1. Introduction
Diabetic nephropathy (DN), the main cause of chronic kidney disease and end-stage kidney disease, is a microvascular complication caused by diabetes with a prevalence of 30–40% [
1,
2]. Traditional Chinese Medicine (TCM) contains a variety of effective components, which can produce comprehensive therapeutic effects through multiple channels and multiple targets, and has obvious advantages and characteristics in the prevention and treatment of chronic metabolic diseases. Moreover, TCM has shown a good effect in the treatment of DN [
3,
4,
5].
Ligustri Lucidi Fructus (LLF) is the dried ripe fruit of
Ligubtrum lucidum W.T. Aiton [Oleaceae]; it is often used to nourish the liver and kidney, improve eyesight, and blacken hair in clinical practice [
6]. Among the clinical prescriptions, LLF is a core drug for the treatment of DN [
7]. In the treatment of DN, LLF is often mixed with other TCMs, which can improve the therapeutic effect of TCMs [
8,
9]. Several pharmacological studies have shown that LLF has anti-inflammatory, antioxidant, antitumor, hepatoprotective, and immune-regulative effects [
10,
11,
12,
13,
14]. In addition, research has suggested that LLF can also reduce hypercholesterolemia [
15]. In terms of the chemical composition, LLF includes phenylethanols, iridoids, triterpenoids, flavonoids, etc [
16], of which phenylethanols and iridoids are the main bioactive components. Phenylethanoid components (including salidroside, tyrosinol, hydroxyltyrosinol, acteoside, and echinacoside) have anti-inflammatory, anti-cancer, hypoglycemic, and lipid effects [
17,
18,
19,
20]. The iridoid glycosides, which mainly include specnuezhenide, ligustroside G13 (G13), oleonuezhenide, nuezhenidic acid, neonuezhenide, 1‴-O-β-
d-glucosylformoside, and oleuropein, were reported for have the effect of lowering blood lipids, anti-oxidation action, and immuno-enhancing activity [
21,
22,
23].
Most TCMs are used clinically after processing to reduce toxicity or enhance the curative effect. According to the Chinese Pharmacopoeia, the processing method of LLF is to soak in rice wine [
6]. According to the TCM theory, LLF can strengthen the function of nourishing the liver and kidney after wine-steaming; thus, wine-steamed Ligustri Lucidi Fructus (WLL) is usually used in the clinic. The results showed that the contents of specnuezhenide, G13, oleonuezhenide, neonuezhenide, oleuropein, and nuezhenoside decreased after wine-steaming, while the content of salidroside, tyrosol, and hydroxytyrosol increased [
24,
25]. According to the pharmacology research, LLF steamed with wine can enhance the protective effects on the oxidative stress damage to the testis of rats with kidney-yin deficiency [
26]. It has been reported that the protective effect of WLL on liver injury induced by carbon tetrachloride (CCl
4) in mice was stronger than LLF [
27]. WLL could improve oxidative stress, inhibit the release of inflammatory factors, and regulate endocrine disorders, which can improve and regulate liver and kidney yin deficiency syndrome caused by anger in rats [
28]. The studies have indicated that LLF could effectively recover metabolic disorder of blood glucose and blood lipids in DN rats and has excellent prevention and curing effects for DN [
29]. However, up to now, the differences in the pharmacodynamics in DN between LLF and WLL extracts have not been researched.
Several studies have investigated the pharmacokinetics and distribution of active components of LLF in rats [
30,
31,
32,
33]. The pharmacokinetic properties of LLF indicated that LLF compounds can be better absorbed by the blood and maintained for a long period of time [
30]. Additionally, the UPLC-MS method was used to study the pharmacokinetics of salidroside in control and ovariectomized rats; the results demonstrated that the t1/2, MRT0–∞, and apparent volume of distribution for salidroside increased in ovariectomized rats compared with control rats [
31]. Recently, our research team established a UPLC-MS/MS method, which revealed differences in the pharmacokinetic characteristics of nine active components in LLF and WLL in the plasma of normal rats [
32]. Zhang et al. studied the distribution of seven active compounds of LLF before and after wine-steaming in the main organs and tissues of rats. The results showed that wine-steaming has an improved effect on the tissue distribution of the active components of LLF in rats [
33]. However, a comparison of the pharmacokinetics and distribution of the main active components among raw and wine-processed products of LLF in diseased rats (DN rats) have not been reported.
In this study, a rat model of DN induced by a high-fat-sugar diet (HFSD)/streptozotocin (STZ) was established. Using this animal model, the differences in the protective effects of LLF and WLL on the kidneys of DN rats were tested. Subsequently, the UPLC-MS/MS method was used to determine the eight components in the plasma and kidney of the DN rats. The differences in the pharmacokinetic properties of LLF and WLL in the plasma and the distribution in the kidney were compared in DN rats. This study will provide a basis for clarifying the mechanism of WLL and further study of the clinical efficacy.
3. Discussion
DN is a chronic kidney disease, which is mainly characterized by renal capillary rupture, and glomerular capillary and renal tubular interstitial damage [
36]. The combination of a HFSD diet combined with STZ injection can induce insulin resistance and significantly increase the levels of blood glucose, which in turn triggers DN [
37,
38]. This model is very similar to type 2 diabetes in human [
39]. Here, insulin resistance was induced by administration of HFSD in rats for 4 weeks, followed by STZ peritoneal injection to establish a successful DN rat model, which is similar to that reported in a previous study. High blood glucose can cause damage to some important tissues and organs in the body [
40], producing loss of renal function associated with the elevation of TC, TG, LDL-C, Scr, BUN, and microalbumin levels [
41,
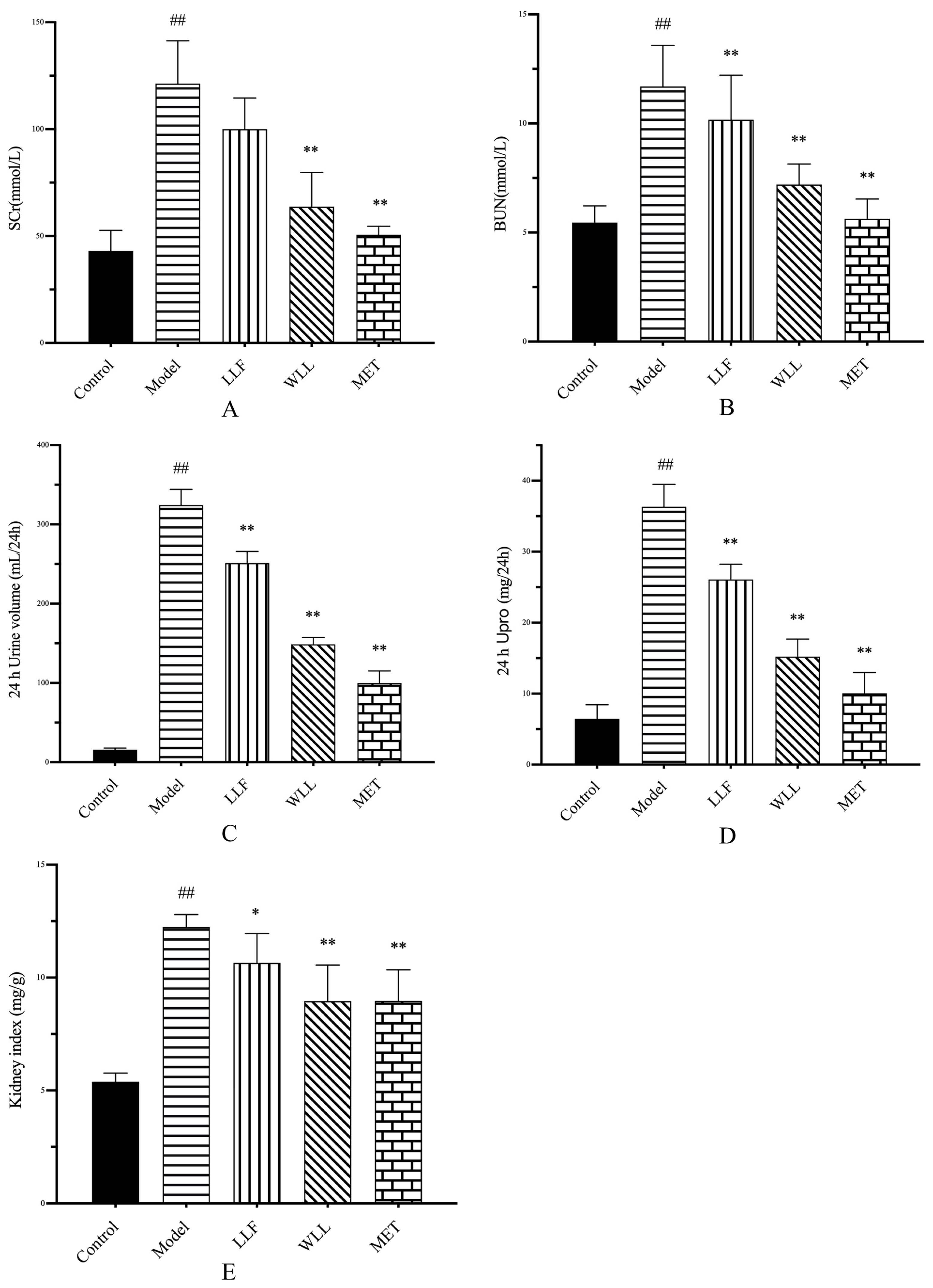
42]. We observed a significant increase in blood glucose levels in the DN rats. However, 8 weeks of treatment with LLF and WLL extracts gradually reduced the level of blood glucose, with the WLL extract showing a better effect than the LLF extract. In addition, DN rats experienced an increase in water intake and food consumption, and a decrease in body weight due to prolonged hyperglycemia. Surprisingly, treatment with LLF and WLL extracts produced body weight gain, and water intake and food consumption decreased. Furthermore, the kidney index of DN rats has increased due to kidney hypertrophy. Significant changes in parameters related to kidney structure and function were observed after administration, revealing the protective effect of WLL and LLF extracts on kidney damage caused by DN. All structural and functional abnormalities were restored following administration of LLF and WLL extracts; the effect of the WLL extract was better than that of the LLF extract.
SCr and BUN are considered as clinical indicators of DN [
43]. High levels of SCr and BUN were found in DN rats, while LLF and WLL extracts reduced these levels. Thus, LLF and WLL extracts exhibited a dramatic protective effect against DN; the effect of the WLL extract was better than that of the LLF extract. Tubular and glomerular injury are often accompanied by high levels of urinary protein [
44]. The 24-h Upro of DN rats increased significantly. After treatment with LLF and WLL extracts, the urine excretion of these proteins was significantly reduced; the effect of the WLL extract was better than that of the LLF extract.
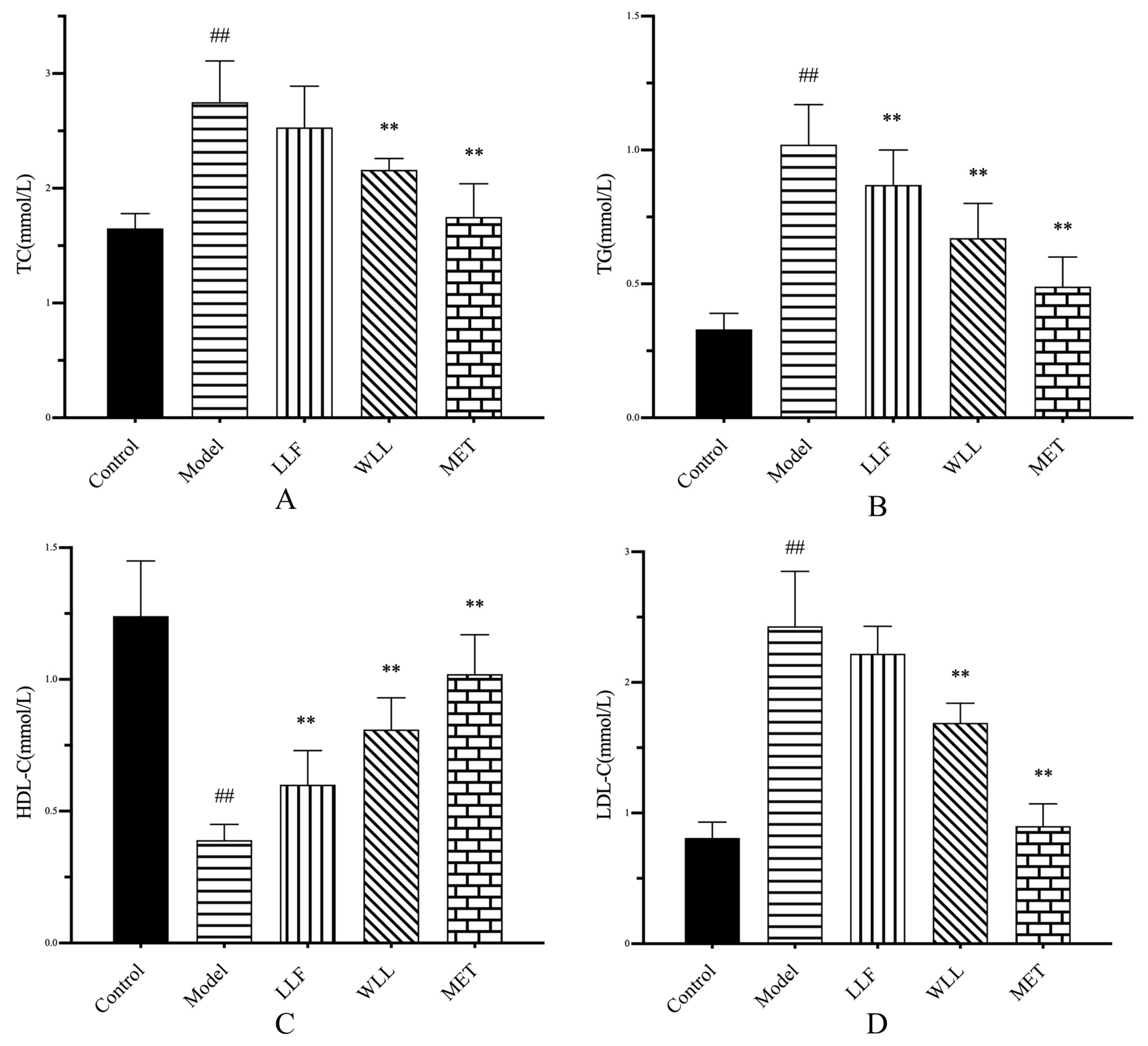
Hyperlipidemia is an important cause of kidney damage. Kidney disease is closely related to high levels of TG, TC, and LDL-C, and a low level of HDL-C [
41]. In this study, we found that LLF and WLL extracts could restore high LDL-C and low TG, TC, and HDL-C levels to different degrees, indicating that the protective role against DN of WLL was better than that of LLF.
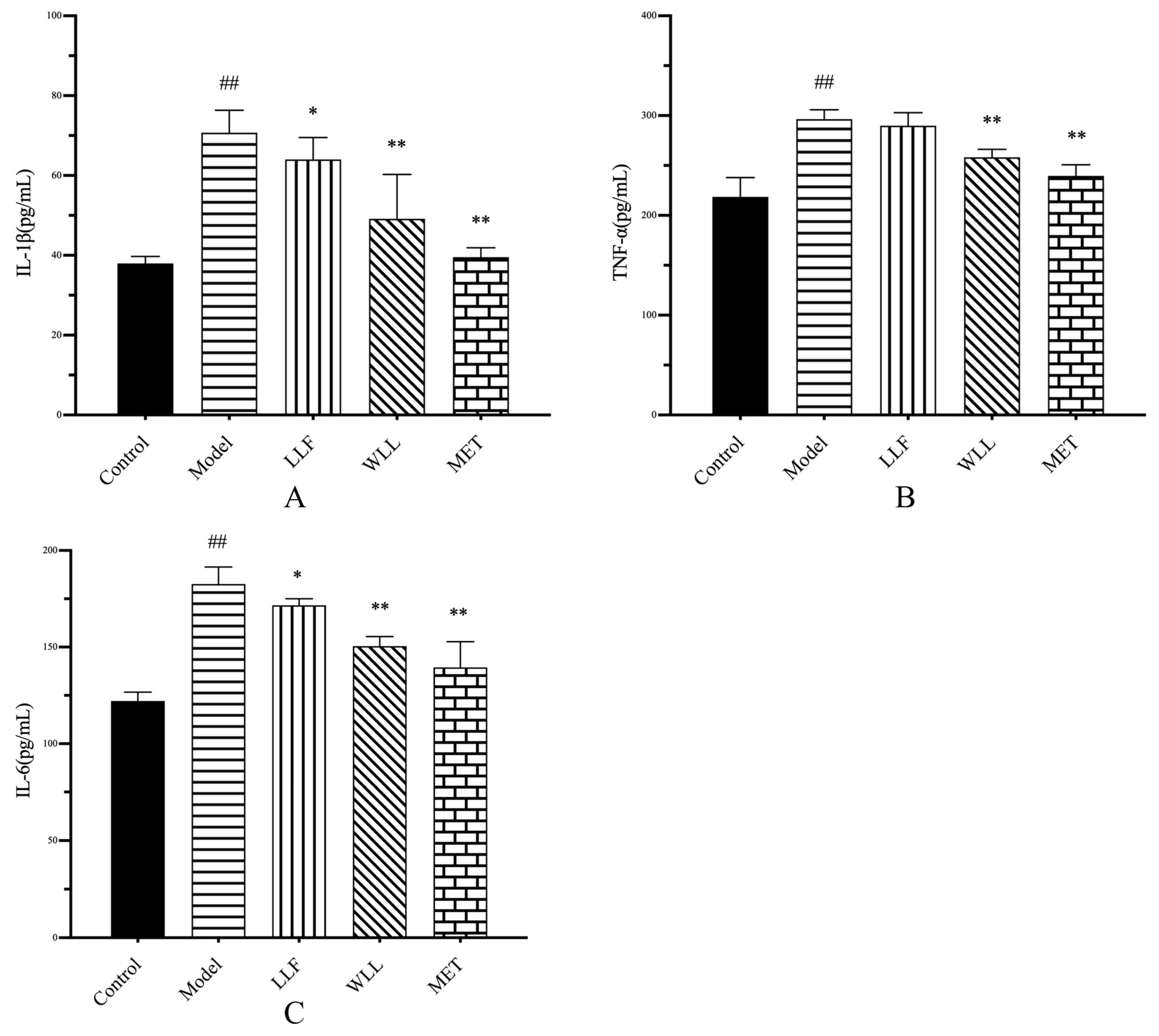
Cytokines are important parameters to identify kidney injury, and several proinflammatory mediators such as TNF-α, IL-6, and IL-1β are closely related to the progression of DN [
45]. Oral administration of LLF and WLL extracts can significantly inhibit the release of pro-inflammatory mediators (TNFα, IL-6, and IL-1β) in the serum of DN rats, indicating that the anti-inflammatory effects of LLF and WLL extracts may be related to kidney protection in DN; meanwhile, the anti-inflammatory effect of the WLL extract was better than that of the LLF extract.
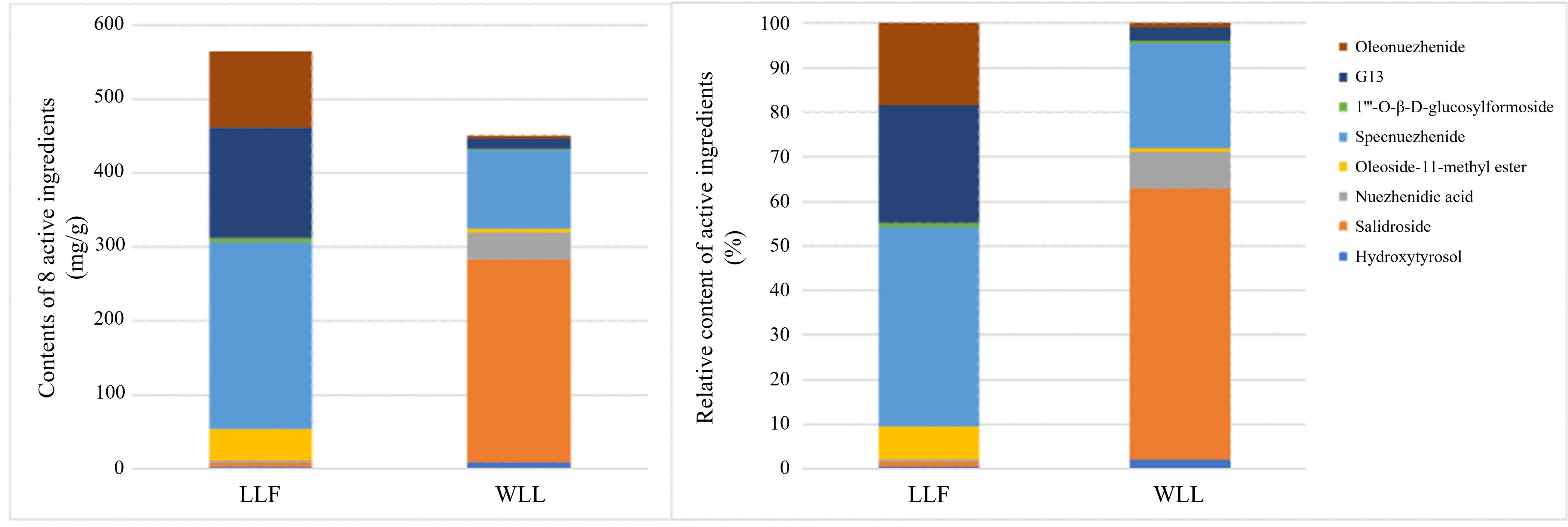
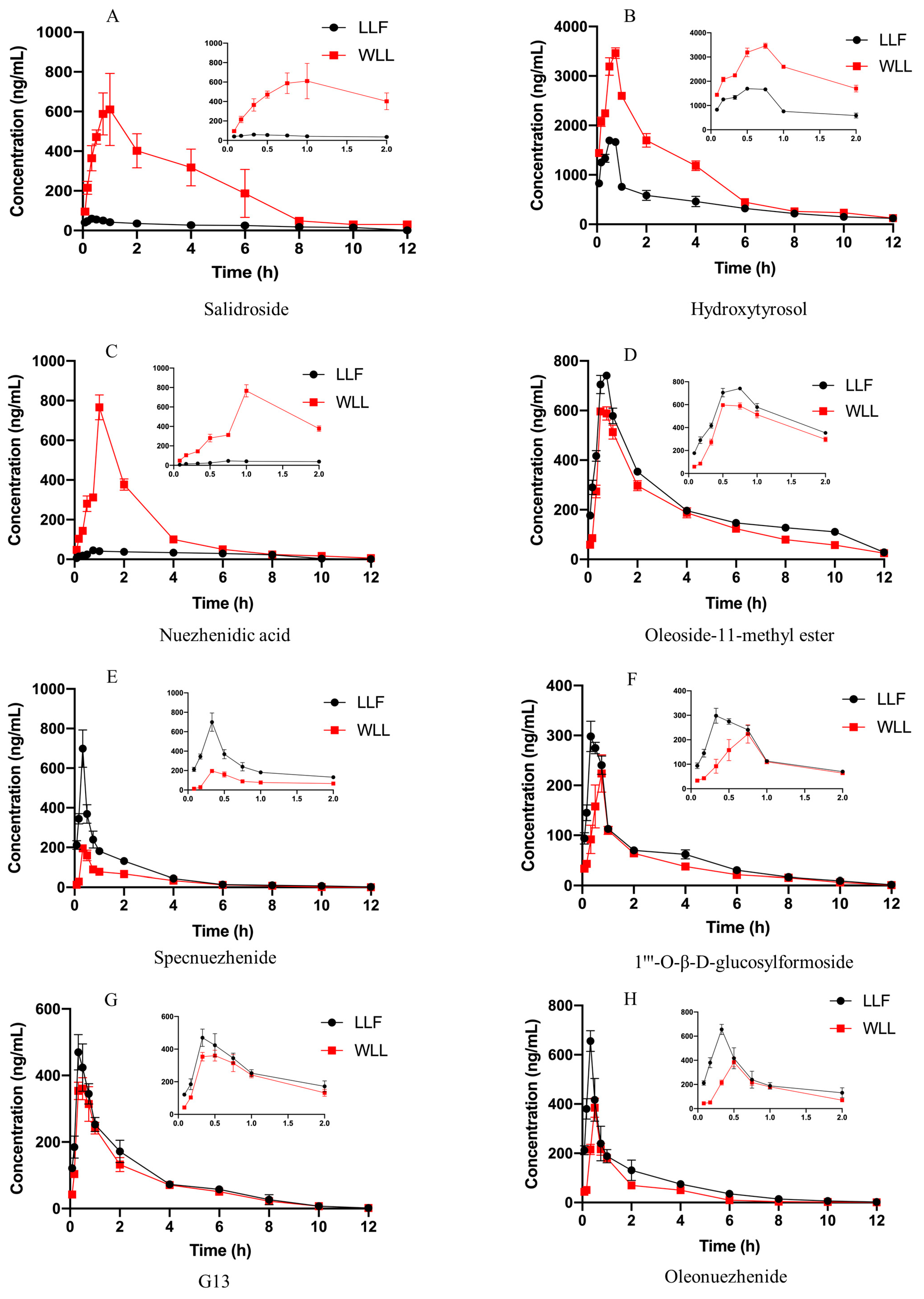
LLF mainly contains iridoids and phenylethanoids, which have good pharmacodynamic activity. Our previous experiments showed that the ethyl acetate and n-butanol components of LLF had protective effects in DN rats. The results of the HPLC demonstrated the effectiveness of hydroxytyrosol, salidroside, nuezhenidic acid, oleoside-11-methylester, specnuezhenide, 1‴-O-β-D-glucosylformoside, G13, and oleonuezhenide, which have a high content [
46]. We speculated that these components might be the effective material basis in the treatment of DN. It has been reported that salidroside has a protective effect on the kidney of DN rats. Salidroside, tyrosol, and hydroxytyrosol have hypoglycemic, anti-inflammatory, and hypolipidemic effects [
47,
48]. Specnuezhenide and salidroside are characteristic components of WLL, which have been used as content determination indexes for quality control of Pharmacopoeia 2020 [
6]. Therefore, we studied the pharmacokinetics and kidney distribution of these eight components in LLF and WLL extracts in DN rats. Firstly, a UPLC-MS/MS method was used for rapid and accurate quantification of the eight major bioactive components in the plasma of DN rats. Then, the main pharmacokinetic parameters and kidney distribution of the eight main active components in LLF and WLL extracts were analyzed. Compared with LLF, the AUC
0–12 h and C
max values of salidroside, hydroxytyrosol and nuezhenidic acid increased, while oleoside-11-methyl ester, 1‴-O-β-
d-glucosylformoside, specnuezhenide, G13, and oleonuezhenide were decreased in WLL. In kidney tissue, the concentration of salidroside, hydroxytyrosol, and nuezhenidic acid increased in WLL compared with LLF, while oleoside-11-methyl ester, 1‴-O-β-
d-glucosylformoside, specnuezhenide, G13, and oleonuezhenide decreased in WLL compared with LLF. The pharmacokinetic characteristic parameters of the AUC
0–12h and C
max of LLF were significantly different from those of WLL, indicating that steaming with wine could improve the bioavailability of LLF. Our previous research showed that the macromolecule iridoid glycosides are transformed into small molecule phenylethanol components, which are more easily absorbed by the body [
49]. The pharmacokinetic study also showed that salidroside had a higher bioavailability and was more conducive to body absorption than specnuezhenide and G13 [
50]. In our experiment, the WLL had an enhanced kidney protective effect in DN. It is speculated that the enhancement of kidney efficacy of LLF after wine-steaming may be related to the conversion of macromolecule iridoid glycosides into small molecular phenylethanol components, which is more conducive to absorption.
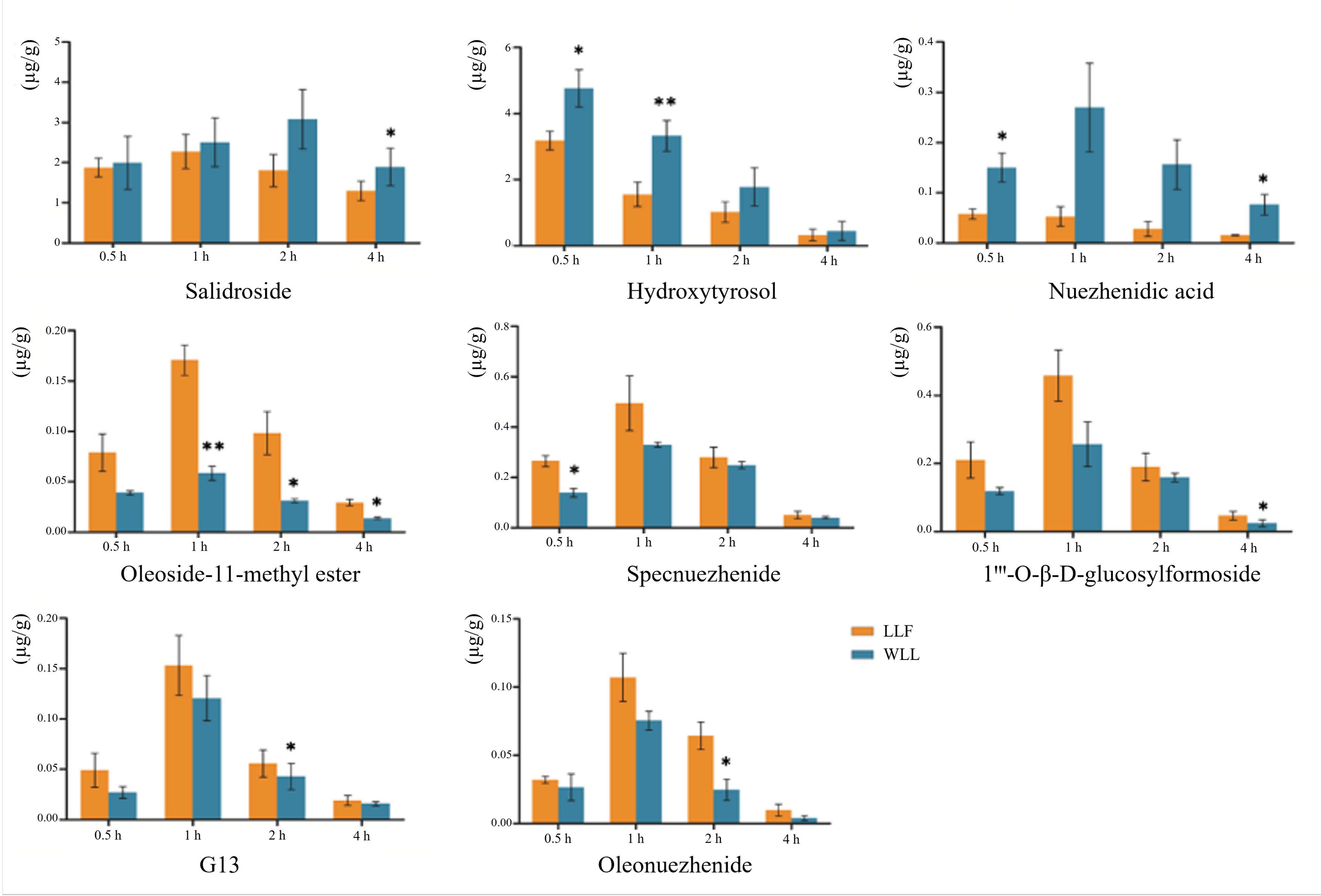
In addition, it was found that after wine-steaming, the contents of oleoside-11-methyl ester, 1‴-O-β-d-glucosylformoside, specnuezhenide, G13, and oleonuezhenide decreased, but the decrease in the AUC0–12h and Cmax in DN rats was much less than the contents in DN rats. The same trend was seen in the distribution of kidney tissue. It is speculated that wine-steaming promoted the absorption of these components and increased the bioavailability of these components in DN rats. The results showed that the effect of WLL was enhanced not only because the macromolecular substances were transformed into easily absorbed small molecules, but also because the wine-steaming promoted the absorption of substances, which further proves the theory of the effect of LLF wine-steaming.
4. Materials and Methods
4.1. Chemicals and Reagents
Crude products of LLF were purchased from Jianlian Shengjia TCM Co. Ltd. (Shandong Province, Jinan, China), which were identified by Professor Li Feng of TCM identification at Shandong University of TCM. Voucher specimen Nos. SDCM-YZ2019040601 were stored in the School of Chinese Materia, Shandong University of TCM (Jinan, China). The specimens were deposited at the laboratory of the author. Acetonitrile, methanol, and formic acid were provided by Fisher Scientific (Waltham, MA, USA). Purified water was purchased from watsons (Jinan, China). Other reagents were of analytical grade. Serum creatinine (SCr), blood urea nitrogen (BUN), 24-h urinary albumin, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterin (LDL-C), triglyceride (TG), and total serum cholesterol (TC) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute. ELISA kits for TNF-α, IL-6, and IL-1β were purchased from Wuhan Genmei Biotechnology Co, Ltd. Streptozocin (STZ) was purchased from Sigma Chemicals Co. (St. Louis, MO, USA). Metformin (MET) was purchased from Shanghai Squibb Pharmaceutical Co. Ltd. (Shanghai, China). Metformin was ground into a powder with a mortar before dissolution.
The standards for G13, oleonuezhenide, nuezhenidic acid, and oleoside-11-methyl ester were purchased from Shanghai Yilin Biotechnology Co., Ltd. (Shanghai, China), specnuezhenide was purchased from Chengdu Biopunify Phytochemicals Co., Ltd. (Chengdu, China), hydroxytyrosol and geniposide were provided by Chengdu Must Biotechnology Co., Ltd. (Chengdu, China), and 1‴-O-β-D-glucosylformoside and salidroside were obtained from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). The purity of these standards was greater than 98%. The chemical structures are presented in
Figure 11.
4.2. Instruments
The HPLC system consisted of a 1430 diode array detector, 1110 pumps with a high-pressure mixer, a 1310 column oven, and 1210 autosampler (Hitachi, Tokyo, Japan). The chromatographic separation of the analyte was achieved by a Kromasil C18 column (5 μm, 250 mm × 4.6 mm, Akzo Nobel N.V, Amsterdam, The Netherlands).
The UPLC system (Agilent 1260 series; Santa Clara, CA, USA) was equipped with a solvent degasser, G1311B quaternary pump, and G1329B automated injector. A Halo® C18 column (2.1 × 100 mm, 2.1 μm) was used for elution. An Agilent 6470 triple quadrupole mass spectrometer (Santa Clara, CA, USA) equipped with ESI source was used for mass detection.
4.3. Preparation of WLL
WLL was prepared in the laboratory. LLF was added with appropriate amount of rice wine and water, soaked for 4 h until soft, placed in a closed container, steamed for 24 h until the surface turned black, and then dried in a 50 °C drying oven.
4.4. Preparation of LLF and WLL Extracts
LLF was powdered by pulverizer, accurately weighed to 1.0 kg, and underwent ultrasonic extraction with 50% ethanol three times for 1 h each time; the ratios of plant/ethanol used were 1/10 (w/w). The filtrate was combined and ethanol was recovered. The total crude extract of LLF was purified by macroporous resin to obtain the LLF extract, which was accurately weighed. The preparation of WLL extract was the same as the above procedure. The two samples obtained were used in subsequent experiments.
4.5. Quantitative Analysis of LLF and WLL Extracts by HPLC
Quantitative determination of the eight ingredients in LLF and WLL extracts were performed. Before analysis, the LLF and WLL extracts (0.10 g) were dissolved in 50 mL of 50% methanol and filtered through a 0.22 μm membrane.
The chromatographic conditions were established by our team in the early stage [
51]. The Kromasil C18 column (250 mm × 4.6 mm i.d., 5 μm particle size) was used, the mobile phase was acetonitrile (solution A) and 0.1% formic acid aqueous solution (solution B). The flow rate was 1 mL/min, the column temperature was 20 °C, and the injection volume was set to 10 μL. The sequence of gradient elution was as follows: 0–10 min with 7–12% A; 10–35 min with 12–25% A; 35–52 min with 25–41% A. The wavelength of the UV detector was fixed at 280 nm (hydroxytyrosol and salidroside) and 240 nm (nuezhenidic acid, oleoside-11-methyl ester, specnuezhenide, 1‴-O-β-
d-glucosylformoside, G13, and oleonuezhenide) for determination.
4.6. Experimental Animals
Five-week-old male Sprague-Dawley (SD) rats, weighing 150 ± 20 g, were purchased from the Jinan Pengyue experimental animal breeding co. Ltd. (Shangdong, Jinan, China). Rats were reared in a 12 h light/dark cycle at 25–30 °C with free access to drinking water and food. Before the experiment, the animals were bred adaptively for 1 week. All animal experiments were performed in accordance with the guidelines established by the Institutional Animal Protection and Use Committee of Shandong University of TCM.
4.7. Establishments of DN Rat Models
A high-fat-sugar diet (HFSD)/streptozotocin (STZ) regimen was used to experimentally induce DN in rats. Before the experiment, the rats were adaptively reared for 1 week. The rats in the control group were maintained on a standard diet, and the model rats were fed a HFSD for 4 weeks. The HFSD was composed of 18% fat, 20% sucrose, 3% cholesterol, and 59% basic food. After 4 weeks, the rats were fasted for 12 h. Then, the rats in the Model groups were intraperitoneally injected STZ at 50 mg/kg dissolved in 0.05 mol/L citrate buffer (pH = 4.5). The rats in the Control group received the same volume of citrate buffer as the body weight standard. The blood glucose level of the rats was measured 3 days after STZ injection. Rats with blood glucose levels over 16.6 mmol/L were used as the follow-up experimental model rats.
4.8. Pharmacodynamic Study of LLF and WLL Extracts in DN Rats
4.8.1. Experimental Procedure
DN rats were randomly divided into the Model group, LLF group, WLL group, and MET group, with 10 rats in each group. Each group was treated as follows: Control group and Model group were treated with an equal volume of distilled water. The LLF group was given 2.12 g/kg extract of LLF and the WLL group was given 1.89 g/kg extract of WLL, both of which were equivalent to 15 g/kg raw LLF. The MET group was treated with 200 mg/kg metformin. All rats were orally administered once every morning. Blood was collected from the tail vein every 2 weeks to measure blood glucose levels. After the last treatment, the food consumption, water consumption, and body weight of the rats were measured. The rats were placed in a metabolic cage to collect urine samples for 24 h. The collected urine samples were centrifuged at 3000 rpm for 5 min, and the supernatant was taken and stored at −20 °C until use. Blood samples were collected through the abdominal aorta after the rats were anesthetized with isoflurane; samples were centrifuged at 3000 rpm for 10 min to separate the serum and stored at −80 °C for biochemical analysis of kidney function. Finally, the two kidneys of each rat were collected and weighed to calculate the kidney/body mass index. A kidney was taken for histopathological evaluation.
4.8.2. Urine Analysis and Measurement of Biochemical Parameters
24-h urinary protein was measured using the auto spectrophotometer (Shimadzu, Kyoto, Japan). The levels of SCr and BUN were measured using the Spectra Max M 5 automatic microplate reader (Molecular Devices, Inc., San Jose, CA, USA).
4.8.3. Analysis of Lipid Profiles
The effect of LLF and WLL extracts on the lipid profiles was measured in serum using automatic biochemical analyzer, according to the manufacturer’s instructions.
4.8.4. Assessment of Inflammatory Cytokines
We investigated the inflammatory cytokines responsible for DN, including TNF-α, interleukin 1 β (IL-1β), and interleukin 6 (IL-6). The concentrations of inflammatory cytokines were measured using ELISA assay kits, according to the manufacturer’s instructions.
4.8.5. Histopathological Examination
Kidney tissue was collected and stored in 10% paraformaldehyde immediately. Paraffin sections of the kidney were used for hematoxylin and eosin (H&E) staining to observe pathological changes of the kidney tissue.
4.9. Plasma Pharmacokinetics in Rats
4.9.1. UPLC-MS/MS Conditions
The mobile phase consisted of 0.1% (v/v) formic acid-water (solvent A) and acetonitrile (solvent B). Gradient elution was carried out according to the following program: 15–25% B (0–3 min), 25–75% B (3–4 min), 75% B (4–6 min), 75–15% B (6–7 min), and 15% B (7–9 min). The column temperature was set at 35 °C, the flow rate was 0.3 mL/min, and the injection volume was 5 μL. The mass spectrometer was set at negative ionization mode. The multiple reaction monitoring (MRM) mass scan mode was implemented. The optimized parameters were programmed as follows: nebulizer gas pressure, 50 psi; capillary voltage, 3500 V; cell acceleration voltage, 4 V; gas temperature, 350 °C; dwell time, 30 ms, and flow rate, 11 L/min. The precursor ion/product ion were m/z 229.1/199.2 for salidroside, 153.1/123.1 for hydroxytyrosol, 433.2/209.1 for nuezhenidic acid, 403.1/223.1 for oleoside-11-methyl ester, 685.2/299.0 for 1‴-O-β-D-glucosylformoside, 685.5/453.3 for specnuezhenide, 1071.3/771.5 for G13, 1071.4/909.3 for oleonuezhenide, and 411.1/217.0 for the internal standard (IS, geniposide).
4.9.2. Preparation of Standard Solutions, Calibration Samples, and Quality Control Samples
An appropriate amount of each standard solution was dissolved in methanol to prepare a mixture containing salidroside, hydroxytyrosol, nuezhenidic acid, oleoside-11-methyl ester, 1‴-O-β-d-glucosylformoside, specnuezhenide, G13, and oleonuezhenide stock standard solutions. The mixed stock solution was serially diluted with methanol to obtain a series of working standard solutions. The internal standard stock standard solution of geniposide in methanol was similarly diluted to produce a working solution of 600 ng/mL. The diluted stock solution was added to blank rat plasma to prepare the calibration curve working solution. Quality control samples of the eight markers were prepared from blank plasma with high, medium, and low concentrations.
4.9.3. Plasma Sample Treatments
One-hundred microliters of plasma sample was placed in a 1.5 mL EP tube and 10 μL of internal standard working solution (600 ng/mL geniposide) and 300 μL of methanol were added.
The mixture was vortexed for 3 min, centrifuged for 10 min, and the supernatant was taken and dried with nitrogen; the residue was reconstituted by adding 100 µL of the initial mobile phase. Sixty microliters of the prepared sample was transferred to a liquid phase vial, and 5 μL of the prepared sample underwent UHPLC-MS/MS analysis.
4.9.4. Plasma Sample Analysis
Before the pharmacokinetic study, the DN rats were fasted for 12 h, during which time they had free access to drinking water. Twelve rats were randomly divided into two groups, with six rats in each group. Rats were orally administered LLF and WLL extracts with a dose equivalent to 15 g/kg raw LLF. At 0, 0.083, 0.167, 0.333, 0.50, 0.75, 1, 2, 4, 6, 8, 10, and 12 h after administration, blood samples (500 μL) were collected from the rat orbit and placed in a 1.5 mL heparin sodium anticoagulant centrifuge tube. The sample was immediately centrifuged at 4000 rpm for 10 min and the plasma was stored at −20 °C for subsequent analysis. Data analysis was performed using the DAS 2.0 pharmacokinetic software (Shanghai, China).
4.10. Kidney Distribution in Rats
4.10.1. Kidney Tissue Collection
DN rats were randomly divided into two groups: the LLF extract group and WLL extract group. The two groups were orally given the same dose of extracts equivalent to raw LLF. Tissues of kidneys were taken at 0.5, 1, 2, and 4 h after oral administration. The blood and contents on the tissue surface were washed with 0.9% saline solution, dried, and stored at −20 °C before analysis. Six batches were determined in parallel.
4.10.2. Kidney Sample Pretreatment
Four-hundred micrograms of kidney tissue was weighed, cut into small pieces, and normal saline was added at a ratio of 1:2 (w/v). Tissue homogenate was prepared under ice bath conditions, centrifuged at 12,000 rpm for 5 min, and 100 µL supernatant was taken; 10 µL internal standard solution and 10 µL methanol solution were added, rotated, and mixed for 3 min. Next, 300 µL of methanol solution was added, vortexed for 3 min, centrifuged at 10,000 rpm for 10 min, followed by removal of the supernatant and drying with nitrogen. Then, 100 µL of the initial mobile phase was added to the residue, vortexed and mixed for 3 min, and 5 µL of sample was taken for analysis.
4.11. Statistical Analyses
All data were expressed as mean ± standard deviation (SD). Analysis was performed with SPSS software 21.0 (IBM, New York, NY, USA). The statistical significance of the pharmacodynamics, pharmacokinetics, and tissue distribution parameters obtained from each group were evaluated. The data were evaluated using one-way analysis of variance (ANOVA). p < 0.05 was considered statistically significant, and p < 0.01 was considered very significant.