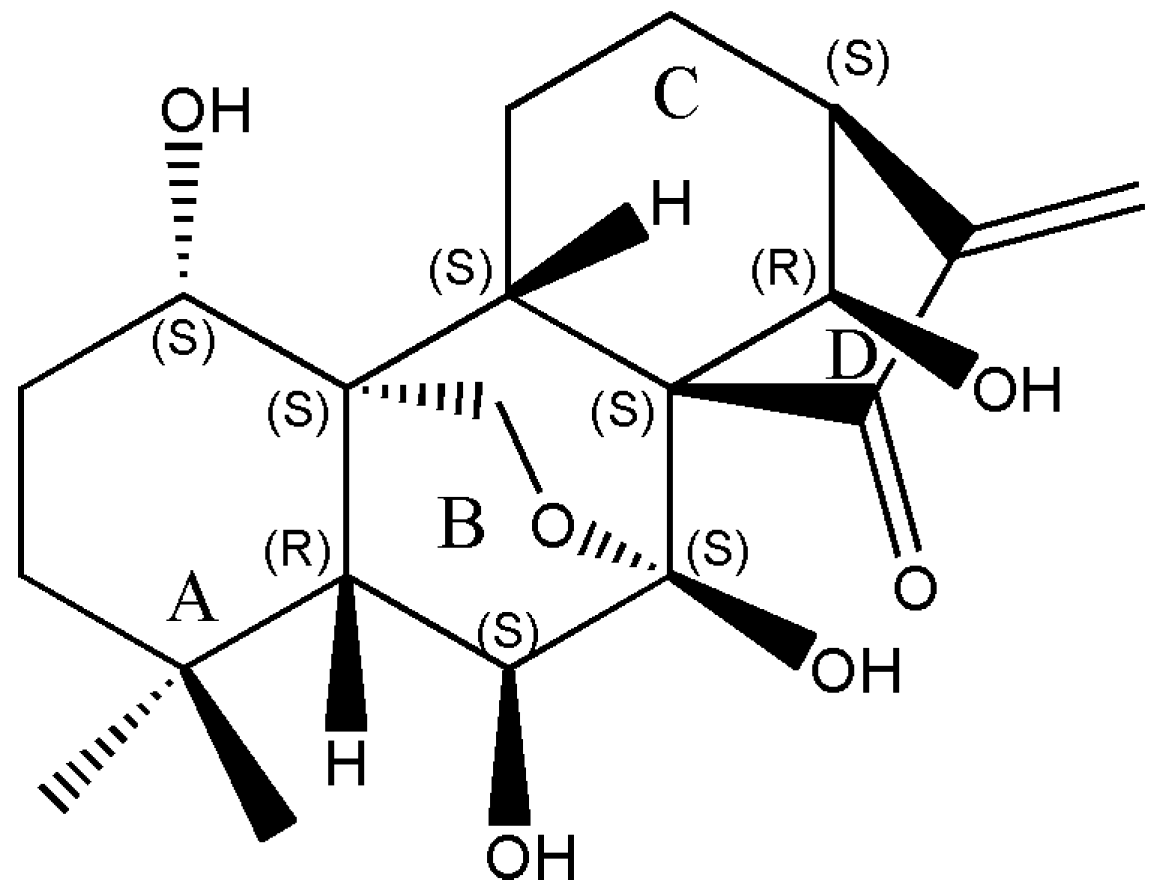
Oridonin Induces Apoptosis in Esophageal Squamous Cell Carcinoma by Inhibiting Cytoskeletal Protein LASP1 and PDLIM1
Abstract
1. Introduction
2. Results
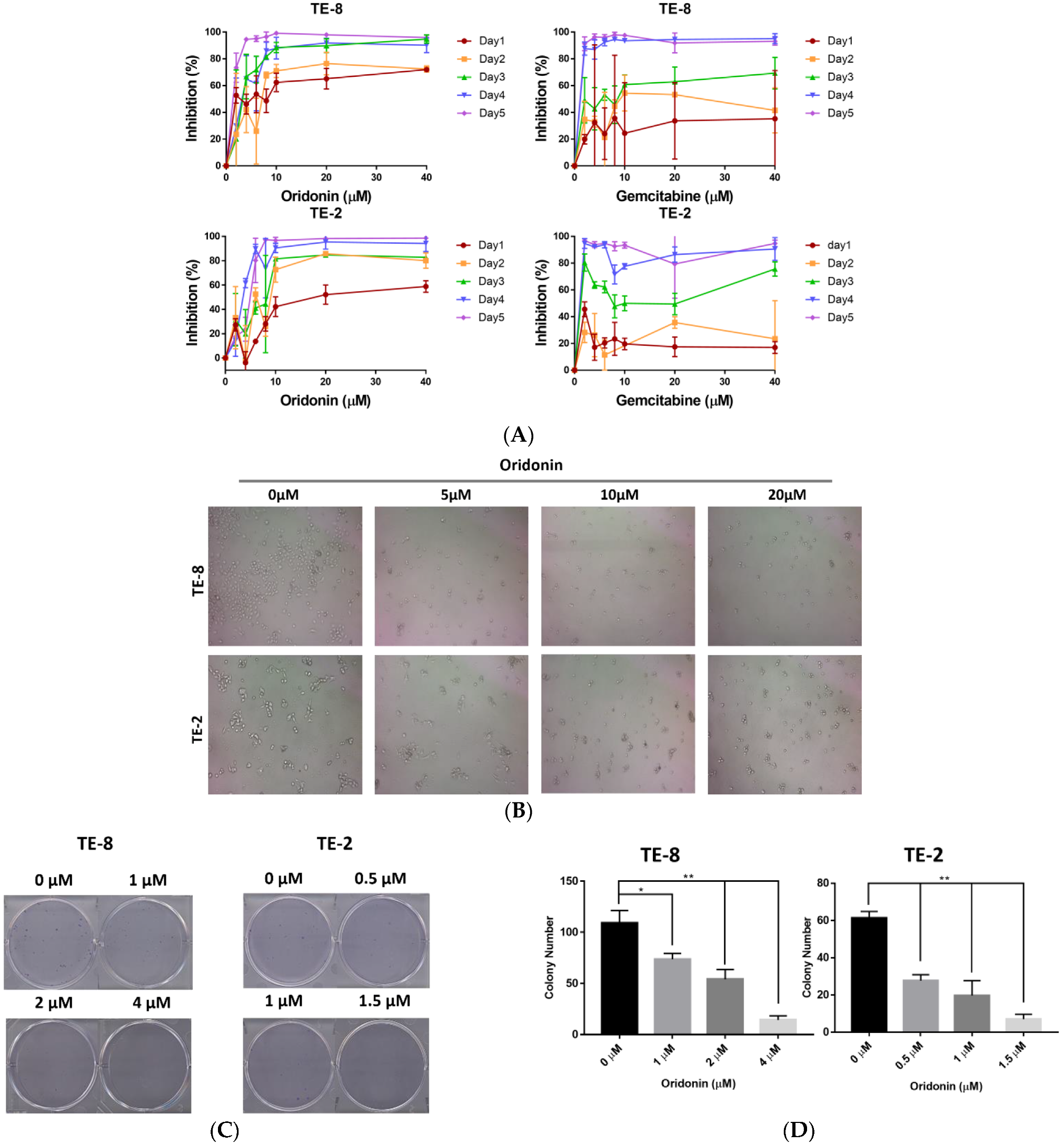
2.1. Oridonin Inhibits the Proliferation and Colony Formation of Esophageal Squamous Cell Carcinoma Cells
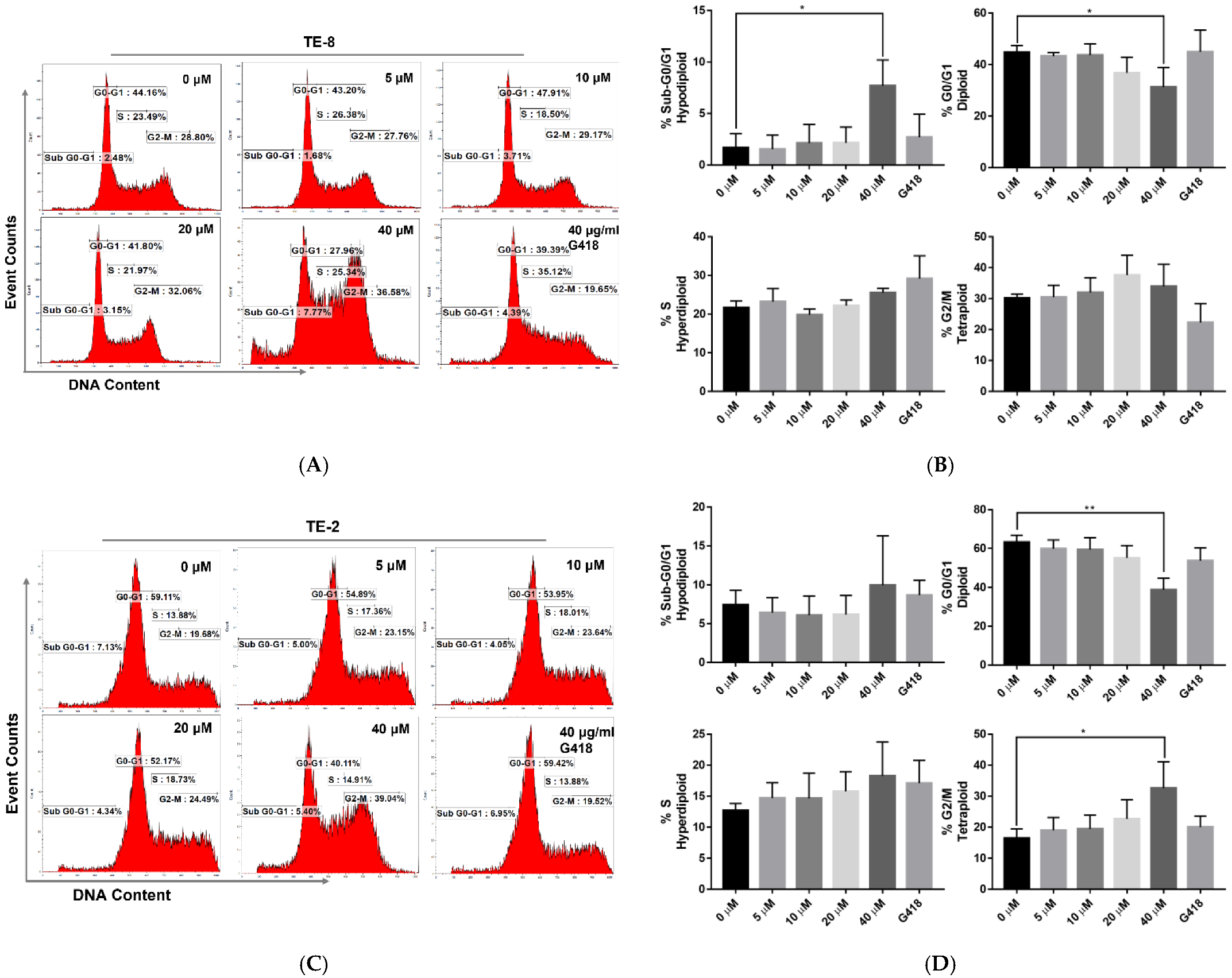
2.2. Oridonin Disturbs the Cell Cycle of Esophageal Squamous Cell Carcinoma Cells
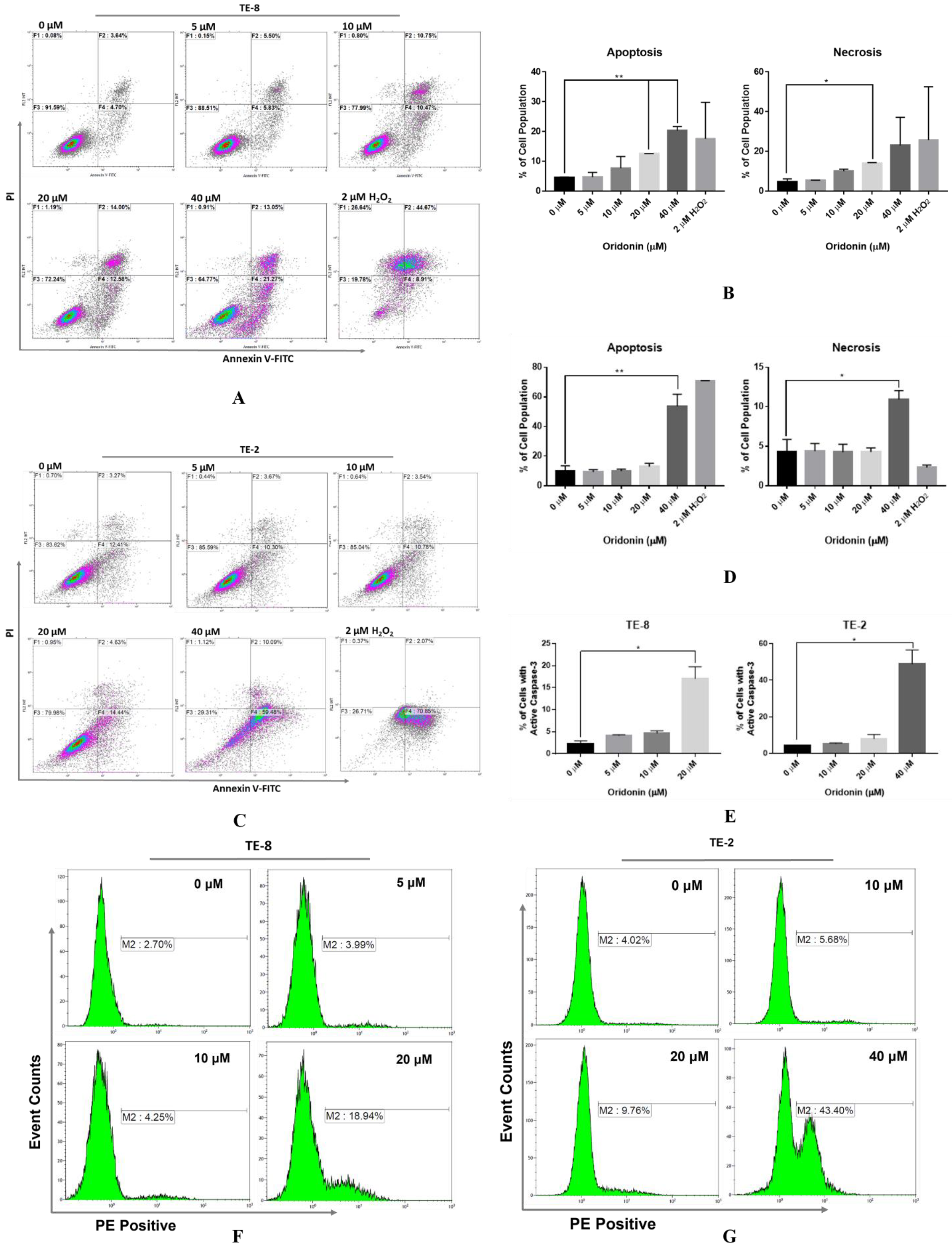
2.3. Oridonin Evokes Phosphatidylserine Externalization and Actives Caspase-3 on Esophageal Squamous Cell Carcinoma Cells
2.4. Two-Dimensional Electrophoresis-Based Proteomics Identifies Proteins That Are Impacted by Oridonin
2.5. Oridonin Treatment Inhibits LASP1 and PDLIM1 Expression on ESCC
3. Conclusions and Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Cell Proliferation and Inhibition Assay
4.3. Colony Formation Assay and Cellular Morphological Changes
4.4. Cell Cycle Profile Analysis
4.5. Analysis of the Phosphatidylserine Distribution via Annexin V-FITC/PI Assay
4.6. Detection of Caspase-3 Activation
4.7. 2-Dimensional Electrophoresis SDS-PAGE Gel Analysis
4.8. Sample In-GEL Trypsin Digestion for Mass Spectrometry
4.9. Liquid Chromatography High-Resolution Mass Spectrometry
4.10. Mass Spectrometry Analysis of Different Expression of Proteins
4.11. Gene Set Enrichment Analysis
4.12. Western Blotting Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Alsop, B.R.; Sharma, P. Esophageal cancer. Gastroenterol. Clin. 2016, 45, 399–412. [Google Scholar] [CrossRef]
- Ohashi, S.; Miyamoto, S.i.; Kikuchi, O.; Goto, T.; Amanuma, Y.; Muto, M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology 2015, 149, 1700–1715. [Google Scholar] [CrossRef]
- González-Plaza, J.J.; Hulak, N.; García-Fuentes, E.; Garrido-Sánchez, L.; Zhumadilov, Z.; Akilzhanova, A. Oesophageal squamous cell carcinoma (ESCC): Advances through omics technologies, towards ESCC salivaomics. Drug Discov. Ther. 2015, 9, 247–257. [Google Scholar] [CrossRef]
- Thun, M.; Linet, M.S.; Cerhan, J.R.; Haiman, C.A.; Schottenfeld, D. Cancer Epidemiology and Prevention; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Testa, U.; Castelli, G.; Pelosi, E. Esophageal cancer: Genomic and molecular characterization, stem cell compartment and clonal evolution. Medicines 2017, 4, 67. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Corvera, C.; Das, P.; Denlinger, C.S.; Enzinger, P.C.; Fanta, P.; Farjah, F. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 855–883. [Google Scholar] [CrossRef]
- Li, B.; Tan, T.; Chu, W.; Zhang, Y.; Ye, Y.; Wang, S.; Qin, Y.; Tang, J.; Cao, X. Co-delivery of paclitaxel (PTX) and docosahexaenoic acid (DHA) by targeting lipid nanoemulsions for cancer therapy. Drug Deliv. 2022, 29, 75–88. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Su, X.; Li, Q.; Chen, S.; Dong, C.; Hu, Y.; Yin, L.; Yang, J. Analysis of the transcriptome of Isodon rubescens and key enzymes involved in terpenoid biosynthesis. Biotechnol. Biotechnol. Equip. 2016, 30, 592–601. [Google Scholar] [CrossRef]
- Owona, B.A.; Schluesener, H.J. Molecular insight in the multifunctional effects of oridonin. Drugs RD 2015, 15, 233–244. [Google Scholar] [CrossRef]
- Tian, W.; Chen, S.-Y. Recent advances in the molecular basis of anti-neoplastic mechanisms of oridonin. Chin. J. Integr. Med. 2013, 19, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.-T.; Ma, W.; Xie, X.; Huang, Q. Oridonin: A review of its pharmacology, pharmacokinetics and toxicity. Front. Pharmacol. 2021, 12, 645824. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, J.; Zhou, J.; Shen, Q. Oridonin and its derivatives for cancer treatment and overcoming therapeutic resistance. Genes Dis. 2021, 8, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, Y.; Pan, S.-Y.; Zhu, G.-Y.; Li, Y.-W.; Fong, D.W.; Yu, Z.-L. Proteomic identification of proteins involved in the anticancer activities of oridonin in HepG2 cells. Phytomedicine 2011, 18, 163–169. [Google Scholar] [CrossRef]
- Dal Piaz, F.; Cotugno, R.; Lepore, L.; Vassallo, A.; Malafronte, N.; Lauro, G.; Bifulco, G.; Belisario, M.A.; De Tommasi, N. Chemical proteomics reveals HSP70 1A as a target for the anticancer diterpene oridonin in Jurkat cells. J. Proteom. 2013, 82, 14–26. [Google Scholar] [CrossRef]
- Vasaturo, M.; Cotugno, R.; Fiengo, L.; Vinegoni, C.; Dal Piaz, F.; De Tommasi, N. The anti-tumor diterpene oridonin is a direct inhibitor of Nucleolin in cancer cells. Sci. Rep. 2018, 8, 16735. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, Y.; Yu, Z.-L. Proteomic and functional analyses demonstrate the involvement of oxidative stress in the anticancer activities of oridonin in HepG2 cells. Oncol. Rep. 2014, 31, 2165–2172. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, M.; He, P.; Zhao, J.; Chen, Y.; Qi, J.; Wang, Y. Proteomic analysis of oridonin-induced apoptosis in multiple myeloma cells. Mol. Med. Rep. 2017, 15, 1807–1815. [Google Scholar] [CrossRef]
- Wang, H.; Ye, Y.; Chu, J.-H.; Zhu, G.-Y.; Fong, W.-F.; Yu, Z.-L. Proteomic and functional analyses reveal the potential involvement of endoplasmic reticulum stress and α-CP1 in the anticancer activities of oridonin in HepG2 cells. Integr. Cancer Ther. 2011, 10, 160–167. [Google Scholar] [CrossRef]
- Ma, Z.; Xue, X. Differentially expressed proteins in the human esophageal cancer cell line Eca-109, in the presence and absence of gemcitabine. Mol. Med. Rep. 2018, 17, 1873–1878. [Google Scholar] [CrossRef]
- Paroha, S.; Verma, J.; Dubey, R.D.; Dewangan, R.P.; Molugulu, N.; Bapat, R.A.; Sahoo, P.K.; Kesharwani, P. Recent advances and prospects in gemcitabine drug delivery systems. Int. J. Pharm. 2021, 592, 120043. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Shi, J.; Ahmed, T.; Liu, H.; Guo, J.; Tang, W.; Guo, Y.; Zhang, Q. Involvement of Glutathione Depletion in Selective Cytotoxicity of Oridonin to p53-Mutant Esophageal Squamous Carcinoma Cells. Front. Oncol. 2020, 9, 1525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-B.; Kang, H.; Wang, L.; Gao, L.; Liu, P.; Xie, J.; Zhang, F.-X.; Weng, X.-Q.; Shen, Z.-X.; Chen, J. Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion protein and shows potent antitumor activity with low adverse effects on t (8; 21) leukemia in vitro and in vivo. Blood 2007, 109, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhu, J.; Wang, X.; Sun, X.; Luo, C.; Zhang, Y.; Ye, Y.; Li, X.; Abulizi, A.; Huang, Z.; et al. Oridonin Delays Aging Through the AKT Signaling Pathway. Front. Pharmacol. 2022, 13, 888247. [Google Scholar] [CrossRef]
- Jiang, J.H.; Pi, J.; Jin, H.; Cai, J.Y. Oridonin-induced mitochondria-dependent apoptosis in esophageal cancer cells by inhibiting PI3K/AKT/mTOR and Ras/Raf pathways. J. Cell. Biochem. 2019, 120, 3736–3746. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.L.; Ghoorun, R.A.; Wu, X.H.; Chen, H.L.; Zhou, Q.; Wu, X.B. Oridonin induces apoptosis in HGC-27 cells by activating the JNK signaling pathway. Oncol. Lett. 2020, 19, 255–260. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Jiang, H.; Chen, Y.; Ye, J.; Wang, A.; Wang, C.; Liu, Q.; Liang, G.; Deng, X.; Jiang, W. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 2018, 9, 2550. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Zhou, Y.; Wang, K.; Sun, Y.; Yan, H.; Han, W.; Wang, X.; Wei, B.; Ke, Y. Oridonin induces ferroptosis by inhibiting gamma-glutamyl cycle in TE1 cells. Phytother. Res. 2021, 35, 494–503. [Google Scholar] [CrossRef]
- Butt, E.; Raman, D. New frontiers for the cytoskeletal protein LASP1. Front. Oncol. 2018, 8, 391. [Google Scholar] [CrossRef]
- Butt, E.; Ebbing, J.; Bubendorf, L.; Ardelt, P. Influence of hematuria and infection on diagnostic accuracy of urinary LASP1: A new biomarker for bladder carcinoma. Biomark. Med. 2017, 11, 347–357. [Google Scholar] [CrossRef]
- Shi, J.; Guo, J.; Li, X. Role of LASP-1, a novel SOX9 transcriptional target, in the progression of lung cancer. Int. J. Oncol. 2018, 52, 179–188. [Google Scholar] [CrossRef]
- Lin, Y.H.; Park, Z.-Y.; Lin, D.; Brahmbhatt, A.A.; Rio, M.-C.; Yates III, J.R.; Klemke, R.L. Regulation of cell migration and survival by focal adhesion targeting of Lasp-1. J. Cell Biol. 2004, 165, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-K.; Fan, X.; Cheng, J.; Liu, W.; Peng, Y. PDLIM1: Structure, function and implication in cancer. Cell Stress 2021, 5, 119. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Duan, Y.; Wang, B.; Shi, J.; Wang, P.; Ye, H.; Dai, L.; Zhang, J.; Wang, X. Serum Anti-PDLIM1 Autoantibody as Diagnostic Marker in Ovarian Cancer. Front. Immunol. 2021, 12, 698312. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Shao, X.; Xu, M.; Zhou, C.; Wang, J. ENO1 acts as a prognostic biomarker candidate and promotes tumor growth and migration ability through the regulation of Rab1A in colorectal cancer. Cancer Manag. Res. 2019, 11, 9969. [Google Scholar] [CrossRef]
- Tsai, S.-T.; Chien, I.-H.; Shen, W.-H.; Kuo, Y.-Z.; Jin, Y.-T.; Wong, T.-Y.; Hsiao, J.-R.; Wang, H.-P.; Shih, N.-Y.; Wu, L.-W. ENO1, a potential prognostic head and neck cancer marker, promotes transformation partly via chemokine CCL20 induction. Eur. J. Cancer 2010, 46, 1712–1723. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Miao, L.; Ding, J. Silencing of ENO1 inhibits the proliferation, migration and invasion of human breast cancer cells. J. Buon 2020, 25, 696–701. [Google Scholar]
- Cappello, P.; Principe, M.; Bulfamante, S.; Novelli, F. Alpha-Enolase (ENO1), a potential target in novel immunotherapies. Front. Biosci. (Landmark Ed.) 2017, 22, 944–959. [Google Scholar]
- Qiao, H.; Wang, Y.; Zhu, B.; Jiang, L.; Yuan, W.; Zhou, Y.; Guan, Q. Enolase1 overexpression regulates the growth of gastric cancer cells and predicts poor survival. J. Cell. Biochem. 2019, 120, 18714–18723. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Chen, Z.; He, Z.; Xu, Y.; Li, Z. CircRNA-ENO1 promoted glycolysis and tumor progression in lung adenocarcinoma through upregulating its host gene ENO1. Cell Death Dis. 2019, 10, 885. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Gidfar, S.; Vu, A.; Schraufnagel, D. Annexins family: Insights into their functions and potential role in pathogenesis of sarcoidosis. J. Transl. Med. 2016, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wang, Q.; Guo, Y.; Chen, Z.; Li, K.; Yang, Y.; Zhang, H.; Dong, H.; Shen, K.; Yang, W. PRSS8 methylation and its significance in esophageal squamous cell carcinoma. Oncotarget 2016, 7, 28540. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.D.; Bi, X.; Song, X.; Pohl, N.M.; Cheng, Y.; Zhou, Y.; Shears, S.; Ansong, E.; Xing, M.; Wang, S. A sequence variant in the phospholipase C epsilon C2 domain is associated with esophageal carcinoma and esophagitis. Mol. Carcinog. 2013, 52, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Francia, G.; Zhang, J.-Y. p62/IMP2 stimulates cell migration and reduces cell adhesion in breast cancer. Oncotarget 2015, 6, 32656. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Li, P.; Wang, X.; Li, J.; Shi, J.; Qin, J.; Zhang, X.; Ma, Y.; Francia, G.; Zhang, J.-Y. Overexpression of p62/IMP2 can Promote Cell Migration in Hepatocellular Carcinoma via Activation of the Wnt/β-Catenin Pathway. Cancers 2020, 12, 7. [Google Scholar] [CrossRef]
- Hu, T.; Li, P.; Luo, Z.; Chen, X.; Zhang, J.; Wang, C.; Chen, P.; Dong, Z. Chloroquine inhibits hepatocellular carcinoma cell growth in vitro and in vivo. Oncol. Rep. 2016, 35, 43–49. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, J.-Y.; Sha, B.-B.; Ma, Y.-E.; Hu, T.; Ma, Y.-C.; Sun, H.; Shi, J.-X.; Dong, Z.-M.; Li, P. Luteolin inhibits cell proliferation and induces cell apoptosis via down-regulation of mitochondrial membrane potential in esophageal carcinoma cells EC1 and KYSE450. Oncotarget 2017, 8, 27471. [Google Scholar] [CrossRef]
- Iglesias-Figueroa, B.F.; Siqueiros-Cendón, T.S.; Gutierrez, D.A.; Aguilera, R.J.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Varela-Ramirez, A.; Rascón-Cruz, Q. Recombinant human lactoferrin induces apoptosis, disruption of F-actin structure and cell cycle arrest with selective cytotoxicity on human triple negative breast cancer cells. Apoptosis 2019, 24, 562–577. [Google Scholar] [CrossRef]
- Robles-Escajeda, E.; Das, U.; Ortega, N.M.; Parra, K.; Francia, G.; Dimmock, J.R.; Varela-Ramirez, A.; Aguilera, R.J. A novel curcumin-like dienone induces apoptosis in triple-negative breast cancer cells. Cell. Oncol. 2016, 39, 265–277. [Google Scholar] [CrossRef]
- Robles-Escajeda, E.; Lerma, D.; Nyakeriga, A.M.; Ross, J.A.; Kirken, R.A.; Aguilera, R.J.; Varela-Ramirez, A. Searching in mother nature for anti-cancer activity: Anti-proliferative and pro-apoptotic effect elicited by green barley on leukemia/lymphoma cells. PLoS ONE 2013, 8, e73508. [Google Scholar] [CrossRef]
- Dai, L.; Li, J.; Xing, M.; Sanchez, T.W.; Casiano, C.A.; Zhang, J.Y. Using serological proteome analysis to identify serum anti-nucleophosmin 1 autoantibody as a potential biomarker in European-American and African-American patients with prostate cancer. Prostate 2016, 76, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Qu, Y.; Li, J.; Wang, X.; Wang, K.; Wang, P.; Jiang, B.-H.; Zhang, J. Serological proteome analysis approach-based identification of ENO1 as a tumor-associated antigen and its autoantibody could enhance the sensitivity of CEA and CYFRA 21-1 in the detection of non-small cell lung cancer. Oncotarget 2017, 8, 36664. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef] [PubMed]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M. AnnotationDbi: Introduction to Bioconductor Annotation Packages. 2015. Available online: http://bioconductor.statistik.tu-dortmund.de/packages/2.11/bioc/vignettes/AnnotationDbi/inst/doc/IntroToAnnotationPackages.pdf (accessed on 11 December 2022).
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]


| Spot No. | Protein Description | MW a (KDa) | pI b | No. of Total Spectra | Coverage (%) | Exclusive Peptides c | MS d Scores (%) | Density Decrease Ratio (%) |
|---|---|---|---|---|---|---|---|---|
| 1 e | Ubiquitin-conjugating enzyme E2 variant 2 | 16 | 7.79 | 57 | 62 | 4 | 100 | 13.1 |
| Ubiquitin-conjugating enzyme E2 variant 1 | 16 | 7.71 | 50 | 57 | 1 | 100 | ||
| 2 e | Annexin A2 | 39 | 7.57 | 23 | 46 | 14 | 100 | 37.3 |
| Outer mitochondrial membrane protein porin 2 | 34 | 7.50 | 20 | 35 | 9 | 100 | ||
| 3 | Alpha-enolase | 47 | 7.01 | 2134 | 76 | 19 | 100 | 60.1 |
| 4 f | Epididymis secretory protein Li 112 | 36 | 6.56 | 86 | 31 | 10 | 100 | 63.9 |
| LIM and SH3 domain protein 1 | 30 | 6.61 | 24 | 39 | 9 | 100 | ||
| Heterogeneous nuclear ribonucleoprotein H3 | 37 | 6.37 | 4 | 10 | 3 | 99 |
| HSPA1A (5) | HSPA1B (4) | LASP1 (4) | PDLIM1 (4) | ENO1 (3) | ANXA2 (3) | ||
|---|---|---|---|---|---|---|---|
| Molecular Function | Cadherin binding | X | X | X | X | X | |
| Cadherin binding involved cell-cell adhesion | X | X | |||||
| Heat shock protein binding | X | X | |||||
| Cellular Content | Focal adhesion | X | X | X | X | ||
| Cell substrate junction | X | X | X | X | |||
| Cell cortex | X | X | X | ||||
| Biological Process | Negative regulation of apoptotic signaling pathway | X | X | X | |||
| Removal of superoxide radicals | |||||||
| Cellular response to oxygen radical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xing, M.; Ma, Y.; Zhang, Z.; Qiu, C.; Wang, X.; Zhao, Z.; Ji, Z.; Zhang, J.-Y. Oridonin Induces Apoptosis in Esophageal Squamous Cell Carcinoma by Inhibiting Cytoskeletal Protein LASP1 and PDLIM1. Molecules 2023, 28, 805. https://doi.org/10.3390/molecules28020805
Zhang X, Xing M, Ma Y, Zhang Z, Qiu C, Wang X, Zhao Z, Ji Z, Zhang J-Y. Oridonin Induces Apoptosis in Esophageal Squamous Cell Carcinoma by Inhibiting Cytoskeletal Protein LASP1 and PDLIM1. Molecules. 2023; 28(2):805. https://doi.org/10.3390/molecules28020805
Chicago/Turabian StyleZhang, Xiaojun, Mengtao Xing, Yangcheng Ma, Zhuangli Zhang, Cuipeng Qiu, Xiao Wang, Zhihong Zhao, Zhenyu Ji, and Jian-Ying Zhang. 2023. "Oridonin Induces Apoptosis in Esophageal Squamous Cell Carcinoma by Inhibiting Cytoskeletal Protein LASP1 and PDLIM1" Molecules 28, no. 2: 805. https://doi.org/10.3390/molecules28020805
APA StyleZhang, X., Xing, M., Ma, Y., Zhang, Z., Qiu, C., Wang, X., Zhao, Z., Ji, Z., & Zhang, J.-Y. (2023). Oridonin Induces Apoptosis in Esophageal Squamous Cell Carcinoma by Inhibiting Cytoskeletal Protein LASP1 and PDLIM1. Molecules, 28(2), 805. https://doi.org/10.3390/molecules28020805





