In Vitro Metabolism and CYP-Modulating Activity of Lavender Oil and Its Major Constituents
Abstract
1. Introduction
2. Results
2.1. In Vitro Metabolism
2.2. CYP Modulating Activity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. LC-MS/MS Analysis
4.3. Assay for Metabolic Stability in Human Liver Microsomes and S9 Fractions
4.4. CYP Inhibition Assay
4.5. Reporter Gene Assay for PXR Activation
4.6. Reporter Gene Assay for AhR Activation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malcolm, B.J.; Tallian, K. Essential oil of lavender in anxiety disorders: Ready for prime time? Ment. Health Clin. 2017, 7, 147–155. [Google Scholar] [CrossRef]
- Koulivand, P.H.; Khaleghi, G.M.; Gorji, A. Lavender and the nervous system. Evid. Based Complement. Altern. Med. 2013, 2013, 681304. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.E.; Brown, T.A. Differentiating generalized anxiety disorder from anxiety disorder not otherwise specified. J. Nerv. Ment. Dis. 2009, 197, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Schuwald, A.M.; Nöldner, M.; Wilmes, T.; Klugbauer, N.; Leuner, K.; Müller, W.E. Lavender oil-potent anxiolytic properties via modulating voltage dependent calcium channels. PLoS One 2013, 8, e59998. [Google Scholar] [CrossRef] [PubMed]
- Kasper, S.; Müller, W.E.; Volz, H.P.; Möller, H.J.; Koch, E.; Dienel, A. Silexan in anxiety disorders: Clinical data and pharmacological background. World J. Biol. Psychiatry 2018, 19, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Samojlik, I.; Petković, S.; Mimica-Dukić, N.; Božin, B. Acute and chronic pretreatment with essential oil of peppermint (Mentha× piperita L., Lamiaceae) influences drug effects. Phytother. Res. 2012, 26, 820–825. [Google Scholar] [CrossRef]
- Samojlik, I.; Petković, S.; Stilinović, N.; Vukmirović, S.; Mijatović, V.; Božin, B. Pharmacokinetic herb–drug interaction between essential oil of aniseed (Pimpinella anisum L., Apiaceae) and acetaminophen and caffeine: A potential risk for clinical practice. Phytother. Res. 2016, 30, 253–259. [Google Scholar] [CrossRef]
- Doroshyenko, O.; Rokitta, D.; Zadoyan, G.; Klement, S.; Schläfke, S.; Dienel, A.; Gramatté, T.; Lück, H.; Fuhr, U. Drug cocktail interaction study on the effect of the orally administered lavender oil preparation silexan on cytochrome P450 enzymes in healthy volunteers. Drug Metab. Dispos. 2013, 41, 987–993. [Google Scholar] [CrossRef]
- Heger-Mahn, D.; Pabst, G.; Dienel, A.; Schläfke, S.; Klipping, C. No interacting influence of lavender oil preparation silexan on oral contraception using an ethinyl estradiol/levonorgestrel combination. Drugs RD 2014, 14, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Shon, J.; Kim, M.J.; Yu, C.; Zhang, L.; Huang, S.M.; Lee, L.; Tran, D.; Li, L. Role of CYP3A in oral contraceptives clearance. Clin. Transl. Sci. 2018, 11, 251. [Google Scholar] [CrossRef]
- Nosková, K.; Dovrtelova, G.; Zendulka, O.; Reminek, R.; Jurica, J. The effect of (-)-linalool on the metabolic activity of liver CYP enzymes in rats. Physiol. Res. 2016, 654, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Meesters, R.J.; Duisken, M.; Hollender, J. Study on the cytochrome P450-mediated oxidative metabolism of the terpene alcohol linalool: Indication of biological epoxidation. Xenobiotica 2007, 37, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Awortwe, C.; Manda, V.K.; Avonto, C.; Khan, S.I.; Khan, I.A.; Walker, L.A.; Bouic, P.J.; Rosenkranz, B. Echinacea purpurea up-regulates CYP1A2, CYP3A4 and MDR1 gene expression by activation of pregnane X receptor pathway. Xenobiotica 2015, 45, 218–229. [Google Scholar] [CrossRef]
- Obach, R.S. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab. Dispos. 1999, 27, 1350–1359. [Google Scholar]
- Obach, R.S.; Baxter, J.G.; Liston, T.E.; Silber, B.M.; Jones, B.C.; Macintyre, F.; Rance, D.J.; Wastall, P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J. Pharmacol. Exp. Therapeutics. 1997, 283, 46–58. [Google Scholar]
- Bialon, M.; Krzyśko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical composition of two different lavender essential oils and their effect on facial skin microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhao, Y.; Firempong, C.K.; Xu, X. Preparation, characterization and pharmacokinetic studies of linalool-loaded nanostructured lipid carriers. Pharm. Biol. 2016, 54, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Nöldner, M.; Germer, S.; Koch, E. Pharmacokinetics of linalool and linalyl acetate, the two main constituents of silexan, an essential oil from Lavandula angustifolia flowers, in rats. Planta Medica. 2011, 77, PM44. [Google Scholar] [CrossRef]
- Paine, M.F.; Hart, H.L.; Ludington, S.S.; Haining, R.L.; Rettie, A.E.; Zeldin, D.C. The human intestinal cytochrome P450 “pie”. Drug Metab. Dispos. 2006, 34, 880–886. [Google Scholar] [CrossRef]
- Wei, Y.; Tang, C.; Sant, V.; Li, S.; Poloyac, S.M.; Xie, W. A molecular aspect in the regulation of drug metabolism: Does PXR-induced enzyme expression always lead to functional changes in drug metabolism? Curr. Pharmacol. Rep. 2016, 2, 187–192. [Google Scholar] [CrossRef]
- Zhou, M.; Maitra, S.R.; Wang, P. The potential role of transcription factor aryl hydrocarbon receptor in downregulation of hepatic cytochrome P-450 during sepsis. Int. J. Mol. Med. 2008, 21, 423–428. [Google Scholar] [CrossRef]
- Thorn, C.F.; Aklillu, E.; Klein, T.E.; Altman, R.B. PharmGKB summary: Very important pharmacogene information for CYP1A2. Pharm. Genomics. 2012, 22, 73. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Ali, Z.; Avonto, C.; Khan, I.A. A novel approach for lavender essential oil authentication and quality assessment. J. Pharm. Biomed. Anal. 2021, 199, 114050. [Google Scholar] [CrossRef] [PubMed]
- Manda, V.K.; Avula, B.; Ali, Z.; Khan, I.A.; Walker, L.A.; Khan, S.I. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Medica 2014, 80, 568–876. [Google Scholar] [CrossRef]
- Husain, I.; Manda, V.; Alhusban, M.; Dale, O.R.; Bae, J.Y.; Avula, B.; Gurley, B.J.; Chittiboyina, A.G.; Khan, I.A.; Khan, S.I. Modulation of CYP3A4 and CYP2C9 activity by Bulbine natalensis and its constituents: An assessment of HDI risk of B. natalensis containing supplements. Phytomedicine 2021, 81, 153416. [Google Scholar] [CrossRef] [PubMed]
- Manda, V.K.; Dale, O.R.; Awortwe, C.; Ali, Z.; Khan, I.A.; Walker, L.A.; Khan, S.I. Evaluation of drug interaction potential of Labisia pumila (Kacip Fatimah) and its constituents. Front. Pharmacol. 2014, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Dale, O.R.; Manda, V.; Ali, Z.; Gurley, B.J.; Chittiboyina, A.G.; Khan, I.A.; Khan, S.I. Bulbine natalensis (currently Bulbine latifolia) and select bulbine knipholones modulate the activity of AhR, CYP1A2, CYP2B6, and P-gp. Planta Medica 2022, 88, 975–984. [Google Scholar] [CrossRef]
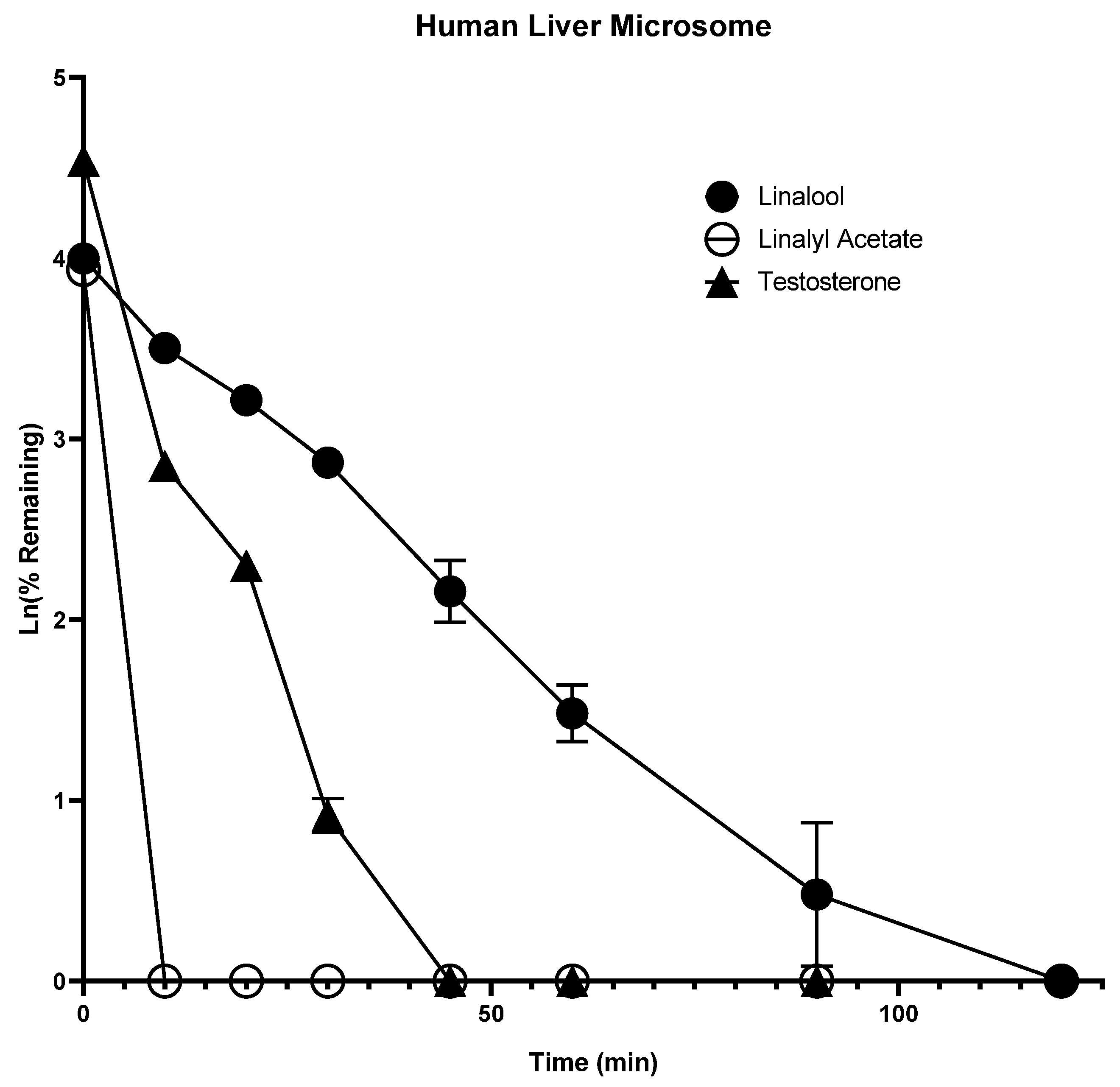
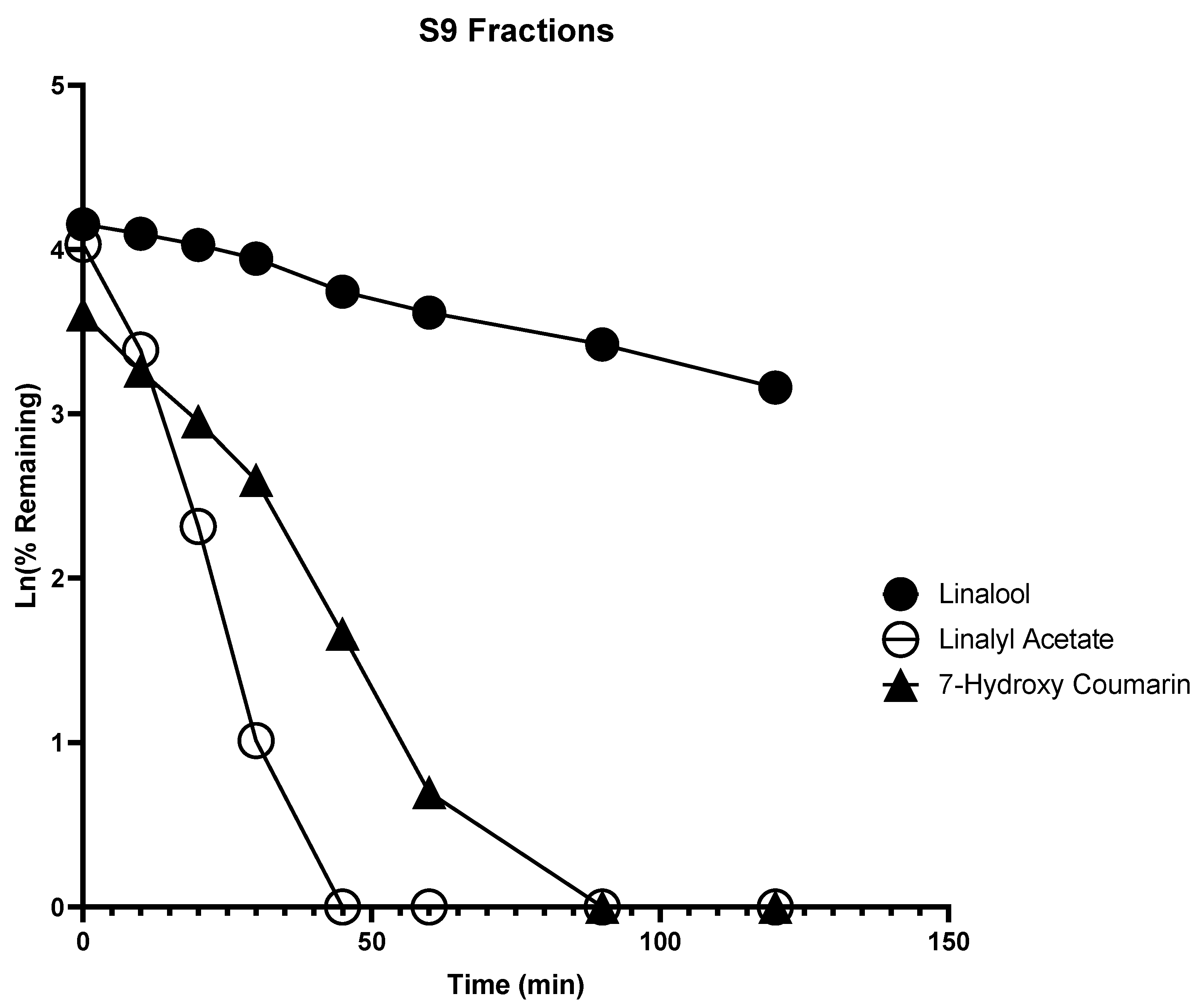
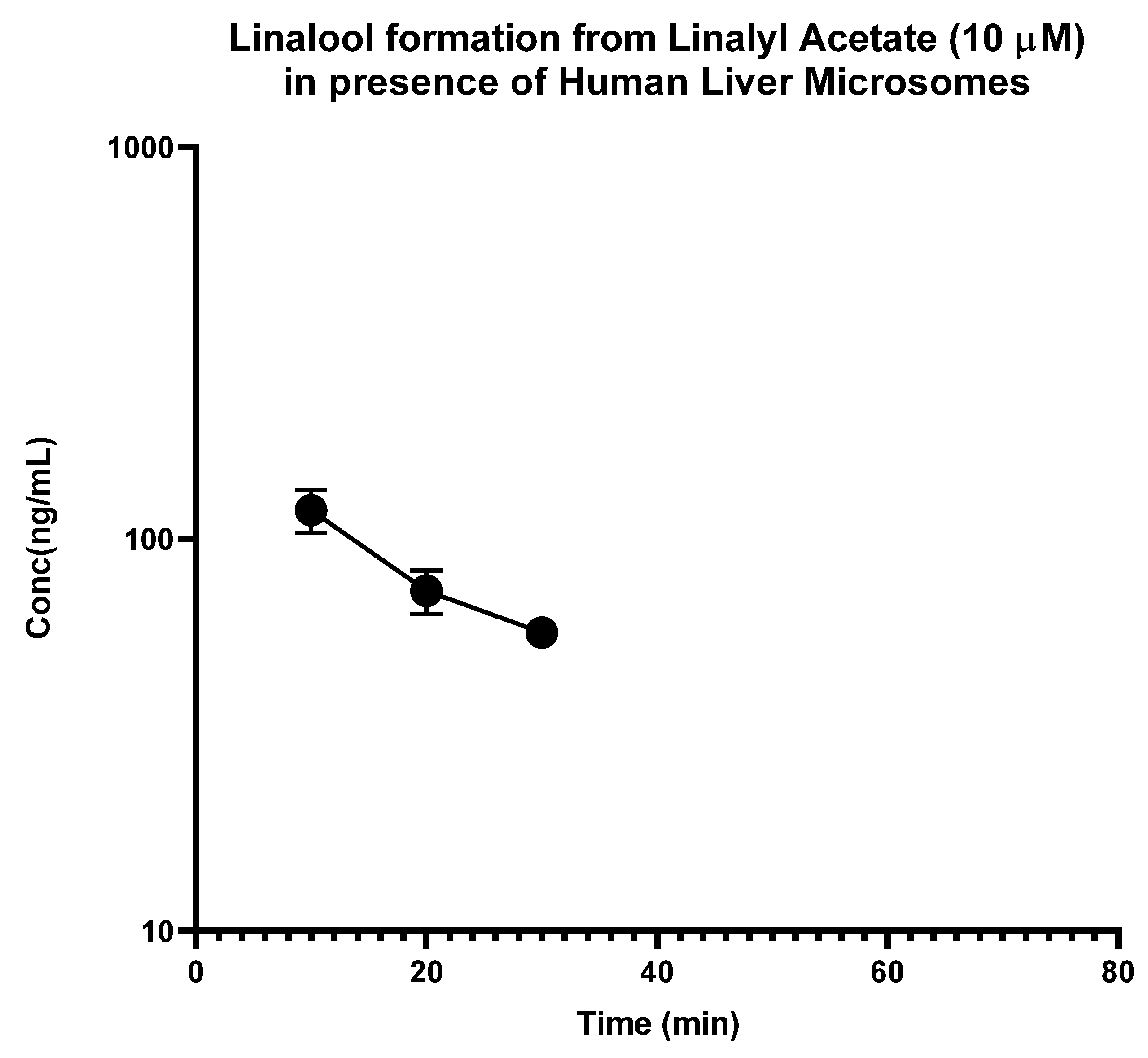
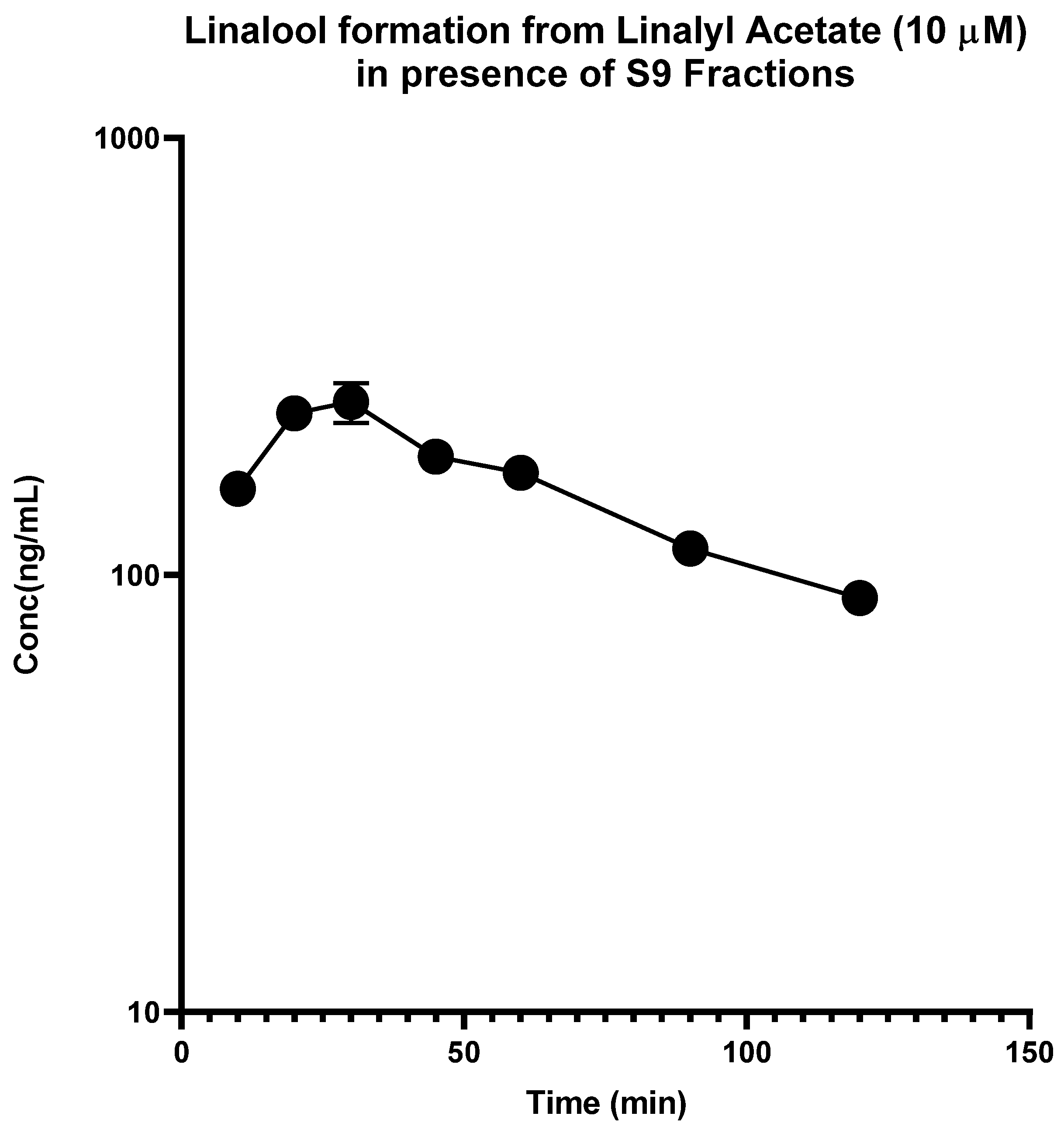
| Compound Name | IC50 (µg/mL) | |
|---|---|---|
| CYP3A4 | CYP1A2 | |
| Lavender oil | 12.0 ± 3.00 | 21.5 ± 0.50 |
| Linalyl acetate | 4.75 ± 0.25 | NI |
| Linalool | NI | NI |
| Ketoconazole * (µM) | 0.05 ± 0.01 | |
| α-naphthoflavone * (µM) | 0.04 ± 0.01 | |
| Compound Name | Concentration | Fold Increase in PXR Activity |
|---|---|---|
| Lavender oil | 60 µg/mL | 1.73 ± 0.14 |
| 20 µg/mL | 1.71 ± 0.15 | |
| 6.7 µg/mL | 1.43 ± 0.23 | |
| Linalyl acetate | 30 µg/mL | 1.83 ± 0.22 |
| 10 µg/mL | 1.63 ± 0.18 | |
| 3.3 µg/mL | 1.45 ± 0.25 | |
| Linalool | 30 µg/mL | 1.67 ± 0.07 |
| 10 µg/mL | 1.34 ± 0.08 | |
| 3.3 µg/mL | 1.33 ± 0.12 | |
| Rifampicin * | 10 µM | 2.82 ± 0.00 |
| 3.3 µM | 2.21 ± 0.04 | |
| 1.1 µM | 1.63 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, G.; Dale, O.R.; Wang, Y.-H.; Khan, S.I.; Khan, I.A.; Yates, C.R. In Vitro Metabolism and CYP-Modulating Activity of Lavender Oil and Its Major Constituents. Molecules 2023, 28, 755. https://doi.org/10.3390/molecules28020755
Mondal G, Dale OR, Wang Y-H, Khan SI, Khan IA, Yates CR. In Vitro Metabolism and CYP-Modulating Activity of Lavender Oil and Its Major Constituents. Molecules. 2023; 28(2):755. https://doi.org/10.3390/molecules28020755
Chicago/Turabian StyleMondal, Goutam, Olivia R. Dale, Yan-Hong Wang, Shabana I. Khan, Ikhlas A. Khan, and Charles R. Yates. 2023. "In Vitro Metabolism and CYP-Modulating Activity of Lavender Oil and Its Major Constituents" Molecules 28, no. 2: 755. https://doi.org/10.3390/molecules28020755
APA StyleMondal, G., Dale, O. R., Wang, Y.-H., Khan, S. I., Khan, I. A., & Yates, C. R. (2023). In Vitro Metabolism and CYP-Modulating Activity of Lavender Oil and Its Major Constituents. Molecules, 28(2), 755. https://doi.org/10.3390/molecules28020755





