Simultaneous Extraction of Oil and Protein from Silkworm (Bombyx mori L.) Pupae (Lueng Parroj var.) and Their In Vitro Skin Moisturization
Abstract
:1. Introduction
2. Results
2.1. Simultaneous Extraction of Oil and Protein from SWP
2.2. Fatty Acid Composition and SWP Oil Properties
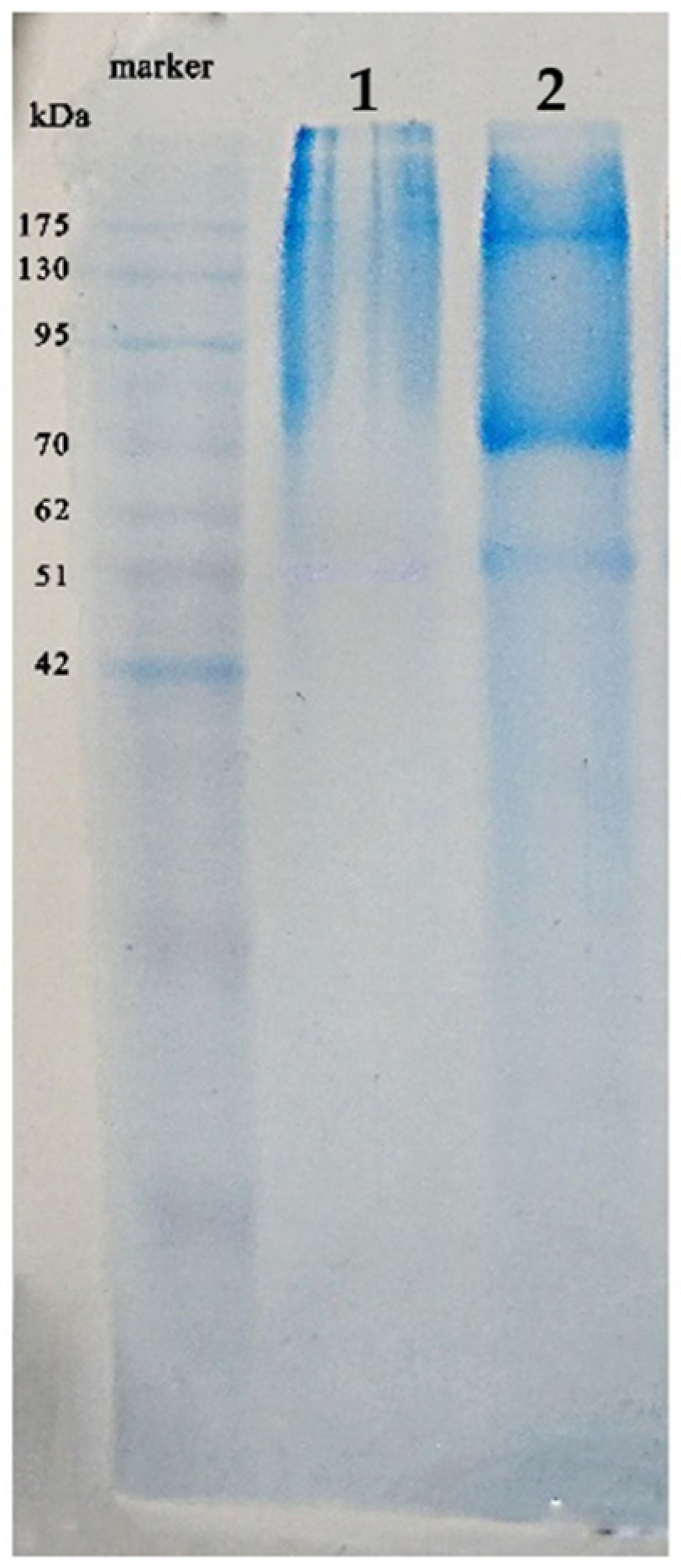
2.3. SDS-PAGE of SWP Protein
2.4. Amino Acid Composition of SWP Protein
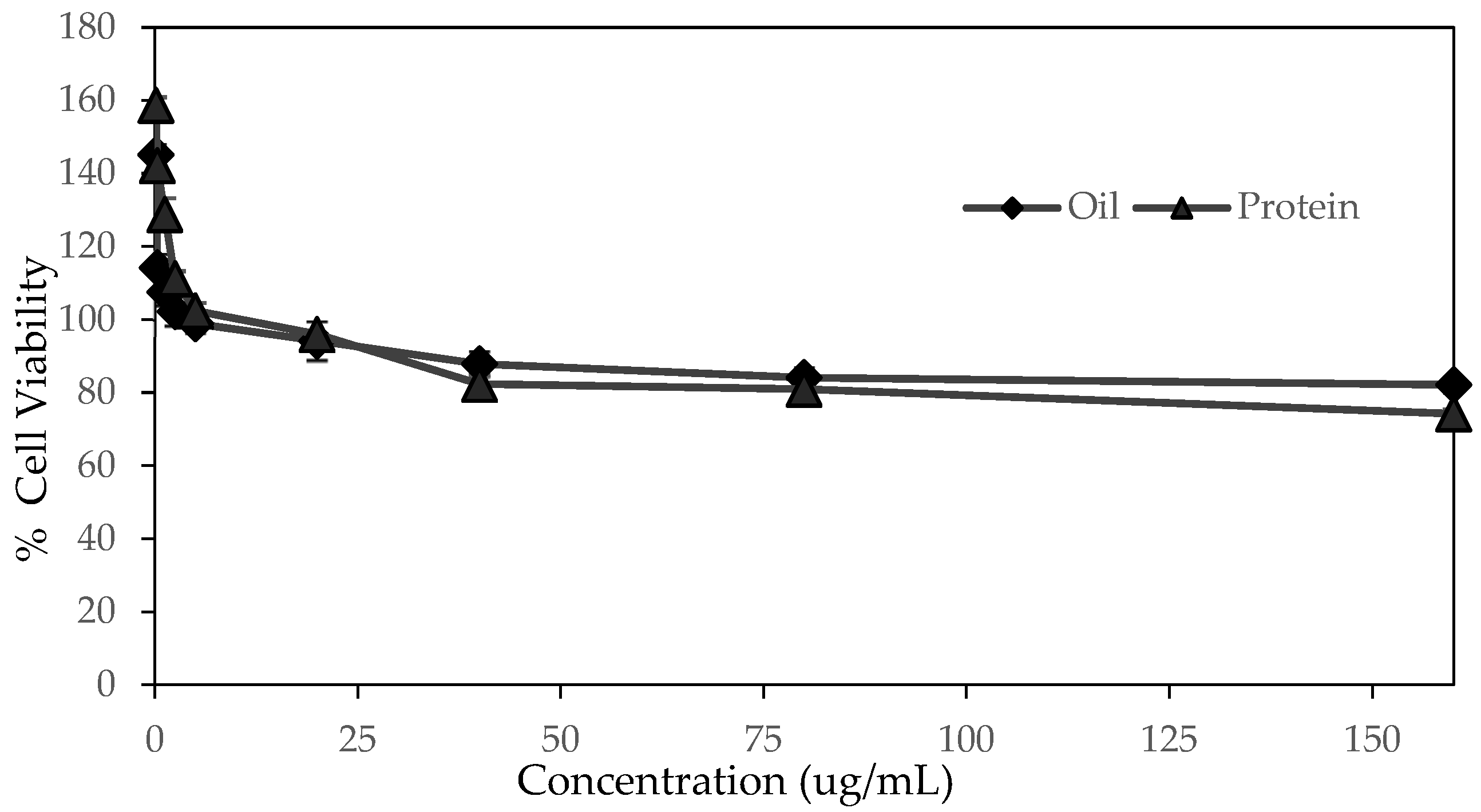
2.5. Cell Cytotoxicity of SWP Oil and Protein
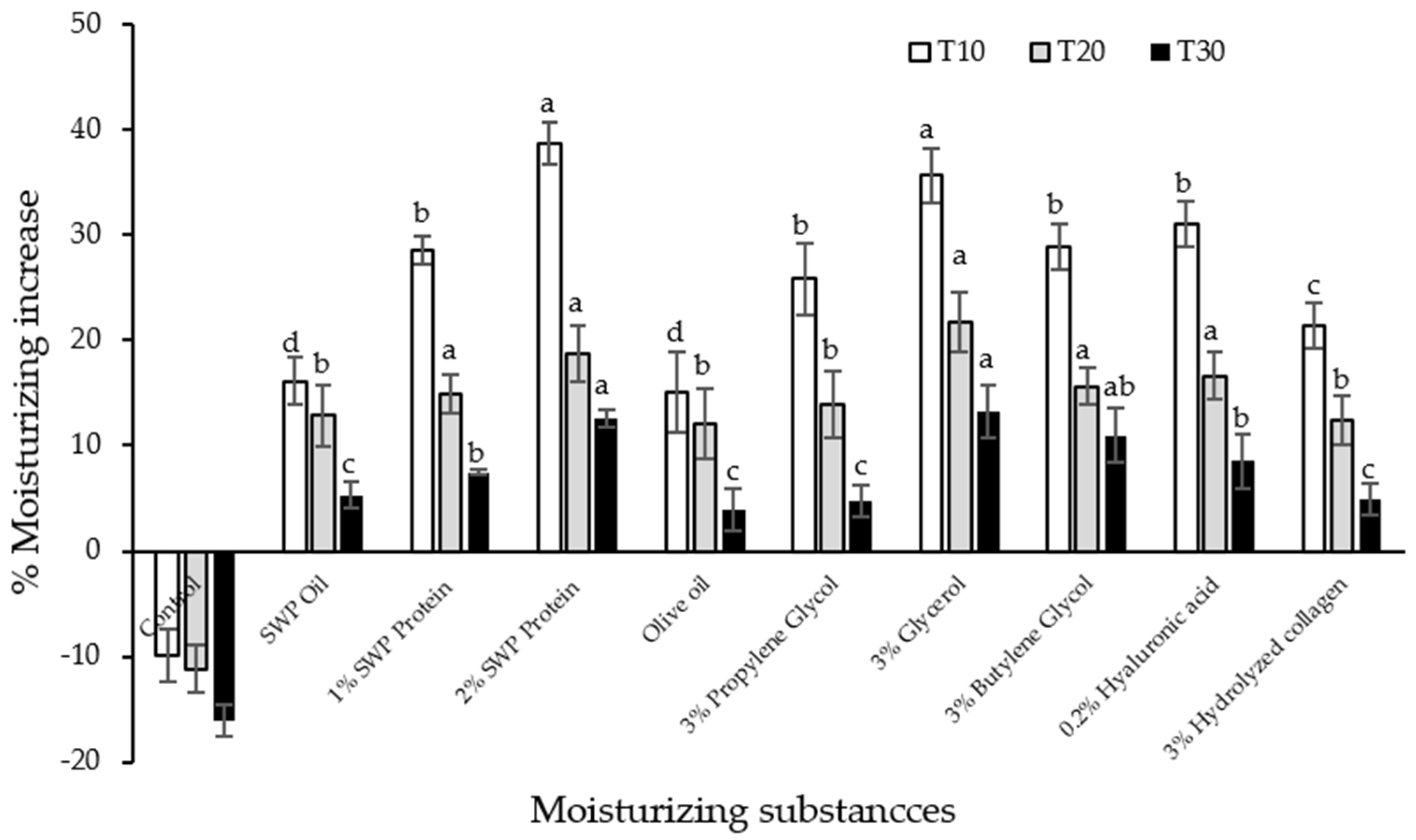
2.6. In Vitro Moisturizing Effect of SWP Oil and Protein
3. Discussion
3.1. Simultaneous Extraction of Oil and Protein from SWP
3.2. Fatty Acid Composition and SWP Oil Properties
3.3. SDS-PAGE of SWP Protein
3.4. Amino Acid Composition of SWP Protein
3.5. Cell Cytotoxicity of SWP Oil and Protein
3.6. In Vitro Moisturizing Effect of SWP Oil and Protein
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of SWP Samples
4.3. Simultaneous Extraction of SWP Oil and Protein Using TPP
4.4. Analysis of SWP Oil
4.4.1. Determination of Fatty Acid Composition
4.4.2. Determination of Oil Properties
4.5. Analysis of SWP Protein
4.5.1. Determination of Protein Content
4.5.2. Determination of Amino Acid Composition
4.5.3. SDS-PAGE Analysis
4.6. Cell Cytotoxicity Test of SWP Oil and Protein
4.7. In Vitro Skin Moisturizing Test
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Altman, G.H.; Farrell, B.D. Sericulture as a sustainable agroindustry. Clean. Circ. Bioecon. 2022, 2, 1000011. [Google Scholar] [CrossRef]
- Akande, A.O.; Jolayemi, O.S.; Adelugba, V.A.; Akande, S.T. Silkworm pupae (Bombyx mori) and locusts as alternative protein sources for high-energy biscuits. J. Asia Pac. Entomol. 2020, 23, 234–241. [Google Scholar] [CrossRef]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- David-Birman, T.; Moshe, H.; Lesmes, U. Impact of thermal processing on physicochemical properties of silk moth pupae (Bombyx mori) flour and in-vitro gastrointestinal proteolysis in adults and seniors. Food Res. Int. 2019, 123, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Rafiullah; Khan, S.; Khan, R.U.; Ullah, Q. Does the gradual replacement of spent silkworm (Bombyx mori) pupae affect the performance, blood metabolites and gut functions in White Leghorn laying hens? Res. Vet. Sci. 2020, 132, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, S.; Hu, S.; Song, K.; Wang, L.; Lu, K.; Wu, R.; Zhang, C. Replacement of fish meal with defatted silkworm (Bombyx mori L.) pupae meal in diets for pacific white shrimp (Litopenaeus vannamei). Aquaculture 2019, 510, 150–159. [Google Scholar] [CrossRef]
- Chukiatsiri, S.; Siriwong, S.; Thumanu, K. Pepae protein extracts exert anticancer effects by downregulating the expression of IL-6, IL-1β and TNF-α through biomolecular changes in human breast cancer cells. Biomed. Pharmacother. 2020, 128, 110278. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Xin, X.; Zhang, B.; Thomas, A.; Charles, A.; Lee, K.S.; Jin, B.R.; Gui, Z. Purification and characterization of a novel immunomodulatory hexapeptide from alcalase hydrolysate of ultramicro-pretreated silkworm (Bombyx mori) pupa protein. J. Asia Pac. Entomol. 2019, 22, 633–637. [Google Scholar] [CrossRef]
- Felix, M.; Bascon, C.; Cermeno, M.; FitzGerald, R.J.; de la Fuente, J.; Carrera-Sanchez, C. Interfacial/foaming properties and an-tioxidant activity of a silkworm (Bombyx mori) pupae protein concentrate. Food Hydrocoll. 2020, 103, 105645. [Google Scholar] [CrossRef]
- Nguyen, P.; Kim, K.Y.; Kim, A.Y.; Kim, N.S.; Kweon, H.Y.; Ji, S.D.; Koh, Y.H. Increased healthspan and resistance to Parkinson’s disease in Drosophila by boiled and freeze-dried mature silk worm larval powders. J. Asia Pac. Entomol. 2016, 19, 551–561. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and characterization of protein fractions from five insect species. Food Chem. 2013, 134, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Dev, P.; Kumar, R.V. Biomedical applications of silkworm pupae proteins. In Biomedical Applications of Natural Proteins; Kumar, D., Kundapur, R.R., Eds.; Springer: New Delhi, India, 2015; pp. 41–49. [Google Scholar] [CrossRef]
- Draelos, Z.D. Clinical situations conductive to proactive skin health and anti-aging improvement. J. Investig. Dermatol. Symp. Proc. 2008, 13, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.-J.; Yang, Z.-Z.; Yi, Y.-J.; Wang, H.-Y.; Zhou, W.-L.; Li, F.-F.; Wang, C.-Y. Extraction of oil from flaxseed (Linum usitatissimum L.) using enzyme-assisted three-phase partitioning. Appl. Biochem. Biotechnol. 2016, 179, 1325–1335. [Google Scholar] [CrossRef]
- Panadare, D.C.; Rathod, V.K. Three phase partitioning for extraction of oil: A review. Trends Food Sci. Technol. 2017, 68, 145–151. [Google Scholar] [CrossRef]
- Patil, S.S.; Rathod, V.K. Synergistic effect of ultrasound and three phase partitioning for the extraction of curcuminoids from Curcuma longa and its bioactivity profile. Proc. Biochem. 2020, 93, 85–93. [Google Scholar] [CrossRef]
- Vital, M.D.; Rathod, V.K. Three phase partitioning a novel technique for purification of peroxidase from orange peels (Citrus sinensis). Food Bioprod. Proc. 2015, 94, 284–289. [Google Scholar] [CrossRef]
- Alam, M.A.; Wu, J.; Xu, J.; Wang, Z. Enhanced isolation of lipids from micro algal biomass with high water content for biodiesel production. Bioresour. Technol. 2019, 291, 121834. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Zhang, X.; Tan, T. Lipid extraction from bon-broken and high water content microalgae Chlorella spp. by three-phase partitioning. Algal Res. 2015, 10, 218–223. [Google Scholar] [CrossRef]
- Tan, Z.-J.; Wan, C.-Y.; Yi, Y.-J.; Wang, H.-Y.; Zhou, W.-L.; Tan, S.-Y.; Li, F.-F. Three phase partitioning for simultaneous purification of aloe polysaccharide and protein using single-step extraction. Proc. Biochem. 2015, 50, 482–486. [Google Scholar] [CrossRef]
- Gaur, R.; Sharma, A.; Khare, S.K.; Gupta, M.N. A novel process for extraction of edible oils. Enzyme assisted three phase parti-tioning (EATPP). Bioresour. Technol. 2007, 98, 696–699. [Google Scholar] [CrossRef]
- Rachana, C.R.; Lyju Jose, V. Three phase partitioning-A novel protein purification method. Inter. J. Chem. Tech. Res. 2014, 6, 3467–3472. [Google Scholar]
- Chatsuwan, N.; Puechkamut, Y.; Pinsirodom, P. Characterization, functionality and antioxidant activity of water-soluble pro-teins extracted from Bombyx mori Linn. Curr. Appl. Sci. Technol. 2018, 18, 83–96. [Google Scholar] [CrossRef]
- Winitchai, S.; Manosroi, J.; Abe, M.; Boonpisuttinant, K.; Manosroi, A. Free radical scavenging activity, tyrosinase inhibition activity and fatty acids composition of oils from pupae of native Thai silkworm (Bombyx mori L.). Kasetsart J. (Nat. Sci.) 2011, 45, 404–412. [Google Scholar]
- Zou, Y.X.; Hu, T.-G.; Shi, Y.; Liu, J.; Mu, L.-X.; Xiao, Y.; Liao, S.-T. Establishment of model to evaluate the nutritional quality of Bombyx mori Linnaeus (Lepidoptera, Bombycidae) pupae lipid based on principles components. J. Asia Pac. Entomol. 2017, 20, 1364–1371. [Google Scholar] [CrossRef]
- Byun, H.J.; Cho, K.H.; Eun, H.C.; Lee, M.-J.; Lee, Y.; Lee, S.; Chung, J.H. Lipid ingredients in moisturizers can modulate skin re-sponses to UV in barrier-disrupted human skin in vivo. J. Dermatol. Sci. 2012, 65, 110–117. [Google Scholar] [CrossRef]
- Sethi, A.; Kaur, T.; Malhotra, S.K.; Gambhir, M.L. Moisturizers: The slippery road. Indian J. Dermatol. 2016, 61, 279–287. [Google Scholar] [CrossRef]
- Gopinath, A.; Puhan, S.; Nagarajan, G. Theoretical modeling of iodine value and saponification value of biodiesel fuels from their fatty acid composition. Renew. Energy 2009, 34, 1806–1811. [Google Scholar] [CrossRef]
- Patil, A.R.; Dip, G.; Meenatchi, R.; Moses, J.A.; Bhuvana, S. Extraction and characterization of silkworm pupae (Bombyx mori) oil by LC-MS/MS method. Inter. J. Pure App. Biosci. 2019, 7, 503–509. [Google Scholar]
- Wang, W.; Wang, N.; Zhou, Y.; Zhang, Y.; Xu, L.; Xu, J.; Feng, F.; He, G. Isolation of a novel peptide from silkworm pupae protein components and interaction characteristic to angiotensin I-converting enzyme. Eur. Food Res. Technol. 2011, 232, 29–38. [Google Scholar] [CrossRef]
- Kang, H.-S.; Park, J.H.; Auh, J.-H. Effects of protein hydrolysate from silkworm (Bombyx mori) pupae on the C2C12 myogenic differentiation. Foods 2023, 12, 2840. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, D.; Ray, M.; Sinha, S. Comparison of amino acid profiles and vitamin contents of male and female prepupae and pupae of eri silkworm, Samia ricini. J. Food Compos. Anal. 2022, 113, 104723. [Google Scholar] [CrossRef]
- Longvah, T.; Mangthya, K.; Ramulu, P. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food Chem. 2011, 128, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, A.; Djerassi, D. Amino acids and peptides: Building blocks for skin proteins. In Nutritional Cosmetics: Beauty; Tobor, A., Blair, R.M., Eds.; William Andrew Inc.: Norwich, NY, USA, 2009; pp. 287–317. [Google Scholar] [CrossRef]
- Barreto, S.M.A.G.; Maia, M.S.; Benica, A.M.; de Assis, H.R.B.S.; Leite-Silva, V.R.; da Rocha-Filho, F.A.; Negreiros, M.; Rocha, H.; Ostrosky, E.; Lopes, P.S.; et al. Evaluation of in vitro and in vivo safety of the by-product of Agave sisalana as a new cosmetic raw material: Development and clinical evaluation of a nanoemulsion to improve skin moisturizing. Ind. Crop. Prod. 2017, 108, 470–479. [Google Scholar] [CrossRef]
- Ji, K.-M.; Zhan, Z.-K.; Chen, J.-J.; Liu, Z.-G. Anaphylactic shock caused by silkworm pupa consumption in China. Allergy 2008, 63, 1407–1408. [Google Scholar] [CrossRef]
- Komane, B.; Vermaak, I.; Summers, B.; Viljoen, A. Safety and efficacy of Sclerocarya birrea (A. Rich.) Hochst (Marula) oil: A clinical perspective. J. Ethonopharm. 2015, 176, 327–335. [Google Scholar] [CrossRef]
- Stamatas, G.N.; de Sterke, J.; Hauser, M.; von Stetten, O.; van der Pol, A. Lipid uptake and skin occlusion following topical application of oils on adult and infant skin. J. Dermatol. Sci. 2008, 50, 135–142. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Leelapornpisid, P.; Mungmai, L.; Sirithunyalug, B.; Jiranusornkul, S.; Peerapornpisal, Y. A novel moisturizer extracted from freshwater macroallga (Rhizoclonium hieroglyphicum (C. Agardh) Kutzing) for skin care cosmetic. Chiang Mai J. Sci. 2014, 41, 1195–1207. [Google Scholar]
- Chaiyana, W.; Leelapornpisid, P.; Jakmunee, J.; Korsamphan, C. Antioxidant and moisturizing effect of Camellia assamica seed oil and its development into microemulsion. Cosmetics 2018, 5, 40. [Google Scholar] [CrossRef]
- Kassakul, W.; Praznik, W.; Viernstein, H.; Hongwiset, D.; Phrutivorapongkul, A.; Leelapornpisid, P. Characterization of the mucilages extracted from Hibiscus rosa-sinensis Lin. and Hibiscus mutabilis Linn and their skin moisturizing effect. Inter. J. Pharm. Pharm. Sci. 2014, 6, 453–457. [Google Scholar]
- Badiu, D.; Luque, R.; Rajendram, R. Effect of olive oil on the skin. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 1125–1132. [Google Scholar] [CrossRef]
- Draelos, Z.D. Proper skin hydration and barrier function. In Nutritional Cosmetics: Beauty; Tabor, A., Blair, R.M., Eds.; William Andrew: Norwich, UK, 2009; pp. 355–363. [Google Scholar] [CrossRef]
- Pintathong, P.; Chaiwut, P.; Thitipramote, N.; Thitilertdecha, N.; Nantitanont, W.; Sangthong, S.; Tiensri, N. Simultaneous ex-traction of oil and protein from perilla seed by three-phase partitioning and their application in serum. J. Appl. Sci. 2018, 17, 73–83. [Google Scholar] [CrossRef]
- Firestone, D. Methods Cd 8-53; Cd 1–25; Ca 5a-40; Ca 6a-40; Cd 3-25; Cc 13e-92; Ca 2c-25; Cc 7-25. In Official Methods and Recommended Practices of the American Oil Chemists’s Society, 5th ed.; AOCS Press: Champaign, IL, USA, 2003. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
| Extraction Time (h) | Oil Yield (% w/w) | Protein Yield (% w/w) | Protein Content (mg/g Extract) |
|---|---|---|---|
| 3 h | 7.63 ± 0.58 b1 | 2.56 ± 0.36 c2 | 267.57 ± 1.69 a3 |
| 6 h | 7.67 ± 0.35 b1 | 3.75 ± 1.23 c2 | 267.36 ± 1.25 a3 |
| 12 h | 7.82 ± 1.01 ab1 | 7.01 ± 0.32 b2 | 268.34 ± 1.71 a3 |
| 18 h | 8.24 ± 0.21 a1 | 8.41 ± 0.26 a2 | 268.25 ± 1.99 a3 |
| Fatty Acids | % Content (w/w) |
|---|---|
| Lauric acid (C12:0) | 0.13 ± 0.01 |
| Myristic acid (C14:0) | 0.49 ± 0.01 |
| Palmitic acid (C16:0) | 21.27 ± 0.05 |
| Stearic acid (C18:0) | 6.60 ± 0.09 |
| Oleic acid (C18:1) | 28.97 ± 0.13 |
| Linoleic acid (C18:2) | 4.73 ± 0.21 |
| Linolenic acid (C18:3) | 37.81 ± 0.34 |
| Properties | Values |
|---|---|
| Refractive index (30 °C) | 1.464 ± 0.001 |
| Viscosity (cP) | 533.73 ± 2.33 |
| Appearance | Clear viscous oil |
| Color | Yellow |
| L* | 55.56 ± 0.16 |
| a* | 5.37 ± 0.16 |
| b* | 23.07 ± 0.08 |
| Saponification value (mg KOH/g oil) | 191.51 ± 0.22 |
| Iodine value (g I2/100 g oil) | 119.37 ± 0.67 |
| Acid value (mg KOH/g oil) | 0.94 ± 0.04 |
| Peroxide value (mEq O2/kg oil) | 2.00 ± 0.01 |
| HLB value | 5–6 |
| Amino Acids | Type Of Amino Acids | Content (g/100 g) |
|---|---|---|
| Glutamic acid | polar, negatively charged | 2.26 ± 0.08 |
| Aspartic acid | polar, negatively charged | 2.78 ± 0.04 |
| Histidine | polar, positively charged | 0.38 ± 0.00 |
| Arginine | polar, positively charged | 1.78 ± 0.02 |
| Lysine | polar, positively charged | 1.48 ± 0.04 |
| Cysteine | polar, uncharged | 0.08 ± 0.00 |
| Tyrosine | polar, uncharged | 1.70 ± 0.11 |
| Serine | polar, uncharged | 2.05 ± 0.07 |
| Glycine | polar, uncharged | 2.39 ± 0.04 |
| Threonine | polar, uncharged | 1.33 ± 0.01 |
| Alanine | non polar | 1.35 ± 0.02 |
| Proline | non polar | 1.40 ± 0.12 |
| Valine | non polar | 1.26 ± 0.10 |
| Methionine | non polar | 0.83 ± 0.04 |
| Isoleucine | non polar | 0.97 ± 0.06 |
| Leucine | non polar | 1.68 ± 0.03 |
| Phenylalanine | non polar | 1.40 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susirirut, P.; Thitipramote, N.; Chaiwut, P. Simultaneous Extraction of Oil and Protein from Silkworm (Bombyx mori L.) Pupae (Lueng Parroj var.) and Their In Vitro Skin Moisturization. Molecules 2023, 28, 7032. https://doi.org/10.3390/molecules28207032
Susirirut P, Thitipramote N, Chaiwut P. Simultaneous Extraction of Oil and Protein from Silkworm (Bombyx mori L.) Pupae (Lueng Parroj var.) and Their In Vitro Skin Moisturization. Molecules. 2023; 28(20):7032. https://doi.org/10.3390/molecules28207032
Chicago/Turabian StyleSusirirut, Pannarasi, Natthawut Thitipramote, and Phanuphong Chaiwut. 2023. "Simultaneous Extraction of Oil and Protein from Silkworm (Bombyx mori L.) Pupae (Lueng Parroj var.) and Their In Vitro Skin Moisturization" Molecules 28, no. 20: 7032. https://doi.org/10.3390/molecules28207032
APA StyleSusirirut, P., Thitipramote, N., & Chaiwut, P. (2023). Simultaneous Extraction of Oil and Protein from Silkworm (Bombyx mori L.) Pupae (Lueng Parroj var.) and Their In Vitro Skin Moisturization. Molecules, 28(20), 7032. https://doi.org/10.3390/molecules28207032







