Exploring the HIV-1 Rev Recognition Element (RRE)–Rev Inhibitory Capacity and Antiretroviral Action of Benfluron Analogs
Abstract
:1. Introduction
2. Results
2.1. Optimization Strategy
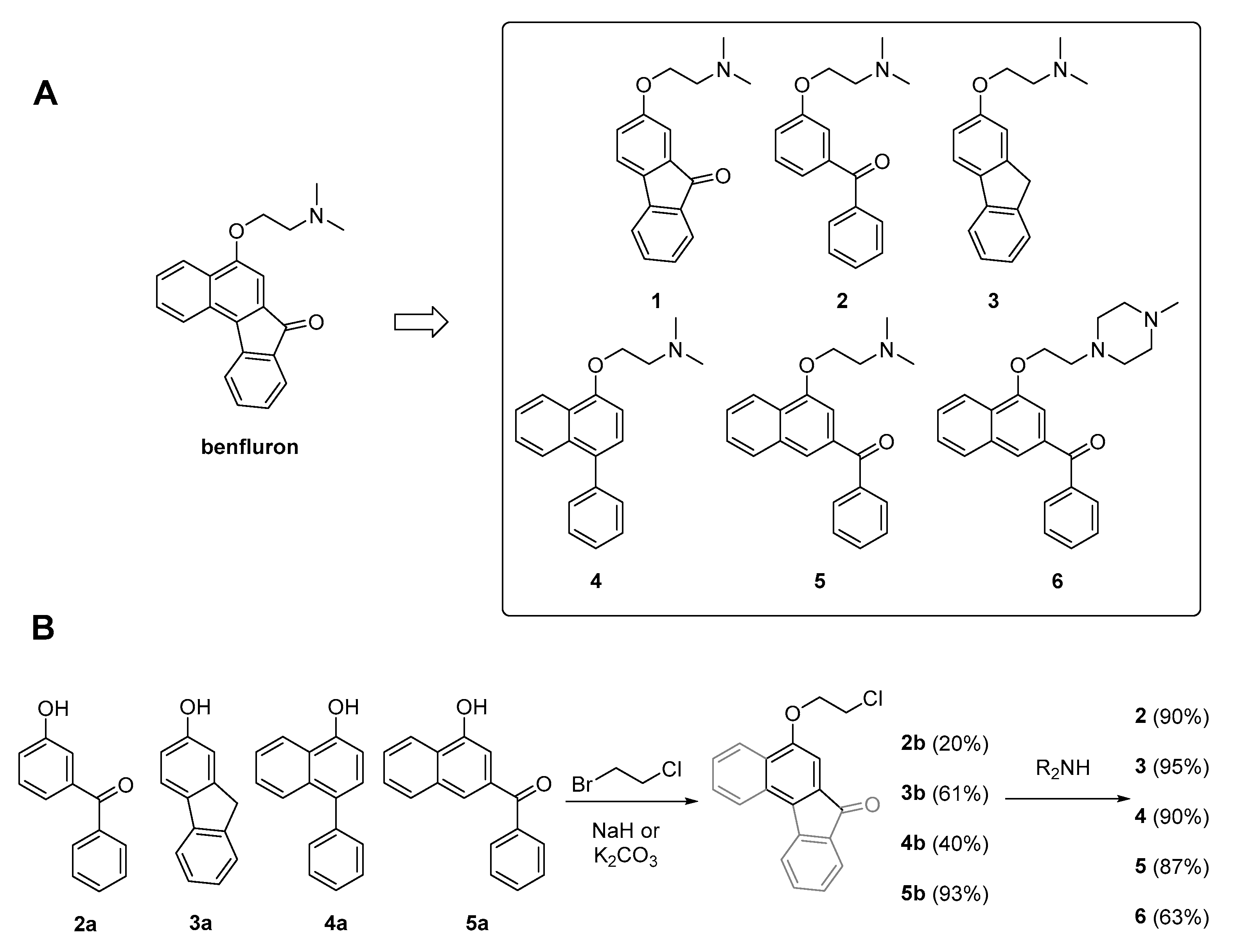
2.2. Benfluron Analogs
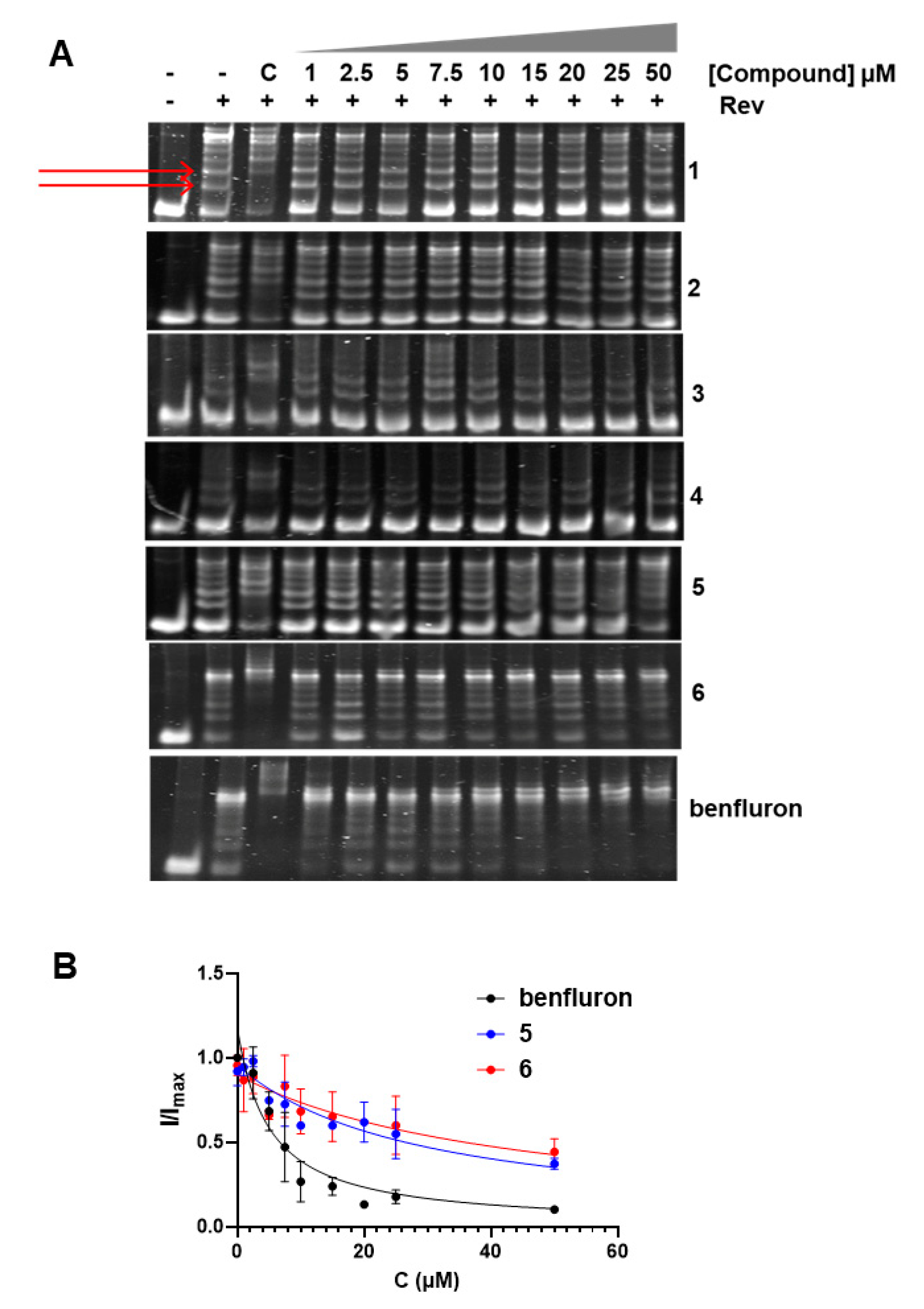
2.3. RRE Affinity and RRE–Rev Inhibition
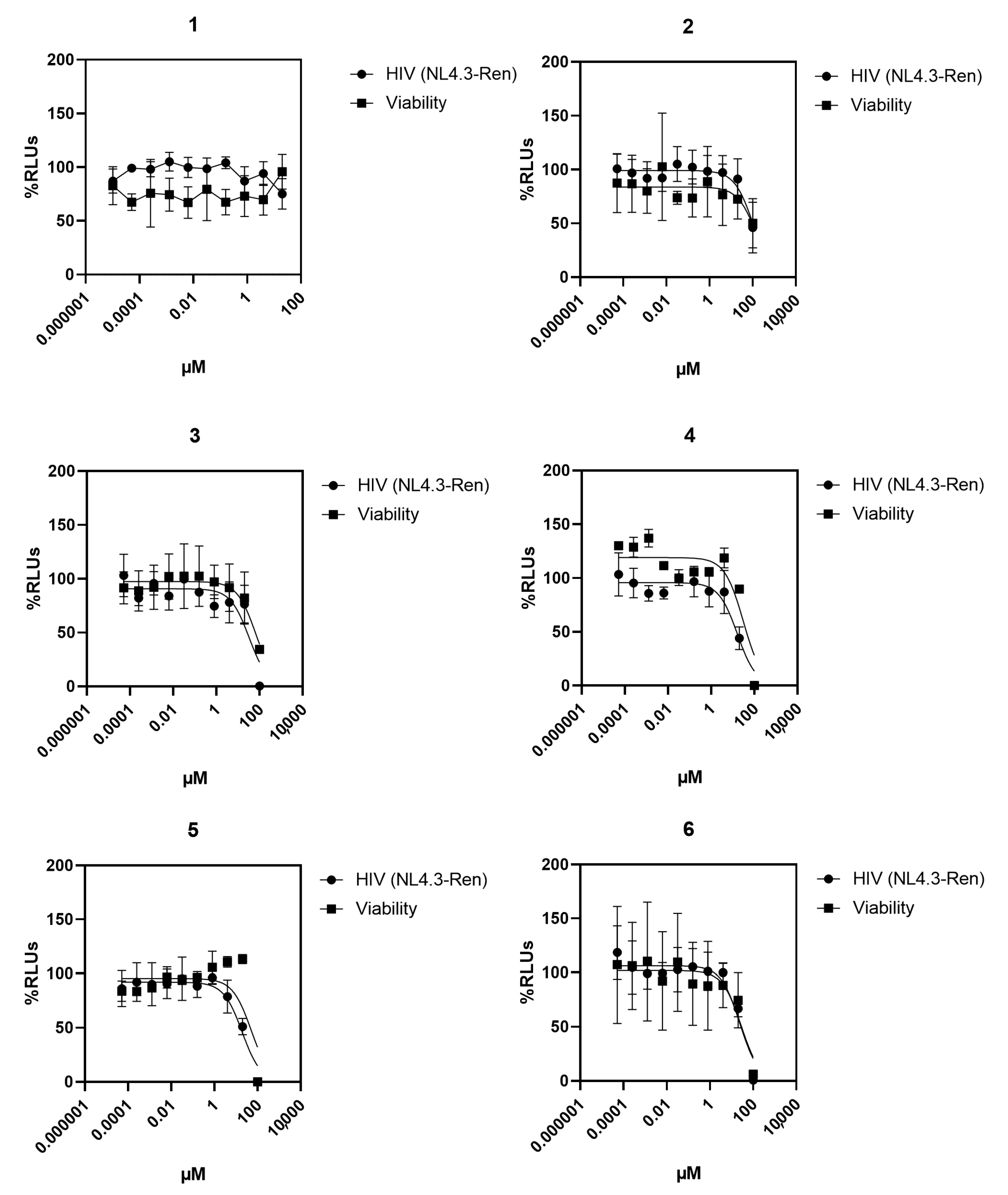
2.4. Antiretroviral Activity and Toxicity
3. Discussion
4. Materials and Methods
4.1. Materials and Methods for Compound Preparation
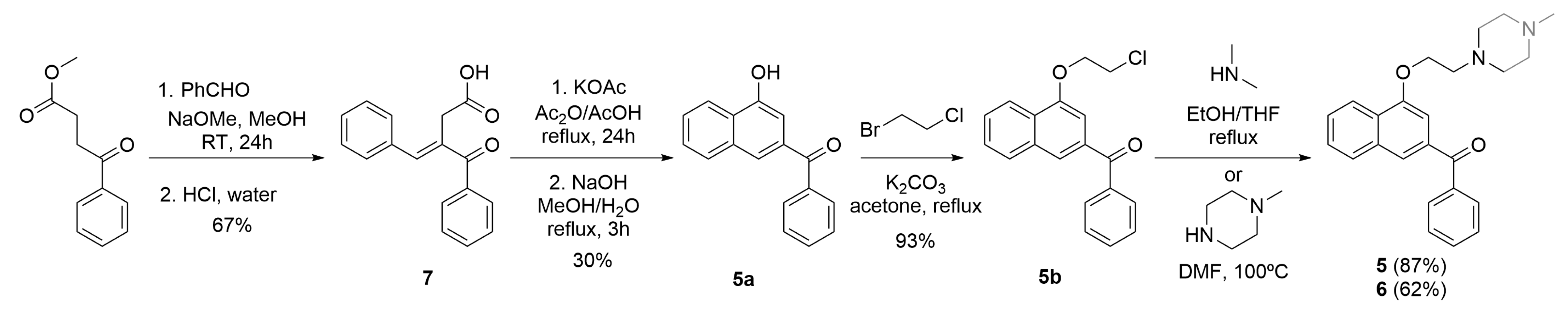
4.2. Synthesis of Benfluron Analogs 2–6
4.3. Fluorescence Binding Experiments
4.4. EMSA
4.5. Antiretroviral Activity and Cellular Toxicity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Margolis, D.M.; Archin, N.M.; Cohen, M.S.; Eron, J.J.; Ferrari, G.; Garcia, J.V.; Gay, C.L.; Goonetilleke, N.; Joseph, S.B.; Swanstrom, R.; et al. Curing HIV: Seeking to Target and Clear Persistent Infection. Cell 2020, 181, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Mudgal, J.; Kamath, B.V.; Pai, K.S.R. An insight on promising strategies hoping to cure HIV-1 infection by targeting Rev protein-short review. Pharmacol. Rep. 2021, 73, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.L.; Stanley, T.B.; Hazen, R.; Garvey, E.P. Small molecule modulators of HIV Rev/Rev response element interaction identified by random screening. Antivir. Res. 2002, 54, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Shuck-Lee, D.; Chen, F.F.; Willard, R.; Raman, S.; Ptak, R.; Hammarskjold, M.L.; Rekosh, D. Heterocyclic compounds that inhibit Rev-RRE function and human immunodeficiency virus type 1 replication. Antimicrob. Agents Chemother. 2008, 52, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.W.; Balachandran, A.; Haaland, M.; Stoilov, P.; Cochrane, A. Characterization of novel inhibitors of HIV-1 replication that function via alteration of viral RNA processing and rev function. Nucleic Acids Res. 2013, 41, 9471–9483. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bulnes, L.; Ibanez, I.; Bedoya, L.M.; Beltran, M.; Catalan, S.; Alcami, J.; Fustero, S.; Gallego, J. Structure-Based Design of an RNA-Binding p-Terphenylene Scaffold that Inhibits HIV-1 Rev Protein Function. Angew. Chem. Int. Ed. 2013, 52, 13405–13409. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Geng, G.; Chen, B.; Pan, T.; Li, Q.; Zhang, H.; Bai, C. Identification of benzenesulfonamide quinoline derivatives as potent HIV-1 replication inhibitors targeting Rev protein. Org. Biomol. Chem. 2015, 13, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Campos, N.; Myburgh, R.; Garcel, A.; Vautrin, A.; Lapasset, L.; Nadal, E.S.; Mahuteau-Betzer, F.; Najman, R.; Fornarelli, P.; Tantale, K.; et al. Long lasting control of viral rebound with a new drug ABX464 targeting Rev-mediated viral RNA biogenesis. Retrovirology 2015, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Prado, S.; Beltrán, M.; Coiras, M.; Bedoya, L.M.; Alcamí, J.; Gallego, J. Bioavailable inhibitors of HIV-1 RNA biogenesis identified through a Rev-based screen. Biochem. Pharmacol. 2016, 107, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.L.; Burlingame, M.A.; Yang, S.; Crosby, D.C.; Talbot, D.J.; Chui, K.; Frankel, A.D.; Renslo, A.R. Identification and Optimization of Thienopyridine Carboxamides as Inhibitors of HIV Regulatory Complexes. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Peralta, A.N.; Wynn, J.E.; Sherpa, C.; Li, H.; Verma, A.; Le Grice, S.F.J.; Santos, W.L. Molecular recognition of a branched peptide with HIV-1 Rev Response Element (RRE) RNA. Bioorg. Med. Chem. 2019, 27, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.D.; Booth, D.S.; Frankel, A.D. A structurally plastic ribonucleoprotein complex mediates post-transcriptional gene regulation in HIV-1. Wiley Interdiscip. Rev. RNA 2016, 7, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Prado, S.; Beltrán, M.; Moreno, A.; Bedoya, L.M.; Alcamí, J.; Gallego, J. A small-molecule inhibitor of HIV-1 Rev function detected by a diversity screen based on RRE-Rev interference. Biochem. Pharmacol. 2018, 156, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Melka, M.; Krepelka, J. Benfluron hydrochloride. Drugs Future 1987, 12, 745–748. [Google Scholar] [CrossRef]
- Yalçin, E.; Obrovac, L.G.; Piantanida, I. Novel fluorene/fluorenone DNA and RNA binders as efficient non-toxic ds-RNA selective fluorescent probes. Tetrahedron 2018, 74, 535–543. [Google Scholar] [CrossRef]
- Simba-Lahuasi, A.; Alcami, J.; Beltrán, M.; Bedoya, L.M.; Gallego, J. Novel HIV-1 RNA biogenesis inhibitors identified by virtual pharmacophore-based screening. Biochem. Pharmacol. 2023, 215, 115734. [Google Scholar] [CrossRef] [PubMed]
- Medina-Trillo, C.; Sedgwick, D.M.; Herrera, L.; Beltrán, M.; Moreno, A.; Barrio, P.; Bedoya, L.M.; Alcamí, A.; Fustero, S.; Gallego, J. Nucleic acid recognition and antiviral activity of 1,4-substituted terphenyl compounds mimicking all faces of the HIV-1 Rev protein positively-charged alpha-helix. Sci. Rep. 2020, 10, 7190. [Google Scholar] [CrossRef] [PubMed]
- Bradrick, T.D.; Marino, J.P. Ligand-induced changes in 2-aminopurine fluorescence as a probe for small molecule binding to HIV-1 TAR RNA. RNA 2004, 10, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, J.; Sanchez-Palomino, S.; Perez-Olmeda, M.; Fernandez, B.; Alcami, J. A new strategy based on recombinant viruses as a tool for assessing drug susceptibility of human immunodeficiency virus type 1. J. Med. Virol. 2007, 79, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Adachi, A.; Gendelman, H.E.; Koenig, S.; Folks, T.; Willey, R.; Rabson, A.; Martin, M.A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 1986, 59, 284–291. [Google Scholar] [CrossRef] [PubMed]

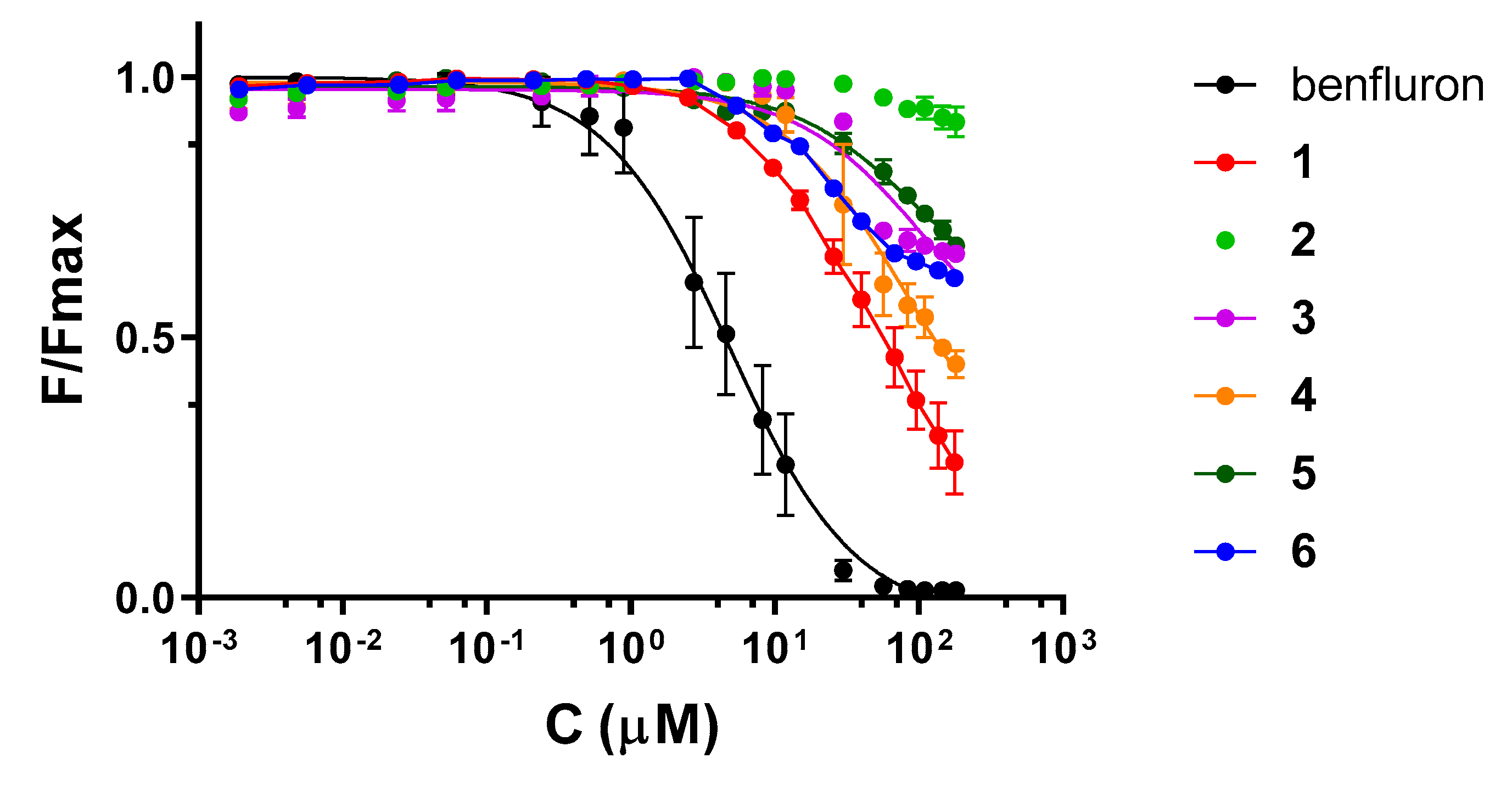
| Compound a | Kd (RRE IIB) (µM) | IC50 (RRE–Rev) (µM) |
|---|---|---|
| benfluron | 4.83 (4.39–5.31, 0.9779) b | 5.0 (3.4–7.5, 0.8340) |
| 1 | 45.6 (39.8–52.5, 0.9863) | >50 |
| 2 | >100 | >50 |
| 3 | 106 (64.1–197, 0.9007) | >50 |
| 4 | 77.5 (55.0–113.1, 0.9591) | >50 |
| 5 | 95.1 (72.3–128, 0.9835) | 29 (20–43, 0.8137) |
| 6 | 34.0 (28.6–40.6, 0.9662) | 45 (27–87, 0.5991) |
| Compound | EC50 (µM) | CC50 (µM) |
|---|---|---|
| Benfluron | 0.830 (0.580–1.17, 0.9707) b | 28.1 (12.0–65.7, 0.8513) b |
| 1 | >100 | >100 |
| 2 | ~100 | ~100 |
| 3 | ~100 | ~100 |
| 4 | 17.0 (10.0–29.1, 0.8180) | 28.7 (16.3–50.8, 0.7588) |
| 5 | 19.4 (11.5–32.9, 0.8178) | 49.7 (23.4–113, 0.5291) |
| 6 | 24.2 (16.2–36.3, 0.7632) | >20 <100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chumillas, S.; Loharch, S.; Beltrán, M.; Szewczyk, M.P.; Bernal, S.; Puertas, M.C.; Martinez-Picado, J.; Alcamí, J.; Bedoya, L.M.; Marchán, V.; et al. Exploring the HIV-1 Rev Recognition Element (RRE)–Rev Inhibitory Capacity and Antiretroviral Action of Benfluron Analogs. Molecules 2023, 28, 7031. https://doi.org/10.3390/molecules28207031
Chumillas S, Loharch S, Beltrán M, Szewczyk MP, Bernal S, Puertas MC, Martinez-Picado J, Alcamí J, Bedoya LM, Marchán V, et al. Exploring the HIV-1 Rev Recognition Element (RRE)–Rev Inhibitory Capacity and Antiretroviral Action of Benfluron Analogs. Molecules. 2023; 28(20):7031. https://doi.org/10.3390/molecules28207031
Chicago/Turabian StyleChumillas, Sergi, Saurabh Loharch, Manuela Beltrán, Mateusz P. Szewczyk, Silvia Bernal, Maria C. Puertas, Javier Martinez-Picado, José Alcamí, Luis M. Bedoya, Vicente Marchán, and et al. 2023. "Exploring the HIV-1 Rev Recognition Element (RRE)–Rev Inhibitory Capacity and Antiretroviral Action of Benfluron Analogs" Molecules 28, no. 20: 7031. https://doi.org/10.3390/molecules28207031
APA StyleChumillas, S., Loharch, S., Beltrán, M., Szewczyk, M. P., Bernal, S., Puertas, M. C., Martinez-Picado, J., Alcamí, J., Bedoya, L. M., Marchán, V., & Gallego, J. (2023). Exploring the HIV-1 Rev Recognition Element (RRE)–Rev Inhibitory Capacity and Antiretroviral Action of Benfluron Analogs. Molecules, 28(20), 7031. https://doi.org/10.3390/molecules28207031






