Advances in Low Pt Loading Membrane Electrode Assembly for Proton Exchange Membrane Fuel Cells
Abstract
1. Introduction
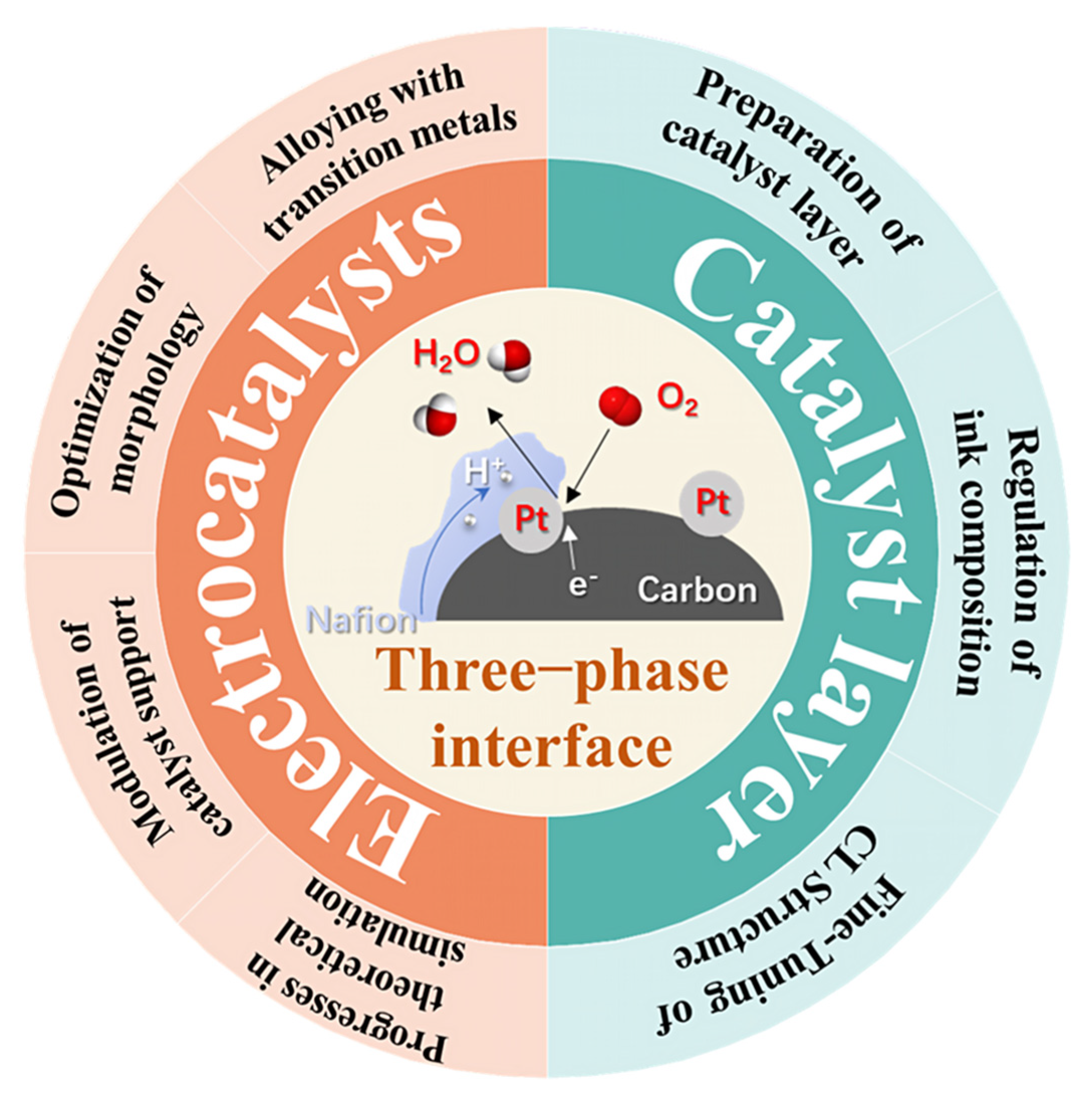
2. Electrocatalysts for ORR in MEA
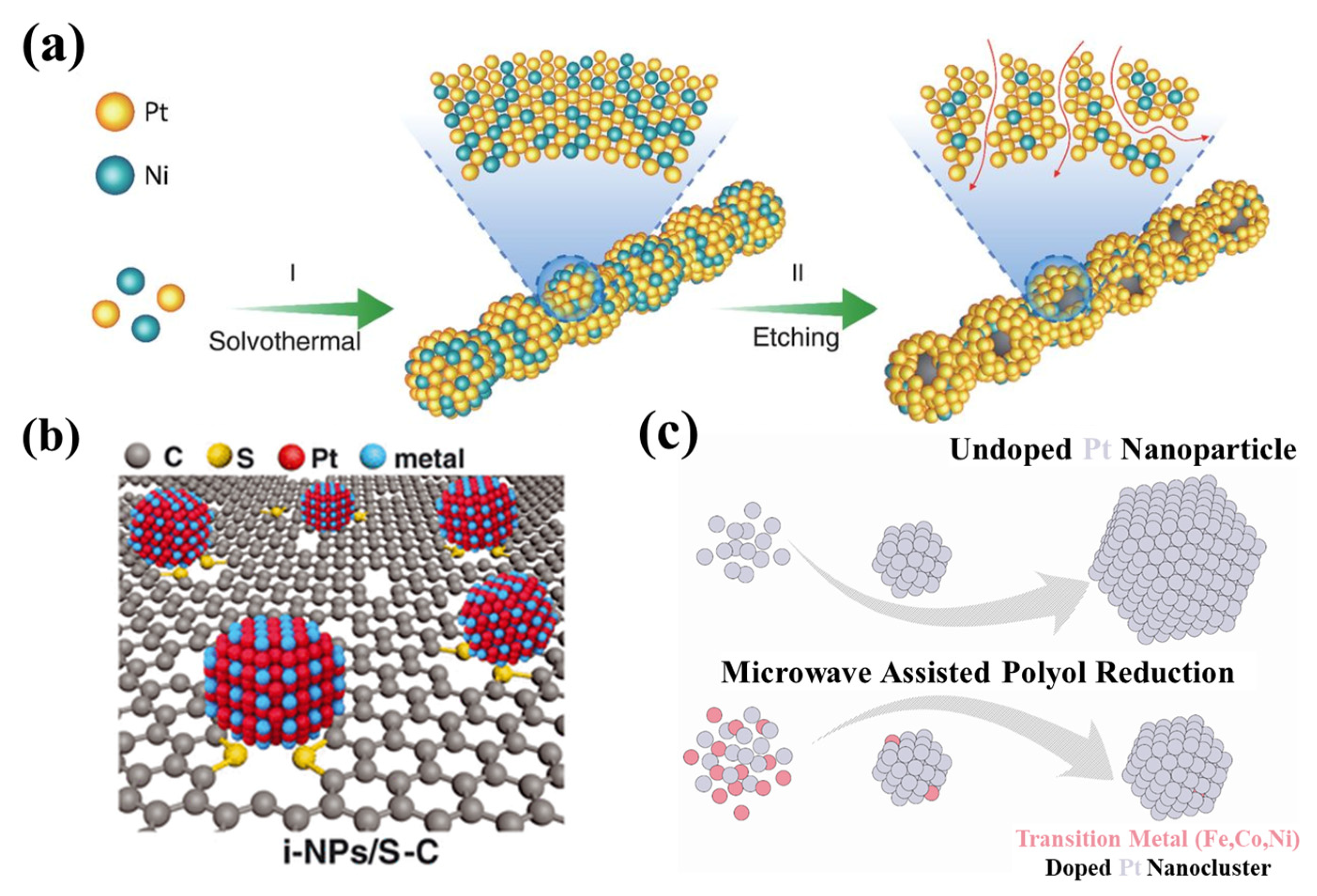
2.1. Alloying with Transition Metals
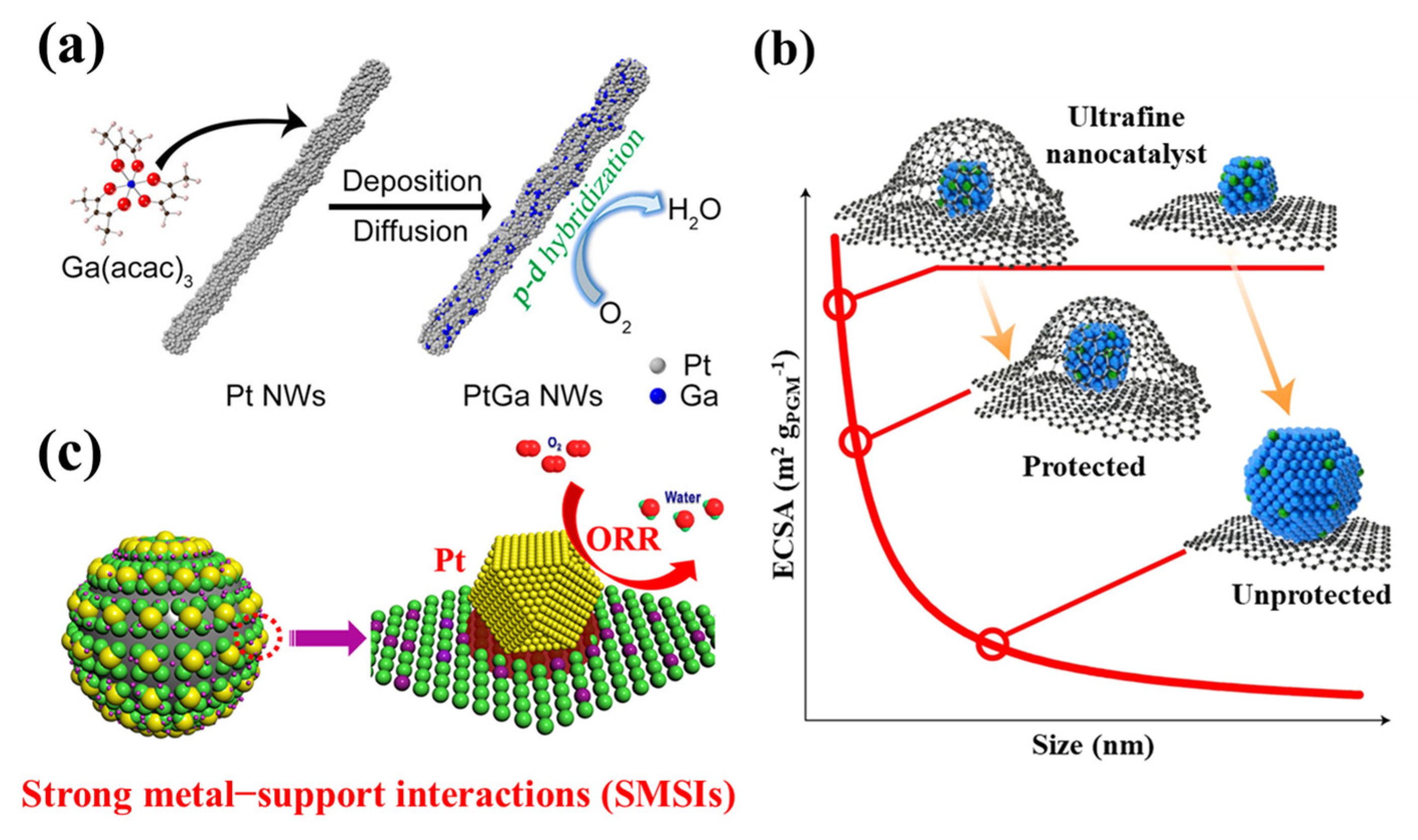
2.2. Optimization of Morphology
2.3. Modulation of Catalyst Support
2.4. Progresses in Theoretical Simulation
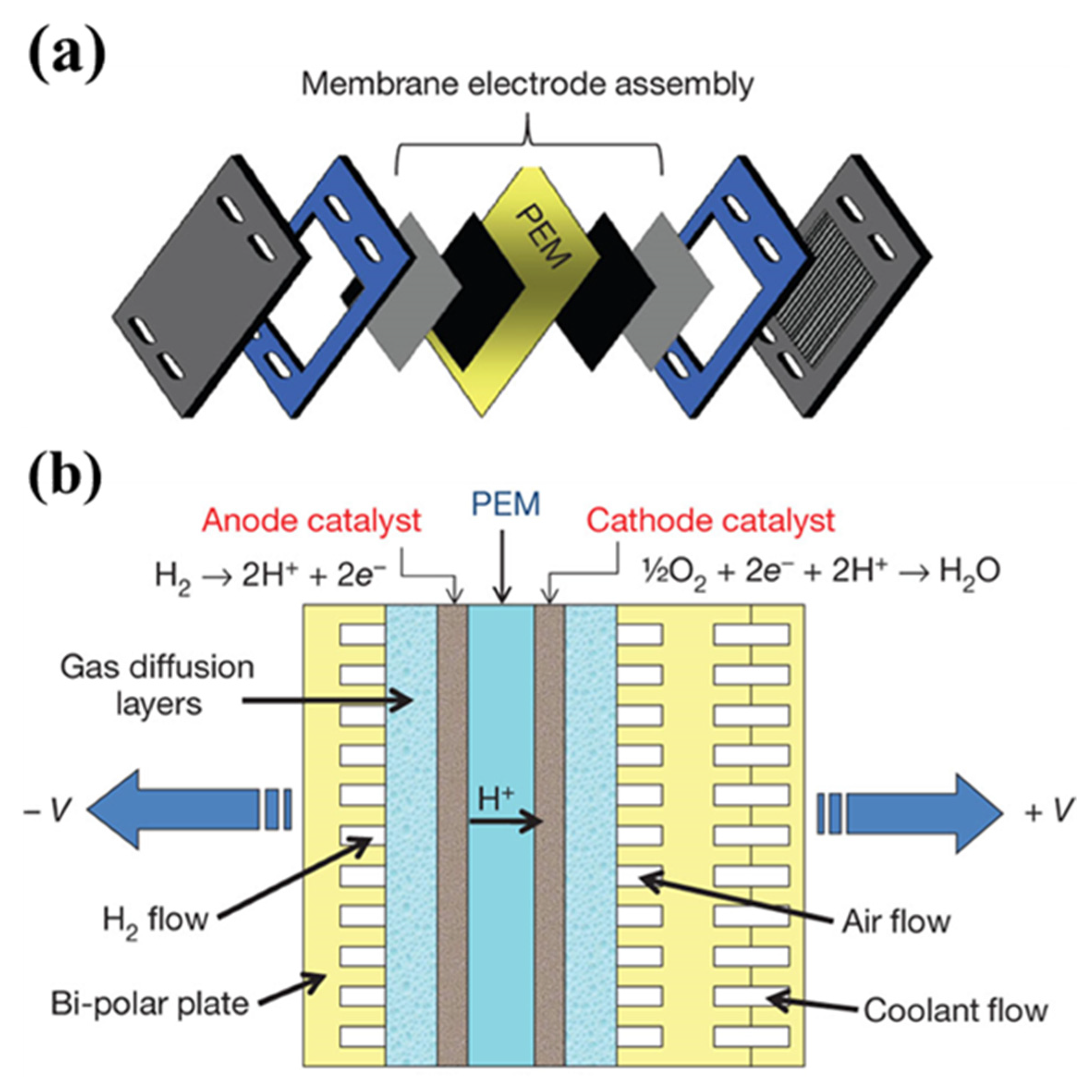
3. CL Structure in MEA
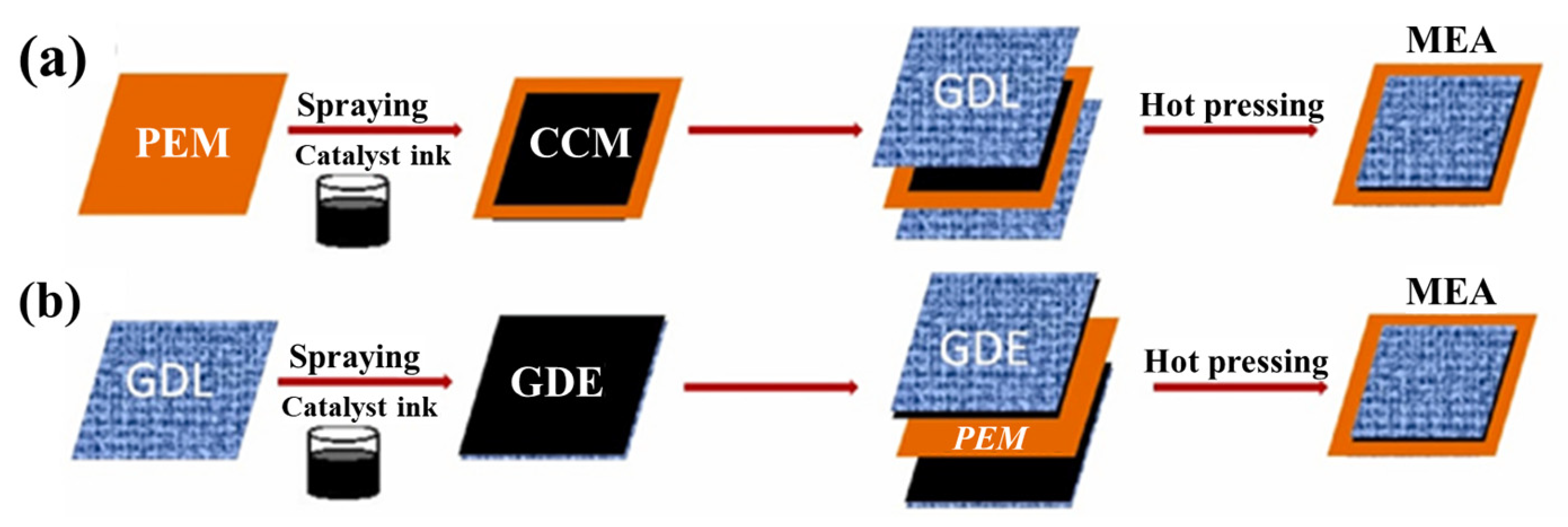
3.1. Preparation of CL
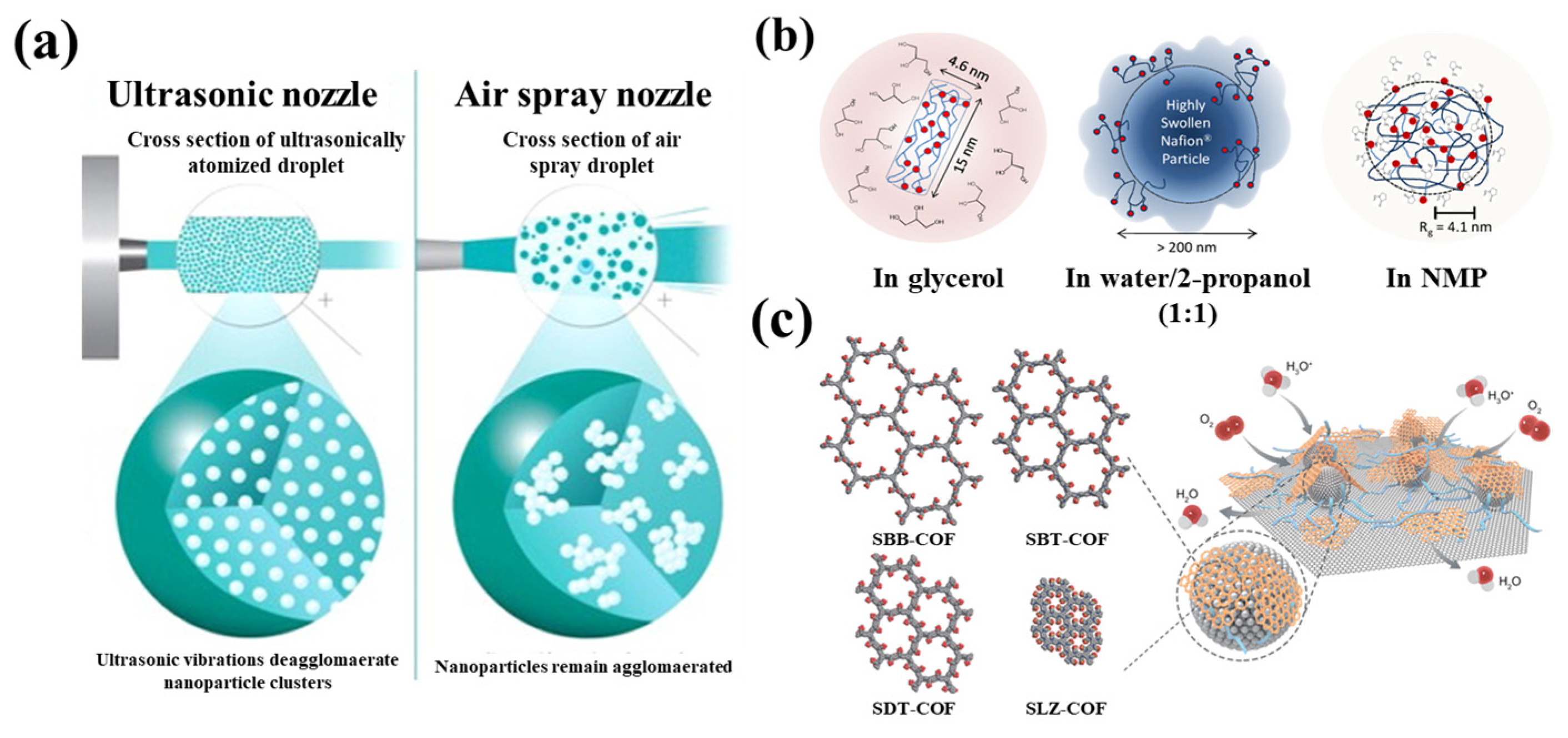
3.2. Regulation of Ink Composition
3.3. Fine-Tuning of CL Structure
4. Summary and Outlook
- In many cases, high activity or Pt utilization efficiency for the catalyst on RDE does not translate to good catalytic performance in MEAs [59,70,120]. This difference is due to different working conditions. Numerous studies continue to focus on RDE electrocatalyst performance due to their low cost. We suggest that electrocatalysts be evaluated and optimized in MEA and single cells. The findings would then be more convincing and valuable for commercial development. Low-Pt loading in MEA needs to be conducted for both catalyst and CL structures. The most pressing issue is MEA durability.
- In developing low-Pt loading MEAs, a large ECSA is needed to address mass transfer issues in current catalysts. Therefore, catalyst particle size needs to be reduced [32,49,52]. However, this reduction causes migration and ripening, leading to reduced durability, and durability is sometimes more important than activity [121,122,123]. Currently, many studies still focus on obtaining enhanced catalytic activity. Stability is rarely the goal. ADTs are often performed after the best sample is chosen at the end. We suggest that researchers consider stability as equally important as activity in their research and development.
- Nonprecious metal electrocatalysts have recently been proven as an alternative to traditional Pt-based electrocatalysts, even in MEA [124,125,126,127]. However, the stability of nonprecious metal electrocatalysts could not meet the demands of practical applications. Additionally, activity and stability are still significant challenges, especially in larger-scale application circumstances, such as in actual stacks [128,129]. Like the Pt-based material system that has been discussed, most studies of nonprecious metal-based electrocatalysts are still on RDE [72]. However, recent reports have indicated that combining nonprecious and precious metal catalysts may benefit from a synergistic effect [130,131,132]. This integrated field might be promising for enhancing ORR catalytic performance while keeping costs down.
- The problem of mass transport severely affects the performance of low-Pt loading MEA [133,134,135,136]. Therefore, for CL, a good structure suitable for mass transfer needs to be developed. However, a cheap and effective solution has not yet emerged. Hence, in-depth studies focusing on suitable pore structures and homogeneous three-phase interfaces are needed.
- It should also be noted that the reduction in noble metal loadings is not only important. Considering the limited crustal reserves, it is very economical to recycle precious metals from obsolete and defunct MEAs [137,138]. Specific manufacturing routes of the MEAs may have used less Pt but would increase the difficulties in recycling. Most researchers have not yet realized that the cost should eventually be “life-long”. Hence, it is suggested that such prospects be concerned in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cullen, D.A.; Neyerlin, K.C.; Ahluwalia, R.K.; Mukundan, R.; More, K.L.; Borup, R.L.; Weber, A.Z.; Myers, D.J.; Kusoglu, A. New roads and challenges for fuel cells in heavy-duty transportation. Nat. Energy 2021, 6, 462–474. [Google Scholar] [CrossRef]
- Shi, F.; Chen, C.; Xu, Z.-L. Recent Advances on Electrospun Nanofiber Materials for Post-lithium Ion Batteries. Adv. Fiber Mater. 2021, 3, 275–301. [Google Scholar] [CrossRef]
- Li, D.; Long, X.; Wu, Y.; Hou, H.; Wang, X.; Ren, J.; Zhang, L.; Yang, D.; Xia, Y. Hierarchically Porous and Defective Carbon Fiber Cathode for Efficient Zn-Air Batteries and Microbial Fuel Cells. Adv. Fiber Mater. 2022, 4, 795–806. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, Z.; Zhang, W.; Zhang, D. High-Efficiency g-C3N4 Based Photocatalysts for CO2 Reduction: Modification Methods. Adv. Fiber Mater. 2022, 4, 342–360. [Google Scholar] [CrossRef]
- Wang, X.X.; Swihart, M.T.; Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 17832–17852. [Google Scholar] [CrossRef]
- Suter, T.A.M.; Smith, K.; Hack, J.; Rasha, L.; Rana, Z.; Angel, G.M.A.; Shearing, P.R.; Miller, T.S.; Brett, D.J.L. Engineering Catalyst Layers for Next-Generation Polymer Electrolyte Fuel Cells: A Review of Design, Materials, and Methods. Adv. Energy Mater. 2021, 11, 2101025. [Google Scholar] [CrossRef]
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 2021, 16, 140–147. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.-C.; Wu, Z.-P.; Chen, G.; Yang, F.; Zhu, S.; Siddharth, K.; Kong, Z.; Lu, A.; Li, J.-C.; et al. Recent Advances in Electrocatalysts for Proton Exchange Membrane Fuel Cells and Alkaline Membrane Fuel Cells. Adv. Mater. 2021, 33, 2006292. [Google Scholar] [CrossRef]
- Ahn, C.-Y.; Park, J.E.; Kim, S.; Kim, O.-H.; Hwang, W.; Her, M.; Kang, S.Y.; Park, S.; Kwon, O.J.; Park, H.S.; et al. Differences in the Electrochemical Performance of Pt-Based Catalysts Used for Polymer Electrolyte Membrane Fuel Cells in Liquid Half- and Full-Cells. Chem. Rev. 2021, 121, 15075–15140. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, S.; Chen, S. Pt Utilization in Proton Exchange Membrane Fuel Cells: Structure Impacting Factors and Mechanistic Insights. Chem. Soc. Rev. 2022, 51, 1529–1546. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, J.; Zheng, Y.; Xiao, M.; Gao, R.; Zhang, Z.; Wen, G.; Dou, H.; Deng, Y.P.; Yu, A.; et al. Materials Engineering toward Durable Electrocatalysts for Proton Exchange Membrane Fuel Cells. Adv. Energy Mater. 2021, 12, 2102665. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Q.; Rui, Z.; Li, J.; Liu, J. High-Loading Pt–Co/C Catalyst with Enhanced Durability toward the Oxygen Reduction Reaction through Surface Au Modification. ACS Appl. Mater. Interfaces 2020, 12, 30381–30389. [Google Scholar] [CrossRef]
- Hua, K.; Rui, Z.; Zhang, T.; Cao, Y.; Shen, X.; Wang, S.; He, P.; Li, J.; Liu, J. Efficient synthesis of Pt3Co/NC alloy catalysts with enhanced durability and activity for the oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 13022–13029. [Google Scholar] [CrossRef]
- Lin, Z.; Sheng, Y.; Li, J.; Rui, Z.; Liu, Y.; Liu, J.; Zou, Z. Ternary heterogeneous Pt–Ni–Au nanowires with enhanced activity and stability for PEMFCs. Chem. Commun. 2020, 56, 4276–4279. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Yang, S.; Fu, C.; Zou, L.; Zou, Z.; Jiang, Z.; Zhang, J.; Yang, H. High-loaded sub-6 nm Pt1Co1 intermetallic compounds with highly efficient performance expression in PEMFCs. Energy Environ. Sci. 2022, 15, 278–286. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Li, X.; Duan, X.; Yuan, M.; Cao, F.; Sun, K.; Zhang, Y.; Wang, Y.; Gu, Z.; et al. A TiN@C core–shell support for improving Pt catalyst corrosion resistance. RSC Adv. 2022, 12, 25035–25040. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Zhang, G.; Liu, H.; Wang, Y.; Sun, S.; Guo, X. Pyrolysis of Self-Assembled Iron Porphyrin on Carbon Black as Core/Shell Structured Electrocatalysts for Highly Efficient Oxygen Reduction in Both Alkaline and Acidic Medium. Adv. Funct. Mater. 2017, 27, 1604356. [Google Scholar] [CrossRef]
- Ehelebe, K.; Knöppel, J.; Bierling, M.; Mayerhöfer, B.; Böhm, T.; Kulyk, N.; Thiele, S.; Mayrhofer, K.J.J.; Cherevko, S. Platinum Dissolution in Realistic Fuel Cell Catalyst Layers. Angew. Chem. Int. Ed. 2021, 60, 8882–8888. [Google Scholar] [CrossRef]
- Zhu, F.; Luo, L.; Wu, A.; Wang, C.; Cheng, X.; Shen, S.; Ke, C.; Yang, H.; Zhang, J. Improving the High-Current-Density Performance of PEMFC through Much Enhanced Utilization of Platinum Electrocatalysts on Carbon. ACS Appl. Mater. Interfaces 2020, 12, 26076–26083. [Google Scholar] [CrossRef]
- Weber, A.Z.; Kusoglu, A. Unexplained transport resistances for low-loaded fuel-cell catalyst layers. J. Mater. Chem. A 2014, 2, 17207–17211. [Google Scholar] [CrossRef]
- Lopez-Haro, M.; Guétaz, L.; Printemps, T.; Morin, A.; Escribano, S.; Jouneau, P.H.; Bayle-Guillemaud, P.; Chandezon, F.; Gebel, G. Three-dimensional analysis of Nafion layers in fuel cell electrodes. Nat. Commun. 2014, 5, 5229. [Google Scholar]
- Ferreira, P.J.; la O’, G.J.; Shao-Horn, Y.; Morgan, D.; Makharia, R.; Kocha, S.; Gasteiger, H.A. Instability of Pt/C Electrocatalysts in Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2005, 152, A2256. [Google Scholar]
- Bing, Y.; Liu, H.; Zhang, L.; Ghosh, D.; Zhang, J. Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction. Chem. Soc. Rev. 2010, 39, 2184–2202. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hady, E.E.; Shaban, M.; Abdel-Hamed, M.O.; Gamal, A.; Yehia, H.; Ahmed, A.M. Synthesis and Characterization of NiCoPt/CNFs Nanoparticles as an Effective Electrocatalyst for Energy Applications. Nanomaterials 2022, 12, 492. [Google Scholar]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Guo, S.; Zhang, X.; Shen, X.; Su, D.; Lu, G.; Zhu, X.; Yao, J.; Guo, J.; Huang, X. Surface engineering of hierarchical platinum-cobalt nanowires for efficient electrocatalysis. Nat. Commun. 2016, 7, 11850. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.M.; Lou, X.W.; et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef]
- Wang, X.X.; Sokolowski, J.; Liu, H.; Wu, G. Pt alloy oxygen-reduction electrocatalysts: Synthesis, structure, and property. Chin. J. Catal. 2020, 41, 739–755. [Google Scholar] [CrossRef]
- Gamler, J.T.L.; Ashberry, H.M.; Skrabalak, S.E.; Koczkur, K.M. Random Alloyed versus Intermetallic Nanoparticles: A Comparison of Electrocatalytic Performance. Adv. Mater. 2018, 30, 1801563. [Google Scholar] [CrossRef] [PubMed]
- Hodnik, N.; Jeyabharathi, C.; Meier, J.C.; Kostka, A.; Phani, K.L.; Rečnik, A.; Bele, M.; Hočevar, S.; Gaberšček, M.; Mayrhofer, K.J.J. Effect of ordering of PtCu3 nanoparticle structure on the activity and stability for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 13610–13615. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xin, H.L.; Hovden, R.; Wang, H.; Yu, Y.; Muller, D.A.; DiSalvo, F.J.; Abruña, H.D. Structurally ordered intermetallic platinum–cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87. [Google Scholar] [CrossRef]
- Yang, C.-L.; Wang, L.-N.; Yin, P.; Liu, J.; Chen, M.-X.; Yan, Q.-Q.; Wang, Z.-S.; Xu, S.-L.; Chu, S.-Q.; Cui, C.; et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459–464. [Google Scholar] [CrossRef]
- Song, T.-W.; Xu, C.; Sheng, Z.-T.; Yan, H.-K.; Tong, L.; Liu, J.; Zeng, W.-J.; Zuo, L.-J.; Yin, P.; Zuo, M.; et al. Small molecule-assisted synthesis of carbon supported platinum intermetallic fuel cell catalysts. Nat. Commun. 2022, 13, 6521. [Google Scholar] [CrossRef]
- Liang, J.; Li, N.; Zhao, Z.; Ma, L.; Wang, X.; Li, S.; Liu, X.; Wang, T.; Du, Y.; Lu, G.; et al. Tungsten-Doped L10-PtCo Ultrasmall Nanoparticles as a High-Performance Fuel Cell Cathode. Angew. Chem. Int. Ed. 2019, 58, 15471–15477. [Google Scholar] [CrossRef]
- Duan, X.; Cao, F.; Ding, R.; Li, X.; Li, Q.; Aisha, R.; Zhang, S.; Hua, K.; Rui, Z.; Wu, Y.; et al. Cobalt-doping stabilized active and durable sub-2 nm Pt nanoclusters for low-Pt-loading PEMFC cathode. Adv. Energy Mater. 2022, 12, 2103144. [Google Scholar] [CrossRef]
- Xia, Z.; Guo, S. Strain engineering of metal-based nanomaterials for energy electrocatalysis. Chem. Soc. Rev. 2019, 48, 3265–3278. [Google Scholar] [CrossRef]
- Zhu, E.; Wu, M.; Xu, H.; Peng, B.; Liu, Z.; Huang, Y.; Li, Y. Stability of Platinum-Group-Metal-Based Electrocatalysts in Proton Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2022, 32, 2203883. [Google Scholar] [CrossRef]
- Chen, S.; Li, M.; Gao, M.; Jin, J.; van Spronsen, M.A.; Salmeron, M.B.; Yang, P. High-Performance Pt-Co Nanoframes for Fuel-Cell Electrocatalysis. Nano Lett. 2020, 20, 1974–1979. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, T.; Goddard Iii, W.A. Atomistic Explanation of the Dramatically Improved Oxygen Reduction Reaction of Jagged Platinum Nanowires, 50 Times Better than Pt. J. Am. Chem. Soc. 2020, 142, 8625–8632. [Google Scholar] [CrossRef]
- Luo, S.; Chen, W.; Cheng, Y.; Song, X.; Wu, Q.; Li, L.; Wu, X.; Wu, T.; Li, M.; Yang, Q.; et al. Trimetallic Synergy in Intermetallic PtSnBi Nanoplates Boosts Formic Acid Oxidation. Adv. Mater. 2019, 31, 1903683. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly Crystalline Multimetallic Nanoframes with Three-Dimensional Electrocatalytic Surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Heggen, M.; O’Malley, R.; Theobald, B.; Strasser, P. Understanding and Controlling Nanoporosity Formation for Improving the Stability of Bimetallic Fuel Cell Catalysts. Nano Lett. 2013, 13, 1131–1138. [Google Scholar] [CrossRef]
- Cui, C.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013, 12, 765–771. [Google Scholar] [CrossRef]
- Huang, L.; Zaman, S.; Tian, X.; Wang, Z.; Fang, W.; Xia, B.Y. Advanced Platinum-Based Oxygen Reduction Electrocatalysts for Fuel Cells. Acc. Chem. Res. 2021, 54, 311–322. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Yao, Z.; Bai, H.; Lu, Y.; Ma, C.; Lu, S.; Peng, Z.; Yang, J.; Pan, A.; et al. Unconventional p–d Hybridization Interaction in PtGa Ultrathin Nanowires Boosts Oxygen Reduction Electrocatalysis. J. Am. Chem. Soc. 2019, 141, 18083–18090. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, X.; Luo, E.; Yang, L.; Chu, Y.; Gao, L.; Jin, Z.; Liu, C.; Ge, J.; Xing, W. Stabilized Pt Cluster-Based Catalysts Used as Low-Loading Cathode in Proton-Exchange Membrane Fuel Cells. ACS Energy Lett. 2020, 5, 3021–3028. [Google Scholar]
- Cheng, H.; Cao, Z.; Chen, Z.; Zhao, M.; Xie, M.; Lyu, Z.; Zhu, Z.; Chi, M.; Xia, Y. Catalytic system based on sub-2 nm Pt particles and its extraordinary activity and durability for oxygen reduction. Nano Lett. 2019, 19, 4997–5002. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Z.; Zhang, A.; Yan, X.; Xue, W.; Peng, B.; Xin, H.L.; Pan, X.; Duan, X.; Huang, Y. Graphene-nanopocket-encaged PtCo nanocatalysts for highly durable fuel cell operation under demanding ultralow-Pt-loading conditions. Nat. Nanotechnol. 2022, 17, 968–975. [Google Scholar] [PubMed]
- Sánchez-Sánchez, C.M.; Solla-Gullón, J.; Vidal-Iglesias, F.J.; Aldaz, A.; Montiel, V.; Herrero, E. Imaging Structure Sensitive Catalysis on Different Shape-Controlled Platinum Nanoparticles. J. Am. Chem. Soc. 2010, 132, 5622–5624. [Google Scholar] [CrossRef] [PubMed]
- Marković, N.M.; Ross, P.N. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 2002, 45, 117–229. [Google Scholar] [CrossRef]
- Choi, S.-I.; Xie, S.; Shao, M.; Odell, J.H.; Lu, N.; Peng, H.-C.; Protsailo, L.; Guerrero, S.; Park, J.; Xia, X.; et al. Synthesis and Characterization of 9 nm Pt–Ni Octahedra with a Record High Activity of 3.3 A/mgPt for the Oxygen Reduction Reaction. Nano Lett. 2013, 13, 3420–3425. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Chen, Y.; Zhu, E.; Li, M.; Duan, X.; Huang, Y. A rational design of carbon-supported dispersive Pt-based octahedra as efficient oxygen reduction reaction catalysts. Energy Environ. Sci. 2014, 7, 2957–2962. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, S.; Fang, Z.; Ding, J.; Sang, W.; Wang, Y.; Zhao, J.; Peng, Z.; Zeng, J. Octahedral Pd@Pt1.8Ni Core–Shell Nanocrystals with Ultrathin PtNi Alloy Shells as Active Catalysts for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 2804–2807. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Marković, N.M. Improved Oxygen Reduction Activity on Pt3Ni(111) via Increased Surface Site Availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Luo, M.; Sun, Y.; Zhang, X.; Qin, Y.; Li, M.; Li, Y.; Li, C.; Yang, Y.; Wang, L.; Gao, P.; et al. Stable High-Index Faceted Pt Skin on Zigzag-Like PtFe Nanowires Enhances Oxygen Reduction Catalysis. Adv. Mater. 2018, 30, 1705515. [Google Scholar] [CrossRef]
- Chattot, R.; Le Bacq, O.; Beermann, V.; Kühl, S.; Herranz, J.; Henning, S.; Kühn, L.; Asset, T.; Guétaz, L.; Renou, G.; et al. Surface distortion as a unifying concept and descriptor in oxygen reduction reaction electrocatalysis. Nat. Mater. 2018, 17, 827–833. [Google Scholar] [CrossRef]
- Cao, F.; Zhang, H.; Duan, X.; Li, X.; Ding, R.; Hua, K.; Rui, Z.; Wu, Y.; Yuan, M.; Wang, J.; et al. Coating Porous TiO2 Films on Carbon Nanotubes to Enhance the Durability of Ultrafine PtCo/CNT Nanocatalysts for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2022, 14, 51975–51982. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Luo, J.; Nan, H.; Zou, H.; Chen, R.; Shu, T.; Li, X.; Li, Y.; Song, H.; Liao, S.; et al. Transition Metal Nitride Coated with Atomic Layers of Pt as a Low-Cost, Highly Stable Electrocatalyst for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2016, 138, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Ling, T.; Ma, T.-Y.; Wang, H.; Hu, Z.; Zhou, Y.; Mao, J.; Du, X.-W.; Jaroniec, M.; Qiao, S.-Z. Atomically and Electronically Coupled Pt and CoO Hybrid Nanocatalysts for Enhanced Electrocatalytic Performance. Adv. Mater. 2017, 29, 1604607. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Tetteh, E.B.; Lee, H.-Y.; Kang, T.-H.; Yu, J.-S. Tailor-Made Pt Catalysts with Improved Oxygen Reduction Reaction Stability/Durability. ACS Catal. 2019, 9, 8622–8645. [Google Scholar] [CrossRef]
- Chen, S.; Wei, Z.; Guo, L.; Ding, W.; Dong, L.; Shen, P.; Qi, X.; Li, L. Enhanced dispersion and durability of Pt nanoparticles on a thiolated CNT support. Chem. Commun. 2011, 47, 10984–10986. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, Y.-j.; Zhao, J.-x.; Ding, Y.-h. High stability and superior catalytic reactivity of nitrogen-doped graphene supporting Pt nanoparticles as a catalyst for the oxygen reduction reaction: A density functional theory study. RSC Adv. 2015, 5, 34070–34077. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Gao, L.; Zeng, G.; Li, M.; Huang, H. Stabilizing Pt-Based Electrocatalysts for Oxygen Reduction Reaction: Fundamental Understanding and Design Strategies. Adv. Mater. 2021, 33, 2006494. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Zhao, J.; Fang, H.; Huang, Q.; Xiao, W.; Li, T.; Wang, D. Anchoring Ultrafine Pt Electrocatalysts on TiO2-C via Photochemical Strategy to Enhance the Stability and Efficiency for Oxygen Reduction Reaction. Appl. Catal. B 2018, 237, 228–236. [Google Scholar] [CrossRef]
- Song, Z.; Banis, M.N.; Zhang, L.; Wang, B.; Yang, L.; Banham, D.; Zhao, Y.; Liang, J.; Zheng, M.; Li, R.; et al. Origin of achieving the enhanced activity and stability of Pt electrocatalysts with strong metal-support interactions via atomic layer deposition. Nano Energy 2018, 53, 716–725. [Google Scholar] [CrossRef]
- Fan, J.; Chen, M.; Zhao, Z.; Zhang, Z.; Ye, S.; Xu, S.; Wang, H.; Li, H. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 2021, 6, 475–486. [Google Scholar]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Ding, R.; Wang, R.; Ding, Y.; Yin, W.; Liu, Y.; Li, J.; Liu, J. Designing AI-Aided Analysis and Prediction Models for Nonprecious Metal Electrocatalyst-Based Proton-Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2020, 59, 19175–19183. [Google Scholar] [CrossRef]
- Ding, R.; Ding, Y.; Zhang, H.; Wang, R.; Xu, Z.; Liu, Y.; Yin, W.; Wang, J.; Li, J.; Liu, J. Applying machine learning to boost the development of high-performance membrane electrode assembly for proton exchange membrane fuel cells. J. Mater. Chem. A 2021, 9, 6841–6850. [Google Scholar] [CrossRef]
- Ding, R.; Yin, W.; Cheng, G.; Chen, Y.; Wang, J.; Wang, R.; Rui, Z.; Li, J.; Liu, J. Boosting the optimization of membrane electrode assembly in proton exchange membrane fuel cells guided by explainable artificial intelligence. Energy AI 2021, 5, 100098. [Google Scholar] [CrossRef]
- Ding, R.; Chen, Y.; Chen, P.; Wang, R.; Wang, J.; Ding, Y.; Yin, W.; Liu, Y.; Li, J.; Liu, J. Machine Learning-Guided Discovery of Underlying Decisive Factors and New Mechanisms for the Design of Nonprecious Metal Electrocatalysts. ACS Catal. 2021, 11, 9798–9808. [Google Scholar] [CrossRef]
- Wang, J.; Ding, R.; Cao, F.; Li, J.; Dong, H.; Shi, T.; Xing, L.; Liu, J. Comparison of state-of-the-art machine learning algorithms and data-driven optimization methods for mitigating nitrogen crossover in PEM fuel cells. Chem. Eng. J. 2022, 442, 136064. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, S.; Chen, Y.; Rui, Z.; Hua, K.; Wu, Y.; Li, X.; Duan, X.; Wang, X.; Li, J.; et al. Application of Machine Learning in Optimizing Proton Exchange Membrane Fuel Cells: A Review. Energy AI 2022, 9, 100170. [Google Scholar] [CrossRef]
- Ding, R.; Chen, Y.; Rui, Z.; Hua, K.; Wu, Y.; Li, X.; Duan, X.; Wang, X.; Li, J.; Liu, J. Guiding the Optimization of Membrane Electrode Assembly in a Proton Exchange Membrane Water Electrolyzer by Machine Learning Modeling and Black-Box Interpretation. ACS Sustain. Chem. Eng. 2022, 10, 4561–4578. [Google Scholar] [CrossRef]
- Ding, R.; Ma, M.; Chen, Y.; Wang, X.; Li, J.; Wang, G.; Liu, J. Inspecting design rules of metal-nitrogen-carbon catalysts for electrochemical CO2 reduction reaction: From a data science perspective. Nano Res. 2022. [Google Scholar] [CrossRef]
- Kang, J.; Noh, S.H.; Hwang, J.; Chun, H.; Kim, H.; Han, B. First-principles database driven computational neural network approach to the discovery of active ternary nanocatalysts for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2018, 20, 24539–24544. [Google Scholar] [CrossRef]
- Rück, M.; Garlyyev, B.; Mayr, F.; Bandarenka, A.S.; Gagliardi, A. Oxygen Reduction Activities of Strained Platinum Core–Shell Electrocatalysts Predicted by Machine Learning. J. Phys. Chem. Lett. 2020, 11, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Z.; Li, B.; Ming, P.; Zhang, C. Understanding the functions and modifications of interfaces in membrane electrode assemblies of proton exchange membrane fuel cells. J. Mater. Chem. A 2021, 9, 15111–15139. [Google Scholar] [CrossRef]
- Gong, X.; Ruan, M.; Song, P.; Li, H.; Cao, J.; Yao, P.; Xu, W. A cost-effective and highly efficient dissymmetric membrane electrode assembly designed for fuel cells. J. Power Sources 2021, 489, 229485. [Google Scholar] [CrossRef]
- Gomes Bezerra, C.A.; Deiner, L.J.; Tremiliosi-Filho, G. Unexpected Performance of Inkjet-Printed Membrane Electrode Assemblies for Proton Exchange Membrane Fuel Cells. Adv. Eng. Mater. 2019, 21, 1900703. [Google Scholar] [CrossRef]
- Pollet, B.G. The use of ultrasound for the fabrication of fuel cell materials. Int. J. Hydrogen Energy 2010, 35, 11986–12004. [Google Scholar] [CrossRef]
- Millington, B.; Whipple, V.; Pollet, B.G. A novel method for preparing proton exchange membrane fuel cell electrodes by the ultrasonic-spray technique. J. Power Sources 2011, 196, 8500–8508. [Google Scholar] [CrossRef]
- Devrim, Y.; Erkan, S.; Baç, N.; Eroglu, I. Improvement of PEMFC performance with Nafion/inorganic nanocomposite membrane electrode assembly prepared by ultrasonic coating technique. Int. J. Hydrogen Energy 2012, 37, 16748–16758. [Google Scholar] [CrossRef]
- Huang, T.-H.; Shen, H.-L.; Jao, T.-C.; Weng, F.-B.; Su, A. Ultra-low Pt loading for proton exchange membrane fuel cells by catalyst coating technique with ultrasonic spray coating machine. Int. J. Hydrogen Energy 2012, 37, 13872–13879. [Google Scholar] [CrossRef]
- Gong, Q.; Li, C.; Liu, Y.; Ilavsky, J.; Guo, F.; Cheng, X.; Xie, J. Effects of Ink Formulation on Construction of Catalyst Layers for High-Performance Polymer Electrolyte Membrane Fuel Cells. ACS Appl. Mater. Interfaces 2021, 13, 37004–37013. [Google Scholar] [CrossRef]
- Suzuki, T.; Tsushima, S.; Hirai, S. Effects of Nafion® ionomer and carbon particles on structure formation in a proton-exchange membrane fuel cell catalyst layer fabricated by the decal-transfer method. Int. J. Hydrogen Energy 2011, 36, 12361–12369. [Google Scholar] [CrossRef]
- Lee, M.R.; Lee, H.Y.; Yim, S.D.; Kim, C.S.; Shul, Y.G.; Kucernak, A.; Shin, D. Effects of Ionomer Carbon Ratio and Ionomer Dispersity on the Performance and Durability of MEAs. Fuel Cells 2018, 18, 129–136. [Google Scholar] [CrossRef]
- Yu, H.; Roller, J.M.; Mustain, W.E.; Maric, R. Influence of the ionomer/carbon ratio for low-Pt loading catalyst layer prepared by reactive spray deposition technology. J. Power Sources 2015, 283, 84–94. [Google Scholar] [CrossRef]
- Alink, R.; Singh, R.; Schneider, P.; Christmann, K.; Schall, J.; Keding, R.; Zamel, N. Full Parametric Study of the Influence of Ionomer Content, Catalyst Loading and Catalyst Type on Oxygen and Ion Transport in PEM Fuel Cell Catalyst Layers. Molecules 2020, 25, 1523. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Yu, T.L.; Lin, H.-L.; Liu, W.-H.; Lai, C.-L. Solution properties of nafion in methanol/water mixture solvent. Polymer 2004, 45, 2853–2862. [Google Scholar] [CrossRef]
- Kim, Y.S.; Welch, C.F.; Mack, N.H.; Hjelm, R.P.; Orler, E.B.; Hawley, M.E.; Lee, K.S.; Yim, S.D.; Johnston, C.M. Highly durable fuel cell electrodes based on ionomers dispersed in glycerol. Phys. Chem. Chem. Phys. 2014, 16, 5927–5932. [Google Scholar] [CrossRef]
- Ding, R.; Yin, W.; Cheng, G.; Chen, Y.; Wang, J.; Wang, X.; Han, M.; Zhang, T.; Cao, Y.; Zhao, H.; et al. Effectively Increasing Pt Utilization Efficiency of the Membrane Electrode Assembly in Proton Exchange Membrane Fuel Cells through Multiparameter Optimization Guided by Machine Learning. ACS Appl. Mater. Interfaces 2022, 14, 8010–8024. [Google Scholar] [CrossRef]
- Orfanidi, A.; Rheinländer, P.J.; Schulte, N.; Gasteiger, H.A. Ink Solvent Dependence of the Ionomer Distribution in the Catalyst Layer of a PEMFC. J. Electrochem. Soc. 2018, 165, F1254. [Google Scholar] [CrossRef]
- Yang, F.; Xin, L.; Uzunoglu, A.; Qiu, Y.; Stanciu, L.; Ilavsky, J.; Li, W.; Xie, J. Investigation of the Interaction between Nafion Ionomer and Surface Functionalized Carbon Black Using Both Ultrasmall Angle X-ray Scattering and Cryo-TEM. ACS Appl. Mater. Interfaces 2017, 9, 6530–6538. [Google Scholar] [CrossRef]
- Ngo, T.T.; Yu, T.L.; Lin, H.-L. Influence of the composition of isopropyl alcohol/water mixture solvents in catalyst ink solutions on proton exchange membrane fuel cell performance. J. Power Sources 2013, 225, 293–303. [Google Scholar] [CrossRef]
- Welch, C.; Labouriau, A.; Hjelm, R.; Orler, B.; Johnston, C.; Kim, Y.S. Nafion in Dilute Solvent Systems: Dispersion or Solution? ACS Macro Lett. 2012, 1, 1403–1407. [Google Scholar] [CrossRef]
- Uchida, M.; Aoyama, Y.; Eda, N.; Ohta, A. New Preparation Method for Polymer-Electrolyte Fuel Cells. J. Electrochem. Soc. 1995, 142, 463. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, H.; Ilavsky, J.; Stanciu, L.; Ho, D.; Justice, M.J.; Petrache, H.I.; Xie, J. Investigation of a Catalyst Ink Dispersion Using Both Ultra-Small-Angle X-ray Scattering and Cryogenic TEM. Langmuir 2010, 26, 19199–19208. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kim, B.-S.; Park, J.-S. Effect of ionomer dispersions on the performance of catalyst layers in proton exchange membrane fuel cells. Electrochim. Acta 2022, 424, 140680. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, S.; Shao, P.; Zhu, Y.; Mu, Z.; Sheng, D.; Zhang, T.; Jiang, X.; Shao, R.; Ren, Z.; et al. Covalent organic framework-based porous ionomers for high-performance fuel cells. Science 2022, 378, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Sassin, M.B.; Garsany, Y.; Gould, B.D.; Swider-Lyons, K.E. Fabrication Method for Laboratory-Scale High-Performance Membrane Electrode Assemblies for Fuel Cells. Anal. Chem. 2017, 89, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Middelman, E. Improved PEM fuel cell electrodes by controlled self-assembly. Fuel Cells Bull. 2002, 2002, 9–12. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, H.; Wang, Z.; Jia, J.; Miao, S.; Song, W.; Xiao, Y.; Yu, H.; Shao, Z.; Yi, B. Nano-engineering of a 3D-ordered membrane electrode assembly with ultrathin Pt skin on open-walled PdCo nanotube arrays for fuel cells. J. Mater. Chem. A 2018, 6, 6521–6533. [Google Scholar] [CrossRef]
- Pan, S.; Qin, J.; Ning, F.; Bai, C.; Wen, Q.; Shen, M.; Li, Y.; Song, Y.; Chen, J.; Huang, Y.; et al. Well-Dispersed Nafion Array Prepared by the Freeze-Drying Method to Effectively Improve the Performance of Proton Exchange Membrane Fuel Cells. ACS Sustainable Chem. Eng. 2021, 9, 16770–16777. [Google Scholar] [CrossRef]
- Liu, H.; Qin, J.; Rockward, T.; Wu, J.; Li, J.; Li, G.; Mao, Q.; Lv, Y.; Wang, X.; Zhang, S.; et al. Photo-driven growth of a monolayer of platinum spherical-nanocrowns uniformly coated on a membrane toward fuel cell applications. J. Mater. Chem. A 2020, 8, 23284–23292. [Google Scholar] [CrossRef]
- van der Vliet, D.F.; Wang, C.; Tripkovic, D.; Strmcnik, D.; Zhang, X.F.; Debe, M.K.; Atanasoski, R.T.; Markovic, N.M.; Stamenkovic, V.R. Mesostructured thin films as electrocatalysts with tunable composition and surface morphology. Nat. Mater. 2012, 11, 1051–1058. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Ahn, C.; You, D.J.; Pak, C.; Hur, S.H.; Kim, J.; Joo, S.H. Ordered mesoporous carbon–carbon nanotube nanocomposites as highly conductive and durable cathode catalyst supports for polymer electrolyte fuel cells. J. Mater. Chem. A 2013, 1, 1270–1283. [Google Scholar] [CrossRef]
- Li, M.; Xiong, Y.; Liu, X.; Han, C.; Zhang, Y.; Bo, X.; Guo, L. Iron and nitrogen co-doped carbon nanotube@hollow carbon fibers derived from plant biomass as efficient catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 9658–9667. [Google Scholar] [CrossRef]
- Sun, R.; Xia, Z.; Shang, L.; Fu, X.; Li, H.; Wang, S.; Sun, G. Hierarchically ordered arrays with platinum coated PANI nanowires for highly efficient fuel cell electrodes. J. Mater. Chem. A 2017, 5, 15260–15265. [Google Scholar] [CrossRef]
- Zhao, J.; He, X.; Wang, L.; Tian, J.; Wan, C.; Jiang, C. Addition of NH4HCO3 as pore-former in membrane electrode assembly for PEMFC. Int. J. Hydrogen Energy 2007, 32, 380–384. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Wang, C.; Lei, Y.-J.; Zhang, J.; Mao, Z.; Mao, Z.-Q.; Guo, J.-W.; Li, J.; Ouyang, M. Gradient design of Pt/C ratio and Nafion content in cathode catalyst layer of PEMFCs. Int. J. Hydrogen Energy 2017, 42, 29960–29965. [Google Scholar] [CrossRef]
- Ye, L.; Gao, Y.; Zhu, S.; Zheng, J.; Li, P.; Zheng, J.P. A Pt content and pore structure gradient distributed catalyst layer to improve the PEMFC performance. Int. J. Hydrogen Energy 2017, 42, 7241–7245. [Google Scholar] [CrossRef]
- Xie, Z.; Navessin, T.; Shi, K.; Chow, R.; Wang, Q.; Song, D.; Andreaus, B.; Eikerling, M.; Liu, Z.; Holdcroft, S. Functionally Graded Cathode Catalyst Layers for Polymer Electrolyte Fuel Cells: II. Experimental Study of the Effect of Nafion Distribution. J. Electrochem. Soc. 2005, 152, A1171. [Google Scholar] [CrossRef]
- Zheng, Z.; Yang, F.; Lin, C.; Zhu, F.; Shen, S.; Wei, G.; Zhang, J. Design of gradient cathode catalyst layer (CCL) structure for mitigating Pt degradation in proton exchange membrane fuel cells (PEMFCs) using mathematical method. J. Power Sources 2020, 451, 227729. [Google Scholar] [CrossRef]
- Ott, S.; Orfanidi, A.; Schmies, H.; Anke, B.; Nong, H.N.; Hübner, J.; Gernert, U.; Gliech, M.; Lerch, M.; Strasser, P. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells. Nat. Mater. 2020, 19, 77–85. [Google Scholar] [CrossRef]
- Sun, Y.; Polani, S.; Luo, F.; Ott, S.; Strasser, P.; Dionigi, F. Advancements in cathode catalyst and cathode layer design for proton exchange membrane fuel cells. Nat. Commun. 2021, 12, 5984. [Google Scholar] [CrossRef]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Sandbeck, D.J.S.; Inaba, M.; Quinson, J.; Bucher, J.; Zana, A.; Arenz, M.; Cherevko, S. Particle Size Effect on Platinum Dissolution: Practical Considerations for Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 25718–25727. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yano, H.; Uchida, H.; Tryk, D.A. Achievement of distinctively high durability at nanosized Pt catalysts supported on carbon black for fuel cell cathodes. J. Electroanal. Chem. 2018, 819, 359–364. [Google Scholar] [CrossRef]
- Ding, R.; Liu, Y.; Rui, Z.; Li, J.; Liu, J.; Zou, Z. Facile grafting strategy synthesis of single-atom electrocatalyst with enhanced ORR performance. Nano Res. 2020, 13, 1519–1526. [Google Scholar] [CrossRef]
- Li, J.; Zhu, X.; Wang, J.; Rui, Z.; Zhang, S.; Li, Y.; Ding, R.; He, W.; Liu, J.; Zou, Z. Iron-Containing Porphyrins Self-Assembled on ZnO Nanoparticles as Electrocatalytic Materials for Oxygen Reduction. ACS Appl. Nano Mater. 2020, 3, 742–751. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, M.; Li, X.; Ding, R.; Duan, X.; Li, J.; Wang, Y.; Li, X.; Zhang, Y.; Liu, J. Assistance of rearrangement of active sites in Fe/N/C catalyst for harvesting ultra-high power density PEMFCs. Appl. Catal., B 2022, 312, 121365. [Google Scholar] [CrossRef]
- Wei, H.; Su, X.; Liu, J.; Tian, J.; Wang, Z.; Sun, K.; Rui, Z.; Yang, W.; Zou, Z. A CeO2 modified phenylenediamine-based Fe/N/C with enhanced durability/stability as non-precious metal catalyst for oxygen reduction reaction. Electrochem. Commun. 2018, 88, 19–23. [Google Scholar] [CrossRef]
- Osmieri, L.; Meyer, Q. Recent advances in integrating platinum group metal-free catalysts in proton exchange membrane fuel cells. Curr. Opin. Electrochem. 2022, 31, 100847. [Google Scholar] [CrossRef]
- Kiani, M.; Tian, X.Q.; Zhang, W. Non-precious metal electrocatalysts design for oxygen reduction reaction in polymer electrolyte membrane fuel cells: Recent advances, challenges and future perspectives. Coord. Chem. Rev. 2021, 441, 213954. [Google Scholar] [CrossRef]
- Chong, L.; Wen, J.; Kubal, J.; Sen, F.G.; Zou, J.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.; Liu, D.-J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281. [Google Scholar] [CrossRef]
- Wang, X.X.; Hwang, S.; Pan, Y.-T.; Chen, K.; He, Y.; Karakalos, S.; Zhang, H.; Spendelow, J.S.; Su, D.; Wu, G. Ordered Pt3Co Intermetallic Nanoparticles Derived from Metal–Organic Frameworks for Oxygen Reduction. Nano Lett. 2018, 18, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wei, M.; Qi, R.; Dong, C.-L.; Dang, D.; Yang, C.-C.; Xia, C.; Chen, C.; Zaman, S.; Li, F.-M.; et al. An integrated platinum-nanocarbon electrocatalyst for efficient oxygen reduction. Nat. Commun. 2022, 13, 6703. [Google Scholar] [CrossRef]
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.R.; Caulk, D.A.; Neyerlin, K.C.; Murphy, M.W. Measurement of Oxygen Transport Resistance in PEM Fuel Cells by Limiting Current Methods. J. Electrochem. Soc. 2009, 156, B991. [Google Scholar] [CrossRef]
- Cheng, X.; Shen, S.; Wei, G.; Wang, C.; Luo, L.; Zhang, J. Perspectives on Challenges and Achievements in Local Oxygen Transport of Low Pt Proton Exchange Membrane Fuel Cells. Adv. Mater. Technol. 2022, 7, 2200228. [Google Scholar] [CrossRef]
- Greszler, T.A.; Caulk, D.; Sinha, P. The Impact of Platinum Loading on Oxygen Transport Resistance. J. Electrochem. Soc. 2012, 159, F831–F840. [Google Scholar] [CrossRef]
- Duclos, L.; Lupsea, M.; Mandil, G.; Svecova, L.; Thivel, P.-X.; Laforest, V. Environmental assessment of proton exchange membrane fuel cell platinum catalyst recycling. J. Cleaner Prod. 2017, 142, 2618–2628. [Google Scholar] [CrossRef]
- Duclos, L.; Svecova, L.; Laforest, V.; Mandil, G.; Thivel, P.X. Process development and optimization for platinum recovery from PEM fuel cell catalyst. Hydrometallurgy 2016, 160, 79–89. [Google Scholar] [CrossRef]

| Catalyst | Specific Activity (mA cm−2) | Mass Activity (A mgPt−1) | Electrochemically Active Surface Area (m2 gPt−1) | Accelerated Durability Test | Mass Activity Retention | Electrolyte | Reference |
|---|---|---|---|---|---|---|---|
| Au– Pt–Co/C-0.015 | 0.535 | 0.386 | 72.2 | 0.6–1.0 V 30 k cycles | 90.6% | 0.1 M HClO4 | [14] |
| Pt3Co/NC | 1.236 | 0.382 | 31.0 | 0.6–1.0 V 30 k cycles | 104.5% | 0.1 M HClO4 | [15] |
| Pt–Ni–Au NWs | 2.59 | 0.651 | 25.1 | 0.6–1.0 V 30 k cycles | 82.3% | 0.1 M HClO4 | [16] |
| 700- Pt1Co1-IMC@Pt/C-2.5 | 1.11 | 0.53 | 43.5 | 0.6–1.1 V 30 k cycles | 76.6% | 0.1 M HClO4 | [17] |
| Pt/TiN@C | 0.155 | 69.2 | 1.2 V constant for 400 h | 95.9% | 0.1 M HClO4 | [18] | |
| Ni4Co2Pt/CNFs Ni6Pt/CNFs | NA | 35.7 A/g @ 799 mV for ethanol 37.4 A/g @ 799 mV for ethanol | NA | 0.8 V constant chronoamperometry | 57.4% (1000 s for methanol) 70.8% (1000 s for urea) | 1 M KOH | [27] |
| Pt3Co NWs/C | 7.12 | 3.71 | 52.1 | 0.6–1.1 V 20 k cycles | 91.9% | 0.1 M HClO4 | [29] |
| PtNi BNCs/C | 5.16 | 3.52 | 68.2 | 0.6–1.1 V 50 k cycles | 98.7% | 0.1 M HClO4 | [30] |
| PtCu3 | 3.8 | 4.18 | 0.1 M HClO4 | [31] | |||
| Co-doped Pt | 0.498 | 0.579 | 116.2 | 0.6–1.0 V 30 k cycles | 91.0% | 0.1 M HClO4 | [32] |
| PtCo | 3.21 | 2.25 | 70.1 | 0.1 M HClO4 | [37] | ||
| L10-W-PtCo/C | 3.60 | 2.21 | 61.4 | 0.6–1.0 V 10 k cycles | 86.4% | 0.1 M HClO4 | [38] |
| PtGa NWs/ C | 3.28 | 1.89 | 53.4 | 0.6–1.1 V 30 k cycles | 84.2% | 0.1 M HClO4 | [48] |
| PtCo@Gnp | 1.62 | 1.19 | 68.7 | 0.1 M HClO4 | [49] | ||
| Pt/N-ALDTa2O5/C | 0.28 | 70.3 | 0.6–1.0 V 10 k cycles | 90% | 0.1 M HClO4 | [50] | |
| Pt2.5Ni/C | 7.3 | 3.3 | 45 | 0.65 V (5 s) 0.95 V (5 s) 5 k cycles | 60% | 0.1 M HClO4 | [55] |
| PtNiCo/C | 3.88 | 2.33 | 61.6 | 0.6–1.1 V 6 k cycles | 43.3% | 0.1 M HClO4 | [56] |
| Pd@Pt1.8Ni | 0.79 | 178.01 | 0.6–1.05 V 6 k cycles | NA (MA retention) 90% (ECSA retention) | 0.1 M HClO4 | [57] | |
| Pt-skin Pt3Fe z-NWs/C | 4.34 | 2.11 | 34.0 | 0.6–1.1 V 50 k cycles | 75.4% | 0.1 M HClO4 | [60] |
| PtCo/TiO2/CNT | 0.628 | 0.476 | 75.8 | 0.6–1.0 V 30 k cycles | 88.8% | 0.1 M HClO4 | [62] |
| TiNiN@Pt | 0.49 | 0.83 | 55.4 | 0.6–1.05 V 10 k cycles | 91.6% | 0.1 M HClO4 | [63] |
| Pt/TiO2-C | 0.21 | 81.7 | 0.6–1.0 V 10 k cycles | 99.1% | 0.1 M HClO4 | [69] |
| Catalyst | Cathode Pt Loading (mgPt cm−2) | Test Conditions | Peak Power Density (W cm−2) | Area (cm2) | Mass Activity (A mgPt−1) | Accelerated Durability Test | Mass Activity Retention | Reference |
|---|---|---|---|---|---|---|---|---|
| Pt–Ni–Au NWs | 0.1 | H2/O2 200/200 sccm 100 kPaabs | 0.714 A cm−2 @ 0.6 V | 1 | 0.6 V (3 s) 0.95 V (3 s) 10 k cycles | 0.520 A cm−2 @ 0.6 V | [16] | |
| Pt1Co1- IMC@Pt/C | 0.2 | H2/O2 1000/400 sccm 100 kPaabs | 1.45 A cm−2 @ 0.65 V | 6.25 | 0.18 | 0.6 V (3 s) 0.95 V (3 s) 30 k cycles | 75.2% | [17] |
| PtNi BNCs/C | 0.15 | H2/Air 150/300 sccm 30 psi | 1.5 A cm−2 @ 0.6 V | 5 | [30] | |||
| PtCo | 0.02 | H2/O2 200/200 sccm 150 kPaabs | 5 | 1.52 | 0.6–1.0 V 30 k cycles | 77% | [31] | |
| PtCo | 0.125 | H2/Air 250 kPaabs | 1.17 | 1.08 | 0.6 V (3 s) 0.95 V (3 s) 30 k cycles | 75% | [37] | |
| L10-W-PtCo/C | 0.11 | H2/O2 200/500 sccm 1.5 kPaabs | 0.57 | 0.6–1.0 V 30 k cycles | 82.5% | [38] | ||
| PtCo@Gnp | 0.07 | H2/O2 835/2000 sccm 150 kPaabs | 1.01 | 5 | 1.21 | 0.6 V (3 s) 0.95 V (3 s) 30 k cycles | 73% | [49] |
| Sigracet SGL 10BC GDL | 0.15 | H2/O2 (stoichiometry 1.3/2.2) 200 kPaabs | 0.53 | 16 | [86] | |||
| Pt/C@SDT-Nafion | 0.07 | H2/Air 150 kPaabs | 1.08 | 5 | 0.35 | 0.6 V (3 s) 0.95 V (3 s) 30 k cycles | 62% | [90] |
| RA1 MEA | H2/Air (stoichiometry 1.5/3) 100 kPaabs | 1.3 A cm−2 @ 0.6 V | 0.6–1.0 V 30 k cycles | 54.3% (ECSA retention) | [93] | |||
| Pt/Vulcan (Cabot) | 0.07 | H2/Air 500/1000 sccm | 0.91 | 25 | 0.23 | [94] | ||
| Ptskin@PdCo NTAs-400 | 3.5 μgPt cm−2 | H2/O2 100/200 sccm 200 kPaabs | 0.78 | 2.56 | 0.6–1.0 V 5 k cycles | 63.5% (power density retention) | [107] | |
| Pt/N-KB | 0.11 | H2/Air 1000/2000 sccm 230 kPaabs | 1.39 | 1.4 | 0.202 | 0.6–1.0 V 1.5 k cycles | 98.5% | [115] |
| Gradient design MEA | 0.2 | H2/Air stoichiometry 1.5/2.5 100 kPaabs | 0.69 | 25 | [117] | |||
| Gradient design MEA | 0.09 | H2/Air 100 kPaabs | 0.48 | 6.25 | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, F.; Ding, R.; Rui, Z.; Wang, X.; Meng, Z.; Zhang, B.; Dong, W.; Li, J.; Liu, J.; Jiang, X. Advances in Low Pt Loading Membrane Electrode Assembly for Proton Exchange Membrane Fuel Cells. Molecules 2023, 28, 773. https://doi.org/10.3390/molecules28020773
Cao F, Ding R, Rui Z, Wang X, Meng Z, Zhang B, Dong W, Li J, Liu J, Jiang X. Advances in Low Pt Loading Membrane Electrode Assembly for Proton Exchange Membrane Fuel Cells. Molecules. 2023; 28(2):773. https://doi.org/10.3390/molecules28020773
Chicago/Turabian StyleCao, Feng, Rui Ding, Zhiyan Rui, Xuebin Wang, Zhen Meng, Bin Zhang, Weiwen Dong, Jia Li, Jianguo Liu, and Xiangfen Jiang. 2023. "Advances in Low Pt Loading Membrane Electrode Assembly for Proton Exchange Membrane Fuel Cells" Molecules 28, no. 2: 773. https://doi.org/10.3390/molecules28020773
APA StyleCao, F., Ding, R., Rui, Z., Wang, X., Meng, Z., Zhang, B., Dong, W., Li, J., Liu, J., & Jiang, X. (2023). Advances in Low Pt Loading Membrane Electrode Assembly for Proton Exchange Membrane Fuel Cells. Molecules, 28(2), 773. https://doi.org/10.3390/molecules28020773








