Small Molecule Drugs That Inhibit Phagocytosis
Abstract
1. Introduction
2. Results
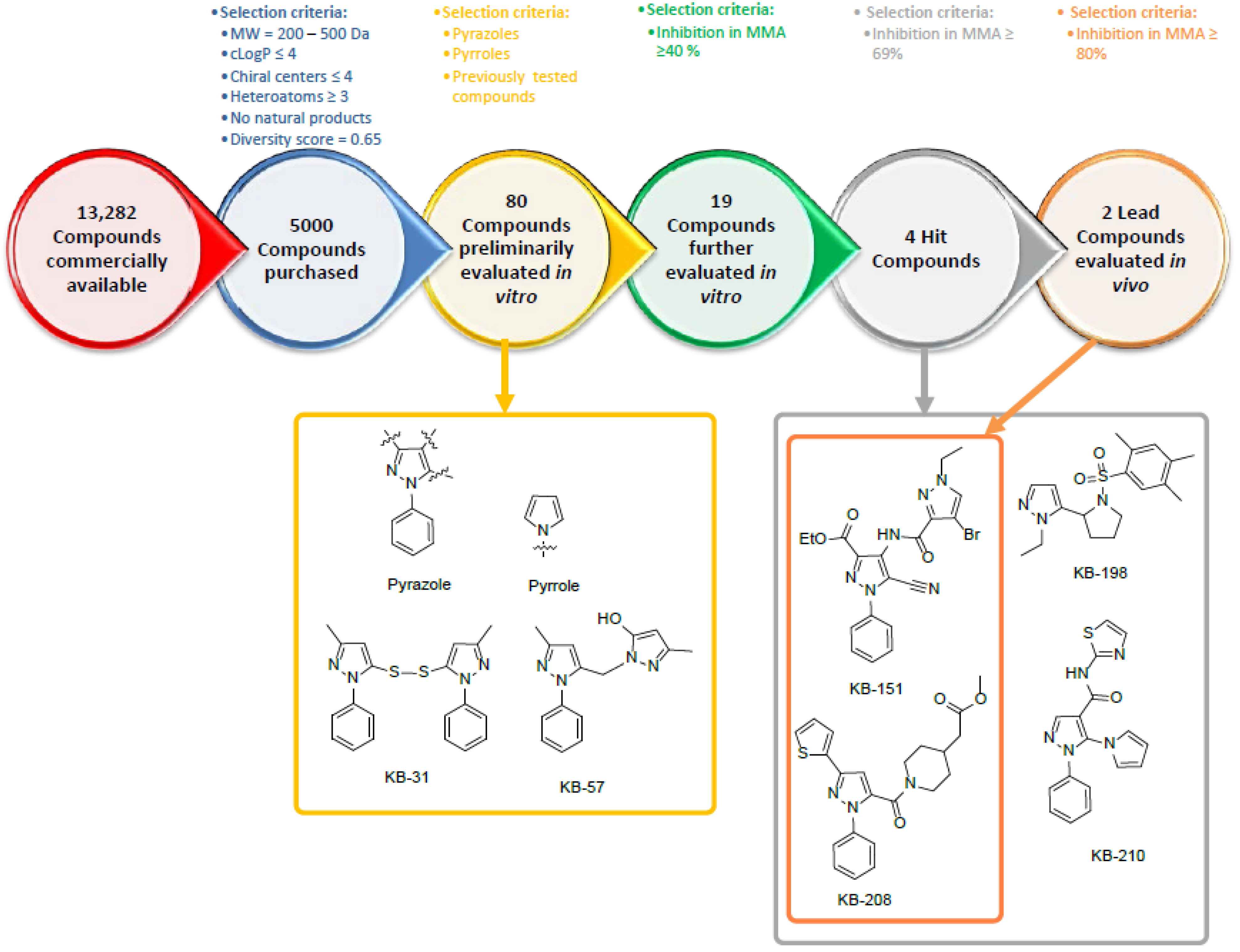
2.1. Selection of Compounds for In Vitro Screening
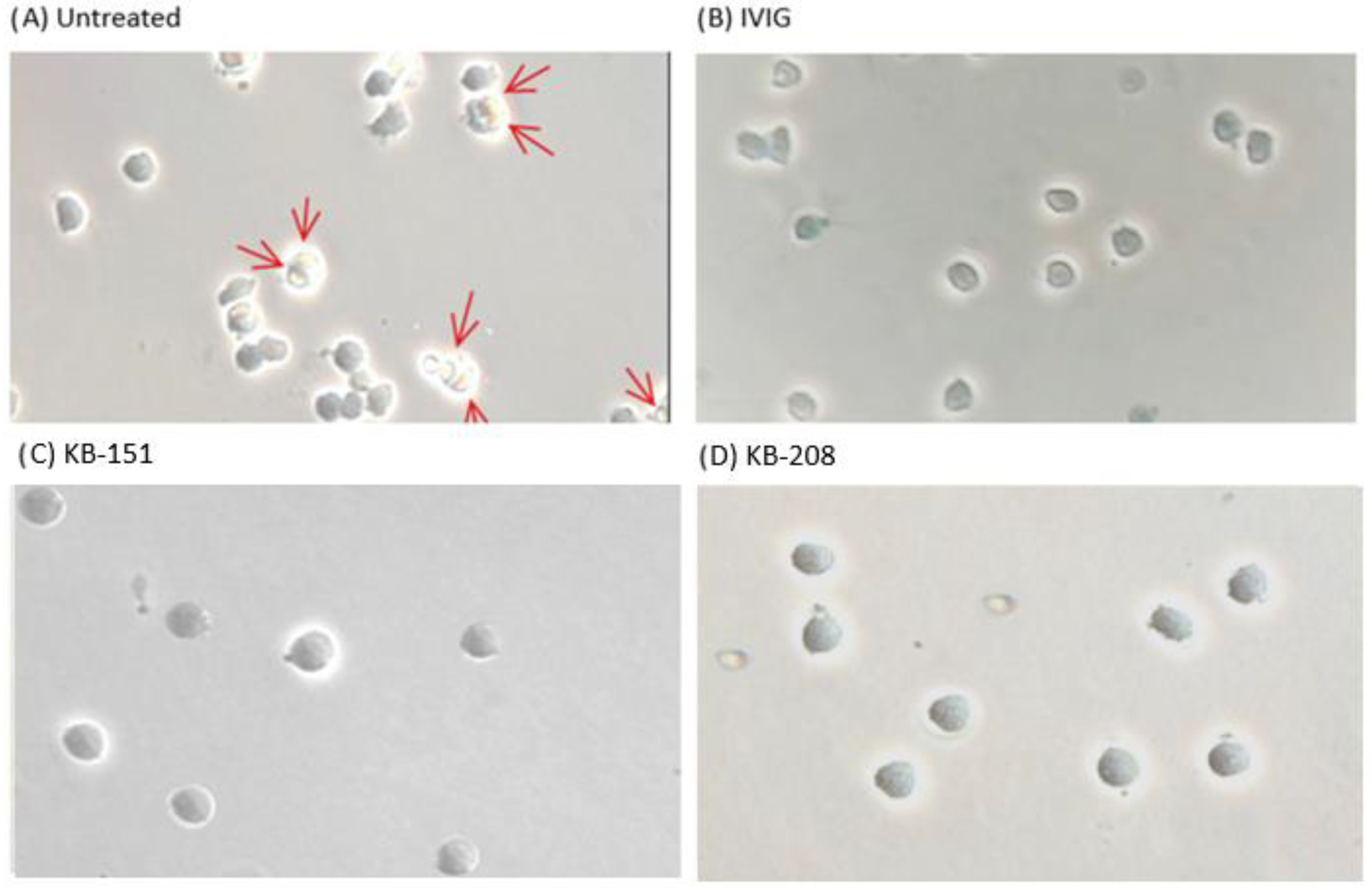
2.2. Monocyte Monolayer Assay (MMA)
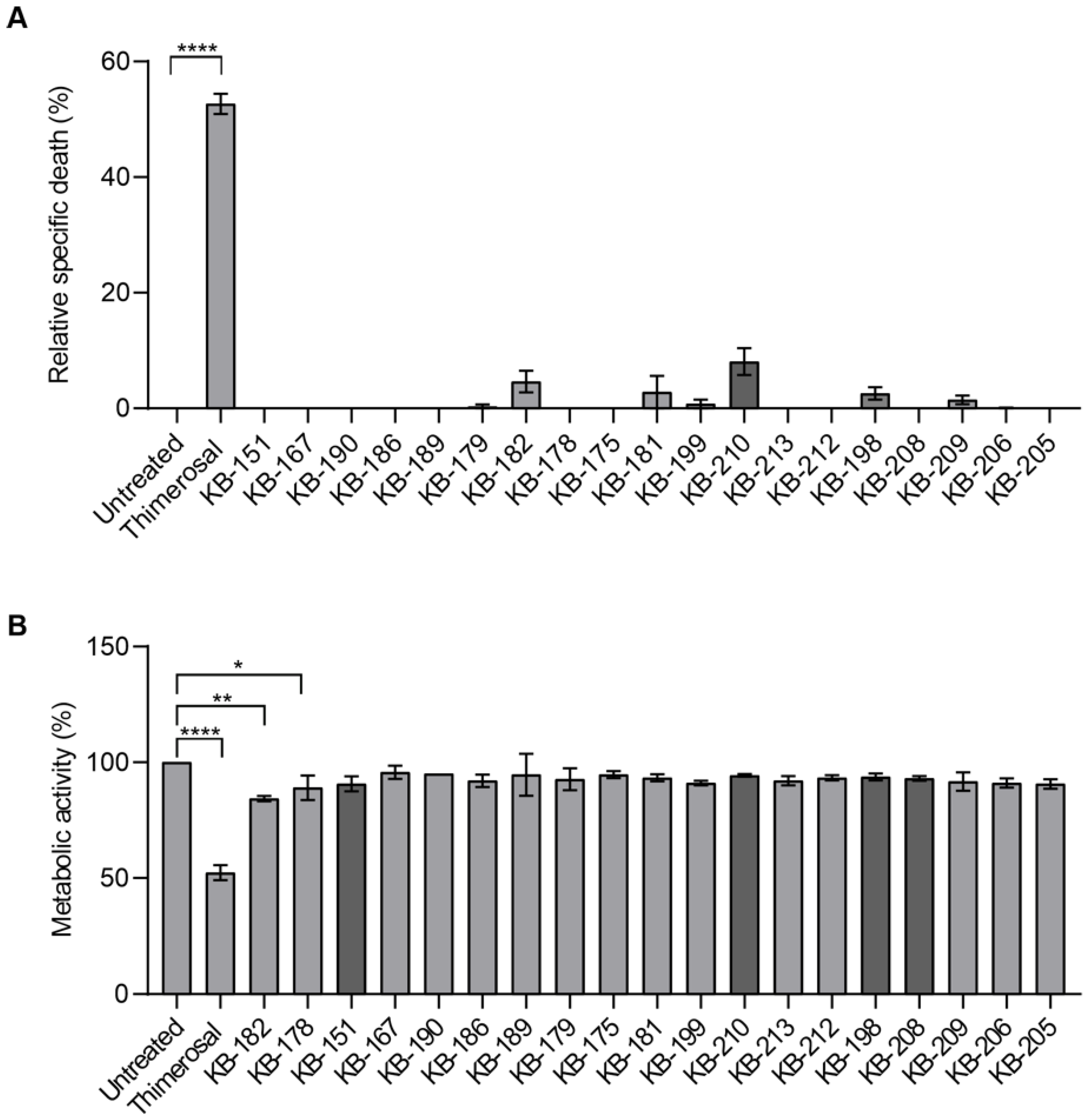
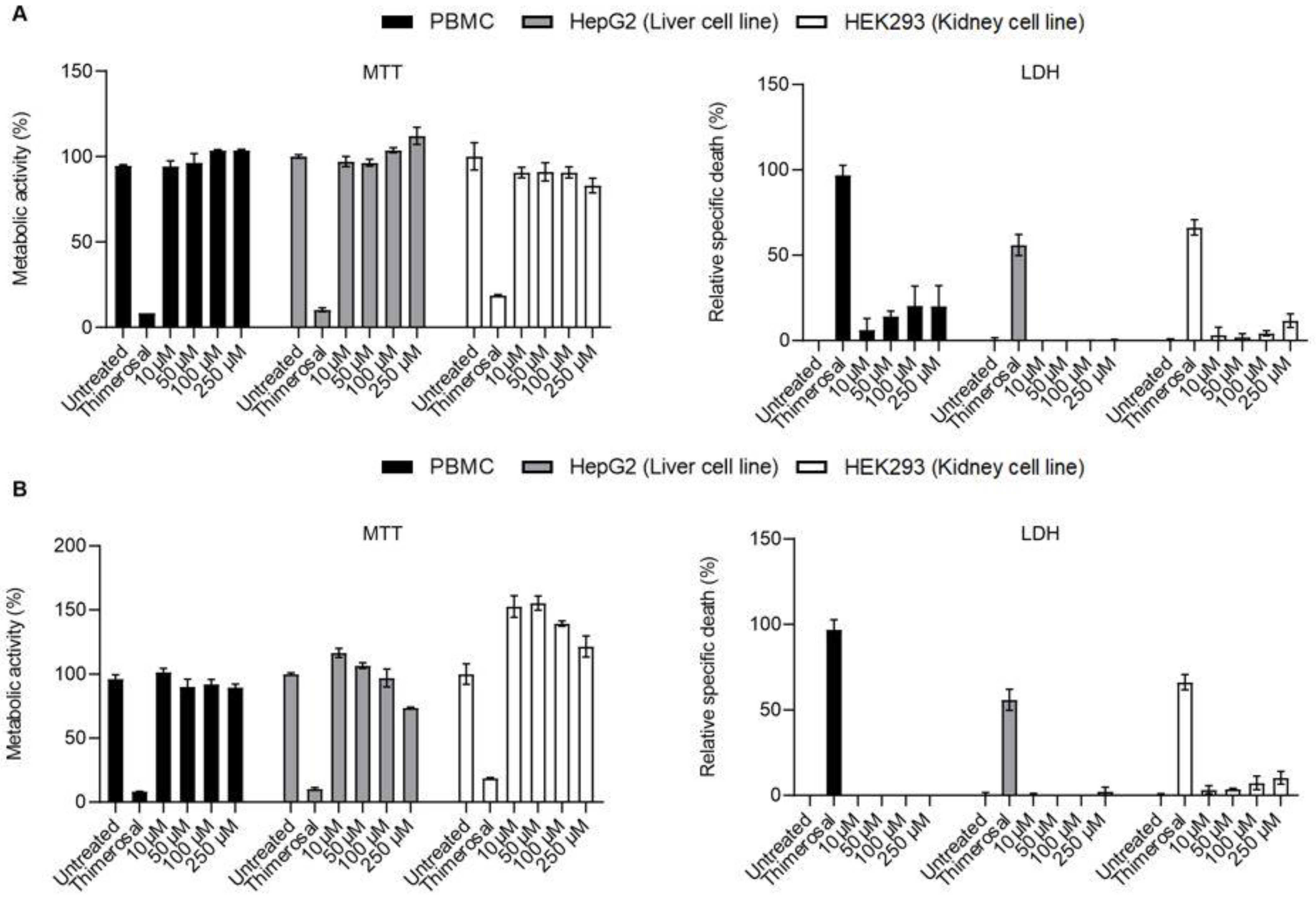
2.3. LDH Release and MTT Assays
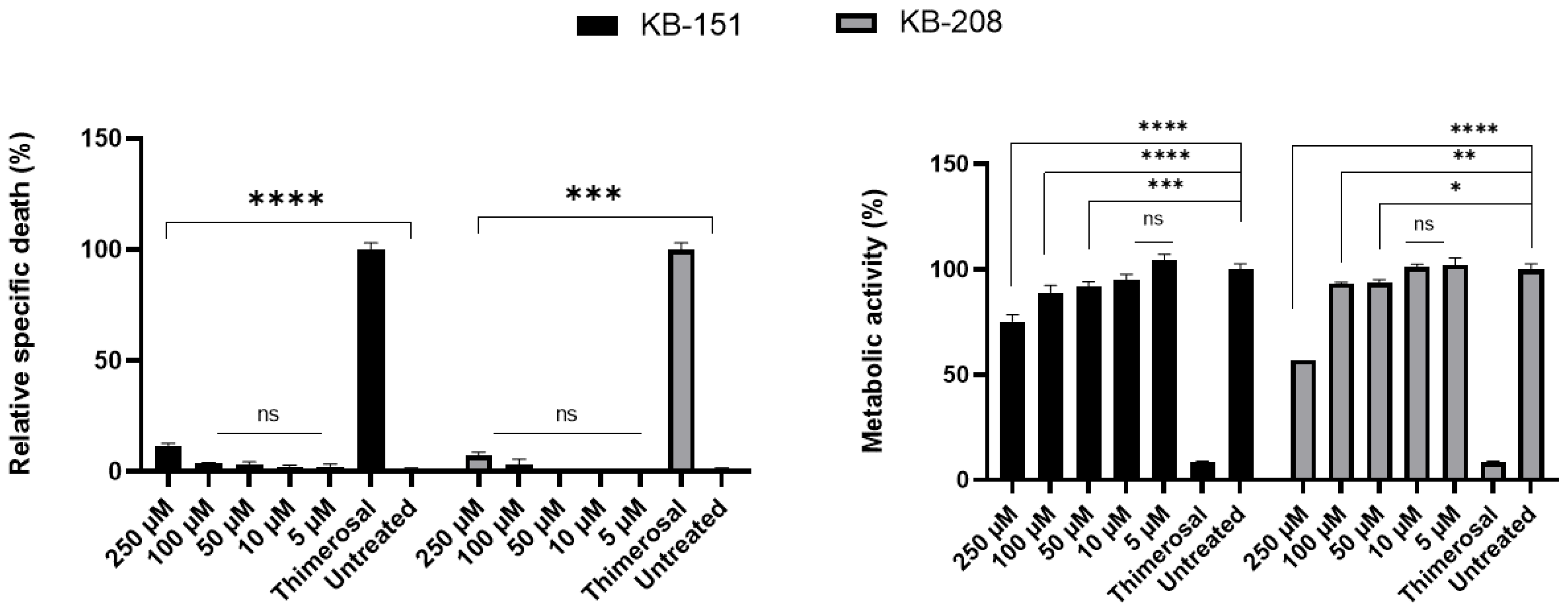
2.4. Dose-Dependent Inhibition
2.5. Additional Toxicity Testing
2.6. Apoptosis Assays
3. Discussion
4. Materials and Methods
4.1. Selection of Compounds for Preliminary In Vitro Screening
4.2. Characterization by 1H-NMR
4.3. Characterization by UHPLC-HRMS/MS
4.4. % Purity and Characterization by PDA
4.5. Spectroscopic Data
4.6. Primary Cells and Cell Lines
4.7. Phagocytosis Assay
4.8. Cell Viability Assays
4.9. Apoptosis Assay
4.10. Dose-Inhibitory Response and IC50
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilliland, B.C.; Evans, R.S. The immune cytopenias. Postgrad. Med. 1973, 54, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Petz, L.D.; Garratty, G. Immune Hemolytic Anemias, 2nd ed.; Petz, L.D., Garratty, G., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2004. [Google Scholar] [CrossRef]
- Connell, N.T.; Berliner, N. Fostamatinib for the treatment of chronic immune thrombocytopenia. Blood 2019, 133, 2027–2030. [Google Scholar] [CrossRef]
- Zuercher, A.W.; Spirig, R.; Baz Morelli, A.; Rowe, T.; Käsermann, F. Next generation Fc receptor-targeting biologics for au-toimmune diseases. Autoimmun. Rev. 2019, 18, 102366. [Google Scholar] [CrossRef]
- Purohit, M.K.; Scovell, I.; Neschadim, A.; Katsman, Y.; Branch, D.R.; Kotra, L.P. Disulfide linked pyrazole derivatives inhibit phagocytosis of opsonized blood cells. Bioorg. Med. Chem. Lett. 2013, 23, 2324–2327. [Google Scholar] [CrossRef] [PubMed]
- Purohit, M.K.; Chakka, S.K.; Scovell, I.; Neschadim, A.; Bello, A.M.; Salum, N.; Katsman, Y.; Bareau, M.C.; Branch, D.R.; Kotra, L.P. Structure-activity relationships of pyrazole derivatives as potential therapeutics for immune thrombocytopenias. Bioorg. Med. Chem. 2014, 22, 2739–2752. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.N.; Branch, D.R. Use of a Monocyte Monolayer Assay to Evaluate Fcγ Receptor-mediated Phagocytosis. J. Vis. Exp. 2017, 119, 55039. [Google Scholar] [CrossRef]
- Tong, T.N.; Cen, S.; Branch, D.R. The Monocyte Monolayer Assay: Past, Present and Future. Transfus. Med. Rev. 2019, 33, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Nance, S.J.; Arndt, P.; Garratty, G. Predicting the clinical significance of red cell alloantibodies using a monocyte monolayer assay. Transfusion 1987, 27, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095497. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, G.C.; Suck, G.; Sakac, D.; Fahim, S.; Foo, A.; Denomme, G.A.; Langler, R.F.; Branch, D.R. Chemical compounds that target thiol-disulfide groups on mononuclear phagocytes inhibit immune mediated phagocytosis of red blood cells. Transfusion 2005, 45, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Branch, D.R.; Guilbert, L.J. Practical in vitro assay systems for the measurement of hematopoietic growth factors. J. Tissue Cult. Methods 1986, 10, 101–107. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Ghanima, W.; Godeau, B.; Cines, D.B.; Bussel, J.B. How I treat immune thrombocytopenia: The choice between splenectomy or a medical therapy as a second-line treatment. Blood 2012, 120, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; Klima, J.; Despotovic, J.M.; O’Brien, S.H. Anti-D immunoglobulin therapy for pediatric ITP: Before and after the FDA’s black box warning. Pediatr. Blood Cancer 2013, 60, E149–E151. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Kalish, L.A.; Neufeld, E.J.; Grace, R.F. Trends in anti-D immune globulin for childhood immune thrombocytopenia: Usage, response rates, and adverse effects. Am. J. Hematol. 2012, 87, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Braselmann, S.; Taylor, V.; Zhao, H.; Wang, S.; Sylvain, C.; Baluom, M.; Qu, K.; Herlaar, E.; Lau, A.; Young, C.; et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharmacol. Exp. Ther. 2006, 319, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K.; Lyseng-Williamson, K.A. Fostamatinib in chronic immune thrombocytopenia: A profile of its use in the USA. Drugs Ther. Perspect. 2018, 34, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Cheloff, A.Z.; Al-Samkari, H. Avatrombopag for the treatment of immune thrombocytopenia and thrombocytopenia of chronic liver disease. J. Blood Med. 2019, 10, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Cohn, C.S.; Bussel, J.B. Romiplostim: A second-generation thrombopoietin agonist. Drugs Today 2009, 45, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, M.A.; Spanbroek, R.; Lottaz, C.; Gautier, E.L.; Frankenberger, M.; Hoffmann, R.; Lang, R.; Haniffa, M.; Collin, M.; Tacke, F.; et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 2010, 115, e10–e19. [Google Scholar] [CrossRef] [PubMed]



| Chemical Compound | PI 1-Average | Inhibition% |
|---|---|---|
| KB-151 | 17 | 80 |
| KB-167 | 14 | 53 |
| KB-175 | 15 | 50 |
| KB-178 | 29 | 53 |
| KB-179 | 14 | 53 |
| KB-181 | 37 | 45 |
| KB-182 | 28 | 55 |
| KB-186 | 28 | 55 |
| KB-189 | 30 | 52 |
| KB-190 | 27 | 56 |
| KB-198 | 9 | 75 |
| KB-199 | 40 | 40 |
| KB-205 | 19 | 47 |
| KB-206 | 18 | 50 |
| KB-208 | 7 | 81 |
| KB-209 | 18 | 50 |
| KB-210 | 11 | 69 |
| KB-212 | 19 | 47 |
| KB-213 | 13 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loriamini, M.; Lewis-Bakker, M.M.; Frias Boligan, K.; Wang, S.; Holton, M.B.; Kotra, L.P.; Branch, D.R. Small Molecule Drugs That Inhibit Phagocytosis. Molecules 2023, 28, 757. https://doi.org/10.3390/molecules28020757
Loriamini M, Lewis-Bakker MM, Frias Boligan K, Wang S, Holton MB, Kotra LP, Branch DR. Small Molecule Drugs That Inhibit Phagocytosis. Molecules. 2023; 28(2):757. https://doi.org/10.3390/molecules28020757
Chicago/Turabian StyleLoriamini, Melika, Melissa M. Lewis-Bakker, Kayluz Frias Boligan, Siming Wang, Mairead B. Holton, Lakshmi P. Kotra, and Donald R. Branch. 2023. "Small Molecule Drugs That Inhibit Phagocytosis" Molecules 28, no. 2: 757. https://doi.org/10.3390/molecules28020757
APA StyleLoriamini, M., Lewis-Bakker, M. M., Frias Boligan, K., Wang, S., Holton, M. B., Kotra, L. P., & Branch, D. R. (2023). Small Molecule Drugs That Inhibit Phagocytosis. Molecules, 28(2), 757. https://doi.org/10.3390/molecules28020757







