Abstract
Annual ryegrass toxicity (ARGT) is an often-fatal poisoning of livestock that consume annual ryegrass infected by the bacterium Rathayibacter toxicus. This bacterium is carried into the ryegrass by a nematode, Anguina funesta, and produces toxins within seed galls that develop during the flowering to seed maturity stages of the plant. The actual mechanism of biochemical transformation of healthy seeds to nematode and bacterial gall-infected seeds remains unclear and no clear-cut information is available on what type of volatile organic compounds accumulate in the respective galls. Therefore, to fill this research gap, the present study was designed to analyze the chemical differences among nematode galls (A. funesta), bacterial galls (R. toxicus) and healthy seeds of annual ryegrass (Lolium rigidum) by using direct immersion solid-phase microextraction (DI-SPME) coupled with gas chromatography–mass spectrometry (GC-MS). The method was optimized and validated by testing its linearity, sensitivity, and reproducibility. Fifty-seven compounds were identified from all three sources (nematode galls, bacterial galls and healthy seed), and 48 compounds were found to be present at significantly different (p < 0.05) levels in the three groups. Five volatile organic compounds (hexanedioic acid, bis(2-ethylhexyl) ester), (carbonic acid, but-2-yn-1-yl eicosyl ester), (fumaric acid, 2-ethylhexyl tridec-2-yn-1-yl ester), (oct-3-enoylamide, N-methyl-N-undecyl) and hexacosanoic acid are the most frequent indicators of R. toxicus bacterial infection in ryegrass, whereas the presence of 15-methylnonacosane, 13-methylheptacosane, ethyl hexacosyl ether, heptacosyl acetate and heptacosyl trifluoroacetate indicates A. funesta nematode infestation. Metabolites occurring in both bacterial and nematode galls included batilol (stearyl monoglyceride) and 9-octadecenoic acid (Z)-, tetradecyl ester. Among the chemical functional group, esters, fatty acids, and alcohols together contributed more than 70% in healthy seed, whereas this contribution was 61% and 58% in nematode and bacterial galls, respectively. This study demonstrated that DI-SPME is a valid technique to study differentially expressed metabolites in infected and healthy ryegrass seed and may help provide better understanding of the biochemical interactions between plant and pathogen to aid in management of ARGT.
Keywords:
annual ryegrass; ARGT; bacteria; DI-SPME; galls; GC-MS; metabolites; nematode; toxicity ryegrass 1. Introduction
Ryegrass is an important pasture and forage plant that is widely cultivated around the world to feed grazing livestock [1,2]. It is highly palatable, protein-rich, and has high productivity and excellent nutritional value [3]. Two types of ryegrasses are routinely cultivated for this purpose: annual ryegrass and perennial ryegrass. Perennial ryegrass (Lolium perenne) is densely tufted, multi-tillered and grows fastest in autumn and spring, whereas annual ryegrass species (Lolium rigidum, L. multiflorum) grow in the winter–spring period [4]. In future, annual ryegrass may replace fodder oat and other forage crops, because of their longer growing period and improved feed quality [5]. A constraint on annual ryegrass adoption is annual ryegrass toxicity (ARGT), present in some regions of South Australia and Western Australia [6]. This fatal disease is caused by association of the nematode Anguina funesta and the bacteria Rathayibacter toxicus (formerly known as Clavibacter toxicus) [7,8]. Plant infestation may result in the formation of two types of galls—one by the nematode and the other by the bacteria—during the period between flowering and seed maturity. The toxin is produced in bacterial galls. Ryegrass containing bacterial galls remains toxic even when senescent and dried off, so hay made from infected ryegrass is also toxic. All grazing animals are susceptible, including horses and pigs [9]. The effects of toxic galls on livestock have been reported in South Africa [10] and Japan [11], and seed galls obtained from New Zealand were positive for the presence of the R. toxicus antigen [12], but it is most serious in Australia, where it has been responsible for the deaths of hundreds of thousands of sheep and cattle [6,13]. In Western Australia, a 2004 report highlighted annual wool production was reduced by 3% because of ARGT [6].
The association of the nematode and bacterium that causes ARGT is widely studied [7,14,15,16,17]. A bacterial inhibition assay was developed to detect toxicity [18,19]. High-performance liquid chromatography (HPLC)-based analytical methods detected corynetoxins [20]. An enzyme-linked immunosorbent assay (ELISA) is widely used to detect water-soluble antigens specific to R. toxicus [21]. Polymerase chain reaction (PCR) in combination with corynetoxin ELISA and real-time PCR is used to detect the presence of R. toxicus and its associated phage [22,23,24]. These assays are used to predict disease occurrence. However, no one method meets all the regulatory requirements [25]. All the above methods focus on the bacteria and none on the nematode, even though the nematode plays an important role in carrying the bacterium into the plant. Therefore, to understand the chemical interaction between nematode and bacteria and the ryegrass plant, information on chemicals present is required from both the bacterial and nematode galls. In this study, direct immersion solid-phase microfiber extraction (DI-SPME) coupled with gas chromatography (GC)–mass spectrometry (MS) (GC-MS) was used to study the chemical profile of bacterial and nematode galls. The advantages of this method include rapid sample preparation, and it is an efficient method to detect and separate analytes. DI-SPME is usually coupled with GC, GC–MS, HPLC or LC–MS for accurate analysis. DI-SPME has advantages over other methods of low solvent use and disposal costs, and improved limits of detection. However, one of the drawbacks of DI-SPME is associated with its handling, such as bending, breaking and stripping of coating that can decrease its life span and that can be overcome by using HS-SPME to a certain limit [26]. DI-SPME has been used in a wide range of applications, including identification of biomarkers, food safety and quality control, food analysis, the pharmaceutical industry, process monitoring, and forensics [27,28,29,30].
This study applied DI-SPME coupled with GC-MS to gain information on metabolic profiles associated with nematode and bacterial galls in annual ryegrass to better understand the biochemical interactions between the nematode, bacterium, and plant. This research aims to provide information for the development of detection assays and improve management of disease.
2. Results
2.1. Identification of Metabolites
Fifty-seven compounds were detected from the three treatment sources: 30 from nematode galls, 25 from bacterial galls and 24 from healthy seed (Table 1). All the annual ryegrass material was collected from a farmer’s field at Bindoon, Western Australia in 2020, and nematode and bacterial galls were separated for analysis. Forty-eight compounds were found to be significantly different (p < 0.05) upon one-way ANOVA and post hoc analysis (Fisher’s LSD) (Figure 1). Some compounds were common to all three treatment sources, while others were differentially expressed in one or two treatment sources (Figure 2). The compounds bicyclo[2.2.1]heptane-2-carboxylic acid, 3,3-dimethyl-, methyl ester; 7-methyl-1-naphthol; 2,4-di-tert-butylphenol; 4-(2-(acryloyloxy)ethoxy)-4-oxobutanoic acid, TMS; carbonic acid, eicosyl vinyl ester; 4′,6′-dimethoxy-2′-hydroxychalcone, and 2-methylpropionate were common to all three treatments, with two compounds, batilol and 9-octadecenoic acid (Z)-, tetradecyl ester, expressed in both the nematode and the bacterial galls. Seven compounds—2-cyclohexen-1-ol; (1R)-1-(2,6-dichloro-3-fluorophenyl) ethanol, methyl ether; n-hexadecanoic acid; pent-4-enoyl amide, 2-methyl-N-dodecyl-; nonadecane, 1-chloro-; 2,4-dihydroxyheptadecyl acetate; and isopropyl hexacosyl ether—were expressed in healthy seed and bacterial galls, but not in nematode galls, and one compound (cis-10-nonadecenoic acid) was expressed in nematode galls and healthy seed, but not in bacterial galls. There were 21, 10 and 10 unique compounds expressed in nematode galls, bacterial galls, and healthy seed, respectively. In nematode galls, some important compounds were found in large quantities compared to others, suggesting a direct connection to the presence of nematodes, i.e., octadecenoic acid; nonadecanoic acid; octadecynoic acid, methyl ester; carbonic acid, octadecyl vinyl ester; fumaric acid, pent-4-en-2-yl tridecyl ester; ethyl hexacosyl ether; 15-methylnonacosane; 13-methylheptacosane; heptacosyl acetate; dotriacontane, 2-methyl-; propyl tetracosyl ether; 1-docosanol, acetate and fumaric acid, hexadecyl 4-heptyl ester were found at significant levels. The compounds hexanedioic acid, bis(2-ethylhexyl) ester; carbonic acid, but-2-yn-1-yl eicosyl ester; hexacosanoic acid; oct-3-enoylamide, N-methyl-N-undecyl-; estra-1,3,5(10)-trien-17β-ol and tetradecanoic acid were present only in bacterial galls. In healthy seed, the most statistically significant (p < 0.05) compounds were hexanedioic acid, dioctyl ester; pent-4-enoyl amide, 2-methyl-N-tetradecyl-; 9-hexadecen-1-ol, (Z)- 2-phosphonopropanoic acid, 3TMS; hexadecyl acrylate. The representative chromatogram and mass spectra of each group are presented in the Supplementary Material (Figures S1–S3).

Table 1.
Significant compounds’ peak areas (one unit corresponds to 104 area) quantified in nematode galls, bacterial galls and healthy seeds of annual ryegrass seed using DI-SPME GC-MS.
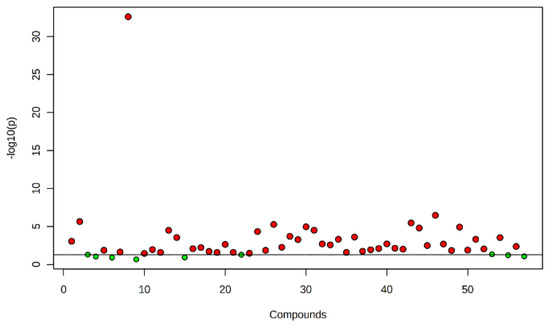
Figure 1.
Metabolites obtained in nematode gall, bacterial gall and healthy seed of annual ryegrass. The points highlighted in red are significant compounds selected based on the p-value threshold (0.05), and green dots represent nonsignificant compounds.
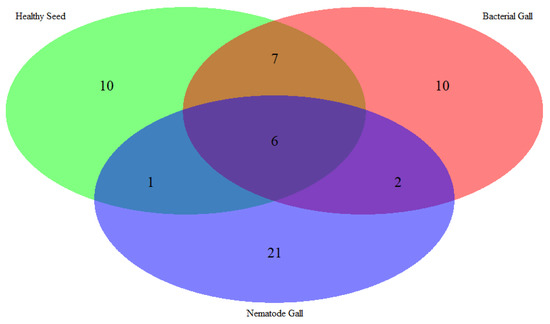
Figure 2.
Venn diagram of the number of metabolites expressed in nematode gall (Anguina funesta), bacterial gall (Rathayibacter toxicus), and healthy seed of ryegrass (Lolium rigidum).
2.2. Multivariate Analysis to Classify Nematode Galls, Bacterial Galls and Healthy Seeds
Distribution of compounds in the three treatment sources was analyzed using principal component analysis (PCA) (Figure 3) and a hierarchical cluster analysis heat map (Figure 4). PCA is an unsupervised method that helps to visualize covariance and correlation among the three treatment sources and metabolites expressed in each.
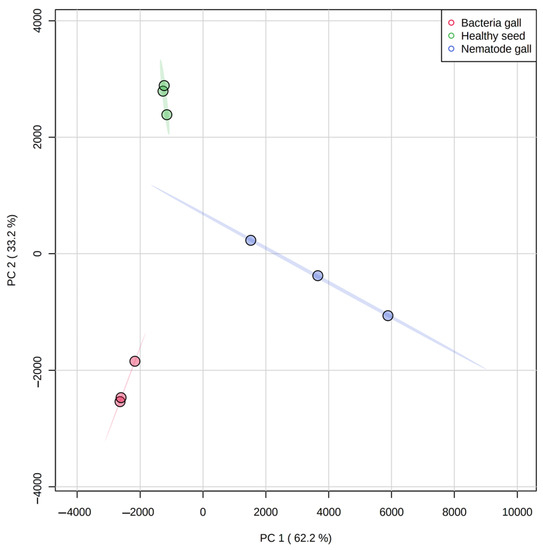
Figure 3.
Principal component analysis (PCA) score plot for separation of metabolites in nematode gall (Anguina funesta), bacterial gall (Rathayibacter toxicus) and healthy seed of ryegrass (Lolium rigidum). The variances (PC1 and PC2) are shown in brackets. The three symbols for each treatment represent three biological replicates.
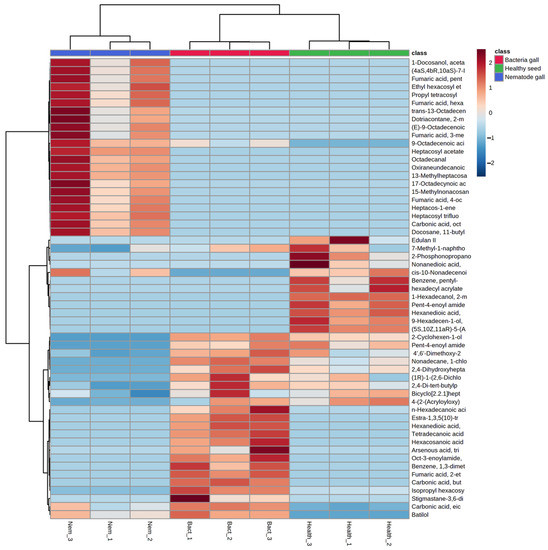
Figure 4.
Heat map of metabolites obtained from nematode gall and bacteria gall in comparison to healthy seed of ryegrass. The three similarly colored columns represent biological replicates of each treatment. Red indicates Z-scores > 0 and blue indicates Z-scores < 0. The saturation threshold is set at ±2 (Z-score −2 to +2 representing low to high values).
There is clustering of metabolites from the three replicates of each treatment source, and the three treatment ellipses do not overlap (Figure 3). This separation indicates that the three treatment sources have different chemical profiling, and this profiling can be used as a marker to detect nematode and bacterial infection in annual ryegrass. The first principal component explains 62.2% of the variance, whereas the second principal component explains 33.2%. Similarly, Figure 4 shows the cluster and heat map of all three treatment sources used in this study. The heat map shows a clear expression level difference between healthy seed, nematode and bacterial galls.
Further, to know the differential compounds between the individual groups, supervised analysis was conducted. Partial least squares-discriminant analysis (PLS-DA) and orthogonal PLS-DA (OPLS-DA) score plots were drawn between nematode gall versus healthy seed and bacterial gall versus healthy seed (Figure 5). The compounds 2-cyclohexen-1-ol and 1-hexadecanol, 2-methyl clearly distinguish healthy seeds from nematode galls by showing their abundance in healthy seeds, whereas due to the abundant nature of carbonic acid, but-2-yn-1-yl eicosyl ester and fumaric acid, 2-ethylhexyl tridec-2-yn-1-yl ester in the bacterial gall, they were separated from the healthy seeds.
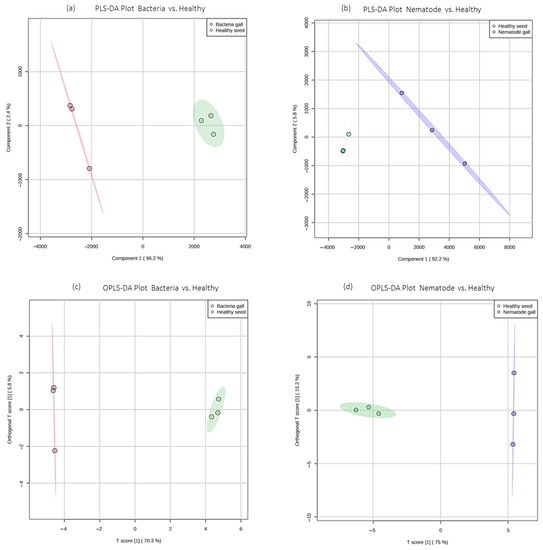
Figure 5.
Partial least squares-discriminant analysis (PLS-DA) and orthogonal PLS-DA (OPLS-DA) score plots of ryegrass samples. (a,b) PLS-DA score plots for bacteria gall versus healthy seed and nematode gall versus healthy seed, respectively; (c,d) OPLS-DA score plots for bacteria gall versus healthy seed and nematode gall versus healthy seed, respectively. Red represents bacterial group, blue represents nematode group, and green represents healthy seeds for bacteria gall versus healthy seed and nematode gall versus healthy seed, respectively.
Chemical functional groups differed among the three. Esters, fatty acids, and alcohols were the dominant groups in healthy seed, representing 34%, 17%, and 13% of the total number of compounds, respectively, followed by amides, ethers, hydrocarbons, and phenols, each at 8%, and heterocyclic compounds at 4% (Figure 6). In nematode galls, the proportions of esters, hydrocarbons, and acids were 48%, 20%, and 10%, respectively, followed by ethers and phenols, each at 7%, and alcohols and aldehydes, each at 3%. In bacterial galls, the proportions of esters, acids, alcohols, hydrocarbons were 36%, 16%, 12%, and 12%, respectively, followed by amides, ethers, and phenols, with 8% in each group. Interestingly, no amides and only the aldehydes were present in the nematode galls, and heterocyclic compounds were found only in healthy seed. In addition to this, esters were highly expressed in each treatment, and the fraction of hydrocarbons was greater in infected seed than healthy seed.
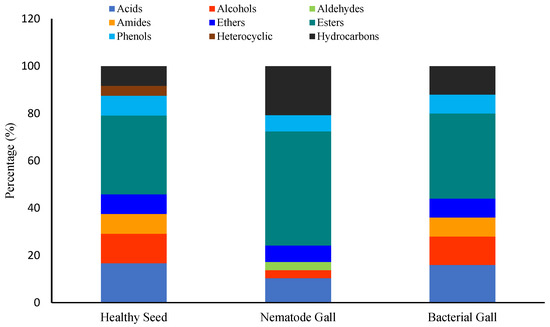
Figure 6.
Ratios of chemical groups expressed in each treatment. Percentage is the ratio of the number of compounds of each group to the total number of compounds.
3. Discussion
To optimize the DI-SPME extraction protocol, the fiber was exposed at room temperature (25 ± 2 °C) for the analyte extraction for different times (30, 45, 60 and 120 min) and desorption time (3 min, 6 min and 10 min). The optimum extraction time, 60 min, and desorption time, 6 min, were optimized based on the number of peaks and peak areas. Similarly conditioned optimization parameters for SPME extraction were reported for VOC detection in barley [31].
Nontargeted metabolite analysis revealed relative expression levels of compounds present in the sample. This enables identification of the prominent compounds in each treatment, information that can be used to develop diagnostic assays and inform breeding efforts. Bacterial gall samples expressed 2,4-dihydroxyheptadecyl acetate derivatives, and this has been described previously [32,33]. Fatty acids play an important role in bacterial lipid membranes, and it has previously been reported that the presence of C-15 saturated branched chain acid, pentadecanoic acid, C-17 saturated branched chain acids and normal saturated fatty acids, such as lauric, myristic, stearic and arachidic, are major components of cell membranes in Rathayibacter species. [34,35,36]. Here, the closely related fatty acids tetradecanoic acid (myristic), hexadecenoic acid, and hexacosanoic acid were identified in bacterial galls. The presence of oct-3-enoyl amide, N-methyl-N-undecyl-, a structural derivative of an alpha-amino acid, and may be responsible in biological pathways for producing 2,4-diaminobutyric acid, which is a characteristic of R. toxicus [37]. Maulidia et al. [38] reported steroidal compounds as the major component in methanolic extracts of Gram-positive Bacillus thuringiensis associated with root knot nematodes, and similarly, this study shows the presence of stigmastane-3,6-dione (5α), which is classified as a steroid compound, in Gram-positive R. toxicus bacterial galls. Fumaric acid is an important intermediate of the tricarboxylic acid (TCA) cycle in plant systems [39]. Expression of fumaric acid, 2-ethylhexyl tridec-2-yn-1-yl ester, in the bacterial galls suggests that this bacterium could be interfering with the host TCA cycle.
There is no published information available on volatile organic compounds generated in nematode-infested cereal seed galls. However, there are reports that showed the protein content is reduced during nematode infestation in ear cockle disease of wheat [40]. Similarly, compounds containing amide groups, such as pent-4-enoyl amide, 2-methyl-N-dodecyl-, pent-4-enoyl amide, 2-methyl-N-tetradecyl- and oct-3-enoylamide, and N-methyl-N-undecyl- were downregulated in nematode gall. Compounds expressed by soybean cyst nematode and analyzed with GC-MS were methyl ester derivatives, a component of nematode pheromones [41]. In our study, the presence of the derivatives of methyl, ethyl and vinyl esters in the nematode galls could indicate the presence of pheromones associated with A. funesta. Nematodes are also known to contain significant quantities of lipids in their body cuticle [42], and fatty acids and fatty aldehydes are major components of the nematode cuticle [43]. Phospholipids play an important role in the formation of nematode cellular membranes and in signal transduction pathways [44]. In our results, the presence of octadecanal, which is a phospholipid derivative [45], could be a potential marker to identify A. funesta. Wei et al. [46] mentioned the role of 17-octadecynoic acid during synthesis of phospholipids in Caenorhabditis elegans, suggesting that 17-octadecynoic acid, methyl ester and (E)-9-octadecenoic acid ethyl ester may play a role in the development of the nematode in the nematode gall. Among the fatty acids, the presence of saturated fatty palmitic (16:0) and stearic (18:0) acids were reported from the parasitic nematode Ascaridia galli [43], whereas we found the unsaturated fatty octadecenoic acid (18:1) and nonadecanoic acid (19:1), which may indicate different requirements for fatty acids in A. funesta.
The high concentration of phenolic compounds in healthy seeds is noteworthy. Hexanedioic acid, dioctyl ester and 9-hexadecen-1-ol, (Z) are considered as bioactive phytochemicals and are known nematicides [47,48].
4. Materials and Methods
4.1. Collection of Infected Plant Materials
Infected annual ryegrass samples were collected in December 2020 from a farmer’s field at Bindoon, Western Australia (31°21′2″ S 116°7′47″ E). The ryegrass seeds along with galls were separated using sieves (1.4 mm, 1.70 mm and 850 µm) by manual threshing in the laboratory. After separation, the bacterial galls, nematode galls and healthy seeds were physically identified and differentiated using a light box [9]. All three were presorted based on their shape and color under the light box (Figure 7). The nematode gall appears dark brown to blackish with a pointed end, bacterial gall looks yellow in color with a piercing end, whereas healthy seed is denser and exhibits normal curvature, as shown in Figure 7.
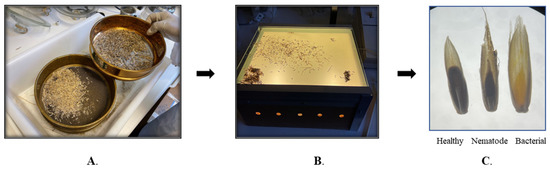
Figure 7.
(A) Separation of heathy seeds and those with galls from ryegrass husk using different size of sieves, (B) light box used for identification and differentiation between infected galls and healthy seeds, (C) healthy seeds, nematode gall, and bacterial gall.
4.2. Sample Preparation and Extraction Using DI-SPME
Twenty-five milligrams of each treatment source of plant sample (10–15 seeds or galls without removal of lamella or the gall walls) was transferred into 2 mL microtube containing 1 mL of HPLC grade acetonitrile. Three separate samples were prepared for each source. The microtube was sealed with a screw cap and shaken in a bedbug homogenizer (Benchmark Scientific, Shanghai, China), using two milling balls for 2 min at 4000 RCF, then another 1mL of acetonitrile was added, shaken for 2 min, and then centrifuged at 4000 RCF for 3 min using a minicentrifuge (Dynamica Velocity 13µ, Techcomp Europe, Livingston, UK). After that, the supernatant (~1.5 mL) was collected in a 2 mL GC vial with septum and stored at 4 °C until further use.
For conducting DI-SPME, a three-phase fiber (50/30 µm DVB/CAR/PDMS, Stableflex 2 cm, Supelco®, Bellefonte, PA, USA) was used. Three replicate sets of each sample were exposed to three different fibers. All new fibers were preconditioned for 60 min at 270 °C as per the manufacturer’s instructions. The SPME fiber was directly immersed by inserting into the GC vial containing the sample for 60 min at room temperature (25 ± 2 °C), and thereafter the fiber was taken out and injected directly into the GC-MS instrument with desorption for 6 min at inlet temperature 290 °C to analyze the sample.
4.3. GC-MS Conditions
The compounds were analyzed using a GC-MS 7890B coupled with a 5977B MSD mass spectrometer based on Agilent Technologies, Santa Clara, CA, USA with an Agilent HP-5MS column (30 m length, 0.25 mm internal diameter, 0.25 µm film thickness with 5% phenyl, and 95% dimethylpolysiloxane stationary phase). The following analytical conditions were used: splitless mode with helium as a carrier gas with a flow of 1 mL/min. The GC conditions were adopted from a previously used method [49], with slight modifications in temperature programming, starting at 50 °C with 5 min hold, then ramped at 6 °C/min to 90 °C, 8 °C/min to 140 °C, 6 °C/min to 190 °C, 4 °C/min to 240 °C, finally to 50 °C/min to 300 °C with hold at 300 °C for 12 min. The detector was operated in electron impact (EI) ionization mode at 70 eV and the spectra were acquired from 3 scan/s in a range from 50 to 550 atomic mass units (amu). The transfer line temperature of the MSD was 280 °C, and the ion source temperature was 230 °C with total run time of 51.95 min.
4.4. Data Processing and Statistical Analysis
For the identification of compounds, the National Institute of Standards and Technology Mass Spectrometry (NIST MS) was used with Kovat’s retention index (RI) of published data. The calculation for Kovat’s index is provided in the supplementary file (Table S1). The data were processed with Agilent Mass Hunter Qualitative Analysis software (Version B.08.00, Build 8.0.8208.0, Santa Clara, CA, USA) [50]. The n-alkane (C7−C40, catalogue number 49451-U; Castle Hill, NSW, Australia) with concentration 100 µg/mL was used as the external standard. Microsoft Excel 2021 was used for data arrangement and other data processing. To perform multivariate analysis and principal component analysis (PCA) with one-way analysis of variance (ANOVA), MetaboAnalyst 5.0 (2022) (https://www.metaboanalyst.ca/MetaboAnalyst/upload/StatUploadView.xhtml; accessed on 20 June 2022) was used. R was used to draw the diagrams. Tukey’s post hoc test (HSD) was performed for statistical differences with p < 0.05).
5. Conclusions
Direct immersion solid-phase microextraction using a three-phase fiber was employed to collect volatile organic compounds from acetonitrile extract from nematode galls, bacterial galls, and healthy ryegrass seed, followed by analysis in GC-MS. The GC-MS analytical data showed a wide spectrum of metabolites in the three treatment sources. For the first time, this study has successfully identified chemical compounds that differ between nematode galls, bacterial galls, and healthy ryegrass seed. These three treatment sources could be distinguished based on their chemical signatures and could form the basis for development of new analytical tools for determining the risk of ARGT occurrence from feedstuff samples.
Supplementary Materials
The following supporting information can be download at: https://www.mdpi.com/article/10.3390/molecules28020828/s1.
Author Contributions
Conceptualization: P.K., S.J.M., Y.R. and M.A.; data curation: P.K.; methodology: P.K., Y.R., M.A., D.K. and X.D.; resources: P.K., Y.R., D.K., S.M. and X.D.; supervision: S.J.M., Y.R., M.A. and D.K.; validation: P.K., Y.R., S.J.M. and S.M.; writing—original draft: P.K.; writing—review and editing: P.K., S.J.M., Y.R., M.A., D.K., S.M. and X.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding except sponsorship of the first author (as below).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article.
Acknowledgments
We would like to thank the Indian government and Indian Council of Agricultural Research (ICAR) for supporting the first author and sponsoring a PhD fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hegarty, M.; Yadav, R.; Lee, M.; Armstead, I.; Sanderson, R.; Scollan, N.; Powell, W.; Skøt, L. Genotyping by RAD sequencing enables mapping of fatty acid composition traits in perennial ryegrass (L olium perenne (L.)). Plant Biotechnol. J. 2013, 11, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Restrepo, C.A.; Waghorn, G.C.; Gillespie, H.; Clark, H. Partition of dietary energy by sheep fed fresh ryegrass (Lolium perenne) with a wide-ranging composition and quality. Anim. Prod. Sci. 2019, 60, 1008–1017. [Google Scholar] [CrossRef]
- Cassol, L.C.; Piva, J.T.; Soares, A.B.; Assmann, A.L. Yield and structural composition of oat and ryegrass subjected to different periods of cutting and nitrogen fertilization. Rev. Ceres 2011, 58, 438–443. [Google Scholar] [CrossRef]
- Warnke, S.E.; Barker, R.E.; Jung, G.; Sim, S.-C.; Mian, R.; Saha, M.C.; Brilman, L.A.; Dupal, M.P.; Forster, J.W. Genetic linkage mapping of an annual× perennial ryegrass population. Theor. Appl. Genet. 2004, 109, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Advanta Advanta Winter Forages. Available online: http://www.nutrifeed.in/ppt/Advanta%20-%20Winter%20Forages.pdf (accessed on 22 May 2021).
- Page, S. Review of Current Management Options and Research Needs; ARGT.001; Meat and Livestock Australia Ltd.: Sydney, Australia, 2004. [Google Scholar]
- Price, P.C. Investigation of a Nematode-Bacterium Disease Complex Affecting Wimmera Ryegrass/by PC Price. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 1973. [Google Scholar]
- Fisher, J.M.; Dube, A.J.; Watson, C.M. Distribution in South Australia of Anguina funesta, the nematode associated with annual ryegrass toxicity. Aust. J. Exp. Agric. 1979, 19, 48–52. [Google Scholar] [CrossRef]
- Allen, J. Annual ryegrass toxicity–an animal disease caused by toxins produced by a bacterial plant pathogen. Microbiol. Aust. 2012, 33, 18–21. [Google Scholar] [CrossRef]
- Schneider, D.J. First report of annual ryegrass toxicity in the Republic of South Africa. Onderstepoort. J. Vet. Res. 1981, 48, 251–255. [Google Scholar]
- Elson, R. Code of Practice for Minimisation of the Risk of Corynetoxins Contamination of Hay and Straw for Export; Department of Agriculture, Fisheries and Forestry—Australia and Australian Quarantine and Inspection Service: Canberra, Australia, 2002.
- Anderton, N.; Beales, K.A.; Cao, Y.; Colegate, S.M.; Edgar, J.A.; Michalewicz, A.; Riley, I.T.; Stewart, P.L.; Than, K.A. The identification of corynetoxin-like tunicaminyluracil-glycolipids from nematode galls in Festuca nigrescens from North America and New Zealand. In Poisonous Plants Related Toxins; Acamovic, T., Stewart, C., Pennycott, T., Eds.; CABI Publishing: Wallingford, UK, 2004; pp. 204–209. [Google Scholar]
- Allen, J.G. Annual Ryegrss Toxicity; El Caballo: Northam, Australia, 2002; pp. 1–11. [Google Scholar]
- Lanigan, G.W.; Payne, A.L.; Frahn, J.L. Letter: Origin of toxicity in parasitised annual ryegrass (Lolium rigidum). Aust. Vet. J. 1976, 52, 244–246. [Google Scholar] [CrossRef]
- Bird, A.F. The Anguina-Corynebacterlum Association. Plant Parasit. Nematodes 1981, 3, 303–323. [Google Scholar]
- Riley, I.T.; Schmitz, A.; de Silva, P. Anguina australis, a vector for Rathayibacter toxicus in Ehrharta longiflora. Australas. Plant Pathol. 2001, 30, 171–175. [Google Scholar]
- Yasuhara-Bell, J.; Stack, J.P. Panel of three loop-mediated isothermal amplification assays differentiates Rathayibacter toxicus populations RT-I, RT-II, RT-III, RT-IV and RT-V. J. Plant Pathol. 2019, 101, 707–717. [Google Scholar] [CrossRef]
- Stynes, B.A.; Vogel, P. A bacterial inhibition assay for corynetoxins from parasitized annual ryegrass. Aust. J. Agric. Res. 1983, 34, 483–489. [Google Scholar] [CrossRef]
- Riley, I.T.; Ophel, K.M. Rapid detection of corynetoxins produced by Clavibacter toxicum. Lett. Appl. Microbiol. 1992, 14, 96–99. [Google Scholar] [CrossRef]
- Cockrum, P.A.; Edgar, J.A. Rapid estimation of corynetoxins in bacterial galls from annual ryegrass (Lolium rigidum Gaudin) by high-performance liquid chromatography. Aust. J. Agric. Res. 1985, 36, 35–41. [Google Scholar] [CrossRef]
- Masters, A.M.; Gregory, A.R.; Evans, R.J.; Speijers, J.E.; Sutherland, S.S. An enzyme-linked immunosorbent assay for the detection of Rathayibacter toxicus, the bacterium involved in annual ryegrass toxicity, in hay. Aust. J. Agric. Res. 2006, 57, 731–742. [Google Scholar] [CrossRef][Green Version]
- Kowalski, M.C.; Cahill, D.; Doran, T.J.; Colegate, S.M. Development and application of polymerase chain reaction-based assays for Rathayibacter toxicus and a bacteriophage associated with annual ryegrass (Lolium rigidum) toxicity. Aust. J. Exp. Agric. 2007, 47, 177–183. [Google Scholar] [CrossRef]
- Arif, M.; Busot, G.Y.; Mann, R.; Rodoni, B.; Stack, J.P. Field-deployable recombinase polymerase amplification assay for specific, sensitive and rapid detection of the US select agent and toxigenic bacterium, Rathayibacter toxicus. Biology 2021, 10, 620. [Google Scholar] [CrossRef]
- Arif, M.; Busot, G.Y.; Mann, R.; Rodoni, B.; Stack, J.P. Multiple internal controls enhance reliability for PCR and real time PCR detection of Rathayibacter toxicus. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Colegate, S.M.; Gardner, D.R.; Lee, S.T. Plant-Associated Natural Food Toxins 23. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 753–783. [Google Scholar]
- Lorenzo-Parodi, N.; Kaziur, W.; Stojanović, N.; Jochmann, M.A.; Schmidt, T.C. Solventless microextraction techniques for water analysis. TrAC Trends Anal. Chem. 2019, 113, 321–331. [Google Scholar] [CrossRef]
- Mondello, L.; Costa, R.; Tranchida, P.Q.; Dugo, P.; Lo Presti, M.; Festa, S.; Fazio, A.; Dugo, G. Reliable characterization of coffee bean aroma profiles by automated headspace solid phase microextraction-gas chromatography-mass spectrometry with the support of a dual-filter mass spectra library. J. Sep. Sci. 2005, 28, 1101–1109. [Google Scholar] [CrossRef]
- Nerin, C.; Salafranca, J.; Aznar, M.; Batlle, R. Critical review on recent developments in solventless techniques for extraction of analytes. Anal. Bioanal. Chem. 2009, 393, 809–833. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, A.; Miekus, N.; Baczek, T. Application of solid-phase microextraction in current biomedical research. J. Sep. Sci. 2019, 42, 285–302. [Google Scholar] [CrossRef]
- Dong, X.; Sun, L.; Maker, G.; Ren, Y.; Yu, X. Ozone Treatment Increases the Release of VOC from Barley, Which Modifies Seed Germination. J. Agric. Food Chem. 2022, 70, 3127–3135. [Google Scholar] [CrossRef]
- Vogel, P.; Petterson, D.S.; Berry, P.H.; Frahn, J.L.; Anderton, N.; Cockrum, P.A.; Edgar, J.A.; Jago, M.V.; Lanigan, G.W.; Payne, A.L.; et al. Isolation of a group of glycolipid toxins from seedheads of annual ryegrass Lolium rigidum Gaud.) infected by Corynebacterium rathayi. Aust. J. Exp. Biol. Med. Sci. 1981, 59, 455–467. [Google Scholar] [CrossRef]
- Frahn, J.L.; Edgar, J.A.; Jones, A.J.; Cockrum, P.A.; Anderton, N.A.; Culvenor, C.C.J. Structure of the corynetoxins, metabolites of Corynebacterium rathayi responsible for toxicity of annual ryegrass (Lolium rigidum) pastures. Aust. J. Chem. 1984, 37, 165–182. [Google Scholar] [CrossRef]
- Moss, C.W.; Dowell, V.R., Jr.; Lewis, V.J.; Schekter, M.A. Cultural characteristics and fatty acid composition of Corynebacterium acnes. J. Bacteriol. 1967, 94, 1300–1305. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; Evtushenko, L.I.; Akimov, V.N.; Kalakoutskii, L.V. Rathayibacter gen. nov., including the species Rathayibacter rathayi comb. nov., Rathayibacter tritici comb. nov., Rathayibacter iranicus comb. nov., and six strains from annual grasses. Int. J. Syst. Evol. Microbiol. 1993, 43, 143–149. [Google Scholar] [CrossRef]
- Herebian, D.; Hanisch, B.; Marner, F.-J. Strategies for gathering structural information on unknown peaks in the GC/MS analysis of Corynebacterium glutamicum cell extracts. Metabolomics 2005, 1, 317–324. [Google Scholar] [CrossRef]
- Sasaki, J.; Chijimatsu, M.; Suzuki, K.-i. Taxonomic significance of 2, 4-diaminobutyric acid isomers in the cell wall peptidoglycan of actinomycetes and reclassification of Clavibacter toxicus as Rathayibacter toxicus comb. nov. Int. J. Syst. Evol. Microbiol. 1998, 48, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Maulidia, V.; Soesanto, L.; Syamsuddin, S.; Khairan, K.; Hamaguchi, T.; Hasegawa, K.; Sriwati, R. Secondary metabolites produced by endophytic bacteria against the Root-Knot Nematode (Meloidogyne sp.). Biodiversitas J. Biol. Divers. 2020, 21, 5270–5275. [Google Scholar] [CrossRef]
- Goldberg, I.; Rokem, J.S. Organic and fatty acid production, microbial. In Encyclopedia of Microbiology; Schaechter, M., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 421–442. [Google Scholar]
- Singh, V.K.; Devi, A.; Pathania, S.; Kumar, V.; Tripathi, D.K.; Sharma, S.; Chauhan, D.K.; Singh, V.K.; Zorba, V. Spectroscopic investigation of wheat grains (Triticum aestivum) infected by wheat seed gall nematodes (Anguina tritici). Biocatal. Agric. Biotechnol. 2017, 9, 58–66. [Google Scholar] [CrossRef]
- Jaffe, H.; Huettel, R.N.; Demilo, A.B.; Hayes, D.K.; Rebois, R.V. Isolation and identification of a compound from soybean cyst nematode, Heterodera glycines, with sex pheromone activity. J. Chem. Ecol. 1989, 15, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, D.J.; Krusberg, L.R. Diacyl, alkylacyl and alkenylacyl phospholipids of the nematode Turbatrix aceti. Comp. Biochem. Physiol. Part B Comp. Biochem. 1981, 69, 115–120. [Google Scholar] [CrossRef]
- Ghosh, A.; Kar, K.; Ghosh, D.; Dey, C.; Misra, K.K. Major lipid classes and their fatty acids in a parasitic nematode, Ascaridia galli. J. Parasit. Dis. 2010, 34, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Jez, J.M. Nematode phospholipid metabolism: An example of closing the genome–structure–function circle. Trends Parasitol. 2014, 30, 241–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gardner, H.K.; Huber, C.S.; Bourland, C.T.; Smith, M.C., Jr. Identification and quantitation of hexadecanal and octadecanal in broiler muscle phospholipids. Poult. Sci. 1972, 51, 1056–1058. [Google Scholar] [CrossRef]
- Wei, L.; Hu, F.; Shen, Y.; Chen, Z.; Yu, Y.; Lin, C.-C.; Wang, M.C.; Min, W. Live-cell imaging of alkyne-tagged small biomolecules by stimulated Raman scattering. Nat. Methods 2014, 11, 410–412. [Google Scholar] [CrossRef]
- Choe, S.; Jung, I. Growth inhibition of freshwater algae by ester compounds released from rotted plants. J. Ind. Eng. Chem. 2002, 8, 297–304. [Google Scholar]
- Lalthanpuii, P.B.; Lalchhandama, K. Chemical composition and broad-spectrum anthelmintic activity of a cultivar of toothache plant, Acmella oleracea, from Mizoram, India. Pharm. Biol. 2020, 58, 393–399. [Google Scholar] [CrossRef]
- Cairns, A.J.; Begley, P.; Sims, I.M. The structure of starch from seeds and leaves of the fructan-accumulating ryegrass, Lolium temulentum L. J. Plant Physiol. 2002, 159, 221–230. [Google Scholar] [CrossRef]
- Agilent. MassHunter Workstation Software Qualitative Analysis Navigator; B.08.00; Agilent Technologies: Santa Clara, CA, USA, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).