Unveiling Various Facades of Tinospora cordifolia Stem in Food: Medicinal and Nutraceutical Aspects
Abstract
:1. Introduction
2. Phytochemistry of T. cordifolia
2.1. Biological Activity
2.2. Pharmacological Properties
2.3. Nutraceuticals
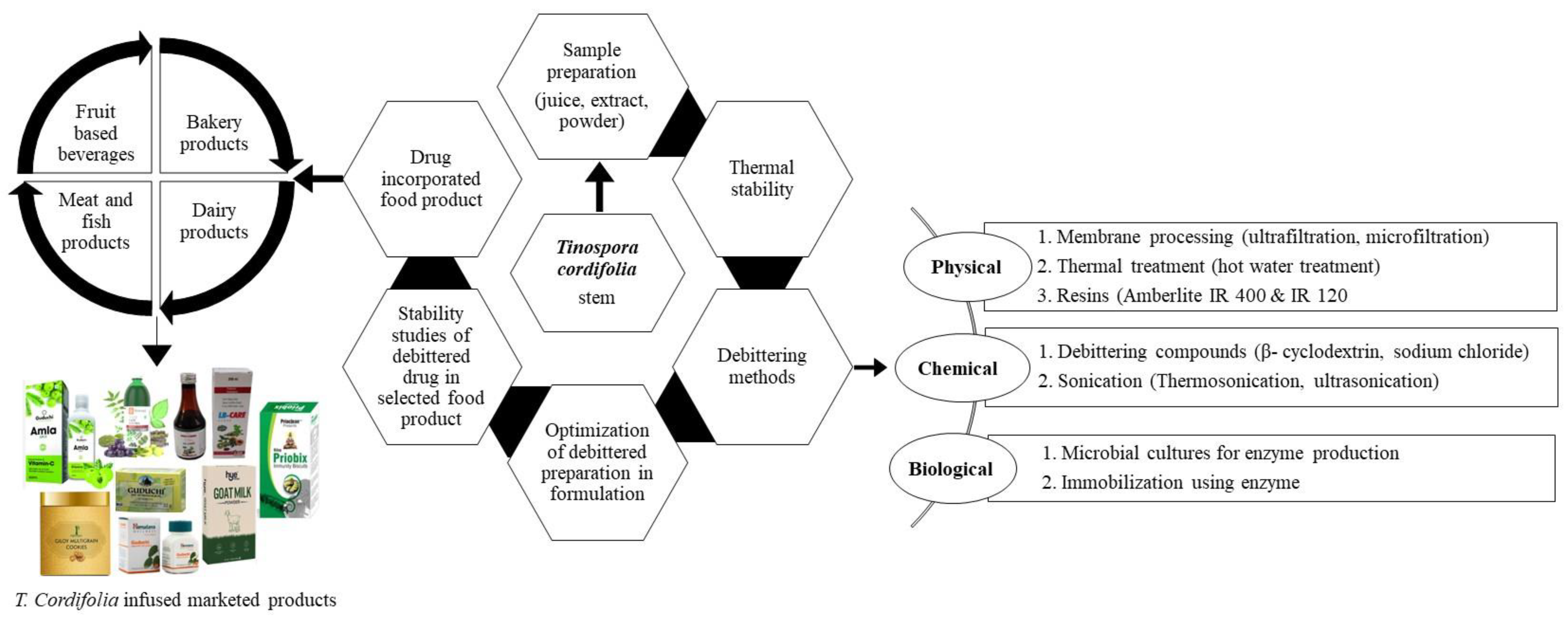
2.4. An Insight for Application in Food Products
2.4.1. Various Ways of Using T. Cordifolia in Foods
2.4.2. Debitter Methodology in Food Products
2.4.3. Functional Ingredient in Food Products
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rani, J.; Singh, L.; Singh, H.; Kapoor, M.; Singh, G. Preliminary phytochemical analysis of different solvent extracts from leaf and stem of Tinospora cordifolia. Int. J. Phyther. 2015, 5, 124–128. [Google Scholar]
- Sharma, P.; Dwivedee, B.P.; Bisht, D.; Dash, A.K.; Kumar, D. The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon 2019, 5, e02437. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, R.; Pandey, R.P.; Sharma, V.; Verma, A.K. Assessment of the multifaceted immunomodulatory potential of the aqueous extract of Tinospora cordifolia. Res. J. Chem. Sci. 2011, 1, 71–79. [Google Scholar]
- Singh, D.; Chaudhuri, P.K. Chemistry and pharmacology of Tinospora cordifolia. Nat. Prod. Commun. 2017, 12, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; She, G.; Han, D.; Wang, W.; Liu, Z.; Liu, B. Genus Tinospora: Ethnopharmacology, phytochemistry, and pharmacology. Evid.-Based Complement. Altern. Med. 2016, 2016, 9232593. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Ashi, H.; Hosmani, J.; Almalki, A.Y.; Alhazmi, Y.A.; Mushtaq, S.; Parveen, S.; Baeshen, H.A.; Varadarajan, S.; Raj, A.T.; et al. Tinospora cordifolia (Thunb.) Miers (Giloy) inhibits oral cancer cells in a dose-dependent manner by inducing apoptosis and attenuating epithelial-mesenchymal transition. Saudi J. Biol. Sci. 2021, 28, 4553–4559. [Google Scholar] [CrossRef]
- Sengupta, M.; Sharma, G.D.; Chakraborty, B. Effect of aqueous extract of Tinospora cordifolia on functions of peritoneal macrophages isolated from CCl4 intoxicated male albino mice. BMC Complement. Altern. Med. 2011, 11, 102. [Google Scholar] [CrossRef]
- Ilaiyaraja, N.; Khanum, F. Antioxidant potential of Tinospora cordifolia extracts and their protective effect on oxidation of biomolecules. Pharmacog. J. 2011, 3, 56–62. [Google Scholar] [CrossRef]
- Gowrishankar, R.; Kumar, M.; Menon, V.; Divi, S.M.; Saravanan, M.; Magudapathy, P.; Panigrahi, B.K.; Nair, K.G.; Venkataramaniah, K. Trace element studies on Tinospora cordifolia (Menispermaceae), Ocimum sanctum (Lamiaceae), Moringa oleifera (Moringaceae), and Phyllanthus niruri (Euphorbiaceae) Using PIXE. Biologic. Trace Elem. Res. 2010, 133, 357–363. [Google Scholar] [CrossRef]
- Tiwari, M.; Dwivedi, U.N.; Kakkar, P. Tinospora cordifolia extract modulates COX-2, iNOS, ICAM-1, pro-inflammatory cytokines and redox status in murine model of asthma. J. Ethnopharmacol. 2014, 153, 326–337. [Google Scholar] [CrossRef]
- Sharma, A.; Shanker, C.; Tyagi, L.K.; Singh, M.; Rao, C.V. Herbal medicine for market potential in India: An overview. Acad. J. Plant Sci. 2008, 1, 26–36. [Google Scholar]
- Sharma, B.; Dutt, V.; Kaur, N.; Mittal, A.; Dabur, R. Tinospora cordifolia protects from skeletal muscle atrophy by alleviating oxidative stress and inflammation induced by sciatic denervation. J. Ethnopharmacol. 2020, 254, 112720. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kaur, S.; Goswami, M.; Singh, S.; Sharma, A.; Mehta, N. Antioxidant and antimicrobial efficacy of giloy (Tinospora cordifolia) stem powder in spent hen meat patties under aerobic packaging at refrigeration temperature (4 ± 1 °C). J. Food Process. Preserv. 2021, 45, e15772. [Google Scholar] [CrossRef]
- Parveen, A.; Wang, Y.H.; Fantoukh, O.; Alhusban, M.; Raman, V.; Ali, Z.; Khan, I.A. Development of a chemical fingerprint as a tool to distinguish closely related Tinospora species and quantitation of marker compounds. J. Pharm. Biomed. Anal. 2020, 178, 112894. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Sachan, S.; Khandia, R.; Munjal, A.; Iqbal, H.M.N.; Latheef, S.K.; Karthik, K.; Samad, H.A.; Tiwari, R.; Dadar, M. Medicinal and beneficial health applications of Tinospora cordifolia (Guduchi): A miraculous herb countering various diseases/disorders and its immunomodulatory effects. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017, 10, 96–111. [Google Scholar] [CrossRef]
- Sharma, A.; Bajaj, P.; Bhandari, A.; Kaur, G. From ayurvedic folk medicine to preclinical neurotherapeutic role of a miraculous herb, Tinospora cordifolia. Neurochem. Int. 2020, 141, 104891. [Google Scholar] [CrossRef]
- Balkrishna, A.; Pokhrel, S.; Tomer, M.; Verma, S.; Kumar, A.; Nain, P.; Gupta, A.; Varshney, A. Anti-acetylcholinesterase activities of mono-herbal extracts and exhibited synergistic effects of the phytoconstituents: A biochemical and computational study. Molecules 2019, 24, 4175. [Google Scholar] [CrossRef]
- Palmieri, A.; Scapoli, L.; Iapichino, A.; Mercolini, L.; Mandrone, M.; Poli, F.; Giannì, A.B.; Baserga, C.; Martinelli, M. Berberine and Tinospora cordifolia exert a potential anticancer effect on colon cancer cells by acting on specific pathways. Int. J. Immunopathol. Pharmacol. 2019, 33, 1–10. [Google Scholar] [CrossRef]
- Parveen, A.; Adams, J.S.; Raman, V.; Budel, J.M.; Zhao, J.; Babu, G.N.M.; Ali, Z.; Khan, I.A. Comparative morpho-anatomical and HPTLC profiling of Tinospora species and dietary supplements. Planta Med. 2020, 86, 470–481. [Google Scholar] [CrossRef]
- Sharma, B.; Yadav, A.; Dabur, R. Interactions of a medicinal climber Tinospora cordifolia with supportive interspecific plants trigger the modulation in its secondary metabolic profiles. Sci. Rep. 2019, 9, 14327. [Google Scholar] [CrossRef]
- Sharma, A.; Saggu, S.K.; Mishra, R.; Kaur, G. Anti-brain cancer activity of chloroform and hexane extracts of Tinospora cordifolia Miers: An in vitro perspective. Ann. Neurosci. 2019, 26, 10–20. [Google Scholar] [CrossRef]
- Gangan, V.D.; Pradhan, P.; Sipahimalani, A.T.; Banerji, A. Cordifolisides A, B, C: Norditerpene furan glycosides from Tinospora cordifolia. Phytochemistry 1994, 37, 781–786. [Google Scholar] [CrossRef]
- Chintalwar, G.; Jain, A.; Sipahimalani, A.; Banerji, A.; Sumariwalla, P.; Ramakrishnan, R.; Sainis, K. An immunologically active arabinogalactan from Tinospora cordifolia. Phytochemistry 1999, 52, 1089–1093. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; Leskovec, J.; Ali kamboh, A.; Kakar, I.; Ullah, K.; Ahmad, F.; Sharif, M.; Javaid, A.; Rauf, M.; et al. Using Guduchi (Tinospora cordifolia) as an eco-friendly feed supplement in human and poultry nutrition. Poult. Sci. 2020, 99, 801–811. [Google Scholar] [CrossRef]
- Bernard, P.K.; Andrea, G.; Nicole, K.L.; Verena, A.; Cristina, G.; Woojoo, K.; Sean, M.K.; Jeffrey, S.B.; Neile, G.; Mingliang, F.; et al. Intestinal bitter taste receptor activation alters hormone secretion and imparts metabolic benefits. Mol. Metab. 2018, 16, 76–87. [Google Scholar]
- Kapil, A.; Sharma, S. Immunopotentiating compounds from Tinospora cordifolia. J. Ethnopharmacol. 1997, 58, 89–95. [Google Scholar] [CrossRef]
- Maurya, R.; Wazir, V.; Tyagi, A.; Kapil, R.S. Clerodane diterpenoids from Tinospora cordifolia. Phytochemistry 1995, 38, 659–661. [Google Scholar] [CrossRef]
- Khan, M.A.; Gray, A.I.; Waterman, P.F. Tinosporaside, an 18-norclerodane glucoside from Tinospora cordifolia. Phytochemistry 1989, 28, 273–275. [Google Scholar] [CrossRef]
- Choudhary, N.; Siddiqui, M.B.; Azmat, S.; Khatoon, S. Tinospora cordifolia: Ethnobotany, phytopharmacology and phytochemistry aspects. Int. J. Pharm. Sci. Res. 2013, 4, 891–899. [Google Scholar]
- Bala, M.; Pratap, K.; Verma, P.K.; Singh, B.; Padwad, Y. Validation of ethnomedicinal potential of Tinospora cordifolia for anticancer and immunomodulatory activities and quantification of bioactive molecules by HPTLC. J. Ethnopharmacol. 2015, 175, 131–137. [Google Scholar] [CrossRef]
- Bisset, N.G.; Nwaiwu, J. Quaternary alkaloids of Tinospora species. Planta Med. 1983, 48, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Hanuman, J.B.; Bhatt, R.K.; Sabata, B.K. A diterpenoid furanolactone from Tinospora cordifolia. Phytochemistry 1986, 25, 1677–1680. [Google Scholar] [CrossRef]
- Hanuman, J.B.; Mishra, A.K.; Sabata, B. A natural phenolic lignan from Tinospora cordifolia Miers. J. Chem. Soc. Perkin Trans. 1 1986, 4, 1181–1185. [Google Scholar] [CrossRef]
- Hanuman, J.B.; Bhatt, R.K.; Sabata, B. A clerodane furano-diterpene from Tinospora cordifolia. J. Nat. Prod. 1989, 51, 197–201. [Google Scholar] [CrossRef]
- Maurya, R.; Wazir, V.; Kapil, A.; Kapil, R.S. Cordifoliosides A and B, two new phenylpropene disaccharides from Tinospora cordifolia possessing immunostimulant activity. Nat. Prod. Lett. 1996, 8, 7–10. [Google Scholar] [CrossRef]
- Wazir, V.; Maurya, R.; Kapil, R.S. Cordioside, a clerodane furano diterpene glucoside from Tinospora cordifolia. Phytochemistry 1995, 38, 447–449. [Google Scholar] [CrossRef]
- Bhatt, R.K.; Sabata, B.K. A furanoid diterpene glucoside from Tinospora cordifolia. Phytochemistry 1989, 28, 2419–2422. [Google Scholar] [CrossRef]
- Pathak, A.K.; Agarwal, P.K.; Jain, D.C.; Sharma, R.P.; Howarth, O.W. NMR studies of 20β-hydroxyecdysone, a steroid; isolated from Tinospora cordifolia. Indian J. Chem. Sec. B 1995, 34, 674–676. [Google Scholar]
- Sharma, A.K.; Kishore, K.; Sharma, D.; Srinivasan, B.P.; Agarwal, S.S.; Sharma, A.; Singh, S.K.; Gaur, S.; Jatav, V.S. Cardioprotective activity of alcoholic extract of Tinospora cordifolia (Willd.) Miers in calcium chloride-induced cardiac arrhythmia in rats. J. Biomed. Res. 2011, 25, 280–286. [Google Scholar] [CrossRef]
- Maurya, R.; Handa, S.S. Tinocordifolin, a sesquiterpene from Tinospora cordifolia. Phytochemistry 1998, 49, 1343–1345. [Google Scholar] [CrossRef]
- Pathak, A.K.; Jain, D.C.; Sharma, R.P. Chemistry and biological activities of the genera Tinaspora. Int. J. Pharmacogn. 1995, 33, 277–287. [Google Scholar] [CrossRef]
- Upadhyay, N.; Ganie, S.A.; Agnihotri, R.K.; Sharma, R. Free Radical Scavenging Activity of Tinospora cordifolia (Willd.) Miers. J. Pharmacogn. Phytochem. 2014, 3, 63–69. [Google Scholar]
- Desai, V.R.; Ramkrishnan, R.; Chintalwar, G.J.; Sainis, K.B. G1-4A, an immunomodulatory polysaccharide from Tinospora cordifolia, modulates macrophage responses and protects mice against lipopolysaccharide induced endotoxic shock. Int. Immunopharmacol. 2007, 7, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Rajan, M.G.R.; Kulkarni, S. Activation of murine macrophages by G1-4A, a polysaccharide from Tinospora cordifolia, in TLR4/MyD88 dependent manner. Int. Immunopharmacol. 2017, 50, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.K.R.; Melnick, S.J.; Ramachandran, R.; Escalon, E.; Ramachandran, C. Mechanism of macrophage activation by (1,4)-alpha-D-glucan isolated from Tinospora cordifolia. Int. Immunopharmacol. 2006, 6, 1815–1824. [Google Scholar] [CrossRef]
- Aranha, I.; Clement, F.; Venkatesh, Y.P. Immunostimulatory properties of the major protein from the stem of the Ayurvedic medicinal herb, guduchi (Tinospora cordifolia). J. Ethnopharmacol. 2012, 139, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Kavya, B.; Kavya, N.; Ramarao, V.; Venkateshwarly, G. Tinospora cordifolia (Willd.) Miers: Nutritional, ethnomedical and therapeutic utility. Int. J. Res. Ayurveda Pharm. 2015, 6, 195–198. [Google Scholar]
- Sangeetha, M.K.; Balaji Raghavendran, H.R.; Gayathri, V.; Vasanthi, H.R. Tinospora cordifolia attenuates oxidative stress and distorted carbohydrate metabolism in experimentally induced type 2 diabetes in rats. J. Nat. Med. 2011, 65, 544–550. [Google Scholar] [CrossRef]
- Dhingra, D.; Goyal, P.K. Evidences for the involvement of monoaminergic and GABAergic systems in antidepressant-like activity of Tinospora cordifolia in mice. Indian J. Pharm. Sci. 2008, 70, 761–767. [Google Scholar] [CrossRef]
- Sudha, P.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement. Altern. Med. 2011, 11, 5. [Google Scholar]
- Patil, K.G. Antidiabetic activity of Tinospora cordifolia (fam: menispermaceae) in alloxan treated albino rats. Appl. Res. J. 2015, 1, 316–319. [Google Scholar]
- Patle, D.; Vyas, M.; Khatik, G.L. A Review on natural products and herbs used in the management of diabetes. Curr. Diabetes Rev. 2021, 17, 186–197. [Google Scholar] [PubMed]
- Sachan, S.; Dhama, K.; Latheef, S.K.; Samad, H.A.; Mariappan, A.K.; Munuswamy, P.; Singh, R.; Singh, K.P.; Malik, Y.S.; Singh, R.K. Immunomodulatory potential of Tinospora cordifolia and CpG ODN (TLR21 Agonist) against the Very Virulent, Infectious Bursal Disease Virus in SPF Chicks. Vaccines 2019, 7, 106. [Google Scholar] [CrossRef]
- Bishayi, B.; Roychowdhury, S.; Ghosh, S.; Sengupta, M. Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl4 intoxicated mature albino rats. J. Toxicol. Sci. 2002, 27, 139–146. [Google Scholar] [CrossRef]
- Subramanian, M.; Chintalwar, G.J.; Chattopadhyay, S. Antioxidant properties of a Tinospora cordifolia polysaccharide against iron-mediated lipid damage and gamma-ray induced protein damage. Redox Rep. 2002, 7, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mittal, J.; Pal, U.; Sharma, L.; Verma, A.K.; Ghosh, M.; Sharma, M.M. Unveiling the cytotoxicity of phytosynthesised silver nanoparticles using Tinospora cordifolia leaves against human lung adenocarcinoma A549 cell line. IET Nanobiotechnol. 2020, 14, 230–238. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; AlYahya, S.; Jalal, M.; Khan, H.M.; Asiri, S.M.M.; Ahmad, W.; Mahdi, A.A.; et al. Biogenic gold nanoparticles as potent antibacterial and antibiofilm nano-antibiotics against Pseudomonas aeruginosa. Antibiotics 2020, 9, 100. [Google Scholar] [CrossRef]
- Rawal, A.; Muddeshwar, M.; Biswas, S. Effect of Rubia cordifolia, Fagonia cretica linn, and Tinospora cordifolia on free radical generation and lipid peroxidation during oxygen-glucose deprivation in rat hippocampal slices. Biochem. Biophys. Res. Commun. 2004, 324, 588–596. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, A.; Goswami, R.; Basu, S. Hypoglycemic activity of the antioxidant saponarin, characterized as alpha-glucosidase inhibitor present in Tinospora cordifolia. J. Enzyme Inhib. Med. Chem. 2009, 24, 684–690. [Google Scholar] [CrossRef]
- Goel, H.C.; Prasad, J.; Singh, S.; Sagar, R.K.; Agrawala, P.K.; Bala, M.; Sinha, A.K.; Dogra, R. Radioprotective potential of an herbal extract of Tinospora cordifolia. J. Radiat. Res. 2004, 45, 61–68. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, V. Ameliorative effects of Tinospora cordifolia root extract on histopathological and biochemical changes induced by aflatoxin-b(1) in mice kidney. Toxicol. Int. 2011, 18, 94–98. [Google Scholar] [PubMed]
- Patgiri, B.; Umretia, B.L.; Vaishnav, P.U.; Prajapati, P.K.; Shukla, V.J.; Ravishankar, B. Anti-inflammatory activity of guduchi ghana (aqueous extract of Tinospora cordifolia Miers.). AYU (An Int. Q. J. Res. Ayurveda) 2014, 35, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Mishra, S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine 2011, 18, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Joseph, L.; Mathew, M.; Minu Mathew, C.; Josepha, L. A research on screening of learning and memory enhancing activity of whole plant extract of Tinospora cordifolia (Willd). Pharma Innov. J. 2016, 5, 104–107. [Google Scholar]
- Jayaprakash, R.; Ramesh, V.; Sridhar, M.P.; Sasikala, C. Antioxidant activity of ethanolic extract of Tinospora cordifolia on N-nitrosodiethylamine (diethylnitrosamine) induced liver cancer in male Wister albino rats. J. Pharm. Bioallied Sci. 2015, 7, S40–S45. [Google Scholar]
- Shwetha, R.J.; Tahareen, S.; Myrene, R.D. Antioxidant and anti-inflammatory activity of Tinospora cordifolia using in vitro models. J. Chem. Biol. Phy. Sci. 2016, 6, 497–512. [Google Scholar]
- Mehra, R.; Naved, T.; Arora, M.; Madan, S. Standardization and evaluation of formulation parameters of Tinospora cordifolia tablet. J. Adv. Pharm. Educ. Res. 2013, 3, 440–449. [Google Scholar]
- Birla, H.; Rai, S.N.; Singh, S.S.; Zahra, W.; Rawat, A.; Tiwari, N.; Singh, R.K.; Pathak, A.; Singh, S.P. Tinospora cordifolia suppresses neuroinflammation in parkinsonian mouse model. Neuromol. Med. 2019, 21, 42–53. [Google Scholar] [CrossRef]
- Javir, G.; Joshi, K. Evaluation of the combinatorial effect of Tinospora cordifolia and Zingiber officinale on human breast cancer cells. 3 Biotech 2019, 9, 428. [Google Scholar] [CrossRef]
- Ansari, J.A.; Rastogi, N.; Ahmad, M.K.; Mahdi, A.A.; Khan, A.R.; Thakur, R.; Srivastava, V.K.; Mishra, D.P.; Fatima, N.; Khan, H.J.; et al. ROS mediated pro-apoptotic effects of Tinospora cordifolia on breast cancer cells. Front. Biosci. Elite 2017, 9, 89–100. [Google Scholar]
- Mishra, R.; Kaur, G. Aqueous ethanolic extract of Tinospora cordifolia as a potential candidate for differentiation based therapy of glioblastomas. PLoS ONE 2013, 8, e78764. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Dixit, S. Extraction optimization of Tinospora cordifolia and assessment of the anticancer activity of its alkaloid palmatine. Sci. World J. 2013, 2013, 376216. [Google Scholar] [CrossRef]
- Verma, R.; Chaudhary, H.S.; Agrawal, R.C. Evaluation of anticarcinogenic and antimutagenic effect of Tinospora cordifolia in experimental animals. J. Chem. Pharm. Res. 2011, 3, 877–881. [Google Scholar]
- Chandrasekaran, C.V.; Mathuram, L.N.; Daivasigamani, P.; Bhatnagar, U. Tinospora cordifolia, a safety evaluation. Toxicol. In Vitro 2009, 23, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Atal, C.K.; Sharma, M.L.; Kaul, A.; Khajuria, A. Immunomodulating agents of plant origin. I: Preliminary screening. J. Ethnopharmacol. 1986, 18, 133–141. [Google Scholar] [CrossRef]
- Pahadiya, S.; Sharma, J. Alteration of lethal effects of gamma rays in Swiss albino mice by Tinospora cordifolia. Phyther. Res. 2003, 17, 552–554. [Google Scholar] [CrossRef]
- Shirolkar, A.; Sharma, B.; Lata, S.; Dabur, R. Guduchi Sawras (Tinospora cordifolia): An Ayurvedic drug treatment modulates the impaired lipid metabolism in alcoholics through dopaminergic neurotransmission and anti-oxidant defense system. Biomed. Pharmacother. 2016, 83, 1265–1277. [Google Scholar] [CrossRef]
- Nema, A.; Gupta, N.; Jain, U.K. Evaluation of Wound healing activity of Tinospora cordifolia Willd. Der Pharm. Sin. 2012, 3, 126–130. [Google Scholar]
- Goel, B.; Pathak, N.; Nim, D.K.; Singh, S.K.; Dixit, R.K.; Chaurasia, R. Clinical evaluation of analgesic activity of Guduchi (Tinospora cordifolia) using animal model. J. Clin. Diagn. Res. 2014, 8, HC01. [Google Scholar] [CrossRef]
- Mathew, S.; Kuttan, G. Immunomodulatory and antitumour activities of Tinospora cordifolia. Fitoterapia 1999, 70, 35–43. [Google Scholar] [CrossRef]
- Sudhakaran, D.S.; Srirekha, P.; Devasree, L.D.; Premsingh, S.; Michael, R.D. Immunostimulatory effect of Tinospora cordifolia Miers leaf extract in Oreochromis mossambicus. Indian J. Exp. Biol. 2006, 44, 726–732. [Google Scholar] [PubMed]
- Sachdeva, H.; Sehgal, R.; Kaur, S. Tinospora cordifolia as a protective and immunomodulatory agent in combination with cisplatin against murine visceral leishmaniasis. Exp. Parasitol. 2014, 137, 53–65. [Google Scholar] [CrossRef]
- Sohni, Y.R.; Bhatt, R.M. Activity of a crude extract formulation in experimental hepatic amoebiasis and in immunomodulation studies. J. Ethnopharmacol. 1996, 54, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chakraborty, P.; Kumar, S.; Singh, P.K.; Rajan, M.G.R.; Sainis, K.B.; Kulkarni, S. G1-4A, a polysaccharide from Tinospora cordifolia inhibits the survival of mycobacterium tuberculosis by modulating host immune responses in TLR4 dependent manner. PLoS ONE 2016, 11, e0154725. [Google Scholar] [CrossRef] [PubMed]
- Pendse, V.K.; Dadhich, A.P.; Mathur, P.N.; Bal, M.S.; Madan, B.R. Antiinflammatory, immunosuppressive and some related pharmacological actions of the water extract of Neem Giloe (Tinospora cordifolia): A preliminary report. Indian J. Pharmacol. 1977, 9, 221–224. [Google Scholar]
- Dhuley, J.N. Effect of some Indian herbs on macrophage functions in ochratoxin A treated mice. J. Ethnopharmacol. 1997, 58, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Diwanay, S.; Chitre, D.; Patwardhan, B. Immunoprotection by botanical drugs in cancer chemotherapy. J. Ethnopharmacol. 2004, 90, 49–55. [Google Scholar] [CrossRef]
- Herowati, R.; Widodo, G.P. Molecular docking studies of chemical constituents of Tinospora cordifolia on glycogen phosphorylase. Procedia Chem. 2014, 13, 63–68. [Google Scholar] [CrossRef]
- Joladarashi, D.; Chilkunda, N.D.; Salimath, P.V. Glucose uptake-stimulatory activity of Tinospora cordifolia stem extracts in Ehrlich ascites tumor cell model system. J. Food Sci. Technol. 2014, 51, 178–182. [Google Scholar] [CrossRef]
- Stanely, P.; Prince, M.; Menon, V.P. Hypoglycaemic and other related actions of Tinospora cordifolia roots in alloxan-induced diabetic rats. J. Ethnopharmacol. 2000, 70, 9–15. [Google Scholar] [CrossRef]
- Puranik, N.; Kammar, K.F.; Devi, S. Anti-diabetic activity of Tinospora cordifolia (Willd.) in streptozotocin diabetic rats; does it act like sulfonylureas? Turk. J. Med. Sci. 2010, 40, 265–270. [Google Scholar] [CrossRef]
- Chougale, A.D.; Ghadyale, V.A.; Panaskar, S.N.; Arvindekar, A.U. Alpha glucosidase inhibition by stem extract of Tinospora cordifolia. J. Enzyme Inhib. Med. Chem. 2009, 24, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Adhvaryu, M.R.; Reddy, N.; Parabia, M.H. Effects of four Indian medicinal herbs on Isoniazid-, Rifampicin- and Pyrazinamide-induced hepatic injury and immunosuppression in guinea pigs. World J. Gastroenterol. 2007, 13, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.K.; Vats, V.; Rathi, S.S.; Dawar, R. Traditional Indian anti-diabetic plants attenuate progression of renal damage in streptozotocin induced diabetic mice. J. Ethnopharmacol. 2001, 76, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Utpalendu, J.; Chattopadhyay, R.N.; Badri, P.S. Preliminary studies on anti-inflammatory activity of Zingiber officinale rosc., Vitex negundo Linn. and Tinospora cordifolia (Willid) miers in albino rats. Indian J. Pharmacol. 1999, 31, 232–233. [Google Scholar]
- Jagetia, G.C.; Nayak, V.; Vidyasagar, M.S. Evaluation of the antineoplastic activity of guduchi (Tinospora cordifolia) in cultured HeLa cells. Cancer Lett. 1998, 127, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Yang, J.S.; Lu, C.C.; Chiang, J.H.; Li, T.C.; Lin, J.J.; Lai, K.C.; Liao, C.L.; Lin, J.G.; Chung, J.G. Berberine inhibits human tongue squamous carcinoma cancer tumor growth in a murine xenograft model. Phytomedicine 2009, 16, 887–890. [Google Scholar] [CrossRef]
- Mishra, R.; Kaur, G. Tinospora cordifolia Induces differentiation and senescence pathways in neuroblastoma cells. Mol. Neurobiol. 2015, 52, 719–733. [Google Scholar] [CrossRef]
- Dhanasekaran, M.; Baskar, A.A.; Ignacimuthu, S.; Agastian, P.; Duraipandiyan, V. Chemopreventive potential of Epoxy clerodane diterpene from Tinospora cordifolia against diethylnitrosamine-induced hepatocellular carcinoma. Investig. New Drugs 2009, 27, 347–355. [Google Scholar] [CrossRef]
- Leyon, P.V.; Kuttan, G. Effect of Tinospora cordifolia on the cytokine profile of angiogenesis-induced animals. Int. Immunopharmacol. 2004, 4, 1569–1575. [Google Scholar] [CrossRef]
- Leyon, P.V.; Kuttan, G. Inhibitory effect of a polysaccharide from Tinospora cordifolia on experimental metastasis. J. Ethnopharmacol. 2004, 90, 233–237. [Google Scholar] [CrossRef]
- Shivananjappa, M.M.; Muralidhara. Abrogation of maternal and fetal oxidative stress in the streptozotocin-induced diabetic rat by dietary supplements of Tinospora cordifolia. Nutrition 2012, 28, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.S.; Ramatholisamma, P.; Karuna, R.; Saralakumari, D. Preventive effect of Tinospora cordifolia against high-fructose diet-induced insulin resistance and oxidative stress in male Wistar rats. Food Chem. Toxicol. 2009, 47, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Devi, M.M.; Pal, S.; Tripathi, R.P.; Khushu, S. Metabolic regulatory variations in rats due to acute cold stress & Tinospora cordifolia intervention: High resolution 1H NMR approach. Metabolomics 2012, 8, 444–453. [Google Scholar]
- Antonisamy, P.; Dhanasekaran, M.; Ignacimuthu, S.; Duraipandiyan, V.; Balthazar, J.D.; Agastian, P.; Kim, J.H. Gastroprotective effect of epoxy clerodane diterpene isolated from Tinospora cordifolia Miers (Guduchi) on indomethacin-induced gastric ulcer in rats. Phytomedicine 2014, 21, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Piao, X.L.; Kim, J.M.; Kwon, S.W.; Park, J.H. Inhibition of cholinesterase and amyloid-beta aggregation by resveratrol oligomers from Vitis amurensis. Phyther. Res. 2008, 22, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, C.; Rahuman, A.A.; Rajakumar, G.; Vishnu Kirthi, A.; Santhoshkumar, T.; Marimuthu, S.; Bagavan, A.; Kamaraj, C.; Zahir, A.A.; Elango, G. Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heartleaf moonseed plant, Tinospora cordifolia Miers. Parasitol. Res. 2011, 109, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, M.; Eliza, J.; Priya, C.E.; Nirmala, A.; Daisy, P. Anti-diabetic properties of Tinospora cordifolia stem extracts on streptozotocin- induced diabetic rats. Afr. J. Pharm. Pharmacol. 2009, 3, 171–180. [Google Scholar]
- Prakash, R.; Sandhya, E.; Ramya, N.; Dhivya, R.; Priyadarshini, M.; Sakthi, P.B. Neuroprotective activity of ethanolic extract of Tinospora cordifolia on LPS induced neuroinflammation. Transl. Biomed. 2017, 8, 135. [Google Scholar]
- Kosaraju, J.; Chinni, S.; Roy, P.D.; Kannan, E.; Antony, A.S.; Kumar, M.N.S. Neuroprotective effect of Tinospora cordifolia ethanol extract on 6-hydroxy dopamine induced parkinsonism. Indian J. Pharmacol. 2014, 46, 176–180. [Google Scholar] [CrossRef]
- Singh, L.; Tyagi, S.; Rizvi, M.A.; Goel, H.C. Effect of Tinospora cordifolia on gamma ray-induced perturbations in macrophages and splenocytes. J. Radiat. Res. 2007, 48, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Sunanda, S.N.; Desai, N.K.; Ainapure, S.S. Antiallergic properties of Tinospora cordifolia in animal models. Indian J. Pharmacol. 1986, 18, 250–252. [Google Scholar]
- Badar, V.A.; Thawani, V.R.; Wakode, P.T.; Shrivastava, M.P.; Gharpure, K.J.; Hingorani, L.L.; Khiyani, R.M. Efficacy of Tinospora cordifolia in allergic rhinitis. J. Ethnopharmacol. 2005, 96, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, G.; Sheela, M.L.; Salimath, B.P. Octacosanol isolated from Tinospora cordifolia downregulates VEGF gene expression by inhibiting nuclear translocation of NF-κB and its DNA binding activity. Eur. J. Pharmacol. 2008, 588, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Rao, S.K. Evaluation of the antineoplastic activity of guduchi (Tinospora cordifolia) in Ehrlich ascites carcinoma bearing mice. Biol. Pharm. Bull. 2006, 29, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Verma, R.K.; Saraf, S.A. Nutraceuticals: New era of medicine and health. Asian J. Pharm. Clin. Res. 2010, 3, 11–15. [Google Scholar]
- Lola, C.; Lucía, F.N.; Iván, C.; Olaia, M.; Susana, R.; Ramón, A.; Ramón, C. Nutrition, Health, and Disease: Role of Selected Marine and Vegetal Nutraceuticals. Nutrients 2020, 12, 747. [Google Scholar]
- Pathak, P.; Vyas, M.; Vyas, H.; Naria, M. Rasayana effect of Guduchi Churna on the life span of Drosophila melanogaster. AYU (An Int. Q. J. Res. Ayurveda) 2016, 37, 67–70. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef]
- Bao, X.; Wang, Z.; Fang, J.; Li, X. Structural features of an immunostimulating and antioxidant acidic polysaccharide from the seeds of Cuscuta chinensis. Planta Med. 2002, 68, 237–243. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Fungal β-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Dong, Q.; Zuo, J.P.; Fang, J.N. Structure and potential immunological activity of a pectin from Centella asiatica (L.) Urban. Carbohydr. Res. 2003, 338, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Raghu, R.; Sharma, D.; Ramakrishnan, R.; Khanam, S.; Chintalwar, G.J.; Sainis, K.B. Molecular events in the activation of B cells and macrophages by a non-microbial TLR4 agonist, G1-4A from Tinospora cordifolia. Immunol. Lett. 2009, 123, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Anjum, V.; Arora, P.; Ansari, S.H.; Najmi, A.K.; Ahmad, S. Antithrombocytopenic and immunomodulatory potential of metabolically characterized aqueous extract of Carica papaya leaves. Pharm. Biol. 2017, 55, 2043–2056. [Google Scholar] [CrossRef]
- Sood, S.; Shilpa. Assessment of storage stability of Tinospora cordifolia (Giloy) based squash. Int. J. Innov. Res. Dev. 2015, 4, 306–315. [Google Scholar]
- Tyagi, P.; Chauhan, A.K.; Singh, S.N. Sensory acceptability of value added cookies incorporated with Tinospora cordifolia (TC) stem powder; improvement in nutritional properties and antioxidant potential. J. Food Sci. Technol. 2020, 57, 2934–2940. [Google Scholar] [CrossRef]
- Sarala, M.; Velu, V.; Anandharamakrishnan, C.; Singh, R.P. Spray drying of Tinospora cordifolia leaf and stem extract and evaluation of antioxidant activity. J. Food Sci. Technol. 2012, 49, 119–122. [Google Scholar] [CrossRef]
- Sharma, H.; Singh, A.K.; Deshwal, G.K.; Rao, P.S.; Kumar, M.D. Functional Tinospora cordifolia (giloy) based pasteurized goat milk beverage: Impact of milk protein-polyphenol interaction on bioactive compounds, anti-oxidant activity and microstructure. Food Biosci. 2021, 42, 101101. [Google Scholar] [CrossRef]
- Siti Rashima, R.; Maizura, M.; Kang, W.M.; Fazilah, A.; Tan, L.X. Influence of sodium chloride treatment and polysaccharides as debittering agent on the physicochemical properties, antioxidant capacity and sensory characteristics of bitter gourd (Momordica charantia) juice. J. Food Sci. Technol. 2017, 54, 228–235. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Patras, A.; Brunton, N.; Brennan, C.; Cullen, P.J.; O’Donnell, C. Effect of thermosonication on bioactive compounds in watermelon juice. Food Res. Int. 2011, 44, 1168–1173. [Google Scholar] [CrossRef]
- Heena, S.; Priyanka, R.; Ashish, K.S. Fifty years of research on Tinospora cordifolia: From botanical plant to functional ingredient in foods. Trends Food Sci. Technol. 2021, 118, 189–206. [Google Scholar]
- Jain, A.; Dasgupta, N.; Ranjan, S.; Singh, V.; Singh, H.; Purohit, S.D.; Mishra, N.C.; Yadav, N.P.; Haque, S.; Mishra, B.N.; et al. Whey protein based electrosprayed nanospheres for encapsulation and controlled release of bioactive compounds from Tinospora cordifolia extract. Innov. Food Sci. Emerg. Technol. 2021, 69, 102671. [Google Scholar] [CrossRef]
- Kalem, I.K.; Bhat, Z.F.; Kumar, S.; Wang, L.; Mudiyanselage, R.J.; Bhat, H.F. Tinospora cordifolia: A novel bioactive ingredient for edible films for improved lipid oxidative and microbial stability of meat products. J. Food Process. Preserv. 2018, 42, e13774. [Google Scholar] [CrossRef]
- Chauhan, P.; Kumar, R.R.; Mendiratta, S.K.; Talukder, S.; Gangwar, M.; Sakunde, D.T.; Meshram, S.K. In-vitro functional efficacy of extracts from Phyllanthus emblica, Eucalyptus globulus, Tinospora cordifolia as pancreatic lipase inhibitor and source of anti-oxidant in goat meat nuggets. Food Chem. 2021, 348, 129087. [Google Scholar] [CrossRef] [PubMed]


| Chemical Class | Active Constituents | Phytoactive (Class) | Activity | References |
|---|---|---|---|---|
| Glycosides (Phenylpropanoid) | Tinocordioside, glucoside 18-norclerodane, Pregnane glycoside, diterpene Furanoid glucoside, Cordioside, Tinocordifolioside, Syringin, Cordifolioside A, B, C, D and E, Palmatosides, Syringinapiosyl glycoside, 2-Methyl-1,2-pyrrolidine, Cordifolioside, N-Formylannonai | Cordioside (Clerodane furano diterpene) Syringin, Cordifolioside, (phenylpropanoid glycoside) | Enhance phagocytosis | [22,26,27,28,29] |
| Alkaloids | Berberine, magnoflorine, aporphine alkaloids, jatrorrhizine, tembetarine, tinosporin, isocolumbin, tetrahydropalmatine, choline, palmatine | N-Formylannonain, Magnoflorine (Benzylisoquinoline alkaloid) | Increment in ROS, phagocytosis | [30,31] |
| Diterpenoid lactone | Furanolactone, Tinosporon, Diterpenoids, Tinosporides, Tinosporon, Jateorine, columbin, Clerodane derivatives | - | - | [32,33,34,35,36,37] |
| Steroids | β-sitosterol, 20 β-hydroxyecdysone, Makisterone A, Giloinsterol | - | - | [38] |
| Sesquiterpenoids | Tinocordifolin, einocordifolin | Tinocordiside, 11-hydroxymuskatone (Cadinane sesquiterpene) | Increased phagocytosis, increase ROS | [30,39,40] |
| Aliphatic compounds | Octacosanol Heptacosanol, Nonacosan-15-one dichloromethane | - | - | [40,41,42] |
| Lignans | 3,(a,4-dihydroxyl-3-methoxy-benzyl)-4-(4-hydroxy-3 methoxy-benzyl)-Tetrahydrofuran | - | - | [32] |
| Other compounds | Tinosporidine, Arabinogalactan, Giloin, Nonscosan-15-one, Jatrorrhizine, Cordifol, Cordifelone, Giloinin, diacetate N-transferuloyltyramine, Tinosporic acid | Arabinogalactan polysac (G1-4A) | Decrease mortality, increase Th1, decrease Th2, increase macrophage activation, increase mitogenesis | [23,41,43,44] |
| Polysaccharide | Alpha-D-glucan | RR1 | Increase phagocytosis; increase Th1 cytokines | [45] |
| Protein | Guduchi immunomodulatory protein (ImP) | - | Increase phagocytosis; increase mitogenesis, increase bactericidal | [46] |
| Extracting Solvent | Bioassay Method | Observation and the Outcome of the Study | References |
|---|---|---|---|
| Aqueous extract (Aqs Ext) | SOD, GPx activity, β-Glucuronidase, catalase, LPO |
| [12] |
| Alcoholic (Alc) and Aqs Ext | Catalase |
| [64] |
| 95% Ethanolic (Eth) Ext | LPO, SOD |
| [65] |
| Aqs and Alc Ext | DPPH |
| [66] |
| Eth and meth Extfrom stem bark | DPPH |
| [42] |
| Meth Ext | DPPH |
| [67] |
| Aqs Ext | Myeloperoxidase (MPO), creatine kinase (CK) assay |
| [16] |
| Aqs Ext | MPTP-intoxicated Parkinsonian mouse model |
| [68] |
| Composite Aqs Ext of T. cordifolia and Z. officinale | MTT assay |
| [69] |
| Meth Ext | Vital dye Presto Blue-based assay |
| [18] |
| Chloroform andhexane extracts | MTT assay |
| [20] |
| Meth Ext | MTT assay |
| [70] |
| Aqs-Eth Ext (50%) | MTT assay |
| [71] |
| Methanol and acetone (70:30) extract | DMBA-induced skincancer model |
| [72] |
| Hydro-ethanolicextract (50%) | Proliferation assays |
| [71] |
| Methanolic extract of fresh stem (50%) | Melanoma assay |
| [73] |
| T. cordifolia Preparation | Animal Model Used | Dose Administered | Study Objective | Experimental Results | References |
|---|---|---|---|---|---|
| Immunomodulatory Activity | |||||
| Aqs Ext | Swiss albino mice | 15 days-100 mg/kg/day | Colony-stimulating activity | Predominant neutrophilia with induced leucocytosis | [60] |
| Arabinogalactan from Aqs Ext | Spleen cells of murine | - | Mitogenic activity | Increased mitogenic activity | [23] |
| Methanolic extract | Balb/c mice | 5 days-200 mg/kg/day | Phagocytic activity antibody production | Enhanced phagocytic activity, increased WBC, increased immune response, increased stem cells maturation | [80] |
| Ethanolic and Petroleum ether extract | Oreochromis mossambicus (vaccinated with heat killed A. hydrophila, 109 cells/fish) | 0.8, 8 or 80 mg/kg | Neutrophil activity antibody response | Prolonged the peak primary antibody titres (1–3 weeks); enhanced 2° antibody responses (8 mg/kg) and neutrophil activity | [81] |
| 08 compounds (N-formylannonain, magnoflorine, jatrorrhizine, palmatine, 11-hydroxymustakone, cordifolioside A, tinocordiside and yangambin) | Primary cells of murine | Isolated molecules of 10, 25, 50 and 100 μM | Splenocyte assay | Immunomodulatory activity possesses by N-formylannonain and 11-hydroxymustakone | [30] |
| Aqs Ext | Cholestasis-induced rats | 7 days-100 mg/kg | Macrophage activity, cellular immune functions, and polymorphonuclear cells | Improved cellular immune function, 16% reduction in mortality rate by E. coli infection, enhanced phagocytic cell functions | [82] |
| T. cordifolia, T. chebula, B. diffusa, B. aristata, and Z. officinale Eth Ext | Golden hamsters (inoculated with E.Histolytica trophozoites) | 4 days-400, 600 and 800 mg/kg | T and B cell counts and hemagglutination titre | No effect on T-cell counts, enhanced cell mediated immunity, increased humoral immunity | [83] |
| Polysaccharide isolation (α-D-glucan) | HEK 293 cell lines, Mouse macrophages (RAW 264.7) | 0, 100, 500 and 1000 μg/mL | NF-κB enhance activated B cells, TNF-α synthesis; opsonic binding and phagocytosis | TNF-α synthesis inhibition of macrophages cell line, phagocyte inhibition, activated NF-κB in dose and time-dependent manner | [45] |
| G1-4A polysaccharide | primary murine macrophages and RAW 264.7 cells from BALB/c mice | Constant 8 h-1.0 mg/mL | NO and cytokines production, M. tuberculosis intracellular survival, and phagocyte assay | Increased NO level, activated macrophage, IL-1, IL-2, and TNF-levels upregulation, increased MHC-II and CD86 | [84] |
| Aqs Ext | Immunized Albino rabbits with Typhoid ‘H’ antigen | 20 days-10 mg/100 g | Antibody titre | Reduced antibody formation and immunosuppressive action | [85] |
| 80% Eth Ext | Induced Ochratoxin A Albino mice of Hindustan Antibiotics strain | 17 weeks-100 mg/kg | Cytokine production, Macrophage chemotaxis | IL-1α and TNF-α production, inhibition of suppressed chemotactic activity | [86] |
| Non-polar, alkaloid-free extract and polar fraction | Ascitic tumour-induced BALB/c mice | 15 days-100 mg/kg | Serological and hematological parameters, antibody titres | Ineffective haematological parameters and myelo-protection, increase antibody titres | [87] |
| Antidiabetic Activity | |||||
| 13 active compounds | computational studies | – | Glycogen phosphorylase activity | Glycogen phosphorylase activity is decreased by magnoflorin, cordiofolioside A, and syringin. | [88] |
| Aqs Ext | Alloxan rats | 21–120 days-dose 400 mg/kg | Antihyperglycemic | Decreased amounts of the substrate and the enzymes hexokinase, phosphofructokinase, and glucokinase | [47] |
| Aqs and Alc Ext | Swiss albino mice (injected with Ehrlich ascites tumour cells) | 10 days-1–100 μg | Glucose uptake under tumour conditions | Ethanolic extract (100 μg) and methanolic extract (40 μg) showed good glucose uptake | [89,90] |
| Alc and Aqs Ext Stem | Streptozotocin-induced diabetic albino rats | 30 days-200 and 400 mg/kg, respectively | Enzymes involvement in glucose metabolism, Serum insulin level | When compared to insulin, it is 40–80% more effective; it also increases hepatic glycogen synthase and decreases glycogen phosphorylase activity. | [91] |
| Extraction using different solvents | Albino rats induced by alloxan | 1.0 day-0.3 mg/g | α-glucosidase inhibition | Reduced post-meal spike in blood sugar, Salivary amylase (75%), -glucosidase (100%), and pancreatic (83%) activity are all non-competitively inhibited. | [92] |
| Alkaloid-rich fraction | Tolbutamide-induced diabetic wistar rats | Magnoflorine, palmatine, and jatrorrhizine (10, 20, and 40 mg/kg each), Isoquinoline (50, 100, and 200 mg/kg) | Antihyperglycemic | Blood glucose levels are kept from increasing with a decreased fasting serum glucose and a glucose supplement of 2.0 g/kg. | [63] |
| Aqs Ext | Albino Wistar rats given with high fructose diet | 60 days-400 mg/kg/day | Carbohydrate metabolism | Reduced the rise in triglycerides (54.12%), insulin (51.5%), glucose-insulin index (59.8%), and blood sugar levels (21.3%). | [93] |
| Alc and Aqs Ext | Streptozotocin diabetic rats | 40 days-400 mg/kg | Antiglycemic effect | 7.45% lessened in plasma glucose level, prevented polyuria | [94] |
| Nephroprotective Activity | |||||
| Meth and Aqs Ext | Cyclophosphamide-induced Swiss albino mice | 5 days-200 mg/kg | Urine protein and urea nitrogen content, serum cytokine level, urinary glutathione content | Reduced TNF-α level, increased glutathione level in bladder and liver, decreased protein level in urine and serum, enhanced IL-2 and IFN-gamma levels | [61] |
| Alc and Aqs Ext | Streptozotocin-induced diabetic rats | 40 days-400 mg/kg | Renal damage assay | Considerably controlled urinary albumin levels; no impact on renal hypertrophy | [47] |
| Anti-Inflammatory Activity | |||||
| Solution of powder in 2% gum acacia | 1.0% suspension of carrageenan in normal saline; sub-plantar injected in albino rats | 6 days-50 mg/kg/oral | Acute and chronic inflammation | 67% inhibition in granulation tissue of paw in both acute and sub-acute inflammation | [95] |
| Aqs Ext | For arthritic syndrome, albino rats were given 1.0% carrageenan and 2.0% croton oil in ground nut oil, respectively, along with 0.1 mL of Freund’s adjuvant. | Acute inflammation-60 mg/100 g, chronic inflammation-20 mg/100 g, arthritic syndrome-10 mg/100 g | Mild analgesic effect, mean volume of edema, percent inhibition of edema | Dose-dependent effect, minimal impact on volume of edema, oral drug administration results in a 63.16% edema suppression compared to intraperitoneal injection’s 49.20% | [85] |
| Antineoplastic Activity | |||||
| Methanolic, aqueous, and methylene chloride stem extract | HeLa S3 cells | 0, 5, 10, 25, 50, and 100 mg/mL | Micronucleus assay, cytotoxicity, Colonogenic assay | Concentration-dependent increased frequency of micronuclei, dose-dependent increase in cell killing by 2.8 (50 mg/mL) and 6.8 (100 mg/mL)-fold, reduction in survival fractions of cells | [96] |
| Berberine-rich extract | Human tongue squamous carcinoma SCC-4 cells injected in BALB/cnu/nu nude mice | 28 days-10 mg/kg | Tumour size and volume | Significantly lower tumour volume and growth (52% tumour inhibition) | [97] |
| Eight isolated compounds from Eth Ext | HT-29 (human colorectal cancer), SiHa, CHOK-1 (hamster ovary), and KB (human oral squamous carcinoma) | Molecular isolates at 10, 25, 50, and 100 μM | Cytotoxicity (Sulforhodamine B assay) | All are effective against KB and CHOK-1 cells, while yangambin, palmatine, and tinocordiside are also effective against KB and HT-29 cells and KB and CHOK-1 cells, respectively. | [30] |
| Eth Ext | C6 glioma cells | 250 μg/mL and 350 μg/mL | Cytotoxic and antiproliferative property | Enhanced production of mortalin, differentiation in C6 glioma cells, cell proliferation reduction in dose-dependent | [98] |
| Epoxy clerodane Diterpene | Diethyl nitrosamine induced liver tumour in Wistar albino rats | 20 weeks-10 mg/kg | Biochemical parameters, liver morphology | Reduced liver weight, decrease in lactate dehydrogenase, catalase, glutamate transaminase and pyruvate transaminase | [99] |
| Meth Ext (70%) | Mice C57BL/6 (angiogenesis induced with metastatic B16F-10 melanoma cells) | 5 days-20 mg/kg | synthesis of cytokines, antiangiogenic compounds, and growth factors | Metalloprotease-1 tissue inhibitor synthesis was increased, and tumour-directed capillary development was suppressed. Pro-inflammatory cytokines such as granulocyte-monocyte-colony-stimulating factor were decreased. | [100] |
| Polysaccharide fraction | Mice C57BL/6 induced with metastatic B16F-10 melanoma cells | 0.5 mg/dose/animal | Enzyme level in serum, biochemical parameters, survival rate, | Decrease in lung collagen’s hydroxyproline, hexosamines, and uronic acids; inhibition of lung metastases (72%); reduction levels of sialic acid and gamma-glutamyl transpeptidase | [101] |
| Antioxidant Activity | |||||
| Stem powder | Streptozotocin-induced CFT-Wistar pregnant diabetic rats | 1.0% and 2.0% | Embryo-lethality, oxidative stress markers in fetal brain and liver | 53% and 48% increment in glutathione and total thiols level, 63% protection against embryo-lethality, 25% and 72% decline in malondialdehyde and reactive oxygen species, respectively | [102] |
| Stem Aqs Ext | High fructose diet given to Albino Wistar rats | 400 mg/kg/day (for 60 days) | Oxidation stage | 34% and 28% reduction in TBARS and protein carbonyl groups, respectively | [103] |
| Antistress Activity | |||||
| Aqs Ext | Sprague Dawley rats | 15 days-100 mg/kg | Urine metabolites | Regulation of glomerular filtration rate, TCA cycle, Gut Microflora activity and catecholamine pathway | [104] |
| Diterpene isolated Epoxy clerodane | Wistar albino rats (indomethacin-induced gastric ulcer) | Single dose-12.50, 25, and 50 mg/kg | ulcer index, myeloperoxidase activity, and gastric mucosal lesions; anti-inflammatory and pro-angiogenic factors | Increased gastric myeloperoxidase activity, 3.01- and 2.26-fold increase in IL4 and IL-10, respectively, whereas 1.67-, 1.72-, and 1.71-fold reduction in TNF-α, IL-1, and IL-6, respectively, 1.5-fold pro-angiogenic growth factors increase, 54.42% apoptotic index, reduction in ulcer index and gastric mucosal lesions (91.80%) | [105] |
| Hepatoprotective Activity | |||||
| Stem decoction | Horse serum injection in rats | 30 days-100 mg/kg | Kupffer cell activity performed by carbon clearance method | Prevention of suppression of Kupffer cells of liver in treated groups by lowest carbon half-life | [82] |
| Stem Aqs Ext | Wistar rats (pyrazinamide, isoniazid, and rifampicin induced hepatic damage) | 90 days-100 mg/kg | Hepatic enzymes, liver morphology | Restriction in weight and liver volume, together with normal levels of bilirubin, alkaline phosphatase, aspartate transaminase, and alanine transaminase | [106] |
| Aqs Ext | Wistar strain of Albino rats (intoxicated with carbon tetrachloride) | 100 mg/kg (for 15 days) | Enzyme level in serum | Reduction in serum glutamate oxaloacetate transaminase, alkaline phosphatase, and bilirubin level (2.2 mg/100 mL) | [54] |
| Antiparasitic Activity | |||||
| Eth Ext | Pediculus humanus capitis, A. subpictus and C. quinquefasciatus | 100 mg/L | Larvicidal, pedulocidal | 75–90% mortality against A. subpictus, C. quinquefasciatus larvae, and in Pediculus humanus capitis adults | [107] |
| Guduchi satwa | Paracetamol induced hepatotoxicity in Wistar albino rats | 4 days-200 and 400 mg/kg | Blood biochemical markers | High levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and total bilirubin were reduced. | [108] |
| Whole plant extract | BALB/c mice (infected with 107 promastigotes of L. donovani) | 15 days-100 mg/kg | Tests for liver and renal function, parasite load, immunoglobulins, cytokines, and delayed hypersensitivity | Significant increment in delayed hypersensitivity, reduction in hepatic parasitic load, increased IgG production with no effect in SGOT and SGPT levels | [82] |
| Hypolipidemic Activity | |||||
| Aqs Ext | Wistar rats (high fructose diet) | 60 days-400 mg/kg/day | Lipid enzymes | Attained adipose tissue fatty acid synthetase, malic enzyme, and lipoprotein lipase levels with reduced impact on hepatic fatty acid synthetase and malic enzyme activity | [103] |
| Neuroprotective Activity | |||||
| Eth Ext | Albino rats (neuro-inflammation induced with lipopolysaccharides) | 14 days-200 mg/kg and 400 mg/kg | Estimate antioxidant enzymatic levels in brain and neuronal damage | Decreased TBARS level associated with increased neural regeneration; decreased cell edema; increased glutathione; superoxide dismutase; and catalase. | [109] |
| Alc Ext | Wistar albino rats (injected with 6-hydroxy dopamine) | 30 days-200 and 400 mg/kg | Anti-Parkinson’s activity | Reduced oxidative stress, increased dopamine (2.45 ng/mg of protein) and complex I activity that restores locomotor function, and decreased iron asymmetry ratio | [110] |
| Radioprotective Activity | |||||
| Hydro-alcoholic stem extract | Gamma radiation-exposed Swiss albino mice | Single dose-200 mg/kg | Cell cycle progression, Spleen colony forming units, micro-nuclei induction | 30-day survival rate of 76.30% compared to 100% mortality in control, raised spleen CFU count to 31.60 (treated) compared to control, and decreased induction of micronuclei in the S-phase cell population | [60] |
| Aqs Ext | Swiss Albino mice (exposed to 60Co gamma radiation) | Single dose for 3, 7, 15 days at a dose of 5, 10, 15 mg/kg | Animal behaviour and survival rate | 50% survival rate in 24 days, 100% mortality in 30 days (15 days), 33% survival rate (single dose) for 30 days, 100% mortality in 3 and 7 days, and 50% survival rate in 24 days | [76] |
| 50% hydro-Alc Ext | Swiss albino strain ‘A’ mice (exposure to 60Co gamma radiation) | 200 mg/kg | Phagocytic activity, splenocyte proliferation assay, macrophage functionality | 19–2% reduction in apoptosis, spleen weight increment, 120% macrophage adherence, splenocytes proliferation | [111] |
| Antiallergic Activity | |||||
| Aqs Ext | Albino rats (Bovine albumen and Freund’s adjuvant), Guinea pigs (histamine-induced bronchospasm), Swiss mice (Bovine albumen and Freund’s adjuvant) | 100 mg/kg (for 24 h) | Bronchospasm and mast cell production response | 95% reduction in bronchoconstriction, capillary permeability, and mast cell number | [112] |
| Hydroalcoholic extract | Ovalbumin administration i.p. and intranasally in BALB/c mice | 7 days-100 mg/kg | Cytokine production, oxidative stress markers | Enhanced superoxide dismutase, glutathione peroxidase, catalase, reductase, reduced airway hyper-responsiveness, IgE and eosinophil level, dwindling level of pro-inflammatory cytokines (COX-2, iNOS, ICAM-1) | [10] |
| Aqs Ext | Clinical patients (suffering from rhinitis) | 60 days-One tablet thrice daily (300 mg extract) | Response to allergic rhinitis | 100% nasal discharge control, 83% sneezing relief, decreased eosinophil and neutrophil in nasal smear, nasal mucosa looked pink against blue colour | [113] |
| Cardioprotective Activity | |||||
| Isolated Octacosanol | Swiss albino mice (injected with Ehrlich ascites tumour cells) | 6 to 14 days-60 μg/alternate day | Micro-vessel density, peritoneal angiogenesis, quantification of vaso-endothelial growth factor (VEGF) | Decreased geletinolytic activity on metalloproteinases in dose-dependent manner, inhibition of Nf-κB in VEGF gene expression | [114] |
| Alc Ext | Myocardial ischemia induced Sprague Dawley rats | 7 days-250, 500, and 1000 mg/kg | Testing of infarct size in heart tissue and lipid peroxide levels in liver | Decreased heart rate, a dose-dependent increase in infarct size, and elevated amounts of malonaldehyde in the blood and heart | [115] |
| Alc Ext | CaCl2 induced cardiac arrhythmia in Wistar albino rats | Single dose-150, 250, and 450 mg/kg | Cardiac responses and mineral level in blood | Reduced heart rate, normalized PQRST waves, elevated K levels, lowered Ca and Na levels in the blood, and regulated atrial and ventricular fibrillation | [39] |
| S. No | Experimentation/ Animal Model | Targeted Phytoconstituent | Mechanism of Action | References |
|---|---|---|---|---|
| 1 | Immunomodulatory activity in murine model | Low molecular wt. phytochemical, polysaccharides as immunostimulatory protein (ImP) |
| [46] |
| 2 | Immunostimulatoryactivity in HEK293 cells | α-glucan, (1,4)-a-D-glucan (RR1) |
| [45] |
| 3 | Immunomodulatory activity in murine model | β-glucans |
| [119,120,121,122] |
| 4 | Examination of B-cells and macrophages as potential target cell populations for G1-4A in murine model | Arabinogalactan polysaccharide G1-4A |
| [43,44,123] |
| 5 | Immunostimulatory studies in Balb/c mice | Glycosides-Cordifoliosides A and B |
| [35] |
| 6 | Immunostimulatory studies in Balb/c mice | Cordifolioside A, syringin, cordiol, cordioside |
| [26] |
| 7 | Immunomodulatory activity in murine model | Arabinogalactan polysaccharide |
| [23,44] |
| 8 | Immunostimulation in murine model of septicemia | G1-4A polysaccharide |
| [43] |
| 9 | Histocompatibility effect in murine model for immunomodulation | Polysaccharide |
| [44] |
| 10 | Hot water/methanol: water followed by fractionation with n-hexane, ethyl acetate, chloroform, n-butanol, water, ethyl acetate investigating immunomodulatory activity in murine model | N-formylannonain, cordiofolioside A, magnoflorine, tinocordiside, syringin, 11-hydroxymuskatone, N-methyl-2-pyrrolidone, magnoflorine, tinocordioside |
| [30,39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjum, V.; Bagale, U.; Kadi, A.; Potoroko, I.; Sonawane, S.H.; Anjum, A. Unveiling Various Facades of Tinospora cordifolia Stem in Food: Medicinal and Nutraceutical Aspects. Molecules 2023, 28, 7073. https://doi.org/10.3390/molecules28207073
Anjum V, Bagale U, Kadi A, Potoroko I, Sonawane SH, Anjum A. Unveiling Various Facades of Tinospora cordifolia Stem in Food: Medicinal and Nutraceutical Aspects. Molecules. 2023; 28(20):7073. https://doi.org/10.3390/molecules28207073
Chicago/Turabian StyleAnjum, Varisha, Uday Bagale, Ammar Kadi, Irina Potoroko, Shirish H. Sonawane, and Areefa Anjum. 2023. "Unveiling Various Facades of Tinospora cordifolia Stem in Food: Medicinal and Nutraceutical Aspects" Molecules 28, no. 20: 7073. https://doi.org/10.3390/molecules28207073
APA StyleAnjum, V., Bagale, U., Kadi, A., Potoroko, I., Sonawane, S. H., & Anjum, A. (2023). Unveiling Various Facades of Tinospora cordifolia Stem in Food: Medicinal and Nutraceutical Aspects. Molecules, 28(20), 7073. https://doi.org/10.3390/molecules28207073







