Fuel Characteristics and Removal of AAEMs in Hydrochars Derived from Sewage Sludge and Corn Straw
Abstract
1. Introduction
2. Results and Discussion
2.1. Model Building
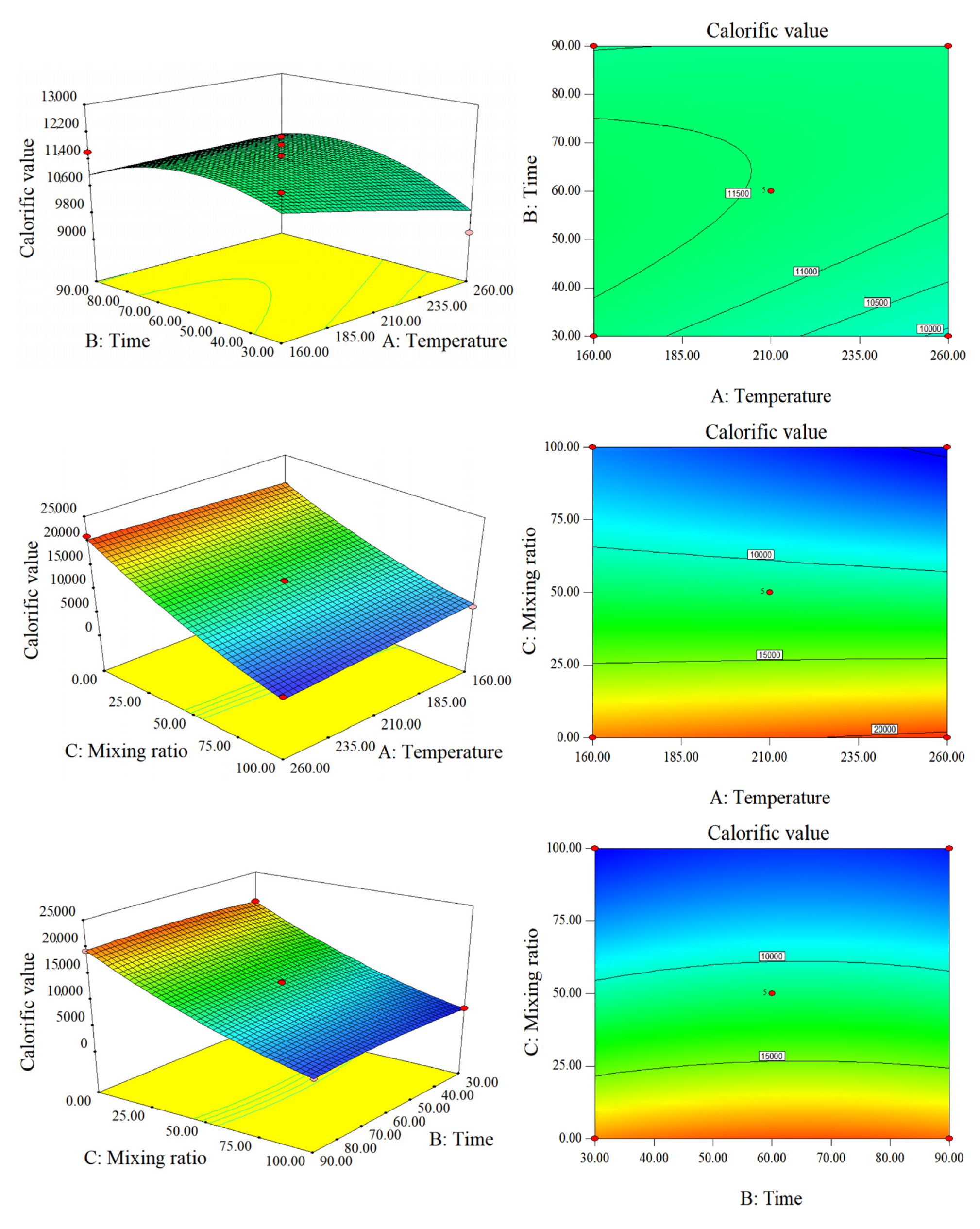
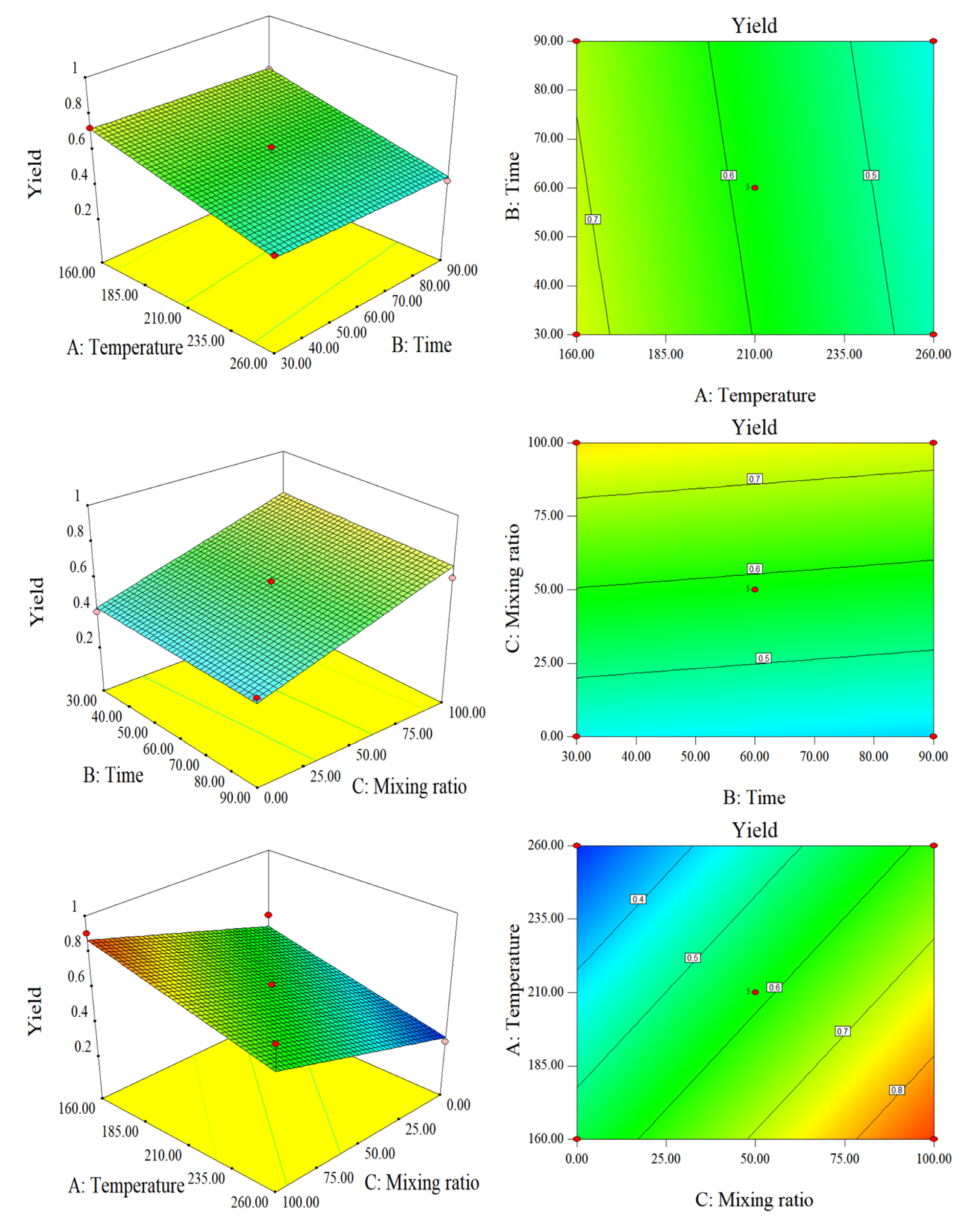
2.2. Response Surface/Contour Plots
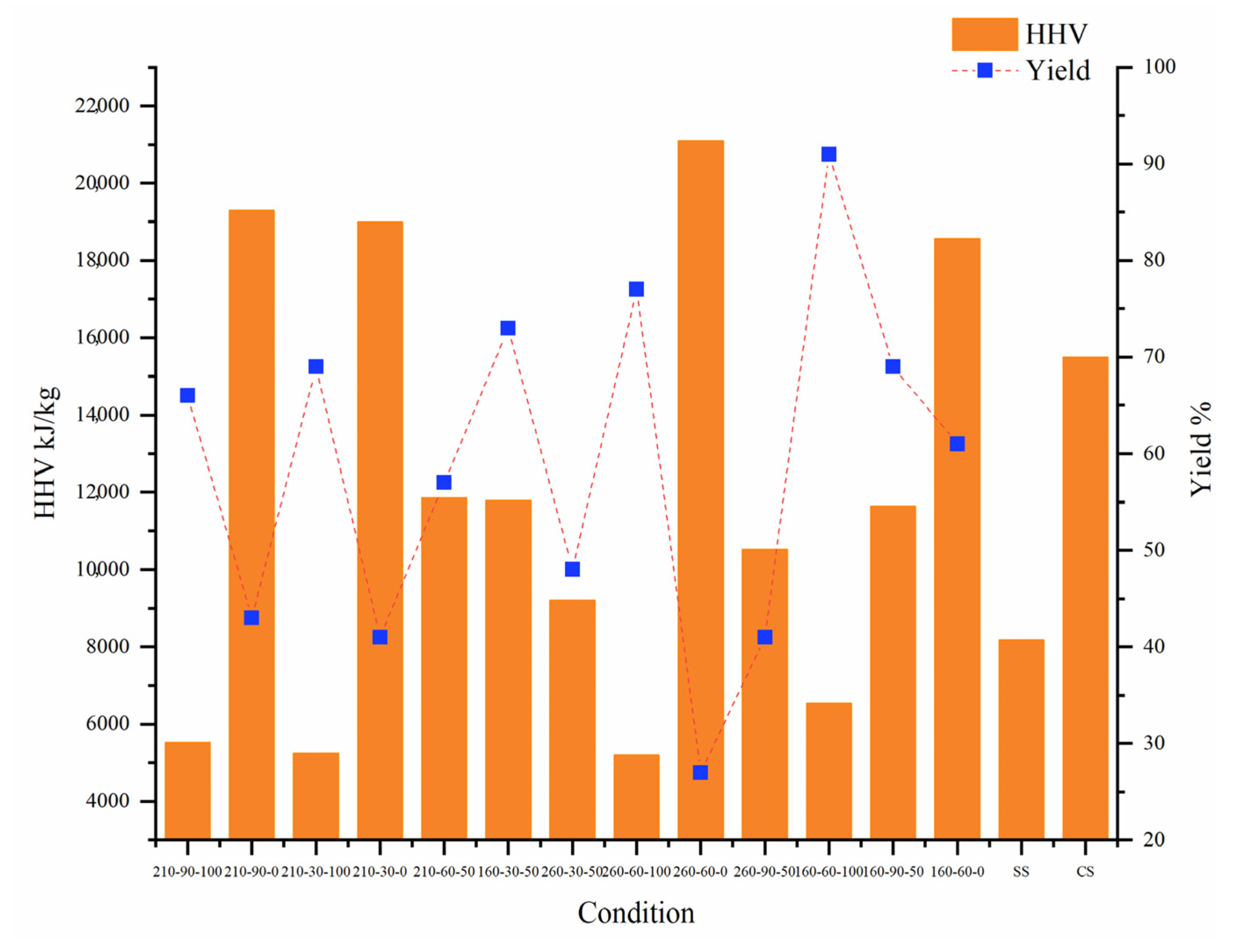
2.2.1. Response Surface Analysis of Fuel Characteristics
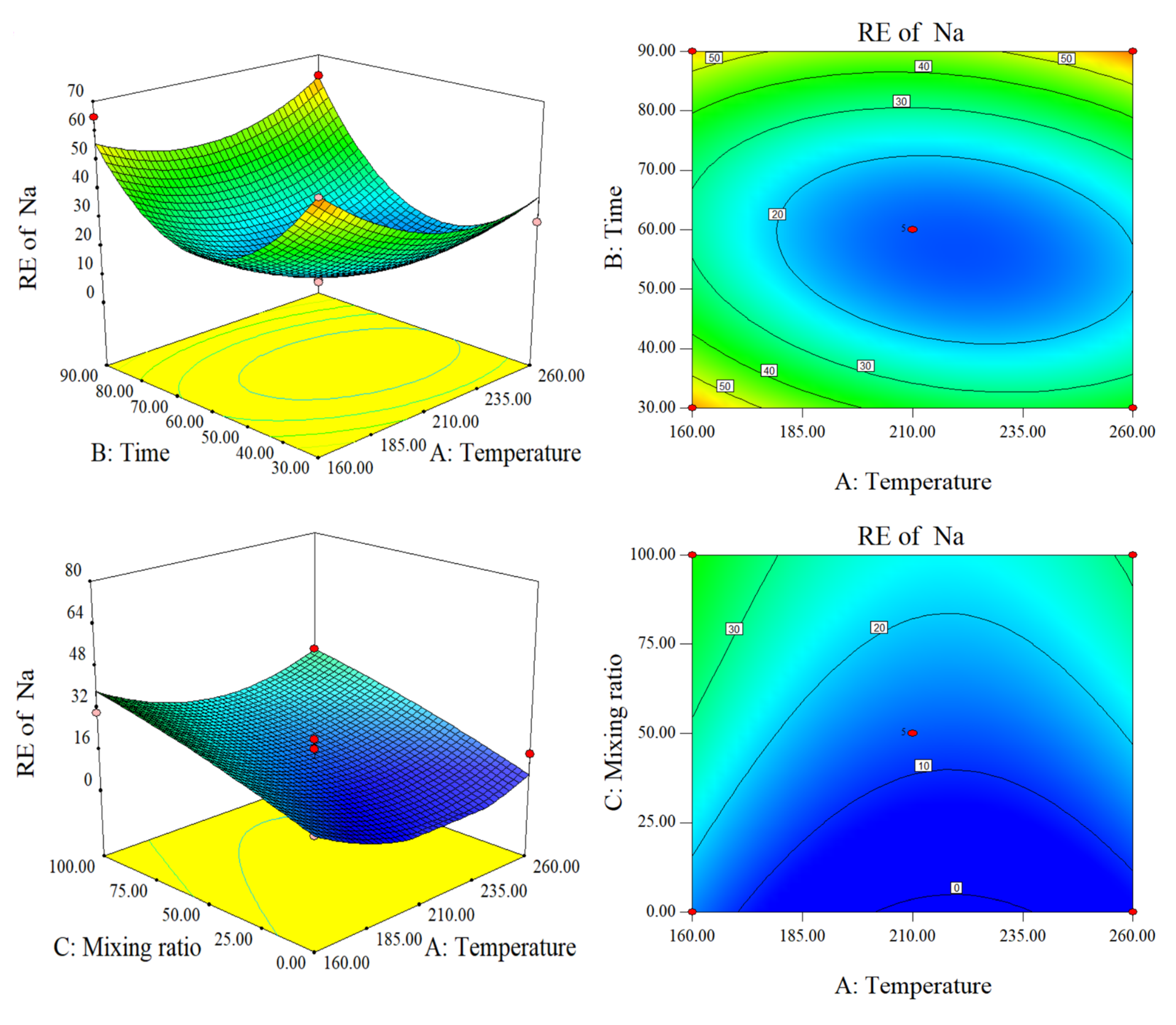
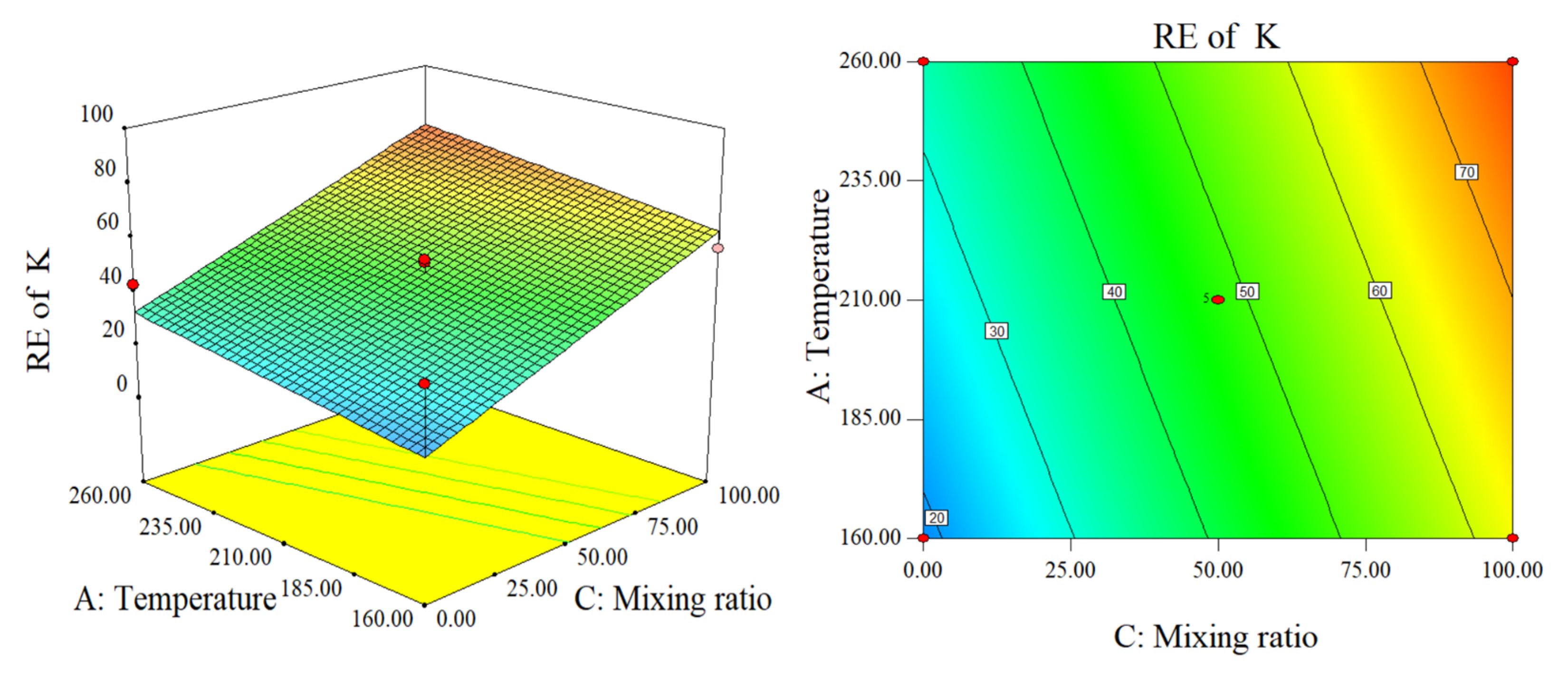
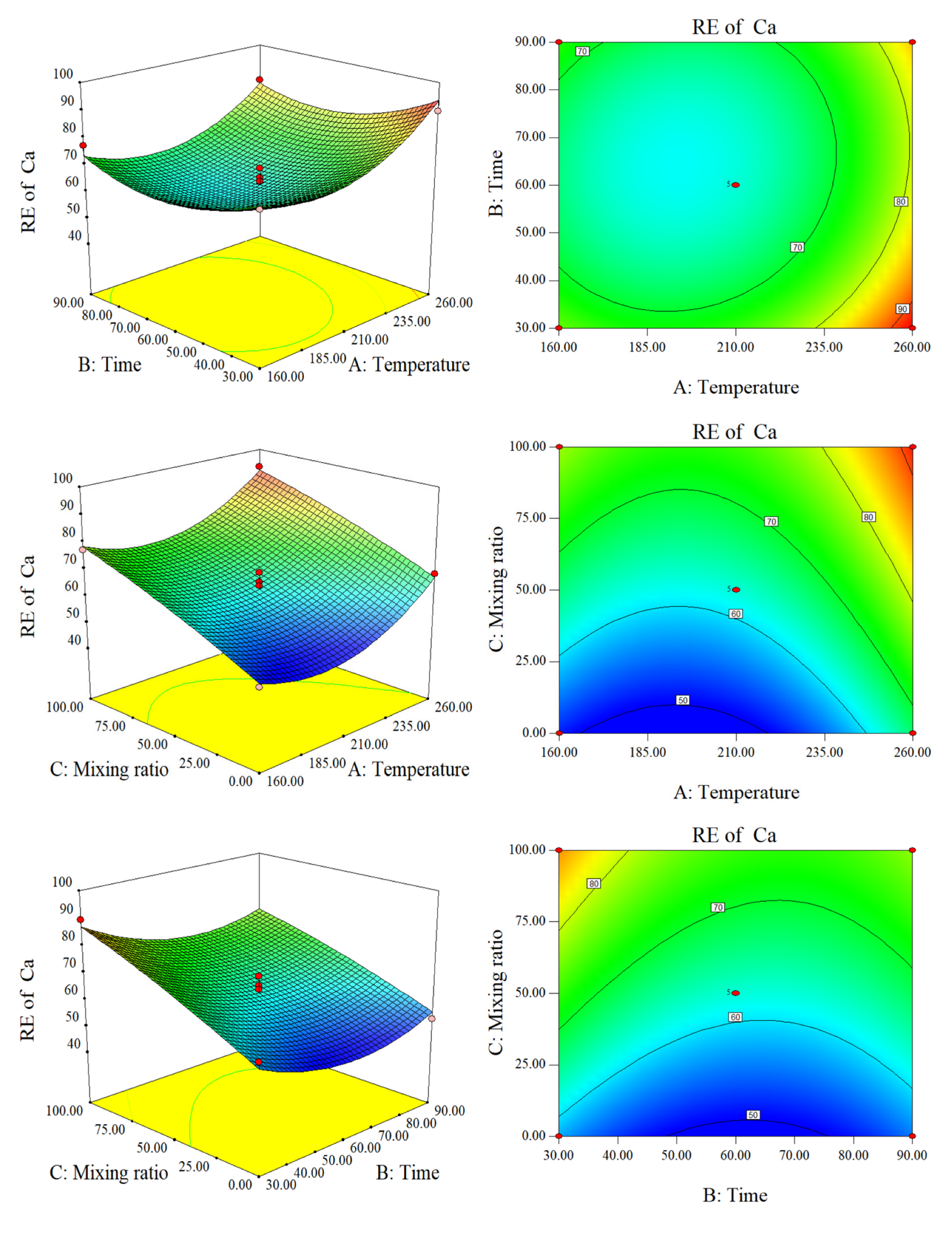
2.2.2. Response Surface Analysis of RE of AAEM and DE
Response Surface Analyses of RE of Na and K
Response Surface Analysis of RE of Ca and Mg
Response Surface Analysis of DE
2.3. Multi-Objective Optimization
3. Materials and Methods
3.1. Materials
3.2. HTC Experiments
3.3. Experimental Design for Process Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, J.; Xiong, Z.; Shi, M. Review of sludge energy utilization technology. Mod. Chem. Ind. 2021, 7, 99–102. [Google Scholar]
- Kim, J.; Jeong, S. Economic and Environmental Cost Analysis of Incineration and Recovery Alternatives for Flammable Industrial Waste: The Case of South Korea. Sustainability 2017, 9, 1638. [Google Scholar] [CrossRef]
- Wang, L.; Sha, L.; Zhang, S.; Cao, F.; Ren, X.; Levendis, Y.A. Preparation of activated coke by carbonization, activation, ammonization and thermal treatment of sewage sludge and waste biomass for SO2 absorption applications. Fuel Process. Technol. 2022, 231, 107233. [Google Scholar] [CrossRef]
- Miricioiu, M.; Zaharioiu, A.; Oancea, S.; Bucura, F.; Raboaca, M.; Filote, C.; Ionete, R.; Niculescu, V.; Constantinescu, M. Sewage Sludge Derived Materials for CO2 Adsorption. Appl. Sci. 2021, 11, 7139. [Google Scholar] [CrossRef]
- Seibert, D.; Quesada, H.; Bergamasco, R.; Borba, F.H.; Pellenz, L. Presence of endocrine disrupting chemicals in sanitary landfill leachate, its treatment and degradation by Fenton based processes: A review. Process. Saf. Environ. Prot. 2019, 131, 255–267. [Google Scholar] [CrossRef]
- LLi, Y.; Li, L.; Liu, Y.; Ren, X.; Chen, J.; Levendis, Y.A. Sulfur and Nitrogen Release From Co-Pyrolysis of Coal and Biomass Under Oxidative and Non-Oxidative Conditions. J. Energy Resour. Technol. 2020, 143, 061304. [Google Scholar] [CrossRef]
- Ren, X.; Sun, R.; Meng, X.; Vorobiev, N.; Schiemann, M.; Levendis, Y.A. Carbon, sulfur and nitrogen oxide emissions from combustion of pulverized raw and torrefied biomass. Fuel 2017, 188, 310–323. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, X.; Yao, Z.; Chen, X. The properties and combustion behaviors of hydrochars derived from cohydrothermal carbonization of sewage sludge and food waste. Bioresour. Technol. 2019, 285, 121347. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, D.; Lee, K.; Park, K.Y. Solid fuel production through hydrothermal carbonization of sewage sludge and microalgae Chlorella sp. from wastewater treatment plant. Chemosphere 2019, 230, 157–163. [Google Scholar] [CrossRef]
- Ma, J.; Luo, H.; Li, Y.; Liu, Z.; Li, D.; Gai, C.; Jiao, W. Pyrolysis kinetics and thermodynamic parameters of the hydrochars derived from co-hydrothermal carbonization of sawdust and sewage sludge using thermogravimetric analysis. Bioresour. Technol. 2019, 282, 133–141. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Li, A. Hydrothermal co-carbonization of sewage sludge and pinewood sawdust for nutrient-rich hydrochar production: Synergistic effects and products characterization. J. Environ. Manag. 2017, 201, 52–62. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Ge, C.; Liu, W.; Tang, Y.; Zhuang, X.; Qiu, R. Synergistic effect of hydrothermal co-carbonization of sewage sludge with fruit and agricultural wastes on hydrochar fuel quality and combustion behavior. Waste Manag. 2019, 100, 171–181. [Google Scholar] [CrossRef]
- Wang, C.; Fan, Y.; Hornung, U.; Zhu, W.; Dahmen, N. Char and tar formation during hydrothermal treatment of sewage sludge in subcritical and supercritical water: Effect of organic matter composition and experiments with model compounds. J. Clean. Prod. 2020, 242, 118586. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, X.; Huang, T.; Zhou, Y.; Tian, Y. Co-hydrothermal carbonization of water hyacinth and polyvinyl chloride: Optimization of process parameters and characterization of hydrochar. Bioresour. Technol. 2020, 314, 123676. [Google Scholar] [CrossRef]
- Huang, N.; Zhao, P.; Ghosh, S.; Fedyukhin, A. Co-hydrothermal carbonization of polyvinyl chloride and moist biomass to remove chlorine and inorganics for clean fuel production. Appl. Energy 2019, 240, 882–892. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass-Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Sun, J.; Xu, Y.; Li, P.; Xu, Y.; Wu, S. Effects of process parameters on the physicochemical properties of corn stalk hydrochar and the removal of alkali and alkaline earth metals. IET Renew. Power Gener. 2021, 15, 1397–1407. [Google Scholar] [CrossRef]
- Khan, N.; Mohan, S.; Dinesha, P. Regimes of hydrochar yield from hydrothermal degradation of various lignocellulosic biomass: A review. J. Clean. Prod. 2020, 288, 125629. [Google Scholar] [CrossRef]
- Shao, Y.; Long, Y.; Wang, H.; Liu, D.; Shen, D.; Chen, T. Hydrochar derived from green waste by microwave hydrothermal carbonization. Renew. Energy 2018, 135, 1327–1334. [Google Scholar] [CrossRef]
- Zhang, J.; An, Y.; Borrion, A.; He, W.; Wang, N.; Chen, Y.; Li, G. Process characteristics for microwave assisted hydrothermal carbonization of cellulose. Bioresour. Technol. 2018, 259, 91–98. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Liu, Q. Fate and distribution of nutrients and heavy metals during hydrothermal carbonization of sewage sludge with implication to land application. J. Clean. Prod. 2019, 225, 972–983. [Google Scholar] [CrossRef]
- Liao, J.; Luo, X. Absorptive and slow-released properties of lignin and its application in fertilizer. Soils Fertil. 2004, 5, 43–45. [Google Scholar]
- Ju, M.; Wen, C.; Liu, J. The application of lignin in agriculture. Mod. Agric. 2011, 1, 27–28. [Google Scholar]
- Yao, Z.; Ma, X. Characteristics of co-hydrothermal carbonization on polyvinyl chloride wastes with bamboo. Bioresour. Technol. 2018, 247, 302–309. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, G.; Shang, Y.; Zhao, P.; Cui, X.; Guo, Q. Chlorine migration during hydrothermal carbonization of recycled paper wastes and fuel performance of hydrochar. Process. Saf. Environ. Prot. 2022, 158, 495–502. [Google Scholar] [CrossRef]
- Zhao, P.; Huang, N.; Li, J.; Cui, X. Fate of sodium and chlorine during the co-hydrothermal carbonization of high-alkali coal and polyvinyl chloride. Fuel Process. Technol. 2020, 199, 106277. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Valenzuela-Medina, D.; Bilbao-Sainz, C.; Klamczynski, A.K.; Avena-Bustillos, R.J.; Milczarek, R.R.; Du, W.-X.; Glenn, G.M.; Orts, W.J. Torrefaction of pomaces and nut shells. Bioresour. Technol. 2015, 177, 58–65. [Google Scholar] [CrossRef]
- Oumabady, S.; Kamaludeen, S.P.; Ramasamy, M.; Kalaiselvi, P.; Parameswari, E. Preparation and Characterization of Optimized Hydrochar from Paper Board Mill Sludge. Sci. Rep. 2020, 10, 773. [Google Scholar] [CrossRef]
- Perendeci, N.; Ciggin, A.; Ünşar, E.K.; Orhon, D. Optimization of alkaline hydrothermal pretreatment of biological sludge for enhanced methane generation under anaerobic conditions. Waste Manag. 2020, 107, 9–19. [Google Scholar] [CrossRef]
- Afolabi, O.O.; Sohail, M.; Cheng, Y.-L. Optimisation and characterisation of hydrochar production from spent coffee grounds by hydrothermal carbonisation. Renew. Energy 2019, 147, 1380–1391. [Google Scholar] [CrossRef]
- Hadhoum, L.; Loubar, K.; Paraschiv, M.; Burnens, G.; Awad, S.; Tazerout, M. Optimization of oleaginous seeds liquefaction using response surface methodology. Biomass- Convers. Biorefinery 2020, 11, 2655–2667. [Google Scholar] [CrossRef]
- Kutlu, O.; Kocar, G. Upgrading lignocellulosic waste to fuel by torrefaction: Characterisation and process optimization by response surface methodology. Int. J. Energy Res. 2018, 42, 4746–4760. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, Y.-H.; Lee, S.-M.; Lee, H.-W. Optimizing the torrefaction of mixed softwood by response surface methodology for biomass upgrading to high energy density. Bioresour. Technol. 2012, 116, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Zhao, S.; Wu, P.; Han, L.; Liu, X. Optimization of a “coal-like” pelletization technique based on the sustainable biomass fuel of hydrothermal carbonization of wheat straw. J. Clean. Prod. 2019, 242, 118426. [Google Scholar] [CrossRef]




| Name | Goal | Lower | Upper | Lower | Upper |
|---|---|---|---|---|---|
| Limit | Limit | Weight | Weight | ||
| A: Temperature | is in range | 160 | 260 | 1 | 1 |
| B: Time | is in range | 30 | 90 | 1 | 1 |
| C: Mixing ratio | is in range | 0 | 100 | 1 | 1 |
| HHV | is in range | 10,000 | 21,102.4 | 1 | 1 |
| Yield | is in range | 50 | 90.6 | 1 | 1 |
| Ca (RE) | maximize | 50.31 | 93.18 | 1 | 1 |
| K (RE) | maximize | 8.82 | 81.91 | 1 | 1 |
| Mg (RE) | maximize | 14.52 | 95.03 | 1 | 1 |
| Na (RE) | maximize | 7.5 | 68.92 | 1 | 1 |
| Cl (DE) | maximize | 46.06 | 95.48 | 1 | 1 |
| Name | Goal Importance (1–5) |
|---|---|
| HHV (kJ/kg) | 3 |
| Yield (%) | 3 |
| K (RE) | 2 |
| Na (RE) | 5 |
| Ca (RE) | 4 |
| Mg (RE) | 2 |
| Cl (DE) | 5 |
| . | Condition | HHV | Yield | K (RE) | Ca (RE) | Na (RE) | Mg (RE) | Cl (DE) | Desirability |
|---|---|---|---|---|---|---|---|---|---|
| Optimization results | 246.14-90-57.18 | 10,000 | 50.00 | 79.6 | 54.2 | 68.2 | 58.9 | 87.1 | 0.74 |
| Experimental results | 246.14-90-57.18 | 9193 | 40.1 | 73.8 | 58.3 | 69.1 | 55.7 | 99.6 | |
| Error | 8.8% | 24.7% | 7.8% | 7.2% | 1.3% | 5.7% | 12.6% |
| Sample | Proximate Analysis (%, Air Dry) | HHV (kJ/kg) | Ultimate Analysis (%, Air Dry) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | A | V | FC | C | H | O | N | S | ||
| SS | 3.84 | 58.80 | 32.66 | 4.70 | 8200 | 18.03 | 2.85 | 13.81 | 2.07 | 0.60 |
| CS | 4.81 | 5.41 | 76.56 | 13.22 | 17,820 | 48.73 | 6.65 | 33.20 | 0.92 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Xiao, W.; Liu, Z.; Zhao, D.; Chen, K.; Zhao, C.; Li, X.; Li, G. Fuel Characteristics and Removal of AAEMs in Hydrochars Derived from Sewage Sludge and Corn Straw. Molecules 2023, 28, 781. https://doi.org/10.3390/molecules28020781
Guo S, Xiao W, Liu Z, Zhao D, Chen K, Zhao C, Li X, Li G. Fuel Characteristics and Removal of AAEMs in Hydrochars Derived from Sewage Sludge and Corn Straw. Molecules. 2023; 28(2):781. https://doi.org/10.3390/molecules28020781
Chicago/Turabian StyleGuo, Shuai, Weinan Xiao, Zhaoyuan Liu, Deng Zhao, Kaixin Chen, Chenchen Zhao, Xingcan Li, and Guangyu Li. 2023. "Fuel Characteristics and Removal of AAEMs in Hydrochars Derived from Sewage Sludge and Corn Straw" Molecules 28, no. 2: 781. https://doi.org/10.3390/molecules28020781
APA StyleGuo, S., Xiao, W., Liu, Z., Zhao, D., Chen, K., Zhao, C., Li, X., & Li, G. (2023). Fuel Characteristics and Removal of AAEMs in Hydrochars Derived from Sewage Sludge and Corn Straw. Molecules, 28(2), 781. https://doi.org/10.3390/molecules28020781






