Thermodynamic Properties of 3- and 4-Ethoxyacetanilides between 80 and 480 K
Abstract
:1. Introduction
2. Results
2.1. Fusion Enthalpies of 3-Ethoxyacetanilide and Phenacetin at Melting Temperature
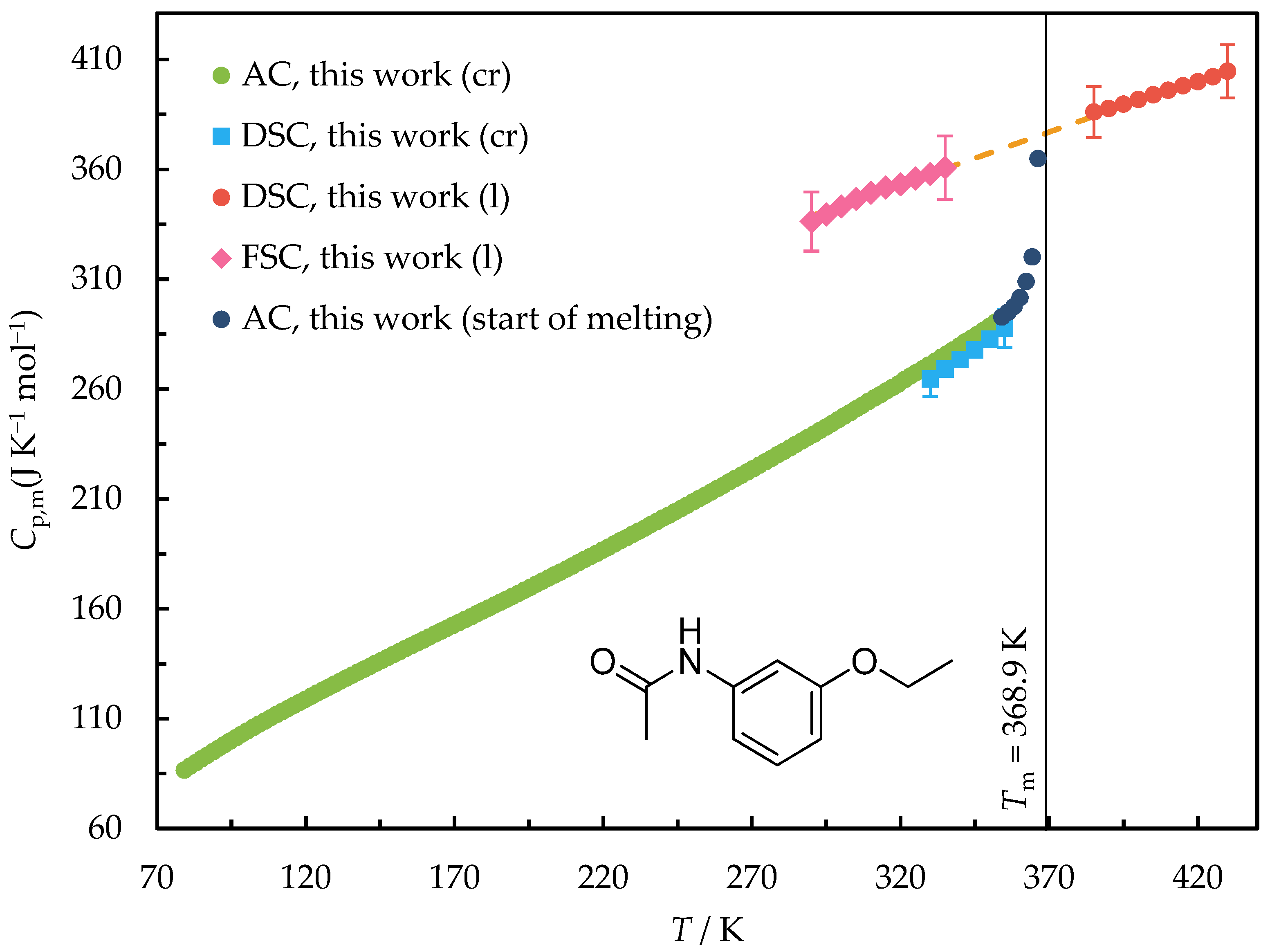
2.2. Heat Capacity and Thermodynamic Properties of 3-Ethoxyacetanilide between 80 and 430 K and Phenacetin between 80 and 475 K
3. Discussion
3.1. Heat Capacities of the Solid and Liquid 3-Ethoxyacetanilide and Phenacetin at 298.15 K
3.2. The Difference between the Heat Capacities of the Solid and Liquid 3-Ethoxyacetanilide and Phenacetin at the Melting Point
3.3. Fusion Enthalpies of 3-Ethoxyacetanilide and Phenacetin at 298.15 K
4. Materials and Methods
4.1. Materials
4.2. Adiabatic Calorimetry
4.3. Differential Scanning Calorimetry
4.4. Fast Scanning Calorimetry
4.5. Solution Calorimetry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Charaya, S.; Bozzelli, J.W. Thermochemistry, Bond Energies and Internal Rotor Potentials of Acetic Acid Hydrazide, Acetamide, N-Methyl Acetamide (NMA) and Radicals. Thermo 2021, 1, 15–31. [Google Scholar] [CrossRef]
- Almeida, A.R.; Monte, M.J. Thermodynamic study of benzamide, N-methylbenzamide, and N, N-dimethylbenzamide: Vapor pressures, phase diagrams, and hydrogen bond enthalpy. J. Chem. Eng. Data 2010, 55, 3507–3512. [Google Scholar] [CrossRef]
- Umnahanant, P.; Chickos, J. Vaporization and sublimation enthalpies of acetanilide and several derivatives by correlation gas chromatography. J. Chem. Eng. Data 2012, 57, 1331–1337. [Google Scholar] [CrossRef]
- Held, C.; Brinkmann, J.; Schröder, A.-D.; Yagofarov, M.I.; Verevkin, S.P. Solubility predictions of acetanilide derivatives in water: Combining thermochemistry and thermodynamic modeling. Fluid Phase Equilibria 2018, 455, 43–53. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Zaitsau, D.H. Thermochemistry of substituted benzamides and substituted benzoic acids: Like tree, like fruit? ChemPhysChem 2018, 19, 619–630. [Google Scholar] [CrossRef]
- Manin, A.N.; Voronin, A.P.; Perlovich, G.L. Thermodynamic and structural aspects of hydroxybenzamide molecular crystals study. Thermochim. Acta 2013, 551, 57–61. [Google Scholar] [CrossRef]
- Vecchio, S.; Tomassetti, M. Vapor pressures and standard molar enthalpies, entropies and Gibbs energies of sublimation of three 4-substituted acetanilide derivatives. Fluid Phase Equilibria 2009, 279, 64–72. [Google Scholar] [CrossRef]
- Clissold, S.P. Paracetamol and phenacetin. Drugs 1986, 32, 46–59. [Google Scholar] [CrossRef]
- Chandrasekharan, N.; Dai, H.; Roos, K.L.T.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Natl. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar] [CrossRef]
- Jensen, C.B.; Jollow, D.J. The role of N-hydroxyphenetidine in phenacetin-induced hemolytic anemia. Toxicol. Appl. Pharmacol. 1991, 111, 1–12. [Google Scholar] [CrossRef]
- Peters, G.; Baechtold-Fowler, N.; Bonjour, J.P.; Chométy-Diézi, F.; Filloux, B.; Guidoux, R.; Guignard, J.; Peters-Haefeli, L.; Roch-Ramel, F.; Schelling, J. General and renal toxicity of phenacetin, paracetamol and some anti-mitotic agents in the rat. Arch. Für Toxikol. 1972, 28, 225–269. [Google Scholar] [CrossRef] [PubMed]
- Easley, J.L.; Condon, B.F. Phenacetin-Induced Methemoglobinemia and Renal Failure. J. Am. Soc. Anesthesiol. 1974, 41, 99–100. [Google Scholar] [CrossRef] [PubMed]
- McCredie, M.; Ford, J.; Stewart, J. Risk Factors for Cancer of the Renal Parenchyma. J. Urol. 1989, 141, 1272–1273. [Google Scholar] [CrossRef]
- Marın, A.; Garcıa, E.; Garcıa, A.; Barbas, C. Validation of a HPLC quantification of acetaminophen, phenylephrine and chlorpheniramine in pharmaceutical formulations: Capsules and sachets. J. Pharm. Biomed. Anal. 2002, 29, 701–714. [Google Scholar] [CrossRef]
- Sagar, K.A.; Smyth, M.R. A comparative bioavailability study of different aspirin formulations using on-line multidimensional chromatography. J. Pharm. Biomed. Anal. 1999, 21, 383–392. [Google Scholar] [CrossRef]
- Cox, G.; Loscombe, C.; Sugden, K. Some applications of bonded-phase high-performance liquid chromatography to the analysis of pharmaceutical formulations. Anal. Chim. Acta 1977, 92, 345–352. [Google Scholar] [CrossRef]
- Starmer, G.; McLean, S.; Thomas, J. Analgesic potency and acute toxicity of substituted anilides and benzamides. Toxicol. Appl. Pharmacol. 1971, 19, 20–28. [Google Scholar] [CrossRef]
- Mantheni, D.R.; Maheswaram, M.; Munigeti, R.; Perera, I.; Riga, A.; Alexander, K. Solid-and liquid-state studies of a wide range of chemicals by isothermal and scanning dielectric thermal analysis. J. Therm. Anal. Calorim. 2014, 115, 2253–2260. [Google Scholar] [CrossRef]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L.S. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef]
- Miyako, Y.; Khalef, N.; Matsuzaki, K.; Pinal, R. Solubility enhancement of hydrophobic compounds by cosolvents: Role of solute hydrophobicity on the solubilization effect. Int. J. Pharm. 2010, 393, 48–54. [Google Scholar] [CrossRef]
- Peña, M.; Escalera, B.; Reíllo, A.; Sánchez, A.; Bustamante, P. Thermodynamics of cosolvent action: Phenacetin, salicylic acid and probenecid. J. Pharm. Sci. 2009, 98, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, M.; Kowalska, A.; Tymorek, N.; Dziaman, T.; Cysewski, P. Thermodynamic characteristics of phenacetin in solid state and saturated solutions in several neat and binary solvents. Molecules 2021, 26, 4078. [Google Scholar] [CrossRef] [PubMed]
- Yagofarov, M.I.; Nagrimanov, R.N.; Solomonov, B.N. Sublimation enthalpies of 9 substituted acetanilides at 298 K estimated by solution calorimetry approach. Thermochim. Acta 2017, 656, 85–89. [Google Scholar] [CrossRef]
- Gerasimov, A.V.; Varfolomeev, M.A.; Ziganshin, M.A.; Gorbatchuk, V.V.; Il’naz, T.R.; Klimovitskii, A.E.; Usmanova, L.S. Thermodynamics of dissolution and infrared-spectroscopy of solid dispersions of phenacetin. J. Adv. Pharm. Technol. Res. 2016, 7, 6. [Google Scholar] [CrossRef]
- Cárdenas, Z.J.; Almanza, O.A.; Jouyban, A.; Martínez, F.; Acree, W.E., Jr. Solubility and preferential solvation of phenacetin in methanol+ water mixtures at 298.15 K. Phys. Chem. Liq. 2018, 56, 16–32. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Wang, L.; Hao, H.; Zhou, L. Measurement and Correlation of Solubility and Thermodynamic Properties of Phenacetin in 12 Pure Solvents from 283.15 to 323.15 K. J. Chem. Eng. Data 2021, 66, 4593–4602. [Google Scholar] [CrossRef]
- Croker, D.M.; Kelly, D.M.; Horgan, D.E.; Hodnett, B.K.; Lawrence, S.E.; Moynihan, H.A.; Rasmuson, Å.C. Demonstrating the influence of solvent choice and crystallization conditions on phenacetin crystal habit and particle size distribution. Org. Process Res. Dev. 2015, 19, 1826–1836. [Google Scholar] [CrossRef]
- Hojjati, H.; Rohani, S. Measurement and prediction of solubility of paracetamol in water−isopropanol solution. Part 2. Prediction. Org. Process Res. Dev. 2006, 10, 1110–1118. [Google Scholar] [CrossRef]
- Wassvik, C.M.; Holmén, A.G.; Bergström, C.A.; Zamora, I.; Artursson, P. Contribution of solid-state properties to the aqueous solubility of drugs. Eur. J. Pharm. Sci. 2006, 29, 294–305. [Google Scholar] [CrossRef]
- Wassvik, C.M.; Holmén, A.G.; Draheim, R.; Artursson, P.; Bergström, C.A. Molecular characteristics for solid-state limited solubility. J. Med. Chem. 2008, 51, 3035–3039. [Google Scholar] [CrossRef]
- Vecchio, S.; Catalani, A.; Rossi, V.; Tomassetti, M. Thermal analysis study on vaporization of some analgesics. Acetanilide and derivatives. Thermochim. Acta 2004, 420, 99–104. [Google Scholar] [CrossRef]
- Manzo, R.H.; Ahumada, A.A. Effects of solvent medium on solubility. V: Enthalpic and entropic contributions to the free energy changes of di-substituted benzene derivatives in ethanol: Water and ethanol: Cyclohexane mixtures. J. Pharm. Sci. 1990, 79, 1109–1115. [Google Scholar] [CrossRef]
- Baena, Y.; Pinzón, J.A.; Barbosa, H.J.; Martínez, F. Temperature-dependence of the solubility of some acetanilide derivatives in several organic and aqueous solvents. Phys. Chem. Liq. 2004, 42, 603–613. [Google Scholar] [CrossRef]
- Perlovich, G.L.; Volkova, T.V.; Bauer-Brandl, A. Towards an understanding of the molecular mechanism of solvation of drug molecules: A thermodynamic approach by crystal lattice energy, sublimation, and solubility exemplified by paracetamol, acetanilide, and phenacetin. J. Pharm. Sci. 2006, 95, 2158–2169. [Google Scholar] [CrossRef] [PubMed]
- Chickos, J.S.; Hesse, D.G.; Liebman, J.F. A group additivity approach for the estimation of heat capacities of organic liquids and solids at 298 K. Struct. Chem. 1993, 4, 261–269. [Google Scholar] [CrossRef]
- Hurst, J.E., Jr.; Keith Harrison, B. Estimation of liquid and solid heat capacities using a modified Kopp’s rule. Chem. Eng. Commun. 1992, 112, 21–30. [Google Scholar] [CrossRef]
- Goodman, B.T.; Wilding, W.V.; Oscarson, J.L.; Rowley, R.L. Use of the DIPPR database for development of quantitative structure− property relationship correlations: Heat capacity of solid organic compounds. J. Chem. Eng. Data 2004, 49, 24–31. [Google Scholar] [CrossRef]
- Hayden, J.G.; O’Connell, J.P. A generalized method for predicting second virial coefficients. Ind. Eng. Chem. Process Des. Dev. 1975, 14, 209–216. [Google Scholar] [CrossRef]
- Quayle, O.R. The Parachors of Organic Compounds. An Interpretation and Catalogue. Chem. Rev. 1953, 53, 439–589. [Google Scholar] [CrossRef]
- Růžička, V., Jr.; Domalski, E.S. Estimation of the heat capacities of organic liquids as a function of temperature using group additivity. I. Hydrocarbon compounds. J. Phys. Chem. Ref. Data 1993, 22, 597–618. [Google Scholar] [CrossRef]
- Růžička, V., Jr.; Domalski, E.S. Estimation of the heat capacities of organic liquids as a function of temperature using group additivity. II. Compounds of carbon, hydrogen, halogens, nitrogen, oxygen, and sulfur. J. Phys. Chem. Ref. Data 1993, 22, 619–657. [Google Scholar] [CrossRef]
- Zábranský, M.; Růžička, V., Jr. Estimation of the heat capacities of organic liquids as a function of temperature using group additivity: An amendment. J. Phys. Chem. Ref. Data 2004, 33, 1071–1081. [Google Scholar] [CrossRef]
- Kolská, Z.; Kukal, J.; Zábranský, M.; Růžička, V. Estimation of the heat capacity of organic liquids as a function of temperature by a three-level group contribution method. Ind. Eng. Chem. Res. 2008, 47, 2075–2085. [Google Scholar] [CrossRef]
- Pappa, G.D.; Voutsas, E.C.; Magoulas, K.; Tassios, D.P. Estimation of the differential molar heat capacities of organic compounds at their melting point. Ind. Eng. Chem. Res. 2005, 44, 3799–3806. [Google Scholar] [CrossRef]
- Mishra, D.S.; Yalkowsky, S.H. Ideal solubility of a solid solute: Effect of heat capacity assumptions. Pharm. Res. 1992, 9, 958–959. [Google Scholar] [CrossRef]
- Prausnitz, J.; Lichtenthaler, R.; Azevedo, E. Molecular Thermodynamics of Fluid Phase Equilibria, 3rd ed.; Prentice Hall PTR: Englewood Cliffs, NJ, USA, 1999. [Google Scholar]
- Abildskov, J.; O’Connell, J.P. Predicting the solubilities of complex chemicals I. Solutes in different solvents. Ind. Eng. Chem. Res. 2003, 42, 5622–5634. [Google Scholar] [CrossRef]
- Silveira, M.; Mayer, D.A.; Rebelatto, E.A.; Araújo, P.H.; Oliveira, J.V. Solubility and thermodynamic parameters of nicotinic acid in different solvents. J. Chem. Thermodyn. 2023, 184, 107084. [Google Scholar] [CrossRef]
- Shakeel, F.; Alshehri, S.; Imran, M.; Haq, N.; Alanazi, A.; Anwer, M.K. Experimental and computational approaches for solubility measurement of pyridazinone derivative in binary (DMSO+ water) systems. Molecules 2019, 25, 171. [Google Scholar] [CrossRef]
- Yagofarov, M.I.; Solomonov, B.N. Calculation of the fusion enthalpy temperature dependence of polyaromatic hydrocarbons from the molecular structure: Old and new approaches. J. Chem. Thermodyn. 2021, 152, 106278. [Google Scholar] [CrossRef]
- Hildebrand, J.H.; Prausnitz, J.M.; Scott, R.L. Regular and Related Solutions: The Solubility of Gases, Liquids, and Solids; (No Title); Van Nostrand Reinhold Co.: New York, NY, USA, 1970. [Google Scholar]
- Mauger, J.W.; Paruta, A.N.; Gerraughty, R.J. Solubilities of sulfadiazine, sulfisomidine, and sulfadimethoxine in several normal alcohols. J. Pharm. Sci. 1972, 61, 94–97. [Google Scholar] [CrossRef]
- Grant, D.; Mehdizadeh, M.; Chow, A.-L.; Fairbrother, J. Non-linear van’t Hoff solubility-temperature plots and their pharmaceutical interpretation. Int. J. Pharm. 1984, 18, 25–38. [Google Scholar] [CrossRef]
- Neau, S.H.; Bhandarkar, S.V.; Hellmuth, E.W. Differential molar heat capacities to test ideal solubility estimations. Pharm. Res. 1997, 14, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yalkowsky, S. Estimation of the molar heat capacity change on melting of organic compounds. Ind. Eng. Chem. Res. 2009, 48, 1063–1066. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Yagofarov, M.I. An approach for the calculation of vaporization enthalpies of aromatic and heteroaromatic compounds at 298.15 K applicable to supercooled liquids. J. Mol. Liq. 2020, 319, 114330. [Google Scholar] [CrossRef]
- Yagofarov, M.I.; Lapuk, S.E.; Mukhametzyanov, T.A.; Ziganshin, M.A.; Valiakhmetov, T.F.; Solomonov, B.N. The fusion thermochemistry of self-associated aromatic compounds at 298.15 K studied by solution calorimetry. J. Chem. Thermodyn. 2019, 137, 43–47. [Google Scholar] [CrossRef]
- Bolmatenkov, D.N.; Yagofarov, M.I.; Sokolov, A.A.; Ziganshin, M.A.; Solomonov, B.N. The heat capacities and fusion thermochemistry of sugar alcohols between 298.15 K and Tm: The study of D-sorbitol, D-mannitol and myo-inositol. J. Mol. Liq. 2021, 330, 115545. [Google Scholar] [CrossRef]
- Yagofarov, M.I.; Sokolov, A.A.; Balakhontsev, I.S.; Nizamov, I.I.; Solomonov, B.N. Thermochemistry of fusion, solution and hydrogen bonding in benzamide, N-methylbenzamide, and acetanilide. Thermochim. Acta 2023, 728, 179579. [Google Scholar] [CrossRef]
- Blokhin, A.V.; Paulechka, Y.U.; Kabo, G.J. Thermodynamic properties of [C6mim][NTf2] in the condensed state. J. Chem. Eng. Data 2006, 51, 1377–1388. [Google Scholar] [CrossRef]
- Yagofarov, M.I.; Sokolov, A.A.; Gerasimov, A.V.; Solomonov, B.N.; Stepurko, E.N.; Yurkshtovich, Y.N. Thermodynamic Properties of Thioxanthone between 80 and 540 K. J. Chem. Eng. Data 2022, 67, 3583–3588. [Google Scholar] [CrossRef]
- Goursot, P.; Girdhar, H.L.; Westrum, E.F., Jr. Thermodynamics of polynuclear aromatic molecules. III. Heat capacities and enthalpies of fusion of anthracene. J. Phys. Chem. 1970, 74, 2538–2541. [Google Scholar] [CrossRef]
- Della Gatta, G.; Richardson, M.J.; Sarge, S.M.; Stølen, S. Standards, calibration, and guidelines in microcalorimetry. Part 2. Calibration standards for differential scanning calorimetry*(IUPAC Technical Report). Pure Appl. Chem. 2006, 78, 1455–1476. [Google Scholar] [CrossRef]
- Yagofarov, M.I.; Lapuk, S.E.; Mukhametzyanov, T.A.; Ziganshin, M.A.; Schick, C.; Solomonov, B.N. Application of fast scanning calorimetry to the fusion thermochemistry of low-molecular-weight organic compounds: Fast-crystallizing m-terphenyl heat capacities in a deeply supercooled liquid state. Thermochim. Acta 2018, 668, 96–102. [Google Scholar] [CrossRef]


| Compound | a | a | Ref. |
|---|---|---|---|
| 3EtOAn | 368.0 ± 0.4 | 28.9 ± 0.4 | [3] |
| 369.8 ± 0.2 | 28.1 ± 0.5 | This work | |
| 368.9 ± 1.3 | 28.5 ± 0.6 | Average b | |
| Phenac | 407.0 ± 0.1 | 32.0 ± 0.1 | [3] |
| 409.6 | 30.0 ± 1.0 | [7] | |
| 407.0 | 28.8 | [18] | |
| 409.0 | 31.5 | [19] | |
| 407.6 | (36.9) | [20] | |
| 408.3 | 28.8 | [21] | |
| 408.1 ± 0.2 | 32.5 ± 0.2 | [22] | |
| 407.7 | 32.3 | [25] | |
| 407.4 ± 0.1 | 34.1 ± 0.9 | [29,30] | |
| (410.2) | (21.4 ± 0.9) | [31] | |
| 407.2 | 31.3 | [32] | |
| 407.7 | 30.7 | [33] | |
| 408.5 ± 0.2 | 31.2 ± 0.5 | This work | |
| 408.1 ± 0.8 | 31.2 ± 1.6 | Average b |
| Crystal | ||||
|---|---|---|---|---|
| 80 | 87.30 ± 0.35 | 0 | 0 | 0 |
| 90 | 95.95 ± 0.38 | 10.79 ± 0.04 | 10.19 ± 0.04 | 0.6016 ± 0.0593 |
| 100 | 104.1 ± 0.4 | 21.32 ± 0.09 | 19.17 ± 0.08 | 2.149 ± 0.115 |
| 110 | 111.6 ± 0.4 | 31.60 ± 0.13 | 27.24 ± 0.11 | 4.361 ± 0.167 |
| 120 | 118.9 ± 0.5 | 41.63 ± 0.17 | 34.58 ± 0.14 | 7.051 ± 0.216 |
| 130 | 125.9 ± 0.5 | 51.42 ± 0.21 | 41.33 ± 0.17 | 10.09 ± 0.26 |
| 140 | 132.7 ± 0.5 | 61.00 ± 0.24 | 47.62 ± 0.19 | 13.38 ± 0.31 |
| 150 | 139.5 ± 0.6 | 70.39 ± 0.28 | 53.52 ± 0.21 | 16.87 ± 0.35 |
| 160 | 146.1 ± 0.6 | 79.60 ± 0.32 | 59.10 ± 0.24 | 20.51 ± 0.40 |
| 170 | 152.7 ± 0.6 | 88.66 ± 0.35 | 64.41 ± 0.26 | 24.25 ± 0.44 |
| 180 | 159.3 ± 0.6 | 97.58 ± 0.39 | 69.50 ± 0.28 | 28.08 ± 0.48 |
| 190 | 166.0 ± 0.7 | 106.4 ± 0.4 | 74.40 ± 0.30 | 31.97 ± 0.52 |
| 200 | 172.8 ± 0.7 | 115.1 ± 0.5 | 79.15 ± 0.32 | 35.90 ± 0.56 |
| 210 | 179.7 ± 0.7 | 123.7 ± 0.5 | 83.78 ± 0.34 | 39.88 ± 0.60 |
| 220 | 186.8 ± 0.7 | 132.2 ± 0.5 | 88.30 ± 0.35 | 43.88 ± 0.64 |
| 230 | 193.9 ± 0.8 | 140.6 ± 0.6 | 92.73 ± 0.37 | 47.90 ± 0.67 |
| 240 | 201.1 ± 0.8 | 149.0 ± 0.6 | 97.10 ± 0.39 | 51.94 ± 0.71 |
| 250 | 208.4 ± 0.8 | 157.4 ± 0.6 | 101.4 ± 0.4 | 55.99 ± 0.75 |
| 260 | 215.8 ± 0.9 | 165.7 ± 0.7 | 105.7 ± 0.4 | 60.05 ± 0.79 |
| 270 | 223.4 ± 0.9 | 174.0 ± 0.7 | 109.9 ± 0.4 | 64.12 ± 0.82 |
| 280 | 231.0 ± 0.9 | 182.3 ± 0.7 | 114.1 ± 0.5 | 68.19 ± 0.86 |
| 290 | 238.8 ± 1.0 | 190.5 ± 0.8 | 118.2 ± 0.5 | 72.27 ± 0.90 |
| 298.15 | 245.3 ± 1.0 | 197.2 ± 0.8 | 121.6 ± 0.5 | 75.59 ± 0.93 |
| 300 | 246.8 ± 1.0 | 198.7 ± 0.8 | 122.4 ± 0.5 | 76.35 ± 0.93 |
| 310 | 254.8 ± 1.0 | 207.0 ± 0.8 | 126.5 ± 0.5 | 80.43 ± 0.97 |
| 320 | 263.0 ± 1.1 | 215.2 ± 0.9 | 130.7 ± 0.5 | 84.51 ± 1.01 |
| 330 | 271.3 ± 1.1 | 223.4 ± 0.9 | 134.8 ± 0.5 | 88.59 ± 1.04 |
| 340 | 279.8 ± 1.1 | 231.6 ± 0.9 | 138.9 ± 0.6 | 92.68 ± 1.08 |
| 350 | 288.4 ± 1.2 | 239.9 ± 1.0 | 143.1 ± 0.6 | 96.77 ± 1.12 |
| 360 b | 297.0 ± 1.2 | 248.1 ± 1.0 | 147.2 ± 0.6 | 100.9 ± 1.2 |
| 368.9 b | 304.7 ± 1.2 | 255.4 ± 1.0 | 151.0 ± 0.6 | 104.5 ± 1.2 |
| Liquid | ||||
| 368.9 b | 376 ± 11 | 332.7 ± 2.9 | 228.2 ± 2.5 | 104.5 ± 3.8 |
| 370 b | 377 ± 11 | 333.8 ± 3.0 | 228.6 ± 2.5 | 105.2 ± 3.9 |
| 380 b | 382 ± 11 | 343.9 ± 3.3 | 232.6 ± 2.6 | 111.3 ± 4.2 |
| 390 | 387 ± 12 | 353.9 ± 3.6 | 236.5 ± 2.8 | 117.4 ± 4.5 |
| 400 | 391 ± 12 | 363.8 ± 3.9 | 240.3 ± 2.9 | 123.5 ± 4.8 |
| 410 | 396 ± 12 | 373.5 ± 4.1 | 244.1 ± 3.0 | 129.4 ± 5.1 |
| 420 | 401 ± 12 | 383.1 ± 4.4 | 247.7 ± 3.1 | 135.4 ± 5.4 |
| 430 | 406 ± 12 | 392.6 ± 4.7 | 251.3 ± 3.2 | 141.2 ± 5.7 |
| Crystal | ||||
|---|---|---|---|---|
| 80 | 87.63 ± 0.35 | 0 | 0 | 0 |
| 90 | 96.88 ± 0.39 | 10.86 ± 0.04 | 10.26 ± 0.04 | 0.6052 ± 0.0598 |
| 100 | 105.6 ± 0.4 | 21.52 ± 0.09 | 19.36 ± 0.08 | 2.166 ± 0.116 |
| 110 | 113.7 ± 0.5 | 31.98 ± 0.13 | 27.57 ± 0.11 | 4.402 ± 0.169 |
| 120 | 121.4 ± 0.5 | 42.20 ± 0.17 | 35.07 ± 0.14 | 7.128 ± 0.220 |
| 130 | 128.9 ± 0.5 | 52.22 ± 0.21 | 42.01 ± 0.17 | 10.21 ± 0.27 |
| 140 | 136.2 ± 0.5 | 62.04 ± 0.25 | 48.48 ± 0.19 | 13.56 ± 0.31 |
| 150 | 143.3 ± 0.6 | 71.69 ± 0.29 | 54.57 ± 0.22 | 17.12 ± 0.36 |
| 160 | 150.3 ± 0.6 | 81.16 ± 0.32 | 60.33 ± 0.24 | 20.83 ± 0.40 |
| 170 | 157.2 ± 0.6 | 90.48 ± 0.36 | 65.83 ± 0.26 | 24.65 ± 0.45 |
| 180 | 164.0 ± 0.7 | 99.66 ± 0.40 | 71.09 ± 0.28 | 28.56 ± 0.49 |
| 190 | 170.8 ± 0.7 | 108.7 ± 0.4 | 76.16 ± 0.30 | 32.54 ± 0.53 |
| 200 | 177.5 ± 0.7 | 117.6 ± 0.5 | 81.06 ± 0.32 | 36.58 ± 0.57 |
| 210 | 184.4 ± 0.7 | 126.5 ± 0.5 | 85.82 ± 0.34 | 40.65 ± 0.61 |
| 220 | 191.3 ± 0.8 | 135.2 ± 0.5 | 90.45 ± 0.36 | 44.75 ± 0.65 |
| 230 | 198.2 ± 0.8 | 143.9 ± 0.6 | 94.99 ± 0.38 | 48.87 ± 0.69 |
| 240 | 205.2 ± 0.8 | 152.4 ± 0.6 | 99.43 ± 0.40 | 53.00 ± 0.73 |
| 250 | 212.3 ± 0.8 | 161.0 ± 0.6 | 103.8 ± 0.4 | 57.15 ± 0.77 |
| 260 | 219.4 ± 0.9 | 169.4 ± 0.7 | 108.1 ± 0.4 | 61.31 ± 0.80 |
| 270 | 226.7 ± 0.9 | 177.8 ± 0.7 | 112.4 ± 0.4 | 65.47 ± 0.84 |
| 280 | 234.0 ± 0.9 | 186.2 ± 0.7 | 116.6 ± 0.5 | 69.63 ± 0.88 |
| 290 | 241.5 ± 1.0 | 194.6 ± 0.8 | 120.8 ± 0.5 | 73.79 ± 0.92 |
| 298.15 | 247.8 ± 1.0 | 201.3 ± 0.8 | 124.1 ± 0.5 | 77.19 ± 0.95 |
| 300 | 249.3 ± 1.0 | 202.9 ± 0.8 | 124.9 ± 0.5 | 77.96 ± 0.95 |
| 310 | 257.1 ± 1.0 | 211.2 ± 0.8 | 129.0 ± 0.5 | 82.12 ± 0.99 |
| 320 | 265.0 ± 1.1 | 219.5 ± 0.9 | 133.2 ± 0.5 | 86.28 ± 1.03 |
| 330 | 273.2 ± 1.1 | 227.7 ± 0.9 | 137.3 ± 0.5 | 90.45 ± 1.06 |
| 340 | 281.6 ± 1.1 | 236.0 ± 0.9 | 141.4 ± 0.6 | 94.60 ± 1.10 |
| 350 | 290.3 ± 1.2 | 244.3 ± 1.0 | 145.5 ± 0.6 | 98.76 ± 1.14 |
| 360 | 299.4 ± 1.2 | 252.6 ± 1.0 | 149.7 ± 0.6 | 102.9 ± 1.2 |
| 370 b | 308.6 ± 1.2 | 260.9 ± 1.0 | 153.9 ± 0.6 | 107.1 ± 1.2 |
| 380 b | 317.8 ± 1.3 | 269.3 ± 1.1 | 158.1 ± 0.6 | 111.2 ± 1.2 |
| 390 b | 327.0 ± 1.3 | 277.7 ± 1.1 | 162.3 ± 0.6 | 115.4 ± 1.3 |
| 400 b | 336.1 ± 1.3 | 286.1 ± 1.1 | 166.5 ± 0.7 | 119.6 ± 1.3 |
| 408.1 b | 343.6 ± 1.4 | 292.9 ± 1.2 | 169.9 ± 0.7 | 122.9 ± 1.4 |
| Liquid | ||||
| 408.1 b | 398 ± 12 | 369.3 ± 5.2 | 246.4 ± 4.8 | 122.9 ± 7.1 |
| 410 b | 399 ± 12 | 371.2 ± 5.3 | 247.1 ± 4.8 | 124.1 ± 7.1 |
| 420 b | 404 ± 12 | 380.9 ± 5.6 | 250.8 ± 4.9 | 130.1 ± 7.4 |
| 430 | 409 ± 12 | 390.4 ± 5.9 | 254.4 ± 5.0 | 136.0 ± 7.7 |
| 440 | 413 ± 12 | 399.9 ± 6.2 | 258.0 ± 5.1 | 141.9 ± 8.0 |
| 450 | 418 ± 13 | 409.2 ± 6.4 | 261.5 ± 5.2 | 147.7 ± 8.3 |
| 460 | 423 ± 13 | 418.5 ± 6.7 | 264.9 ± 5.3 | 153.5 ± 8.6 |
| 470 | 427 ± 13 | 427.6 ± 7.0 | 268.3 ± 5.4 | 159.3 ± 8.8 |
| Compound A | ()/J K−1 mol−1 | ||||
|---|---|---|---|---|---|
| Method | This work | Chickos | Hurst | Goodman (PL) | Goodman (PF) |
| 3EtOAn | 245.3 ± 1.0 | 281.3 | 252.8 | 231.6 | 222.7 |
| Phenac | 247.8 ± 1.0 | 281.3 | 252.8 | 231.6 | 222.7 |
| Compound A | ()/J K−1 mol−1 | ||||
| Method | This work | Chickos | Kolska | ||
| 3EtOAn | 342.6 ± 13.7 | 329.2 | 352.6 | ||
| Phenac | 347.0 ± 13.9 | 329.2 | 352.2 | ||
| Approach | 3EtOAn | Phenac | ||
|---|---|---|---|---|
| 0 | 0.110 | 0 | 0.034 | |
| Pappa, Ia a | 109.0 | 0.151 | 106.9 | 0.068 |
| Pappa, Ib a | 116.9 | 0.155 | 114.6 | 0.072 |
| Pappa, II a | 77.3 | 0.138 | 76.5 | 0.056 |
| Wu b | 49.5 | 0.127 | 49.5 | 0.047 |
| This work (Tm) c | 71.8 | 0.136 | 54.9 | 0.048 |
| This work d | 212.2–0.381 (T/K) | 0.140 | 238.2–0.449 (T/K) | 0.054 |
| Compound A | /kJ mol−1 | /kJ mol−1 | |
|---|---|---|---|
| Method | DSC | Kirchhoff’s law a | solution calorimetry |
| 3EtOAn | 28.5 ± 0.6 | 22.4 ± 1.2 | 23.3 ± 1.0 |
| Phenac | 31.2 ± 1.6 | 22.5 ± 2.2 | 24.9 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, A.A.; Yagofarov, M.I.; Balakhontsev, I.S.; Nizamov, I.I.; Mukhametzyanov, T.A.; Solomonov, B.N.; Yurkshtovich, Y.N.; Stepurko, E.N. Thermodynamic Properties of 3- and 4-Ethoxyacetanilides between 80 and 480 K. Molecules 2023, 28, 7027. https://doi.org/10.3390/molecules28207027
Sokolov AA, Yagofarov MI, Balakhontsev IS, Nizamov II, Mukhametzyanov TA, Solomonov BN, Yurkshtovich YN, Stepurko EN. Thermodynamic Properties of 3- and 4-Ethoxyacetanilides between 80 and 480 K. Molecules. 2023; 28(20):7027. https://doi.org/10.3390/molecules28207027
Chicago/Turabian StyleSokolov, Andrey A., Mikhail I. Yagofarov, Ilya S. Balakhontsev, Ilyas I. Nizamov, Timur A. Mukhametzyanov, Boris N. Solomonov, Yana N. Yurkshtovich, and Elena N. Stepurko. 2023. "Thermodynamic Properties of 3- and 4-Ethoxyacetanilides between 80 and 480 K" Molecules 28, no. 20: 7027. https://doi.org/10.3390/molecules28207027
APA StyleSokolov, A. A., Yagofarov, M. I., Balakhontsev, I. S., Nizamov, I. I., Mukhametzyanov, T. A., Solomonov, B. N., Yurkshtovich, Y. N., & Stepurko, E. N. (2023). Thermodynamic Properties of 3- and 4-Ethoxyacetanilides between 80 and 480 K. Molecules, 28(20), 7027. https://doi.org/10.3390/molecules28207027










