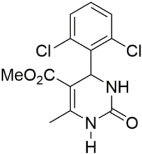
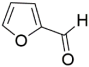
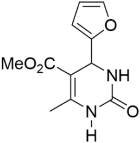
Abstract
Hypertension and cardiovascular diseases related to it remain the leading medical challenges globally. Several drugs have been synthesized and commercialized to manage hypertension. Some of these drugs have a dihydropyrimidine skeleton structure, act as efficient calcium channel blockers, and affect the calcium ions’ intake in vascular smooth muscle, hence managing hypertension. The synthesis of such moieties is crucial, and documenting their structure–activity relationship, their evolved and advanced synthetic procedures, and future opportunities in this area is currently a priority. Tremendous efforts have been made after the discovery of the Biginelli condensation reaction in the synthesis of dihydropyrimidines. From the specific selection of Biginelli adducts to the variation in the formed intermediates to achieve target compounds containing heterocylic rings, aldehydes, a variety of ketones, halogens, and many other desired functionalities, extensive studies have been carried out. Several substitutions at the C3, C4, and C5 positions of dihydropyrimidines have been explored, aiming to produce feasible derivatives with acceptable yields as well as antihypertensive activity. The current review aims to cover this requirement in detail.
1. Introduction
Currently, the whole world is still recovering from the COVID-19 (coronavirus disease, 2019) pandemic, which has led to millions of deaths. Hypertension has been recognized as one of the major and most common risk factor leading to adverse and severe outcomes in COVID-19 patients [1]. However, years prior to the outbreak of COVID-19, in the year 2003, “The Global Burden of Disease Study” from the World Health Organization (WHO) identified hypertension as a leading risk factor worldwide for morbidity as well as mortality [2]. Several cardiovascular diseases (CVDs) and even diabetes have been associated with hypertension, and serious concerns have been raised in the research community regarding the treatment and management of hypertension in human beings. Amongst the drugs developed for pharmacological applications, the contribution of heterocycles is tremendous. The base pairs of RNA and DNA are composed of heterocyclic structures, such as pyrimidine and purine [3]. Pyrimidines have established therapeutic properties in various cases, such as anti-inflammatory, antimicrobial, anticancer, and anti-analgesic effects.
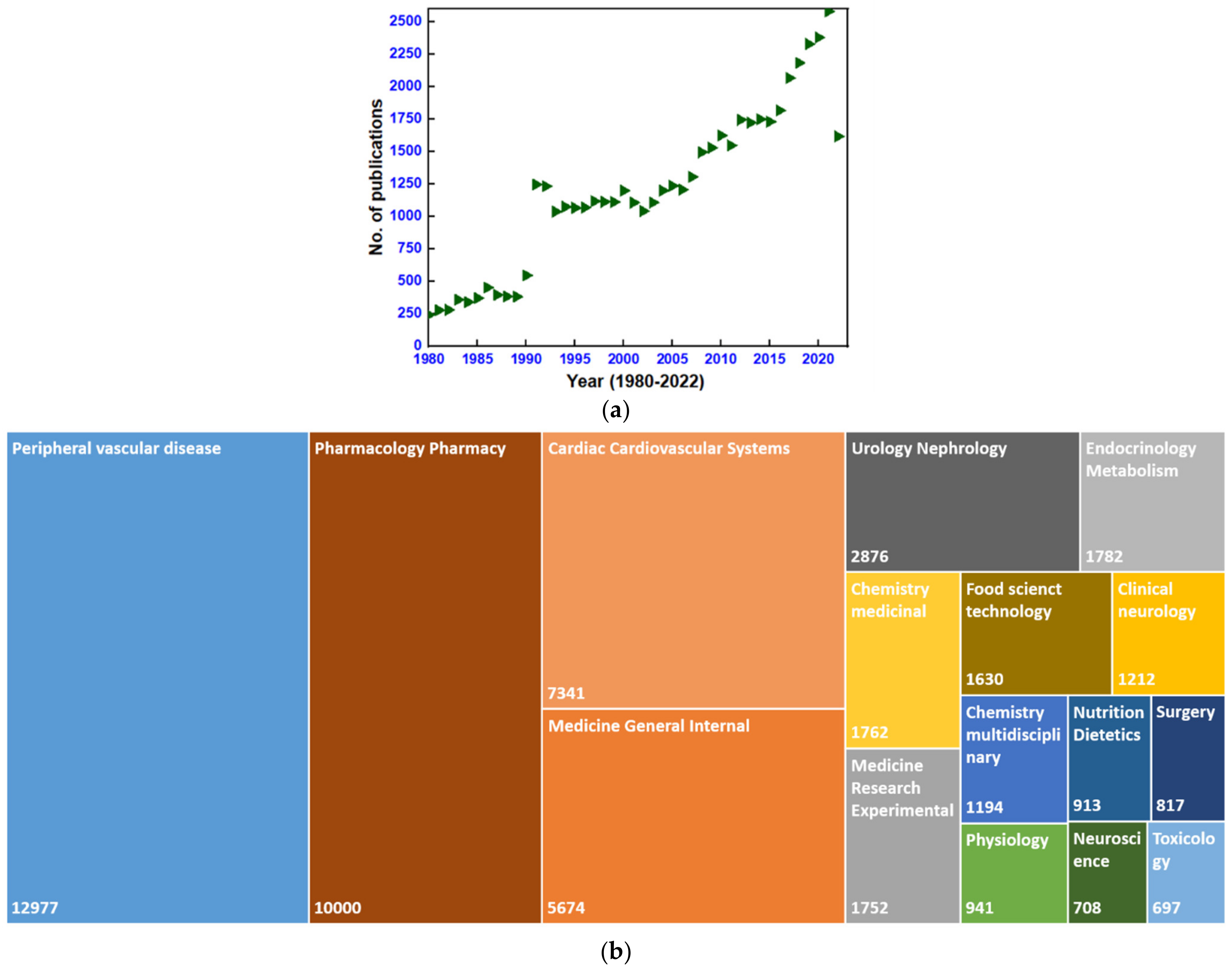
Remarkably, since the synthesis of dihydropyrimidines (DHPMs) by the Italian chemist, Pietro Biginelli, in the year 1893, the determination of passionate researchers has led to modifications of the Biginelli condensation reaction, leading to the synthesis of DHPMs which can be used to achieve feasible yields and hassle-free experimental conditions. The efforts made to increase the diversity in the pharmacological activities of the derivatives of DHPMs containing pyrimidine scaffold have been tremendous [4]. However, after identifying DHPM scaffolds with antihypertensive activity as calcium channel antagonists, an abundance of literature has been produced. Plentiful research and review articles concentrating on calcium channel blockers [5,6,7,8,9], antihypertensive drugs [10,11,12], and the synthesis of DHPMs derivatives [13,14,15,16] with different uses are available from various platforms. The Web of Science (WOS) database was searched on 12 October 2022 with the keywords dihydropyrimidines or calcium channel blockers from the year 1980 to date, indicating a total of 51,647 publications on this topic. Figure 1 summarizes the number of publications produced per year as well as publications released in various fields of research. The current review, however, is focused on the same data due to the lack of review publications describing the synthesis of DHPMs in recent years. The present review article mainly considers the synthesis of DHPMs which are isosteres of nifedipine, providing an introduction of the different types of calcium channels and their modulators.
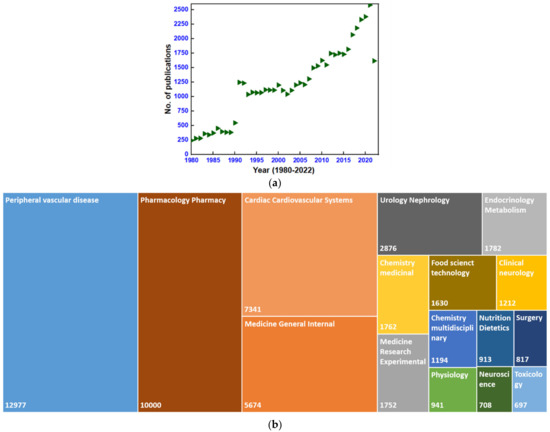
Figure 1.
(a) Point graph presenting yearly publications and (b) a tree graph presenting publications in different areas of research (Web of Science search data obtained on 22 October 2022 using keywords dihydropyrimidines or calcium channel blockers, from January 1980 to October 2022).
2. Calcium Channels
The calcium channels play a vital role in the normal functioning of the heart. Variations in their functioning disrupt the plateau phase of cardiac operations and may trigger irregular conduction cycles, resulting in cardiac dysfunction [17,18]. The primary purpose of the calcium channel is to carry calcium ions into cells [19,20]. The influx of calcium ions from the extracellular space to cells increases the positive potential of cell membranes, and the calcium ions released from the sarcoplasmic reticulum indicate the contraction of cardiac myocytes [21]. This process is widely known as “calcium-induced calcium release” (CICR) [22]. In anomalous cases, calcium channel modulators serve as significant pharmacological objectives, for which the study of the structural and functional features of the cardiac tissues is crucial. Depending on the pharmacological sensitivity and activation rate, so far, six types of calcium channels have been observed. These include T, L, N, P, Q, and R types [23], which are named according to their responses to the voltage applied. The most significant calcium channel subtypes are L-type and T-type, which play key roles in regulating heart functions. Both L-type and T-type are voltage-gated. L-type channels are long-lasting and their activation requires resilient depolarization, whereas T-type channels are transient [23]. A comprehensive understanding of the structural properties of these channels opens a wider pathway through which to identify their importance in cardiovascular pathologies.
L-type channels possess a long opening with a greater number of voltage-gated channels. In the course of membrane depolarization, the calcium influx occurs and offers an “excitation-contraction coupling”, in which L-type calcium channels are the crucial contributors [24,25]. These channels are expressed at the developing and adult stages of the heart. These channels are made of one pore-forming α sub-unit and another three β, δ, and γ accessory subunits. All these four subunits together will contribute to managing the current kinetics, membrane trafficking, and gating features [26]. In an early developmental stage, dilated left ventricles and restricted out-flux tracts are the result of the blocking of L-type channels.
In contrast to L-type channels, T-type channels are transient and feature low-voltage depolarization. With the development of the stages, the functionality of T-type channels decreases [27]. These are expressed in atrial cells rather than ventricular myocytes in the hearts of adults [28]. Similar to the L-type, T-type channels are composed of four homologous subunits containing S1 to S6 transmembrane helices [29]. Contributing to the pacemaker activity, their dysfunction would lead to bradycardia [30]. Some pathological complications are caused when T-type channels are present in atrial as well as ventricular myocytes [27]. When aberrant T-type channels are present, hypertrophied myocytes are seen, and blocking these channels increases cardiac fibrosis and impairs relaxation [30]. In order to rectify the issues associated with calcium channels, relying on the situation and demand, calcium channel modulators or blockers are designed and employed.
3. Calcium Channel Blockers
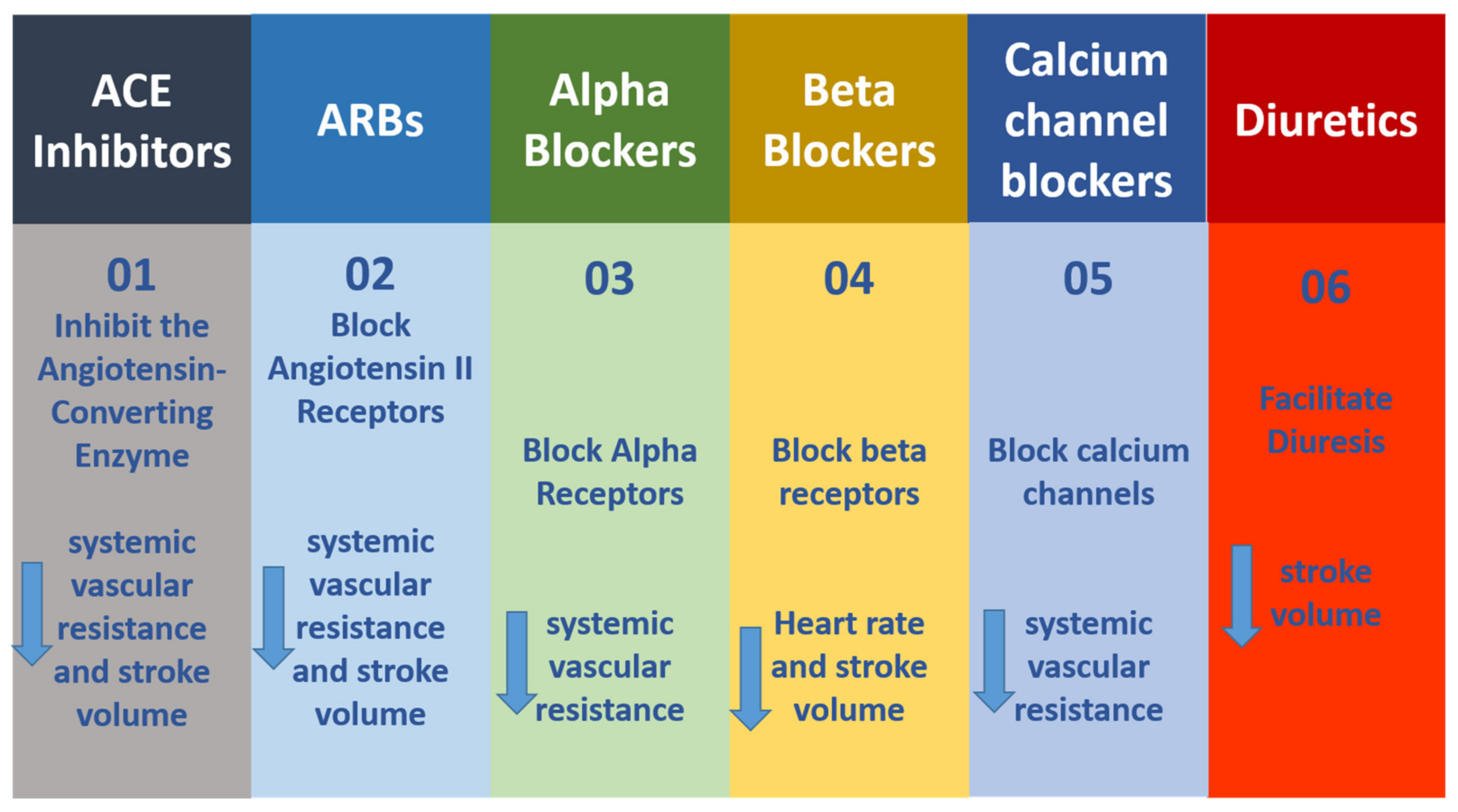
One in three persons faces hypertension, one of the primary heart-related disorders. Hypertension is a condition in which the heart pumps the blood with a force greater than the normal, pushing against the artery walls. There are seven types or classes of antihypertensive agents, and these are detailed in Figure 2. Each class has a different mechanism of action in hypertension reduction. The calcium channel blockers (CCBs) or calcium channel antagonists are such agents used to manage hypertensive conditions. These blockers avert the entry of calcium ions into the conducting cells and reduce the heart relaxation and contraction rate.
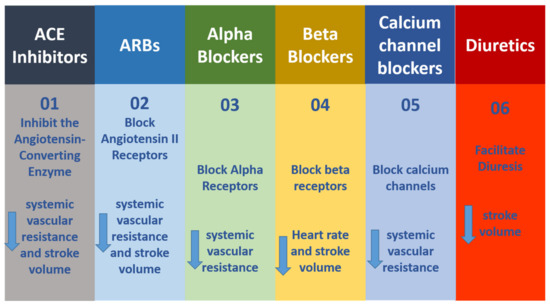
Figure 2.
Seven classes of antihypertensive agents and their mechanisms of action. (01) ACE inhibitors; (02) Block Angiotensin II Receptors; (03) Alpha Blockers; (04) Beta Blockers; (05) Calcium channel blockers; (06) Diuretics.
Under CCBs, there are two main categories: one is dihydropryridines and the other is non-dihydropyridines. Dihydropyridines mainly work in blood vessels and show lesser activity in the heart, whereas non-dihydropyridines act in the opposite way. As this review intends to focus on the dihydropyridimins as CCBs, the next sections will explain their mechanism of action and the procedures used for their synthesis.
4. Dihydropyrimidines as Calcium Channel Blockers
Three classes of CCBs can be identified: dihydropyridines, benzothiazepines, and phenylalkylamines. All of these vary in terms of their fundamental chemical skeleton as well as their relative selectivity for cardiac and vascular L-type channels. However, dihydropyridines are the most hassle-free and smooth muscle-selective CCBs. They are primarily used as hypertensive drugs, as they decrease the arterial pressure and systemic vascular resistance. This mode of action leads to reflux cardia stimulation and strongly enhances the demand of myocardial oxygen. The drug interaction at the cellular levels was studied extensively for the receptor sites of 1,4-dihydropyridines [31]. Nifedipine, nicardipine, nitredipine, amlodipine, and felodipine are some of the major CCBs which are used in clinical settings to treat cardiovascular disorders related to angina and hypertension [32]. Several SAR (structure–activity relationships) have been established for these molecules [33]. Aryl rings of these molecules substituted with different functionalities are positioned axially in the case of receptor-bound conformation. The synperiplanar orientation is preferred by C-4 aryl substituents. A cis-orientation is preferred by the ester group with respect to the C-5 and C-6 double bond. If the C-2 or C-6 substituents and the C-3 and C-5 esters are equivalent, then the molecule is said to have nonchiral Cs symmetry. However, position C-4 is a chiral center with unsymmetrical substitutions. Moreover, unsymmetrical dihydropyridine enantiomers normally differ in their biological actions. Now and then, they can also show opposite actions similar to that of CCBs (calcium antagonists) v/s calcium agonists [34]. Although nifedipine is a highly successful drug, its short plasma half-life [35], which is due to the pyridine metabolic oxidation, is its major limitation [36]. Due to this, the frequent administration of the drug is crucial in achieving clinical efficacy. Substituted 2-chloro-1,4 dihydropryridine exhibited prolonged CCB activity compared to C-2 and C-6 methyl substituted 1,4 dihydropyridines [37].
Pyrimidines, which have similar structural skeletons of pyridines, are identified as the vital component of nucleic acid and play an integral role as biologically significant antitumor, antiviral, and cardiovascular agents. Unlike light-sensitive and aromatic 1,4-dihydropyridines, the N-3 substituted DHPMs are shown to possess promising stability [38]. Analogous to 1,4 dihydropyridines, DHPMs have been shown to have a boat conformation for the modulation of their activity. The ester carbonyl cis orientation and the up and down orientation of pseudoaxial aryl moieties substituted at the C-4 position of DHPM receptors suggest CCB as well as calcium channel agonistic activity, respectively [33].
The “functional interplay between DHPM receptor L-type calcium channels and ryanodine receptor calcium-release channel (RyR)” is highly important in SAR studies. The α1S subunit of the DHPM receptor acts as a voltage sensor and signals the information directly to the RyR1 subtype, without the requirement of Ca2+ influx [39,40]. This kind of DCCR, a directly coupled calcium-release mechanism, is predicted to require physical contact between two associates. The coimmunoprecipitation of DHPM receptor containing α1S and skeletal muscles solubilized by RYR1 interaction is a strong basis for the above prediction [41]. Nevertheless, for this DCCR, the II-III cytoplasmic loop of α1S is acknowledged as the most significant domain [42,43]. The opening of RYR2 is induced by the opening of the α1C subunit of the DHPM receptor due to the entry of extracellular Ca2+ ions upon depolarization [44]. This kind of calcium release induced by calcium ions again requires two partners at two positions, as the migration of Ca2+ towards RyR2 causes diffusion and buffering, which decreases the Ca2+ wave efficiency. It is assumed that α1C is significantly engaged in the CICR coupling and, hence, not many other interactions between α1C and RYRS have been explored. Meanwhile, there are few studies reporting the bidirectional strong coupling of DHPM receptors with RYR in the cardiac muscle [45,46] as well as neurons [47]. The structural diversity of the DHPMs and their receptor subunits widely enriches their calcium blocking ability; therefore, their versatile synthesis has been investigated extensively.
5. Synthesis of Dihydropyrimidines
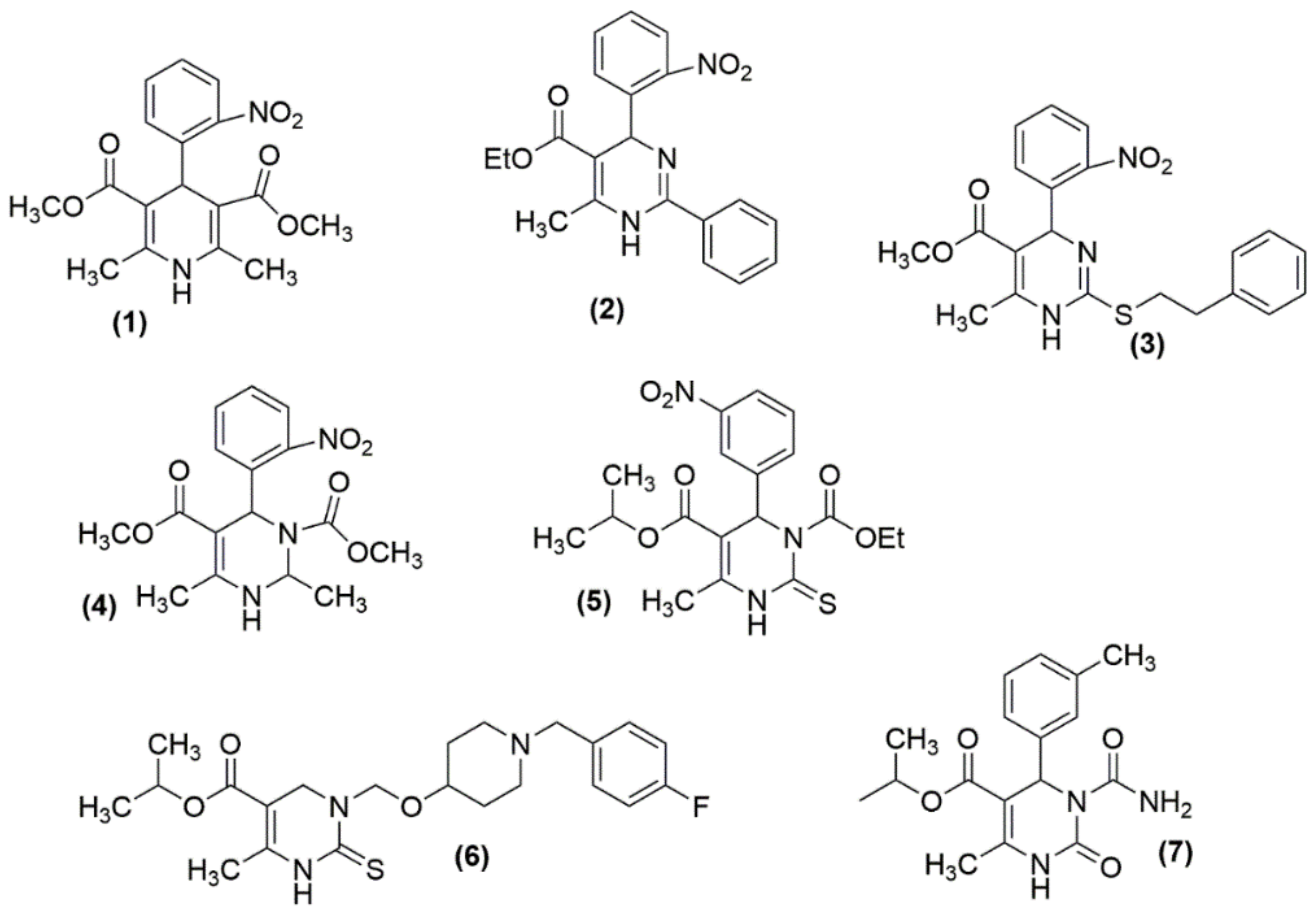
The investigation of dihydropyrimidines as CCBs was started in the mid-1980s with the examination of analogs 2 and 3, which closely mimic nifedipine structure 1, which is a scaffold of dihyropyridine (as shown in Figure 3) [48,49]. Although these molecules showed potential CCB action, their failure in in vivo antihypertensive activity was observed [50]. By then, there had been several attempts to structurally modify those analogues and to enhance their antihypertensive potency. Atwal et al. modified these molecules, obtained ester groups at N3 positions (4, 5), and validated their antihypertensive activity [48]. However, the synthesized compounds were not orally active and upon further modifications, orally active compounds 6 and 7 were obtained.
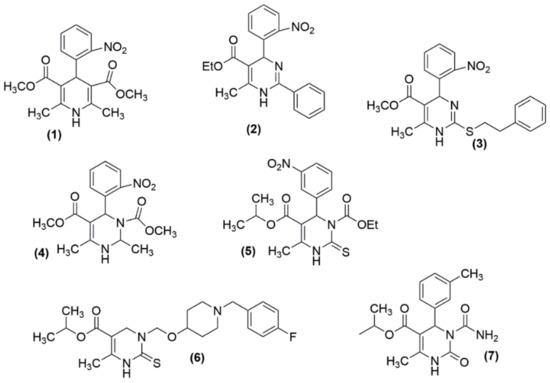
Figure 3.
Some of the DHPMs initially tested for their antihypertensive activity: (1) nifedipine; (2)–(7) analogues of nifedipine.
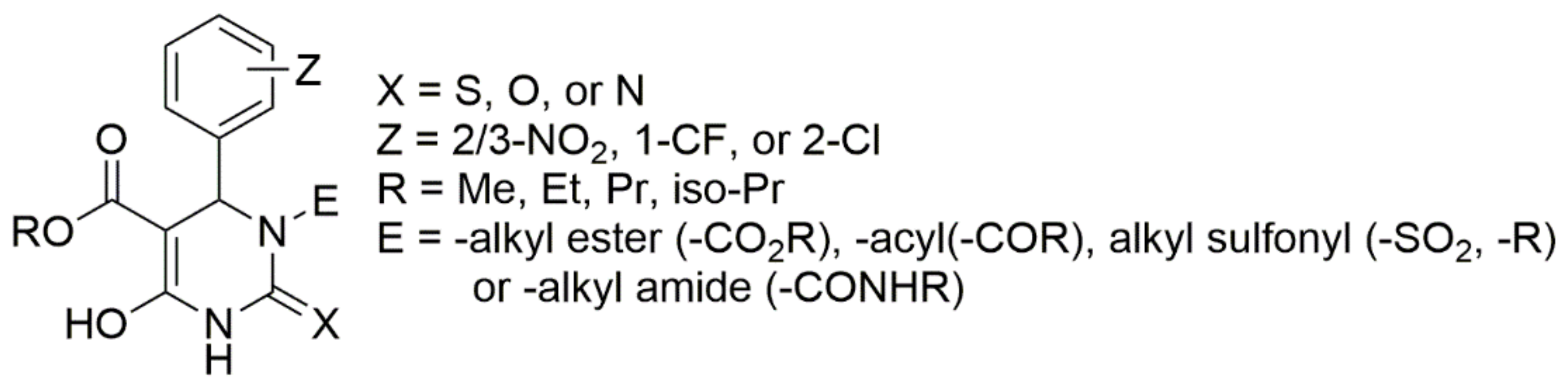
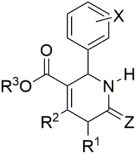
Over several investigations, researchers have finally generalized the structure of DHPMs which showed potential against hypertension. The general structure is detailed in Figure 4. The structure–activity relationship studies reveal that ortho or meta substitutions in the phenyl ring contribute enormously to their in vitro potency. Aromatic substituents with electron-withdrawing groups and the ester alkyl group at C5 show the increasing potency in the order iso-propyl moieties > ethyl > methyl groups. Group “E” substituted at the nitrogen position is a firm requirement, with the N-alkyl carboxamido group in particular demonstrating better oral activity. Similarly, the order for X was S > O > N [51]. Though the generalized structure was investigated in the mid 1980s, the three-component reaction between aromatic aldehydes, urea, and acetoacetic esters was discovered by the Italian chemist P. Biginelli in 1893 to obtain 3,4-dihydropyrimidine-2(1H)-ones.
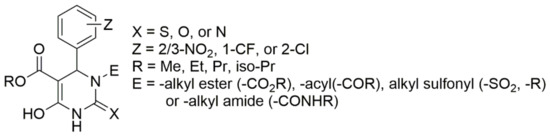
Figure 4.
Generalized structure for antihypertensive DHPMs.
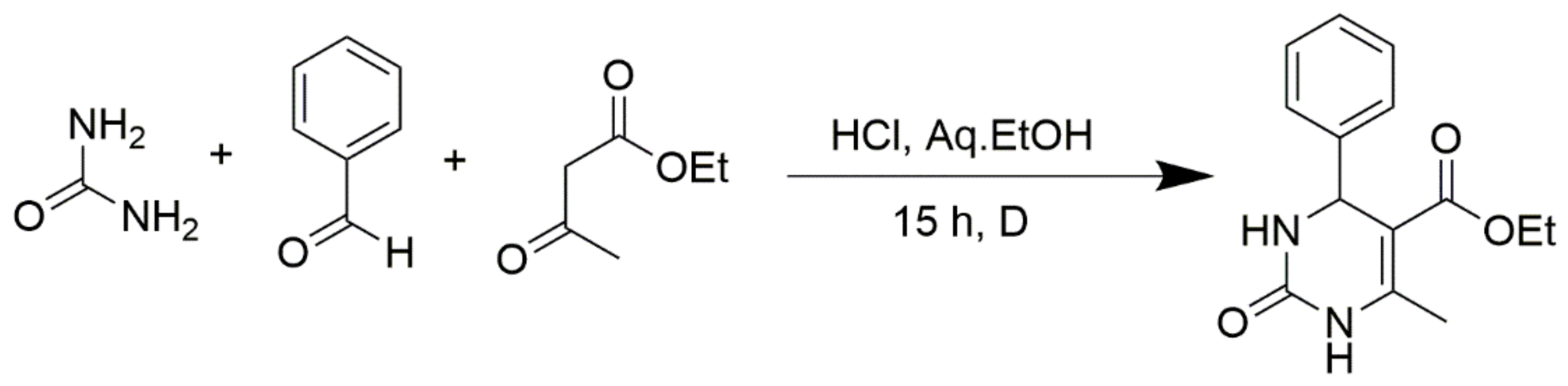
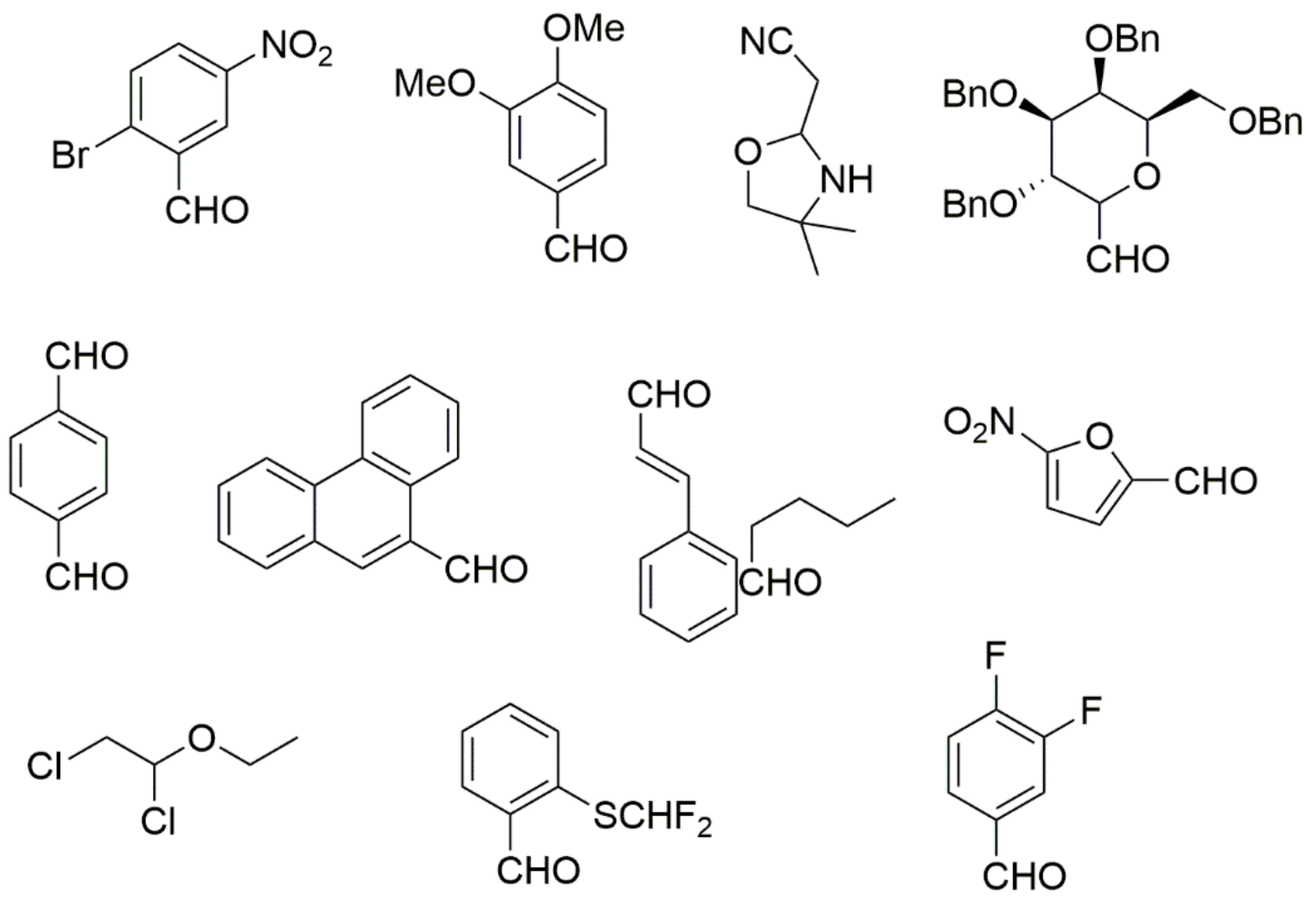
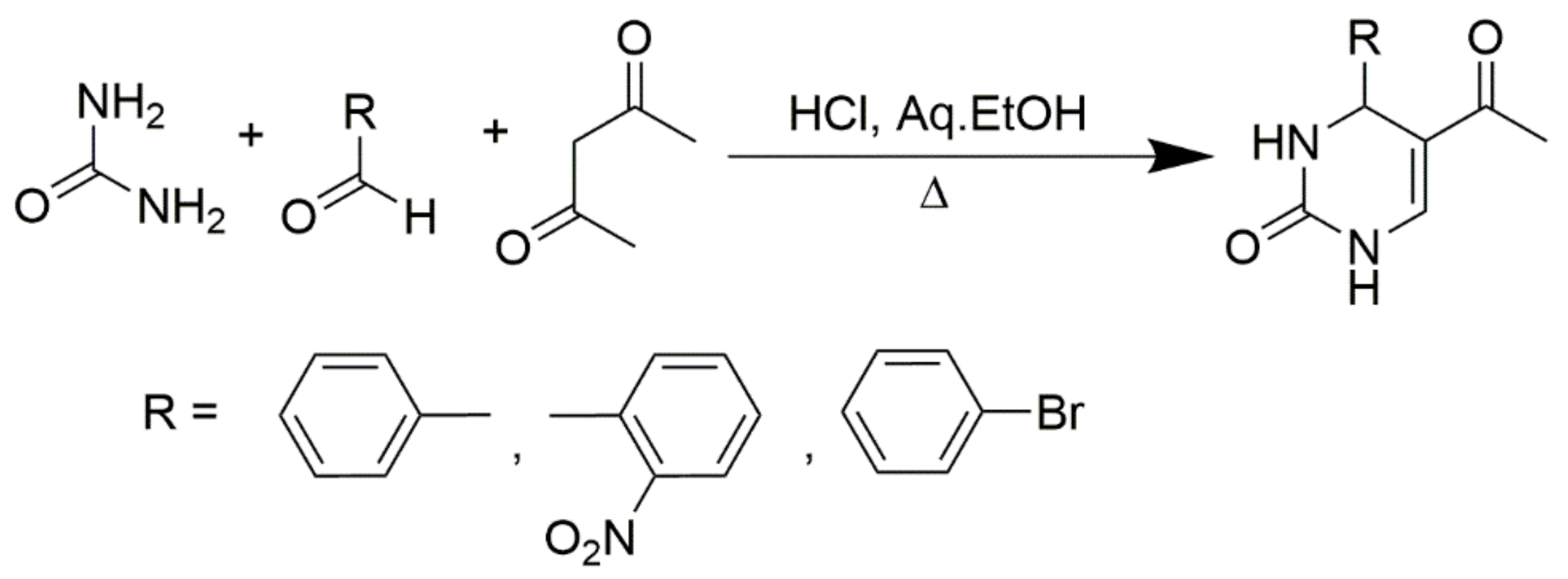
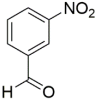
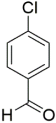
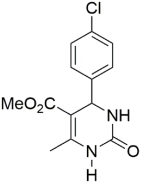
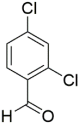
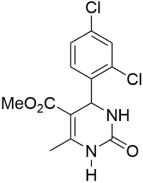
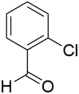
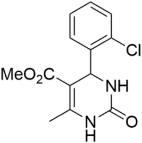
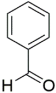
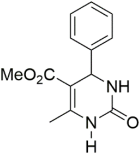
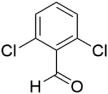
However, the reaction proceeds with low yields and requires a relatively long time (15–20 h) (Scheme 1). A significant number of works have been devoted to the optimization of reaction conditions in order to increase the yields of target DHPMs. The effect of solvents and catalysts on the yields of the target products obtained in the Biginelli reaction have been studied recently. One approach used is the optimization of solvents (acetic acid, acetonitrile, THF, DMF, etc.) and the selection of appropriate catalyst systems (organic and inorganic acids, Lewis acids, ionic liquids, and many more). In order to accelerate the reaction, experiments have been performed with microwave irradiation, infrared irradiation, and ultrasonication, thereby reducing the reaction time to a few minutes and increasing the yield up to 98%. Huge numbers of aldehydes and protected aldehydes, some of them shown in Figure 5, were used in the cyclocondensation of Biginelli dihydropyrimidine synthesis.
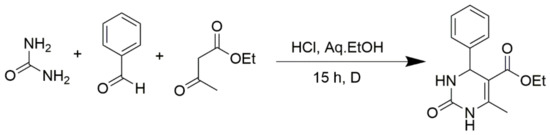
Scheme 1.
Classic Biginelli reaction conditions.
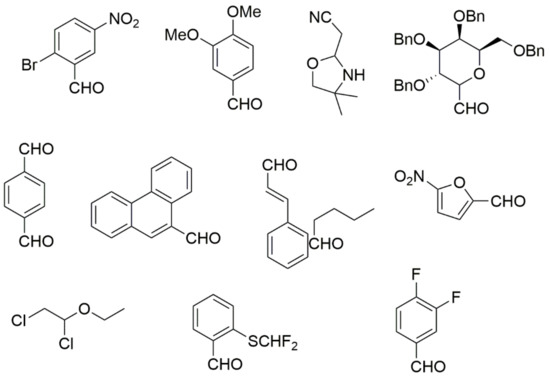
Figure 5.
Classic aldehyde precursors used for Biginelli reactions.
In general, the reaction works best with aromatic aldehydes substituted in the o-,-m-, or p-positions with either electron-withdrawing or -donating groups [52]. Good yields are usually obtained with m- or p-substituted aromatic aldehydes [52]. For o-substituted benzaldehydes with bulky substituents, the yields can be significantly lower. Heterocyclic aldehydes derived from furan, thiophene, and pyridine rings generally provide acceptable yields of DHPM products [53]. Aliphatic aldehydes typically provide only moderate yields in the Biginelli reaction unless special reaction conditions are employed—i.e., Lewis acid catalysts/solvent-free methods—or when using the aldehydes in protected form. The C4 unsubstituted DHPM can be prepared in a similar manner by employing suitable formaldehyde synthons [54].
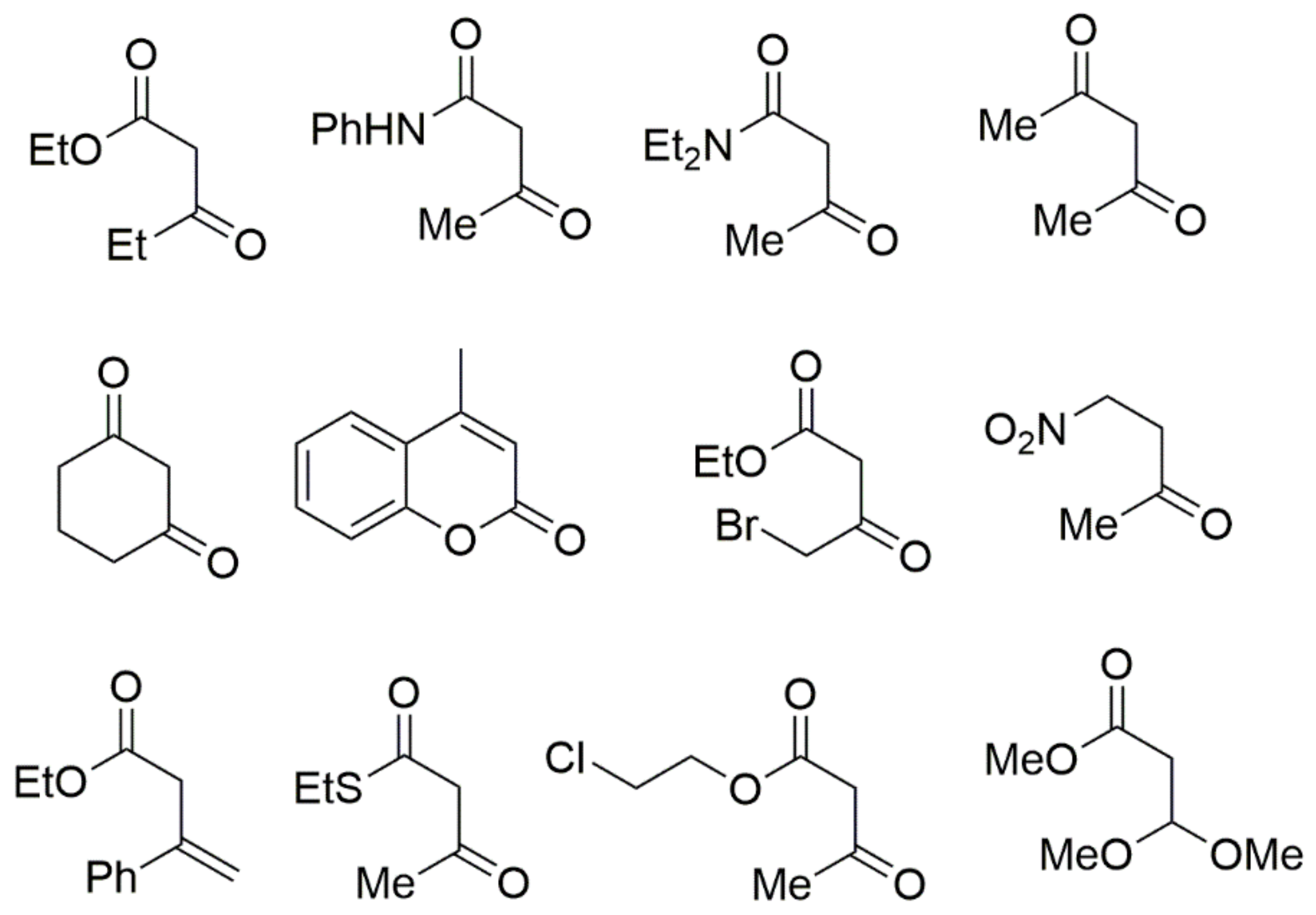
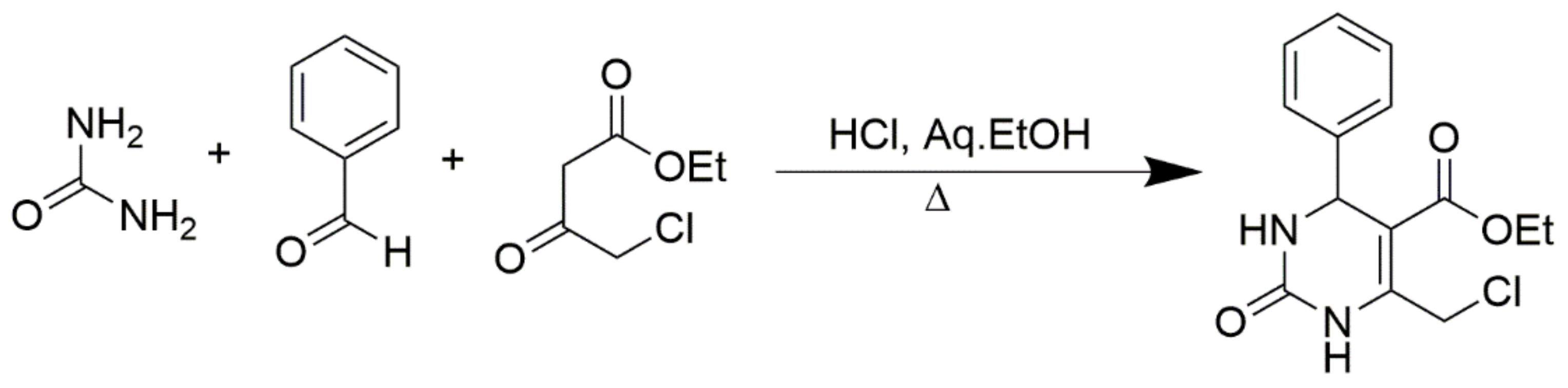
In addition, numerous CH-acidic carbonyl building blocks such as ethyl 3-oxopentanoate, 3-oxo-N-phenylbutanamide, N,N-diethyl-3-oxobutanamide, pentane-2,4-dione, 1-nitropropan-2-one, ethyl 4-bromo-3-oxobutanoate, 4-methyl-2H-1-benzopyran-2-one, cyclohexane-1,3-dione, ethyl 3-oxo-3-phenylpropanoate, 2-ethyl 3-oxobutanethioate, 2-chloroethyl 3-oxobutanoate, and methyl 3,3-dimethoxypropanoate can also be used in Biginelli reactions (Figure 6). Traditionally, simple alkyl acetoacetates are employed as CH-acidic carbonyl building blocks, but other types of 3-oxoalkanoic esters or thioesters can also be used successfully. For instance, the equivalent 6-chloromethyl-substituted DHPMs with 4-chloroacetate can act as important templates for additional synthetic transformations, as shown in Scheme 2.
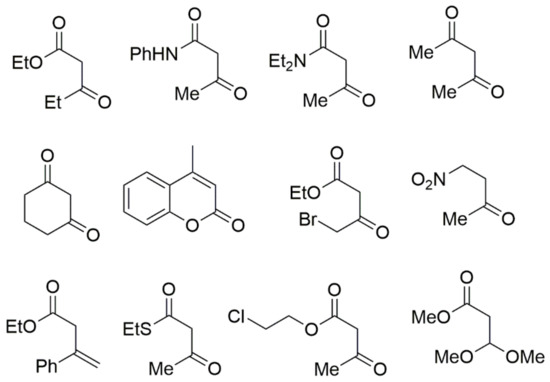
Figure 6.
Typical CH-acidic carbonyl building blocks employed in Biginelli reaction.
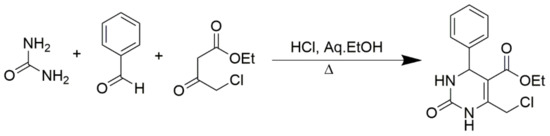
Scheme 2.
Synthesis of 6-chloromethyl-substituted DHPMs using 4-chloroacetate.
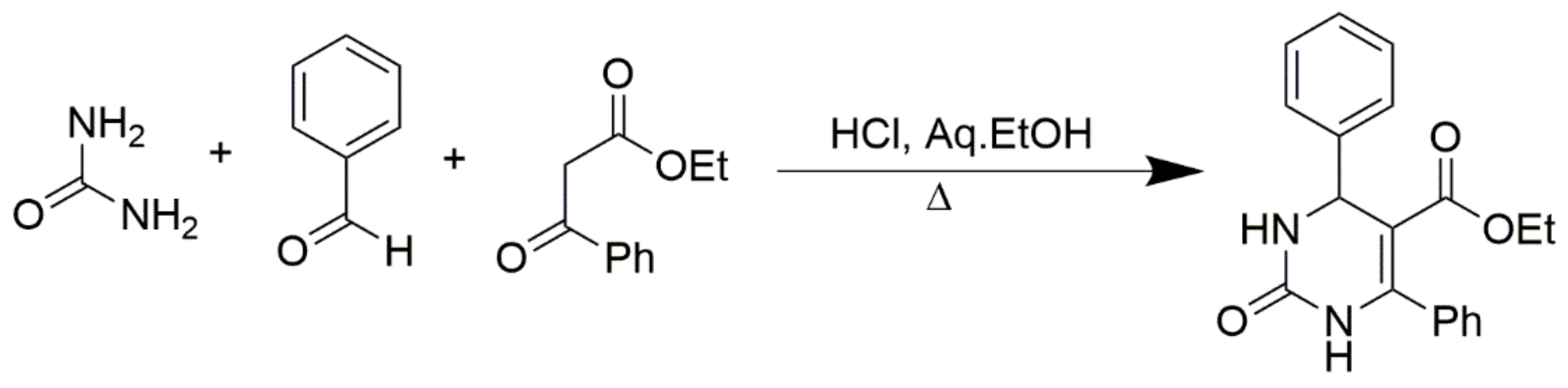
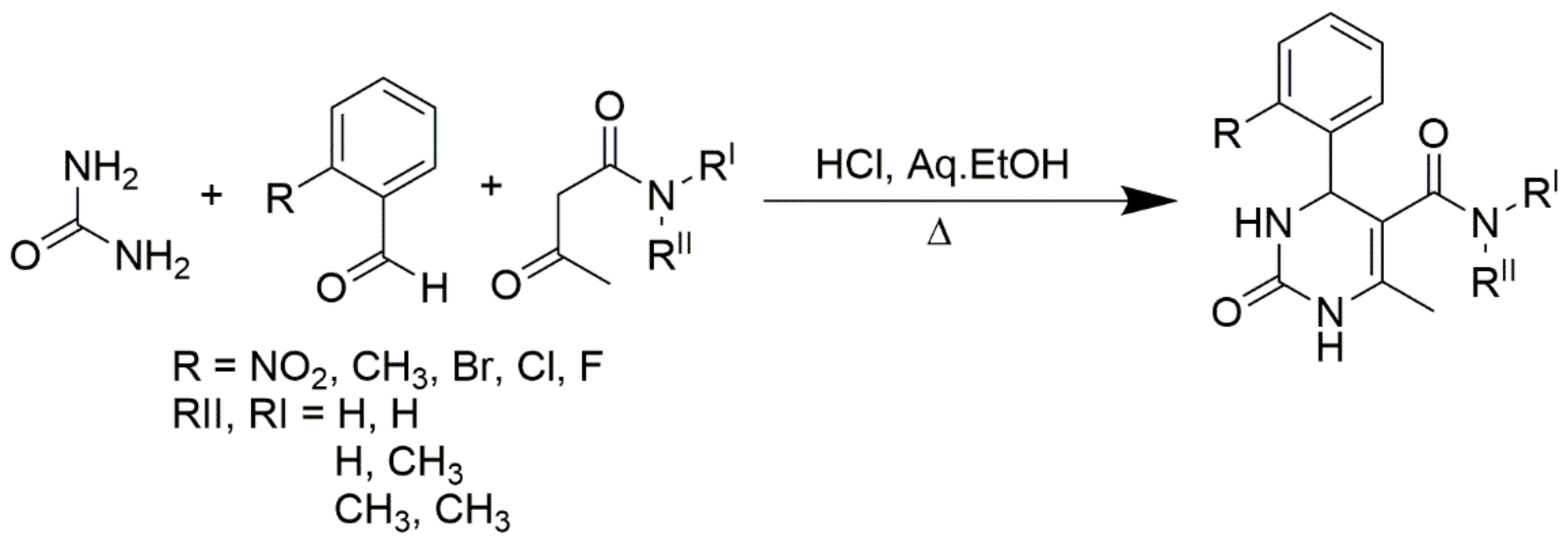
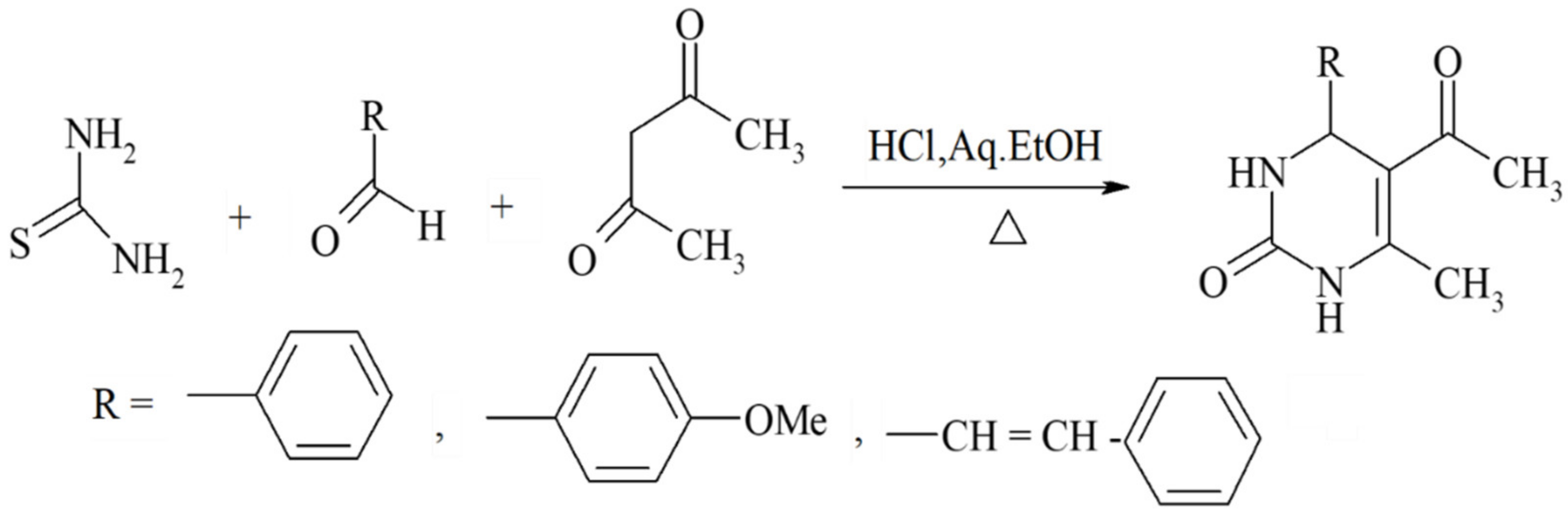
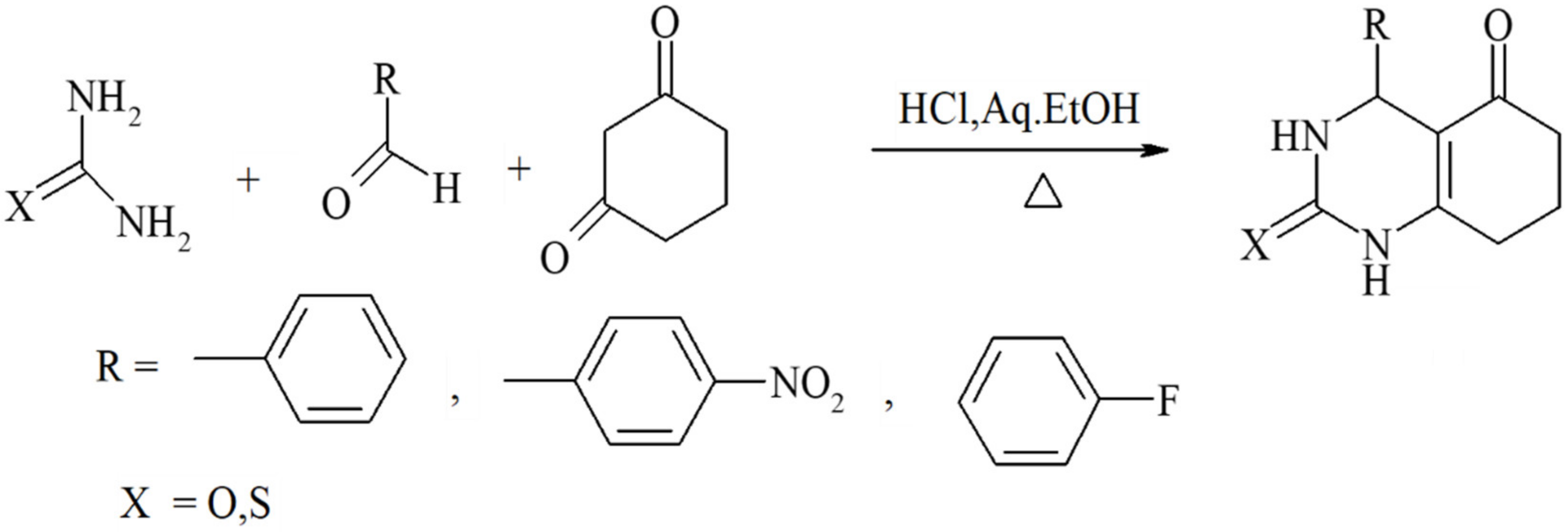
Benzoylacetic esters react analogously, but the yields are significantly lower and the overall condensation process is more sluggish (Scheme 3). Primary, secondary, and tertiary acetoacetamides can be used in place of esters to produce pyrimidine-5-carboxamides, as predicted in Scheme 4. In addition, ß-diketones serve as valuable substrates in Biginelli reactions. As shown in Zainab et al. 2012, the prepared compounds, for which the schemes are provided in Scheme 5, showed promising anti-inflammatory activities [52]. Condensation can also be achieved via employing cyclic ß-diketones such as cyclohexane-1,3-dione and other cyclic ß-dicarbonyl compounds, as described in Scheme 6. If a C6-unsubstituted DHPM derivatives need to be synthesized, the corresponding 3-oxopropanoic ester derivative in which the aldehyde function is masked as an acetal can be employed (Scheme 7).
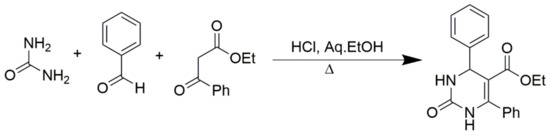
Scheme 3.
Synthesis of DHPMs using benzoylacetic ester as one of the reactants.
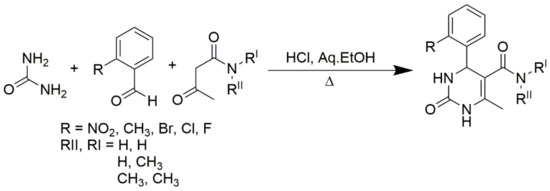
Scheme 4.
Primary, secondary, and tertiary acetoacetamides used in place of esters to produce pyrimidine-5-carboxamides.
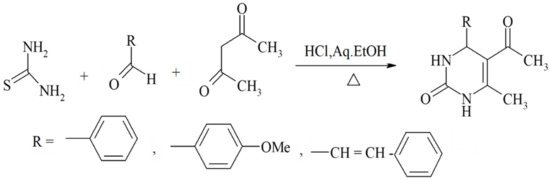
Scheme 5.
ß-diketones serving as valuable substrates in Biginelli reactions.
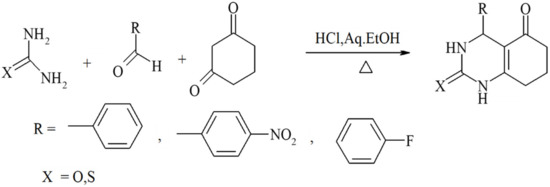
Scheme 6.
Condensations achieved by employing cyclic ß-diketones such as cyclohexane-1,3-dione and other cyclic ß-dicarbonyl compounds.
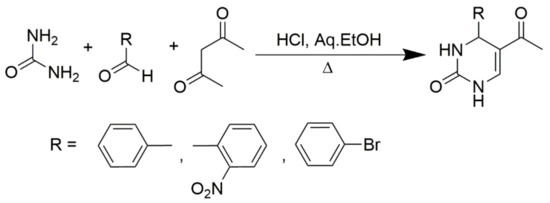
Scheme 7.
Synthesis of C6-unsubstituted DHPM derivatives.
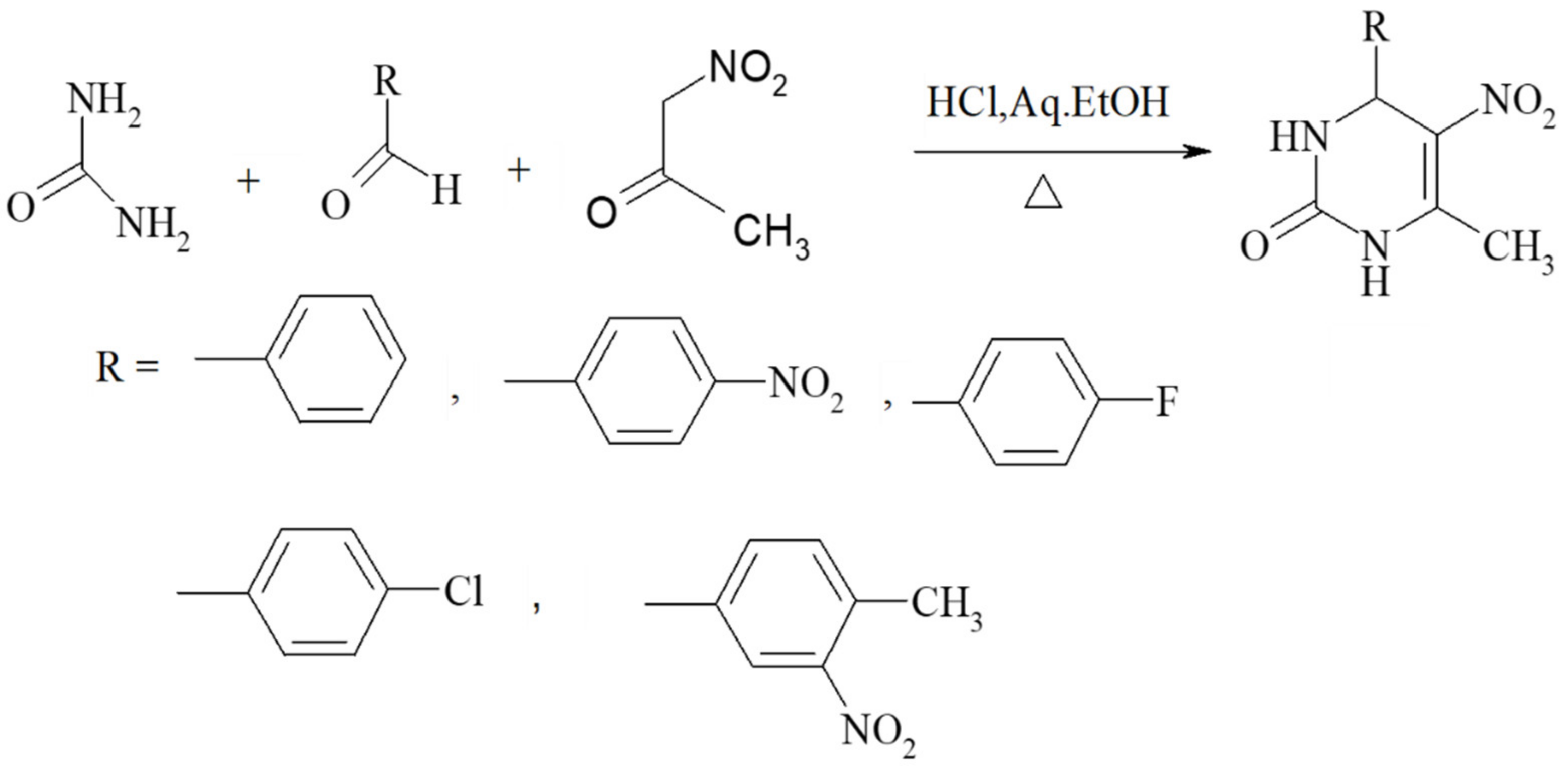
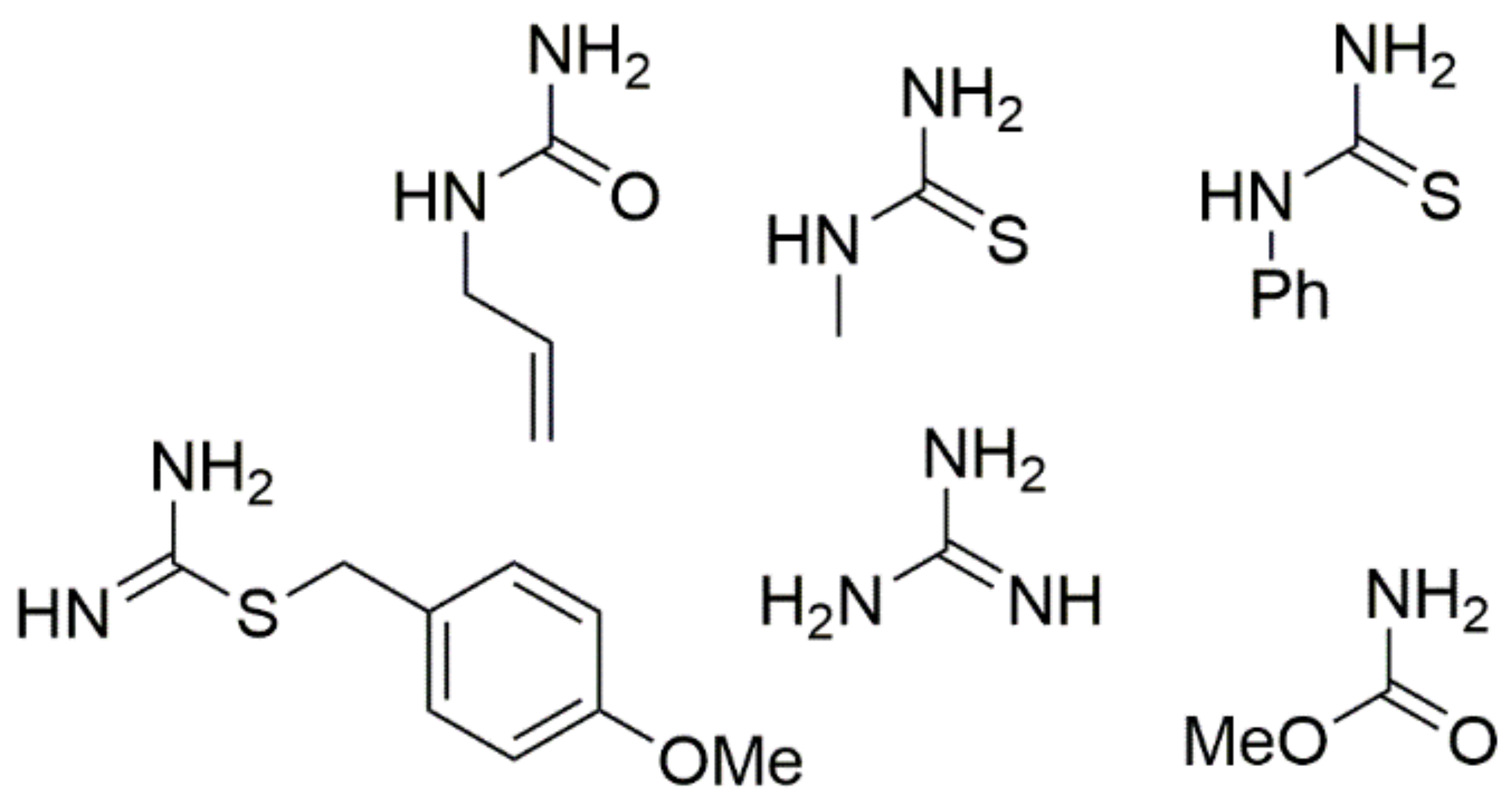
Nitroacetone is a useful building block for creating 5-nitro-substituted DHPM derivatives with good yields, in addition to ester-derived CH-acidic carbonyl compounds. The corresponding method is shown in Scheme 8. Furthermore, the urea component in the Biginelli reaction faces the most restrictions in terms of the structural diversity allowed. Therefore, most of the published examples involve urea itself as a building block. However, simple monosubstituted alkyl urea generally reacts equally well in a regiospecific manner to provide good yields of N1-substituted DHPMs. Some similar units such as N-(prop-2-en-1-yl)urea, N-methylthiourea, N-phenylthiourea, methyl carbamate, (4-methoxyphenyl)methyl carbamimidothioate, and guanidine are provided in Figure 7.
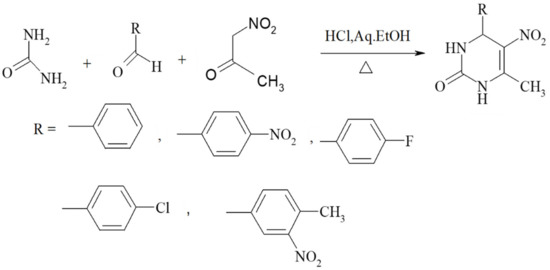
Scheme 8.
Nitroacetone serving as a good building block, leading to 5-nitro-substituted DHPM derivatives.
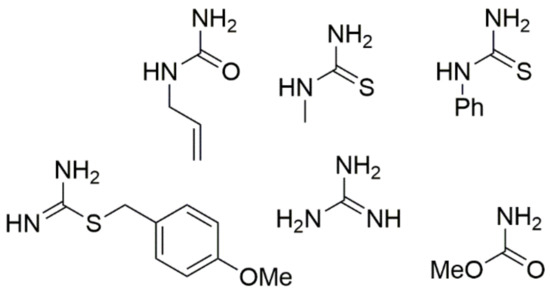
Figure 7.
Structures of monosubstituted alkyl urea used for the synthesis of N1-substituted DHPMs.
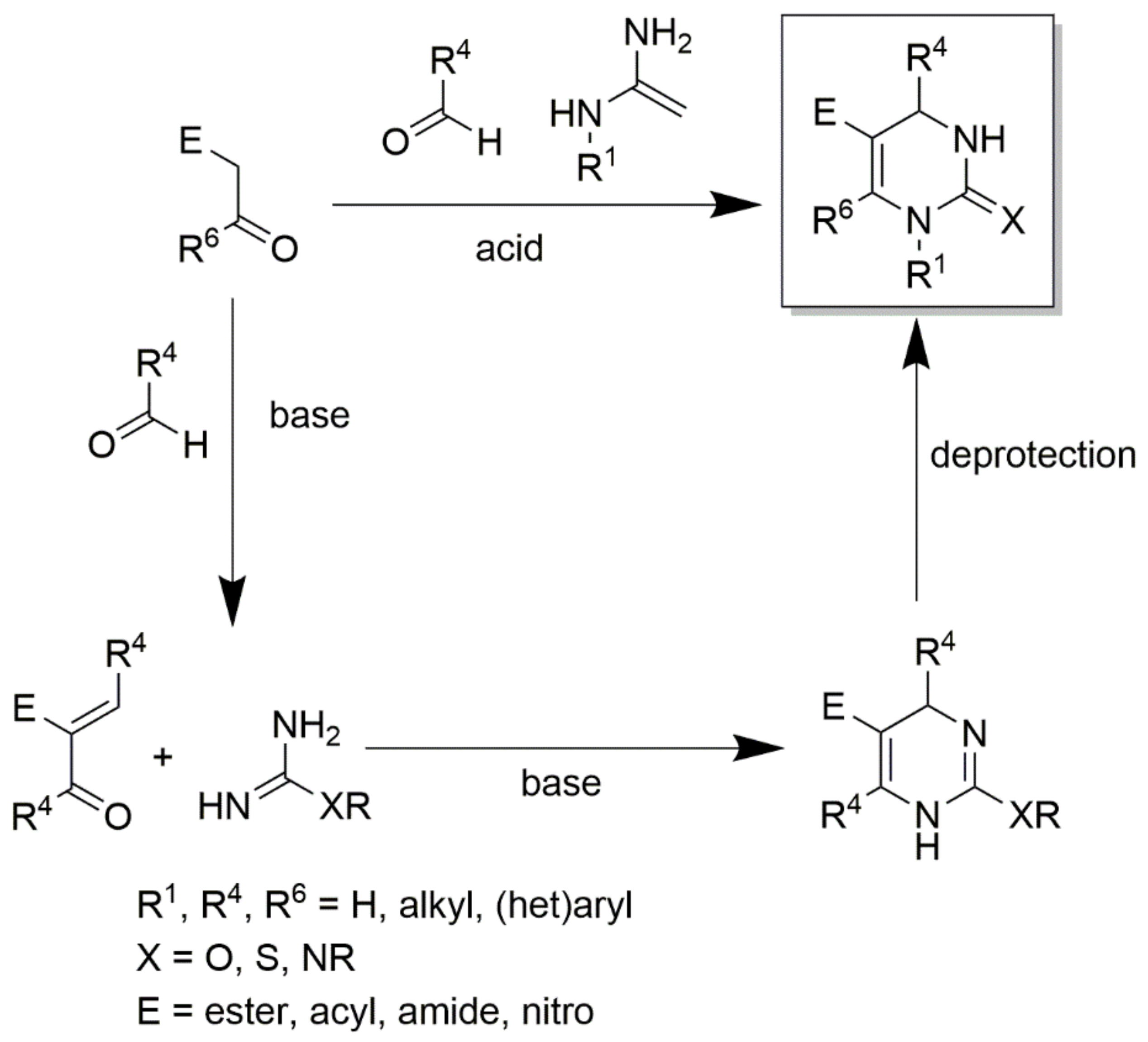
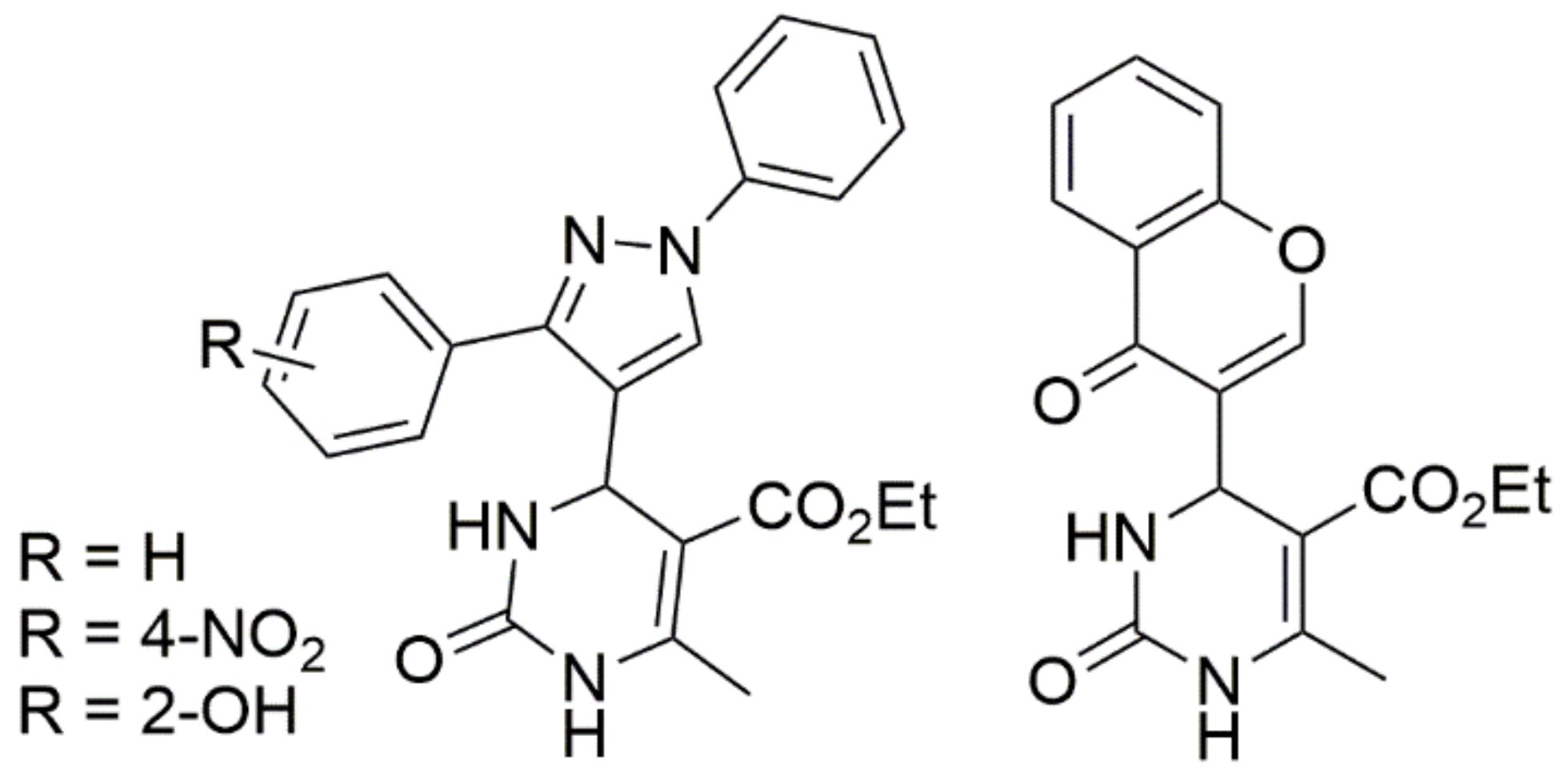
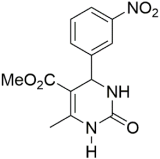
Thiourea and substituted thioureas follow the same general rules. However, longer reaction times are required to achieve good conversions. In some instances, it is also possible to use protected urea, thioureas, or guanidines under weak basic conditions with the aldehyde and CH-acidic carbonyl component (or with a precondensed Knoevenagel-type enone) to yield the corresponding protected DHPMs, as shown in Scheme 9. This latter method using precondensed enones has been frequently referred to as the ‘‘Atwal modification of the Biginelli reaction”. Twenty years ago, a huge number of DHPMs were synthesized and evaluated to determine their biological activities as antiviral; anti-tumor; antibacterial; anti-inflammatory; and, more recently, anti-hypertensive agents. In 2003, some Biginelli products were synthesized by Wageeh S. El-Hamouly et al. and screened to determine their antihypertensive activity (Figure 8). The findings obtained demonstrated that there was a strong anti-hypertensive activity in the series created using 4-chromonyl derivative. Among the synthesized compounds, the 4-chromonyl derivative showed a better anti-hypertensive potency than 4-pyrazolyl analogues and even than the standard drug nifedipine. With the aid of non-selective cat models, the group concluded that the dihydropyrimidines substituted with aryl functionalities were more potent than the dihydropyrimidones substituted with the same aryl functionalities.
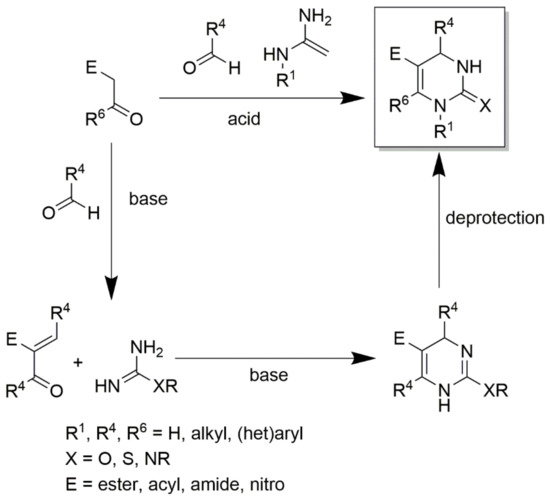
Scheme 9.
An illustration of obtaining protected DHPMs using protected urea or thioureas or guanidines under weak basic conditions with the aldehyde and CH-acidic carbonyl components.
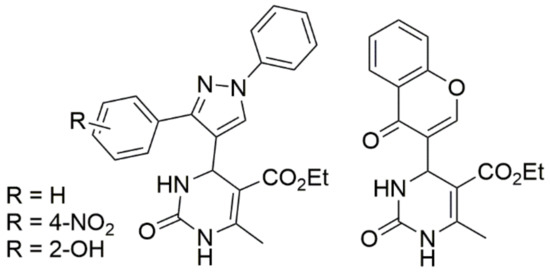
Figure 8.
Biginelli products synthesized by Wageeh S. El-Hamouly et al. for their anti-hypertensive activity.
Recently, some DHPMS were prepared as nifedipine isosteres and evaluated to determine their antihypertensive activity as calcium channel blockers. The current review is not exhaustive; therefore, recent attempts at synthesis with respectable levels of activity have been made. Mohamed Teleb et al. substituted N3 of DHPM and synthesized several derivatives for their CCB activity. Nifedipine was considered as the model structure and several modifications were made to improve the activity, safety profile, and pharmacokinetic activities of DHPMs [55,56,57]. Larger lipophilic alkyl and aryl groups positioned at the third or fifth in the form of esters show greater antihypertensivity [58].
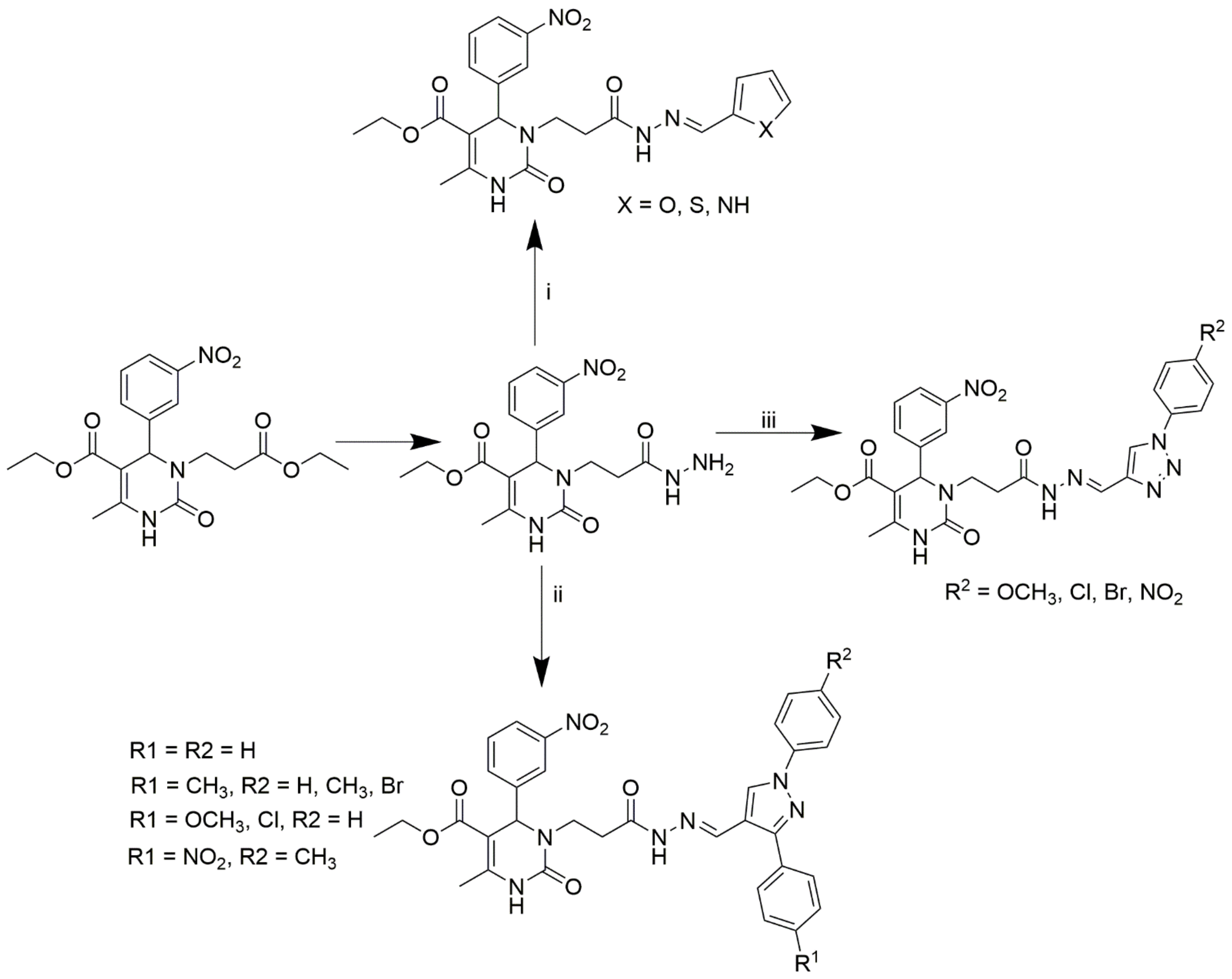
Marwa and group synthesized 14 novel N3-substituted DHPMs and investigated their CCB activity [59]. Among them, the triazole-based DHPMs showed promising efficiency. The scheme used for the synthesis of all the compounds is provided in Scheme 10. Ester compound A was hydrazinolysed to obtain compound B, which was used as the starting material for the synthesis. The compounds were further synthesized by heating the starting material with the corresponding 1,3-diaryl-1H-pyrazole-4-carbaldehydes, 1H-pyrrole-2-carbaldehyde, thiophen-2-carbaldehyde, heteroaromatic furfural, and 1-aryl1H-1,2,3-triazole-4-carbaldehydes in DMSO.
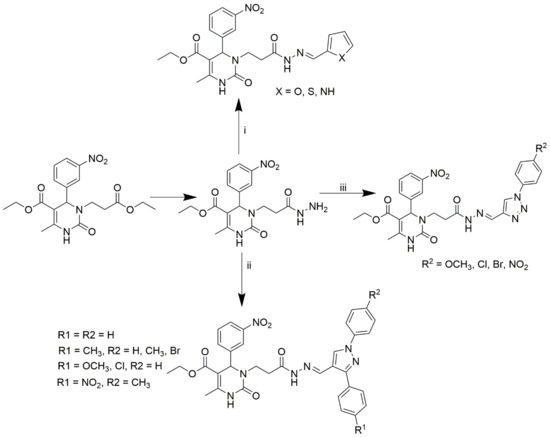
Scheme 10.
Synthesis of the target compounds C1-14. Reagents and conditions: (i) furfural or thiophen-2-carbaldehyde or pyrrole-2-carbaldehyde/DMF/90 °C; (ii) appropriate substituted pyrazole-4-carbaldehyde/DMF/90 °C; (iii) appropriate 1-aryl-1H-1,2,3-triazole-4-carbaldehyde/DMF/90 °C.
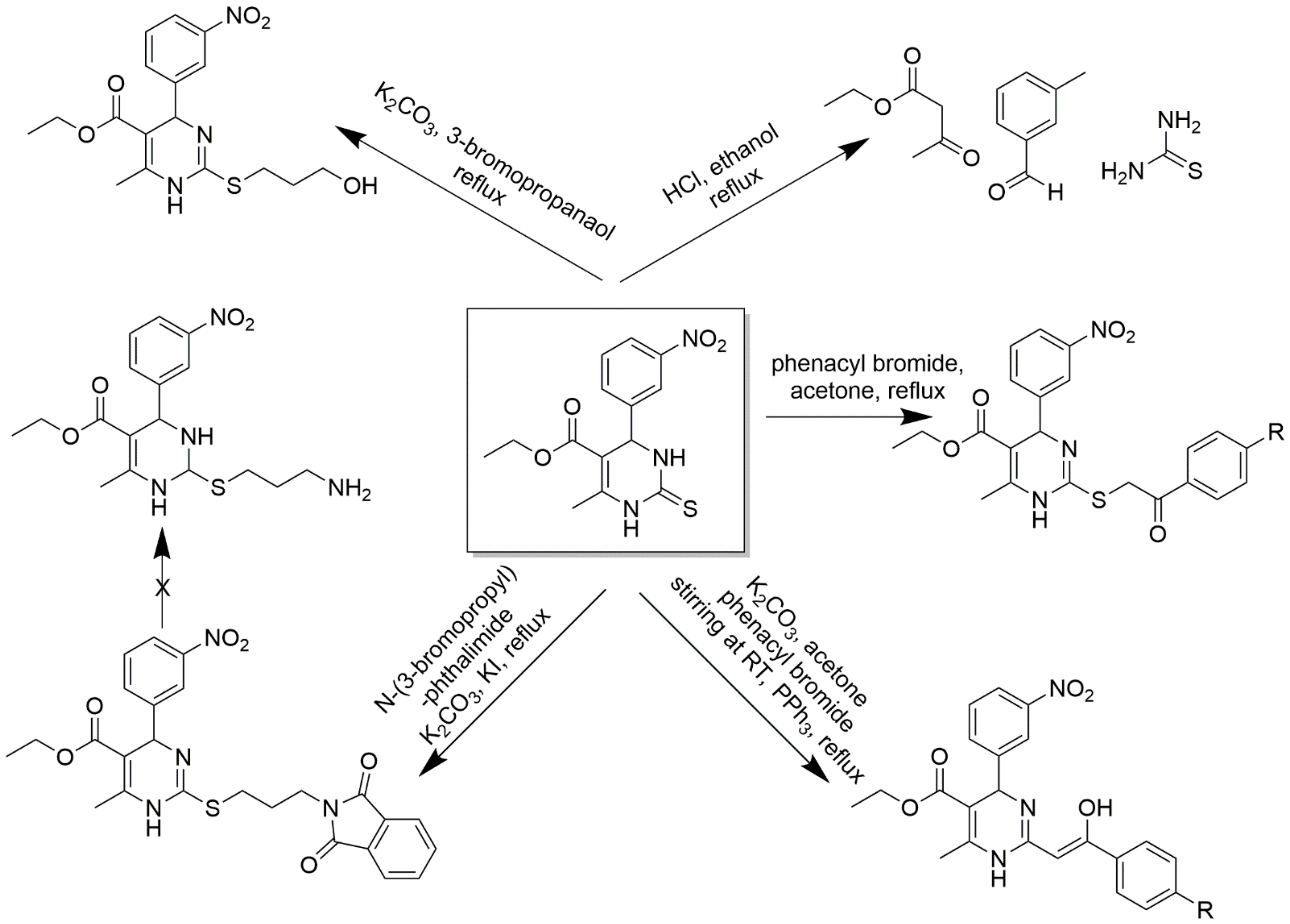
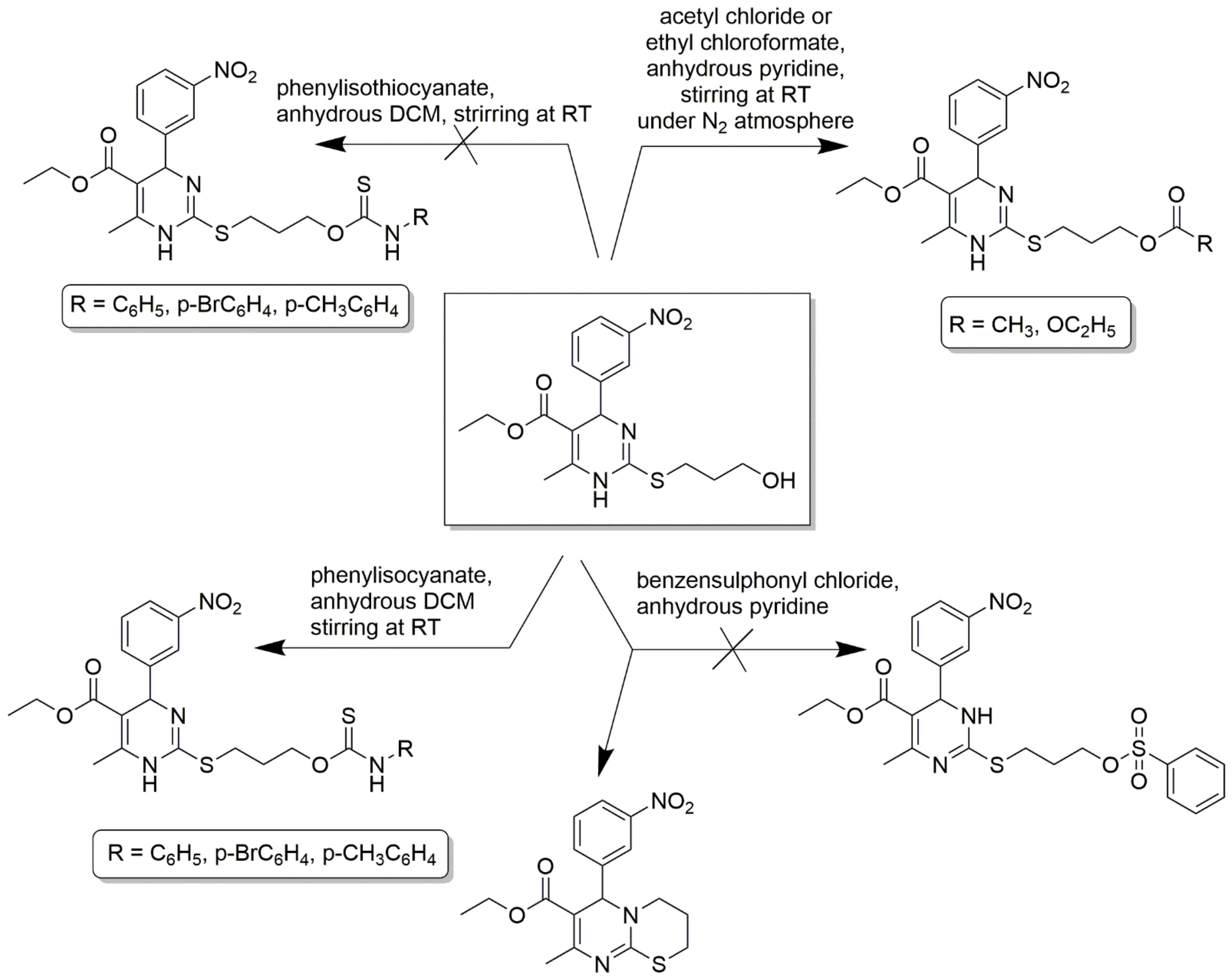
C2-substituted DHPMs are known to show optimal and desired pharmacokinetic activities. A 2(3H)-thioxo derivative of 1,4- 4 dihydropyrimidinethione was converted to 2-aroylmethylsulfanyl derivatives using phenacyl bromides in dry acetone [60]. Free amino derivatives, 3-hydroxypropylsulfanyl derivatives, were also synthesized correspondingly, as shown in Scheme 11. 3-hydroxypropylsulfanyl derivative stirred in anhydrous pyridine and acetyl chloride offered an acetyl derivate and, upon reaction with 1.2 equivalents of ethyl chloroformate, gave a carbonate derivative. The same alcohol derivative was treated with benzenesulfonyl chloride in dry pyridine to give phenylsulfonate ester derivative and many more. The detailed synthesis scheme is provided in Scheme 12. Several functionalities at C2 linked to the DHPM core could be synthesized by a variable spacer nature and size. These compounds act as CCBs via the whole-cell path clamp technique.
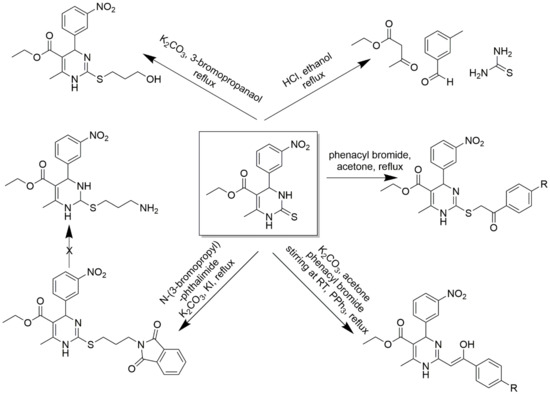
Scheme 11.
Synthetic pathway for the synthesis of an intermediate 1,4-dihydropyrimidinethione by multi-component reaction involving thiourea, 3-nitrobenzaldehyde, and ethyl acetoacetate boiled under ethanol and 37% HCl conditions. The intermediate was further used to synthesize DHPM derivatives to obtain 2-aroylmethylsulfanyl derivative, 2-aryl-2-hydrozyvinyl derivative, and 1,3-dioxoisoindolin-2-ylpropylsulfanylderivatives (the reagents and the experimental conditions are mentioned).
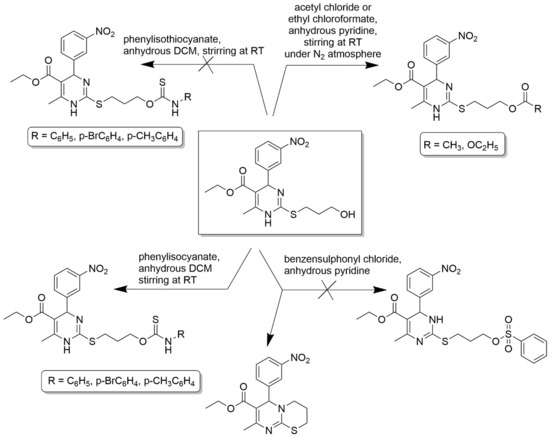
Scheme 12.
3- hydroxypropylsulfanyl derivative used as an intermediate for the synthesis of several other derivatives, such as corresponding acetyl, carbonate, ethyl 8-methyl-6-(3-nitrophenyl)- 3,4-dihydro-2H,6H-pyrimido [2,1-b]thiazine-7-carboxylate derivatives, and their corresponding experimental conditions are provided. However, phenylsulfonate ester derivative was not obtained from the reaction between 3- hydroxypropylsulfanylderivative and benzenesulfonyl chloride in dry pyridine, which was stirred overnight.
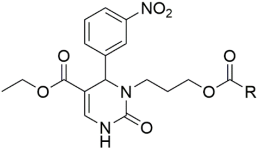
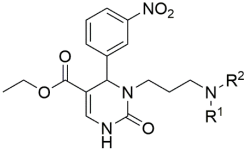
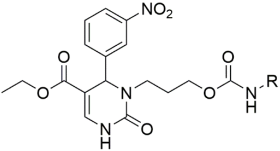
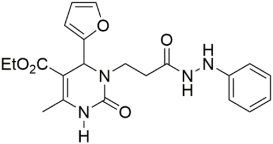
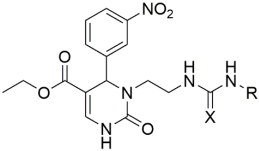
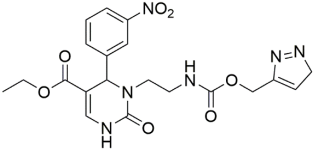
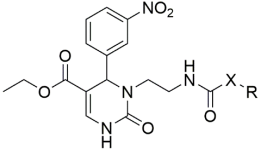
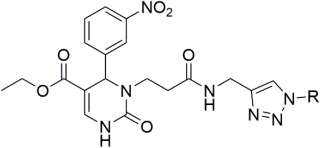
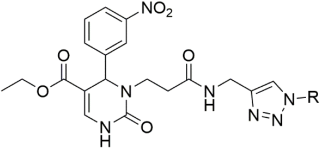
Introducing larger lipophilic alkyl as well as aryl DHPMs has interestingly been demonstrated to enhance safety and pharmacokinetic profiles [55,56,57]. These modifications are also able to harmonize the selectivity of tissues [61,62,63]. The same group synthesized several other target CCB moieties. Starting from three regular components of the Biginelli reaction, several target materials were synthesized via treatment with ethyl acrylate, KF/Al2O3, LiAlH4, THF, alcohols, and several other reagents, as shown in Table 1 [64]. The same research group has contributed enormously to the synthesis of DHPs and DHPMs [65,66]. They have attempted to synthesize CCBs, which could mimic ones which are of clinical use [4,21,22,23,24,25,27]. Several other modifications, such as triazole ligation, which introduce nitrile, carboxylic, and hydrazide groups and amino acid coupling reactions were investigated in order to obtain the compounds listed in Table 2 [67]. The DHPMs with hydrazine functionalities encaged in planar heterocylicpyrazole rings were found to be more efficient calcium channel blockers, while the nitro group derivatives presented a lesser efficiency [66].

Table 1.
Synthesis of several CCBs created by lipophilic alkyl and aryl substitutions.

Table 2.
Triazole ligation introducing nitrile, carboxylic, and hydrazide groups. Amino acid coupling reactions were conducted to obtain the compounds listed in the table.
6. Advanced Pathways
Along with the standard one-pot synthesis of DHPMs using the aforementioned three components and their variations under various reaction circumstances, more complex pathways were also investigated. Microwave-assisted synthesis is one such major success, and some microwave-assisted solvent-free reactions were also attempted. This section aims to highlight those advanced pathways.
6.1. Microwave-Assisted DHPMs Synthesis
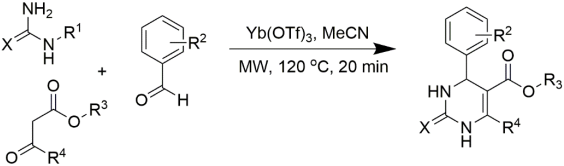
The microwave-assisted synthesis of organic molecules and the combination of regular organic synthesis including high-throughput screening profiles are helpful in the quick synthesis of organic moieties with considerable levels of purity and yield [68,69,70,71,72,73,74]. A different set of aldehydes, urea, and β-ketoesters was created for the microwave-assisted synthesis of multifunctionalized DHPMs. The library generated by “automated sequential microwave-assisted Biginelli multicomponent condensation” was found to be efficient enough in terms of its diversity and combining speed [75,76]. Bimbisar Desai and group synthesized eleven compounds using microwave technology and Yb(OTf)3 as a Lewis acid [77]. Most of the substitutions were obtained at the C5 position of DHPM. The corresponding reaction conditions, obtained DHPMs, and their yields are listed in Table 3. The microwave-assisted deprotection of esters gave made it possible for the carboxylic acid bonds investigated to form multifunctionalized DHPMs. With the aid of polyphosphate ester (PPE) as the mediator of the reaction and coupled with microwave irradiation, various substituted DHPMs were synthesized with good yields, as described in Table 4 [78]. The same group has explored the replacement of C2-methylsulfonyl groups [79]. However, before these investigations, few publications had reported the displacement of the C2-sulphonyl group of pyrimidines with nucleophiles [80]. Additionally, there are some publications on the displacement of nucleophiles that are limited to primary and secondary amines [81]. There are plenty of DHPMs which are biologically active and which have been synthesized by microwave irradiation performed by C. Oliver Kappe and group [75,82,83,84]

Table 3.
Yb(OTf)3 used in the Lewis acid microwave-assisted synthesis of DHPMs offering substitution at the C5 position.

Table 4.
PPE as the mediator of the reaction and coupling with microwave irradiation in order to synthesize various substituted DHPMs.
6.2. Solvent-Free Synthesis of DHPMs
These simple Biginelli reactions have undergone numerous revisions. Microwave-assisted, solvent-free syntheses of DHPMs catalyzed by several efficient catalysts are trending at present. These are reactions carried out in dry media and can offer several benefits. The use of solvents in conventional Biginelli reactions makes it expensive or even toxic, and in some cases solvent removal is difficult, especially in the case of reactions based on aprotic solvents [85].
Ferric chloride hexahydrate has been identified as the effectual catalyst in C-C bond formation [86,87,88]. Maryam and group used this catalyst for revising the Biginelli reaction while keeping the three-component one-pot synthesis strategy. The group was successful in synthesizing microwave-assisted solvent-free DHPMs with considerably high yields [87]. In a typical DHPM synthesis, the corresponding three components of the Biginelli reaction were added to FeCl3·6H2O and the mixture was subjected to microwave irradiation at different time points. Water was added once the reaction mixture had reached room temperature. Ethyl acetate was used to extract the product and Na2SO4 was used for the drying procedure. Recrystallization was performed with ethylacetate to obtain pure products.
Researchers were also successful in solvent-free DHPM synthesis, without using any catalysts. Stefani and Gatti synthesized seven DHPMs using different aldehydes, as shown in Table 5; however, this was carried out without any solvents and catalysts [89]. Stefani presented a 30% yield of DHPM without adding any additives [89]. Mukut and group used CuCl2.2H2O as the catalyst, and the yield was increased from 20–50% [90] to 80–99%, while the reaction time reduced from 18–48 h to 1–1.5 min [91]. Xue Song et al. made an attempt to use different catalysts such as concentrated HCl, FeCl3.6H2O, SnCl2, ZnCl2, and CuCl2.2H2O [92]. Among all the catalysts, Lewis acids showed the most accelerated condensation (89–95% yields); however, the addition of one or two drops of concentrated HCl led to increased yields.

Table 5.
DHPMs synthesized using different aldehydes without any solvents and catalysts.
The use of dry acetic acid as a catalyst has produced enhanced efficiency due to its high polarity. The dry acid with a greater polarity helps in the adsorption of microwaves and the generation of heat energy. Yadav and group attempted to synthesize DHPMs using the same strategy and were successful in producing nearly 13 DHPM derivatives with a yield varying from 82 to 90% [93]. Likewise, NiCl2.H2O, LaCl3.7H2O [94], MgBr2 [95], iodine [96], and MgCl2.6H2O [97,98] are some other catalysts used for the DHPM synthesis.
6.3. Computational Investigations
Regardless of the increased number of patients with hypertension and associated CVDs, the efforts made in drug discovery in the present area are significant. Several SAR investigations strongly recommend the stereochemical and conformational requirements of the DHPMs to be used as calcium channel blockers [99,100]. A revisit of the literature helps us to determine the four significant parameters that play an important role in the biological activity of DHPMs. (1) The extent of the distortion of the aryl groups attached at the C-4 positions; (2) the cis and trans orientation of carbonyl groups positioned at C-3 and C-5, with respect to their subsequent double bonds; (3) the positioning of the aryl group and orthogonal arrangement; (4) the orientation of substitutions at the ortho- and meta- positions of the aryl group or any other equivalent position in the heterocycle ring with respect to C4-hydrogen [62,101]. Afshin Fassihi et al. have synthesized twenty symmetric as well as asymmetric DHPMs and analyzed their conformations using PM3 and DFT investigations [102]. Most of the structures were confirmed to have boat-like conformations. The PM3 method revealed the deviation of 54% of the molecules from their planes, but DFT calculations showed perfect flattened boat confirmations, similar to the nifedipine.
The quantity–structure–activity relationship (QSAR) and docking studies performed by Asghar Davood and group concluded that the symmetrical nature of the DHPM is not important, as only one imidazole nitrogen will interact with the receptor via hydrogen bonds [103]. The alterations in the imidazole rings and the addition of lipophilic groups to the ortho- or meta- positions of the heterocyclic ring enhance the CCB activity. Similar types of investigations were performed by Kappe and Walter; while ta similar conclusion was drawn, the structural conformations were analyzed by three different models—namely, ab initio, semi-empirical, and X-ray crystallographic models [104]. A greatly promising set of twenty-four training compounds was studied using pharmacophore models. Hydrogen donor–acceptor bonds and hydrophobic groups were the major components of that particular set, and these groups enhanced the calcium channel blocking [105]. Along with ab initio and semi-emperical models, molecular mechanic calculations were used for the comparative analysis of conformations of various derivatives of DHPM-based CCBs by Bahram and group [106]. Semi-empirical and molecular mechanic methods have shown the similar 3D structures and conformations of DHPMs, while the results provided by ab intio methods were very different. Most of the analyzed structures showed flattened boat-like conformations for DHPM rings, and very few molecules showed less deviated planarity. Studies did, however, confirm that the stability of molecules decreased when protons were added.
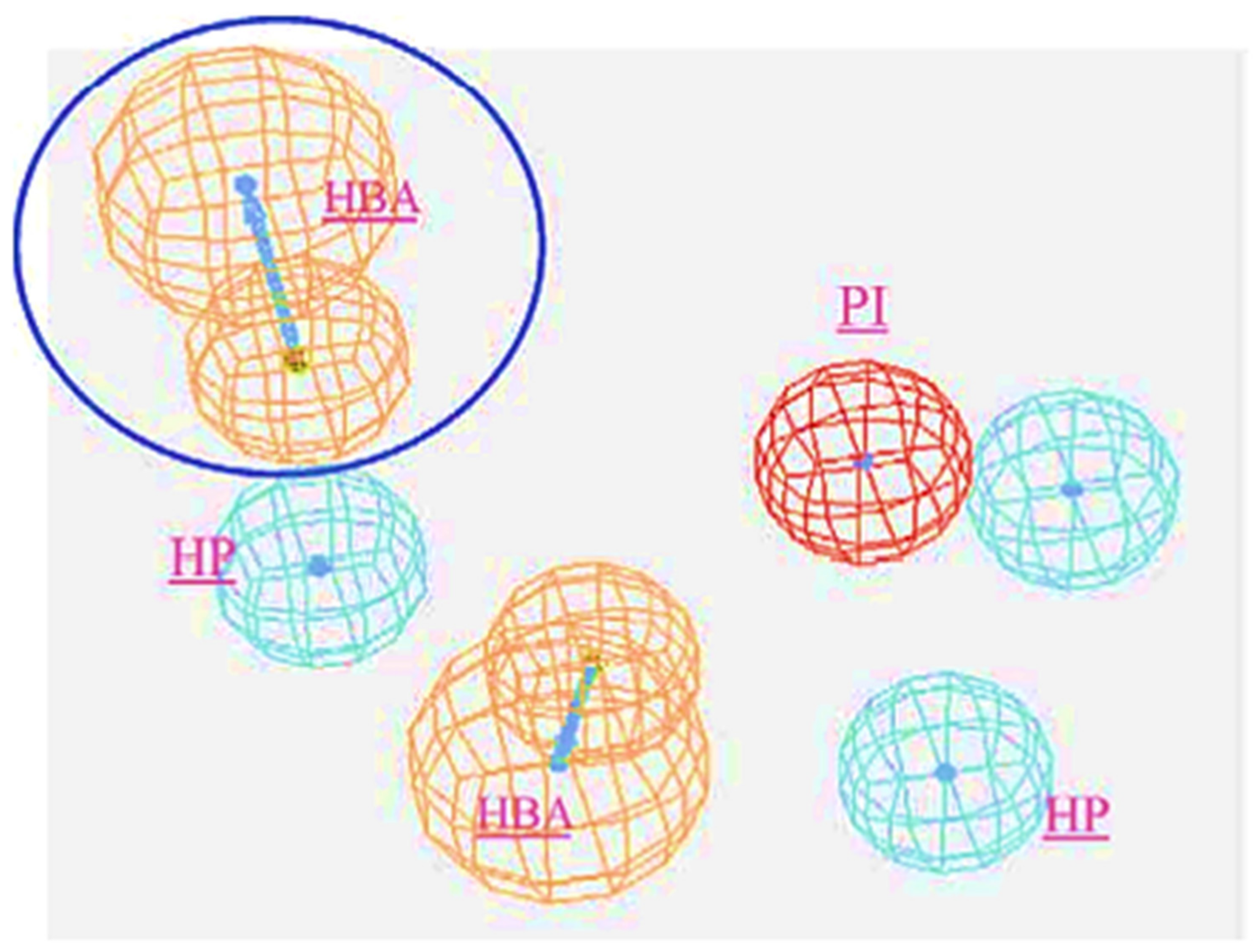
The in silico identification of selective and non-selective T-type calcium channel blockers was mapped via a ligand-pharmacophore approach for the first time in 2004 by Manikumar and group via the CTALYST software [107]. Authors have reported two important strategies and considered 10 test compounds for the validation of the results. In Strategy I, the hypothesis was generated by assuming that “all compounds are equally important and all contain important features”. Among the generated hypotheses, the mapping related to all the features of the active molecules to the maximum extent was considered as the best hypothesis. In strategy II, the hypothesis generation involved the bias between the most active compounds and the ones which presented poor fit values. The best fit hypothesis is as provided in Figure 9.
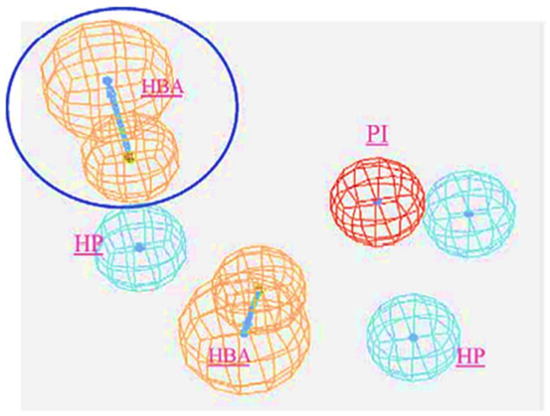
Figure 9.
Statistically best-feature hypothesis (HBA: Hydrogen Bond Acceptor; HP: Hydrophopic; PI: Positive Ionizable). The best fit with HBA interactions is highlighted with circle. Reprinted with permission from Ref. [107]. Copyright 2004, Elsvier.
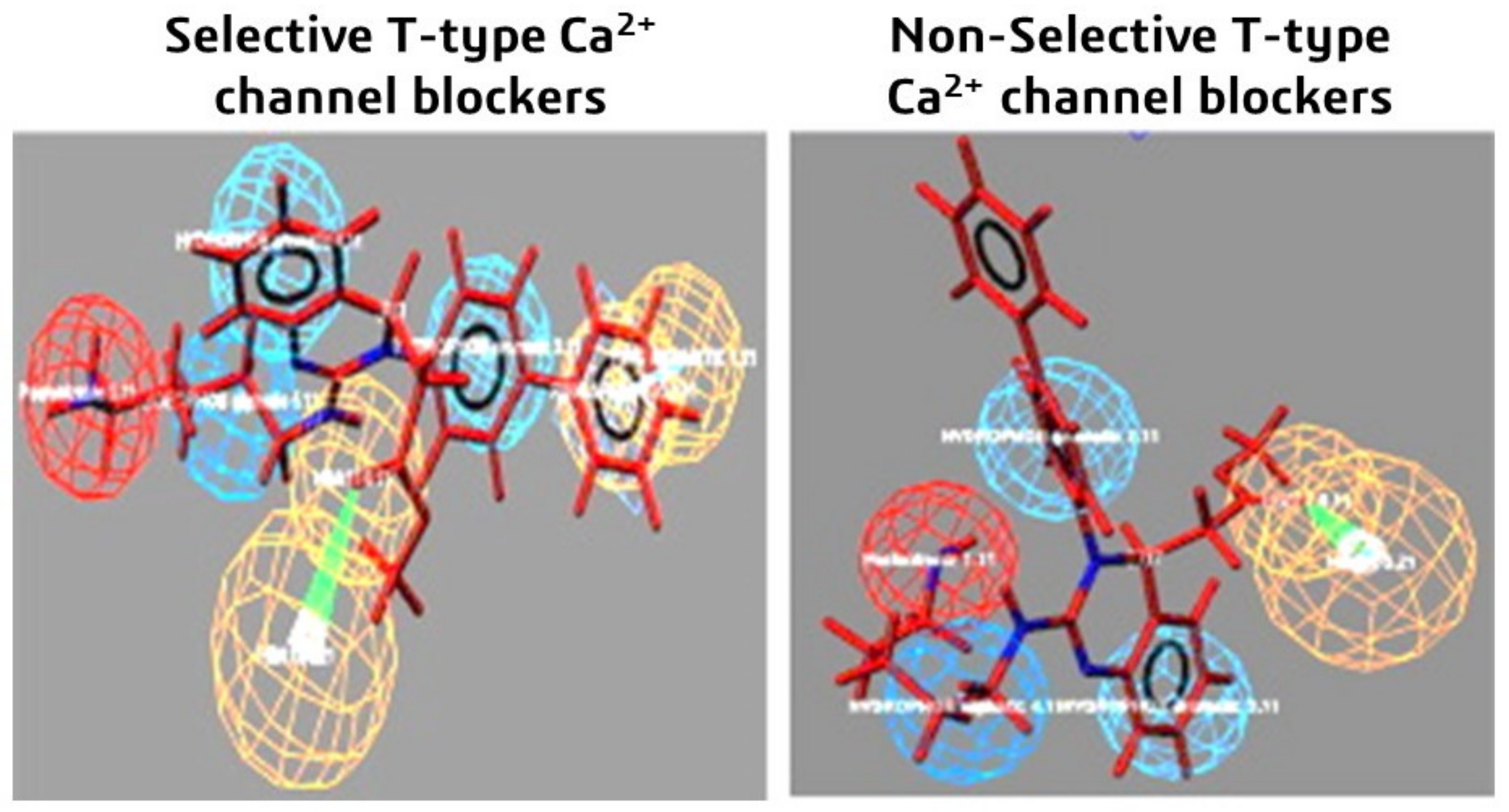
Similarly, six different chemical properties are considered in this study: aromatic ring, hydrophobic aliphatic, two hydrophobic aromatics, hydrogen bond donor and acceptor, and finally positive ionizable characteristics [108]. The molecules which are selective CCBs showed all six features. Meanwhile, the non-selective molecules showed five features but not the aromatic ring property, as shown in Figure 10.
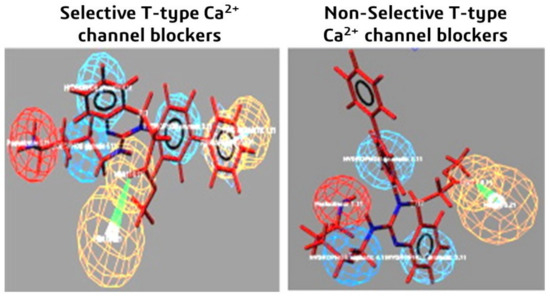
Figure 10.
Predictive selective and non-selective 3D pharmacophore models used for the design and development of better and more safe T-type calcium channel blockers. Reprinted with permission from Ref. [108]. Copyright 2016, Elsevier.
7. Other Therapeutic Accomplishments of Dihydroyrimidines
While DHPMs have been explored extensively as calcium channel modulators, humankind is fortunate to have a scaffold such as DHPMs, as it possesses other diverse pharmacological applications. Along with calcium channel modulators, the appropriate decoration of various functional groups furthers its applications for the blockage of K+ ions activated by Ca2+ ions in human erythrocytes, for which the best examples are nifedipine and its analogues [109]. (+) Niguldipine has been proved to activate Ca2+-dependent, large conductance K channels (BKCa) in “human mesenteric vascular smooth muscles”. Some of these DHPMs have also been identified to block bladder KATP channels and used in the case of urinary issues [110]. Calcium channel antagonists are identified as anticonvulsant agents and could encourage seizures with respect to their dosage [111,112].
The contribution of the lipophilic derivatives of DHPMs to the selective delivery of drugs to the brain has been revealed [111,113]. The delivery of valproic and valeric acids to the brain was investigated by Yiu and Knaus [111]. An equilibrium between DHPM and the pyridinium salt-type redox system was used by El-Sherbeny et al. to deliver the cytotoxic drugs to the brain [114]. The antitubercular activity of DHPMs containing lipophilic dicarbamoyl groups against Mycobacterium tuberculosis H37Rv was investigated [115,116]. Neovascularization induced by TNF-α was found to be inhibited by nifidepine and its analogues [117]. The antioxidant features, GABA receptor inhibition, and analgesic properties [118]; hepatoprotective [119,120]; antifungal [121,122]; antiplatelet [123,124]; and bronchodilatory activities [125] are other features of DHPMs depending on their decorated functionalities and substituents.
8. Conclusions and Future Perspective
Several modifications and revisions of Biginelli reactions have been developed and evolved by enthusiastic researchers and an enormous amount of literature is available. Yet, HMPMs continue to be a promising field, as the pyrimidine scaffold offers diverse pharmacological applications for the DHPM derivatives. The presence of several other heterocycles, such as imidazole, thiazole, pyrazole, benzothiazole, phenothiazine, indole, and many more, along with pyrimidine scaffold, have increased the anti-hypertensive property of the DHPMs. The investigations also suggest that functional groups such as –NO2, -F, -NHCO, -Cl, -Br, -CF3, -COOCH3, -OCH3, -OC2H5, and several groups are found to enhance the biological features of DHPM derivatives. Combinatorial syntheses, efforts towards the optimization of yields, accomplishing green catalysts, and the creation of synthetic methods to reduce the danger of emerging hazardous chemicals are still topics worthy of investigation. Additionally, the recognition of suitable substitutions leading to lower toxicity and the best pharmacological activity would be a first step towards overcoming the toxicity issues. The deprotection of esters of the Biginelli adducts assisted by micro-waves led to the efficient synthesis of the DHPM derivatives. In the case of such solvent-free synthesis, the addition of small amount of acids resulted in a greater yield. The pharmacophore models suggest that the hydrogen donor and acceptor substituents and hydrophobic substituents present good calcium channel blocking abilities. The employment of green chemistry and catalysts; avoiding solvents; and the synthesis of new skeletons containing bi- and tricyclic structural parameters such as “benzo [4,5] imidazo [1,2-a]-pyrimidine, 4H-pyrimido [2,1-b]benzothiazole, and 5,8-dihydro-[1,2,4]triazolo [4,3-a]pyrimidine” could be explored. In order to establish the promising activity of synthesized drugs, QSAR techniques must be improved in order to achieve more accurate binding studies.
This literature review of the use of Biginelli reactions for the synthesis of DHPM derivatives suggests that enantioselective reaction adducts and conditions result in efficient anti-hypertensive products. The synthesis of optically active DHPMs is popular, in addition to the above-mentioned features and techniques; however, this remains unexplored in the present review.
Author Contributions
Conceptualization, methodology, original draft preparation, Y.M.Z.; Original draft preparation, review and editing, Y.M.Z., S.M.A.; visualization, data curation, M.A.R.; resources, validation, software, O.A.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC was funded by Deputyship for Research & lnnovation, Ministry of Education in Saudi Arabia, project number IFP2021-105.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & lnnovation, Ministry of Education in Saudi Arabia, for funding this research work through the project number IFP2021-105.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tadic, M.; Saeed, S.; Grassi, G.; Taddei, S.; Mancia, G.; Cuspidi, C. Hypertension and COVID-19: Ongoing controversies. Front. Cardiovasc. Med. 2021, 8, 639222. [Google Scholar] [CrossRef]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef]
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review. Eur. J. Med. Chem. 2017, 132, 108–134. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M. Biginelli Reaction Mediated Synthesis of Antimicrobial Pyrimidine Derivatives and Their Therapeutic Properties. Molecules 2021, 26, 6022. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B. Glucagon in β-blocker and calcium channel blocker overdoses: A systematic review. J. Toxicol. Clin. Toxicol. 2003, 41, 595–602. [Google Scholar] [CrossRef]
- Eisenberg, M.J.; Brox, A.; Bestawros, A.N. Calcium channel blockers: An update. Am. J. Med. 2004, 116, 35–43. [Google Scholar] [CrossRef]
- Nassar, A.H.; Aoun, J.; Usta, I.M. Calcium channel blockers for the management of preterm birth: A review. Am. J. Perinatol. 2011, 28, 57–66. [Google Scholar] [CrossRef]
- Kow, C.S.; Ramachandram, D.S.; Hasan, S.S. Clinical outcomes of hypertensive patients with COVID-19 receiving calcium channel blockers: A systematic review and meta-analsysis. Hypertens. Res. 2022, 45, 360–363. [Google Scholar] [CrossRef]
- Kow, C.S.; Ramachandram, D.S.; Hasan, S.S. Use of Calcium Channel Blockers and the Risk of All-cause Mortality and Severe Illness in Patients With COVID-19: A Systematic Review and Meta-analysis. J. Cardiovasc. Pharmacol. 2022, 79, 199–205. [Google Scholar] [CrossRef]
- Den Brok, M.G.; van Dalen, J.W.; Abdulrahman, H.; Larson, E.B.; van Middelaar, T.; van Gool, W.A.; van Charante, E.P.M.; Richard, E. Antihypertensive medication classes and the risk of dementia: A systematic review and network meta-analysis. J. Am. Med. Dir. Assoc. 2021, 22, 1386–1395.e1315. [Google Scholar] [CrossRef] [PubMed]
- Sahney, S. A review of calcium channel antagonists in the treatment of pediatric hypertension. Pediatr. Drugs 2006, 8, 357–373. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lin, J.-W.; Wu, M.-S.; Chen, K.-C.; Peng, C.-C.; Kang, Y.-N. Effects of calcium channel blockers comparing to angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with hypertension and chronic kidney disease stage 3 to 5 and dialysis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0188975. [Google Scholar] [CrossRef]
- Bull, J.A.; Mousseau, J.J.; Pelletier, G.; Charette, A.B. Synthesis of pyridine and dihydropyridine derivatives by regio-and stereoselective addition to N-activated pyridines. Chem. Rev. 2012, 112, 2642–2713. [Google Scholar] [CrossRef]
- Gore, R.P.; Rajput, A.P. A review on recent progress in multicomponent reactions of pyrimidine synthesis. Drug Inven. Today 2013, 5, 148–152. [Google Scholar] [CrossRef]
- Ling, Y.; Hao, Z.-Y.; Liang, D.; Zhang, C.-L.; Liu, Y.-F.; Wang, Y. The expanding role of pyridine and dihydropyridine scaffolds in drug design. Drug Des. Devel. Ther. 2021, 15, 4289. [Google Scholar] [CrossRef]
- Borah, B.; Patat, M.; Swain, S.; Chowhan, L.R. Recent Advances and Prospects in the Transition-Metal-Free Synthesis of 1,4-Dihydropyridines. ChemistrySelect 2022, 7, e202202484. [Google Scholar] [CrossRef]
- Betzenhauser, M.; Pitt, G.; Antzelevitch, C. Calcium channel mutations in cardiac arrhythmia syndromes. Curr. Mol. Pharmacol. 2015, 8, 133–142. [Google Scholar] [CrossRef]
- Lam, A.; Karekar, P.; Shah, K.; Hariharan, G.; Fleyshman, M.; Kaur, H.; Singh, H.; Gururaja Rao, S. Drosophila Voltage-Gated Calcium Channel α1-Subunits Regulate Cardiac Function in the Aging Heart. Sci. Rep. 2018, 8, 6910. [Google Scholar] [CrossRef]
- Bers, D.M.; Perez-Reyes, E. Ca channels in cardiac myocytes: Structure and function in Ca influx and intracellular Ca release. Cardiovasc. Res. 1999, 42, 339–360. [Google Scholar] [CrossRef]
- Pachòn Angona, I.; Daniel, S.; Martin, H.; Bonet, A.; Wnorowski, A.; Maj, M.; Jóźwiak, K.; Silva, T.B.; Refouvelet, B.; Borges, F.; et al. Design, Synthesis and Biological Evaluation of New Antioxidant and Neuroprotective Multitarget Directed Ligands Able to Block Calcium Channels. Molecules 2020, 25, 1329. [Google Scholar] [CrossRef]
- Kowalska, M.; Fijałkowski, Ł.; Kubacka, M.; Sałat, K.; Grześk, G.; Nowaczyk, J.; Nowaczyk, A. Antiepileptic Drug Tiagabine Does Not Directly Target Key Cardiac Ion Channels Kv11.1, Nav1.5 and Cav1.2. Molecules 2021, 26, 3522. [Google Scholar] [CrossRef] [PubMed]
- Endo, M. Calcium release from the sarcoplasmic reticulum. Physiol. Rev. 1977, 57, 71–108. [Google Scholar] [CrossRef]
- Kushner, J.; Ferrer, X.; Marx, S.O. Roles and Regulation of Voltage-gated Calcium Channels in Arrhythmias. J. Innov. Card. Rhythm Manag. 2019, 10, 3874. [Google Scholar] [CrossRef]
- Nargeot, J.; Lory, P.; Richard, S. Molecular basis of the diversity of calcium channels in cardiovascular tissues. Eur. Heart J. 1997, 18, 15–26. [Google Scholar] [CrossRef]
- Andrade, F.; Rangel-Sandoval, C.; Rodríguez-Hernández, A.; López-Dyck, E.; Elizalde, A.; Virgen-Ortiz, A.; Bonales-Alatorre, E.; Valencia-Cruz, G.; Sánchez-Pastor, E. Capsaicin Causes Vasorelaxation of Rat Aorta through Blocking of L-type Ca2+ Channels and Activation of CB1 Receptors. Molecules 2020, 25, 3957. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, Z.; Li, Z.; Yan, C.; Lu, S.; Dong, M.; Yan, N. Structure of the voltage-gated calcium channel Cav1. 1 complex. Science 2015, 350, aad2395. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Iijima, T. Cardiac T-type Ca2+ channels in the heart. J. Mol. Cell. Cardiol. 2010, 48, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Zamponi, G.W. T-type calcium channels: From molecule to therapeutic opportunities. Int. J. Biochem. Cell Biol. 2019, 108, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.P.; Marx, S.O. Novel approaches to examine the regulation of voltage-gated calcium channels in the heart. Curr. Mol. Pharmacol. 2015, 8, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.B. Functional importance of T-type voltage-gated calcium channels in the cardiovascular and renal system: News from the world of knockout mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 308, R227–R237. [Google Scholar] [CrossRef]
- Mason, R.; Marche, P.; Hintze, T. Novel vascular biology of third-generation L-type calcium channel antagonists: Ancillary actions of amlodipine. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2155–2163. [Google Scholar] [CrossRef]
- Triggle, D.; Janis, R. Calcium channel ligands. Annu. Rev. Pharmacol. Toxicol. 1987, 27, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Rovnyak, G.C.; Kimball, S.D.; Beyer, B.; Cucinotta, G.; DiMarco, J.D.; Gougoutas, J.; Hedberg, A.; Malley, M.; McCarthy, J.P. Calcium entry blockers and activators: Conformational and structural determinants of dihydropyrimidine calcium channel modulators. J. Med. Chem. 1995, 38, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Gjörstrup, P.; Hårding, H.; Isaksson, R.; Westerlund, C. The enantiomers of the dihydropyridine derivative H 160/51 show opposite effects of stimulation and inhibition. Eur. J. Pharmacol. 1986, 122, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Bühler, F.R.; Hulthén, U.L.; Kiowski, W.; Müller, F.B.; Bolli, P. The place of the calcium antagonist verapamil in antihypertensive therapy. J. Cardiovasc. Pharmacol. 1982, 4, S350–S357. [Google Scholar]
- Morad, M.; Goldman, Y.; Trentham, D. Rapid photochemical inactivation of Ca2+-antagonists shows that Ca2+ entry directly activates contraction in frog heart. Nature 1983, 304, 635–638. [Google Scholar] [CrossRef]
- Cho, H.; Ueda, M.; Mizuno, A.; Ishihara, T.; Aisaka, K.; Noguchi, T. Synthesis of novel 2-chloro-1, 4-dihydropyridines by chlorination of 2-hydroxy-1, 4-dihydropyridines with phosphorus oxychloride. Chem. Pharm. Bull. 1989, 37, 2117–2121. [Google Scholar] [CrossRef]
- Singh, K.; Arora, D.; Singh, K.; Singh, S. Genesis of dihydropyrimidinonep calcium channel blockers: Recent progress in structure-activity relationships and other effects. Mini Rev. Med. Chem. 2009, 9, 95. [Google Scholar]
- Miledi, R.; Parker, I.; Zhu, P. Extracellular ions and excitation-contraction coupling in frog twitch muscle fibres. J. Physiol. 1984, 351, 687–710. [Google Scholar] [CrossRef] [PubMed]
- Brum, G.; Stefani, E.; Rios, E. Simultaneous measurements of Ca2+ currents and intracellular Ca2+ concentrations in single skeletal muscle fibers of the frog. Can. J. Physiol. Pharmacol. 1987, 65, 681–685. [Google Scholar] [CrossRef]
- Marty, I.; Robert, M.; Villaz, M.; De Jongh, K.; Lai, Y.; Catterall, W.A.; Ronjat, M. Biochemical evidence for a complex involving dihydropyridine receptor and ryanodine receptor in triad junctions of skeletal muscle. Proc. Natl. Acad. Sci. USA 1994, 91, 2270–2274. [Google Scholar] [CrossRef]
- Tanabe, T.; Beam, K.G.; Powell, J.A.; Numa, S. Restoration of excitation—Contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 1988, 336, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Beam, K.G.; Adams, B.A.; Niidome, T.; Numa, S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation–contraction coupling. Nature 1990, 346, 567–569. [Google Scholar] [CrossRef]
- Fabiato, A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol.-Cell Physiol. 1983, 245, C1–C14. [Google Scholar] [CrossRef] [PubMed]
- Suda, N.; Penner, R. Membrane repolarization stops caffeine-induced Ca2+ release in skeletal muscle cells. Proc. Natl. Acad. Sci. USA 1994, 91, 5725–5729. [Google Scholar] [CrossRef]
- Howlett, S.E.; Ferrier, G.R. The voltage-sensitive release mechanism: A new trigger for cardiac contraction. Can. J. Physiol. Pharmacol. 1997, 75, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Chavis, P.; Fagni, L.; Lansman, J.; Bockaert, J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature 1996, 382, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Atwal, K.; O’reilly, B.; Gougoutas, J.; Malley, M. Synthesis of substituted 1, 2, 3, 4-tetrahydro-6-methyl-2-thioxo-5-pyrimidinecarboxylic acid esters. Heterocycles 1987, 26, 1189–1192. [Google Scholar] [CrossRef]
- Mishina, T.; Tsuda, N.; Inui, A.; Miura, Y. Jpn. Kokai Tokkyo Koho (1987) JP 62169793. Chem. Abstr. 1988, 108, 56120e. [Google Scholar]
- Atwal, K.S.; Rovnyak, G.C.; Schwartz, J.; Moreland, S.; Hedberg, A.; Gougoutas, J.Z.; Malley, M.F.; Floyd, D.M. Dihydropyrimidine calcium channel blockers: 2-heterosubstituted 4-aryl-1, 4-dihydro-6-methyl-5-pyrimidinecarboxylic acid esters as potent mimics of dihydropyridines. J. Med. Chem. 1990, 33, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Rovnyak, G.C.; Atwal, K.S.; Hedberg, A.; Kimball, S.D.; Moreland, S.; Gougoutas, J.Z.; O’Reilly, B.C.; Schwartz, J.; Malley, M.F. Dihydropyrimidine calcium channel blockers. 4. Basic 3-substituted-4-aryl-1, 4-dihydropyrimidine-5-carboxylic acid esters. Potent antihypertensive agents. J. Med. Chem. 1992, 35, 3254–3263. [Google Scholar] [CrossRef]
- Mahgoub, S.; Kotb El-Sayed, M.-I.; El-Shehry, M.F.; Mohamed Awad, S.; Mansour, Y.E.; Fatahala, S.S. Synthesis of novel calcium channel blockers with ACE2 inhibition and dual antihypertensive/anti-inflammatory effects: A possible therapeutic tool for COVID-19. Bioorg. Chem. 2021, 116, 105272. [Google Scholar] [CrossRef]
- Irshad, N.; Khan, A.-u.; Alamgeer; Khan, S.-U.-D.; Iqbal, M.S. Antihypertensive potential of selected pyrimidine derivatives: Explanation of underlying mechanistic pathways. Biomed. Pharmacother. 2021, 139, 111567. [Google Scholar] [CrossRef]
- Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J.N. Biginelli reaction: An overview. Tetrahedron Lett. 2016, 57, 5135–5149. [Google Scholar] [CrossRef]
- Takenaka, T.; Usuda, S.; Nomura, T.; Maeno, H.; Sado, T. Vasodilator profile of a new 1, 4-dihydropyridine derivative, 2, 6-dimethyl-4-(3-nitrophenyl)-1, 4-dihydropyridine-3, 5-dicarboxylic acid 3-[2-(N-benzyl-N-methylamino)]-ethyl ester 5-methyl ester hydrochloride (YC-93). Arzneimittelforschung 1976, 26, 2172–2178. [Google Scholar]
- Arrowsmith, J.E.; Campbell, S.F.; Cross, P.E.; Stubbs, J.K.; Burges, R.A.; Gardiner, D.G.; Blackburn, K.J. Long-acting dihydropyridine calcium antagonists. 1. 2-Alkoxymethyl derivatives incorporating basic substituents. J. Med. Chem. 1986, 29, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Motta, G.; Pennini, R.; Testa, R.; Sironi, G.; Catto, A.; Cerri, A.; Zappa, M.; Bianchi, G.; Nardi, D. Asymmetric N-(3, 3-diphenylpropyl) aminoalkyl esters of 4-aryl-2, 6-dimethyl-1, 4-dihydropyridine-3, 5-dicarboxylic acids with antihypertensive activity. Eur. J. Med. Chem. 1998, 33, 399–420. [Google Scholar] [CrossRef]
- Meguro, K.; Aizawa, M.; Sohda, T.; Kawamatsu, Y.; Nagaoka, A. New 1, 4-dihydropyridine derivatives with potent and long-lasting hypotensive effect. Chem. Pharm. Bull. 1985, 33, 3787–3797. [Google Scholar] [CrossRef] [PubMed]
- El-Wakil, M.H.; Teleb, M.; Abu-Serie, M.M.; Huang, S.; Zamponi, G.W.; Fahmy, H. Structural optimization, synthesis and in vitro synergistic anticancer activities of combinations of new N3-substituted dihydropyrimidine calcium channel blockers with cisplatin and etoposide. Bioorg. Chem. 2021, 115, 105262. [Google Scholar] [CrossRef] [PubMed]
- Teleb, M.; Rizk, O.H.; Zhang, F.-X.; Fronczek, F.R.; Zamponi, G.W.; Fahmy, H. Synthesis of some new C2 substituted dihydropyrimidines and their electrophysiological evaluation as L-/T-type calcium channel blockers. Bioorg. Chem. 2019, 88, 102915. [Google Scholar] [CrossRef]
- Gordeev, M.F.; Patel, D.V.; England, B.P.; Jonnalagadda, S.; Combs, J.D.; Gordon, E.M. Combinatorial synthesis and screening of a chemical library of 1, 4-dihydropyridine calcium channel blockers. Bioorg. Med. Chem. 1998, 6, 883–889. [Google Scholar] [CrossRef]
- Goldmann, S.; Stoltefuss, J. 1, 4-dihydropyridines: Effects of chirality and conformation on the calcium antagonist and calcium agonist activities. Angew. Chem. Int. Ed. Engl. 1991, 30, 1559–1578. [Google Scholar] [CrossRef]
- Ioan, P.; Carosati, E.; Micucci, M.; Cruciani, G. Broccatelli. F.; Zhorov, B.S.; Chiarini, A.; Budriesi, R. 1, 4-Dihydropyridine scaffold in medicinal chemistry, the story so far and perspectives (part 1): Action in ion channels and GPCRs. Curr. Med. Chem 2011, 18, 4901–4922. [Google Scholar] [CrossRef] [PubMed]
- Teleb, M.; Rizk, O.H.; Zhang, F.-X.; Fronczek, F.R.; Zamponi, G.W.; Fahmy, H. Design, synthesis and pharmacological evaluation of some substituted dihydropyrimidines with L-/T-type calcium channel blocking activities. Bioorg. Chem. 2019, 83, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Teleb, M.; Zhang, F.-X.; Huang, J.; Gadotti, V.M.; Farghaly, A.M.; AboulWafa, O.M.; Zamponi, G.W.; Fahmy, H. Synthesis and biological evaluation of novel N3-substituted dihydropyrimidine derivatives as T-type calcium channel blockers and their efficacy as analgesics in mouse models of inflammatory pain. Bioorg. Med. Chem. 2017, 25, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, A.M.; Rizk, O.H.; Darwish, I.; Hamza, M.; Altowyan, M.S.; Barakat, A.; Teleb, M. Design, Synthesis, Pharmacodynamic and In Silico Pharmacokinetic Evaluation of Some Novel Biginelli-Derived Pyrimidines and Fused Pyrimidines as Calcium Channel Blockers. Molecules 2022, 27, 2240. [Google Scholar] [CrossRef] [PubMed]
- Teleb, M.; Zhang, F.-X.; Farghaly, A.M.; Wafa, O.M.A.; Fronczek, F.R.; Zamponi, G.W.; Fahmy, H. Synthesis of new N3-substituted dihydropyrimidine derivatives as L-/T-type calcium channel blockers. Eur. J. Med. Chem. 2017, 134, 52–61. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A.; Dallinger, D. Microwaves in Organic and Medicinal Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 52. [Google Scholar]
- Kappe, C.O.; Dallinger, D. Controlled microwave heating in modern organic synthesis: Highlights from the 2004–2008 literature. Mol. Divers. 2009, 13, 71–193. [Google Scholar]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Lidstrom, P.; Westman, J.; Lewis, A. Enhancement of combinatorial chemistry by microwave-assisted organic synthesis. Comb. Chem. High Throughput Screen. 2002, 5, 441–458. [Google Scholar] [CrossRef]
- Wilson, N.S.; Sarko, C.R.; Roth, G.P. Development and applications of a practical continuous flow microwave cell. Org. Process Res. Dev. 2004, 8, 535–538. [Google Scholar] [CrossRef]
- Kappe, C.O.; Dallinger, D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug Discov. 2006, 5, 51–63. [Google Scholar] [CrossRef]
- Wathey, B.; Tierney, J.; Lidström, P.; Westman, J. The impact of microwave-assisted organic chemistry on drug discovery. Drug Discov. Today 2002, 7, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Stadler, A.; Kappe, C.O. Automated library generation using sequential microwave-assisted chemistry. Application toward the Biginelli multicomponent condensation. J. Comb. Chem. 2001, 3, 624–630. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A. Building dihydropyrimidine libraries via microwave-assisted Biginelli multicomponent reactions. Methods Enzymol. 2003, 369, 197–223. [Google Scholar]
- Desai, B.; Dallinger, D.; Kappe, C.O. Microwave-assisted solution phase synthesis of dihydropyrimidine C5 amides and esters. Tetrahedron 2006, 62, 4651–4664. [Google Scholar] [CrossRef]
- Kappe, C.O.; Kumar, D.; Varma, R.S. Microwave-assisted high-speed parallel synthesis of 4-aryl-3, 4-dihydropyrimidin-2 (1H)-ones using a solventless Biginelli condensation protocol. Synthesis 1999, 1999, 1799–1803. [Google Scholar] [CrossRef]
- Matloobi, M.; Kappe, C.O. Microwave-assisted solution-and solid-phase synthesis of 2-amino-4-arylpyrimidine derivatives. J. Comb. Chem. 2007, 9, 275–284. [Google Scholar] [CrossRef]
- Watanabe, M.; Koike, H.; Ishiba, T.; Okada, T.; Seo, S.; Hirai, K. Synthesis and biological activity of methane sulfonyl pyrrole-substituted 3.5-dihydroxy-6 heptanoates: A novel series of HMG-CoA reductase inhibitors. Bioorg. Med. Chem. 1997, 5, 437–444. [Google Scholar] [CrossRef]
- Kappe, C.O. 100 years of the Biginelli dihydropyrimidine synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Dallinger, D.; Gorobets, N.Y.; Kappe, C.O. Microwave-assisted scavenging of electrophiles utilizing polymer-supported sequestration reagents. Application to the synthesis of N3-acylated dihydropyrimidine libraries. Mol. Divers. 2003, 7, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, D.; Gorobets, N.Y.; Kappe, C.O. High-Throughput Synthesis of N 3-Acylated Dihydropyrimidines Combining Microwave-Assisted Synthesis and Scavenging Techniques. Org. Lett. 2003, 5, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Prokopcová, H.; Kremsner, J.M.; Kappe, C.O. 5-Aroyl-3,4-dihydropyrimidin-2-one Library Generation via Automated Sequential and Parallel Microwave-assisted Synthesis Techniques. J. Comb. Chem. 2007, 9, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Petit, A.; Hamelin, J.; Texier-Boullet, F.; Jacquault, P.; Mathe, D. New solvent-free organic synthesis using focused microwaves. Synthesis 1998, 1998, 1213–1234. [Google Scholar] [CrossRef]
- Christoffers, J. Iron (III) catalysis of the Michael reaction of 1, 3-dicarbonyl compounds and enones. Chem. Commun. 1997, 10, 943–944. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Bolourtchian, M.; Hosseini, M. Microwave-assisted efficient synthesis of dihydropyrimidines in solvent-free condition. Synth. Commun. 2004, 34, 3335–3341. [Google Scholar] [CrossRef]
- Heravi, M.M.; Aghayan, M.M. Microwave-assisted oxidation of alcohols using wet alumina supported ammonium chlorochromate in solventless system. Zeitschrift für Naturforschung B 1999, 54, 815–817. [Google Scholar] [CrossRef]
- Stefani, H.A.; Gatti, P.M. 3, 4-Dihydropyrimidin-2 (1H)-ones: Fast synthesis under microwave irradiation in solvent free conditions. Synth. Commun. 2000, 30, 2165–2173. [Google Scholar] [CrossRef]
- Hu, E.H.; Sidler, D.R.; Dolling, U.-H. Unprecedented catalytic three component one-pot condensation reaction: An efficient synthesis of 5-alkoxycarbonyl-4-aryl-3, 4-dihydropyrimidin-2 (1H)-ones. J. Org. Chem. 1998, 63, 3454–3457. [Google Scholar] [CrossRef]
- Gohain, M.; Prajapati, D.; Sandhu, J.S. A novel Cu-catalysed three-component one-pot synthesis of dihydropyrimidin-2 (1H)-ones using microwaves under solvent-free conditions. Synlett 2004, 2004, 0235–0238. [Google Scholar]
- Xue, S.; Shen, Y.C.; Li, Y.L.; Shen, X.M.; Guo, Q.X. Synthesis of 4-aryl-3, 4-dihydropyrimidinones using microwave-assisted solventless Biginelli reaction. Chin. J. Chem. 2002, 20, 385–389. [Google Scholar] [CrossRef]
- Yadav, J.; Reddy, B.S.; Reddy, E.J.; Ramalingam, T. Microwave-assisted efficient synthesis of dihydro pyrimidines: Improved high yielding protocol for the Biginelli reaction. J. Chem. Res. 2000, 2000, 354–355. [Google Scholar] [CrossRef]
- Basha, S.; Kalla, R.M.N.; Varalakshmi, M.; Sudhamani, H.; Appa, R.M.; Chul Hong, S.; Raju, C.N. Heterogeneous catalyst SiO2–LaCl3 7H2O: Characterization and microwave-assisted green synthesis of α-aminophosphonates and their antimicrobial activity. Mol. Divers. 2022, 26, 2703–2715. [Google Scholar] [CrossRef] [PubMed]
- Salehi, H.; Guo, Q.X. A facile and efficient one-pot synthesis of dihydropyrimidinones catalyzed by magnesium bromide under solvent-free conditions. Synth. Commun. 2004, 34, 171–179. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, N.; Xu, J.; Zhang, W.; Kong, F. Microwave-assisted and iodine-catalyzed synthesis of dihydropyrimidin-2-thiones via biginelli reaction under solvent-free conditions. Synth. Commun. 2013, 43, 139–146. [Google Scholar] [CrossRef]
- Zhang, G.L.; Cai, X.H. Magnesium Chloride Hexahydrate Catalyzed One-Pot Synthesis of 3, 4-Dihydropyrimidin-2-(1 H) ones Under Solvent-Free Conditions. Synth. Commun. 2005, 35, 829–833. [Google Scholar] [CrossRef]
- Ladole, C.A.; Salunkhe, N.G.; Bhaskar, R.S.; Aswar, A.S. A microwave-assisted synthesis of 3, 4-dihydropyrimidin-2 (1H)-one/thione derivatives using nanocrystalline MgFe2O4 as catalyst. Eur. J. Chem. 2014, 5, 122–126. [Google Scholar] [CrossRef]
- Bikker, J.A.; Weaver, D.F. Theoretical studies applicable to the design of novel anticonvulsants: Part 2. A comparison of AM1, MNDO, and PM3 semiempirical molecular orbital conformational analyses of dihydropyridine calcium channel blockers. J. Mol. Struct. THEOCHEM 1993, 281, 173–184. [Google Scholar] [CrossRef]
- Liepina, I.; Blanco, M.; Duburs, G.; Liwo, A. Spatial structure of dihydropyridines and similarity of dihydropyridines with some amino acids. Mol. Eng. 1997, 7, 401–427. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Richards, W.G. A conformational distinction between dihydropyridine calcium agonists and antagonists. J. Chem. Soc. Chem. Commun. 1986, 10, 739–741. [Google Scholar] [CrossRef]
- Fassihi, A.; Mahnam, K.; Moeinifard, B.; Bahmanziari, M.; Aliabadi, H.S.; Zarghi, A.; Sabet, R.; Salimi, M.; Mansourian, M. Synthesis, calcium-channel blocking activity, and conformational analysis of some novel 1, 4-dihydropyridines: Application of PM3 and DFT computational methods. Med. Chem. Res. 2012, 21, 2749–2761. [Google Scholar] [CrossRef]
- Davood, A.; Nematollahi, A.; Iman, M.; Shafiee, A. Computational studies of new 1, 4-dihydropyridines containing 4-(5)-chloro-2-ethyl-5-(4)-imidazolyl substituent: QSAR and docking. Med. Chem. Res. 2010, 19, 58–70. [Google Scholar] [CrossRef]
- Kappe, C.O.; Fabian, W.M.; Semones, M.A. Conformational analysis of 4-aryl-dihydropyrimidine calcium channel modulators. A comparison of ab initio, semiempirical and X-ray crystallographic studies. Tetrahedron 1997, 53, 2803–2816. [Google Scholar] [CrossRef]
- Choudhari, P.B.; Bhatia, M.S.; Jadhav, S.D. Pharmacophore modelling, quantitative structure activity relationship (QSAR) and docking studies of pyrimidine analogs as potential calcium channel blockers. J. Korean Chem. Soc. 2013, 57, 99–103. [Google Scholar] [CrossRef]
- Hemmateenejad, B.; Miri, R.; Safarpour, M.A.; Khoshneviszadeh, M.; Edraki, N. Conformational analysis of some new derivatives of 4-nitroimidazolyl-1,4-dihydropyridine-based calcium channel blockers. J. Mol. Struct. THEOCHEM 2005, 717, 139–152. [Google Scholar] [CrossRef]
- Doddareddy, M.R.; Jung, H.K.; Lee, J.Y.; Lee, Y.S.; Cho, Y.S.; Koh, H.Y.; Pae, A.N. First pharmacophoric hypothesis for T-type calcium channel blockers. Bioorg. Med. Chem. 2004, 12, 1605–1611. [Google Scholar] [CrossRef]
- Gandhi, T.; Melge, A.R.; Gopi Mohan, C. In silico identification of T-type calcium channel blockers: A ligand-based pharmacophore mapping approach. J. Adv. Res. 2016, 7, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Triggle, D.J. 1, 4-Dihydropyridines as calcium channel ligands and privileged structures. Cell. Mol. Neurobiol. 2003, 23, 293–303. [Google Scholar] [CrossRef]
- Mannhold, R. KATP channel openers: Structure-activity relationships and therapeutic potential. Med. Res. Rev. 2004, 24, 213–266. [Google Scholar] [CrossRef] [PubMed]
- Yiu, S.h.; Knaus, E.E. Synthesis of valproate, valerate, and 1-methyl-1, 4-dihydropyridyl-3-carbonyloxy ester derivatives of Hantzsch 1, 4-dihydropyridines as potential prodrugs and their evaluation as calcium channel antagonist and anticonvulsant agents. Drug Dev. Res. 1999, 48, 26–37. [Google Scholar] [CrossRef]
- Kamiński, R.M.; Mazurek, M.; Turski, W.A.; Kleinrok, Z.; Czuczwar, S.J. Amlodipine enhances the activity of antiepileptic drugs against pentylenetetrazole-induced seizures. Pharmacol. Biochem. Behav. 2001, 68, 661–668. [Google Scholar] [CrossRef]
- Raghavan, K.; Shek, E.; Bodor, N. Improved delivery through biological membranes. XXX. Synthesis and biological aspects of a 1, 4-dihydropyridine based chemical delivery system for brain-sustained delivery of hydroxy CCNU. Anticancer Drug Des. 1987, 2, 25–36. [Google Scholar]
- El-Sherbeny, M.A.; Al-Salem, H.S.; Sultan, M.A.; Radwan, M.A.; Farag, H.A.; El-Subbagh, H.I. Synthesis, in vitro and in vivo evaluation of a delivery system for targeting anticancer drugs to the brain. Arch. Der Pharm. Int. J. Pharm. Med. Chem. 2003, 336, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Desai, B.; Sureja, D.; Naliapara, Y.; Shah, A.; Saxena, A.K. Synthesis and QSAR studies of 4-substituted phenyl-2, 6-dimethyl-3, 5-bis-N-(substituted phenyl) carbamoyl-1, 4-dihydropyridines as potential antitubercular agents. Bioorg. Med. Chem. 2001, 9, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Khoshneviszadeh, M.; Edraki, N.; Javidnia, K.; Alborzi, A.; Pourabbas, B.; Mardaneh, J.; Miri, R. Synthesis and biological evaluation of some new 1, 4-dihydropyridines containing different ester substitute and diethyl carbamoyl group as anti-tubercular agents. Bioorg. Med. Chem. 2009, 17, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Briede, J.; Stivriņa, M.; Stoldere, D.; Bisenieks, E.; Uldriķis, J.; Poikāns, J.; Makarova, N.; Duburs, G. Effect of new and known 1, 4-dihydropyridine derivatives on blood glucose levels in normal and streptozotocin-induced diabetic rats. Cell Biochem. Funct. Cell. Biochem. Its Modul. Act. Agents Dis. 2004, 22, 219–224. [Google Scholar] [CrossRef]
- Das, P.; Bell-Horner, C.; Huang, R.; Raut, A.; Gonzales, E.; Chen, Z.; Covey, D.; Dillon, G. Inhibition of type A GABA receptors by L-type calcium channel blockers. Neuroscience 2004, 124, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Vyshtakalyuk, A.; Nazarov, N.; Zobov, V.; Abdulkhakov, S.; Minnekhanova, O.; Semenov, V.; Galyametdinova, I.; Cherepnev, G.; Reznik, V. Evaluation of the hepatoprotective effect of L-ascorbate 1-(2-hydroxyethyl)-4, 6-dimethyl-1, 2-dihydropyrimidine-2-one upon exposure to carbon tetrachloride. Bull. Exp. Biol. Med. 2017, 162, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Lu, Y.; Zhu, Y.; Zheng, W.; Guo, F.; Yao, B.; Xu, S.; Wang, Y.; Jin, L.; Li, Y. Structural basis for the hepatoprotective effects of antihypertensive 1, 4-dihydropyridine drugs. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 2261–2270. [Google Scholar] [CrossRef]
- Rami, C.; Patel, L.; Patel, C.N.; Parmar, J.P. Synthesis, antifungal activity, and QSAR studies of 1, 6-dihydropyrimidine derivatives. J. Pharm. Bioallied Sci. 2013, 5, 277. [Google Scholar] [CrossRef] [PubMed]
- Abu-Melha, H. Synthesis, antibacterial and antifungal evaluation of novel 1, 4-dihydropyridine derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 113, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Durstberger, M.; Eichelberger, B.; Koppensteiner, R.; Panzer, S. Calcium-channel blockers attenuate the antiplatelet effect of clopidogrel. Cardiovasc. Ther. 2015, 33, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Rha, S.-W.; Shin, W.-Y.; Lee, S.-J.; Jin, D.-K.; Lee, S.-W.; Park, J.Y.; Choi, B.G.; Poddar, K.L.; Kumari, M. AS-160 Calcium channel blockers of dihydropyridine class reduce the antiplatelet effect of Clopidogrel?: What is clinical impact. Am. J. Cardiol. 2011, 107, 47A. [Google Scholar] [CrossRef]
- Chhillar, A.K.; Arya, P.; Mukherjee, C.; Kumar, P.; Yadav, Y.; Sharma, A.K.; Yadav, V.; Gupta, J.; Dabur, R.; Jha, H.N. Microwave-assisted synthesis of antimicrobial dihydropyridines and tetrahydropyrimidin-2-ones: Novel compounds against aspergillosis. Bioorg. Med. Chem. 2006, 14, 973–981. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).