Realizing Scalable Nano-SiO2-Aerogel-Reinforced Composite Polymer Electrolytes with High Ionic Conductivity via Rheology-Tuning UV Polymerization
Abstract
1. Introduction
2. Results and Discussion
2.1. Rheology-Tuning UV Polymerization
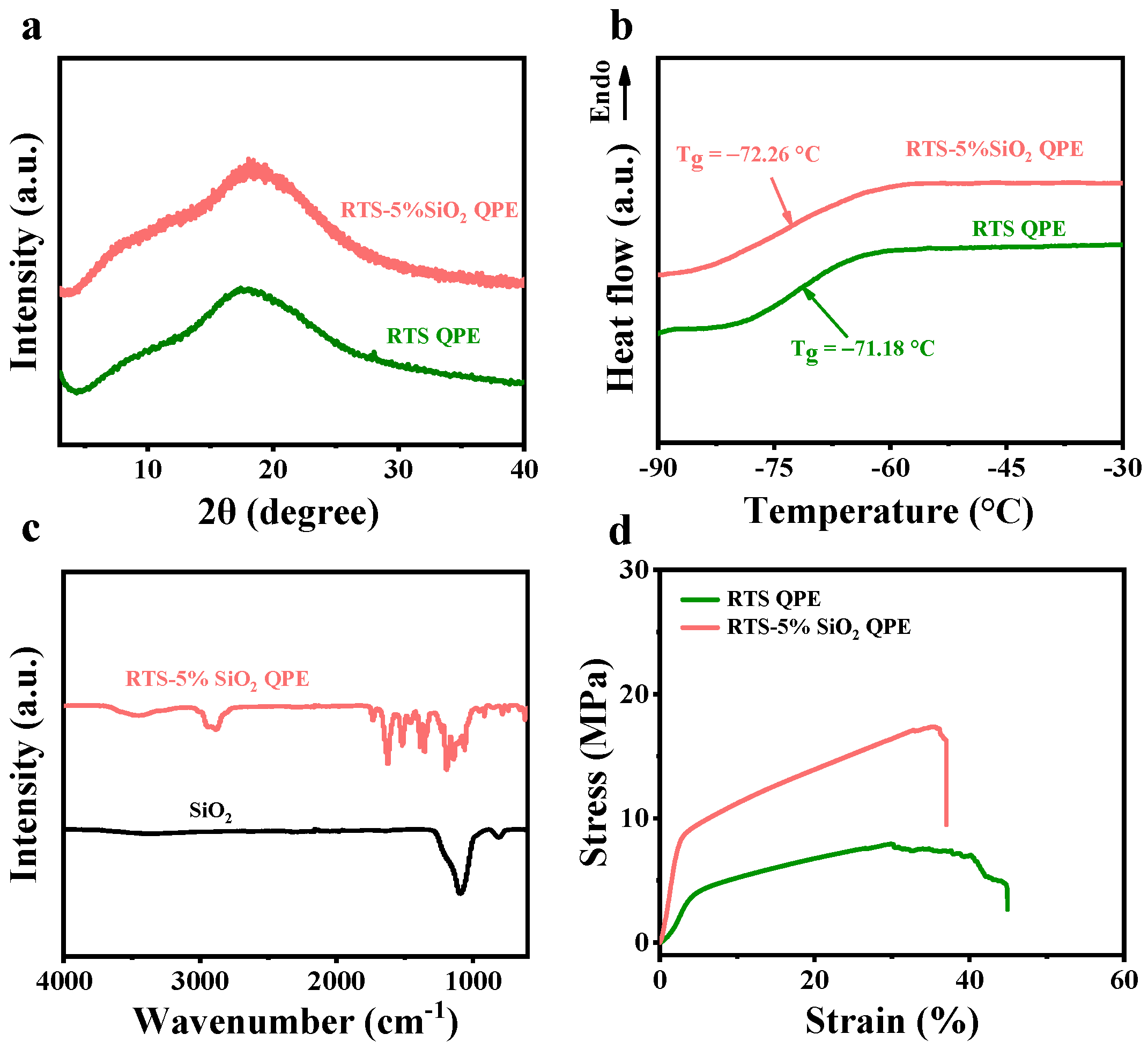
2.2. Physicochemical Characterization
2.3. Electrochemical Behaviors of RTS-5% SiO2 QPE
2.4. Electrochemical Performance of RTS-5%-SiO2-QPE-Based FullCells
3. Materials and Methods
3.1. Preparation of RTS Recipe Slurry
3.2. Preparation of SiO2-Modified Quasi-Solid Polymer Electrolyte Membrane
3.2.1. RTS-5% SiO2 Recipe Slurry
3.2.2. RTS-5% SiO2 QPE
3.3. Preparation of LiFePO4 Cathode
3.4. Physical Characterization
3.5. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Goodenough, J.B. Electrochemical energy storage in a sustainable modern society. Energy Environ. Sci. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, e1800561. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, J.; Liu, D.-s.; Ye, M.; Zhang, Y.; Qin, Y.; Li, C.C. Suppressing vanadium dissolution by modulating aqueous electrolyte structure for ultralong lifespan zinc ion batteries at low current density. Energy Storage Mater. 2022, 49, 93–101. [Google Scholar] [CrossRef]
- Liao, H.; Chen, H.; Zhou, F.; Zhang, Z. A novel SiO2 nanofiber-supported organic–inorganic gel polymer electrolyte for dendrite-free lithium metal batteries. J. Mater. Sci. 2020, 55, 9504–9515. [Google Scholar] [CrossRef]
- Liu, W.; Lin, D.; Sun, J.; Zhou, G.; Cui, Y. Improved Lithium Ionic Conductivity in Composite Polymer Electrolytes with Oxide-Ion Conducting Nanowires. ACS Nano 2016, 10, 11407–11413. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, R.; Jia, D.; Cui, Y.; Liu, Q.; Liu, S.; Wu, D. Ultrathin Yet Robust Single Lithium-Ion Conducting Quasi-Solid-State Polymer-Brush Electrolytes Enable Ultralong-Life and Dendrite-Free Lithium-Metal Batteries. Adv. Mater. 2021, 33, e2100943. [Google Scholar] [CrossRef]
- Lin, D.; Yuen, P.Y.; Liu, Y.; Liu, W.; Liu, N.; Dauskardt, R.H.; Cui, Y. A Silica-Aerogel-Reinforced Composite Polymer Electrolyte with High Ionic Conductivity and High Modulus. Adv. Mater. 2018, 30, e1802661. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Yang, K.; Wang, H.; Yu, C.; Xu, D.; Xu, B.; Wang, L.M. Superior Blends Solid Polymer Electrolyte with Integrated Hierarchical Architectures for All-Solid-State Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 36886–36896. [Google Scholar] [CrossRef]
- Xue, Z.G.; He, D.; Xie, X.L. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Q.; Zhao, C.; Gao, X.; Adair, K.R.; Liu, Y.; Luo, J.; Lin, X.; Liang, J.; Huang, H.; et al. High-areal-capacity all-solid-state lithium batteries enabled by rational design of fast ion transport channels in vertically-aligned composite polymer electrodes. Nano Energy 2019, 61, 567–575. [Google Scholar] [CrossRef]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in Solid Polymer Electrolytes for Lithium-Ion Batteries and Beyond. Small 2022, 18, e2103617. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Fan, L.Z.; Hu, Y.S.; Bhattacharyya, A.J.; Maier, J. Succinonitrile as a Versatile Additive for Polymer Electrolytes. Adv. Funct. Mater. 2007, 17, 2800–2807. [Google Scholar] [CrossRef]
- Guan, T.; Rong, Z.; Cheng, F.; Zhang, W.; Chen, J. UV-Cured Interpenetrating Networks of Single-ion Conducting Polymer Electrolytes for Rechargeable Lithium Metal Batteries. ACS Appl. Energy Mater. 2020, 3, 12532–12539. [Google Scholar] [CrossRef]
- Ju, S.H.; Lee, Y.-S.; Sun, Y.-K.; Kim, D.-W. Unique core–shell structured SiO2(Li+) nanoparticles for high-performance composite polymer electrolytes. J. Mater. Chem. A 2013, 1, 395–401. [Google Scholar] [CrossRef]
- Huang, H.; Ding, F.; Zhong, H.; Li, H.; Zhang, W.; Liu, X.; Xu, Q. Nano-SiO2-embedded poly(propylene carbonate)-based composite gel polymer electrolyte for lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 9539–9549. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, L.; Lin, Z.; Tang, M.; Ding, P.; Guo, X.; Zhang, Z.; Liu, S.; Wang, B.; Yin, X.; et al. Hydrogen bonds enhanced composite polymer electrolyte for high-voltage cathode of solid-state lithium battery. Nano Energy 2022, 96, 107105. [Google Scholar] [CrossRef]
- Shin, W.-K.; Yoo, J.H.; Choi, W.; Chung, K.Y.; Jang, S.S.; Kim, D.-W. Cycling performance of lithium-ion polymer cells assembled with a cross-linked composite polymer electrolyte using a fibrous polyacrylonitrile membrane and vinyl-functionalized SiO2 nanoparticles. J. Mater. Chem. A 2015, 3, 12163–12170. [Google Scholar] [CrossRef]
- Li, Z.; Xie, H.-X.; Zhang, X.-Y.; Guo, X. In situ thermally polymerized solid composite electrolytes with a broad electrochemical window for all-solid-state lithium metal batteries. J. Mater. Chem. A 2020, 8, 3892–3900. [Google Scholar] [CrossRef]
- Xu, H.; Ye, W.; Wang, Q.; Han, B.; Wang, J.; Wang, C.; Deng, Y. An in situ photopolymerized composite solid electrolyte from halloysite nanotubes and comb-like polycaprolactone for high voltage lithium metal batteries. J. Mater. Chem. A 2021, 9, 9826–9836. [Google Scholar] [CrossRef]
- Qi, S.; Li, S.; Zou, W.; Zhang, W.; Wang, X.; Du, L.; Liu, S.; Zhao, J. Enabling Scalable Polymer Electrolyte with Synergetic Ion Conductive Channels via a Two Stage Rheology Tuning UV Polymerization Strategy. Small 2022, 18, e2202013. [Google Scholar] [CrossRef]
- Li, W.-l.; Tang, J.-j.; Li, B.-t. Preparation and Characterization of Composite Microporous Gel Polymer Electrolytes Containing SiO2(Li+). J. Inorg. Organomet. Polym. Mater. 2013, 23, 831–838. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Ju, S.H.; Kim, J.-H.; Hwang, S.S.; Choi, J.-M.; Sun, Y.-K.; Kim, H.; Scrosati, B.; Kim, D.-W. Composite gel polymer electrolytes containing core-shell structured SiO2(Li+) particles for lithium-ion polymer batteries. Electrochem. Commun. 2012, 17, 18–21. [Google Scholar] [CrossRef]
- Shin, W.K.; Cho, J.; Kannan, A.G.; Lee, Y.S.; Kim, D.W. Cross-linked Composite Gel Polymer Electrolyte using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries. Sci. Rep. 2016, 6, 26332. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Ba, D.; Zhao, Y.; Ye, Y.; Li, Y.; Liu, J. Filler-Integrated Composite Polymer Electrolyte for Solid-State Lithium Batteries. Adv. Mater. 2022, 2110423. [Google Scholar] [CrossRef] [PubMed]
- Nagajothi, A.J.; Kannan, R.; Rajashabala, S. Lithium ion conduction in plasticizer based composite gel polymer electrolytes with the addition of SiO2. Mater. Res. Innov. 2017, 22, 226–230. [Google Scholar] [CrossRef]
- Yuan, B.; Luo, G.; Liang, J.; Cheng, F.; Zhang, W.; Chen, J. Self-assembly synthesis of solid polymer electrolyte with carbonate terminated poly(ethylene glycol) matrix and its application for solid state lithium battery. J. Energy Chem. 2019, 38, 55–59. [Google Scholar] [CrossRef]
- Pei, D.; Ma, R.; Yang, G.; Li, Y.; Huang, C.; Liu, Z.; Huang, S.; Cao, G.; Jin, H. Enhanced ion transport behaviors in composite polymer electrolyte: The case of a looser chain folding structure. J. Mater. Chem. A 2022, 10, 3226–3232. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Liu, D.; Gao, Y.; Wang, Y.; Bu, H.; Li, M.; Zhang, Y.; Gao, G.; Ding, S. A composite solid polymer electrolyte incorporating MnO2 nanosheets with reinforced mechanical properties and electrochemical stability for lithium metal batteries. J. Mater. Chem. A 2019, 8, 2021–2032. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Hsiang, S.-H.; Tsao, C.-H.; Teng, H.; Hou, S.-S.; Jan, J.-S. In situ formation of polymer electrolytes using a dicationic imidazolium cross-linker for high-performance lithium ion batteries. J. Mater. Chem. A 2021, 9, 5796–5806. [Google Scholar] [CrossRef]
- Atik, J.; Diddens, D.; Thienenkamp, J.H.; Brunklaus, G.; Winter, M.; Paillard, E. Cation-Assisted Lithium-Ion Transport for High-Performance PEO-based Ternary Solid Polymer Electrolytes. Angew. Chem. Int. Ed. 2021, 60, 11919–11927. [Google Scholar] [CrossRef]
- Porcarelli, L.; Shaplov, A.S.; Bella, F.; Nair, J.R.; Mecerreyes, D.; Gerbaldi, C. Single-Ion Conducting Polymer Electrolytes for Lithium Metal Polymer Batteries that Operate at Ambient Temperature. ACS Energy Lett. 2016, 1, 678–682. [Google Scholar] [CrossRef]
- Huo, H.; Zhao, N.; Sun, J.; Du, F.; Li, Y.; Guo, X. Composite electrolytes of polyethylene oxides/garnets interfacially wetted by ionic liquid for room-temperature solid-state lithium battery. J. Power Sources 2017, 372, 1–7. [Google Scholar] [CrossRef]




| RTS QPE | RTS-5% SiO2 QPE | |
|---|---|---|
| Tensile strength (MPa) | 7.29 | 17.38 |
| Breaking elongation (%) | 40.93 | 37.03 |
| Maximum load (MPa) | 9.01 | 23.47 |
| Elastic modulus (MPa) | 60 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Qi, S.; Li, S.; Du, L. Realizing Scalable Nano-SiO2-Aerogel-Reinforced Composite Polymer Electrolytes with High Ionic Conductivity via Rheology-Tuning UV Polymerization. Molecules 2023, 28, 756. https://doi.org/10.3390/molecules28020756
Li M, Qi S, Li S, Du L. Realizing Scalable Nano-SiO2-Aerogel-Reinforced Composite Polymer Electrolytes with High Ionic Conductivity via Rheology-Tuning UV Polymerization. Molecules. 2023; 28(2):756. https://doi.org/10.3390/molecules28020756
Chicago/Turabian StyleLi, Mianrui, Shengguang Qi, Shulian Li, and Li Du. 2023. "Realizing Scalable Nano-SiO2-Aerogel-Reinforced Composite Polymer Electrolytes with High Ionic Conductivity via Rheology-Tuning UV Polymerization" Molecules 28, no. 2: 756. https://doi.org/10.3390/molecules28020756
APA StyleLi, M., Qi, S., Li, S., & Du, L. (2023). Realizing Scalable Nano-SiO2-Aerogel-Reinforced Composite Polymer Electrolytes with High Ionic Conductivity via Rheology-Tuning UV Polymerization. Molecules, 28(2), 756. https://doi.org/10.3390/molecules28020756





