Metabolite Variation between Nematode and Bacterial Seed Galls in Comparison to Healthy Seeds of Ryegrass Using Direct Immersion Solid-Phase Microextraction (DI-SPME) Coupled with GC-MS
Abstract
1. Introduction
2. Results
2.1. Identification of Metabolites
2.2. Multivariate Analysis to Classify Nematode Galls, Bacterial Galls and Healthy Seeds
3. Discussion
4. Materials and Methods
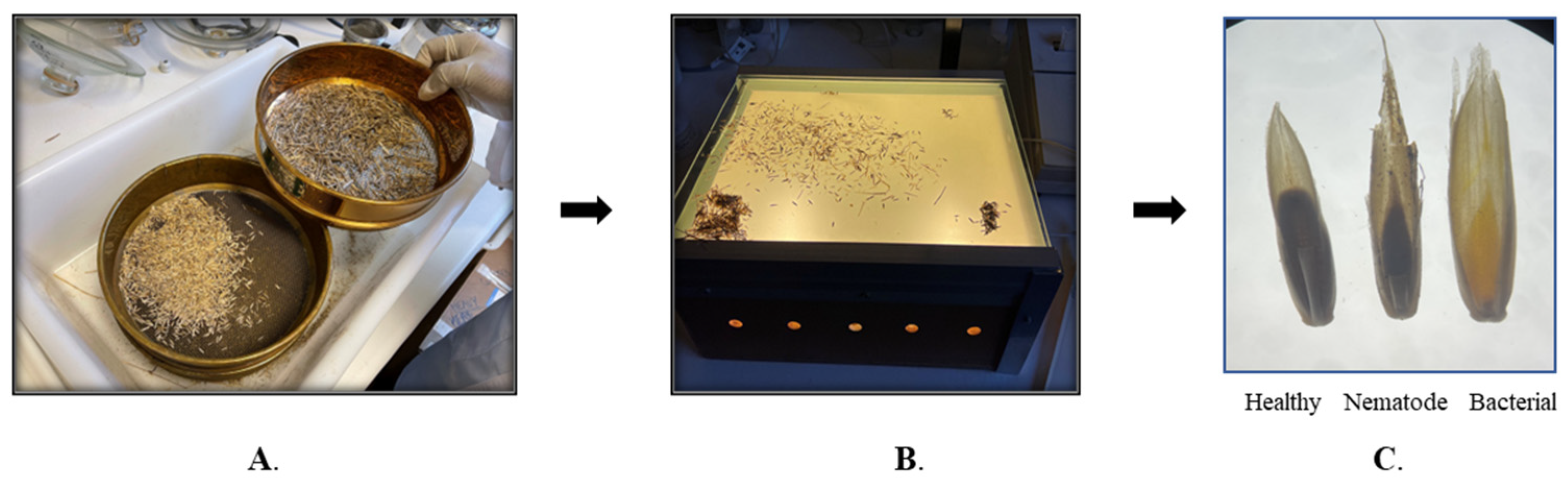
4.1. Collection of Infected Plant Materials
4.2. Sample Preparation and Extraction Using DI-SPME
4.3. GC-MS Conditions
4.4. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hegarty, M.; Yadav, R.; Lee, M.; Armstead, I.; Sanderson, R.; Scollan, N.; Powell, W.; Skøt, L. Genotyping by RAD sequencing enables mapping of fatty acid composition traits in perennial ryegrass (L olium perenne (L.)). Plant Biotechnol. J. 2013, 11, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Restrepo, C.A.; Waghorn, G.C.; Gillespie, H.; Clark, H. Partition of dietary energy by sheep fed fresh ryegrass (Lolium perenne) with a wide-ranging composition and quality. Anim. Prod. Sci. 2019, 60, 1008–1017. [Google Scholar] [CrossRef]
- Cassol, L.C.; Piva, J.T.; Soares, A.B.; Assmann, A.L. Yield and structural composition of oat and ryegrass subjected to different periods of cutting and nitrogen fertilization. Rev. Ceres 2011, 58, 438–443. [Google Scholar] [CrossRef]
- Warnke, S.E.; Barker, R.E.; Jung, G.; Sim, S.-C.; Mian, R.; Saha, M.C.; Brilman, L.A.; Dupal, M.P.; Forster, J.W. Genetic linkage mapping of an annual× perennial ryegrass population. Theor. Appl. Genet. 2004, 109, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Advanta Advanta Winter Forages. Available online: http://www.nutrifeed.in/ppt/Advanta%20-%20Winter%20Forages.pdf (accessed on 22 May 2021).
- Page, S. Review of Current Management Options and Research Needs; ARGT.001; Meat and Livestock Australia Ltd.: Sydney, Australia, 2004. [Google Scholar]
- Price, P.C. Investigation of a Nematode-Bacterium Disease Complex Affecting Wimmera Ryegrass/by PC Price. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 1973. [Google Scholar]
- Fisher, J.M.; Dube, A.J.; Watson, C.M. Distribution in South Australia of Anguina funesta, the nematode associated with annual ryegrass toxicity. Aust. J. Exp. Agric. 1979, 19, 48–52. [Google Scholar] [CrossRef]
- Allen, J. Annual ryegrass toxicity–an animal disease caused by toxins produced by a bacterial plant pathogen. Microbiol. Aust. 2012, 33, 18–21. [Google Scholar] [CrossRef]
- Schneider, D.J. First report of annual ryegrass toxicity in the Republic of South Africa. Onderstepoort. J. Vet. Res. 1981, 48, 251–255. [Google Scholar]
- Elson, R. Code of Practice for Minimisation of the Risk of Corynetoxins Contamination of Hay and Straw for Export; Department of Agriculture, Fisheries and Forestry—Australia and Australian Quarantine and Inspection Service: Canberra, Australia, 2002.
- Anderton, N.; Beales, K.A.; Cao, Y.; Colegate, S.M.; Edgar, J.A.; Michalewicz, A.; Riley, I.T.; Stewart, P.L.; Than, K.A. The identification of corynetoxin-like tunicaminyluracil-glycolipids from nematode galls in Festuca nigrescens from North America and New Zealand. In Poisonous Plants Related Toxins; Acamovic, T., Stewart, C., Pennycott, T., Eds.; CABI Publishing: Wallingford, UK, 2004; pp. 204–209. [Google Scholar]
- Allen, J.G. Annual Ryegrss Toxicity; El Caballo: Northam, Australia, 2002; pp. 1–11. [Google Scholar]
- Lanigan, G.W.; Payne, A.L.; Frahn, J.L. Letter: Origin of toxicity in parasitised annual ryegrass (Lolium rigidum). Aust. Vet. J. 1976, 52, 244–246. [Google Scholar] [CrossRef]
- Bird, A.F. The Anguina-Corynebacterlum Association. Plant Parasit. Nematodes 1981, 3, 303–323. [Google Scholar]
- Riley, I.T.; Schmitz, A.; de Silva, P. Anguina australis, a vector for Rathayibacter toxicus in Ehrharta longiflora. Australas. Plant Pathol. 2001, 30, 171–175. [Google Scholar]
- Yasuhara-Bell, J.; Stack, J.P. Panel of three loop-mediated isothermal amplification assays differentiates Rathayibacter toxicus populations RT-I, RT-II, RT-III, RT-IV and RT-V. J. Plant Pathol. 2019, 101, 707–717. [Google Scholar] [CrossRef]
- Stynes, B.A.; Vogel, P. A bacterial inhibition assay for corynetoxins from parasitized annual ryegrass. Aust. J. Agric. Res. 1983, 34, 483–489. [Google Scholar] [CrossRef]
- Riley, I.T.; Ophel, K.M. Rapid detection of corynetoxins produced by Clavibacter toxicum. Lett. Appl. Microbiol. 1992, 14, 96–99. [Google Scholar] [CrossRef]
- Cockrum, P.A.; Edgar, J.A. Rapid estimation of corynetoxins in bacterial galls from annual ryegrass (Lolium rigidum Gaudin) by high-performance liquid chromatography. Aust. J. Agric. Res. 1985, 36, 35–41. [Google Scholar] [CrossRef]
- Masters, A.M.; Gregory, A.R.; Evans, R.J.; Speijers, J.E.; Sutherland, S.S. An enzyme-linked immunosorbent assay for the detection of Rathayibacter toxicus, the bacterium involved in annual ryegrass toxicity, in hay. Aust. J. Agric. Res. 2006, 57, 731–742. [Google Scholar] [CrossRef][Green Version]
- Kowalski, M.C.; Cahill, D.; Doran, T.J.; Colegate, S.M. Development and application of polymerase chain reaction-based assays for Rathayibacter toxicus and a bacteriophage associated with annual ryegrass (Lolium rigidum) toxicity. Aust. J. Exp. Agric. 2007, 47, 177–183. [Google Scholar] [CrossRef]
- Arif, M.; Busot, G.Y.; Mann, R.; Rodoni, B.; Stack, J.P. Field-deployable recombinase polymerase amplification assay for specific, sensitive and rapid detection of the US select agent and toxigenic bacterium, Rathayibacter toxicus. Biology 2021, 10, 620. [Google Scholar] [CrossRef]
- Arif, M.; Busot, G.Y.; Mann, R.; Rodoni, B.; Stack, J.P. Multiple internal controls enhance reliability for PCR and real time PCR detection of Rathayibacter toxicus. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Colegate, S.M.; Gardner, D.R.; Lee, S.T. Plant-Associated Natural Food Toxins 23. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 753–783. [Google Scholar]
- Lorenzo-Parodi, N.; Kaziur, W.; Stojanović, N.; Jochmann, M.A.; Schmidt, T.C. Solventless microextraction techniques for water analysis. TrAC Trends Anal. Chem. 2019, 113, 321–331. [Google Scholar] [CrossRef]
- Mondello, L.; Costa, R.; Tranchida, P.Q.; Dugo, P.; Lo Presti, M.; Festa, S.; Fazio, A.; Dugo, G. Reliable characterization of coffee bean aroma profiles by automated headspace solid phase microextraction-gas chromatography-mass spectrometry with the support of a dual-filter mass spectra library. J. Sep. Sci. 2005, 28, 1101–1109. [Google Scholar] [CrossRef]
- Nerin, C.; Salafranca, J.; Aznar, M.; Batlle, R. Critical review on recent developments in solventless techniques for extraction of analytes. Anal. Bioanal. Chem. 2009, 393, 809–833. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, A.; Miekus, N.; Baczek, T. Application of solid-phase microextraction in current biomedical research. J. Sep. Sci. 2019, 42, 285–302. [Google Scholar] [CrossRef]
- Dong, X.; Sun, L.; Maker, G.; Ren, Y.; Yu, X. Ozone Treatment Increases the Release of VOC from Barley, Which Modifies Seed Germination. J. Agric. Food Chem. 2022, 70, 3127–3135. [Google Scholar] [CrossRef]
- Vogel, P.; Petterson, D.S.; Berry, P.H.; Frahn, J.L.; Anderton, N.; Cockrum, P.A.; Edgar, J.A.; Jago, M.V.; Lanigan, G.W.; Payne, A.L.; et al. Isolation of a group of glycolipid toxins from seedheads of annual ryegrass Lolium rigidum Gaud.) infected by Corynebacterium rathayi. Aust. J. Exp. Biol. Med. Sci. 1981, 59, 455–467. [Google Scholar] [CrossRef]
- Frahn, J.L.; Edgar, J.A.; Jones, A.J.; Cockrum, P.A.; Anderton, N.A.; Culvenor, C.C.J. Structure of the corynetoxins, metabolites of Corynebacterium rathayi responsible for toxicity of annual ryegrass (Lolium rigidum) pastures. Aust. J. Chem. 1984, 37, 165–182. [Google Scholar] [CrossRef]
- Moss, C.W.; Dowell, V.R., Jr.; Lewis, V.J.; Schekter, M.A. Cultural characteristics and fatty acid composition of Corynebacterium acnes. J. Bacteriol. 1967, 94, 1300–1305. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; Evtushenko, L.I.; Akimov, V.N.; Kalakoutskii, L.V. Rathayibacter gen. nov., including the species Rathayibacter rathayi comb. nov., Rathayibacter tritici comb. nov., Rathayibacter iranicus comb. nov., and six strains from annual grasses. Int. J. Syst. Evol. Microbiol. 1993, 43, 143–149. [Google Scholar] [CrossRef]
- Herebian, D.; Hanisch, B.; Marner, F.-J. Strategies for gathering structural information on unknown peaks in the GC/MS analysis of Corynebacterium glutamicum cell extracts. Metabolomics 2005, 1, 317–324. [Google Scholar] [CrossRef]
- Sasaki, J.; Chijimatsu, M.; Suzuki, K.-i. Taxonomic significance of 2, 4-diaminobutyric acid isomers in the cell wall peptidoglycan of actinomycetes and reclassification of Clavibacter toxicus as Rathayibacter toxicus comb. nov. Int. J. Syst. Evol. Microbiol. 1998, 48, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Maulidia, V.; Soesanto, L.; Syamsuddin, S.; Khairan, K.; Hamaguchi, T.; Hasegawa, K.; Sriwati, R. Secondary metabolites produced by endophytic bacteria against the Root-Knot Nematode (Meloidogyne sp.). Biodiversitas J. Biol. Divers. 2020, 21, 5270–5275. [Google Scholar] [CrossRef]
- Goldberg, I.; Rokem, J.S. Organic and fatty acid production, microbial. In Encyclopedia of Microbiology; Schaechter, M., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 421–442. [Google Scholar]
- Singh, V.K.; Devi, A.; Pathania, S.; Kumar, V.; Tripathi, D.K.; Sharma, S.; Chauhan, D.K.; Singh, V.K.; Zorba, V. Spectroscopic investigation of wheat grains (Triticum aestivum) infected by wheat seed gall nematodes (Anguina tritici). Biocatal. Agric. Biotechnol. 2017, 9, 58–66. [Google Scholar] [CrossRef]
- Jaffe, H.; Huettel, R.N.; Demilo, A.B.; Hayes, D.K.; Rebois, R.V. Isolation and identification of a compound from soybean cyst nematode, Heterodera glycines, with sex pheromone activity. J. Chem. Ecol. 1989, 15, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, D.J.; Krusberg, L.R. Diacyl, alkylacyl and alkenylacyl phospholipids of the nematode Turbatrix aceti. Comp. Biochem. Physiol. Part B Comp. Biochem. 1981, 69, 115–120. [Google Scholar] [CrossRef]
- Ghosh, A.; Kar, K.; Ghosh, D.; Dey, C.; Misra, K.K. Major lipid classes and their fatty acids in a parasitic nematode, Ascaridia galli. J. Parasit. Dis. 2010, 34, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Jez, J.M. Nematode phospholipid metabolism: An example of closing the genome–structure–function circle. Trends Parasitol. 2014, 30, 241–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gardner, H.K.; Huber, C.S.; Bourland, C.T.; Smith, M.C., Jr. Identification and quantitation of hexadecanal and octadecanal in broiler muscle phospholipids. Poult. Sci. 1972, 51, 1056–1058. [Google Scholar] [CrossRef]
- Wei, L.; Hu, F.; Shen, Y.; Chen, Z.; Yu, Y.; Lin, C.-C.; Wang, M.C.; Min, W. Live-cell imaging of alkyne-tagged small biomolecules by stimulated Raman scattering. Nat. Methods 2014, 11, 410–412. [Google Scholar] [CrossRef]
- Choe, S.; Jung, I. Growth inhibition of freshwater algae by ester compounds released from rotted plants. J. Ind. Eng. Chem. 2002, 8, 297–304. [Google Scholar]
- Lalthanpuii, P.B.; Lalchhandama, K. Chemical composition and broad-spectrum anthelmintic activity of a cultivar of toothache plant, Acmella oleracea, from Mizoram, India. Pharm. Biol. 2020, 58, 393–399. [Google Scholar] [CrossRef]
- Cairns, A.J.; Begley, P.; Sims, I.M. The structure of starch from seeds and leaves of the fructan-accumulating ryegrass, Lolium temulentum L. J. Plant Physiol. 2002, 159, 221–230. [Google Scholar] [CrossRef]
- Agilent. MassHunter Workstation Software Qualitative Analysis Navigator; B.08.00; Agilent Technologies: Santa Clara, CA, USA, 2016. [Google Scholar]
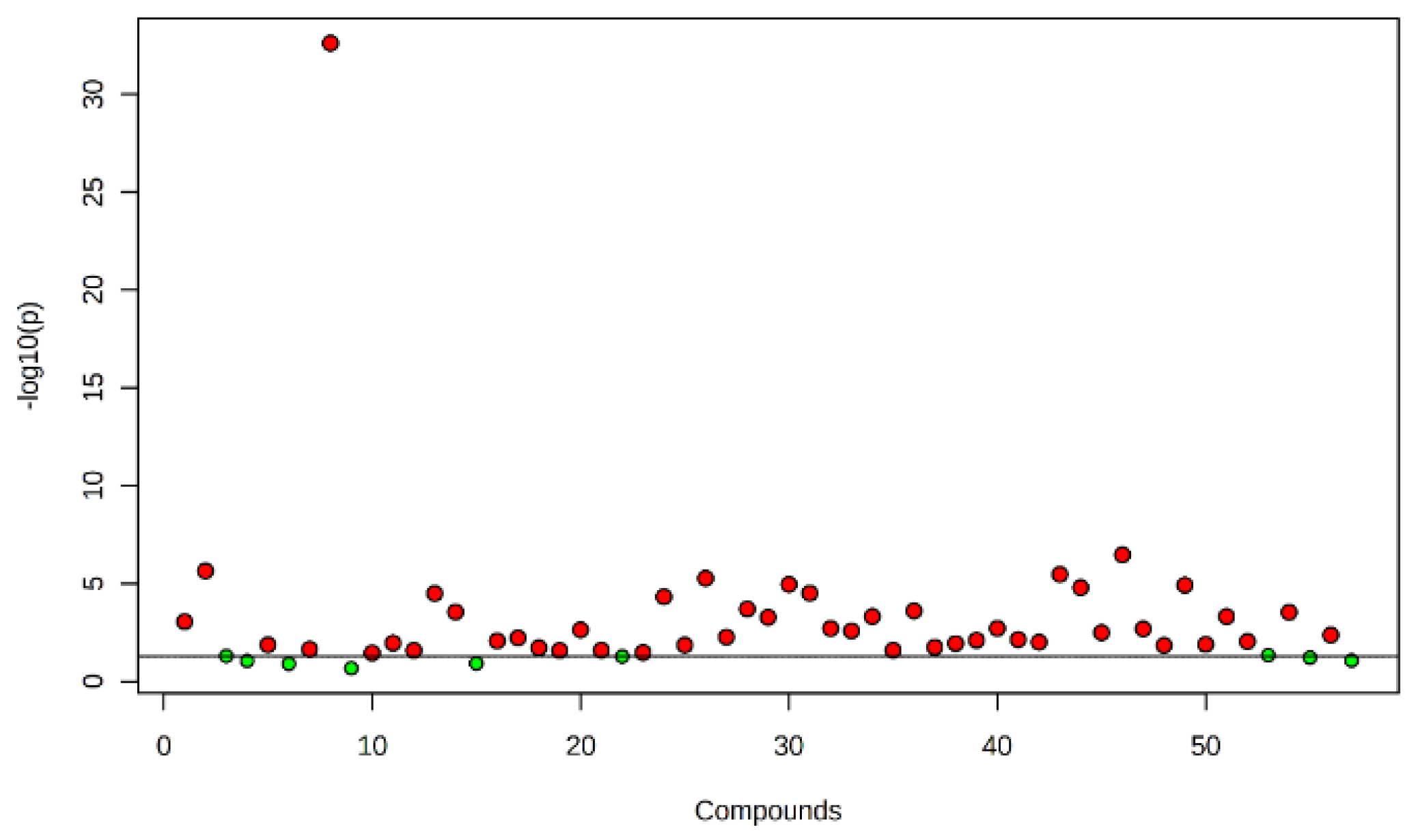
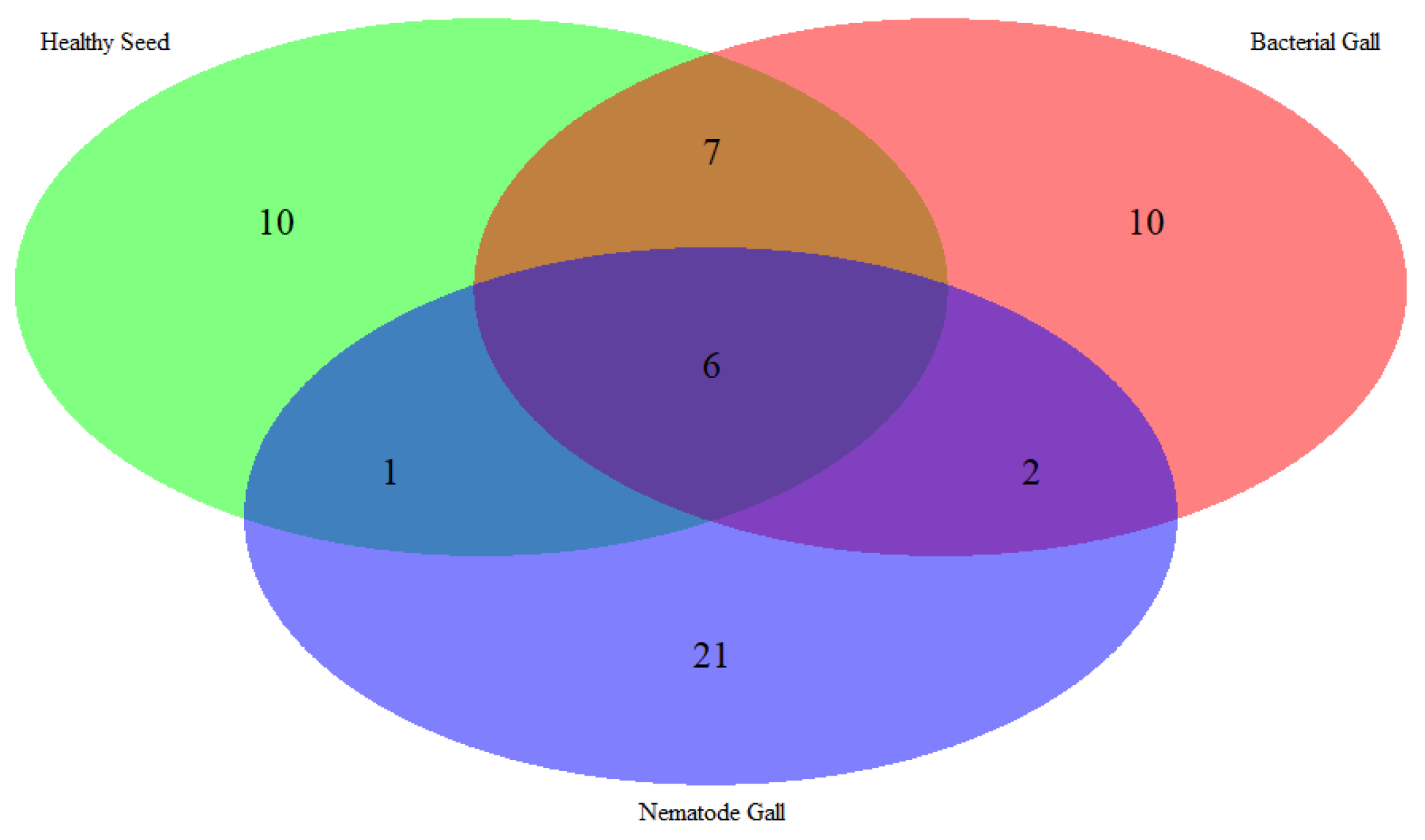
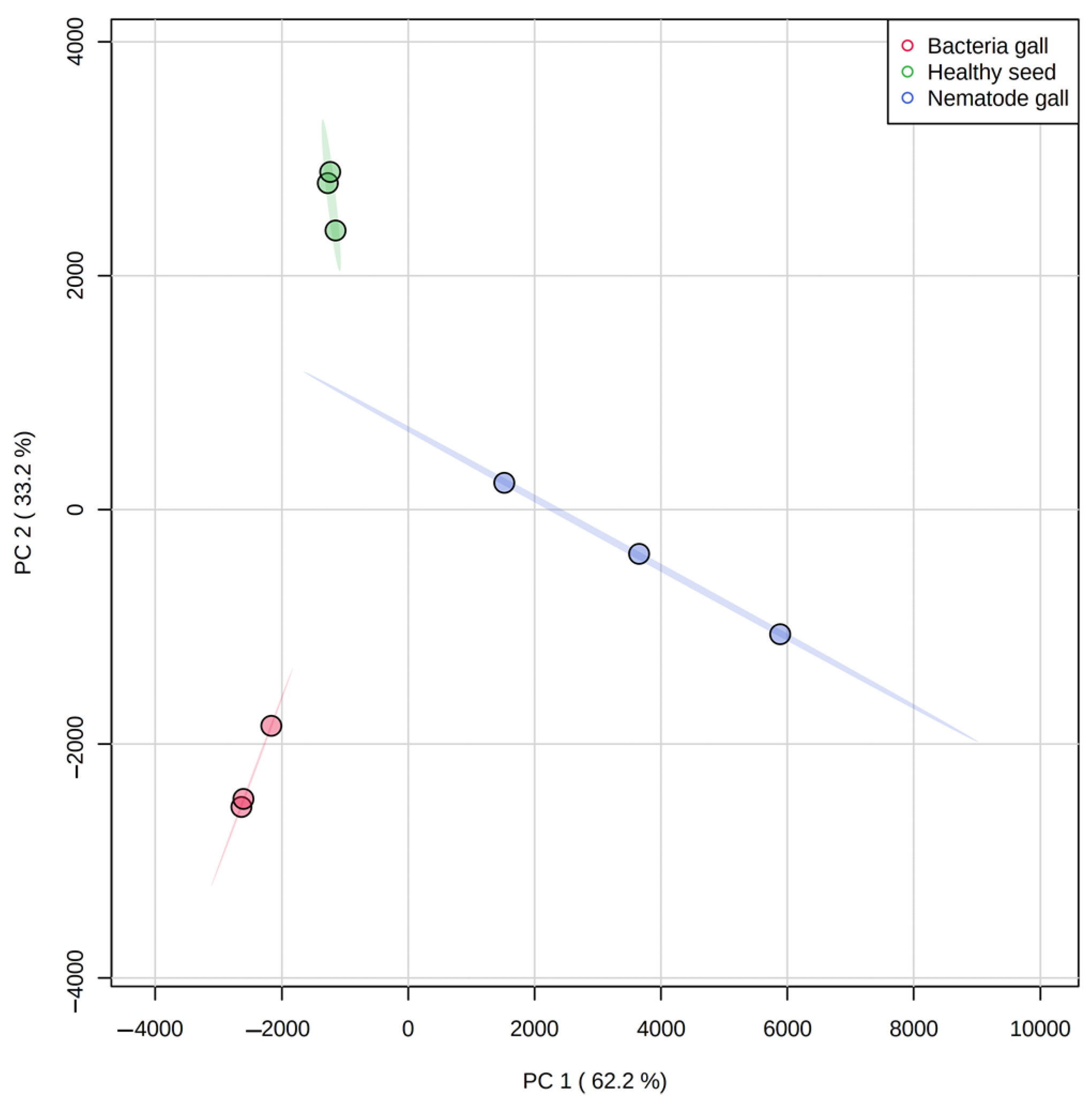

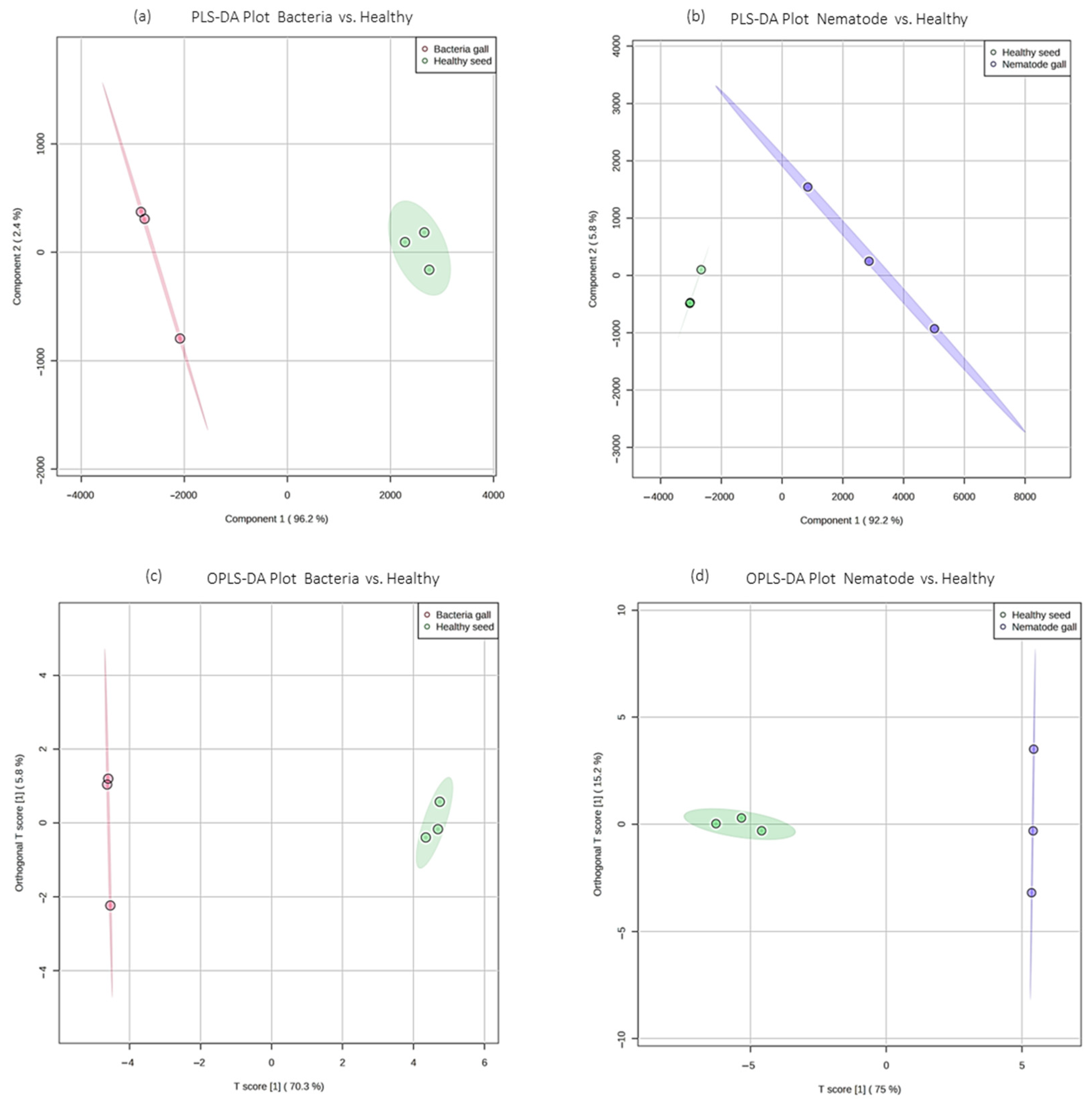
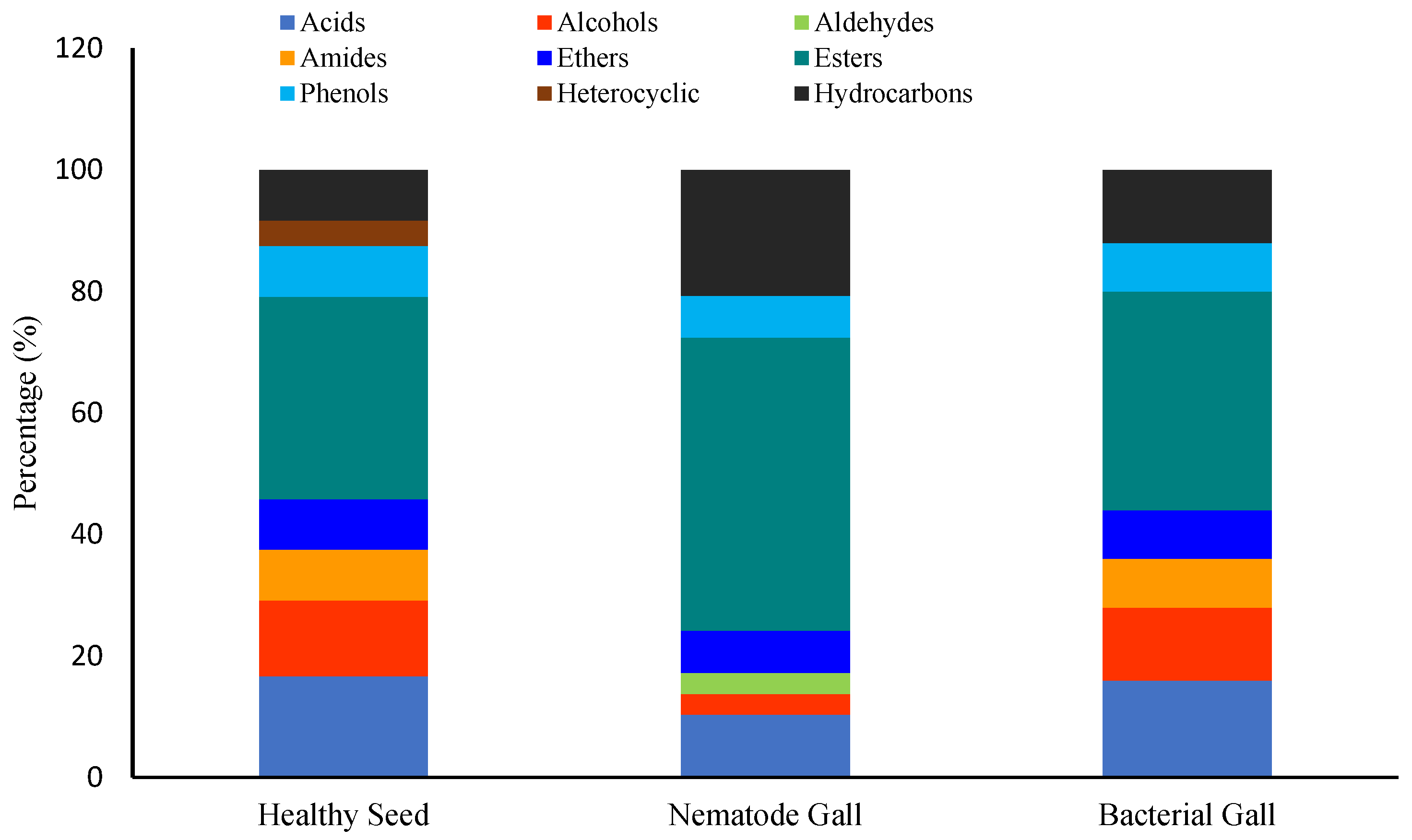
| Compounds | RI | RT | Treatment Sources | FDR | Molecular Formula | MW | ||
|---|---|---|---|---|---|---|---|---|
| Nematode Gall | Bacterial Gall | Healthy Seed | ||||||
| Benzene, 1,3-dimethyl- | 876.8 | 8.09 | N.D. b | 20.662 a | N.D. b | 0.003 | C8H10 | 106.08 |
| 2-Cyclohexen-1-ol | 898.5 | 8.81 | N.D. c | 10.620 a | 8.139 b | 0.000 | C6H10O | 98.07 |
| Benzene, pentyl- | 1161.7 | 16.27 | N.D. b | N.D. b | 20.270 a | 0.021 | C11H16 | 148.13 |
| (1R)-1-(2,6-Dichloro-3-fluorophenyl) ethanol, methyl ether | 1296.6 | 19.05 | N.D. b | 25.209 a | 13.113 ab | 0.031 | C9H9Cl2FO | 222.00 |
| 1-Hexadecanol, 2-methyl- | 1315.9 | 19.43 | N.D. b | N.D. b | 13.948 a | 0.000 | C17H36O | 256.28 |
| 2-Phosphonopropanoic acid, 3TMS | 1435.7 | 22.44 | N.D. b | N.D. b | 67.509 a | 0.042 | C12H31O5PSi3 | 370.12 |
| 2,4-Di-tert-butylphenol | 1519.6 | 23.26 | 27.074 b | 46.585 a | 39.439 a | 0.018 | C14H22O | 206.17 |
| 4-(2-(Acryloyloxy) ethoxy)-4-oxobutanoic acid, TMS | 1648.9 | 25.77 | 48.480 b | 696.220 a | 856.414 a | 0.032 | C12H20O6Si | 288.10 |
| Tetradecanoic acid | 1758.0 | 27.84 | N.D. b | 61.926 a | N.D. b | 0.000 | C14H28O2 | 228.21 |
| 9-Hexadecen-1-ol, (Z)- | 1860.2 | 29.82 | N.D. b | N.D. b | 105.752 a | 0.001 | C16H32O | 240.25 |
| n-Hexadecanoic acid | 1966.7 | 31.97 | N.D. b | 255.530 a | 27.134 b | 0.016 | C16H32O2 | 256.24 |
| Octadecanal | 2021.2 | 33.08 | 43.277 a | N.D. b | N.D. b | 0.012 | C18H36O | 268.28 |
| (4aS,4bR,10aS)-7-Isopropyl-1,1,4a-trimethyl-1,2,3,4,4a,4b,5,6,10,10a-decahydrophenanthrene | 2067.6 | 34.03 | 74.366 a | N.D. b | N.D. b | 0.026 | C20H32 | 272.25 |
| hexadecyl acrylate | 2098.4 | 34.66 | N.D. b | N.D. b | 16.190 a | 0.032 | C19H36O2 | 296.27 |
| Pent-4-enoyl amide, 2-methyl-N-dodecyl- | 2141.7 | 35.53 | N.D. b | 271.343 a | 271.581 a | 0.006 | C18H35NO | 281.27 |
| 17-Octadecynoic acid, methyl ester | 2163.8 | 35.99 | 19.769 a | N.D. b | N.D. b | 0.032 | C19H34O2 | 282.26 |
| (E)-9-Octadecenoic acid ethyl ester | 2175.0 | 36.22 | 101.540 a | N.D. b | N.D. b | 0.041 | C20H38O2 | 310.29 |
| Estra-1,3,5(10)-trien-17β-ol | 2257.5 | 37.89 | N.D. b | 68.765 a | N.D. b | 0.000 | C18H24O | 256.18 |
| cis-10-Nonadecenoic acid | 2258.0 | 37.91 | 168.520 a | N.D. b | 196.594 a | 0.021 | C19H36O2 | 296.27 |
| Nonadecane, 1-chloro- | 2298.1 | 38.71 | N.D.c | 104.389 a | 51.739 b | 0.000 | C19H39Cl | 302.27 |
| Carbonic acid, octadecyl vinyl ester | 2299.3 | 38.74 | 93.518 a | N.D. b | N.D. b | 0.011 | C21H40O3 | 340.30 |
| Pent-4-enoyl amide, 2-methyl-N-tetradecyl- | 2356.4 | 39.38 | N.D. b | N.D. b | 286.189 a | 0.001 | C20H39NO | 309.30 |
| Oct-3-enoylamide, N-methyl-N-undecyl- | 2356.4 | 39.38 | N.D. b | 291.407 a | N.D. b | 0.002 | C20H39NO | 309.30 |
| Hexanedioic acid, dioctyl ester | 2401.2 | 39.87 | N.D. b | N.D. b | 3569.522 a | 0.000 | C22H42O4 | 370.31 |
| Hexanedioic acid, bis(2-ethylhexyl) ester | 2402.7 | 39.88 | N.D. b | 4438.417 a | N.D. b | 0.000 | C22H42O4 | 370.31 |
| Oxiraneundecanoic acid, 3-pentyl-, methyl ester, cis- | 2433.4 | 40.09 | 67.235 a | N.D. b | N.D. b | 0.005 | C19H36O3 | 312.27 |
| 2,4-Dihydroxyheptadecyl acetate | 2466.5 | 40.31 | N.D.c | 78.065 a | 46.500 b | 0.006 | C19H38O4 | 330.28 |
| Carbonic acid, eicosyl vinyl ester | 2499.6 | 40.54 | 744.725 b | 1370.199 a | 182.234c | 0.002 | C23H44O3 | 368.33 |
| Fumaric acid, pent-4-en-2-yl tridecyl ester | 2515.1 | 40.65 | 122.261 a | N.D. b | N.D. b | 0.032 | C22H38O4 | 366.28 |
| Batilol | 2598.6 | 41.21 | 126.647 b | 187.007 a | N.D.c | 0.001 | C21H44O3 | 344.33 |
| 1-Docosanol, acetate | 2623.3 | 41.37 | 502.454 a | N.D. b | N.D. b | 0.025 | C24H48O2 | 368.37 |
| Fumaric acid, 4-octyl dodec-2-en-1-yl ester | 2636.0 | 41.46 | 81.578 a | N.D. b | N.D. b | 0.019 | C24H42O4 | 394.31 |
| Heptacos-1-ene | 2677.5 | 41.74 | 245.258 a | N.D. b | N.D. b | 0.015 | C27H54 | 378.42 |
| 13-Methylheptacosane | 2701.9 | 41.91 | 2675.940 a | N.D. b | N.D. b | 0.005 | C28H58 | 394.45 |
| 4′,6′-Dimethoxy-2′-hydroxychalcone, 2-methylpropionate | 2731.6 | 42.12 | 80.654 b | 232.646 a | 166.662 a | 0.014 | C21H22O5 | 354.15 |
| Propyl tetracosyl ether | 2783.5 | 42.52 | 600.788 a | N.D. b | N.D. b | 0.017 | C27H56O | 396.43 |
| Fumaric acid, 2-ethylhexyl tridec-2-yn-1-yl ester | 2798.8 | 42.60 | N.D. b | 328.787 a | N.D. b | 0.000 | C25H42O4 | 406.31 |
| (5S,10Z,11aR)-5-(Acetyloxy)-6,10-bis(hydroxymethyl)-3-methylidene-2-oxo-2,3,3a,4,5,8,9,11a-octahydrocyclodeca[b]furan-4-yl 2-methylbutanoate, 2TMS derivative | 2796.4 | 42.62 | N.D. b | N.D. b | 135.028 a | 0.000 | C28H46O8Si2 | 566.27 |
| Docosane, 11-butyl- | 2799.5 | 42.64 | 340.807 a | N.D. b | N.D. b | 0.007 | C26H54 | 366.42 |
| Carbonic acid, but-2-yn-1-yl eicosyl ester | 2819.3 | 42.80 | N.D. b | 1032.923 a | N.D. b | 0.000 | C25H46O3 | 394.34 |
| Heptacosyl trifluoroacetate | 2879.9 | 43.31 | 708.013 a | N.D. b | N.D. b | 0.005 | C29H55F3O2 | 492.42 |
| Ethyl hexacosyl ether | 2888.7 | 43.39 | 2961.463 a | N.D. b | N.D. b | 0.021 | C28H58O | 410.45 |
| Isopropyl hexacosyl ether | 2900.8 | 43.47 | N.D.c | 1546.260 a | 426.273 b | 0.000 | C29H60O | 424.46 |
| 15-Methylnonacosane | 2902.0 | 43.50 | 3105.581 a | N.D. b | N.D. b | 0.020 | C30H62 | 422.49 |
| Hexacosanoic acid | 2944.3 | 43.91 | N.D. b | 213.320 a | N.D. b | 0.002 | C26H52O2 | 396.40 |
| Fumaric acid, hexadecyl 4-heptyl ester | 2986.6 | 44.33 | 223.079 a | N.D. b | N.D. b | 0.016 | C27H50O4 | 438.37 |
| Heptacosyl acetate | 3081.7 | 45.39 | 1160.147 a | N.D. b | N.D. b | 0.001 | C29H58O2 | 438.44 |
| 9-Octadecenoic acid (Z)-, tetradecyl ester | 3478.0 | 48.29 | 256.767 a | 123.793 b | N.D. c | 0.009 | C32H62O | 478.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koli, P.; Agarwal, M.; Kessell, D.; Mahawar, S.; Du, X.; Ren, Y.; McKirdy, S.J. Metabolite Variation between Nematode and Bacterial Seed Galls in Comparison to Healthy Seeds of Ryegrass Using Direct Immersion Solid-Phase Microextraction (DI-SPME) Coupled with GC-MS. Molecules 2023, 28, 828. https://doi.org/10.3390/molecules28020828
Koli P, Agarwal M, Kessell D, Mahawar S, Du X, Ren Y, McKirdy SJ. Metabolite Variation between Nematode and Bacterial Seed Galls in Comparison to Healthy Seeds of Ryegrass Using Direct Immersion Solid-Phase Microextraction (DI-SPME) Coupled with GC-MS. Molecules. 2023; 28(2):828. https://doi.org/10.3390/molecules28020828
Chicago/Turabian StyleKoli, Pushpendra, Manjree Agarwal, David Kessell, Shalini Mahawar, Xin Du, Yonglin Ren, and Simon J. McKirdy. 2023. "Metabolite Variation between Nematode and Bacterial Seed Galls in Comparison to Healthy Seeds of Ryegrass Using Direct Immersion Solid-Phase Microextraction (DI-SPME) Coupled with GC-MS" Molecules 28, no. 2: 828. https://doi.org/10.3390/molecules28020828
APA StyleKoli, P., Agarwal, M., Kessell, D., Mahawar, S., Du, X., Ren, Y., & McKirdy, S. J. (2023). Metabolite Variation between Nematode and Bacterial Seed Galls in Comparison to Healthy Seeds of Ryegrass Using Direct Immersion Solid-Phase Microextraction (DI-SPME) Coupled with GC-MS. Molecules, 28(2), 828. https://doi.org/10.3390/molecules28020828







