Design and Performance of an Adsorption Bed with Activated Carbons for Biogas Purification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Evaluation of Textural Properties
2.3. Evaluation of the Adsorption Capacity
2.4. Design of Experiments
3. Results and Discussion
3.1. Textural Properties Effect
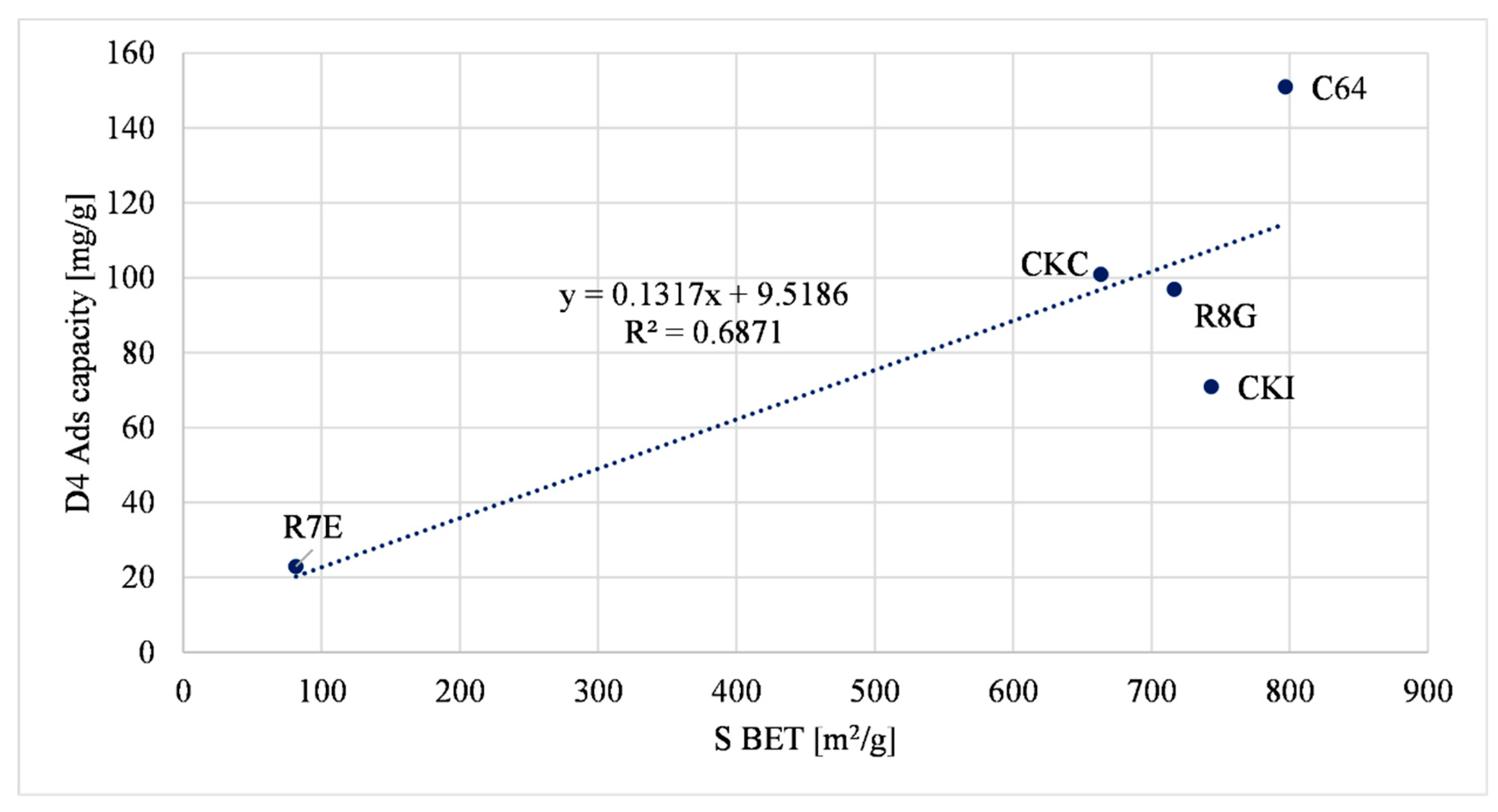
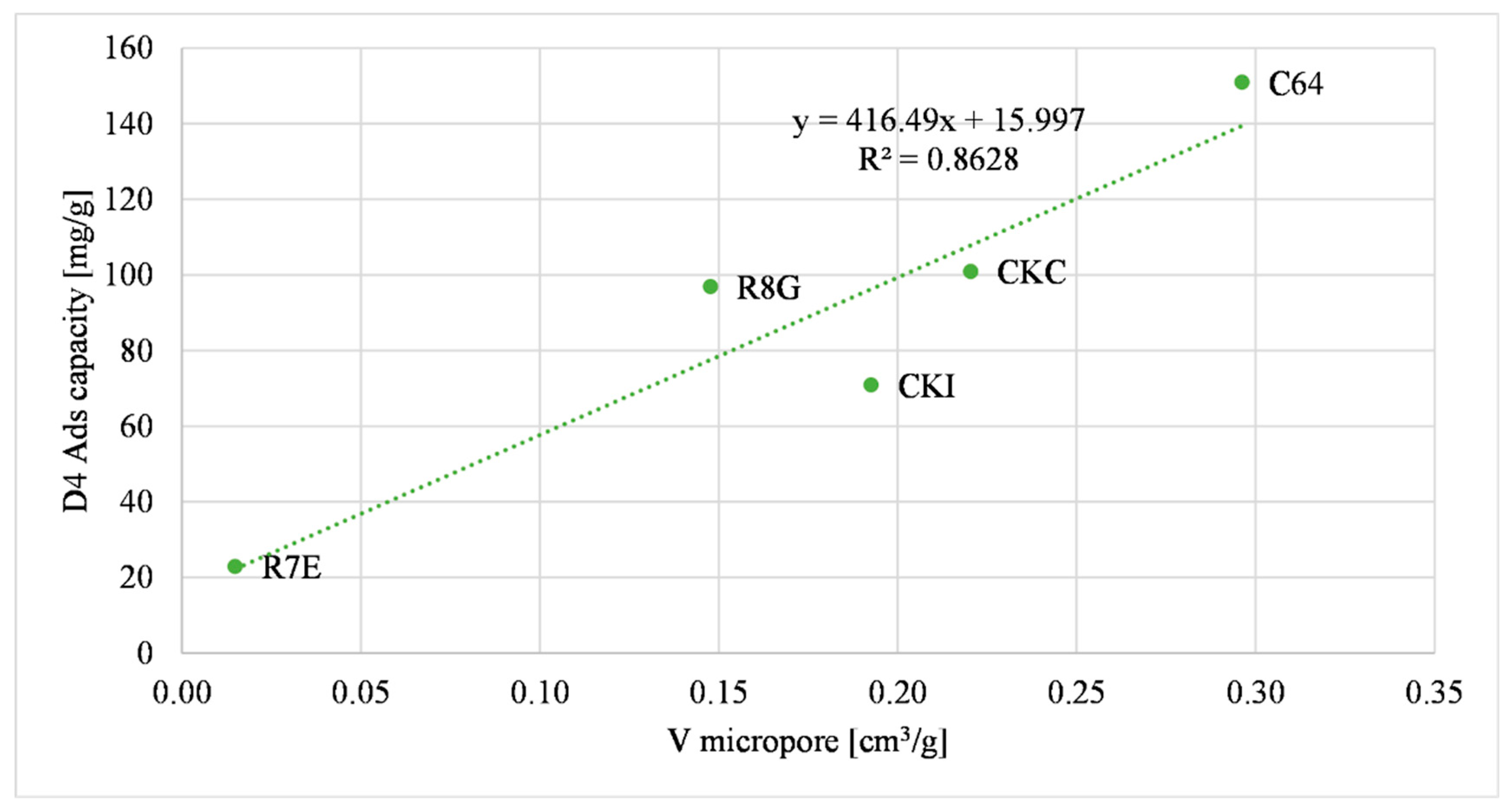
3.1.1. D4 Adsorption Capacity Correlations
3.1.2. H2S Adsorption Capacity Correlations
Microreactor Data: AC Powders
Large Reactor Data: AC As-Received
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Activated Carbons |
| ADG | Anaerobic Digester Gas |
| Ads Cap | Adsorption capacity |
| BET | Brunauer Emmett Teller method |
| CKC | AirDep CKC activated carbon of mineral origin |
| D4 | Octamethylcyclotetrasiloxane |
| DFT | Density Functional Theory |
| EDS | Energy-dispersive X-ray spectroscopy |
| FC | Fuel Cell |
| GHG | Greenhouse Gas |
| GPU | Gas Processing Unit |
| ICE | Internal Combustion Engine |
| MCFC | Molten Carbonate Fuel Cell |
| MTZ | Mass Transfer Zone |
| NLDFT | Nonlocal Density Functional Theory |
| SEM | Scanning Electron Microscopy |
| SOFC | Solid Oxide Fuel Cell |
| VOC | Volatile Organic Compounds |
| WWTP | Waste-Water Treatment Plant |
| Nomenclature | |
| is the H2S concentration in the gas phase, | |
| is the H2S inlet concentration, | |
| is the sulfur adsorption capacity, | |
| is the biogas volumetric flow, | |
| is the sulfur volumetric concentration, | |
| is the sulfur molecular weight, | |
| is the breakthrough time, | |
| is the weight of AC contained inside the sample, | |
Appendix A
| Atomic Concentration (EDS) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Region | O | Si | Al | K | Ca | Fe | S | Mg | Na | Pt | I | Ti | Cu | Mn |
| C64 | 1 | 11.37 | 0.47 | 0.4 | 0.33 | 0.29 | 0.18 | 0.15 | 0.08 | 0 | 0 | ||||
| 2 | 11.27 | 0.48 | 0.36 | 0.36 | 0.29 | 0.19 | 0.15 | 0.09 | 0 | 0 | |||||
| 3 | 11.68 | 0.37 | 0.32 | 0 | 0.18 | 0.16 | 0.07 | 0.06 | 0.05 | 0 | |||||
| 4 | 8.77 | 0.50 | 0.44 | 0.03 | 0.22 | 0.14 | 0.11 | 0.12 | 0.10 | 0.41 | |||||
| mean value | 10.77 | 0.46 | 0.38 | 0.18 | 0.25 | 0.17 | 0.12 | 0.09 | 0.04 | 0.10 | |||||
| CKC | region | ||||||||||||||
| 1 | 11.37 | 0.47 | 0.4 | 0.33 | 0.29 | 0.18 | 0.15 | 0.08 | |||||||
| 2 | 11.27 | 0.48 | 0.36 | 0.36 | 0.29 | 0.19 | 0.15 | 0.09 | |||||||
| mean value | 11.32 | 0.475 | 0.38 | 0.345 | 0.29 | 0.185 | 0.15 | 0.085 | |||||||
| CKI | region | ||||||||||||||
| 1 | 6.44 | 0.54 | 0.33 | 0.39 | 0.63 | 0.48 | 0.63 | 0.05 | 0.4 | 0.06 | |||||
| 2 | 8.96 | 0.34 | 0.52 | 0.23 | 0.06 | 0.21 | 0.79 | 0.13 | 0.14 | 0.03 | |||||
| mean value | 7.7 | 0.44 | 0.425 | 0.31 | 0.345 | 0.345 | 0.71 | 0.09 | 0.27 | 0.045 | |||||
| R8G | region | ||||||||||||||
| 1 | 39.1 | 0.49 | 0.26 | 2.79 | 4.5 | 5.62 | |||||||||
| 2 | 46.05 | 0.32 | 0.26 | 1.11 | 3.77 | 9.46 | |||||||||
| mean value | 42.575 | 0.405 | 0.26 | 1.95 | 4.135 | 7.54 | |||||||||
| R7E | region | ||||||||||||||
| 1 | 61.32 | 3.71 | 5.69 | 22.87 | 6.4 | ||||||||||
| 2 | 60.46 | 4.88 | 6.04 | 21.99 | 6.63 | ||||||||||
| mean value | 60.89 | 4.295 | 5.865 | 22.43 | 6.515 | ||||||||||
References
- Lanzini, A.; Madi, H.; Chiodo, V.; Papurello, D.; Maisano, S.; Santarelli, M.; van herle, J. Dealing with fuel contaminants in biogas-fed solid oxide fuel cell (SOFC) and molten carbonate fuel cell (MCFC) plants: Degradation of catalytic and electro-catalytic active surfaces and related gas purification methods. Prog. Energy Combust. Sci. 2017, 61, 150–188. [Google Scholar] [CrossRef]
- Tait, S.; Harris, P.W.; McCabe, B.K. Biogas recovery by anaerobic digestion of Australian agro-industry waste: A review. J. Clean. Prod. 2021, 299, 126876. [Google Scholar] [CrossRef]
- Chew, K.R.; Leong, H.Y.; Khoo, K.S.; Vo, D.-V.N.; Anjum, H.; Chang, C.-K.; Show, P.L. Effects of anaerobic digestion of food waste on biogas production and environmental impacts: A review. Environ. Chem. Lett. 2021, 19, 2921–2939. [Google Scholar] [CrossRef]
- Carnevale, E.; Lombardi, L. Comparison of different possibilities for biogas use by Life Cycle Assessment. Energy Procedia 2015, 81, 215–226. [Google Scholar] [CrossRef][Green Version]
- Santarelli, M.; Briesemeister, L.; Gandiglio, M.; Herrmann, S.; Kuczynski, P.; Kupecki, J.; Lanzini, A.; Llovelld, F.; Papurello, D.; Spliethoff, H.; et al. Carbon recovery and re-utilization (CRR) from the exhaust of a solid oxide fuel cell (SOFC): Analysis through a proof-of-concept. J. CO2 Util. 2017, 18, 206–221. [Google Scholar] [CrossRef]
- Aravind, P.V.; Ouweltjes, J.P.; Woudstra, N.; Rietveld, G. Impact of Biomass-Derived Contaminants on SOFCs with Ni/Gadolinia-Doped Ceria Anodes. Electrochem. Solid-State Lett. 2008, 11, B24–B28. [Google Scholar] [CrossRef]
- Din, Z.U.; Zainal, Z.A. The fate of SOFC anodes under biomass producer gas contaminants. Renew. Sustain. Energy Rev. 2017, 72, 1050–1066. [Google Scholar] [CrossRef]
- Papurello, D.; Silvestri, S.; Modena, S. Biogas trace compounds impact on high-temperature fuel cells short stack performance. Int. J. Hydrogen Energy 2021, 46, 8792–8801. [Google Scholar] [CrossRef]
- Kuramoto, K.; Hosokai, S.; Matsuoka, K.; Ishiyama, T.; Kishimoto, H.; Yamaji, K. Degradation behaviors of SOFC due to chemical interaction between Ni-YSZ anode and trace gaseous impurities in coal syngas. Fuel Process. Technol. 2017, 160, 8–18. [Google Scholar] [CrossRef]
- Escudero, M.J.; Maffiotte, C.A.; Serrano, J.L. Long-term operation of a solid oxide fuel cell with MoNi–CeO2 as anode directly fed by biogas containing simultaneously sulphur and siloxane. J. Power Sources 2021, 481, 229048. [Google Scholar] [CrossRef]
- Li, Y.; Pang, Y.; Tu, H.; Torrigino, F.; Biollaz, S.M.A.; Li, Z.; Huang, Y.; Yin, X.; Grimm, F.; Karl, J. Impact of syngas from biomass gasification on solid oxide fuel cells: A review study for the energy transition. Energy Convers. Manag. 2021, 250, 114894. [Google Scholar] [CrossRef]
- Aravind, P.V.; De Jong, W. Evaluation of high temperature gas cleaning options for biomass gasification product gas for Solid Oxide Fuel Cells. Prog. Energy Combust. Sci. 2012, 38, 737–764. [Google Scholar] [CrossRef]
- Paolini, V.; Petracchini, F.; Carnevale, M.; Gallucci, F.; Perilli, M.; Esposito, G.; Segreto, M.; Occulti, L.G.; Scaglione, D.; Ianniello, A.; et al. Characterisation and cleaning of biogas from sewage sludge for biomethane production. J. Environ. Manag. 2018, 217, 288–296. [Google Scholar] [CrossRef]
- Sisani, E.; Cinti, G.; Discepoli, G.; Penchini, D.; Desideri, U.; Marmottini, F. Adsorptive removal of H2S in biogas conditions for high temperature fuel cell systems. Int. J. Hydrogen Energy 2014, 39, 21753–21766. [Google Scholar] [CrossRef]
- Coppola, G.; Papurello, D.; Coppola, G.; Papurello, D. Biogas Cleaning: Activated Carbon Regeneration for H2S Removal. Clean Technol. 2018, 1, 40–57. [Google Scholar] [CrossRef]
- Brightman, E.; Ivey, D.G.; Brett, D.J.L.; Brandon, N.P. The effect of current density on H2S-poisoning of nickel-based solid oxide fuel cell anodes. J. Power Sources 2011, 196, 7182–7187. [Google Scholar] [CrossRef]
- Kromp, A.; Dierickx, S.; Leonide, A.; Weber, A.; Ivers-Tiffée, E. Electrochemical Analysis of Sulphur-Poisoning in Anode-Supported SOFCs under Reformate Operation. ECS Trans. 2012, 41, 161–169. [Google Scholar] [CrossRef]
- Kwaśny, J.; Balcerzak, W. Sorbents used for biogas desulfurization in the adsorption process. Pol. J. Environ. Stud. 2016, 25, 37–43. [Google Scholar] [CrossRef]
- Ramírez-Sáenz, D.; Zarate-Segura, P.B.; Guerrero-Barajas, C.; García-Peña, E.I. H2S and volatile fatty acids elimination by biofiltration: Clean-up process for biogas potential use. J. Hazard. Mater. 2009, 163, 1272–1281. [Google Scholar] [CrossRef]
- Haga, K.; Adachi, S.; Shiratori, Y.; Itoh, K.; Sasaki, K. Poisoning of SOFC anodes by various fuel impurities. Solid State Ion. 2008, 179, 1427–1431. [Google Scholar] [CrossRef]
- Błesznowski, M.; Jewulski, J.; Zieleniak, A. Determination of H2S and HCl concentration limits in the fuel for anode supported SOFC operation. Cent. Eur. J. Chem. 2013, 11, 960–967. [Google Scholar] [CrossRef]
- Micoli, L.; Bagnasco, G.; Turco, M. HCl removal from biogas for feeding MCFCs: Adsorption on microporous materials. Int. J. Hydrogen Energy 2013, 38, 447–452. [Google Scholar] [CrossRef]
- Papadias, D.D.; Ahmed, S.; Kumar, R. Fuel quality issues with biogas energy - An economic analysis for a stationary fuel cell system. Energy 2012, 44, 257–277. [Google Scholar] [CrossRef]
- Rasi, S.; Veijanen, A.; Rintala, J. Trace compounds of biogas from different biogas production plants. Energy 2007, 32, 1375–1380. [Google Scholar] [CrossRef]
- Rey, M.D.; Font, R.; Aracil, I. Biogas from MSW landfill: Composition and determination of chlorine content with the AOX (adsorbable organically bound halogens) technique. Energy 2013, 63, 161–167. [Google Scholar] [CrossRef]
- Calì, G.; Deiana, P.; Bassano, C.; Meloni, S.; Maggio, E.; Mascia, M.; Pettinau, A. Syngas Production, Clean-Up and Wastewater Management in a Demo-Scale Fixed-Bed Updraft Biomass Gasification Unit. Energies 2020, 13, 2594. [Google Scholar] [CrossRef]
- Ahmed, S.; Papadias, D.D. Database of Trace Contaminants in LFG and ADG. Argonne National Laboratory. 2012. Available online: http://www.cse.anl.gov/FCs_on_biogas/ (accessed on 22 April 2022).
- Spiegel, R.J.; Preston, J.L. Technical assessment of fuel cell operation on anaerobic digester gas at the Yonkers, NY, wastewater treatment plant. Waste Manag. 2003, 23, 709–717. [Google Scholar] [CrossRef]
- Lens, P.; Westermann, P.; Haberbauer, M.; Moreno, A. Biofuels for Fuel Cells: Renewable Energy from Biomass Fermentation; Iwa Publishing: London, UK, 2006. [Google Scholar]
- De Arespacochaga, N.; Valderrama, C.; Peregrina, C.; Mesa, C.; Bouchy, L.; Cortina, J.L. Evaluation of a pilot-scale sewage biogas powered 2.8 kWe Solid Oxide Fuel Cell: Assessment of heat-to-power ratio and influence of oxygen content. J. Power Sources 2015, 300, 325–335. [Google Scholar] [CrossRef]
- Santarelli, M. DEMOSOFC project to install first European plant to produce clean energy from waste water. Fuel Cells Bull. 2020, 2015, 14–15. [Google Scholar] [CrossRef]
- Dannesboe, C.; Hansen, J.B.; Johannsen, I. Removal of sulfur contaminants from biogas to enable direct catalytic methanation. Biomass Convers. Biorefinery 2021, 11, 1823–1834. [Google Scholar] [CrossRef]
- Schweigkofler, M.; Niessner, R. Removal of siloxanes in biogases. J. Hazard. Mater. 2001, 83, 183–196. [Google Scholar] [CrossRef]
- De Arespacochaga, N.; Valderrama, C.; Mesa, C.; Bouchy, L.; Cortina, J.L. Biogas deep clean-up based on adsorption technologies for Solid Oxide Fuel Cell applications. Chem. Eng. J. 2014, 255, 593–603. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Boivin, S. A review of biogas purification processes. Biofuels Bioprod. Biorefining 2009, 3, 42–71. [Google Scholar] [CrossRef]
- Marcantonio, V.; del Zotto, L.; Ouweltjes, J.P.; Bocci, E. Main issues of the impact of tar, H2S, HCl and alkali metal from biomass-gasification derived syngas on the SOFC anode and the related gas cleaning technologies for feeding a SOFC system: A review. Int. J. Hydrogen Energy 2022, 47, 517–539. [Google Scholar] [CrossRef]
- Biocell Project, BIOCELL-Energy Self-Sustaining and Environmental Footprint Reduction on Wastewater Treatment Plants via fuel cells, (n.d.). Available online: http://ec.europa.eu/environment/life/project/Projects/index.cfm?fuseaction=search.dspPage&n_proj_id=3279 (accessed on 23 July 2019).
- Bak, C.U.; Lim, C.J.; Lee, J.G.; Kim, Y.D.; Kim, W.S. Removal of sulfur compounds and siloxanes by physical and chemical sorption. Sep. Purif. Technol. 2019, 209, 542–549. [Google Scholar] [CrossRef]
- Rasi, S.; Seppälä, M.; Rintala, J. Organic silicon compounds in biogases produced from grass silage, grass and maize in laboratory batch assays. Energy 2013, 52, 137–142. [Google Scholar] [CrossRef]
- AirDep, AirDep-Impianti di Depurazione aria e Biogas San Bonifacio-Verona, 2019. (n.d.). Available online: https://www.airdep.eu/ (accessed on 8 May 2019).
- Sulfatrap, Desulfurization–SulfaTrap–Global Leader in Sulfur Removal Sorbents, 2019. (n.d.). Available online: https://sulfatrap.com/ (accessed on 8 May 2019).
- Alptekin, G.O.; Jayaraman, A.; Schaefer, M.; Ware, M.; Jerry, W.G.P.; Destefano, M.S. Novel Sorbent to Clean Up Biogas for CHPs; Interim Report # 19; TDA Research, Incorporated: Wheat Ridge, CO, USA, 2015. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Papurello, D.; Schuhfried, E.; Lanzini, A.; Romano, A.; Cappellin, L.; Märk, T.D.; Silvestri, S.; Biasioli, F. Influence of co-vapors on biogas filtration for fuel cells monitored with PTR-MS (Proton Transfer Reaction-Mass Spectrometry). Fuel Process. Technol. 2014, 118, 133–140. [Google Scholar] [CrossRef]
- Papurello, D.; Gandiglio, M.; Kafashan, J.; Lanzini, A. Biogas Purification: A Comparison of Adsorption Wood-Derived Char Using Isotherm Equations. Processes 2019, 7, 774. [Google Scholar] [CrossRef]
- Boulinguiez, B.; le Cloirec, P. Biogas pre-upgrading by adsorption of trace compounds onto granular activated carbons and an activated carbon fiber-cloth. Water Sci. Technol. 2009, 59, 935–944. [Google Scholar] [CrossRef]
- Shang, G.; Li, Q.; Liu, L.; Chen, P.; Huang, X. Adsorption of hydrogen sulfide by biochars derived from pyrolysis of different agricultural/forestry wastes. J. Air Waste Manag. Assoc. 2016, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Papurello, D.; Tomasi, L.; Silvestri, S. Proton transfer reaction mass spectrometry for the gas cleaning using commercial and waste-derived materials: Focus on the siloxane removal for SOFC applications. Int. J. Mass Spectrom. 2018, 430, 69–79. [Google Scholar] [CrossRef]
- Finocchio, E.; Montanari, T.; Garuti, G.; Pistarino, C.; Federici, F.; Cugino, M.; Busca, G. Purification of biogases from siloxanes by adsorption: On the regenerability of activated carbon sorbents. Energy Fuels 2009, 23, 4156–4159. [Google Scholar] [CrossRef]
- Cabrera-Codony, A.; Montes-Morán, M.A.; Sánchez-Polo, M.; Martín, M.J.; Gonzalez-Olmos, R. Biogas Upgrading: Optimal Activated Carbon Properties for Siloxane Removal. Environ. Sci. Technol. 2014, 48, 7187–7195. [Google Scholar] [CrossRef]
- Matsui, T.; Imamura, S. Removal of siloxane from digestion gas of sewage sludge. In Bioresource Technology; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; pp. S29–S32. [Google Scholar] [CrossRef]
- Agnihotri, R.; Chauk, S.S.; Mahuli, S.K.; Fan, L.-S. Mechanism of CaO reaction with H2S: Diffusion through CaS product layer. Chem. Eng. Sci. 1999, 54, 3443–3453. [Google Scholar] [CrossRef]
- Papurello, D.; Silvestri, S.; Lanzini, A. Biogas cleaning: Trace compounds removal with model validation. Sep. Purif. Technol. 2018, 210, 80–92. [Google Scholar] [CrossRef]
- Cristiano, D.M.; Mohedano, R.D.A.; Nadaleti, W.C.; de Castilhos Junior, A.B.; Lourenço, V.A.; Gonçalves, D.F.; Belli Filho, P. H2S adsorption on nanostructured iron oxide at room temperature for biogas purification: Application of renewable energy. Renew. Energy 2020, 154, 151–160. [Google Scholar] [CrossRef]
- Papurello, D.; Santarelli, M.; Fiorilli, S. Physical Activation of Waste-Derived Materials for Biogas Cleaning. Energies 2018, 11, 2338. [Google Scholar] [CrossRef]
- Chauk, S.S.; Agnihotri, R.; Jadhav, R.A.; Misro, S.K.; Fan, L.-S. Kinetics of high-pressure removal of hydrogen sulfide using calcium oxide powder. AIChE J. 2000, 46, 1157–1167. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; Wu, J.; Liu, Y. A review of sorbents for high-temperature hydrogen sulfide removal from hot coal gas. Environ. Chem. Lett. 2019, 17, 259–276. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.S.; Gantenbein, A.; Schneebeli, J.; Frei, A.; Knorpp, A.J.; Schildhauer, T.J.; Biollaz, S.M.A. Deep removal of sulfur and trace organic compounds from biogas to protect a catalytic methanation reactor. Chem. Eng. J. 2019, 360, 577–590. [Google Scholar] [CrossRef]
- Lee, S.; Lee, T.; Kim, D. Adsorption of Hydrogen Sulfide from Gas Streams Using the Amorphous Composite of α-FeOOH and Activated Carbon Powder. Ind. Eng. Chem. Res. 2017, 56, 3116–3122. [Google Scholar] [CrossRef]
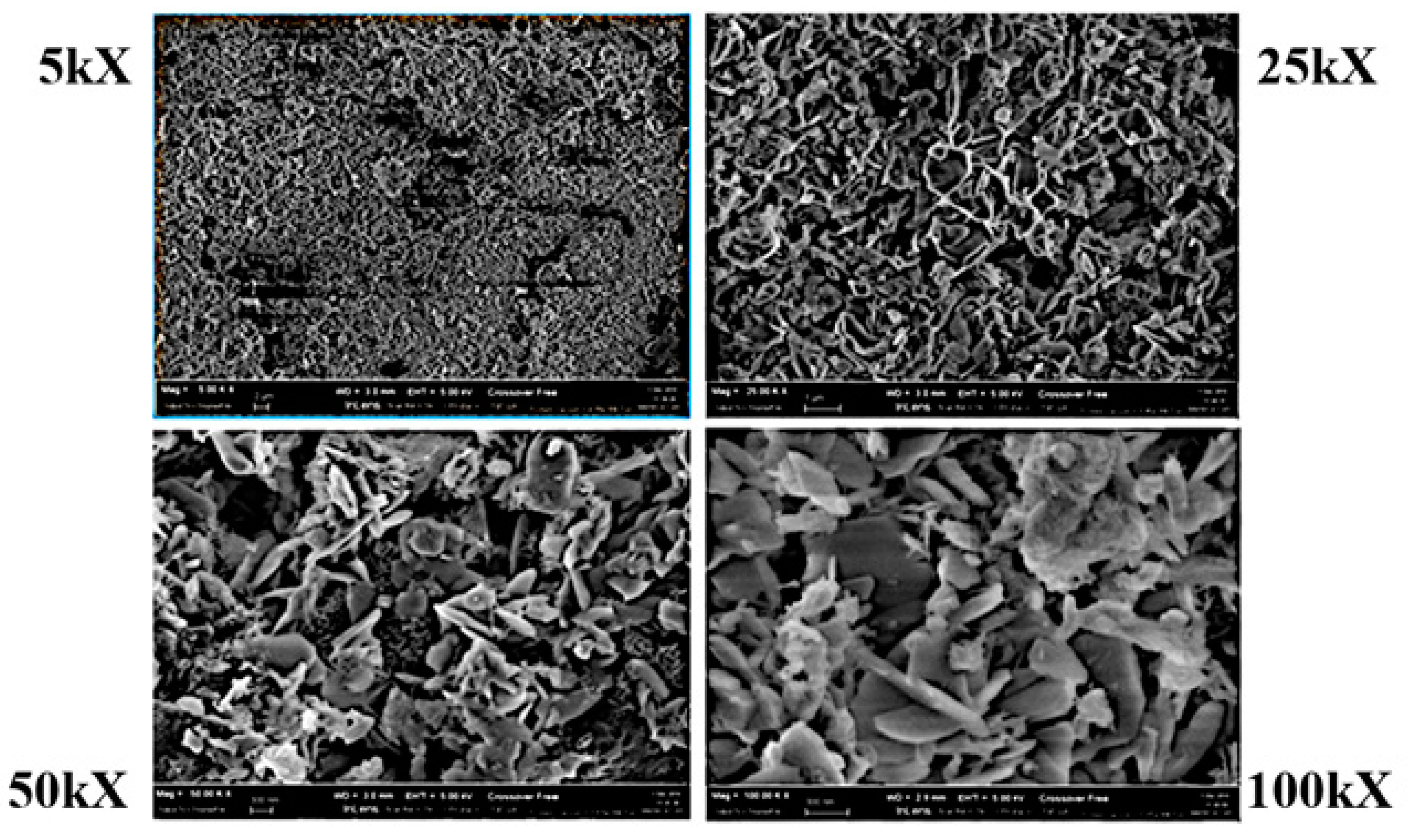
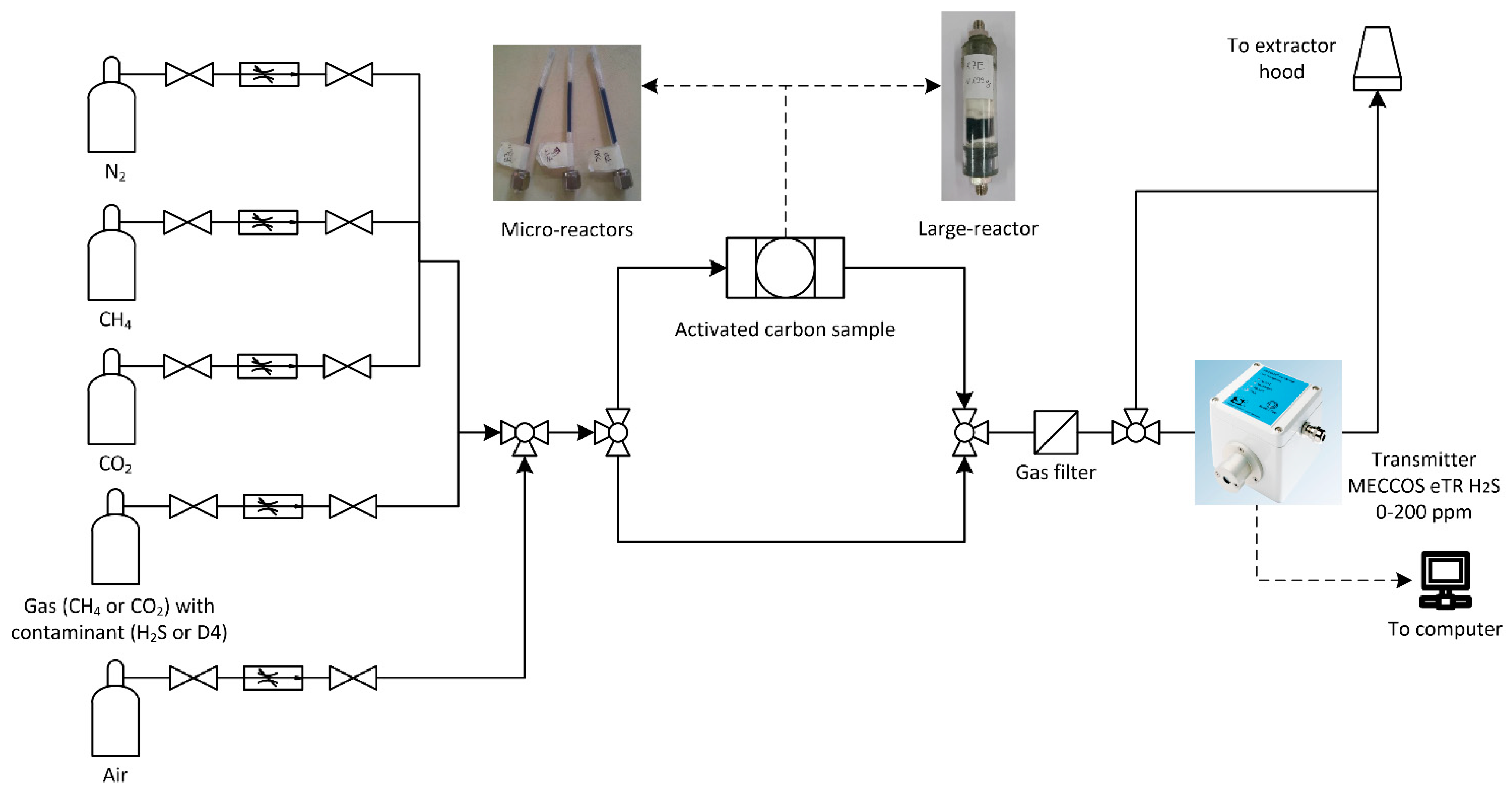



| Test Set #1 | Test Set #2 | Test Set #3 | Test Set #4 | |
|---|---|---|---|---|
| Reactor type | Microreactor 4 mm | Large reactor | Microreactor 4 mm | Microreactor 4 and 10 mm |
| Experiment | Sulfur | Sulfur | Siloxane | Sulfur, speed experiment |
| Samples | CKC, CKI, R8G | C64, CKC, CKI, R8G, R7E | C64, CKC, CKI, R8G, R7E | R8G |
| Flow rate ** | 750 mL/min | 950 mL/min | 200 mL/min | 500–750 mL/min |
| Gas composition | 62.5% CH4–37.5% CO2 | 62.5% CH4–37.5% CO2 | 62.5% CH4–37.5% CO2 | 62.5% CH4–37.5% CO2 |
| Contaminant | H2S | H2S | D4 | H2S |
| Contaminant concentration | 20 ppmv | 20 ppmv | 20 ppmv | 20 ppmv |
| Gas linear velocity | 0.995 m/s | 0.032 m/s | 0.27 m/s | Range 0.1–1 m/s |
| Pression | Ambient pressure | Ambient pressure | Ambient pressure | Ambient pressure |
| Temperature | Ambient temperature | Ambient temperature | Ambient temperature | Ambient temperature |
| Sample weight inside filter | 0.15 g ± 0.01 g | 7.5 g ± 0.5 g 11 g ± 0.5 g * | 0.15 ± 0.05 g | 0.07–0.92 g |
| GHSV | 154.8–190.8 kh−1 | 2520–6624 h−1 | 20.2–35.36 kh−1 | 20.10–325.54 kh−1 |
| Sample | Micropore Volume (cm3/g) | Total Pore Volume (cm3/g) | Surface Area (m2/g) | D4 Ads. Capacity (mg/g) |
|---|---|---|---|---|
| t-Plot | DFT | BET | ||
| R8G | 0.15 | 0.36 | 716.5 | 97 ± 3.1 |
| R7E | 0.015 | 0.21 | 81.5 | 23 ± 1.1 |
| C64 | 0.3 | 0.36 | 796.8 | 151 ± 5.3 |
| CKC | 0.22 | 0.41 | 663.4 | 101 ± 3.8 |
| CKI | 0.19 | 0.39 | 743.2 | 71 ± 2.4 |
| Sample | O | Si | Al | K | Ca | Fe | S | Mg | Na | Pt | I | Ti | Cu | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C64 | 10.77 | 0.46 | 0.38 | 0.18 | 0.25 | 0.17 | 0.12 | 0.09 | 0.04 | 0.10 | ||||
| CKC | 11.32 | 0.48 | 0.38 | 0.35 | 0.29 | 0.19 | 0.15 | 0.09 | ||||||
| CKI | 7.70 | 0.44 | 0.43 | 0.31 | 0.35 | 0.35 | 0.71 | 0.09 | 0.27 | 0.05 | ||||
| R8G | 42.58 | 0.41 | 0.26 | 1.95 | 4.14 | 7.54 | ||||||||
| R7E | 60.89 | 4.30 | 5.865 | 22.43 | 6.515 |
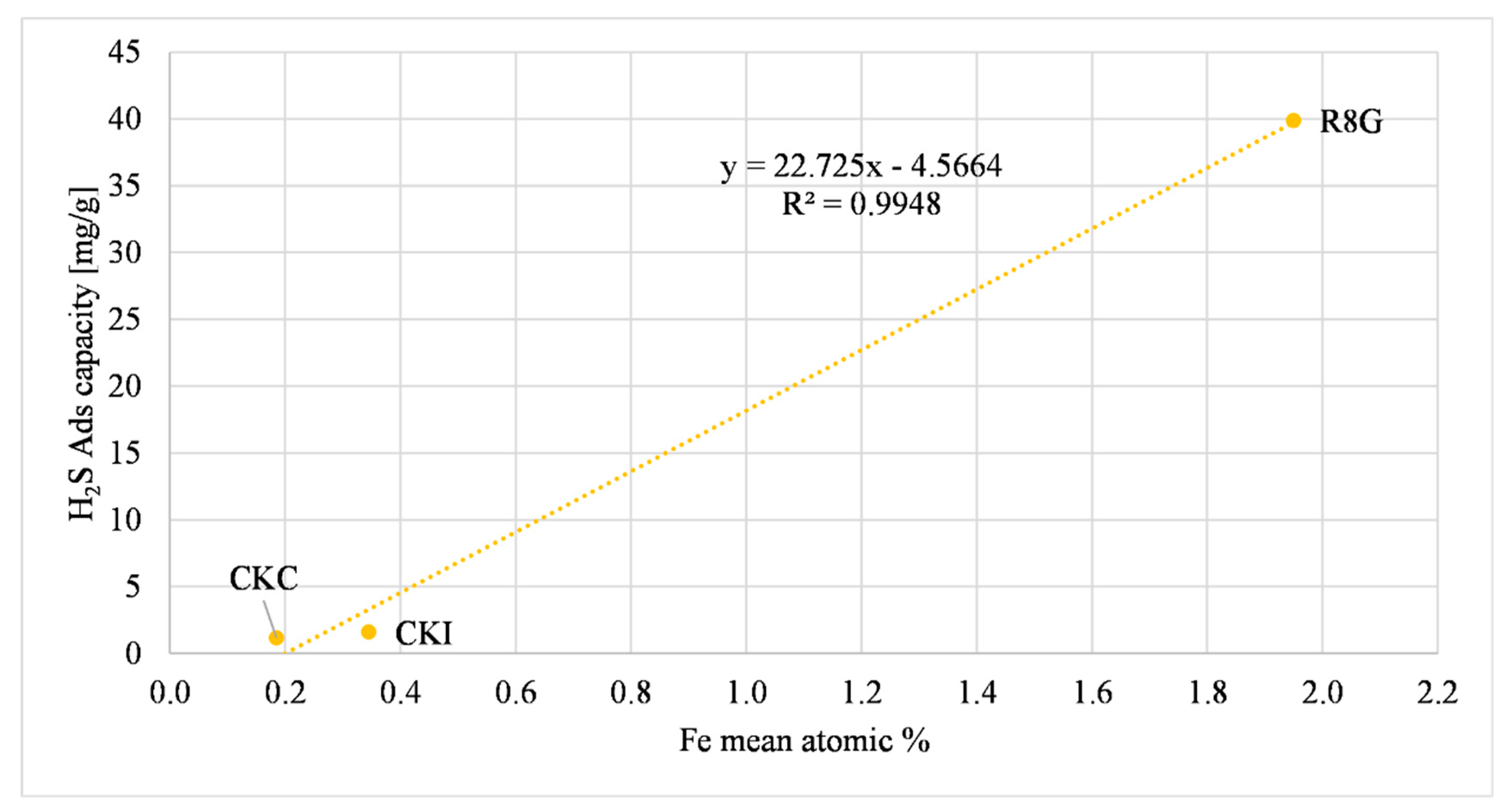
| Sample | Ca Atomic Concentration | Fe Atomic Concentration | H2S Ads Capacity Microreactor (mg/g) |
|---|---|---|---|
| CKC | 0.29 | 0.19 | 1.16 +/− 0.05 |
| CKI | 0.35 | 0.35 | 1.60 +/− 0.06 |
| R8G | - | 1.95 | 39.9 +/− 1.2 |
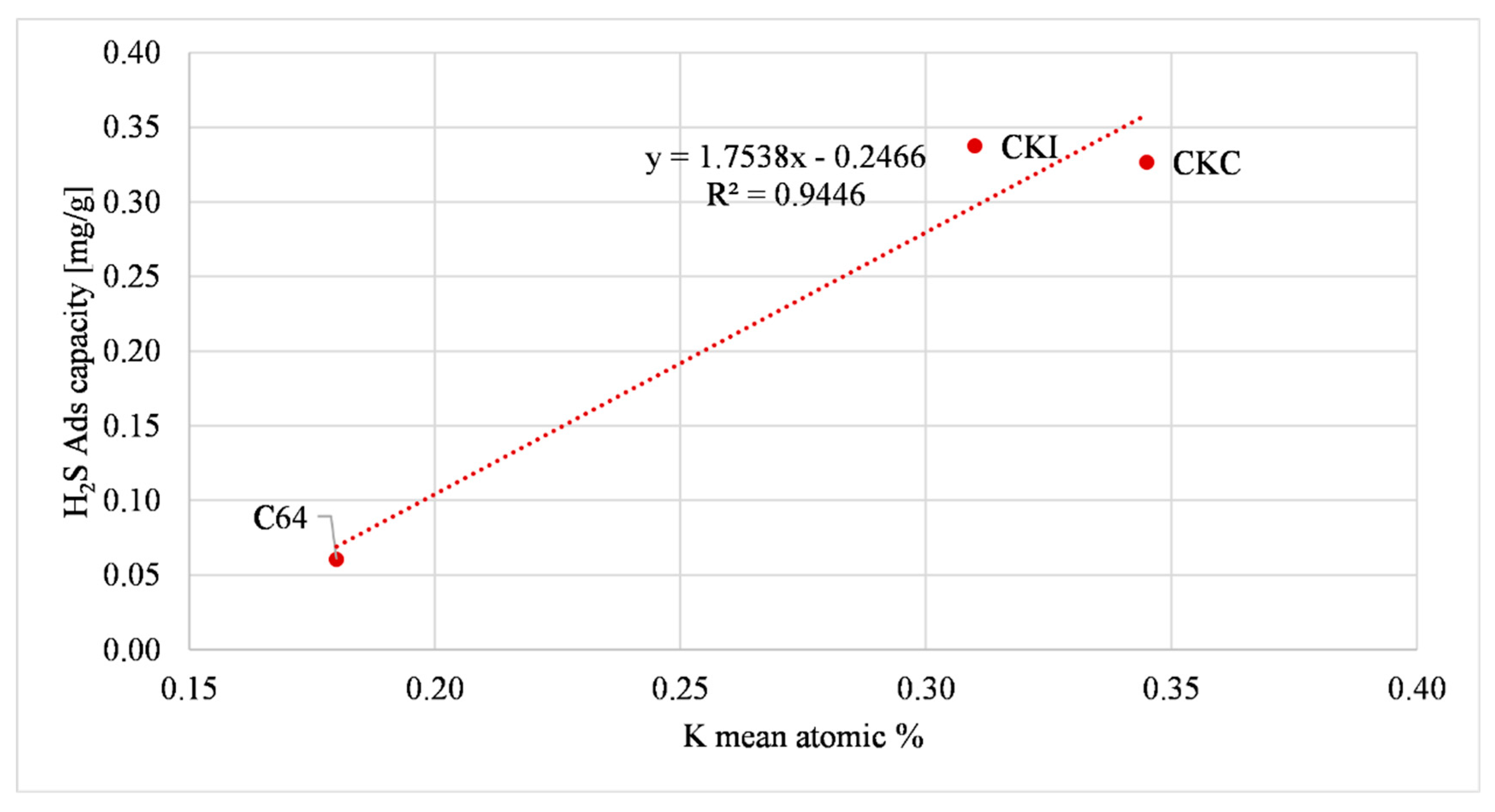
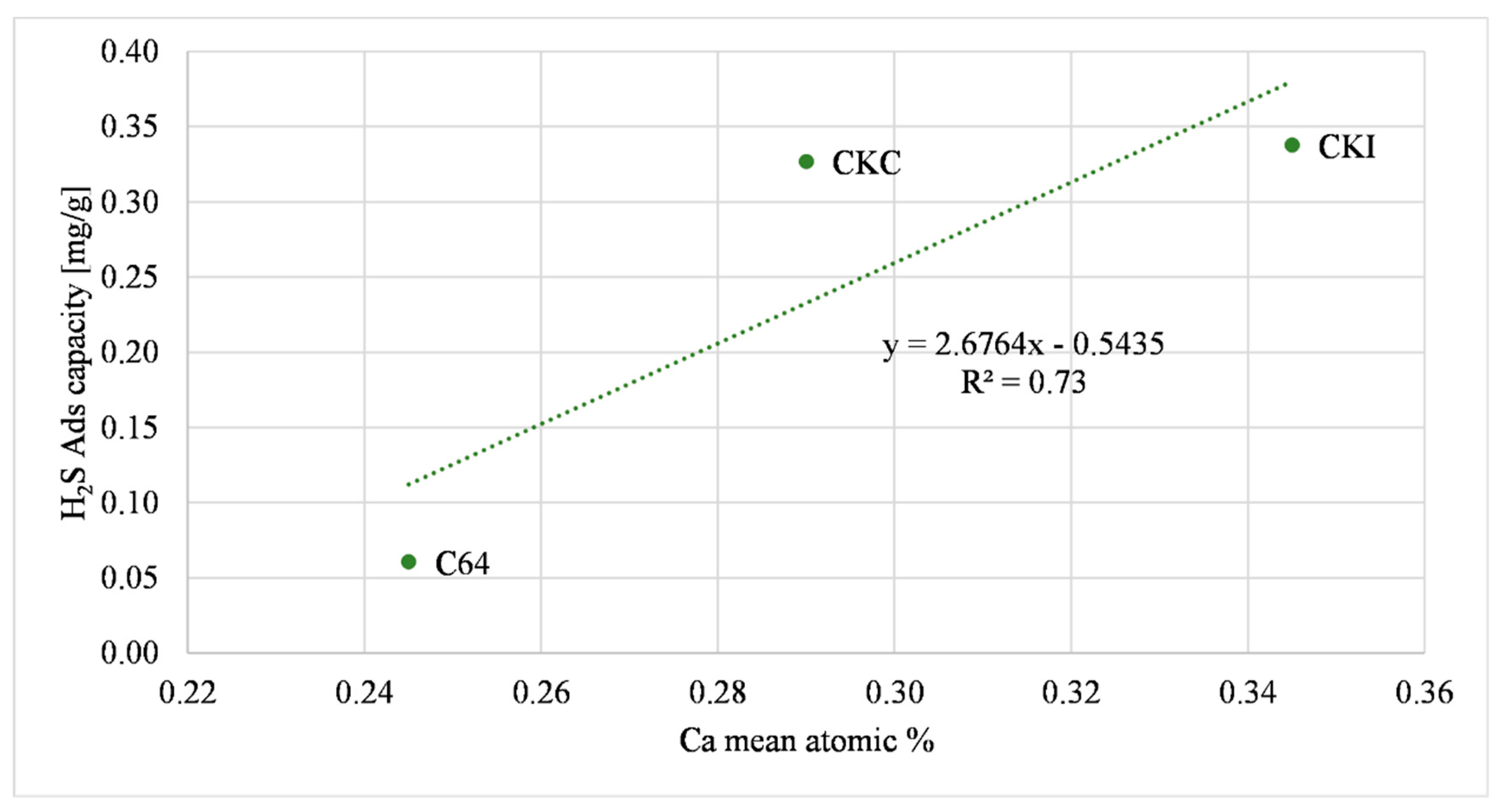
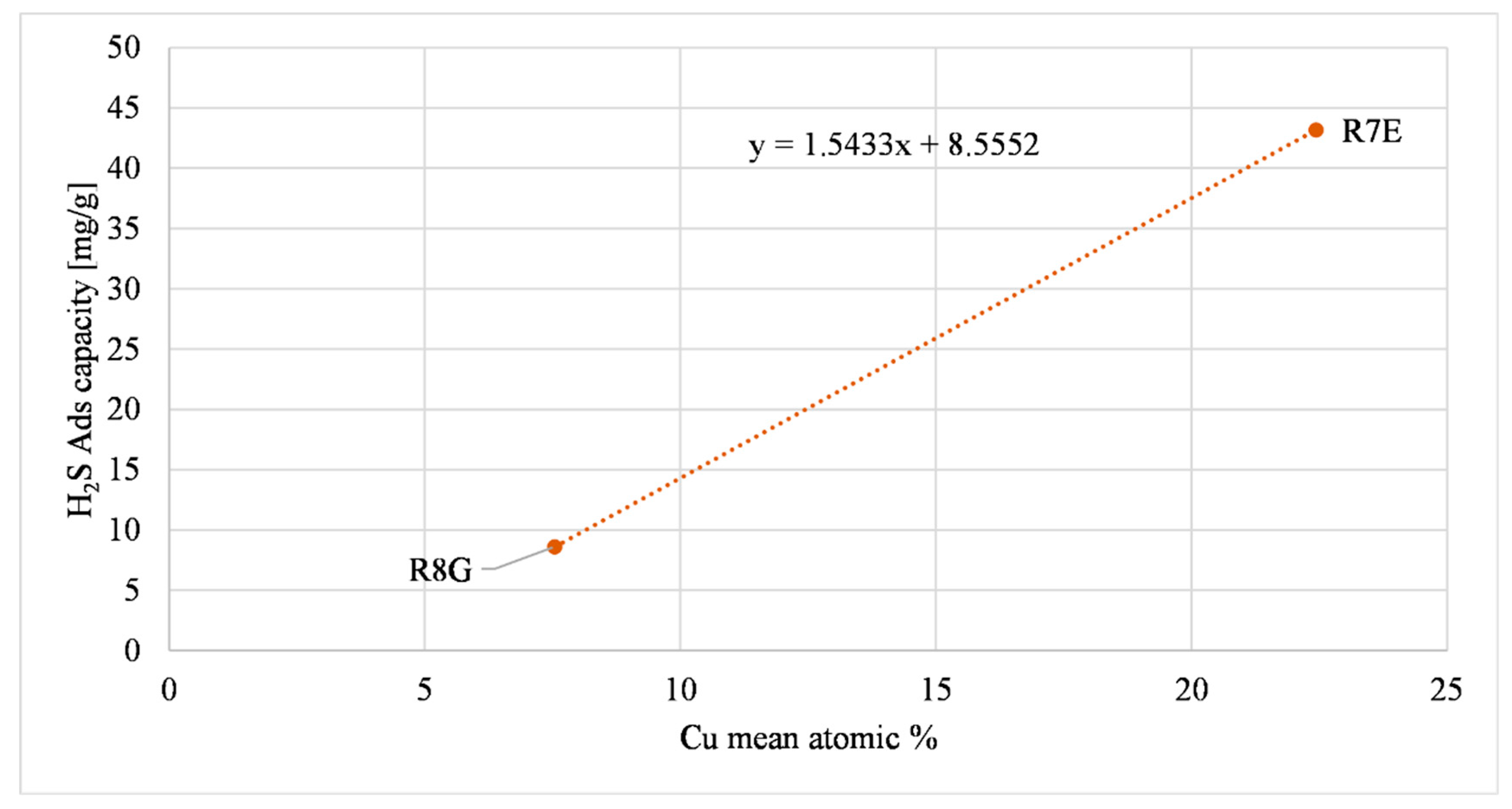
| Sample | K | Ca | Fe | Cu | H2S Ads Capacity Large Reactor (mg/g) (AC as Received) |
|---|---|---|---|---|---|
| C64 | 0.18 | 0.25 | 0.17 | - | 0.06 +/− 0.005 |
| CKC | 0.35 | 0.29 | 0.19 | - | 0.33 +/− 0.01 |
| CKI | 0.31 | 0.345 | 0.35 | - | 0.34 +/− 0.015 |
| R8G | - | - | 1.95 | 7.54 | 8.59 +/− 0.4 |
| R7E | - | - | - | 22.43 | 43.17 +/− 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molino, G.; Gandiglio, M.; Fiorilli, S.; Lanzini, A.; Drago, D.; Papurello, D. Design and Performance of an Adsorption Bed with Activated Carbons for Biogas Purification. Molecules 2022, 27, 7882. https://doi.org/10.3390/molecules27227882
Molino G, Gandiglio M, Fiorilli S, Lanzini A, Drago D, Papurello D. Design and Performance of an Adsorption Bed with Activated Carbons for Biogas Purification. Molecules. 2022; 27(22):7882. https://doi.org/10.3390/molecules27227882
Chicago/Turabian StyleMolino, Giulia, Marta Gandiglio, Sonia Fiorilli, Andrea Lanzini, Davide Drago, and Davide Papurello. 2022. "Design and Performance of an Adsorption Bed with Activated Carbons for Biogas Purification" Molecules 27, no. 22: 7882. https://doi.org/10.3390/molecules27227882
APA StyleMolino, G., Gandiglio, M., Fiorilli, S., Lanzini, A., Drago, D., & Papurello, D. (2022). Design and Performance of an Adsorption Bed with Activated Carbons for Biogas Purification. Molecules, 27(22), 7882. https://doi.org/10.3390/molecules27227882








