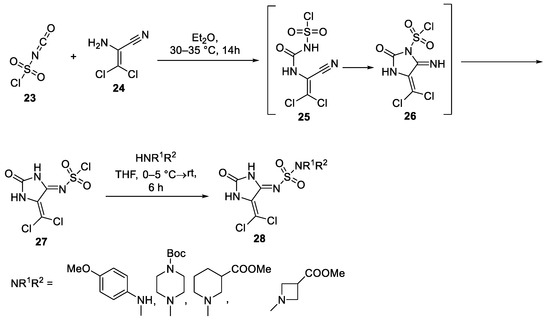
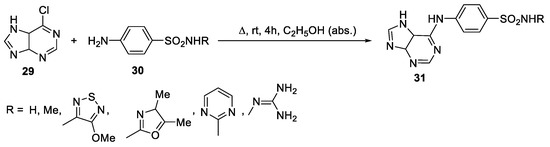
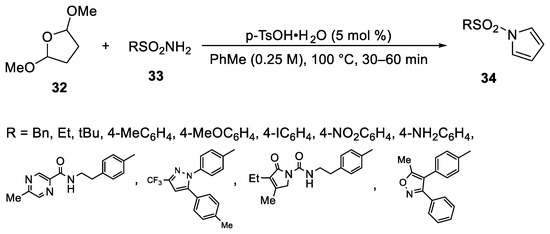
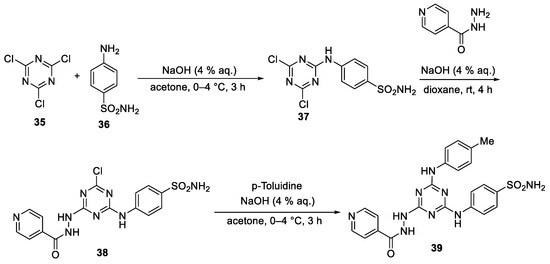
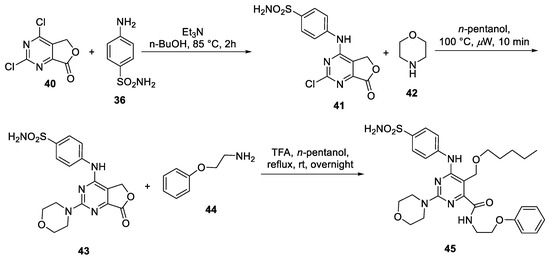
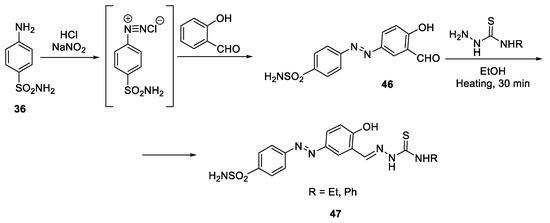
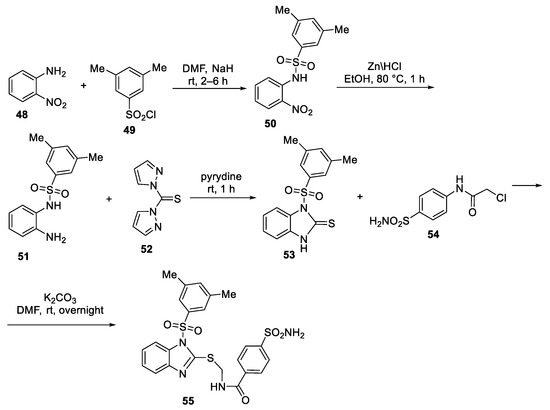
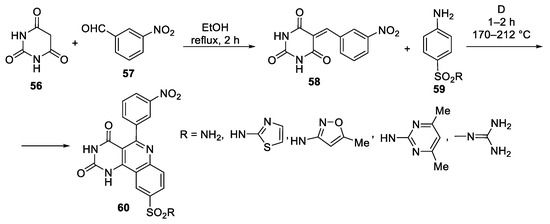
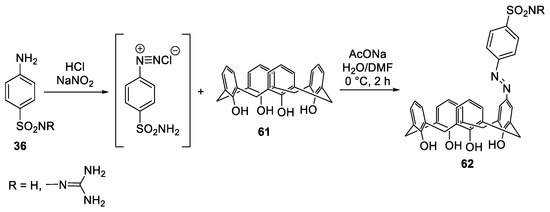
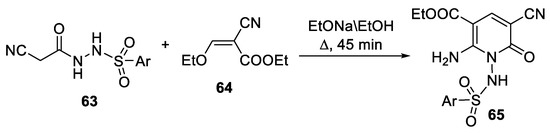
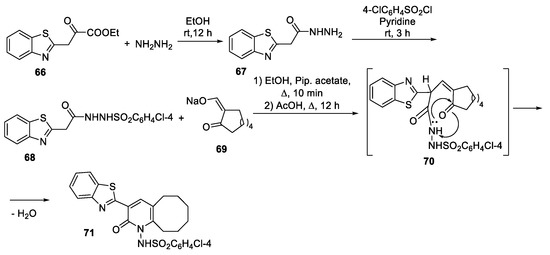
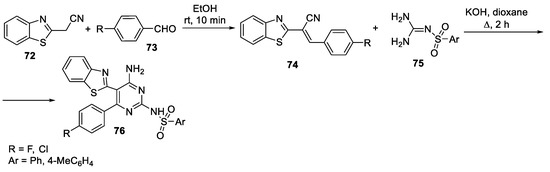
Abstract
Sulfonamides are the basic motifs for a whole generation of drugs from a large group of antibiotics. Currently, research in the field of the new sulfonamide synthesis has received a “second wind”, due to the increase in the synthetic capabilities of organic chemistry and the study of their medical and biological properties of a wide spectrum of biological activity. New reagents and new reactions make it possible to significantly increase the number of compounds with a sulfonamide fragment in combination with other important pharmacophore groups, such as, for example, a wide class of N-containing heterocycles. The result of these synthetic possibilities is the extension of the activity spectrum—along with antibacterial activity, many of them exhibit other types of biological activity. Antiviral activity is also observed in a wide range of sulfonamide derivatives. This review provides examples of the synthesis of sulfonamide compounds with antiviral properties that can be used to develop drugs against coxsackievirus B, enteroviruses, encephalomyocarditis viruses, adenoviruses, human parainfluenza viruses, Ebola virus, Marburg virus, SARS-CoV-2, HIV and others. Since over the past three years, viral infections have become a special problem for public health throughout the world, the development of new broad-spectrum antiviral drugs is an extremely important task for synthetic organic and medicinal chemistry. Sulfonamides can be both sources of nitrogen for building a nitrogen-containing heterocyclic core and the side chain substituents of a biologically active substance. The formation of the sulfonamide group is often achieved by the reaction of the N-nucleophilic center in the substrate molecule with the corresponding sulfonylchloride. Another approach involves the use of sulfonamides as the reagents for building a nitrogen-containing framework.
Keywords:
sulfonamide; N-heterocycles; antiviral activity; multi-step syntheses; piperidine; morpholine; azoles; azines 1. Introduction
N-heterocycles and linear products synthesized from sulfonamides or containing a sulfonamide fragment in the side chain are important objects for studying biological activity. Traditionally, sulfonamides are primarily considered as antibacterial agents; however, among these substances one can find not only effective antibiotics [1,2], but also compounds with very different activities: oral hypoglycemic [3,4], antitumor [3,5], antiviral [6,7,8,9], antiepileptic [10], antihypertensive [11], antiprotozoal [12], antifungal [13], anticancer [14,15,16], anti-inflammatory [17], diuretic [18], butyrylcholinesterase inhibitors (anti-Alzheimer’s disease activity) [19], MAO-B specific inhibitors (activity in the treatment of neurodegenerative disorders such as Parkinson’s disease) [20], COX-2 inhibitors (therapy of inflammatory disease) [21], etc. Recent advances in the medicinal chemistry of sulfonamides showed the possibilities of new unique drug design. Sulfonamides are of particular importance in the synthesis of carbonic anhydrase inhibitors, which are used, for example, in combined chemotherapy for cancer [22]. Heterocyclic sulfonamides acts as hCA IV (as drug targets) inhibitors [23]. Pyrrolidine-containing sulfonamides (hCA IV inhibitors) are promising drugs for the treatment of glioma [23]. Sulfonamide hCA VII inhibitors are used in the complex therapy of HIV-infection. The N-acylsulfonamide form of the prodrug Elsulfavirin selectively inhibits hCA VII to treat neurological complications of HIV infection [24]. Sulfonamide-containing 1,3-oxazoles and thiophenes show cytosolic CA I and CA II inhibition in the picomolar concentrations with extremely high selectivity [25]. Obtained compounds can currently be considered as the most potential basic structures of CA inhibitors for the synthesis of a wide range of drugs [25]. The authors of [26] obtained next-generation sulfonamide-containing carbonic anhydrase inhibitors that demonstrated high and selective inhibition effect of glaucoma-related hCA II, high hydrophilicity and the possibility of conjugation to sustained-release nanoparticles. Even at the initial stage of research, 5-(sulfamoyl)thien-2-yl 1,3-oxazoles (eye drops, 1% solution) showed an intraocular pressure reduction similar to the clinically used 2% solution of dorzolamide [26]. Sulfonamide derivatives often do not require complex functionalization of substituents [27], that greatly facilitates the synthesis of compounds used in the treatment of a wide range of diseases. It is widely known that nitrogen-containing heterocycles are the part of a large number of drugs of synthetic and natural origin [28,29]. Combining sulfonamide and N-heterocyclic pharmacophore groups in a molecule is one of the effective approaches to the synthesis of broad-spectrum drugs.
3. Conclusions
Viral infections have attracted the close attention of the entire scientific community in every corner of the planet. The COVID-19 epidemic has confirmed the danger of drug-resistant strains and made the task of developing new antiviral drugs more urgent than ever. The presented short review showed the main methodologies for the synthesis of antiviral derivatives of sulfonamides, which were obtained on the basis of N-containing heterocyclic structures. There is no doubt about the effect of the sulfonamide fragment on the antiviral properties of the obtained compounds. Moreover, sulfonamides can be both sources of nitrogen for building a nitrogen-containing heterocyclic core and the side chain substituents of a biologically active substance. The formation of the sulfonamide group is often achieved by the reaction of the N-nucleophilic center in the substrate molecule with the corresponding sulfonylchloride. This review provides examples of the synthesis of sulfonamide compounds with antiviral properties that can be used to develop drugs against EMCV, AdV5, HPIV, EBOV, MARV, APMV, SARS-CoV-2, HIV, DENV, ZIKV, BVDV, NDV, IBDV, H9N2, AIV, IBV, HSV, CBV, HAV, VYG, HCVcc and HAdV.
Funding
This work was supported by the Russian Science Foundation (project 22-13-00036).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| MAO-B | monoamine oxidase-B |
| COX-2 | cyclooxygenase-2 |
| hCA | human carbonic anhydrase |
| DCM | dichloromethane |
| THF | tetrahydrofuran |
| BOC | tert-butyloxycarbonyl |
| DIPEA | N,N-diisopropylethylamine |
| DMF | dimethylformamide |
| 447-52D | human monoclonal antibody isolated from a heterohybridoma derived from an HIV-1-infected individual |
| gp120 V3 loop | the V3 loop of the human immunodeficiency virus type 1 (HIV-1) gp120 exterior envelope glycoprotein |
| ADME | absorption, distribution, metabolism, and excretion |
| DNA | deoxyribonucleic acid |
| RNA | ribonucleic acid |
| NS2B-NS3 protease | NS3 serine protease in the complex with the small activator protein NS2B |
| EMCV | encephalomyocarditis virus |
| AdV5 | human adenovirus 5 |
| HPIV | human parainfluenza virus |
| EBOV | Zaire ebolavirus |
| MARV | Marburg virus |
| APMV | apple mosaic virus |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| HIV | human immunodeficiency virus |
| DENV | dengue virus |
| ZIKV | Zika virus |
| BVDV | Bovine viral diarrhea virus |
| NDV | Newcastle disease virus |
| IBDV | infectious bursal disease virus |
| H9N2 | influenza A virus subtype H9N2 |
| AIV | avian (bird) influenza (flu) Type A virus |
| IBV | infectious bronchitis virus |
| HSV | herpes simplex virus |
| CBV | Coxsackie B virus |
| HAV | hepatitis A virus |
| HCV | hepatitis C virus |
| HAdV | human adenovirus |
References
- Mansour, O.; Herbali, J.; Yousef, F. Sulfonamides: Historical Discovery Development (Structure-Activity Relationship Notes). Vitr. -Vivo -Silico J. 2018, 1, 1–15. [Google Scholar]
- Scozzafava, A.; Owa, T.; Mastrolorenzo, A.; Supuran, C. Anticancer and Antiviral Sulfonamides. Curr. Med. Chem. 2003, 10, 925–953. [Google Scholar] [CrossRef] [PubMed]
- Chinthakindi, P.K.; Naicker, T.; Thota, N.; Govender, T.; Kruger, H.G.; Arvidsson, P.I. Sulfonimidamides in Medicinal and Agricultural Chemistry. Angew. Chem. Int. Ed. 2017, 56, 4100–4109. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Karim, S.S.; Anwar, M.M.; Syam, Y.M.; Nael, M.A.; Ali, H.F.; Motaleb, M.A. Rational design and synthesis of new tetralin-sulfonamide derivatives as potent anti-diabetics and DPP-4 inhibitors: 2D & 3D QSAR, in vivo radiolabeling and bio distribution studies. Bioorg. Chem. 2018, 81, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Said, M.A.; Eldehna, W.M.; Nocentini, A.; Fahim, S.H.; Bonardi, A.; Elgazar, A.A.; Kryštof, V.; Soliman, D.H.; Abdel-Aziz, H.A.; Gratteri, P.; et al. Sulfonamide-based ring-fused analogues for CAN508 as novel carbonic anhydrase inhibitors endowed with antitumor activity: Design, synthesis, and in vitro biological evaluation. Eur. J. Med. Chem. 2020, 189, 112019. [Google Scholar] [CrossRef]
- He, F.; Shi, J.; Wang, Y.; Wang, S.; Chen, J.; Gan, X.; Song, B.; Hu, D. Synthesis, Antiviral Activity, and Mechanisms of Purine Nucleoside Derivatives Containing a Sulfonamide Moiety. J. Agric. Food Chem. 2019, 67, 8459–8467. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, J.; Zan, N.; Li, C.; Hu, D.; Song, B. Discovery of Novel Chromone Derivatives Containing a Sulfonamide Moiety as Anti-ToCV Agents through the Tomato Chlorosis Virus Coat Protein-Oriented Screening Method. J. Agric. Food Chem. 2021, 69, 12126–12134. [Google Scholar] [CrossRef]
- Delijewski, M.; Haneczok, J. AI drug discovery screening for COVID-19 reveals zafirlukast as a repurposing candidate. Med. Drug Discov. 2021, 9, 100077. [Google Scholar] [CrossRef]
- White, K.; Esparza, M.; Liang, J.; Bhat, P.; Naidoo, J.; McGovern, B.L.; Williams, M.A.P.; Alabi, B.R.; Shay, J.; Niederstrasser, H.; et al. Aryl Sulfonamide Inhibits Entry and Replication of Diverse Influenza Viruses via the Hemagglutinin Protein. J. Med. Chem. 2021, 64, 10951–10966. [Google Scholar] [CrossRef]
- Van Berkel, M.A.; Elefritz, J.L. Evaluating off-label uses of acetazolamide. Am. J. Health-Sys. Pharm. 2018, 75, 524–531. [Google Scholar] [CrossRef]
- Masaret, G.S. Synthesis, Docking and Antihypertensive Activity of Pyridone Derivatives. Chem. Select 2020, 5, 13995–14003. [Google Scholar] [CrossRef]
- Dolensky, J.; Hinteregger, C.; Leitner, A.; Seebacher, W.; Saf, R.; Belaj, F.; Mäser, P.; Kaiser, M.; Weis, R. Antiprotozoal Activity of Azabicyclo-Nonanes Linked to Tetrazole or Sulfonamide Cores. Molecules 2022, 27, 6217. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Mushtaq, S.; Naz, S.; Farooq, U.; Zaidi, A.; Bukhari, S.; Rauf, A.; Mubarak, M. Sulfonamides as potential bioactive scaffolds. Curr. Org. Chem. 2018, 22, 818–830. [Google Scholar] [CrossRef]
- Wan, Y.; Fang, G.; Chen, H.; Deng, X.; Tang, Z. Sulfonamide derivatives as potential anti-cancer agents and their SARs elucidation. Eur. J. Med. Chem. 2021, 226, 113837. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.I.; Yamali, C.; Sakagami, H.; Angeli, A.; Leitans, J.; Kazaks, A.; Tars, K.; Ozgun, D.O.; Supuran, C.T. New anticancer drug candidates sulfonamides as selective hCA IX or hCA XII inhibitors. Bioorg. Chem. 2018, 77, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Eisvand, F.; Hadizadeh, F.; Mosaffa, F.; Ghasemi, A.; Ghodsi, R. Design, synthesis and biological evaluation of novel 5,6,7-trimethoxy-N-aryl-2-styrylquinolin-4-amines as potential anticancer agents and tubulin polymerization inhibitors. Bioorg. Chem. 2020, 98, 103711. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.A.M.; Angeli, A.; El-Azab, A.S.; Hammouda, M.E.A.; El-Sherbeny, M.A.; Supuran, C.T. Synthesis and anti-inflammatory activity of sulfonamides and carboxylates incorporating trimellitimides: Dual cyclooxygenase/carbonic anhydrase inhibitory actions. Bioorg. Chem. 2019, 84, 260–268. [Google Scholar] [CrossRef]
- Ferraroni, M.; Angeli, A.; Pinteala, M.; Supuran, C.T. Sulfonamide diuretic azosemide as an efficient carbonic anhydrase inhibitor. J. Mol. Struct. 2022, 1268, 133672. [Google Scholar] [CrossRef]
- Košak, U.; Brus, B.; Knez, D.; Žakelj, S.; Trontelj, J.; Pišlar, A.; Šink, R.; Jukič, M.; Živin, M.; Podkowa, A.; et al. The Magic of Crystal Structure-Based Inhibitor Optimization: Development of a Butyrylcholinesterase Inhibitor with Picomolar Affinity and in Vivo Activity. J. Med. Chem. 2018, 61, 119–139. [Google Scholar] [CrossRef]
- Shetnev, A.; Shlenev, R.; Efimova, J.; Ivanovskii, S.; Tarasov, A.; Petzer, A.; Petzer, J.P. 1,3,4-Oxadiazol-2-ylbenzenesulfonamides as privileged structures for the inhibition of monoamine oxidase B. Bioorg. Med. Chem. Lett. 2019, 29, 126677. [Google Scholar] [CrossRef]
- Sarnpitak, P.; Mujumdar, P.; Morisseau, C.; Hwang, S.H.; Hammock, B.; Iurchenko, V.; Zozulya, S.; Gavalas, A.; Geronikaki, A.; Ivanenkov, Y.; et al. Potent, orally available, selective COX-2 inhibitors based on 2-imidazoline core. Eur. J. Med. Chem. 2014, 84, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, S.; Malkova, A.; Sharonova, T.; Sharoyko, V.; Bunev, A.; Supuran, C.T.; Krasavin, M. Carbonic Anhydrase IX Inhibitors as Candidates for Combination Therapy of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 13405. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, S.; Nocentini, A.; Kovalenko, A.; Sharoyko, V.; Bonardi, A.; Angeli, A.; Gratteri, P.; Tennikova, T.B.; Supuran, C.T.; Krasavin, M. From random to rational: A discovery approach to selective subnanomolar inhibitors of human carbonic anhydrase IV based on the Castagnoli-Cushman multicomponent reaction. Eur. J. Med. Chem. 2019, 182, 111642. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Nocentini, A.; Yakubova, E.; Savchuk, N.; Kalinin, S.; Krasavin, M. Biochemical profiling of anti-HIV prodrug Elsulfavirine (Elpida®) and its active form VM1500A against a panel of twelve human carbonic anhydrase isoforms. J. Enzyme Inhib. Med. Chem. 2021, 36, 1056–1060. [Google Scholar] [CrossRef]
- Krasavin, M.; Korsakov, M.; Dorogov, M.; Tuccinardi, T.; Dedeoglu, N.; Supuran, C.T. Probing the ‘bipolar’ nature of the carbonic anhydrase active site: Aromatic sulfonamides containing 1,3-oxazol-5-yl moiety as picomolar inhibitors of cytosolic CA I and CA II isoforms. Eur. J. Med. Chem. 2015, 101, 334–347. [Google Scholar] [CrossRef]
- Kalinin, S.; Kovalenko, A.; Valtari, A.; Nocentini, A.; Gureev, M.; Urtti, A.; Korsakov, M.; Supuran, C.T.; Krasavin, M. 5-(Sulfamoyl)thien-2-yl 1,3-oxazole inhibitors of carbonic anhydrase II with hydrophilic periphery. J. Enzyme Inhib. Med. Chem. 2022, 37, 1005–1011. [Google Scholar] [CrossRef]
- Moskalik, M.Y.; Astakhova, V.V.; Shainyan, B.A. Oxidative sulfamidation as a route to N-heterocycles and unsaturated sulfonamides. Pure Appl. Chem. 2019, 92, 123–149. [Google Scholar] [CrossRef]
- Naito, T. Development of new synthetic reactions for nitrogen-containing compounds and their application. Chem. Pharm. Bull. 2008, 56, 1367–1383. [Google Scholar] [CrossRef][Green Version]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
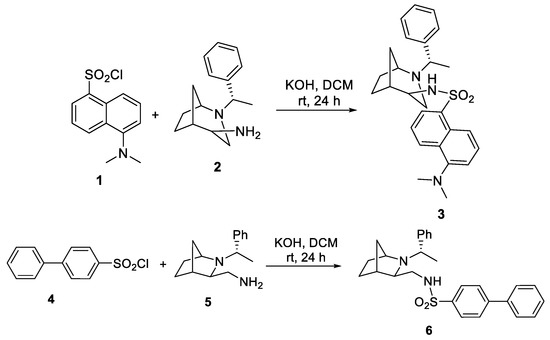
- Iwan, D.; Kamińska, K.; Denel-Bobrowska, M.; Olejniczak, A.B.; Wojaczyńska, E. Chiral sulfonamides with various N-heterocyclic and aromatic units—Synthesis and antiviral activity evaluation. Biomed. Pharmacother. 2022, 153, 113473. [Google Scholar] [CrossRef]
- Wojaczyńska, E.; Turowska-Tyrk, I.; Skarżewski, J. Novel chiral bridged azepanes: Stereoselective ring expansion of 2-azanorbornan-3-yl methanols. Tetrahedron 2012, 68, 7848–7854. [Google Scholar] [CrossRef]
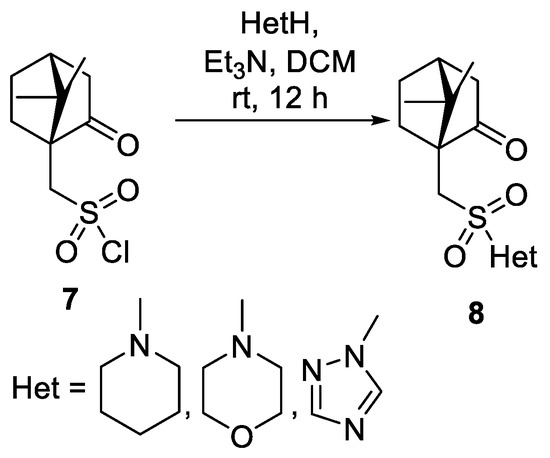
- Sokolova, A.S.; Baranova, D.V.; Yarovaya, O.I.; Baev, D.S.; Polezhaeva, O.A.; Zybkina, A.V.; Shcherbakov, D.N.; Tolstikova, T.G.; Salakhutdinov, N.F. Synthesis of (1S)-(+)-camphor-10-sulfonic acid derivatives and investigations in vitro and in silico of their antiviral activity as the inhibitors of fi lovirus infections. Russ. Chem. Bull. 2019, 68, 1041–1046. [Google Scholar] [CrossRef]
- Kononova, A.A.; Sokolova, A.S.; Cheresiz, S.V.; Yarovaya, O.I.; Nikitina, R.A.; Chepurnov, A.A.; Pokrovsky, A.G.; Salakhutdinov, N.F. N-Heterocyclic borneol derivatives as inhibitors of Marburg virus glycoprotein-mediated VSIV pseudotype entry. Med. Chem. Commun. 2017, 8, 2233–2237. [Google Scholar] [CrossRef] [PubMed]
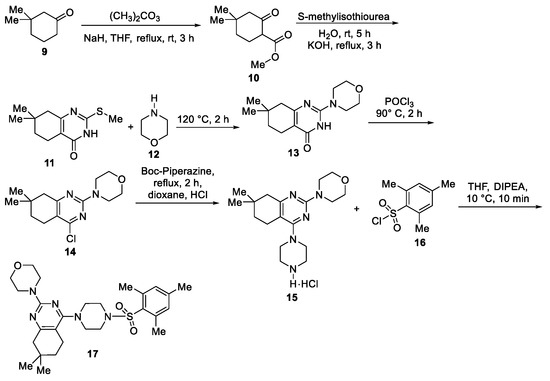
- Selvakumar, B.; Gujjar, N.; Subbiah, M.; Elango, K.P. Synthesis and antiviral study of 4-(7,7-dimethyl-4-(piperazin-1-yl)-5,6,7,8-tetrahydroquinazolin-2-yl) morpholine derivatives. Med. Chem. Res. 2018, 27, 512–519. [Google Scholar] [CrossRef]
- Sekiya, T.; Hiranuma, H.; Uchide, M.; Hata, S.; Yamada, S. Pyrimidine Derivatives. II. New Synthesis and Reactions of 4-Amino-2-methylthiopyrimidine Derivatives. Chem. Pharm. Bull. 1981, 29, 948–954. [Google Scholar] [CrossRef]
- Koenig, J.R.; Liu, H.; Drizin, I.; Witte, D.G.; Carr, T.L.; Manelli, A.M.; Milicic, I.; Strakhova, M.I.; Miller, T.R.; Esbenshade, T.A.; et al. Rigidified 2-aminopyrimidines as histamine H4 receptor antagonists: Effects of substitution about the rigidifying ring. Biorg. Med. Chem. Lett. 2010, 20, 1900–1904. [Google Scholar] [CrossRef]
- Matsuno, K.; Seishi, T.; Nakajima, T.; Ichimura, M.; Giese, N.A.; Yu, J.-C.; Oda, S.; Nomoto, Y. Potent and selective inhibitors of platelet-derived growth factor receptor phosphorylation. Part 4: Structure–activity relationships for substituents on the quinazoline moiety of 4-[4-(N-substituted(thio)carbamoyl)-1-piperazinyl]-6,7-dimethoxyquinazoline derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 3001–3004. [Google Scholar] [CrossRef]
- Selvakumar, B.; Vaidyanathan, S.P.; Subbiah, M.; Elango, K.P. Synthesis and antiviral activity of 4-(7,7-dimethyl-4-[4-{N-aroyl/benzyl}1-piperazinyl]-5,6,7,8-tetrahydroquinazolin-2-yl)morpholine derivatives. Arkivoc 2017, 2017, 353–364. [Google Scholar] [CrossRef]
- Shin, Y.S.; Lee, J.Y.; Noh, S.; Kwak, Y.; Jeon, S.; Kwon, S.; Jin, Y.-h.; Jang, M.S.; Kim, S.; Song, J.H.; et al. Discovery of cyclic sulfonamide derivatives as potent inhibitors of SARS-CoV-2. Bioorg. Med. Chem. Lett. 2021, 31, 127667. [Google Scholar] [CrossRef]
- Lehmann, S.V.; Hoeck, U.; Breinholdt, J.; Olsen, C.E.; Kreilgaard, B. Characterization and chemistry of imidazolidinyl urea and diazolidinyl urea. Contact Dermat. 2006, 54, 50–58. [Google Scholar] [CrossRef]
- Cachet, N.; Genta-Jouve, G.; Regalado, E.L.; Mokrini, R.; Amade, P.; Culioli, G.; Thomas, O.P. Parazoanthines A−E, Hydantoin Alkaloids from the Mediterranean Sea Anemone Parazoanthus axinellae. J. Nat. Prod. 2009, 72, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- Meusel, M.; Gütschow, M. Recent Developments in Hydantoin Chemistry. A Review. Org. Prep. Proced. Int. 2004, 36, 391–443. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.-H.; Shin, D. Recent applications of hydantoin and thiohydantoin in medicinal chemistry. Eur. J. Med. Chem. 2019, 164, 517–545. [Google Scholar] [CrossRef]
- Kornii, Y.; Chumachenko, S.; Shablykin, O.; Prichard, M.N.; James, S.H.; Hartline, C.; Zhirnov, V.; Brovarets, V. New 2-Oxoimidazolidine Derivatives: Design, Synthesis and Evaluation of Anti-BK Virus Activities in Vitro. Chem. Biodivers. 2019, 16, e1900391. [Google Scholar] [CrossRef] [PubMed]
- Kornii, Y.; Shablykin, O.; Shablykina, O.; Brovarets, V. New 4-iminohydantoin sulfamide derivatives with antiviral and anticancer activity. Ukr. Bioorg. Acta 2021, 16, 10–17. [Google Scholar] [CrossRef]
- Bhat, M.A.; Tüzün, B.; Alsaif, N.A.; Ali Khan, A.; Naglah, A.M. Synthesis, characterization, molecular modeling against EGFR target and ADME/T analysis of novel purine derivatives of sulfonamides. J. Mol. Struct. 2022, 1257, 132600. [Google Scholar] [CrossRef]
- McLaren, C.; Datema, R.; Knupp, C.A.; Buroker, R.A. Didanosine. Antivir. Chem. Chemother. 1991, 2, 321–328. [Google Scholar] [CrossRef]
- Valiaeva, N.; Beadle, J.R.; Aldern, K.A.; Trahan, J.; Hostetler, K.Y. Synthesis and antiviral evaluation of alkoxyalkyl esters of acyclic purine and pyrimidine nucleoside phosphonates against HIV-1 in vitro. Antivir. Res. 2006, 72, 10–19. [Google Scholar] [CrossRef]
- Lee, K.; Choi, Y.; Gullen, E.; Schlueter-Wirtz, S.; Schinazi, R.F.; Cheng, Y.C.; Chu, C.K. Synthesis and anti-HIV and anti-HBV activities of 2’-fluoro-2’,3’- unsaturated L-nucleosides. J. Med. Chem. 1999, 42, 1320–1328. [Google Scholar] [CrossRef]
- Kmoníčková, E.; Potměšil, P.; Holý, A.; Zídek, Z. Purine P1 receptor-dependent immunostimulatory effects of antiviral acyclic analogues of adenine and 2,6-diaminopurine. Eur. J. Pharmacol. 2006, 530, 179–187. [Google Scholar] [CrossRef]
- Ashry, E.S.H.E.; Rashed, N.; Abdel-Rahman, A.; Awad, L.F.; Rasheed, H.A. Synthesis of 2-Bromomethyl-3-Hydroxy-2-Hydroxymethyl-Propyl Pyrimidine and Theophylline Nucleosides Under Microwave Irradiation. Evaluation of Their Activity Against Hepatitis B Virus. Nucleosides Nucleotides Nucleic Acids 2006, 25, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Kascatan-Nebioglu, A.; Melaiye, A.; Hindi, K.; Durmus, S.; Panzner, M.J.; Hogue, L.A.; Mallett, R.J.; Hovis, C.E.; Coughenour, M.; Crosby, S.D.; et al. Synthesis from Caffeine of a Mixed N-Heterocyclic Carbene−Silver Acetate Complex Active against Resistant Respiratory Pathogens. J. Med. Chem. 2006, 49, 6811–6818. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Yorimitsu, H.; Perry, G.J.P. Late-stage sulfonic acid/sulfonate formation from sulfonamides via sulfonyl pyrroles. Tetrahedron 2022, 117–118, 132830. [Google Scholar] [CrossRef]
- Gómez-Palomino, A.; Cornella, J. Selective Late-Stage Sulfonyl Chloride Formation from Sulfonamides Enabled by Pyry-BF4. Angew. Chem. Int. Ed. 2019, 58, 18235–18239. [Google Scholar] [CrossRef] [PubMed]
- Famiglini, V.; Castellano, S.; Silvestri, R. N-Pyrrylarylsulfones with High Therapeutic Potential. Molecules 2017, 22, 434. [Google Scholar] [CrossRef] [PubMed]
- Senapathi, J.; Bommakanti, A.; Vangara, S.; Kondapi, A.K. Design, synthesis, and evaluation of HIV-1 entry inhibitors based on broadly neutralizing antibody 447-52D and gp120 V3loop interactions. Bioorg. Chem. 2021, 116, 105313. [Google Scholar] [CrossRef] [PubMed]
- Vincetti, P.; Kaptein, S.J.F.; Costantino, G.; Neyts, J.; Radi, M. Scaffold Morphing Approach To Expand the Toolbox of Broad-Spectrum Antivirals Blocking Dengue/Zika Replication. ACS Med. Chem. Lett. 2019, 10, 558–563. [Google Scholar] [CrossRef]
- Del Sarto, J.L.; Rocha, R.d.P.F.; Bassit, L.; Olmo, I.G.; Valiate, B.; Queiroz-Junior, C.M.; Pedrosa, C.d.S.G.; Ribeiro, F.M.; Guimarães, M.Z.; Rehen, S.; et al. 7-Deaza-7-fluoro-2′-C-methyladenosine inhibits Zika virus infection and viral-induced neuroinflammation. Antivir. Res. 2020, 180, 104855. [Google Scholar] [CrossRef]
- Kesel, A.J. Broad-spectrum antiviral activity including human immunodeficiency and hepatitis C viruses mediated by a novel retinoid thiosemicarbazone derivative. Eur. J. Med. Chem. 2011, 46, 1656–1664. [Google Scholar] [CrossRef]
- Abbas, S.Y.; Basyouni, W.M.; El-Bayouki, K.A.M.; Dawood, R.M.; Abdelhafez, T.H.; Elawady, M.K. Efficient synthesis and anti-bovine viral diarrhea virus evaluation of 5-(aryldiazo)salicylaldehyde thiosemicarbazone derivatives. Synth. Commun. 2019, 49, 2411–2416. [Google Scholar] [CrossRef]
- Soraires Santacruz, M.C.; Fabiani, M.; Castro, E.F.; Cavallaro, L.V.; Finkielsztein, L.M. Synthesis, antiviral evaluation and molecular docking studies of N4-aryl substituted/unsubstituted thiosemicarbazones derived from 1-indanones as potent anti-bovine viral diarrhea virus agents. Bioorg. Med. Chem. 2017, 25, 4055–4063. [Google Scholar] [CrossRef] [PubMed]
- Basyouni, W.M.; Abbas, S.Y.; El-Bayouki, K.A.M.; Daawod, R.M.; Elawady, M.K. Synthesis and antiviral evaluation of 5-(arylazo)salicylaldehyde thiosemicarbazone derivatives as potent anti-bovine viral diarrhea virus agents. Synth. Commun. 2021, 51, 2168–2174. [Google Scholar] [CrossRef]
- Monforte, A.M.; De Luca, L.; Buemi, M.R.; Agharbaoui, F.E.; Pannecouque, C.; Ferro, S. Structural optimization of N1-aryl-benzimidazoles for the discovery of new non-nucleoside reverse transcriptase inhibitors active against wild-type and mutant HIV-1 strains. Bioorg. Med. Chem. 2018, 26, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, S.; Rauf, A.; Qureshi, A. Synthesis of new quinoline scaffolds via a solvent-free fusion method and their anti-microbial properties. Tropical J. Pharm. Res. 2018, 17, 1853. [Google Scholar] [CrossRef]
- Oliphant, C.; Green, G. Quinolones: A Comprehensive Review. Am. Fam. Physician 2002, 65, 455–464. [Google Scholar]
- Shinkai, S. Calixarenes—The third generation of supramolecules. Tetrahedron 1993, 49, 8933–8968. [Google Scholar] [CrossRef]
- Hamid, S.; Muhamad Bunnori, N.; Ishola, A.; Ali, Y. Applications of calixarenes in cancer chemotherapy: Facts and perspectives. Drug Des. Dev. Therapy 2015, 9, 2831. [Google Scholar] [CrossRef]
- Zadmard, R.; Schrader, T. Amino-acid, Peptide and Protein Sensing. In Calixarenes in the Nanoworld; Vicens, J., Harrowfield, J., Baklouti, L., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 287–309. [Google Scholar]
- Ali, Y.; Muhamad Bunnori, N.; Susanti, D.; Muhammad Alhassan, A.; Abd Hamid, S. Synthesis, in-Vitro and in Silico Studies of Azo-Based Calix[4]arenes as Antibacterial Agent and Neuraminidase Inhibitor: A New Look Into an Old Scaffold. Front. Chem. 2018, 6, 210. [Google Scholar] [CrossRef]
- Mkpenie, V.; Ebong, G.; Obot, I.B.; Abasiekong, B. Evaluation of the Effect of Azo Group on the Biological Activity of 1-(4-Methylphenylazo)-2-naphthol. J. Chem. 2008, 5, 438946. [Google Scholar] [CrossRef]
- Azzam, R.A.; Elsayed, R.E.; Elgemeie, G.H. Design and Synthesis of a New Class of Pyridine-Based N-Sulfonamides Exhibiting Antiviral, Antimicrobial, and Enzyme Inhibition Characteristics. ACS Omega 2020, 5, 26182–26194. [Google Scholar] [CrossRef]
- Triantafilou, K.; Triantafilou, M. Coxsackievirus B4-Induced Cytokine Production in Pancreatic Cells Is Mediated through Toll-Like Receptor 4. J. Virol. 2004, 78, 11313–11320. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.-K.; Olsson, A.; Korsgren, O.; Frisk, G. Antiviral treatment of Coxsackie B virus infection in human pancreatic islets. Antivir. Res. 2007, 74, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tian, X. Vaccine development for human mastadenovirus. J. Thorac. Dis. 2018, 10, S2280–S2294. [Google Scholar] [CrossRef] [PubMed]
- Azzam, R.A.; Elboshi, H.A.; Elgemeie, G.H. Novel Synthesis and Antiviral Evaluation of New Benzothiazole-Bearing N-Sulfonamide 2-Pyridone Derivatives as USP7 Enzyme Inhibitors. ACS Omega 2020, 5, 30023–30036. [Google Scholar] [CrossRef]
- Azzam, R.A.; Osman, R.R.; Elgemeie, G.H. Efficient Synthesis and Docking Studies of Novel Benzothiazole-Based Pyrimidinesulfonamide Scaffolds as New Antiviral Agents and Hsp90α Inhibitors. ACS Omega 2020, 5, 1640–1655. [Google Scholar] [CrossRef]
- Timiri, A.K.; Selvarasu, S.; Kesherwani, M.; Vijayan, V.; Sinha, B.N.; Devadasan, V.; Jayaprakash, V. Synthesis and molecular modelling studies of novel sulphonamide derivatives as dengue virus 2 protease inhibitors. Bioorg. Chem. 2015, 62, 74–82. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Geraets, J.A.; Ma, Y.; Mirabelli, C.; Flatt, J.W.; Domanska, A.; Delang, L.; Jochmans, D.; Kumar, T.A.; Jayaprakash, V.; et al. A novel druggable interprotomer pocket in the capsid of rhino- and enteroviruses. PLoS Biol. 2019, 17, e3000281. [Google Scholar] [CrossRef]
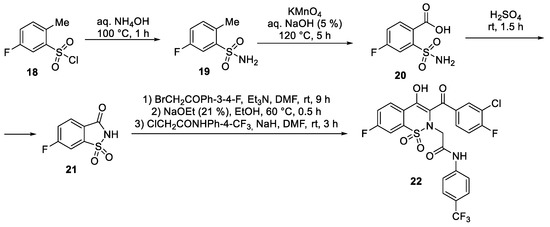
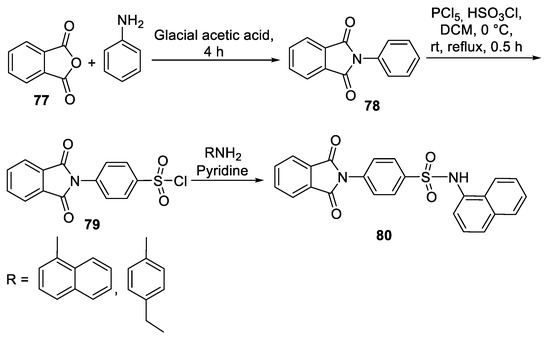
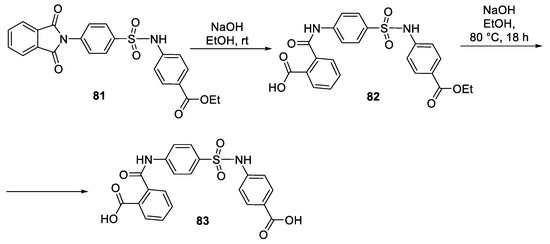
- Shetnev, A.A.; Volobueva, A.S.; Panova, V.A.; Zarubaev, V.V.; Baykov, S.V. Design of 4-Substituted Sulfonamidobenzoic Acid Derivatives Targeting Coxsackievirus B3. Life 2022, 12, 1832. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).