Comprehensive Analyses of Advanced Glycation end Products and Heterocyclic Amines in Peanuts during the Roasting Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Validations of AGEs, HAs, GO and MGO
2.2. Determination of AGEs and HAs Contents in Roasted Peanuts
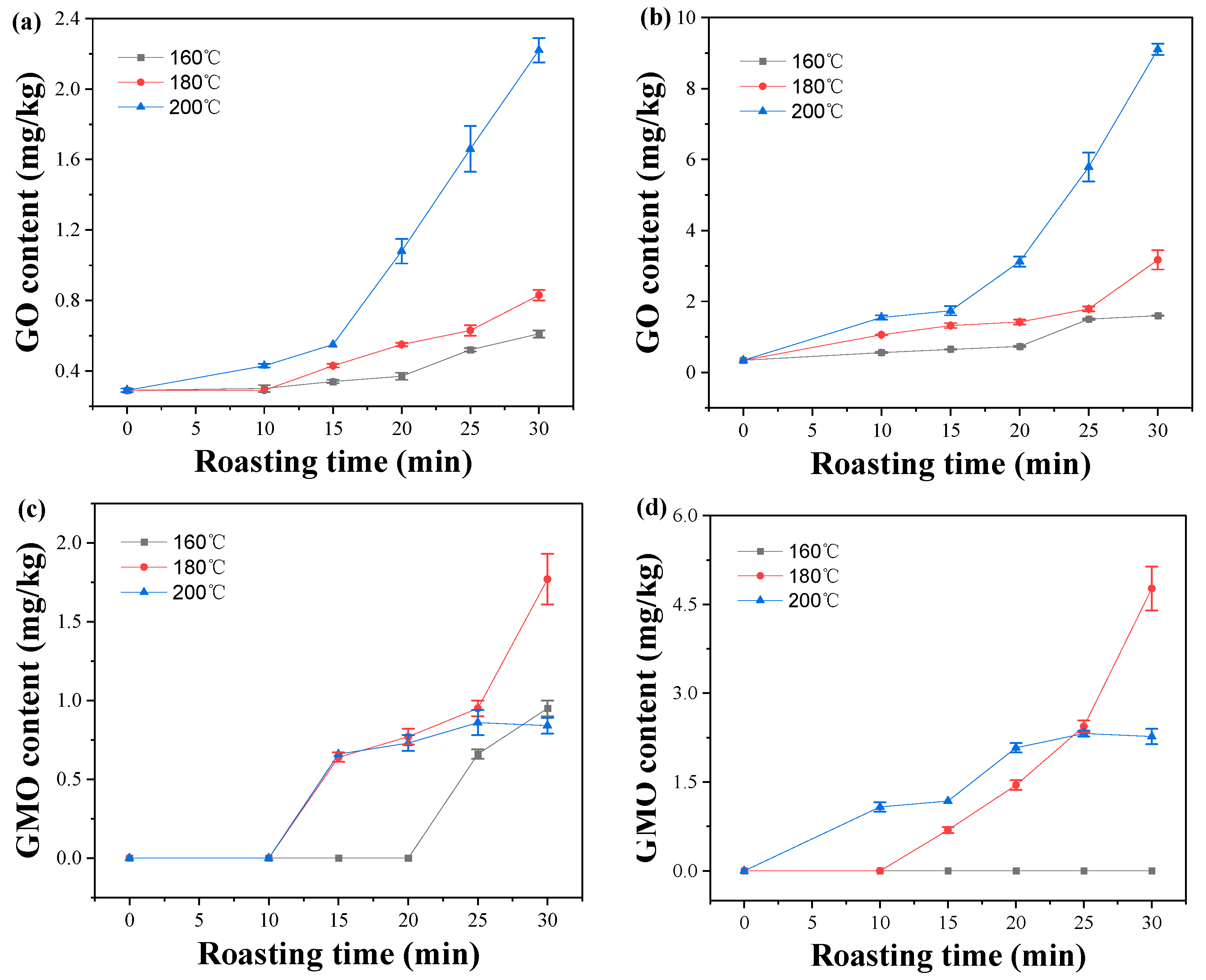
2.3. Effect of Roasting Conditions on GO and MGO Contents
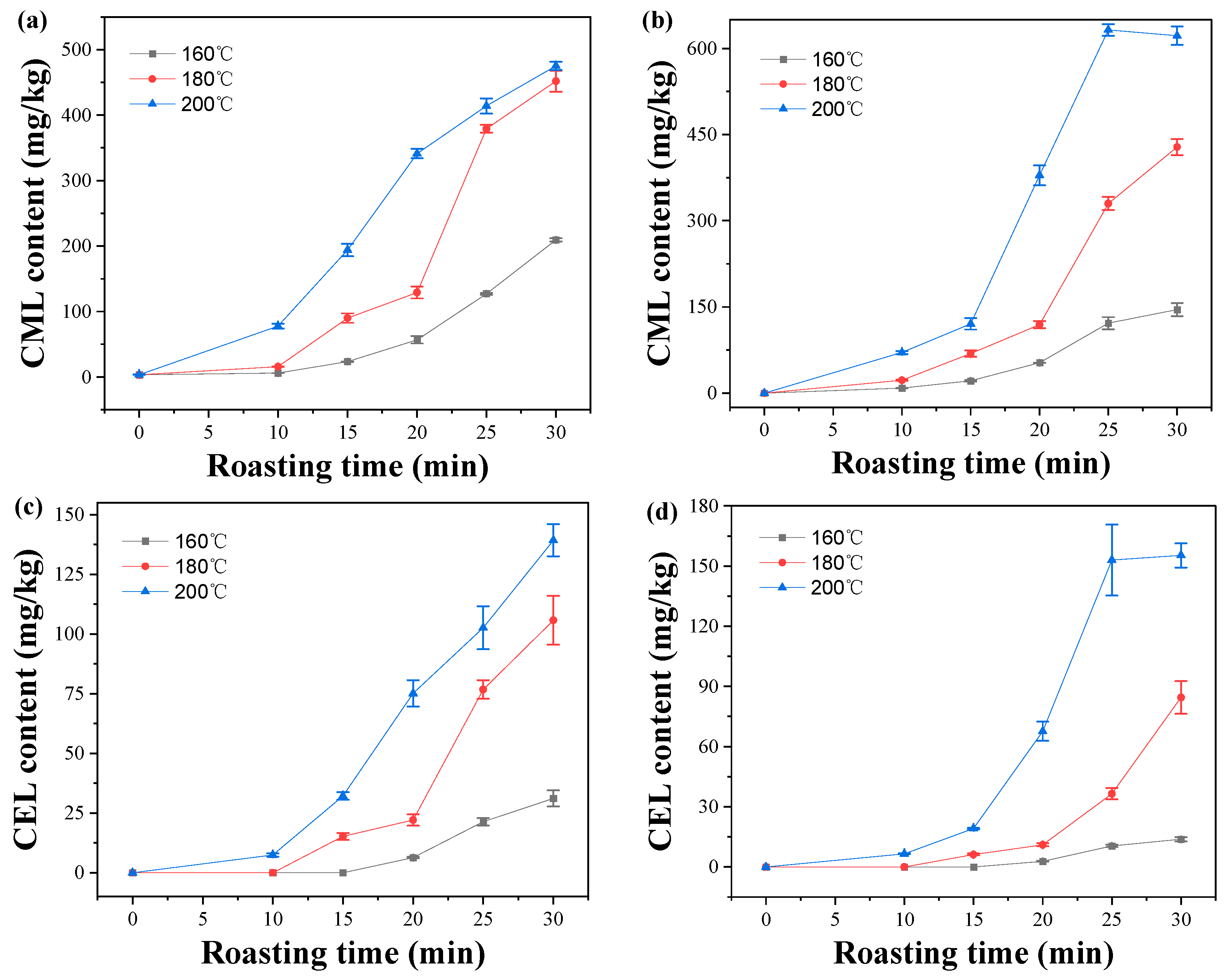
2.4. Effect of Roasting Conditions on CML and CEL Contents in Peanuts
2.5. Effect of Roasting Conditions on HAs Contents in Peanuts
2.6. Correlation Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Roasted Peanuts
3.3. Determination of GO and MGO Contents in Peanuts
3.4. Determination of AGEs Contents in Peanuts
3.5. Determination of HAs Content in Peanuts
3.6. Methodology Validation
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Win, M.M.; Abdul-Hamid, A.; Baharin, B.S.; Anwar, F.; Saari, N. Effects of roasting on phenolics composition and antioxidant activity of peanut (Arachis hypogaea L.) kernel flour. Eur. Food Res. Technol. 2011, 233, 599–608. [Google Scholar] [CrossRef]
- Cabanillas, B.; Maleki, S.J.; Rodriguez, J.; Burbano, C.; Muzquiz, M.; Aranzazu Jimenez, M.; Pedrosa, M.M.; Cuadrado, C.; Crespo, J.F. Heat and pressure treatments effects on peanut allergenicity. Food Chem. 2012, 132, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.S.; Sreedharan, A.; Schneider, K.R. Peanut and peanut products: A food safety perspective. Food Control 2013, 32, 296–303. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Q.; Liu, Y.; Huang, J.; Wang, X.; Mao, W.; Wang, S. Changes in Volatile Compounds of Peanut Oil during the Roasting Process for Production of Aromatic Roasted Peanut Oil. J. Food Sci. 2011, 76, C404–C412. [Google Scholar] [CrossRef]
- Lykomitros, D.; Fogliano, V.; Capuano, E. Flavor of roasted peanuts (Arachis hypogaea)—Part I: Effect of raw material and processing technology on flavor, color and fatty acid composition of peanuts. Food Res. Int. 2016, 89, 860–869. [Google Scholar] [CrossRef]
- Maleki, S.J.; Chung, S.Y.; Champagne, E.T.; Raufman, J.P. The effects of roasting on the allergenic properties of peanut proteins. J. Allergy Clin. Immun. 2000, 106, 763–768. [Google Scholar] [CrossRef]
- Shi, X.; Dean, L.O.; Davis, J.P.; Sandeep, K.P.; Sanders, T.H. The effects of different dry roast parameters on peanut quality using an industrial belt-type roaster simulator. Food Chem. 2018, 240, 974–979. [Google Scholar] [CrossRef]
- Smith, A.L.; Barringer, S.A. Color and Volatile Analysis of Peanuts Roasted Using Oven and Microwave Technologies. J. Food Sci. 2014, 79, C1895–C1906. [Google Scholar] [CrossRef]
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019, 280, 240–254. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bugel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.J.; Barcelo-Barrachina, E.; Toribio, F.; Puignou, L.; Galceran, M.T.; Persson, E.; Skog, K.; Messner, C.; Murkovic, M.; Nabinger, U.; et al. Analysis of heterocyclic amines in food products: Interlaboratory studies. J. Chromatogr. B 2004, 802, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Liu, T.; Sun, D.-W. Advanced glycation end-products (AGEs) in foods and their detecting techniques and methods: A review. Trends Food Sci. Technol. 2018, 82, 32–45. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Jing, X.; Chen, L.-J.; Yu, J.; Wang, H.-J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy. Cell. Physiol. Biochem. 2018, 48, 705–717. [Google Scholar]
- Hellwig, M.; Humpf, H.-U.; Hengstler, J.; Mally, A.; Vieths, S.; Henle, T. Quality Criteria for Studies on Dietary Glycation Compounds and Human Health. J. Agric. Food Chem. 2019, 67, 11307–11311. [Google Scholar] [CrossRef] [PubMed]
- Sanz Alaejos, M.; Ayala, J.H.; Gonzalez, V.; Afonso, A.M. Analytical methods applied to the determination of heterocyclic aromatic amines in foods. J. Chromatogr. B 2008, 862, 15–42. [Google Scholar] [CrossRef]
- Meurillon, M.; Engel, E. Mitigation strategies to reduce the impact of heterocyclic aromatic amines in proteinaceous foods. Trends Food Sci. Technol. 2016, 50, 70–84. [Google Scholar] [CrossRef]
- Cengiz, S.; Kismiroglu, C.; Cebi, N.; Catak, J.; Yaman, M. Determination of the most potent precursors of advanced glycation end products (AGEs) in chips, crackers, and breakfast cereals by high performance liquid chromatography (HPLC) using precolumn derivatization with 4-nitro-1,2-phenlenediamine. Microchem. J. 2020, 158, 105170. [Google Scholar] [CrossRef]
- Alaejos, M.S.; Pino, V.; Afonso, A.M. Metabolism and toxicology of heterocyclic aromatic amines when consumed in diet: Influence of the genetic susceptibility to develop human cancer. A review. Food Res. Int. 2008, 41, 327–340. [Google Scholar] [CrossRef]
- He, J.; Zeng, M.; Zheng, Z.; He, Z.; Chen, J. Simultaneous determination of N (epsilon)-(carboxymethyl) lysine and N (epsilon)-(carboxyethyl) lysine in cereal foods by LC-MS/MS. Eur. Food Res. Technol. 2014, 238, 367–374. [Google Scholar] [CrossRef]
- Hsiao, H.-Y.; Chen, B.-H.; Kao, T.-H. Analysis of Heterocyclic Amines in Meat by the Quick, Easy, Cheap, Effective, Rugged, and Safe Method Coupled with LC-DAD-MS-MS. J. Agric. Food Chem. 2017, 65, 9360–9368. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.L.J.M.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.A.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, K.; Wang, S.; Guo, J.; Wang, X.; Ding, L.; Zhao, J.; Wang, B.; Cai, J.; Chen, L.; et al. Analytical determination of CML and CEL in oral use tobacco products by HPLC-MS/MS method. Microchem. J. 2021, 168, 106338. [Google Scholar] [CrossRef]
- Quan, W.; Li, Y.; Jiao, Y.; Xue, C.; Liu, G.; Wang, Z.; He, Z.; Qin, F.; Zeng, M.; Chen, J. Simultaneous generation of acrylamide, beta-carboline heterocyclic amines and advanced glycation ends products in an aqueous Maillard reaction model system. Food Chem. 2020, 332, 127387. [Google Scholar] [CrossRef]
- Xue, C.; Quan, W.; Li, Y.; He, Z.; Qin, F.; Wang, Z.; Chen, J.; Zeng, M. Mitigative capacity of Kaempferia galanga L. and kaempferol on heterocyclic amines and advanced glycation end products in roasted beef patties and related mechanistic analysis by density functional theory. Food Chem. 2022, 385, 132660. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Z.; Shi, L.; Li, Y. Bioactive beta-Carbolines Harman and Norharman in Sesame Seed Oils in China. Molecules 2022, 27, 402. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Lavado-Tena, C.M.; Zamora, R. Identification of acrolein as the reactive carbonyl responsible for the formation of 2-amino-3,8-dimethylimidazo 4,5-f quinoxaline (MeIQx). Food Chem. 2021, 343, 128478. [Google Scholar] [CrossRef]
- Gibis, M. Heterocyclic Aromatic Amines in Cooked Meat Products: Causes, Formation, Occurrence, and Risk Assessment. Compr. Rev. Food Sci. 2016, 15, 269–302. [Google Scholar] [CrossRef]
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: A comprehensive review. Food Res. Int. 2020, 130, 108933. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, M.; Wang, J.; Jiang, X.; Lu, Y.; Qiu, C.; Lv, L.; Dong, W. Inhibitory Activity on the Formation of Reactive Carbonyl Species in Edible Oil by Synthetic Polyphenol Antioxidants. J. Agric. Food Chem. 2021, 69, 9025–9033. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Henning, C.; Heymann, T.; Glomb, M. Comprehensive Analyses of Carbohydrates, 1,2-Dicarbonyl Compounds, and Advanced Glycation End Products in Industrial Bread Making. J. Agric. Food Chem. 2021, 69, 3721–3731. [Google Scholar] [CrossRef] [PubMed]
- Szterk, A. Heterocyclic aromatic amines in grilled beef: The influence of free amino acids, nitrogenous bases, nucleosides, protein and glucose on HAAs content. J. Food Compos. Anal. 2015, 40, 39–46. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Z.; Shi, L.; Cui, Z.; Li, Y. Degradation of beta-Carbolines Harman and Norharman in Edible Oils during Heating. Molecules 2021, 26, 7018. [Google Scholar] [CrossRef]
- Smuda, M.; Glomb, M.A. Novel insights into the Maillard catalyzed degradation of maltose. J. Agric. Food Chem. 2011, 59, 13254–13264. [Google Scholar] [CrossRef] [PubMed]

| Compound | Linear Range (ng/mL) | Calibration Curve Measurement Points | Linear Regression Equation | R2 | LOD (mg/kg) | LOQ (mg/kg) | Recovery (%) | Precision (RSD,%, n = 6) |
|---|---|---|---|---|---|---|---|---|
| CML | 50–3000 | 7 | y = 0.0097x + 0.1760 | 0.9999 | 0.1050 | 0.3225 | 104.00 ± 3.08 a | 5.62 |
| CEL | 25–1000 | 5 | y = 0.0081x + 0.0508 | 0.9997 | 0.0875 | 0.2375 | 115.00 ± 4.23 | 5.19 |
| AαC | 0.1–20 | 7 | y = 1.47x | 0.9974 | 0.0001 | 0.0003 | 86.50 ± 2.03 | 9.43 |
| DMIP | 0.57–20 | 5 | y = 0.69x | 0.9972 | 0.0009 | 0.0029 | 91.17 ± 3.12 | 5.25 |
| Harman | 0.1–20 | 7 | y = 1.82x | 0.9988 | 0.0001 | 0.0002 | 81.91 ± 2.34 | 4.18 |
| Norharman | 0.1–20 | 7 | y = 2.72x | 0.9992 | 0.0002 | 0.0005 | 83.76 ± 1.98 | 4.10 |
| GO | 100–2000 | 6 | Y = 0.0901x − 0.0246 | 0.9999 | 0.0160 | 0.0533 | 98.88 ± 2.01 | 3.45 |
| MGO | 100–2000 | 6 | Y = 0.0225x − 0.6743 | 0.9997 | 0.0180 | 0.0600 | 96.24 ± 1.75 | 4.25 |
| Sample | CML (mg/kg) | CEL (mg/kg) | DMIP (µg/kg) | Harman (µg/kg) | Norharman (µg/kg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Roasted | Raw | Roasted | Raw | Roasted | Raw | Roasted | Raw | Roasted | |
| 1 | 6.73 ± 0.65 b | 377.56 ± 11.49 de | ND h | 83.25 ± 2.83 e | ND | 4.18 ± 0.04 d | ND | 3.98 ± 0.18 e | ND | 6.05 ± 0.21 e |
| 2 | 3.48 ± 0.28 c | 451.84 ± 15.94 bc | ND | 105.75 ± 10.25 abc | ND | 3.70 ± 0.31 e | ND | 7.60 ± 0.71 b | ND | 9.60 ± 0.00 c |
| 3 | 6.42 ± 0.23 b | 302.98 ± 27.75 f | ND | 83.13 ± 5.48 e | ND | 6.53 ± 0.39 c | ND | 5.50 ± 0.21 d | ND | 8.08 ± 0.39 d |
| 4 | 6.81 ± 0.48 b | 339.14 ± 19.45 ef | ND | 81.38 ± 3.71 e | ND | 6.18 ± 0.32 c | ND | 5.45 ± 0.57 d | ND | 6.73 ± 0.39 e |
| 5 | 6.22 ± 0.72 b | 491.80 ± 53.03 b | ND | 114.63 ± 5.83 a | ND | 11.90 ± 0.07 b | ND | 5.95 ± 0.49 cd | ND | 7.74 ± 0.46 d |
| 6 | 6.27 ± 0.67 b | 540.47 ± 27.40 a | ND | 108.63 ± 3.01 ab | ND | 12.45 ± 0.21 a | ND | 8.28 ± 0.53 b | ND | 11.45 ± 0.42 b |
| 7 | 4.34 ± 0.28 c | 454.92 ± 24.75 bc | ND | 88.25 ± 0.09 de | ND | 3.95 ± 0.21 de | ND | 3.48 ± 0.39 e | ND | 4.03 ± 0.39 f |
| 8 | 6.10 ± 0.34 b | 422.13 ± 14.14 cd | ND | 98.63 ± 5.48 bc | ND | 7.48 ± 0.18 c | ND | 16.20 ± 0.21 a | ND | 15.23 ± 0.81 a |
| 9 | 8.02 ± 0.94 a | 381.15 ± 28.28 de | ND | 95.63 ± 5.83 cd | ND | 2.85 ± 0.21 f | ND | 5.80 ± 0.49 d | ND | 4.83 ± 0.32 f |
| 10 | ND | 428.28 ± 11.59 c | ND | 84.50 ± 8.13 e | ND | 2.78 ± 0.11 f | ND | 6.63 ± 0.46 c | ND | 6.68 ± 0.04 e |
| Condition | AαC | DMIP | Harman | Norharman | Total | |
|---|---|---|---|---|---|---|
| 160 °C | 10 min | ND | ND | ND | ND | ND |
| 20 min | ND | ND | 1.05 ± 0.07 b | 1.88 ± 0.05 b | 2.93 ± 0.12 b | |
| 25 min | ND | ND | 1.20 ± 0.14 b | 2.14 ± 0.19 b | 3.34 ± 0.33 b | |
| 30 min | ND | 0.82 ± 0.07 | 2.13 ± 0.11 a | 3.93 ± 0.12 a | 6.87 ± 0.30 a | |
| 180 °C | 10 min | ND | 0.44 ± 0.00 c | 0.65 ± 0.07 d | 2.79 ± 0.25 d | 4.58 ± 0.32 d |
| 20 min | ND | 1.10 ± 0.08 b | 2.35 ± 0.21 c | 3.75 ± 0.12 c | 7.20 ± 0.25 c | |
| 25 min | ND | 2.61 ± 0.00 a | 4.35 ± 0.07 b | 6.02 ± 0.62 b | 12.98 ± 0.69 b | |
| 30 min | ND | 3.70 ± 0.31 a | 7.60 ± 0.71 a | 9.60 ± 0.00 a | 20.90 ± 1.01 a | |
| 200 °C | 10 min | ND | 0.47 ± 0.05 d | 1.35 ± 0.07 d | 1.57 ± 0.00 d | 2.69 ± 0.12 d |
| 20 min | ND | 2.69 ± 0.10 c | 5.36 ± 0.49 c | 6.98 ± 0.25 c | 15.01 ± 0.64 c | |
| 25 min | ND | 5.00 ± 0.31 b | 10.20 ± 0.14 b | 19.37 ± 0.49 b | 34.57 ± 0.94 b | |
| 30 min | 0.80 ± 0.07 | 8.41 ± 0.62 a | 33.95 ± 2.76 a | 83.76 ± 7.90 a | 126.92 ± 10.11 a | |
| Condition | AαC | DMIP | Harman | Norharman | Total | |
|---|---|---|---|---|---|---|
| 160 °C | 10 min | ND | ND | ND | ND | ND |
| 20 min | ND | ND | ND | ND | ND | |
| 25 min | ND | ND | 0.60 ± 0.07 | 0.63 ± 0.04 | 1.23 ± 0.04 | |
| 30 min | ND | ND | 0.75 ± 0.07 | 0.80 ± 0.07 | 1.55 ± 0.00 | |
| 180 °C | 10 min | ND | ND | ND | ND | ND |
| 20 min | ND | ND | 0.75 ± 0.00 c | 0.90 ± 0.07 c | 1.65 ± 0.07 c | |
| 25 min | ND | 0.95 ± 0.07 | 2.18 ± 0.18 b | 1.65 ± 0.07 b | 4.78 ± 0.18 b | |
| 30 min | ND | 2.78 ± 0.11 | 6.63 ± 0.46 a | 6.68 ± 0.04 a | 16.08 ± 0.53 a | |
| 200 °C | 10 min | ND | ND | ND | ND | ND |
| 20 min | ND | 1.35 ± 0.07 c | 3.23 ± 0.11 c | 3.05 ± 0.14 c | 7.63 ± 0.00 c | |
| 25 min | ND | 3.75 ± 0.35 b | 7.95 ± 0.28 b | 12.35 ± 0.71 b | 24.05 ± 1.34 b | |
| 30 min | 0.55 ± 0.00 | 4.78 ± 0.25 a | 20.63 ± 1.17 a | 45.95 ± 1.56 a | 71.90 ± 2.97 a | |
| Analytes | Precursor Ion (m/z) | Production Ion (m/z) | CE (v) | DP (v) | Dwell Time (ms) |
|---|---|---|---|---|---|
| CML | 205.0 | 130.0 d | 56 | 17 | 50 |
| 84.0 | 56 | 25 | 50 | ||
| CEL | 219.0 | 130.0 | 67 | 18 | 50 |
| 84.0 | 66 | 29 | 50 | ||
| CML-d4 | 209.0 | 134.0 | 50 | 19 | 50 |
| 88.0 | 70 | 29 | 50 | ||
| CEL-d4 | 223.0 | 134.0 | 70 | 19 | 50 |
| 88.0 | 70 | 29 | 50 | ||
| AαC | 184.0 | 167.2 | 108 | 32 | 30 |
| 140.0 | 108 | 32 | 30 | ||
| DMIP | 162.9 | 147.3 | 90 | 45 | 30 |
| 105.0 | 90 | 45 | 30 | ||
| 4,7,8-TriMeIQx | 242.0 | 227.1 | 120 | 40 | 30 |
| 145.0 | 120 | 50 | 30 | ||
| Harman | 183.0 | 115.0 | 120 | 50 | 30 |
| 168.3 | 120 | 40 | 30 | ||
| Norharman | 169.2 | 115.0 | 100 | 45 | 30 |
| 142.0 | 100 | 40 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Yu, X.; Shi, L.; Liu, W. Comprehensive Analyses of Advanced Glycation end Products and Heterocyclic Amines in Peanuts during the Roasting Process. Molecules 2023, 28, 7012. https://doi.org/10.3390/molecules28207012
Yu J, Yu X, Shi L, Liu W. Comprehensive Analyses of Advanced Glycation end Products and Heterocyclic Amines in Peanuts during the Roasting Process. Molecules. 2023; 28(20):7012. https://doi.org/10.3390/molecules28207012
Chicago/Turabian StyleYu, Jingjing, Xiaohui Yu, Lili Shi, and Wei Liu. 2023. "Comprehensive Analyses of Advanced Glycation end Products and Heterocyclic Amines in Peanuts during the Roasting Process" Molecules 28, no. 20: 7012. https://doi.org/10.3390/molecules28207012
APA StyleYu, J., Yu, X., Shi, L., & Liu, W. (2023). Comprehensive Analyses of Advanced Glycation end Products and Heterocyclic Amines in Peanuts during the Roasting Process. Molecules, 28(20), 7012. https://doi.org/10.3390/molecules28207012






