10.3390/molecules28207210
APA StyleJiang, Z., Sun, K., Wu, H., Dong, W., Ma, J., & Jiang, M. (2023). Preparation and Characterization of a Novel Morphosis of Dextran and Its Derivatization with Polyethyleneimine. Molecules, 28(20), 7210. https://doi.org/10.3390/molecules28207210






