Impact of Exposure to Pyraclostrobin and to a Pyraclostrobin/Boscalid Mixture on the Mitochondrial Function of Human Hepatocytes
Abstract
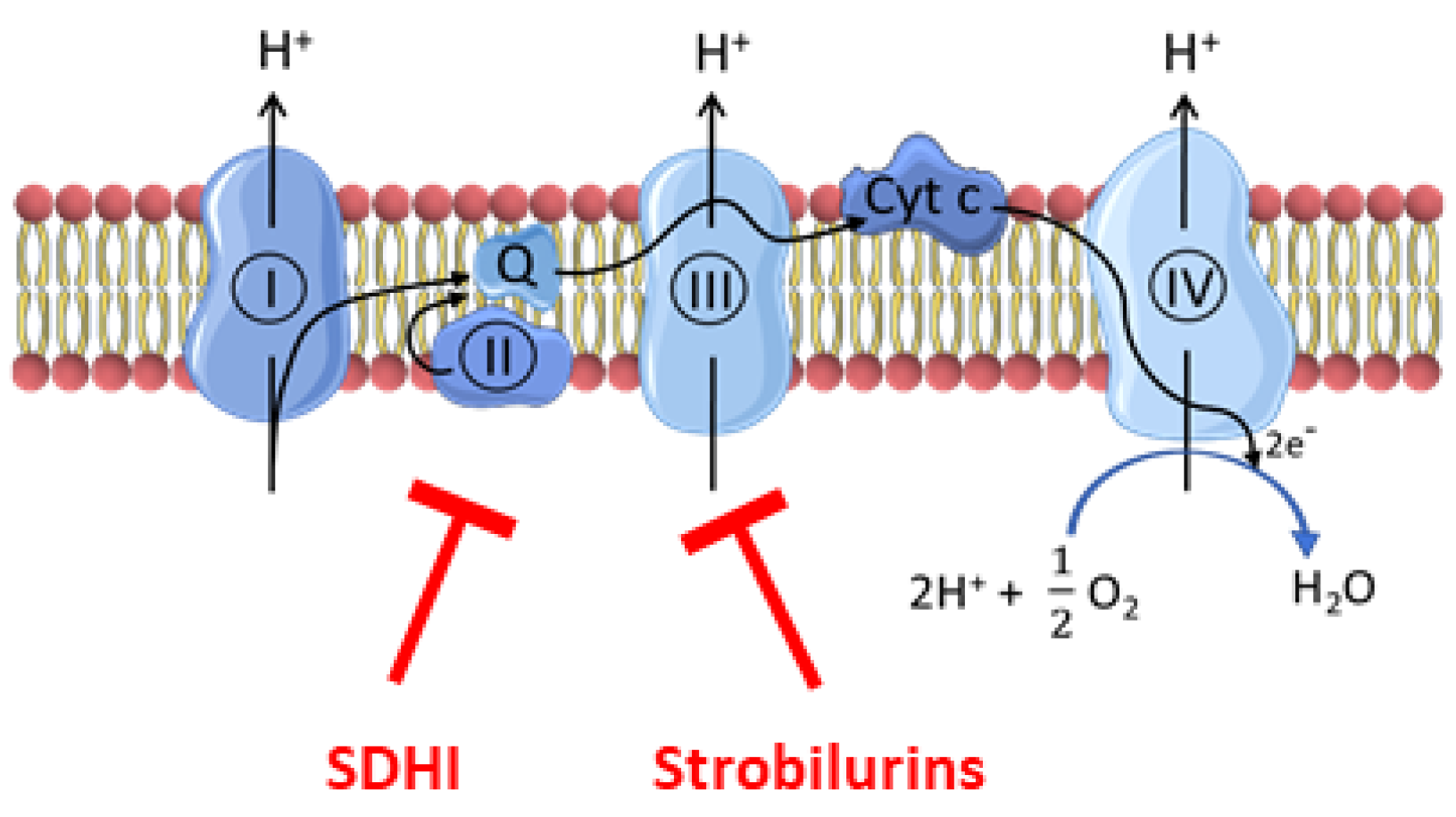
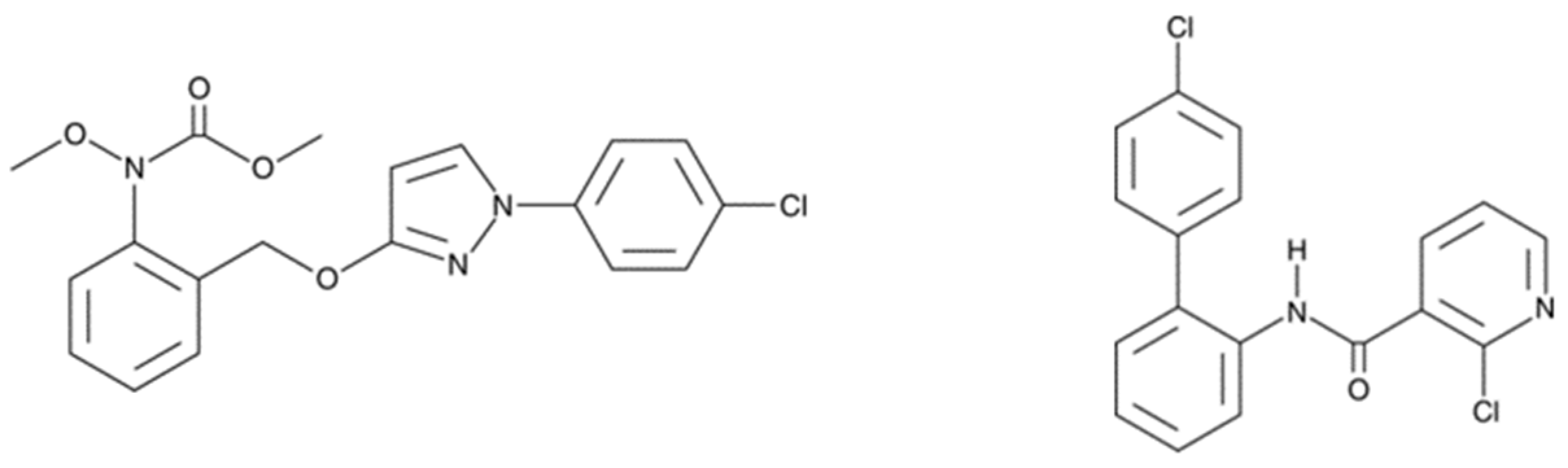
:1. Introduction
2. Results
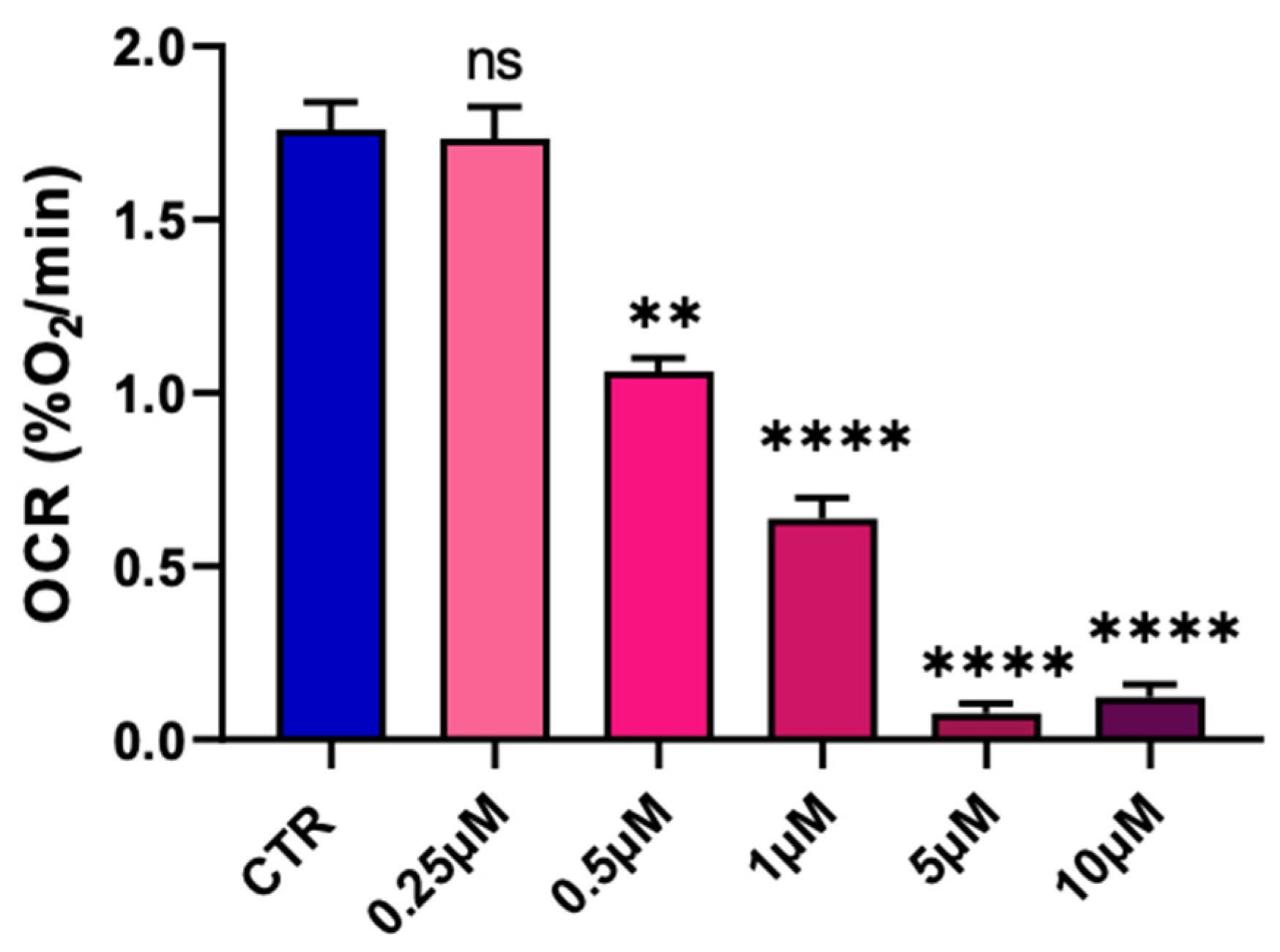
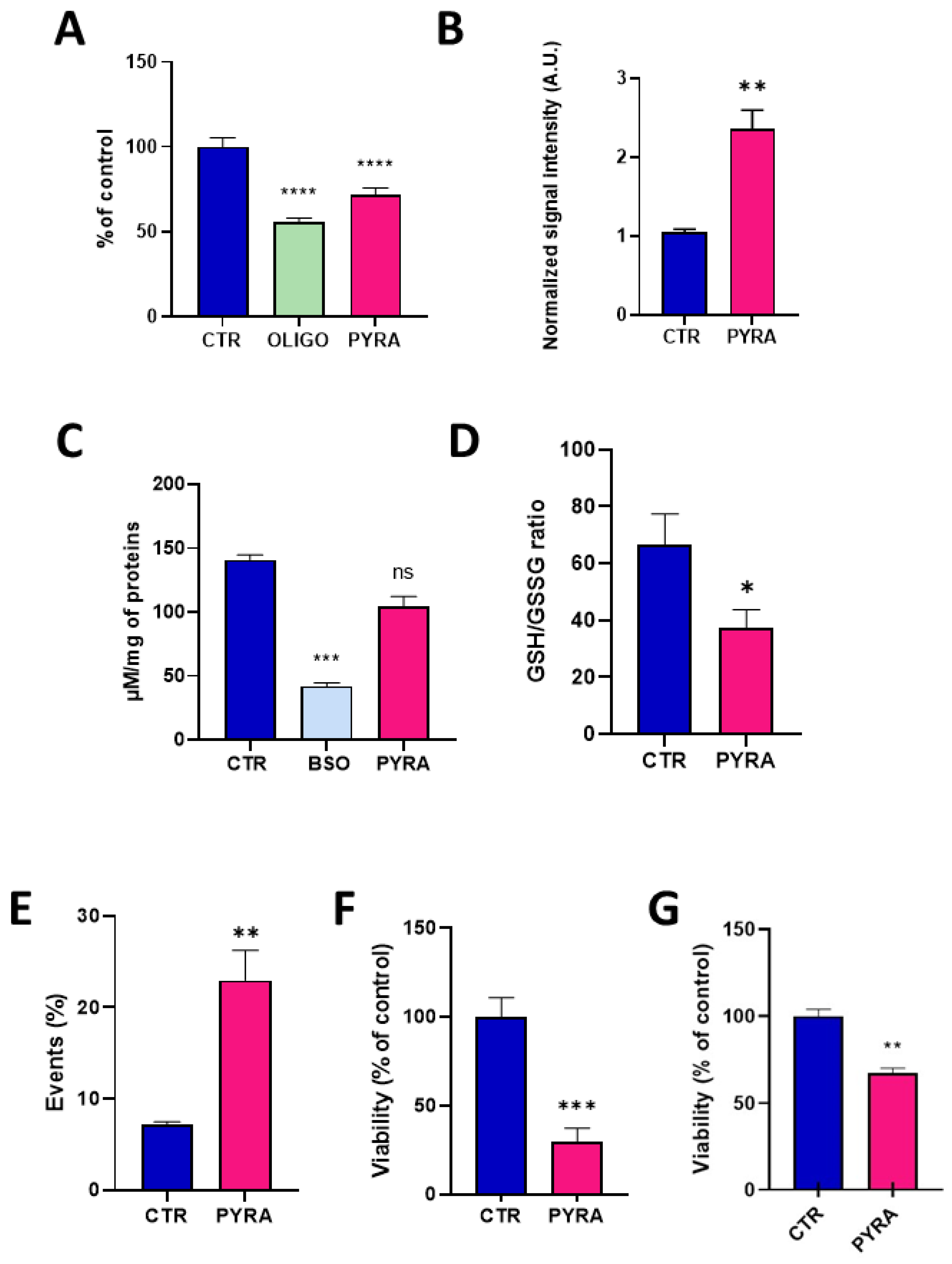
2.1. Pyraclostrobin Induces an Impairment of the Mitochondrial Function of Human Hepatocytes
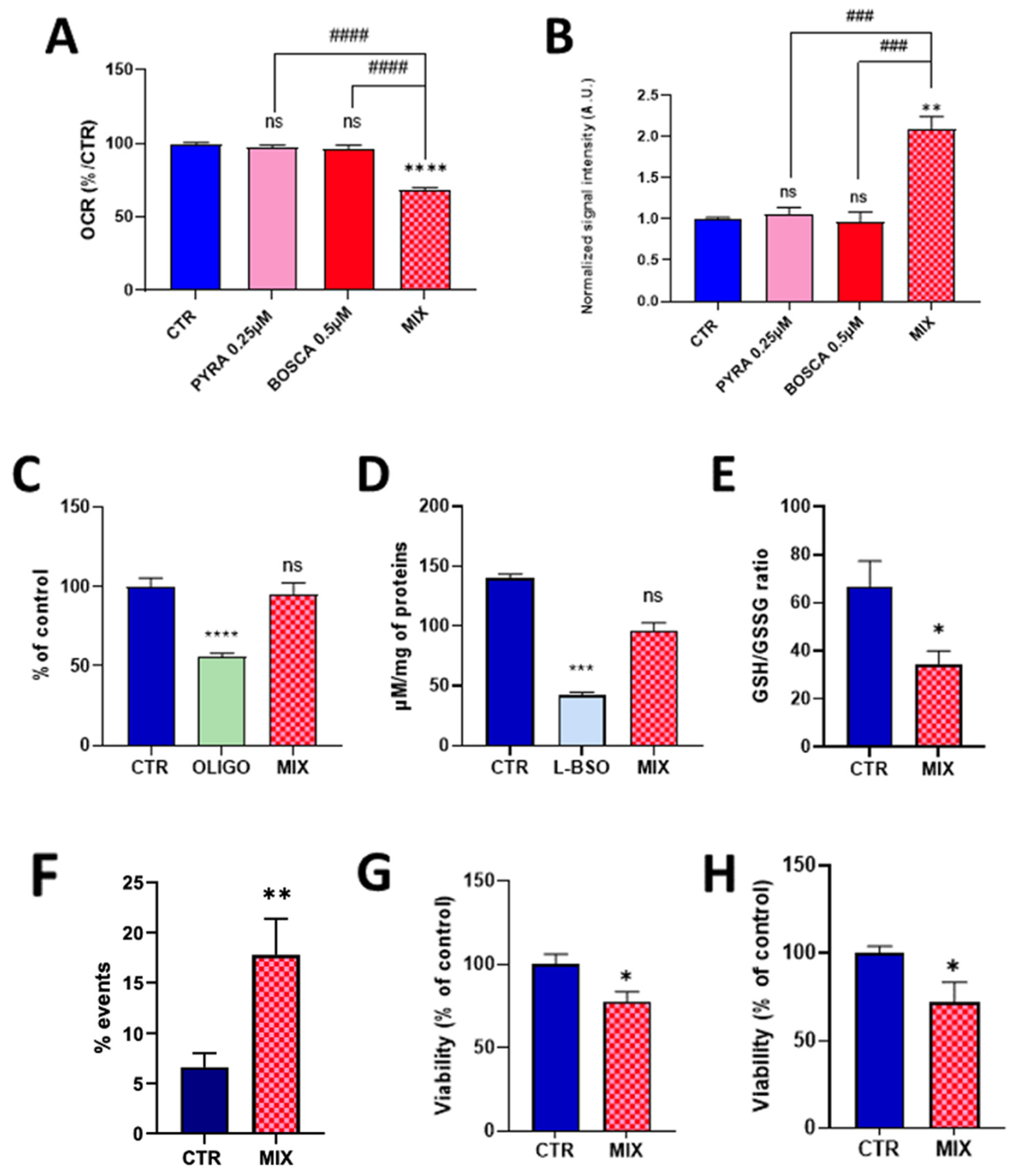
2.2. Mixing Pyraclostrobin and Boscalid Potentiates a Mitochondrial Dysfunction in Human Hepatocytes
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. EPR Oximetry
4.4. EPR Mitochondrial Superoxide Measurement
4.5. Intracellular ATP Quantification
4.6. Intracellular Glutathione Quantification
4.7. Apoptotic Changes by Flow Cytometry
4.8. Cell Viability by PrestoBlue
4.9. Cell Viability by Crystal Violet
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Davies, C.R.; Wohlgemuth, F.; Young, T.; Violet, J.; Dickinson, M.; Sanders, J.W.; Vallieres, C.; Avery, S.V. Evolving challenges and strategies for fungal control in the food supply chain. Fungal Biol. Rev. 2021, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21th century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, B.A.; Bayon, C.; Atkins, S.; Cools, H.J.; Lucas, J.A.; Fraaije, M.W. Risk assessment studies on succinate dehydrogenase inhibitors, the new weapons in the battle to control Septoria leaf blotch in wheat. Mol. Plant Pathol. 2012, 13, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Esser, L.; Zhou, F.; Yu, C.A.; Xia, D. Crystal structure of bacterial cytochrome bc1 in complex with azoxystrobin reveals a conformational switch of the Rieske iron-sulfur protein subunit. J. Biol. Chem. 2019, 294, 12007–12019. [Google Scholar] [CrossRef] [PubMed]
- Bénit, P.; Kahn, A.; Chretien, D.; Bortoli, S.; Huc, L.; Schiff, M.; Gimenez-Roqueplo, A.P.; Favier, J.; Gressens, P.; Rak, M.; et al. Evolutionarily conserved susceptibility of the mitochondrial respiratory chain to SDHI pesticides and its consequence on the impact of SDHIs on human cultured cells. PLoS ONE 2019, 14, e0224132. [Google Scholar] [CrossRef]
- Bénit, P.; Goncalves, J.; El Khoury, R.; Rak, M.; Favier, J.; Gimenez-Roqueplo, A.P.; Rustin, P. Succinate Dehydrogenase, Succinate, and Superoxides: A Genetic, Epigenetic, Metabolic, Environmental Explosive Crossroad. Biomedicines 2022, 10, 1788. [Google Scholar] [CrossRef]
- Mowery, P.C.; Ackrell, B.A.; Singer, T.P. Carboxins: Powerful selective inhibitors of succinate oxidation in animal tissues. Biochem. Biophys. Res. Commun. 1976, 71, 354–361. [Google Scholar] [CrossRef]
- Mowery, P.C.; Steenkamp, D.J.; Ackrell, A.C.; Singer, T.P.; White, G.A. Inhibition of mammalian succinate dehydrogenase by carboxins. Arch. Biochem. Biophys. 1977, 178, 495–506. [Google Scholar] [CrossRef]
- Coles, C.J.; Singer, T.P.; White, G.A.; Thorn, G.D. Studies on the binding of carboxin analogs to succinate dehydrogenase. J. Biol. Chem. 1978, 253, 5573–5578. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Ackrell, B.A.; Coles, C.J.; Singer, T.P.; White, G.A.; Thorn, G.D. Reaction site of carboxanilides and of thenoyltrifluoroacetone in complex II. Proc. Natl. Acad. Sci. USA 1981, 78, 825–828. [Google Scholar] [CrossRef]
- Brenet, A.; Hassan-Abdi, R.; Soussi-Yanicostas, N. Bixafen, a succinate dehydrogenase inhibitor fungicide, causes microcephaly and motor neuron axon defects during development. Chemosphere 2021, 265, 128781. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Meng, Z.; Liu, F.; Zhou, L.; Su, M.; Meng, Y.; Zhang, S.; Liao, X.; Cao, Z.; Lu, H. Characterization of boscalid-induced oxidative stress and neurodevelopmental toxicity in zebrafish embryos. Chemosphere 2020, 238, 124753. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Li, W.; Xiao, P. Bixafen causes cardiac toxicity in zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. Res. Int. 2021, 28, 36303–36313. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, J.; Chen, X.; Qi, S.; Wu, P.; Wang, C.; Wang, C. Toxic effects of boscalid in adult zebrafish (Danio rerio) on carbohydrate and lipid metabolism. Environ. Pollut. 2019, 247, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, F.; Zhao, F.; Yang, Y.; Teng, M.; Wang, C.; Qiu, L. Developmental toxicity, oxidative stress and immunotoxicity induced by three strobilurins (pyraclostrobin, trifloxystrobin and picoxystrobin) in zebrafish embryos. Chemosphere 2018, 207, 781–790. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Zhang, S.; Zhu, L.; Du, Z.; Wang, J. Acute and subchronic toxicity of pyraclostrobin in zebrafish (Danio rerio). Chemosphere 2017, 188, 510–516. [Google Scholar] [CrossRef]
- Jiang, J.; Lv, L.; Wu, S.; An, X.; Wang, F.; Liu, X.; Zhao, X. Developmental toxicity of kresoxim-methyl during zebrafish (Danio rerio) larval development. Chemosphere 2019, 219, 517–525. [Google Scholar] [CrossRef]
- Cayir, A.; Coskun, M.; Coskun, M. Micronuclei, nucleoplasmic bridges, and nuclear buds induced in human lymphocytes by the fungicide signum and its active ingredients (boscalid and pyraclostrobin). Environ. Toxicol. 2014, 29, 723–732. [Google Scholar] [CrossRef]
- Graillot, V.; Tomasetig, F.; Cravedi, J.P.; Audebert, M. Evidence of the in vitro genotoxicity of methyl-pyrazole pesticides in human cells. Mutat. Res. 2012, 748, 8–16. [Google Scholar] [CrossRef]
- Shi, X.K.; Bian, X.B.; Huang, T.; Wen, B.; Zhao, L.; Mu, H.X.; Fatima, S.; Fan, B.M.; Bian, Z.X.; Huang, L.F.; et al. Azoxystrobin Induces Apoptosis of Human Esophageal Squamous Cell Carcinoma KYSE-150 Cells through Triggering of the Mitochondrial Pathway. Front. Pharmacol. 2017, 8, 277. [Google Scholar] [CrossRef]
- van der Stel, W.; Carta, G.; Eakins, J.; Darici, S.; Delp, J.; Forsby, A.; Bennekou, S.H.; Gardner, I.; Leist, M.; Danen, E.H.J.; et al. Multiparametric assessment of mitochondrial respiratory inhibition in HepG2 and RPTEC/TERT1 cells using a panel of mitochondrial targeting agrochemicals. Arch. Toxicol. 2020, 94, 2707–2729. [Google Scholar] [CrossRef]
- d’Hose, D.; Isenborghs, P.; Brusa, D.; Jordan, B.F.; Gallez, B. The Short-Term Exposure to SDHI Fungicides Boscalid and Bixafen Induces a Mitochondrial Dysfunction in Selective Human Cell Lines. Molecules 2021, 26, 5842. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, W.G.; de Roos, J.A.; Westerink, W.M.; Debiton, E. Cytotoxic effects of 110 reference compounds on HepG2 cells and for 60 compounds on HeLa, ECC-1 and CHO cells. II mechanistic assays on NAD(P)H, ATP and DNA contents. Toxicol. Vitr. 2005, 19, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Gerets, H.H.; Hanon, E.; Cornet, M.; Dhalluin, S.; Depelchin, O.; Canning, M.; Atienzar, F.A. Selection of cytotoxicity markers for the screening of new chemical entities in a pharmaceutical context: A preliminary study using a multiplexing approach. Toxicol. Vitr. 2009, 23, 319–332. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Irwin, W.; Diaz, D.; Howard-Cofield, E.; Krejsa, C.M.; Slaughter, M.R.; Gao, B.; Kaludercic, N.; Angeline, A.; Bernardi, P.; et al. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch. Toxicol. 2006, 80, 580–604. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.echa.europa.eu/documents/10162/f8f08b3e-0226-812c-3472-55e78e0b6ac2 (accessed on 8 June 2023).
- Diepart, C.; Verrax, J.; Calderon, P.B.; Feron, O.; Jordan, B.F.; Gallez, B. Comparison of methods for measuring oxygen consumption in tumor cells in vitro. Anal. Biochem. 2010, 396, 250–256. [Google Scholar] [CrossRef] [PubMed]
- d’Hose, D.; Danhier, P.; Northshield, H.; Isenborghs, P.; Jordan, B.F.; Gallez, B. A versatile EPR toolbox for the simultaneous measurement of oxygen consumption and superoxide production. Redox Biol. 2021, 40, 101852. [Google Scholar]
- d’Hose, D.; Gallez, B. Measurement of mitochondrial (dys)function in cellular systems using Electron Paramagnetic Resonance (EPR): Oxygen consumption rate and superoxide production. Methods Mol. Biol. 2022, 2497, 83–95. [Google Scholar]
- Dikalov, S.I.; Harrison, D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef]
- Scheinok, S.; Leveque, P.; Sonveaux, P.; Driesschaert, B.; Gallez, B. Comparison of different methods for measuring the superoxide radical by EPR spectroscopy in buffer, cell lysates and cells. Free Radic. Res. 2018, 52, 1182–1196. [Google Scholar] [CrossRef]
- European Food Safety Authority. Review of the existing maximum residue levels (MRLs) for pyraclostrobin according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2011, 9, 2344. [Google Scholar] [CrossRef]
- European Food Safety Authority. The 2020 European Union report on pesticide residues in food. EFSA J. 2022, 20, 7215. [Google Scholar]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pyraclostrobin#section=EU-Pesticides-Data (accessed on 14 June 2023).
- Pesticide Residues in Food—2003—Joint FAO/WHO Meeting on Pesticide Residues—Pyraclostrobin. Available online: https://inchem.org/documents/jmpr/jmpmono/v2003pr10.htm (accessed on 14 June 2023).
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Boscalid (accessed on 14 June 2023).
- Pesticide Residues in Food—2006: Joint FAO/WHO Meeting on Pesticide Residues—Toxicological Evaluations—Boscalid. Available online: https://inchem.org/documents/jmpr/jmpmono/v2006pr01.pdf (accessed on 14 June 2023).
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Kumar, D.; Sinha, S.N.; Rajendra, S.; Sharma, K. Assessing farmer’s exposure to pesticides and the risk for non-communicable diseases: A biomonitoring study. Sci. Total Environ. 2023, 891, 164429. [Google Scholar] [CrossRef]
- Ramachandran, A.; Jaeschke, H. A mitochondrial journey through acetaminophen hepatotoxicity. Food Chem. Toxicol. 2020, 140, 111282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, M.; Mathieu, B.; Vandensande, Y.; Gallez, B. Impact of Exposure to Pyraclostrobin and to a Pyraclostrobin/Boscalid Mixture on the Mitochondrial Function of Human Hepatocytes. Molecules 2023, 28, 7013. https://doi.org/10.3390/molecules28207013
Carbone M, Mathieu B, Vandensande Y, Gallez B. Impact of Exposure to Pyraclostrobin and to a Pyraclostrobin/Boscalid Mixture on the Mitochondrial Function of Human Hepatocytes. Molecules. 2023; 28(20):7013. https://doi.org/10.3390/molecules28207013
Chicago/Turabian StyleCarbone, Mélina, Barbara Mathieu, Yasmine Vandensande, and Bernard Gallez. 2023. "Impact of Exposure to Pyraclostrobin and to a Pyraclostrobin/Boscalid Mixture on the Mitochondrial Function of Human Hepatocytes" Molecules 28, no. 20: 7013. https://doi.org/10.3390/molecules28207013
APA StyleCarbone, M., Mathieu, B., Vandensande, Y., & Gallez, B. (2023). Impact of Exposure to Pyraclostrobin and to a Pyraclostrobin/Boscalid Mixture on the Mitochondrial Function of Human Hepatocytes. Molecules, 28(20), 7013. https://doi.org/10.3390/molecules28207013






