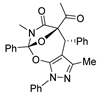
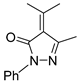
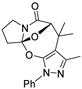
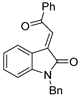
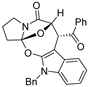
Abstract
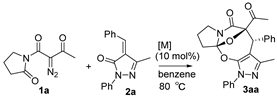
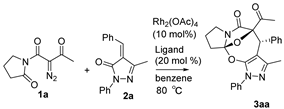
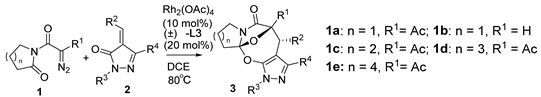
Under the catalysis of Rh2(OAc)4 (10 mol%) and binapbisphosphine ligand (±)-L3 (20 mol%) in DCE at 80 °C, the cascade cyclization of diazoimides with alkylidenepyrazolones underwent stereoselectively (dr > 20:1), affording pyrazole-fused oxa-bridged oxazocines in reasonable chemical yields. The chemical structure and relative configuration of title products were firmly identified by X-ray diffraction analysis.
1. Introduction
Oxazocines belong to a class of medicinally and biologically important chemical skeletons and have a wide range of biological activities such as anticancer, antibacterial, antidiabetic, and anti-Alzheimer properties [1,2] (Scheme 1(1)). Due to the variety of the significant bioactive potentials with oxazocine scaffolds, organic and medicinal chemists have created a variety of synthetic methodologies for their efficient and concise constructions [3,4,5,6,7,8]. Moreover, in this context, non-stereoselective and stereoselective cycloadditions have functioned as the crucial protocols for preparing a variety of structurally diverse and complex bridged and non-bridged oxazocines [9,10,11,12,13,14]. Importantly, elegant and powerful synthetic methodologies have appeared to prepare heterocycle-fused bridged oxazocines, but this area has witnessed slow development in recent years [15]. So, designing and exploring new synthetic protocols is urgently needed to obtain structurally new and unique oxa-bridged oxazocines fused with heterocyclic motifs.
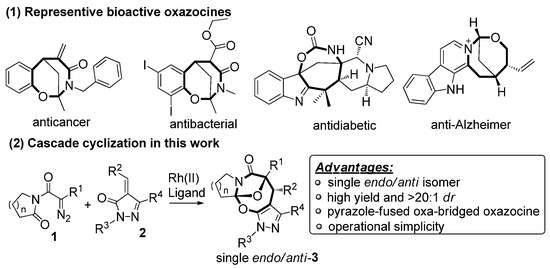
Scheme 1.
Representative bioactive oxazocines and the cascade cyclization in this work.
Diazoimides constitute a kind of robust and versatile building block and they are broadly applied to prepare bioactive natural products and drug scaffolds by means of their cycloadditions [16,17,18,19,20,21,22,23]. Normally, when treated with transition metals, diazoimides can deliver structurally diverse acyclic or cyclic carbonyl ylides, enabling many types of cycloadditions with a wide range of dipolarophiles [24,25,26,27,28,29,30]. Primarily, the cycloaddition of cyclic carbonyl ylides derived from diazoimides with structurally various dipolarophiles can be used to more efficiently and concisely deliver oxa-bridged heterocyclic scaffolds [31,32,33,34,35]. On the basis of the previous advances concerning the cyclization of carbonyl yildes [36,37,38,39,40], we envisioned the transition-metal catalyzed novel cascade cyclization of diazoimides 1 with alkylidenepyrazolones 2, which found a few examples of the cycloadditions centering on their enone moieties [41,42,43,44] (Scheme 1(2)). Pleasingly, in the presence of Rh2(OAc)4 (10 mol%) and binapbisphosphine ligand (±)-L3 (20 mol%) in DCE at 80 °C, the newly designed cascade cyclization proceeded readily and diastereoselectively furnished pyrazole-fused oxa-bridged products 3 in reasonable chemical yields with excellent diastereoselectivities (>20:1 dr). Presumably, the formation of the aromatic pyrazole ring in the products substantially facilitates the cascade cyclization explored in our work. To the best of our knowledge, such a work has not been reported in the literature to date.
2. Results and Discussion
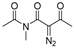
Initially, in benzene at 80 °C we scrutinized several transition-metal catalysts for their effects on the cascade cyclization of diazoimide 1a with alkylidenepyrazolone 2a, as described in Table 1. Generally, all the tested transition-metal catalysts provided product 3aa with excellent diastereoselectivity (>20:1 dr). By comparison, the chemical yield of 3aa highly depended on the screened transition-metal catalysts. In the presence of Ru(bpy)3Cl2.6H2O, the cascade cyclization delivered product 3aa in a trace amount (entry 1). As for catalysts Ru(OAc)3 and Rh2(Oct)4, in contrast with Ru(bpy)3Cl2.6H2O, they behaved well to provide product 3aa in 43% chemical yield (entries 1 vs. 2 and 3). In the case of Pd2(dba)3, Pd(PPh3)2Cl2, and Rh(OAc)3 as catalysts, they differently increased the chemical yield of the cascade cyclization (entries 1–3 vs. 4–6). Finally, we observed that catalyst Rh2(OAc)4 acted most efficiently to furnish product 3aa in the highest chemical yield (entries 1–6 vs. 7).

Table 1.
Screening of metal-catalysts [a].
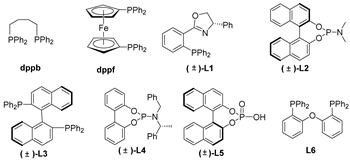
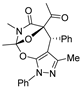
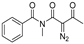
Next, together with Rh2(OAc)4 as the catalyst in benzene at 80 °C, we examined a few mono- and bisphosphine ligands to clarify their effects on the cascade cyclization of diazoimide 1a with alkylidenepyrazolone 2a, as depicted in Table 2. In the absence of a ligand, the cascade cyclization generated product 3aa in 82% chemical yield with >20:1 dr (entry 1). Regarding ligands dppf, (±)-L1, (±)-L4, and (±)-L6, they hugely inhibited the occurrence of the cascade cyclization and furnished product 3aa in a trace amount after 10 h (entries 3–4, 7, and 9). Regarding ligands dppb, (±)-L2, and (±)-L5, they performed well to deliver product 3aa in reasonable chemical yields with excellent diastereoselectivities (entries 2, 5, and 8). Lastly, we identified that ligand (±)-L3 behaved quite efficiently to afford product 3aa in a chemical yield of 88% (entry 6).

Table 2.
Screening of mono- and bisphosphine ligands [a].
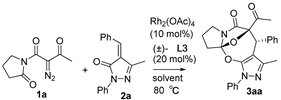
Moreover, with the use of Rh2(OAc)4 and (±)-L3 at 80 °C, we examined a variety of differently polar solvents for their effects on the cascade cyclization of diazoimide 1a with alkylidenepyrazolone 2a, as outlined in Table 3. Generally, in the screened solvents, the cascade cyclization formed product 3aa with excellent diastereoselectivity (>20:1 dr) (entries 1–8). However, the attempted solvents largely influenced the chemical yield of the cascade cyclization. Choosing DMF as the reaction solvent completely inhibited the occurrence of the cascade cyclization (entry 6). As for solvents HFIP and DCM, they afforded product 3aa in a trace amount after 10 h (entries 7 and 9). Using 1,4-dioxane and THF as the reaction solvents, cascade cyclization generated product 3aa in lower chemical yields (entries 2–3). Satisfyingly, we observed that in the aromatic solvents such as benzene, toluene, and PhCF3, the [4+3] cycloaddition produced product 3aa in reasonable chemical yields (entries 1, 4–5). In the end, we found the cascade cyclization in solvent 1,2-DCE performed very well in terms of reactivity, chemical yield, and diastereoselectivity (entry 8). So, at this point, we determined the optimal reaction conditions for the cascade cyclization, as shown below: 1a/2a = 1.5:1.0 (mmol/mmol), Rh2(OAc)4 (10 mol%), (±)-L3 (20 mol%), DCE, 80 °C.

Table 3.
Screening of solvents [a].
Finally, under optimal reaction conditions, we broadened the reaction scope of the cascade cyclization via diversifying substrates 1 and 2, as summarized in Table 4. Basically, most of the explored cascade cyclizations behaved quite well in stereocontrols, and provided products 3 with excellent diastereoselectivities (>20:1 dr). By comparison, the chemical yield of the screened [4+3] cycloadditions ranged widely with the used substrates 1 and 2. In the cascade cyclization with diazoimide 1a, the substrates 2 with Me as the R4 group allowed for the introduction of electron-poor or electron-rich aryls at R2 or R3 positions, and delivered products 3aa–3ag in 65–98% chemical yields (entries 1–7). Generally, in this context, the 2 substrates bearing an electron-rich aryl as the R2 or R3 group acted more efficiently than the 2 substrates possessing an electron-poor aryl as the R2 or R3 group, and gave rise to 3 products in higher chemical yields (e.g., entries 2–3 vs. 4; 6 vs. 7). Concerning the cascade cyclization with diazoimide 1a, alkylidenepyrazolone 2h (where R2 = R3 = R4 = Ph) resulted in the formation of 3ah in 88% chemical yield (entry 8). Unfortunately, the substrates 2i (R2 = Ph, R3 = Me, R4 = Ph), 2j (R2 = Ph, R3 = Me, R4 = Me), and 2k (R2 = Ph, R3 = Me, R4 = Ph) completely failed to conduct the [4+3] cycloaddition with diazoimide 1a (entries 9–11). So, in this series, positioning an aryl group at the R2 position is indispensable to maintain the reactivity of the cascade cyclizations (entries 1 vs. 9; 8 vs. 9).

Table 4.
Substrate scope [a].
As for the cascade cyclization with alkylidenepyrazolone 2a, substrate 1 tolerated the variation in the R1 group and the ring size of the lactam motif, and provided products 3ba–3ea in 70–82% chemical yields (entries 12–15). Basically, in this series, the 1 substrates possessing a bigger ring-sized lactam subunit commonly worked better than the 1 substrates incorporating a smaller ring-sized lactam subunit with respect to the chemical yield of the cascade cyclization (entries 13 vs. 14 vs. 15). As for substrate 1f containing a N-methylacetamide motif, it performed poorly in the cascade cyclization with substrate 2a and produced product 3fa in a lower chemical yield (entry 16). Even worse, substrate 1g bearing a N-methylbenzamide subunit failed to conduct the cascade cyclization with substrate 2a (entry 17). Therefore, in this context, the 1 substrates containing a lactam motif generally have higher reactivities than the 1 substrates bearing a N-methylamide moiety (entries 12–15 vs. 16–17).
Moreover, with an aim of enriching the structural diversity and complexity of 3 products, we accomplished the crossed cascade cyclizations between substrates 1b–1e with a broad spectrum of 2 substrates, as presented in entries 18–41 (Table 4). Apparently, in this regard, all the conducted cascade cyclizations showed excellent diastereoselectivities (>20:1 dr). In the case of the chemical yields of the cascade cyclizations, they highly depended on the selected 1 and 2 substrates and ranged widely from 50% to 95%. Mostly, the crossed cascade cyclizations behaved well, and thus delivered 3 products in moderate to high chemical yields (entries 18–39 and 41). Only the cascade cyclization between 1e and 2n behaved poorly, and provided product 3ej in a quite low chemical yield (entry 40). Moreover, the oxindole-based enone 2q reacted well with diazoimide 1b, thus affording cycloadduct 3bl in excellent chemical yield and diastereoselectivity (entry 41). Meanwhile, we firmly identified the chemical structure and relative stereo-configuration of the desired product 3aa using single crystal X-ray analysis (CCDC 2260380) [45]; see details in SI) and assigned those of other title compounds by inference. In order to show the synthetic potentials of title 3 compounds, we prepared 3aa at gram scale and completed several chemical transformations of title compounds 3ba, 3aa, and 3cg, as described in Scheme 2. In the scale-up cascade cyclization, we isolated product 3aa at a chemical yield of 94% (Scheme 2(1)). Upon treatment with TMSCl in CH2Cl2 at rt, compound 3ba easily converted into its exo/syn stereoisomer 4 (CCDC 2260381) [45]; see details in Supplementary Materials) at a chemical yield of 85% (Scheme 2(2)). Furthermore, the reduction reaction of 3aa with LiAlH4 in anhydrous THF at 0 °C gave 5 at a chemical yield of 92%; and the addition reaction of 3aa with vinylmagnesium bromide in anhydrous THF at 0 °C produced 6 at a chemical yield of 75% (Scheme 2(3)). Lastly, in the presence of pyridine in EtOH at 60 °C, the condensation reaction between 3cg and H2NOMe·HCl furnished 7 at a chemical yield of 88% (Scheme 2(4)).
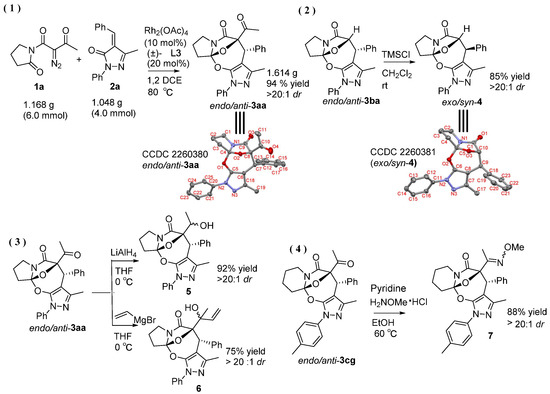
Scheme 2.
Gram-scale synthesis of 3aa and chemical transformations of 3ba, 3aa, and 3cg.
For the sake of clarifying the diastereoselective formation of product 3, we proposed the reaction mechanism for the cascade cyclization between diazoimide 1 and alkylidenepyrazolone 2, as illustrated in Scheme 3. Under the catalysis of Rh2(OAc)4 and (±)-L3, by liberating one molecule of N2, diazoimide 1 decomposes into Rh(II)-carbenoid Int-1. Then, Int-1 transforms into cyclic carbonyl ylide Int-2 through transition state TS-1. Concerning Int-2, which was formed in situ, it can conduct the cascade cyclization with alkylidenepyrazolone 2 by adopting transition states TS-2 or TS-3. In TS-3, there exists strong steric repulsion between R1 and R2 motifs; in contrast, TS-2 fully avoids this kind of unfavorable interaction. So, TS-2 is thermodynamically more stable than TS-3 and mainly accounts for the formation of single endo/anti-3.
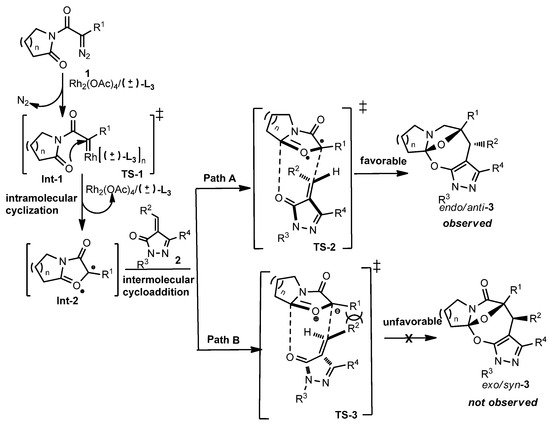
Scheme 3.
Proposed mechanism for formation of product 3.
3. Materials and Methods
Proton (1H), carbon (13C), and fluorine (19F) NMR spectra were recorded on a Bruker Avance HD III spectrometer (400 MHz for 1H NMR, 100 MHz for 13C NMR, and 376 MHz for 19F NMR) and calibrated using tetramethylsilane (TMS) as internal reference. High resolution mass spectra (HRMS) were obtained on an Agilent Technologies LC/MSD TOF spectrometer under electrospray ionization (ESI) conditions. The melting point of compounds was determined using a melting point instrument. Flash column chromatography was performed on silica gel (0.035–0.070 mm) using compressed air. X-ray single crystals were obtained on a Rigaku 002 Saturn 944 spectrometer. Thin layer chromatography (TLC) was carried out on 0.25 mm SDS silica gel coated glass plates (60F254). Eluted plates were visualized using a 254 nm UV lamp. Unless otherwise indicated, all reagents were commercially available and used without further purification. All solvents were distilled from the appropriate drying agents immediately before use. Diazoimides 1a–1g were synthesized according to the reported procedures [10,46,47]. Alkylidenepyrazolones 2a–2q were prepared according to literature procedures [48,49,50].
3.1. General Procedure for Cascade Cycloaddition Reaction
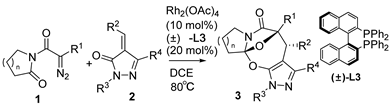
A mixture of diazoimides 1 (1.5 equiv, 0.15 mmol), alkylidenepyrazolones 2 (1.0 equiv, 0.1 mmol), Rh2(OAc)4 (10.0 mol%), and (±)-L3 (20.0 mol%) in DCE (1.5 mL) was stirred at 80 °C. After the reaction was completed as indicated by the TLC plate, the solvent was concentrated under reduced pressure and the resulting crude products were purified by flash column chromatography on silica gel (petroleum ether/ethyl acetate = 6:1~2:1) to afford 3 products (35–98% yields).
3.2. Gram-Scale Synthesis of Compound 3aa
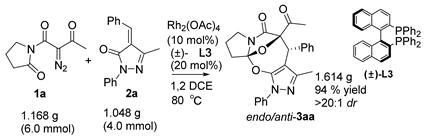
A mixture of diazoimide 1a (1.5 equiv, 6.0 mmol, 1.168 g), alkylidenepyrazolone 2a (1.0 equiv, 4.0 mmol, 1.048 g), Rh2(OAc)4 (10.0 mol%, 0.176 g), and (±)-L3 (20.0 mol%, 0.497 g) in DCE (15 mL) was stirred at 80 °C. After the reaction was completed as indicated by the TLC plate, the solvent was concentrated under reduced pressure and the resulting crude product was purified by flash column chromatography on silica gel (petroleum ether/ethyl acetate = 4:1) to afford product 3aa as a white solid (1.614 g, 94% yield).
3.3. Chemical Transformations of 3ba, 3aa, and 3cg
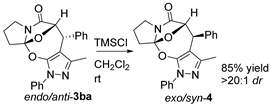
To a well-stirred solution of 3ba (1.0 equiv, 0.1 mmol, 38.7 mg) in CH2Cl2 (1.0 mL), TMSCl (1.0 equiv, 0.1 mmol, 10.8 mg) was added. Then, the reaction mixture was stirred at room temperature for 12 h. After the reaction was finished as indicated by the TLC plate, the reaction mixture was concentrated under reduced pressure. The resulting reaction residue was purified using flash column chromatography on silica gel (petroleum ether/ethyl acetate = 2:1) to afford product 4 as a white solid (32.8 mg, 85% yield). M.P. = 75.3–75.8 °C; 1H NMR (400 MHz, CDCl3): δ 7.81 (d, J = 7.8 Hz, 2H), 7.44 (t, J = 7.6 Hz, 2H), 7.35–7.24 (m, 4H), 7.13 (d, J = 6.3 Hz, 2H), 5.69 (d, J = 1.8 Hz, 1H), 4.43 (d, J = 1.7 Hz, 1H), 3.96–3.75 (m, 2H), 2.87–2.62 (m, 2H), 2.30–2.16 (m, 2H), 2.10 (s, 3H) ppm;13C NMR (100 MHz, CDCl3): δ171.5, 148.3, 146.6, 140.2, 138.7, 128.9, 128.8, 128.0, 127.4, 126.4, 122.7, 122.5, 121.6, 100.4, 89.1, 45.7, 41.4, 34.4, 25.0, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H21N3O3 388.1656; Found 388.1655.
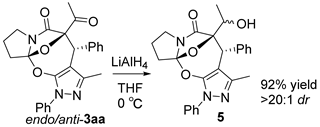
To a well-stirred solution of 3aa (1.0 equiv, 0.1 mmol, 42.9 mg) in dry THF (1.0 mL), LiAlH4 (2.0 equiv, 0.2 mmol, 7.6 mg) was added. Then, the reaction mixture was stirred at 0 °C for 1 h. After the reaction was finished as indicated by the TLC plate, the reaction mixture was concentrated under reduced pressure. The resulting reaction residue was purified using flash column chromatography on silica gel (petroleum ether/ethyl acetate = 1:1) to afford product 5 as a white solid (39.7 mg, 92% yield). M.P. = 215.7–216.1 °C; 1H NMR (400 MHz, CDCl3): δ7.64 (d, J = 7.8 Hz, 2H), 7.47 (d, J = 7.1 Hz, 1H), 7.44 (t, J = 7.7 Hz, 2H), 7.36–7.25 (m, 4H), 7.19 (d, J = 4.8 Hz, 1H), 5.15 (s, 1H), 4.03–3.91 (m, 1H), 3.77–3.67 (m, 1H), 3.41–3.29 (m, 1H), 2.60–2.47 (m, 1H), 2.41–2.25 (m, 1H), 2.25–2.12 (m, 3H) 1.51 (s, 3H), 1.13 (d, J = 6.3 Hz, 3H) ppm;13C NMR (100 MHz, CDCl3): δ 171.5, 149.1, 145.8, 138.8, 136.8, 130.6, 130.0, 128.8, 128.1, 128.0, 127.7, 126.2, 122.6, 119.1, 100.7, 94.3, 66.3, 47.3, 42.4, 35.0, 24.9, 17.9, 14.7, 14.2 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H25N3O4 432.1917; Found 432.1872.
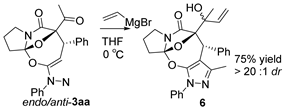
To a well-stirred solution of 3aa (1.0 equiv, 0.1 mmol, 42.9 mg) in anhydrous THF (1.0 mL), 1.0 M vinylmagnesium bromide (2.0 equiv, 0.2 mmol, 0.2 mL) was added under N2. Then, the reaction mixture was stirred at 0 °C for 1 h. After the reaction was finished as indicated by the TLC plate, saturated NH4Cl solution was added to quench the reaction at 0 °C. Then, the reaction mixture was extracted with ethyl acetate, and the combined organic layers were dried over anhydrous Na2SO4. After filtration, the organic layers were concentrated under reduced pressure to give crude product. Finally, the crude product was purified using flash column chromatography on silica gel (petroleum ether/ethyl acetate = 2:1) to afford product 6 as a white solid (34.3 mg, 75% yield). M.P. = 92.7–93.1 °C; 1H NMR (400 MHz, CDCl3): δ 7.64 (dd, J = 8.7, 1.2 Hz, 2H), 7.44 (t, J = 7.6 Hz, 2H), 7.28 (td, J = 7.4,1.1 Hz, 3H), 7.26–7.12 (br, 3H), 6.06 (q, J = 10.8 Hz, 1H), 5.28 (dd, J = 17.3, 1.1 Hz, 1H), 5.14 (dd, J = 10.8, 1.2 Hz, 1H), 5.06 (s, 1H), 3.82–3.68 (m, 1H), 3.42 (s, 1H), 3.39–3.30 (m, 1H), 2.59–2.45 (m, 1H), 2.37–2.13 (m, 3H), 1.58 (s, 3H) 1.53 (s, 3H) ppm;13C NMR (100 MHz, CDCl3): δ173.4, 149.0, 145.1, 141.1, 138.8, 138.4, 128.8, 127.2, 126.2, 122.6, 119.4, 115.2, 101.7, 92.3, 48.2, 42.4, 35.1, 25.0, 22.5, 14.3 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C27H27N3O4 458.2074; Found 458.2082.
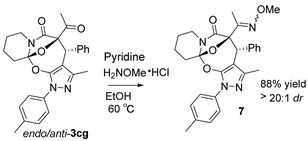
To a well-stirred solution of 3cg (1.0 equiv, 0.1 mmol, 45.7 mg) in dry EtOH (1.0 mL), H2NOMe·HCl (2.0 equiv, 0.2 mmol, 16.6 mg) and pyridine (2.0 equiv, 0.2 mmol, 15.8 mg) were added. Then, the reaction mixture was stirred at 60 °C for 2 h. After the reaction was finished as indicated by the TLC plate, the reaction mixture was concentrated under reduced pressure. The resulting reaction residue was purified using flash column chromatography on silica gel (petroleum ether/ethyl acetate = 6:1) to afford product 7 as a white solid (42.7 mg, 88% yield). M.P. = 195.4–196.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.55 (d, J = 8.2 Hz, 2H), 7.41 (d, J = 7.2 Hz, 2H), 7.29–7.17 (m, 5H), 4.70 (s, 1H), 3.91 (s, 3H), 3.86 (dd, J = 13.4, 4.4 Hz, 1H), 2.71 (d, J = 12.5 Hz, 1H), 2.52 (td, J = 12.8, 2.6 Hz, 1H), 2.39 (s, 3H), 2.04 (s, 3H), 1.96 (d, J = 12.7 Hz, 1H), 1.86 (td, J = 13.0, 4.0 Hz, 1H), 1.81–1.70 (m, 3H), 1.67 (s, 3H), 1.52–1.41 (m, 3H) ppm;13C NMR (100 MHz, CDCl3): δ168.6, 151.3, 147.9, 145.0, 138.4, 136.2, 136.0, 130.4, 129.4, 127.8, 127.0, 122.3, 110.5, 102.4, 87.3, 62.1, 47.5, 38.7, 34.2, 23.3, 21.0, 20.9, 12.9, 11.1 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C28H30N4O4 487.2340; Found 487.2350.
3.4. Charaterization of 3 Products
- 5-acetyl-3-methyl-1,4-diphenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3aa): White solid (yield: 42.0 mg, 98%). M.P. = 152.9–153.1 °C; 1H NMR (400 MHz, CDCl3): δ 7.64 (d, J = 7.7 Hz, 2H), 7.54–7.48 (br, 1H), 7.45 (t, J = 7.6 Hz, 2H), 7.30 (t, J = 7.4 Hz, 3H), 7.28–7.25 (br, 1H)), 7.20–7.14 (br, 1H), 5.12 (s, 1H), 3.92–3.81 (m, 1H), 3.50–3.38 (m, 1H), 2.68–2.56 (m, 1H), 2.47–2.24 (m, 3H), 2.16 (s, 3H), 1.56 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.3, 169.4, 148.9, 145.3, 138.6, 135.8, 128.8, 128.0, 127.7, 126.3, 122.6, 119.8, 100.2, 93.4, 47.5, 42.8, 35.4, 26.7, 25.0, 14.3 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H23N3O4 430.1761; Found 430.1759.
- 5-acetyl-4-(4-bromophenyl)-3-methyl-1-phenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3ab): White solid (yield: 37.0 mg, 73%). M.P. = 93.7–93.9 °C; 1H NMR (400 MHz, CDCl3): δ 7.63 (d, J = 8.7 Hz, 2H), 7.47 (s, 1H), 7.46 (s, 2H), 7.44 (s, 1H), 7.38 (d, J = 8.2 Hz, 1H), 7.32 (t, J = 7.4 Hz, 1H), 7.02 (d, J = 8.3 Hz, 1H), 5.12 (s, 1H), 3.93–3.84 (m, 1H), 3.47–3.38 (m, 1H), 2.67–2.56 (m, 1H), 2.46–2.25 (m, 3H), 2.59 (s, 3H), 1.60 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.1, 169.3, 148.6, 145.3, 138.5, 135.2, 131.2, 131.1, 128.8, 126.4, 122.6, 121.8, 120.0, 99.7, 93.2, 46.4, 43.0, 35.5, 26.5, 25.1, 14.4 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H22BrN3O4 508.0866; Found 508.0867.
- 5-acetyl-3-methyl-4-(4-nitrophenyl)-1-phenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3ac): White solid (yield: 30.8 mg, 65%). M.P. = 109.9–110.3 °C; 1H NMR (400 MHz, CDCl3): δ 8.20 (d, J = 7.6 Hz, 1H), 8.09 (d, J = 8.1 Hz, 1H), 7.83 (d, J = 7.9 Hz, 1H), 7.63 (dd, J = 8.7, 1.2 Hz, 2H), 7.47 (t, J = 7.5 Hz, 2H), 7.36–7.27 (m, 2H), 5.30 (s, 1H), 3.92–3.86 (m, 1H), 3.49–3.42 (m, 1H), 2.67–2.62 (m, 1H), 2.48–2.34 (m, 3H), 2.21 (s, 3H), 1.58 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 197.9, 169.1, 148.2, 147.2, 145.4, 144.2, 138.4, 132.7, 130.6, 129.0, 126.7, 123.1, 122.7, 120.5, 99.2, 93.2, 46.3, 43.1, 35.5, 26.2, 25.2, 14.3ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H22N4O6 475.1612; Found 475.1631.
- 5-acetyl-4-(4-methoxyphenyl)-3-methyl-1-phenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3ad): White solid (yield:37.6 mg, 82%). M.P. = 87.7–88.1 °C; 1H NMR (400 MHz, CDCl3): δ7.63 (dd, J = 8.7, 1.2 Hz, 2H), 7.45 (t, J = 7.5 Hz, 2H), 7.40 (d, J = 7.6 Hz, 1H), 7.30 (t, J = 7.7 Hz, 1H), 7.07 (d, J = 8.0 Hz, 1H), 6.82 (t, J = 4.4 Hz 2H), 5.06 (s, 1H), 3.90–3.83 (m, 1H), 3.80 (s, 3H), 3.45–3.39 (m, 1H), 2.65–2.58 (m, 1H), 2.42–2.31 (m, 3H), 2.16 (s, 3H), 1.58 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.6, 169.5, 159.0, 149.0, 145.2, 138.7, 132.5, 130.7, 128.9, 127.6, 126.3, 122.6, 119.6, 113.7, 113.1, 100.4, 93.4, 55.1, 46.9, 42.8, 35.5, 26.8, 25.0, 14.4 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C26H25N3O5 460.1867; Found 460.1864.
- 5-acetyl-3-methyl-4-(naphthalen-2-yl)-1-phenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3ae): White solid (yield: 35.9 mg, 75%). M.P. = 168.5–168.8 °C; 1H NMR (400 MHz, CDCl3): δ 7.89–7.68 (m, 3H), 7.51–7.47 (m, 3H), 7.48 (t, J = 7.4 Hz, 4H), 7.33 (t, J = 7.4 Hz, 2H), 5.36 (s, 1H), 3.90–3.85 (m, 1H), 3.49–3.42 (m, 1H), 2.66–2.59 (m, 1H), 2.48–2.24 (m, 3H), 2.16 (s, 3H), 1.53 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.6, 198.3, 169.5, 148.9, 145.4, 138.7, 132.8, 128.9, 128.1, 127.7, 127.6, 126.4, 126.1, 126.0, 122.6, 119.8, 99.8, 93.2, 47.6, 42.9, 35.5, 26.8, 25.0, 14.4 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C29H25N3O4 480.1917; Found 480.1922.
- 5-acetyl-1-(4-chlorophenyl)-3-methyl-4-phenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3af): White solid (yield: 33.3 mg, 72%). M.P. = 143.2–143.5 °C; 1H NMR (400 MHz, CDCl3): δ 7.60 (dd, J = 8.8, 2.0 Hz, 2H), 7.51–7.47 (br, 1H), 7.41 (dt, J = 8.9, 2.7 Hz, 2H), 7.34–7.22 (br, 3H), 7.18–7.08 (br, 1H), 5.12 (s, 1H), 3.91–3.81 (m, 1H), 3.46–3.37 (m, 1H), 2.64–2.56 (m, 1H), 2.42–2.56 (m, 3H), 2.16 (s, 3H), 1.55 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.2, 169.3, 149.3, 145.4, 137.2, 135.6, 131.7, 131.5, 128.9, 128.0, 127.8, 123.6, 119.8, 100.4, 93.4, 47.4, 42.9, 35.5, 26.7, 25.0, 14.3 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H22ClN3O4 464.1371; Found 464.1369.
- 5-acetyl-3-methyl-4-phenyl-1-(p-tolyl)-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3ag): White solid (yield: 35.4 mg, 80%). M.P. = 149.2–149.6 °C; 1H NMR (400 MHz, CDCl3): δ 7.50 (d, J = 8.4 Hz, 3H), 7.36–7.26 (br, 2H), 7.26 (t, J = 7.8 Hz, 3H), 7.21–7.12 (br, 1H), 5.12 (s, 1H), 3.90–3.82 (m, 1H), 3.45–3.37 (m, 1H), 2.65–2.55 (m, 1H), 2.41 (s, 3H), 2.40–2.25 (m, 3H), 2.15 (s, 3H), 1.55 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.5, 169.4, 148.6, 145.3, 136.2, 135.9, 131.5, 129.7, 129.4, 128.6, 127.7, 122.7, 120.0, 100.0, 93.5, 47.6, 42.8, 35.5, 26.8, 25.0, 21.1, 14.3 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C26H25N3O4 444.1917; Found 444.1920.
- 5-acetyl-1,3,4-triphenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3ah): White solid (yield: 42.2 mg, 86%). M.P. = 76.2–76.6 °C; 1H NMR (400 MHz, CDCl3): δ 7.75 (d, J = 5.7 Hz, 2H), 7.49 (t, J = 8.3 Hz, 2H), 7.34 (t, J = 7.4 Hz, 1H), 7.14–7.01 (m, 7H), 6.99 (d, J = 1.8 Hz, 3H), 5.39 (s, 1H), 3.90–3.82 (m, 1H), 3.48–3.39 (m, 1H), 2.67–2.55 (m, 1H), 2.46–2.25 (m, 3H), 2.14 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ198.5, 169.4, 152.0, 145.7, 138.7, 135.6, 133.7, 128.9, 128.5, 127.5, 127.4, 127.2, 126.7, 123.0, 119.9, 100.7, 93.9, 47.4, 42.9, 35.5, 26.8, 25.0 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C30H25N3O4 492.1918; Found 492.1932.
- 3-methyl-1,4-diphenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3ba): White solid (yield: 29.8 mg, 77%). M.P. = 146.8–147.0 °C; 1H NMR (400 MHz, CDCl3): δ7.64 (d, J = 7.9 Hz, 2H), 7.46 (t, J = 7.6 Hz, 2H), 7.40–7.32 (m, 4H), 7.30–7.26 (m, 2H), 4.78 (dd, J = 13.6, 6.6 Hz, 2H), 3.78–3.71 (m, 1H), 3.35–3.29 (m, 1H), 2.55–2.51 (m, 1H), 3.34–2.21 (m, 3H), 1.61 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 171.0, 148.6, 146.5, 138.7, 135.0, 129.5, 129.5, 128.9, 128.8, 128.8, 128.5, 128.3, 128.0, 126.4, 122.7, 122.5, 120.8, 99.8, 86.1, 47.8, 42.2, 35.1, 24.8, 14.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H21N3O3 388.1656; Found 388.1622.
- 5-acetyl-3-methyl-1,4-diphenyl-4,5,8,9,10,11-hexahydro-5,11a-epoxypyrazolo[4,3-g]pyrido[2,1-b][1,3]oxazocin-6(1H)-one (3ca): White solid (yield: 31.0 mg, 70%). M.P. = 213.7–213.9 °C; 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 7.6 Hz, 2H), 7.16 (t, J = 7.6 Hz, 2H), 7.35–7.21 (m, 6H), 4.80 (s, 1H), 3.89 (dd, J = 13.3, 4.6 Hz, 1H), 2.76 (d, J = 12.8 Hz, 1H), 2.55 (td, J = 12.9, 3.0 Hz, 1H), 2.00–1.95 (br, 1H), 1.99 (s, 3H), 1.93 (s, 3H), 1.90–1.83 (m, 1H), 1.82–1.70 (m, 2H), 1.53–1.39 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.5, 165.5, 148.3, 145.1, 138.3, 136.7, 129.7, 128.9, 128.4, 127.7, 126.6, 122.4, 110.4, 101.2, 91.9, 48.0, 38.9, 34.4, 27.4, 23.3, 20.8, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C26H25N3O4 444.1918; Found 444.1872.
- 5-acetyl-3-methyl-1,4-diphenyl-4,5,9,10,11,12-hexahydro-8H-5,12a-epoxyazepino[2,1-b]pyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3da): White solid (yield: 34.2 mg, 75%). M.P. = 195.0–195.3 °C; 1H NMR (400 MHz, CDCl3): δ 7.64 (d, J = 7.7 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.34–7.21 (m, 6H), 4.79 (s, 1H), 3.85 (d, J = 13.9 Hz, 1H), 2.90–2.79 (m, 2H), 2.40–2.31 (m, 1H), 1.98 (s, 3H), 1.96 (s, 3H) 1.94–1.85 (m, 2H), 1.78–1.69 (br, 1H), 1.53–1.33 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.6, 166.4, 148.1, 145.3, 138.4, 136.9, 129.6, 128.9, 128.4, 127.7, 126.5, 122.5, 115.4, 100.8, 91.6, 48.0, 40.5, 38.4, 29.5, 28.5, 27.6, 22.5, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C27H27N3O4 458.2074; Found 458.2039.
- 5-acetyl-3-methyl-1,4-diphenyl-4,5,8,9,10,11,12,13-octahydro-5,13a-epoxyazocino[2,1-b]pyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3ea): White solid (yield: 38.6 mg, 82%). M.P. = 149.5–149.8 °C; 1H NMR (400 MHz, CDCl3): δ 7.64 (d, J = 7.7 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.34–7.21 (m, 6H), 4.80 (s, 1H), 3.70 (dt, J = 14.3, 4.0 Hz, 1H), 2.80 (td, J = 11.9, 3.6 Hz, 1H), 2.66 (dt, J = 16.1, 4.4 Hz, 1H), 2.50 (td, J = 10.8, 4.4 Hz, 1H), 1.98 (s, 3H), 1.96 (s, 3H), 1.86–1.75 (m, 3H), 1.70–1.55 (m, 3H), 1.53–1.35 (m, 2H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.6, 167.0, 148.2, 145.2, 138.5, 136.8, 129.6, 128.9, 128.4, 127.7, 126.4, 122.3, 114.1, 100.9, 91.4, 48.4, 40.4, 31.7, 27.6, 27.0, 25.0, 23.8, 22.1, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C28H29N3O4 472.2230; Found 472.2236.
- 5-acetyl-3,7,8-trimethyl-1,4-diphenyl-4,5,7,8-tetrahydro-5,8-epoxypyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3fa): White solid (yield: 14.6 mg, 35%). M.P. = 192.5–192.9 °C; 1H NMR (400 MHz, CDCl3): δ 7.64 (dd, J = 8.7, 1.2 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.38–7.25 (m, 6H), 4.80 (s, 1H), 2.76 (s, 3H), 2.09 (s, 3H), 1.98 (s, 3H), 1.93 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ 200.2, 167.3, 148.2, 145.1, 138.3, 136.7, 129.7, 129.0, 128.4, 127.7, 126.6, 122.4, 112.5, 101.0, 91.8, 48.1, 27.3, 25.9, 23.0, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H23N3O4 418.1761; Found 418.1767.
- 4-(4-bromophenyl)-3-methyl-1-phenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3bb): White solid (yield: 41.8 mg, 90%). M.P. = 106.6–106.9 °C; 1H NMR (400 MHz, CDCl3): δ 7.63 (d, J = 7.8 Hz, 2H), 7.53 (dd, J = 8.1, 1.8 Hz, 1H), 7.50–7.41 (m, 3H), 7.31 (d, J = 7.4 Hz, 1H), 7.25 (dd, J = 8.0, 2.0 Hz, 1H), 7.15 (dd, J = 8.4, 2.1 Hz, 1H), 4.74 (dd, J = 14.9, 6.6 Hz, 2H), 3.78–3.69 (m, 1H), 3.35–3.27 (m, 1H), 2.57–2.45 (m, 1H), 2.36–2.21 (m, 3H), 1.63 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 170.8, 148.3, 146.5, 138.6, 134.2, 131.7, 131.6, 131.3, 131.0, 128.9, 126.5, 122.7, 122.0, 120.9, 99.3, 85.7, 47.2, 42.2, 35.0, 26.9, 24.8, 15.0 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H20BrN3O3 466.0760; Found 466.0769.
- 3-methyl-4-(4-nitrophenyl)-1-phenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3bc): White solid (yield: 34.5 mg, 80%). M.P. = 122.3–122.7 °C; 1H NMR (400 MHz, CDCl3): δ 8.28 (dd, J = 8.3, 2.2 Hz, 1H), 8.20 (dd, J = 8.3, 2.2 Hz, 1H), 7.63 (d, J = 8.0 Hz, 2H), 7.56 (dd, J = 8.4, 1.4 Hz, 1H), 7.46 (t, J = 7.4 Hz, 3H), 7.33 (t, J = 7.4 Hz, 1H), 4.93 (d, J = 6.6 Hz, 1H), 4.77 (d, J = 6.6 Hz, 1H), 3.80–3.69 (m, 1H), 3.38–3.27 (m, 1H), 2.57–2.45 (m, 1H), 2.36–2.21 (m, 3H), 1.62 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 171.2, 159.2, 148.7, 146.4, 138.7, 130.6, 130.5, 129.0 128.9 128.8, 127.0, 126.3, 122.7, 122.4, 120.7, 114.1, 113.8, 113.8, 100.1, 86.2, 55.2, 47.0, 42.1, 35.0, 24.8, 14.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C23H20N4O5 433.1506; Found 433.1510.
- 3-methyl-1-phenyl-4-(3,4,5-trimethoxyphenyl)-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3bl): White solid (yield: 36.7 mg, 77%). M.P. = 89.7–90.0 °C; 1H NMR (400 MHz, CDCl3): δ 7.63 (d, J = 7.7 Hz, 2H), 7.44 (t, J = 7.6 Hz, 2H), 7.30 (d, J = 7.4 Hz, 1H), 6.54 (dd, J = 28.8, 1.6, Hz, 2H), 4.73 (d, J = 6.0 Hz, 1H), 4.65 (d, J = 6.0 Hz, 1H), 3.91 (s, 3H), 3.89 (s, 3H), 3.78 (s, 3H), 3.76–3.69 (m, 1H), 3.39–3.30 (m, 1H), 2.54–2.48 (m, 1H), 2.35–2.20 (m, 3H), 1.67 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 171.2, 153.3, 153.0 148.6, 146.5, 138.7, 137.7, 130.6, 128.8, 126.4, 122.6, 122.4, 120.6, 107.1, 106.8, 100.0, 86.0 61.0, 56.4, 56.2, 48.3, 42.3, 35.0, 24.9, 14.8 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C26H27N3O6478.1972; Found 478.1980.
- 3-methyl-1-phenyl-4-(4-(trifluoromethyl)phenyl)-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3bm): White solid (yield: 40.0 mg, 88%). M.P. = 168.7–168.9 °C; 1H NMR (400 MHz, CDCl3): δ 7.70 -7.56 (m, 4H),7.52–7.42 (m, 3H), 7.40 (d, J = 8.2 Hz, 1H), 7.32 (t, J = 7.4 Hz, 1H), 4.87 (d, J = 6.6 Hz, 1H), 4.76 (d, J = 6.6 Hz, 1H), 3.80–3.69 (m, 1H), 3.39–3.28 (m, 1H), 2.57–2.48 (m, 1H), 2.35–2.20 (m, 3H), 1.60 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 170.6, 148.1, 146.6, 139.3, 138.6, 130.3, 130.0, 130.0, 129.7, 128.9, 126.6, 125.6, 125.3, 125.3, 122.8, 121.0, 99.0, 85.7, 47.6, 42.2, 35.0, 26.9, 24.8, 15.0 ppm; 19F NMR (376 MHz, CDCl3): δ 62.4 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H20F3N3O3 456.1529; Found 456.1534.
- 4-(2-methoxyphenyl)-3-methyl-1-phenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3bn): White solid (yield: 38.7 mg, 93%). M.P. = 158.2–158.5 °C; 1H NMR (400 MHz, CDCl3): δ7.66 (d, J = 7.6 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.34–7.26 (m, 2H), 7.18 (dd, J = 7.6, 1.5 Hz, 1H), 6.95 (t, J = 7.6 Hz, 2H), 5.35 (d, J = 7.0 Hz, 1H), 4.91 (d, J = 7.0 Hz, 1H), 3.93 (s, 3H), 3.78–3.65 (m, 1H), 3.35–3.27 (m, 1H), 2.57–2.46 (m, 1H), 2.35–2.17 (m, 3H), 1.69 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 171.5, 156.8, 148.6, 146.9, 138.9, 130.5, 129.5, 128.8, 128.5, 126.2, 123.1, 122.6, 122.4, 122.3, 121.6, 120.9, 120.6, 120.4, 110.1, 99.7, 87.0, 84.1, 55.4, 42.1, 39.4, 35.2, 24.8, 14.7 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H23N3O4 418.1761; Found 418.1763.
- 3,4,4-trimethyl-1-phenyl-4,5,9,10-tetrahydro-8H-5,10a-epoxypyrazolo[4,3-g]pyrrolo[2,1-b][1,3]oxazocin-6(1H)-one (3bo): White solid (yield: 32.2 mg, 95%). M.P. = 94.4–94.9 °C; 1H NMR (400 MHz, CDCl3): δ7.54 (dd, J = 8.7, 1.2 Hz, 2H), 7.40 (t, J = 8.2 Hz, 2H), 7.27 (d, J = 7.2 Hz, 1H), 4.25 (s, 1H), 3.77–3.56 (m, 1H), 3.30–3.06 (m, 1H), 2.50–2.40 (m, 1H), 2.37 (s, 3H), 2.29–2.17 (m, 3H), 1.57 (s, 3H), 1.41 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 171.7, 146.9, 144.7, 138.6, 128.7, 126.4, 123.0, 120.0, 105.9, 92.2, 41.5, 39.5, 34.4, 28.6, 24.9, 22.1, 16.5 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H21N3O3 340.1656; Found 340.1660.
- 5-acetyl-3-methyl-4-(4-nitrophenyl)-1-phenyl-4,5,8,9,10,11-hexahydro-5,11a-epoxypyrazolo[4,3-g]pyrido[2,1-b][1,3]oxazocin-6(1H)-one (3cc): White solid (yield: 39.0 mg, 80%). M.P. = 231.6–231.9 °C); 1H NMR (400 MHz, CDCl3): δ 8.15 (d, J = 8.7 Hz, 2H), 7.66 (d, J = 8.0 Hz, 2H), 7.53 (d, J = 8.7 Hz, 2H), 7.47 (t, J = 7.7 Hz, 2H), 7.34 (t, J = 7.4 Hz, 1H), 4.95 (s, 1H), 3.91 (dd, J = 13.2, 4.5 Hz, 1H), 2.79 (d, J = 13.2 Hz, 1H), 2.56 (td, J = 13.0, 3.0 Hz, 1H), 2.03 (d, J = 13.5 Hz, 1H), 1.98 (s, 6H), 1.91 (dd, J = 13.3, 4.1 Hz, 1H), 1.86–1.68 (m, 2H), 1.56–1.42 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.0, 164.9, 147.9, 147.4, 145.2, 144.5, 138.0, 130.8, 129.0, 126.9, 123.5, 122.5, 110.8, 100.2, 91.5, 46.8, 39.2, 34.4, 27.2, 23.2, 20.8, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C26H24N4O6 489.1768; Found 489.1773.
- 5-acetyl-4-(4-methoxyphenyl)-3-methyl-1-phenyl-4,5,8,9,10,11-hexahydro-5,11a-epoxypyrazolo[4,3-g]pyrido[2,1-b][1,3]oxazocin-6(1H)-one (3cd): White solid (yield: 42.5 mg, 90%). M.P. = 155.4–155.8 °C; 1H NMR (400 MHz, CDCl3): δ 7.66 (d, J = 7.6 Hz, 2H), 7.50 (t, J = 5.8 Hz, 2H), 7.30 (t, J = 7.4 Hz, 1H), 7.23 (d, J = 8.7 Hz, 2H), 6.80 (d, J = 8.7 Hz, 2H), 4.76 (s, 1H), 3.87 (dd, J = 13.3, 4.6 Hz, 1H), 3.77 (s, 3H), 2.75 (d, J = 13.2 Hz, 1H), 2.55 (td, J = 13.0, 3.0 Hz, 1H), 2.03–1.99 (m, 1H), 1.98 (s, 3H), 1.94 (s, 3H), 1.86 (dd, J = 13.3, 4.1 Hz, 1H), 1.82–1.67 (m, 2H), 1.52–1.41 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.7, 165.5, 158.9, 148.3, 145.0, 138.3, 130.7, 128.9, 128.8, 126.5, 122.4, 113.7, 110.3, 101.4, 92.0, 55.2, 47.3, 38.9, 34.4, 27.5, 23.2, 20.8, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C27H27N3O5 474.2023; Found 474.2032.
- 5-acetyl-3-methyl-4-(naphthalen-2-yl)-1-phenyl-4,5,8,9,10,11-hexahydro-5,11a-epoxypyrazolo[4,3-g]pyrido[2,1-b][1,3]oxazocin-6(1H)-one (3ce): White solid. (yield: 46.8 mg, 95%). M.P. = 202.2–202.5 °C; 1H NMR (400 MHz, CDCl3): δ 7.87 -7.78 (m, 2H), 7.77 (t, J = 4.5 Hz, 2H), 7.71 (d, J = 7.6 Hz, 2H), 7.52–7.45 (m, 5H), 7.33 (t, J = 7.4 Hz, 1H), 5.00 (s, 1H), 3.93 (dd, J = 13.3, 4.6 Hz, 1H), 2.81 (d, J = 13.2 Hz, 1H), 2.60 (td, J = 13.0, 3.0 Hz, 1H), 2.06–2.01 (m, 1H), 2.00 (s, 3H), 1.92 (s, 3H), 1.89 (d, J = 3.8 Hz, 1H), 1.86–1.74 (m, 2H), 1.55–1.43 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.5, 165.5, 148.4, 145.2, 138.3, 134.3, 133.0, 132.8, 129.0, 128.8, 128.2, 128.1, 127.6, 127.4, 126.6, 126.2, 126.1, 122.4, 110.5, 101.2, 92.1, 48.0, 39.0, 34.5, 27.5, 23.3, 20.9, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C30H27N3O4 494.2074; Found 494.2079.
- 5-acetyl-1-(4-chlorophenyl)-3-methyl-4-phenyl-4,5,8,9,10,11-hexahydro-5,11a-epoxypyrazolo[4,3-g]pyrido[2,1-b][1,3]oxazocin-6(1H)-one (3cf): White solid (yield: 38.1 mg, 80%). M.P. = 113.3–113.7 °C; 1H NMR (400 MHz, CDCl3): δ 7.64 (d, J = 8.8 Hz, 2H), 7.44 (d, J = 8.8 Hz, 2H), 7.33–7.22 (m, 5H), 4.80 (s, 1H), 3.89 (dd, J = 13.3, 4.6 Hz, 1H), 2.76 (d, J = 13.2 Hz, 1H), 2.52 (td, J = 13.0, 3.0 Hz, 1H), 2.08 (d, J = 13.4 Hz, 1H), 1.98 (s, 3H), 1.94 (s, 3H), 1.92–1.64 (m, 3H), 1.57–1.39 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.3, 165.4, 148.7, 145.2, 136.9, 136.5, 132.0, 129.6, 129.1, 128.4, 127.7, 123.3, 110.6, 101.5, 91.8, 48.0, 39.0, 34.4, 27.4, 23.2, 20.9, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C26H24ClN3O4 478.1528; Found 478.1532.
- 5-acetyl-3-methyl-4-phenyl-1-(p-tolyl)-4,5,8,9,10,11-hexahydro-5,11a-epoxypyrazolo[4,3-g]pyrido[2,1-b][1,3]oxazocin-6(1H)-one (3cg): White solid (yield: 38.3 mg, 84%). M.P. = 144.7–148.1 °C; 1H NMR (400 MHz, CDCl3): δ 7.53 (d, J = 8.4 Hz, 2H), 7.31 (td, J = 8.5, 1.6 Hz, 2H), 7.29–7.22 (m, 5H), 4.80 (s, 1H), 3.89 (dd, J = 13.3, 4.6 Hz, 1H), 2.75 (d, J = 13.2 Hz, 1H), 2.52 (td, J = 13.0, 3.0 Hz, 1H), 2.41 (s, 3H), 2.04–1.99 (m, 1H), 1.98 (s, 3H), 1.94 (s, 3H), 1.92–1.68 (m, 3H), 1.53–1.39 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.6, 165.5, 147.9, 145.0, 136.8, 136.4, 135.8, 129.7, 129.5, 128.3, 127.6, 122.4, 110.3, 101.0, 91.9, 48.0, 38.9, 34.4, 27.4, 23.3, 21.1, 20.8, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C27H27N3O4 458.2074; Found 458.2079.
- 5-acetyl-3-methyl-1-phenyl-4-(3,4,5-trimethoxyphenyl)-4,5,8,9,10,11-hexahydro-5,11a-epoxypyrazolo[4,3-g]pyrido[2,1-b][1,3]oxazocin-6(1H)-one (3cl): White solid (yield: 39.9 mg, 75%). M.P. = 238.3–238.7 °C; 1H NMR (400 MHz, CDCl3): δ 7.66 (d, J = 7.6 Hz, 2H), 7.46 (t, J = 7.6 Hz, 2H), 7.32 (t, J = 7.4 Hz, 1H), 6.54 (s, 2H), 4.74 (s, 1H), 3.89 (dd, J = 13.3, 4.6 Hz, 1H), 3.83 (s, 6H), 3.82 (s, 3H), 2.74 (d, J = 13.2 Hz, 1H), 2.57 (td, J = 13.0, 3.0 Hz, 1H), 2.04 (s, 3H), 1.98 (s, 3H), 1.90 (td, J = 13.1, 3.9 Hz, 1H), 1.85–1.66 (m, 1H),1.56–1.41 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.6, 165.4, 152.8, 148.4, 145.0, 138.3, 137.5, 132.4, 129.0, 126.6, 122.4, 110.4, 106.9, 101.0, 91.9, 60.8, 56.2, 48.2, 39.0, 34.4, 27.6, 23.2, 20.8, 13.0 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C29H31N3O7534.2234; Found 534.2234.
- 5-acetyl-4-(2-methoxyphenyl)-3-methyl-1-phenyl-4,5,8,9,10,11-hexahydro-5,11a-epoxypyrazolo[4,3-g]pyrido[2,1-b][1,3]oxazocin-6(1H)-one (3cn): White solid (yield: 40.6 mg, 86%). M.P. = 162.3–162.5 °C; 1H NMR (400 MHz, CDCl3): δ 7.66 (d, J = 7.6 Hz, 2H), 7.45 (t, J = 5.8 Hz, 3H), 7.30 (t, J = 7.4 Hz, 1H), 7.21 (td, J = 8.5, 1.6 Hz, 1H), 6.88 (m, 2H), 5.51 (s, 1H), 3.92 (s, 3H), 3.89 (dd, J = 13.3, 4.6 Hz, 1H), 2.74 (d, J = 13.2 Hz, 1H), 2.56 (td, J = 13.0, 3.0 Hz, 1H), 2.20 (s, 3H), 2.00 (s, 3H), 1.96–1.87 (m, 2H), 1.81–1.68 (m, 2H), 1.52–1.37 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.9, 166.2, 155.5, 148.4, 145.1, 138.4 130.9, 129.0, 128.9, 126.4, 125.3, 122.4, 121.0, 110.5, 110.2, 102.1, 92.0, 55.5, 39.1, 38.8, 34.4, 26.8, 23.2, 20.8, 12.7 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C27H27N3O5. 474.2023; Found 474.2026.
- 5-acetyl-3-methyl-1-phenyl-4-(thiophen-2-yl)-4,5,8,9,10,11-hexahydro-5,11a-epoxypyrazolo[4,3-g]pyrido[2,1-b][1,3]oxazocin-6(1H)-one (3cp): White solid (yield: 38.1 mg, 85%). M.P. = 181.6–181.9 °C; 1H NMR (400 MHz, CDCl3): δ 7.65 (d, J = 8.7 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.31 (t, J = 7.5 Hz, 1H), 7.19 (d, J = 5.0 Hz, 1H), 6.96 (d, J = 3.0 Hz, 1H), 6.90 (t, J = 14.0 Hz, 2H), 5.17 (s, 1H), 3.87 (dd, J = 13.3, 4.6 Hz, 1H), 2.76 (d, J = 13.2 Hz, 1H), 2.52 (td, J = 13.0, 3.0 Hz, 1H), 2.10 (s, 3H), 2.09 (s, 3H), 2.04–1.84 (m, 2H), 1.82–1.65 (m, 2H), 1.54–1.39 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.3, 164.9, 148.2, 144.4, 139.9, 138.2, 128.9, 127.2, 126.7, 126.5, 125.7, 122.5, 110.5, 101.3, 91.7, 43.1, 39.0, 34.4, 27.3, 23.2, 20.8, 12.8 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H23N3O4S 450.1482; Found 450.1486.
- 5-acetyl-4-(4-bromophenyl)-3-methyl-1-phenyl-4,5,9,10,11,12-hexahydro-8H-5,12a-epoxyazepino[2,1-b]pyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3db): White solid (yield: 32.1 mg, 60%). M.P. = 212.0–212.2 °C; 1H NMR (400 MHz, CDCl3): δ7.63 (d, J = 7.5 Hz, 2H), 7.47 (t, J = 7.6 Hz, 2H), 7.40 (d, J = 8.4 Hz, 2H),7.32 (t, J = 7.4 Hz, 1H), 7.20 (d, J = 8.5 Hz, 2H), 4.77 (s, 1H), 3.86 (dd, J = 13.3, 4.6 Hz, 1H), 2.91–2.75 (m, 2H), 2.37–2.29 (m, 1H), 2.00 (s, 3H), 1.97 (s, 3H), 1.96–1.88 (m, 2H), 1.79–1.71 (m, 1H), 1.54–1.39 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.5, 166.1, 148.0, 145.3, 138.3, 136.1, 131.5, 131.4, 129.0, 126.6, 122.5, 121.8, 115.6, 100.3, 91.4, 47.1, 40.6, 38.4, 29.5, 28.5, 27.7, 22.6, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C27H26BrN3O4 536.1179; Found 536.1189.
- 5-acetyl-4-(4-methoxyphenyl)-3-methyl-1-phenyl-4,5,9,10,11,12-hexahydro-8H-5,12a-epoxyazepino[2,1-b]pyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3dd): White solid (yield: 37.9 mg, 78%). M.P. = 161.0–161.4 °C; 1H NMR (400 MHz, CDCl3): δ 7.63 (d, J = 7.5 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.30 (t, J = 7.5 Hz, 1H),7.22 (dd, J = 8.7, 1.9 Hz, 2H), 6.80 (d, J = 8.7 Hz, 2H), 4.75 (s, 1H), 3.84 (d, J = 10.6 Hz, 1H), 3.77 (s, 3H), 2.92–2.74 (m, 2H), 2.40–2.30 (m, 1H), 1.97 (s, 6H), 1.96–1.88 (m, 2H), 1.81–1.71 (m, 1H), 1.54–1.27 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.8, 166.4, 158.9, 148.1, 145.2, 138.5, 130.7, 128.9, 126.5, 122.5, 115.4, 113.7, 100.9, 91.7, 55.2, 47.4, 40.5, 38.4, 29.5, 28.5, 27.7, 22.5, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C28H29N3O5 488.2180; Found 488.2187.
- 5-acetyl-3-methyl-4-(naphthalen-1-yl)-1-phenyl-4,5,9,10,11,12-hexahydro-8H-5,12a-epoxyazepino[2,1-b]pyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3de): White solid (yield: 46.1 mg, 91%). M.P. = 183.5–183.8 °C; 1H NMR (400 MHz, CDCl3): δ 7.86–7.78 (m, 2H), 7.76 (d, J = 8.4 Hz, 2H), 7.68 (d, J = 7.6 Hz, 2H), 7.52–7.41 (m, 5H), 7.32 (t, J = 7.4 Hz, 2H), 4.98 (s, 1H), 3.89 (d, J = 10.6 Hz, 1H), 2.98–2.77 (m, 2H), 2.40–2.30 (m, 1H), 1.99 (s, 3H), 1.98–1.94 (br, 1H), 1.95 (s, 3H), 1.94–1.91 (br, 1H), 1.81–1.71 (m, 1H), 1.54–1.27 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.5, 166.4, 148.3, 145.4, 138.5, 134.5, 133.0, 132.8, 129.0, 128.8, 128.2, 128.1, 127.6, 127.3, 126.5, 126.1, 126.1, 122.5, 115.5, 100.7, 91.8, 48.1, 40.6, 38.4, 29.6, 28.5, 27.7, 22.6, 13.0 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C31H29N3O4 508.2230; Found 508.2239.
- 5-acetyl-3-methyl-4-phenyl-1-(p-tolyl)-4,5,9,10,11,12-hexahydro-8H-5,12a-epoxyazepino[2,1-b]pyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3dg): White solid (yield: 42.3 mg, 90%). M.P. = 191.0–191.4 °C; 1H NMR (400 MHz, CDCl3): δ 7.50 (d, J = 8.4 Hz, 2H), 7.30 (t, J = 6.8 Hz, 3H), 7.27–7.21 (m, 4H), 4.79 (s, 1H), 3.84 (d, J = 14.0 Hz, 1H), 2.90–2.74 (m, 2H), 2.40 (s, 3H), 2.34 (dd, J = 15.6, 10.6 Hz, 1H), 1.97 (s, 3H), 1.96 (s, 3H), 1.94–1.86 (m, 2H), 1.77–1.70 (br, 1H), 1.51–1.38 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.7, 166.4, 147.8, 145.2, 137.0, 136.3, 136.0, 129.6, 129.5, 128.3, 127.6, 122.5, 115.3, 100.6, 91.6, 48.0, 40.5, 38.4, 29.5, 28.5, 27.6, 22.5, 21.1, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C28H29N3O4 472.2230; Found 472.2236.
- 5-acetyl-3-methyl-1-phenyl-4-(3,4,5-trimethoxyphenyl)-4,5,9,10,11,12-hexahydro-8H-5,12a-epoxyazepino[2,1-b]pyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3dl): White solid (yield: 44.8 mg, 82%). M.P. = 215.2–215.5 °C; 1H NMR (400 MHz, CDCl3): δ 7.61 (d, J = 7.6 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.30 (t, J = 7.4 Hz, 1H), 6.52 (s, 2H), 4.71 (s, 1H), 3.89–3.82 (br, 1H), 3.81 (s, 6H), 3.80 (s, 3H), 2.89–2.74 (m, 2H), 2.35 (dd, J = 15.6, 10.6 Hz, 1H), 2.02 (s, 3H), 1.99 (s, 3H), 1.96–1.86 (m, 2H), 1.77–1.69 (m, 1H), 1.51–1.32 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.7, 166.3, 152.8, 148.2, 145.2, 138.4, 137.4, 132.5, 129.0, 126.6, 122.4, 115.5, 106.8, 100.6, 91.6, 60.8, 56.2, 48.3, 40.6, 38.4, 29.5, 28.5, 27.8, 22.6, 13.0 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C30H33N3O7 548.2391; Found 548.2404.
- 5-acetyl-4-(2-methoxyphenyl)-3-methyl-1-phenyl-4,5,9,10,11,12-hexahydro-8H-5,12a-epoxyazepino[2,1-b]pyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3dn): White solid (yield: 39.4 mg, 81%). M.P. = 190.9–191.3 °C; 1H NMR (400 MHz, CDCl3): δ 7.62 (d, J = 7.5 Hz, 2H), 7.43 (t, J = 6.9 Hz, 3H), 7.28 (t, J = 7.4 Hz, 1H), 7.20 (td, J = 8.7, 1.9 Hz, 1H), 6.88 (t, J = 6.1 Hz, 2H), 5.53 (s, 1H), 3.92 (s, 3H), 3.85 (dd, J = 10.6, 3.0 Hz, 1H), 2.96–2.74 (m, 2H), 2.37–2.27 (m, 1H), 2.26 (s, 3H), 2.00 (s, 3H), 1.96–1.88 (m, 2H), 1.81–1.71 (m, 1H), 1.54–1.27 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 199.0, 167.2, 155.4, 148.3, 145.2, 138.5, 130.9, 129.0, 128.9, 126.4, 125.5, 122.5, 121.1, 115.3, 110.5, 101.7, 91.8, 55.4, 40.4, 38.8, 38.2, 29.5, 28.5, 26.8, 22.4, 12.7 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C28H29N3O5 488.2180; Found 488.2186.
- 5-acetyl-4-(4-methoxyphenyl)-3-methyl-1-phenyl-4,5,8,9,10,11,12,13-octahydro-5,13a-epoxyazocino[2,1-b]pyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3ed): White solid (yield: 42.5 mg, 85%). M.P. = 106.5–106.9 °C; 1H NMR (400 MHz, CDCl3): δ 7.62 (d, J = 7.5 Hz, 2H), 7.43 (t, J = 7.6 Hz, 2H), 7.28 (t, J = 7.4 Hz, 1H), 7.21 (d, J = 8.4 Hz, 2H), 6.80 (d, J = 11.6 Hz, 2H), 4.75 (s, 1H), 3.76 (s, 3H), 3.85 (dt, J = 14.4, 4.0 Hz, 1H), 2.78 (td, J = 11.9, 3.5 Hz, 1H), 2.64 (dt, J = 15.7, 4.3 Hz, 1H), 2.48 (td, J = 10.9, 4.3 Hz, 1H), 1.98 (s, 3H), 1.97 (s, 3H), 1.86–1.71 (m, 3H), 1.70–1.55 (m, 3H), 1.54–1.33 (m, 2H) ppm; 13C NMR (100 MHz, CDCl3): δ 200.8, 167.0, 158.9, 148.1, 145.1, 138.5, 130.7, 128.9, 128.8, 126.4, 122.3, 114.1, 113.7, 101.1, 91.6, 55.2, 47.7, 40.3, 31.7, 27.7, 27.0, 25.0, 23.8, 22.1, 12.9 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C29H31N3O5 502.2336; Found 502.2341.
- 5-acetyl-3-methyl-1-phenyl-4-(3,4,5-trimethoxyphenyl)-4,5,8,9,10,11,12,13-octahydro-5,13a-epoxyazocino[2,1-b]pyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3el): White solid (yield: 51.0 mg, 90%). M.P. = 223.2–223.6 °C; 1H NMR (400 MHz, CDCl3): δ 7.60 (d, J = 7.8 Hz, 2H), 7.43 (t, J = 7.6 Hz, 2H), 7.28 (t, J = 7.4 Hz, 1H), 7.21 (d, J = 8.4 Hz, 2H), 6.50 (s, 2H), 4.71 (s, 1H), 3.81 (s, 6H), 3.80 (s, 3H), 3.64 (dt, J = 14.4, 3.9 Hz, 1H), 2.76 (td, J = 11.9, 3.5 Hz, 1H), 2.60 (dt, J = 15.7, 4.3 Hz, 1H), 2.53–2.41 (m, 1H), 2.01 (s, 3H), 1.98 (s, 3H), 1.84–1.70 (m, 3H), 1.68–1.53 (m, 3H), 1.52–1.35 (m, 2H) ppm; 13C NMR (100 MHz, CDCl3): δ200.7, 166.9, 152.8, 148.2, 145.1, 138.4, 137.4, 132.4, 129.0, 126.5, 122.2, 114.2, 106.8, 100.8, 91.4, 60.8, 56.2, 48.5, 40.4, 31.8, 27.8, 27.0, 25.0, 23.8, 22.1, 13.0 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C31H35N3O7 562.2547; Found 562.2556.
- 5-acetyl-4-(2-methoxyphenyl)-3-methyl-1-phenyl-4,5,8,9,10,11,12,13-octahydro-5,13a-epoxyazocino[2,1-b]pyrazolo[4,3-g][1,3]oxazocin-6(1H)-one (3en): White solid (yield: 25.0 mg, 50%). M.P. = 69.6–69.9 °C; 1H NMR (400 MHz, CDCl3): δ 7.62 (d, J = 7.5 Hz, 2H), 7.43 (t, J = 7.7 Hz, 3H), 7.28 (t, J = 7.4 Hz, 1H), 7.21 (td, J = 8.4, 1.6 Hz, 1H), 6.92–6.85 (m, 2H), 5.51 (s, 1H), 3.93 (s, 3H), 3.67 (dt, J = 14.4, 4.0 Hz, 1H), 2.80 (td, J = 11.9, 3.5 Hz, 1H), 2.65 (dq, J = 15.7, 3.6 Hz, 1H), 2.44 (td, J = 10.9, 4.3 Hz, 1H), 2.24 (s, 3H), 1.99 (s, 3H), 1.86–1.69 (m, 3H), 1.64–1.55 (m, 3H), 1.54–1.39 (m, 2H) ppm; 13C NMR (100 MHz, CDCl3): δ 198.7, 167.9, 155.5, 148.3, 145.1, 138.6, 131.0, 128.9, 128.9, 126.3, 125.3, 122.3, 121.1, 114.0, 110.5, 101.9, 91.5, 55.4, 40.1, 39.3, 32.0, 26.9, 26.8, 25.3, 23.9, 22.1, 12.7 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C21H31N3O5 502.2336; Found 502.2338.
- 7-benzoyl-12-benzyl-2,3,7,12-tetrahydro-1H-6,13a-epoxypyrrolo[2′,1′:2,3][1,3]oxazocino[8,7-b]indol-5(6H)-one (3bq): White solid (yield: 42.2 mg, 91%). M.P. = 114.5–114.8 °C; 1H NMR (400 MHz, CDCl3): δ 7.38–7.29 (m, 6H), 7.22 (t, J = 3.56 Hz, 2H), 7.14 (dd, J = 7.4, 0.7 Hz, 1H), 7.07 (d, J = 7.8 Hz, 1H), 7.01 (td, J = 7.7, 1.2 Hz, 1H), 6.89 (td, J = 7.7, 1.2Hz, 1H), 6.47 (d, J = 7.8 Hz, 2H), 5.37 (s, 1H), 5.03 (d, J = 15.1 Hz, 1H), 4.63 (d, J = 15.4 Hz, 1H), 4.59 (s, 1H), 3.83–3.75 (m, 1H), 3.22–3.15 (m, 1H), 2.12–2.03 (m, 1H), 1.89–1.80 (m, 2H), 1.26–1.19 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 195.4, 173.5, 173.5, 142.5, 136.3, 135.5, 133.0, 128.9, 128.9, 128.3, 127.9, 127.6, 127.4, 126.3, 125.4, 123.0, 108.5, 108.4, 81.4, 59.6, 56.6, 44.3, 42.6, 26.3, 25.8 ppm; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C29H24N2O4 465.1809; Found 465.1761.
4. Conclusions
Conclusively, under the catalysis of Rh2(OAc)4 and binapbisphosphine ligand (±)-L3 in DCE at 80 °C, the cascade cyclization of diazoimides with alkylidenepyrazolones underwent diastereoselectively (dr > 20:1), affording pyrazole-fused oxa-bridged oxazocines in reasonable chemical yields. Moreover, the design and exploration of other novel cyclic carbonyl-ylide-based cyloadditions is ongoing in our organic lab, and will be reported in due course.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28207021/s1, NMR spectral copies of products 3–7. X-ray crystal data of products 3aa and 4.
Author Contributions
Funding acquisition, project administration, supervision, writing—original draft preparation, H.-W.Z.; investigation, methodology, formal analysis, data curation, writing—review and editing, K.W.; investigation, methodology, Y.Z., L.-Y.C., X.-Q.S., C.W., J.-R.Z. and Y.-F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Beijing Municipal Commission of Education (No. JC015001200902), Beijing Municipal Natural Science Foundation (No. 7102010, No. 2122008, No. 2172003, No. 2222002), Basic Research Foundation of Beijing University of Technology (X4015001201101), Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality (No. PHR201008025), Doctoral Scientific Research Start-up Foundation of Beijing University of Technology (No. 52015001200701) for financial supports.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Parrino, B.; Cascioferro, S.; Carbone, D.; Cirrincione, G.; Diana, P. Eight-membered heterocycles with two heteroatoms in a 1,5-relationship of interest in medicinal chemistry. Adv. Heterocycl. Chem. 2020, 132, 135–239. [Google Scholar]
- Parrino, B.; Cascioferro, S.; Carbone, D.; Pecoraro, C.; Cirrincione, G.; Diana, P. Eight-membered heterocycles with two heteroatoms in a 1,3-relationship of interest in medicinal chemistry. Adv. Heterocycl. Chem. 2022, 136, 1–57. [Google Scholar]
- Bera, M.; Roy, S. Silver(I)-catalyzed dual activation of propargylic alcohol and aziridine/azetidine: Triggering ring-opening and endo-selective ring-closing in a cascade. J. Org. Chem. 2009, 74, 8814–8817. [Google Scholar] [CrossRef]
- Ghosh, A.; Hegde, R.V.; Rode, H.B.; Ambre, R.; Mane, M.V.; Patil, S.A.; Sridhar, B.; Dateer, R.B. Catalyst- and additive-free approach to constructing benzo-oxazine, benzo-oxazepine, and benzo-oxazocine: O atom transfer and C horizontal lineo, C-N, and C-O bond formation at room temperature. Org. Lett. 2021, 23, 8189–8193. [Google Scholar] [CrossRef]
- Mondal, S.; Paira, R.; Maity, A.; Naskar, S.; Sahu, K.B.; Hazra, A.; Saha, P.; Banerjee, S.; Mondal, N.B. Basic alumina supported tandem synthesis of bridged polycyclic quinolino/isoquinolinooxazocines under microwave irradiation. Tetrahedron Lett. 2011, 52, 4697–4700. [Google Scholar] [CrossRef]
- Neogi, A.; Majhi, T.P.; Mukhopadhyay, R.; Chattopadhyay, P. Palladium-mediated intramolecular aryl amination on furanose derivatives: An expedient approach to the synthesis of chiral benzoxazocine derivatives and tricyclic nucleosides. J. Org. Chem. 2006, 71, 3291–3294. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.S.; Cheng, Y. Construction of novel bridged aromatic ring-fused oxazocine frameworks via an N-heterocyclic Carbene-catalyzed azabenzoin reaction and radical-initiated cascade cyclization. Org. Chem. Front. 2021, 8, 4192–4201. [Google Scholar] [CrossRef]
- Pramthaisong, C.; Worayuthakarn, R.; Pharikronburee, V.; Duangthongyou, T.; Rattanakam, R.; Ruchirawat, S.; Thasana, N. Base-mediated cascade cyclization: Stereoselective synthesis of benzooxazocinone. Org. Lett. 2018, 20, 4015–4019. [Google Scholar] [CrossRef]
- Goutham, K.; Ashok Kumar, D.; Suresh, S.; Sridhar, B.; Narender, R.; Karunakar, G.V. Gold-catalyzed intramolecular cyclization of N-propargylic beta-enaminones for the synthesis of 1,4-oxazepine derivatives. J. Org. Chem. 2015, 80, 11162–11168. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.; Qureshi, Z.; Wegmann, M.; Lautens, M. Transition-metal-free [4+3]-cycloaddition of ortho-quinonemethides and isomünchnones: Catalytic and diastereoselective assembly of oxa-bridged oxazocine scaffolds. Angew. Chem. 2018, 130, 16417–16421. [Google Scholar] [CrossRef]
- Li, Q.; Pan, R.; Wang, M.; Yao, H.; Lin, A. Ligand-controlled, palladium-catalyzed asymmetric [4+4] and [2+4] cycloadditions. Org. Lett. 2021, 23, 2292–2297. [Google Scholar] [CrossRef] [PubMed]
- Ramachary, D.B.; Narayana, V.V.; Prasad, M.S.; Ramakumar, K. High-yielding synthesis of nefopam analogues (functionalizedbenzoxazocines) by sequential one-pot cascade operations. Org. Biomol. Chem. 2009, 7, 3372–3378. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, L.; Han, Z.; Yang, Z.; Sun, J.; Huang, H. Pd-catalyzed ligand-directed divergent cycloaddition of cyclic 1-azadienes with oxo-1,4-dipoles. Org. Lett. 2023, 25, 5011–5016. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, K.; Li, D.; Lin, L.; Feng, X. Asymmetric synthesis of oxa-bridged oxazocines through a catalytic Rh(II)/Zn(II) relay [4 + 3] cycloaddition reaction. Angew. Chem. 2019, 58, 18438–18442. [Google Scholar] [CrossRef] [PubMed]
- Doxsee, K.M. Eight-membered rings with two heteroatoms 1,3. Comprehensive Heterocyclic Chemistry II; Elsevier: Amsterdam, The Netherlands, 1996; pp. 495–525. [Google Scholar]
- Ford, A.; Miel, H.; Ring, A.; Slattery, C.N.; Maguire, A.R.; McKervey, M.A. Modern organic synthesis with alpha-diazocarbonyl compounds. Chem. Rev. 2015, 115, 9981–10080. [Google Scholar] [CrossRef]
- Padwa, A. Domino reactions of Rhodium(II) carbenoids for alkaloid synthesis. Chem. Soc. Rev. 2009, 38, 3072–3081. [Google Scholar] [CrossRef]
- Anderson, R.J.; Raolji, G.B.; Kanazawa, A.; Greene, A.E. A novel, expeditious synthesis of racemic camptothecin. Org. Lett. 2005, 7, 2989–2991. [Google Scholar] [CrossRef]
- Padwa, A. Cycloaddition chemistry of carbonyl ylides for alkaloid synthesis. Russ. Chem. Bull. 2017, 65, 2183–2194. [Google Scholar] [CrossRef]
- Padwa, A.; Austin, D.J.; Price, A.T.; David Weingarten, M. Studies on the intramolecularcycloaddition reaction of isomünchnones derived from n-alkenyl substituted diazoimides. Tetrahedron 1996, 52, 3247–3260. [Google Scholar] [CrossRef]
- Padwa, A.; Lynch, S.M.; Mejia-Oneto, J.M.; Zhang, H. Cycloaddition chemistry of 2-vinyl-substituted indoles and related heteroaromatic systems. J. Org. Chem. 2005, 70, 2206–2218. [Google Scholar] [CrossRef]
- Padwa, A.; Prein, M. Acyclic stereocontrol in [3 + 2]-cycloadditions of amino acid-derived isomünchnone dipoles. Tetrahedron 1998, 54, 6957–6976. [Google Scholar] [CrossRef]
- Straub, C.S.; Padwa, A. Synthesis of the angiotensin converting enzyme inhibitor (−)-a58365a via an isomunchnonecycloaddition reaction. Org. Lett. 1999, 1, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.C.; Wright, D. Carbonyl ylides. In Chemistry of Heterocyclic Compounds; Springer: Berlin/Heidelberg, Germany, 2003; pp. 253–314. [Google Scholar]
- Padwa, A.; Weingarten, M.D. Cascade processes of metallocarbenoids. Chem. Rev. 1996, 96, 223–270. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Pieters, R.J.; Taunton, J.; Pho, H.Q.; Padwa, A.; Hertzog, D.L.; Precedo, L. Synthesis of nitrogen-containing polycycles via Rhodium(II)-induced cyclization-cycloaddition and insertion reactions of n-(diazoacetoacetyl)amides. Conformational control of reaction selectivity. J. Org. Chem. 2002, 56, 820–829. [Google Scholar] [CrossRef]
- Gonzalez, R.; Knight, B.W.; Wudl, F.; Semones, M.A.; Padwa, A. The reversible cycloaddition of isomunchnones to C60. J. Org. Chem. 2002, 59, 7949–7951. [Google Scholar] [CrossRef]
- Padwa, A.; Hertzog, D.L.; Nadler, W.R.; Osterhout, M.H.; Price, A.T. Studies on the intramolecularcycloaddition reaction of mesoionics derived from the Rhodium(II)-catalyzed cyclization of diazoimides. J. Org. Chem. 2002, 59, 1418–1427. [Google Scholar] [CrossRef]
- Prein, M.; Manley, P.J.; Padwa, A. Site selectivity in the Rhodium(II)-catalyzed reaction of α-diazoimides. Ligand and substituent effects. Tetrahedron 1997, 53, 7777–7794. [Google Scholar] [CrossRef]
- Sheehan, S.M.; Padwa, A. New synthetic route to 2-pyridones and its application toward the synthesis of (+/−)-ipalbidine. J. Org. Chem. 1997, 62, 438–439. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.M.; Labande, A.H.; Muthusamy, S. Cycloadditions of carbonyl ylides derived from diazocarbonyl compounds. Org. React. 2013, 80, 133–496. [Google Scholar]
- Mehta, G.; Muthusamy, S. Tandem cyclization–cycloaddition reactions of Rhodium generated carbenoids from α-Diazo carbonyl compounds. Tetrahedron 2002, 58, 9477–9504. [Google Scholar] [CrossRef]
- Padwa, A.; Hornbuckle, S.F. Ylide formation from the reaction of carbenes and carbenoids with heteroatom lone pairs. Chem. Rev. 2002, 91, 263–309. [Google Scholar] [CrossRef]
- Marino, J.P.; Osterhout, M.H.; Price, A.T.; Semones, M.A.; Padwa, A. Synthesis of tricyclic nitrogen compounds via a tandem cyclization-cycloaddition-cationic cyclization sequence. J. Org. Chem. 2002, 59, 5518–5520. [Google Scholar] [CrossRef]
- Miah, S.; Slawin, A.M.Z.; Moody, C.J.; Sheehan, S.M.; Marino, J.P.; Semones, M.A.; Padwa, A.; Richards, I.C. Ligand effects in the Rhodium(II) catalysed reactions of diazoamides and diazoimides. Tetrahedron 1996, 52, 2489–2514. [Google Scholar] [CrossRef]
- De, N.; Ko, D.; Yoo, E.J. Rhodium(II)-catalyzed 1,3- and 1,5-dipolar cycloaddition. Rhodium Catal. Org. Synth. 2019, 471–486. [Google Scholar] [CrossRef]
- Suga, H.; Itoh, K. Recent advances in catalytic asymmetric 1,3-dipolar cycloadditions of azomethine imines, nitrile oxides, diazoalkanes, and carbonyl ylides. Methods Appl. Cycloaddit. React. Org. Synth. 2014, 175–204. [Google Scholar] [CrossRef]
- Padwa, A.; Hertzog, D.L.; Chinn, R.L. Synthesis of aza substituted polycycles via Rhodium (II) carboxylate induced cyclization of diazoimides. Tetrahedron Lett. 1989, 30, 4077–4080. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.-J.; Chen, D.-Z.; Liu, J.-B. Mechanistic insights into catalyst-dependent divergent cycloaddition reactions via discrimination between diazo compounds. Org. Chem. Front. 2023, 10, 1606–1616. [Google Scholar] [CrossRef]
- Wang, K.; Xu, C.; Hu, X.; Zhou, Y.; Lin, L.; Feng, X. Catalytic asymmetric [3+2] cycloaddition of isomunchnones with methyleneindolinones. Chem. Commun. 2021, 57, 8917–8920. [Google Scholar] [CrossRef]
- Bania, N.; Barman, D.; Pan, S.C. Organocatalytic asymmetric inverse-electron-demand diels-alder reaction between alkylidenepyrazolones and allyl ketones: Access to tetrahydropyrano[2,3-c]pyrazoles. J. Org. Chem. 2023, 88, 9584–9593. [Google Scholar] [CrossRef]
- Guo, J.M.; Fan, X.Z.; Wu, H.H.; Tang, Z.; Bi, X.F.; Zhang, H.; Cai, L.Y.; Zhao, H.W.; Zhong, Q.D. Asymmetric synthesis of spiropyrazolones via chiral Pd(0)/ligand complex-catalyzed formal [4+2] cycloaddition of vinyl benzoxazinanones with alkylidenepyrazolones. J. Org. Chem. 2021, 86, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Meninno, S.; Roselli, A.; Capobianco, A.; Overgaard, J.; Lattanzi, A. Diastereodivergent and enantioselective access to spiroepoxides via organocatalyticepoxidation of unsaturated pyrazolones. Org. Lett. 2017, 19, 5030–5033. [Google Scholar] [CrossRef]
- Xu, H.; Hong, R.; Weng, M.Y.; Huang, R.L.; Wang, G.W.; Zhang, Z. Regiodivergent synthesis of 4,5′- and 4,4′-imidazolinyl spiropyrazolones from 4-alkylidene pyrazolones and amidines. Org. Lett. 2021, 23, 5305–5310. [Google Scholar] [CrossRef]
- CCDC 2260380 (3aa) and CCDC 2260381 (4) Contain the Supplementary Crystallographic Data for this Paper. These Data can be Obtained Free of Charge from The Cambridge Crystallographic Data Center. X-ray Single Crystal Structures of 3aa and 4 were Shown with Thermal Ellipsoils Shown at the 50% Probability Level. Available online: http://www.ccdc.camac.uk/data_request/cif (accessed on 5 March 2023).
- Padwa, A.; Hornbuckle, S.F.; Fryxell, G.E.; Stull, P.D. Reactivity patterns in the rhodium carbenoid induced tandem cyclization-cycloaddition reaction. J. Org. Chem. 1989, 54, 817–824. [Google Scholar] [CrossRef]
- Padwa, A.; Hertzog, D.L. Bimolecular cycloaddition reactions of isomünchnones derived from the rhodium(II) catalyzed cyclization of diazopyrrolidinones. Tetrahedron 1993, 49, 2589–2600. [Google Scholar] [CrossRef]
- Sheibani, H.; Babaie, M. Three-component reaction to form 1,4-dihydropyrano[2,3-c]pyrazol-5-yl cyanides. Synth. Commun. 2009, 40, 257–265. [Google Scholar] [CrossRef]
- Khairnar, P.V.; Su, Y.H.; Edukondalu, A.; Lin, W. Enantioselective synthesis of spiropyrazolone-fused cyclopenta[c]chromen-4-ones bearing five contiguous stereocenters via [3+2] cycloaddition. J. Org. Chem. 2021, 86, 12326–12335. [Google Scholar] [CrossRef]
- Zhao, C.; Shi, K.; He, G.; Gu, Q.; Ru, Z.; Yang, L.; Zhong, G. Nhc-catalyzed asymmetric formal [4+2] annulation to construct spirocyclohexanepyrazolone skeletons. Org. Lett. 2019, 21, 7943–7947. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).