Paraquat and Diquat: Recent Updates on Their Pretreatment and Analysis Methods since 2010 in Biological Samples
Abstract
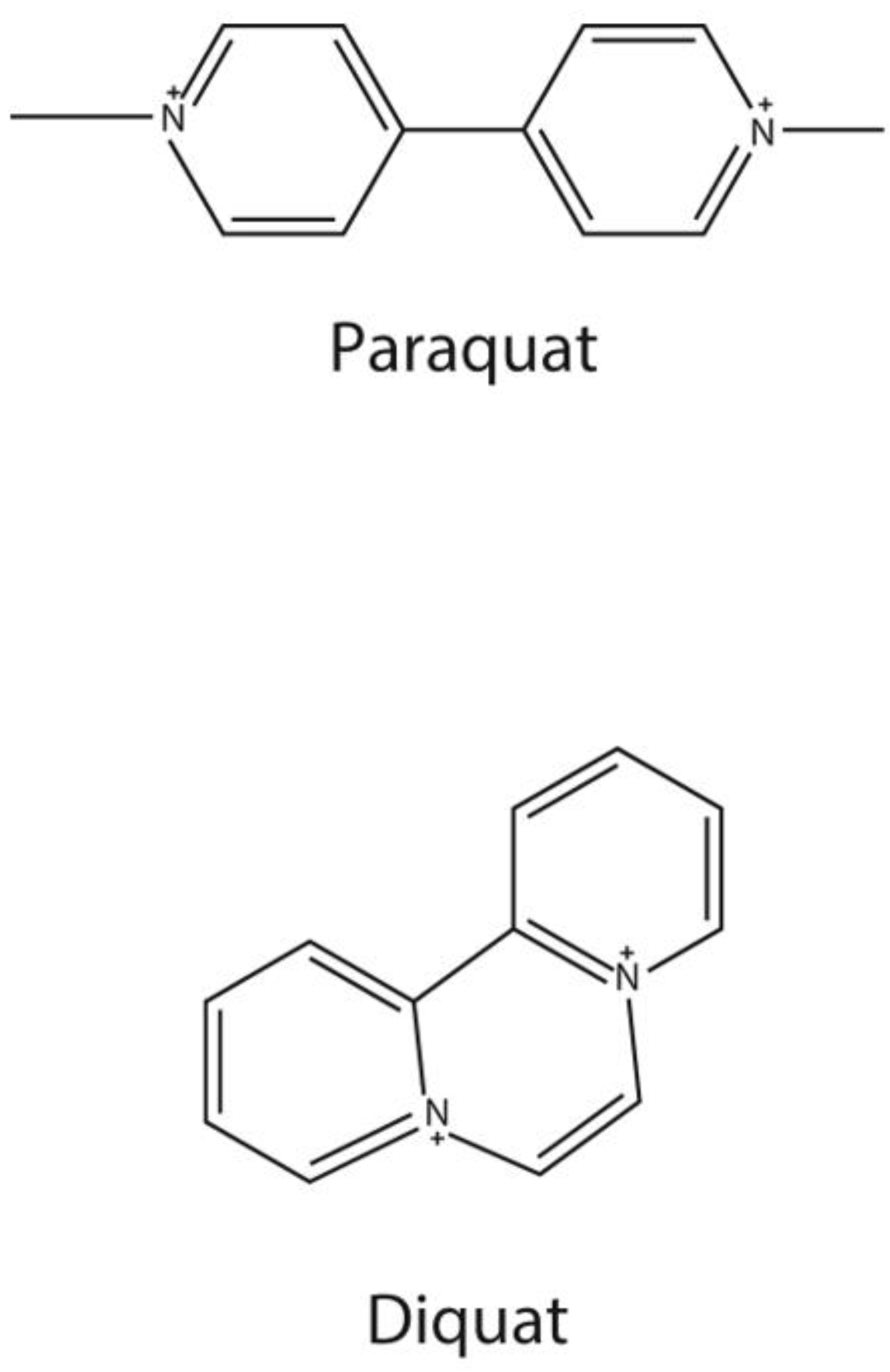
1. Introduction
2. Sample Treatment Methods
2.1. Protein Precipitation
2.2. Liquid–Liquid Extraction (LLE)
2.3. Solid-Phase Extraction (SPE)
2.4. Microextraction Methods
2.5. Other Treatment Methods
2.6. Summary
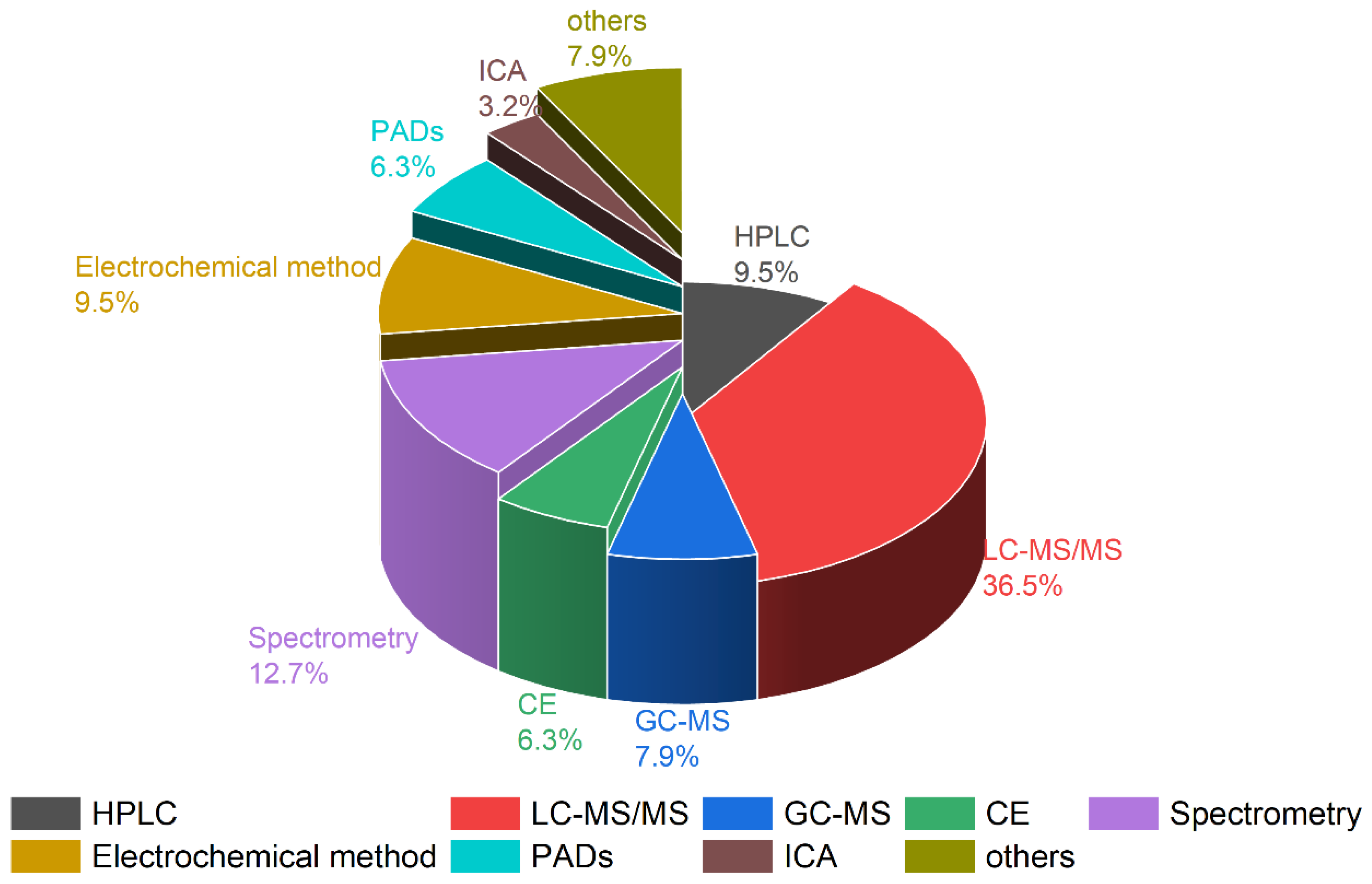
3. Analytical Methods
3.1. Liquid Chromatography
3.1.1. High-Performance Liquid Chromatography
3.1.2. Liquid Chromatography–Mass Spectrometry (LC–MS)
3.2. Gas Chromatography–Mass Spectrometry (GC–MS)
3.3. Capillary Electrophoresis (CE)
3.4. Elcetrochemical Sensors
3.5. Surface-Enhanced Raman Spectroscopy (SERS)
3.6. Immunochromatographic Assay (ICA)
3.7. Paper-Based Analytical Devices (PADs)
3.8. Other Analytical Methods
3.9. Summary
4. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| APPI | atmospheric pressure photoionization |
| C4D | capacitively coupled contactless conductively detection |
| CE | electrophoresis |
| CE-DAD | capillary electrophoresis and diode-array detection |
| DBS | dried blood spot |
| DQ | diquat |
| ELISA | enzyme-linked immunosorbent assay |
| EPQ | ethyl paraquat |
| ESI | electrospray ionization |
| GC | gas chromatography |
| GC–MS | gas chromatography–mass spectrometry |
| HESI | heated electrospray ionization source |
| HFBA | heptafluorobutyric acid |
| HILIC | hydrophilic interaction liquid chromatography |
| HPLC | high-performance liquid chromatography |
| HPLC–UV | high-performance liquid chromatography–ultraviolet detection |
| HRMS | high-resolution mass spectrometer |
| HS-SPME | headspace solid-phase microextraction |
| ICA | immunochromatographic assay |
| IS–SERS | internal standard–SERS |
| LC/TOF–MS | liquid chromatography/time-of-flight mass spectrometry |
| LC–MS | liquid chromatography–mass spectrometry |
| LLE | liquid–liquid extraction |
| LOD | limits of detection |
| LOQ | limits of quantification |
| LPME | liquid-phase microextraction |
| MALDI–FTICR–MS | matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry |
| MCS | microcapillary sampling |
| MDSPE | magnetic dispersed solid-phase extraction |
| MODS | multi-organ dysfunction syndrome |
| MS | mass spectrometer |
| MSWCNTs | magnetic single-walled carbon nanotubes |
| NaBH4–NiCl2 | sodium borohydride–nickel chloride |
| NP | nanoparticle |
| PAD | paper-based analytical devices |
| PFs | preconcentration factors |
| PQ | paraquat |
| PRM | parallel reaction monitoring |
| QqQ | triple quadrupole |
| SALDI-TOF MS | surface-assisted laser desorption/ionization time-of-flight mass spectrometry |
| SERS | surface-enhanced Raman spectroscopy |
| SHS | switchable–hydrophilicity solvent |
| SPE | solid-phase extraction |
| SPME | solid-phase microextraction |
| TEA | triethylamine |
| TRFICA | time-resolved fluorescence immunochromatographic assay |
| UA–SHS–HLLME | ultrasound-assisted switchable-hydrophilicity solvent-based homogeneous liquid–liquid microextraction |
| UPLC–MS | ultra-high-performance liquid chromatography–mass spectrometry |
References
- Fortenberry, G.Z.; Beckman, J.; Schwartz, A.; Prado, J.B.; Graham, L.S.; Higgins, S.; Lackovic, M.; Mulay, P.; Bojes, H.; Waltz, J.; et al. Magnitude and characteristics of acute paraquat- and diquat-related illnesses in the US: 1998–2013. Environ. Res. 2016, 146, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Velez-Pardo, C.; Jimenez-Del-Rio, M.; Lores-Arnaiz, S.; Bustamante, J. Protective Effects of the Synthetic Cannabinoids CP55,940 and JWH-015 on Rat Brain Mitochondria upon Paraquat Exposure. Neurochem. Res. 2010, 35, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Anusha, J.; Moudgil, K. Accidental paraquat induced hypersalivation: A case report. Daru J. Pharm. Sci. 2019, 27, 885–888. [Google Scholar] [CrossRef]
- Chen, F.; Ye, Y.; Jin, B.; Yi, B.; Wei, Q.; Liao, L. Homicidal Paraquat Poisoning. J. Forensic Sci. 2019, 64, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.X.; Zhao, Y.; Gao, J.; Feng, S.Y.; Wu, C.P.; Zhai, Y.Z.; Zhang, M.; Nie, S.; Li, Y. Comparison of severity index and plasma paraquat concentration for predicting survival after paraquat poisoning A meta-analysis. Medicine 2020, 99, e19063. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-H.; Tung, K.-H.; Gu, P.-W.; Yen, T.-H.; Cheng, C.-M. Rapid Simultaneous Determination of Paraquat and Creatinine in Human Serum Using a Piece of Paper. Micromachines 2018, 9, 586. [Google Scholar] [CrossRef]
- Sun, L.; Yan, P.; Liu, Y.; Wei, L.; Li, G. [Analysis of prognostic value of initial serum paraquat concentration in patients with paraquat poisoning]. Zhonghua Laodong Wei Sheng Zhiyebing Zazhi Chin. J. Ind. Hyg. Occup. Dis. 2015, 33, 697–700. [Google Scholar]
- Sawada, Y.; Yamamoto, I.; Hirokane, T.; Nagai, Y.; Satoh, Y.; Ueyama, M. Severity index of paraquat poisoning. Lancet 1988, 331, 1333. [Google Scholar] [CrossRef]
- Proudfoot, A.T.; Stewart, M.S.; Levitt, T.; Widdop, B. Paraquat poisoning: Significance of plasma-paraquat concentrations. Lancet 1979, 314, 330–332. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, N.; Cao, W.; Hong, Z. Advances in dispersive liquid-liquid microextraction and its application to analysis of biological samples. Se Pu Chin. J. Chromatogr. 2020, 38, 491–501. [Google Scholar]
- Rajaram, R.; Neelakantan, L. Recent advances in estimation of paraquat using various analytical techniques: A review. Results Chem. 2023, 5, 10703. [Google Scholar] [CrossRef]
- Lamei, N.; Ezoddin, M.; Kakavandi, N.R.; Abdi, K.; Ghazi-khansari, M. Ultrasound-Assisted Switchable Solvent in Determination of Quaternary Ammonium Herbicide Paraquat in Biological, Environmental Water, and Apple Juice Samples Using Chemical Reduction Process Coupled to GC-MS Detection. Chromatographia 2018, 81, 923–930. [Google Scholar] [CrossRef]
- Lu, H.; Yu, J.; Wu, L.; Xing, J.; Wang, J.; Huang, P.; Zhang, J.; Xiao, H.; Gao, R. Optimized ultra performance liquid chromatography tandem high resolution mass spectrometry method for the quantification of paraquat in plasma and urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1027, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Tsao, Y.-C.; Lai, Y.-C.; Liu, H.-C.; Liu, R.H.; Lin, D.-L. Simultaneous Determination and Quantitation of Paraquat, Diquat, Glufosinate and Glyphosate in Postmortem Blood and Urine by LC-MS-MS. J. Anal. Toxicol. 2016, 40, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Chen, Y. A simple and rapid method for detection of paraquat in human plasma by high-performance liquid chromatography. Int. J. Clin. Exp. Med. 2015, 8, 17067–17071. [Google Scholar] [PubMed]
- Li, P.; Fan, J.; Zhang, X.; Du, P.; Liu, H.; Liu, H.; Liu, L. Uncertainty evaluation on the determination of paraquat concentration in plasma by HPLC-MS/MS. Chin. J. Clin. Pharmacol. 2020, 36, 3938–3942. [Google Scholar]
- Hong, G.; Hu, L.; Tang, Y.; Zhang, T.; Kang, X.; Zhao, G.; Lu, Z. Prognosis and survival analysis of paraquat poisoned patients based on improved HPLC-UV method. J. Pharmacol. Toxicol. Methods 2016, 80, 75–81. [Google Scholar] [CrossRef]
- Mahmood, W.M.A.W.; Khan, A.A.M.; Shamsuddin, S.A.; Zaini, N.S.A.; Mohamed, K.; Rashid, R.A. Paraquat Dichloride Detection From Forensic Blowfly Samples. Malays. Appl. Biol. 2015, 44, 133–138. [Google Scholar]
- Yang, Y.; Zhang, X.G.; Yu, F.; Shi, Y.; Qin, X.X.; Miao, X.G.; Dong, M.; Wen, D.; Ma, C.L. Analysis of Common Herbicides in Blood by UPLC-HRMS. Fa Yi Xue Za Zhi 2018, 34, 590–594. [Google Scholar]
- Wunnapuk, K.; Medley, G.A.; Liu, X.; Grice, J.E.; Jayasinghe, S.; Gawarammana, I.; Buckley, N.A.; Roberts, M.S. Simple and sensitive liquid chromatography-tandem mass spectrometry methods for quantification of paraquat in plasma and urine: Application to experimental and clinical toxicological studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 3047–3052. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, Y.; Bai, Y.; Tang, J.; Chen, Y.; Wang, L. An improved approach for extraction and high-performance liquid chromatography analysis of paraquat in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, X.; Li, R. Simultaneous determination of paraquat and diquat in plasma and urine by ion chromatography-triple quadrupole mass spectrometry. Se Pu Chin. J. Chromatogr. 2020, 38, 1294–1301. [Google Scholar]
- Sha, O.; Cui, B.; Liu, H.; Wang, Y.; Chen, X.; Chen, L.; Wang, S. A simple and rapid method for determination of paraquat in human urine and plasma by improved solid adsorption using equipment built in-house. J. Iran. Chem. Soc. 2019, 16, 2071–2080. [Google Scholar] [CrossRef]
- Yuan, G.; Li, R.; Zhao, Q.; Kong, X.; Wang, Y.; Wang, X.; Guo, R. Simultaneous determination of paraquat and diquat in human plasma by HPLC-DAD: Its application in acute poisoning patients induced by these two herbicides. J. Clin. Lab. Anal. 2021, 35, e23669. [Google Scholar] [CrossRef]
- Guo, X.; Li, T.D.; Tian, D.C.; Ma, C.H.; Lin, Y.R.; Yun, J.P. Liquid chromatography-tandem mass spectrometry method for the determination of paraquat and diquat in plasma and urine. Zhonghua Laodong Weisheng Zhiyebing Zazhi Chin. J. Ind. Hyg. Occup. Dis. 2021, 39, 612–616. [Google Scholar]
- Saito, T.; Fukushima, T.; Yui, Y.; Miyazaki, S.; Nakamoto, A.; Namera, A.; Inokuchi, S. Monolithic spin column extraction and GC-MS for the simultaneous assay of diquat, paraquat, and fenitrothion in human serum and urine. Anal. Bioanal. Chem. 2011, 400, 25–31. [Google Scholar] [CrossRef]
- Whitehead, R.D.J.; Montesano, M.A.; Jayatilaka, N.K.; Buckley, B.; Winnik, B.; Needham, L.L.; Barr, D.B. Method for measurement of the quaternary amine compounds paraquat and diquat in human urine using high-performance liquid chromatography—tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2548–2553. [Google Scholar] [CrossRef]
- Ghavidel, F.; Shahtaheri, S.J.; Torabbeigi, M.; Froushani, A.R. Optimization of solid phase microextraction procedure for determination of paraquat using reduction process. J. Anal. Chem. 2016, 71, 648–652. [Google Scholar] [CrossRef]
- Gao, L.; Liu, J.; Wang, C.; Liu, G.; Niu, X.; Shu, C.; Zhu, J. Fast determination of paraquat in plasma and urine samples by solid-phase microextraction and gas chromatography—mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 944, 136–140. [Google Scholar] [CrossRef]
- Kakavandi, N.R.; Ezoddin, M.; Abdi, K.; Ghazi-Khansari, M.; Amini, M.; Shahtaheri, S.J. Ion-pair switchable-hydrophilicity solvent-based homogeneous liquid-liquid microextraction for the determination of paraquat in environmental and biological samples before high-performance liquid chromatography. J. Sep. Sci. 2017, 40, 3703–3709. [Google Scholar] [CrossRef]
- Kumari, R.; Jha, R.R.; Singh, M.P.; Patel, D.K. Whirling agitated single drop microextraction technique for the simultaneous analysis of Paraquat and Maneb in tissue samples of treated mice. J. Sep. Sci. 2016, 39, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Huang, Z.; Shang, C.; Wang, L.; Qiu, Q.; Xu, Z.; Zhang, D. Design and synthesis of amphiphilic carboxyl-functionalized magnetic polymer microspheres for fast determination of paraquat and its four metabolites in human urine samples prior to ultra-high performance liquid chromatography-high resolution mass spectrometry. J. Chromatogr. A 2022, 1670, 462998. [Google Scholar] [PubMed]
- Pan, S.; Zhang, J.; He, Q.; Chen, X.; Jin, M. Fabrication of benzenesulfonic acid groups modified magnetic microspheres as an MSPE adsorbent for fast determination of paraquat and diquat in human urine combined with UPLC-HRMS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1136, 121880. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.-L.; Qiu, J.-J.; Wu, C.; Huang, T.; Meng, R.-B.; Lai, Y.-Q. Magnetic single-walled carbon nanotubes—dispersive solid-phase extraction method combined with liquid chromatography—tandem mass spectrometry for the determination of paraquat in urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 965, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Sha, O.; Cui, B.; Chen, X.; Liu, H.; Yao, J.; Zhu, Y. Separation and Determination of Paraquat and Diquat in Human Plasma and Urine by Magnetic Dispersive Solid Phase Extraction Coupled with High-Performance Liquid Chromatography. J. Anal. Methods Chem. 2020, 2020, 7359582. [Google Scholar] [CrossRef]
- Sha, O.; Wang, Y.; Yin, X.; Chen, X.; Chen, L.; Wang, S. Magnetic Solid-Phase Extraction Using Fe3O4@SiO2 Magnetic Nanoparticles Followed by UV-Vis Spectrometry for Determination of Paraquat in Plasma and Urine Samples. J. Anal. Methods Chem. 2017, 2017, 8704639. [Google Scholar] [CrossRef]
- Baeck, S.K.; Shin, Y.S.; Chung, H.S.; Pyo, M.Y. Comparison study of the extraction methods of paraquat in post-mortem human blood samples. Arch. Pharmacal Res. 2007, 30, 235–239. [Google Scholar] [CrossRef]
- Tajik, M.; Yamini, Y.; Esrafili, A.; Ebrahimpour, B. On-line extraction and determination of two herbicides: Comparison between two modes of three-phase hollow fiber microextraction. J. Sep. Sci. 2015, 38, 649–655. [Google Scholar] [CrossRef]
- Ezoddin, M.; Abdi, K. Monitoring of antifungal drugs in biological samples using ultrasonic-assisted supramolecular dispersive liquid—liquid microextraction based on solidification of a floating organic droplet. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1027, 74–80. [Google Scholar] [CrossRef]
- Lasarte-Aragonés, G.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Use of switchable hydrophilicity solvents for the homogeneous liquid-liquid microextraction of triazine herbicides from environmental water samples. J. Sep. Sci. 2015, 38, 990–995. [Google Scholar] [CrossRef]
- Ezoddin, M.; Abdi, K.; Lamei, N. Development of air assisted liquid phase microextraction based on switchable-hydrophilicity solvent for the determination of palladium in environmental samples. Talanta 2016, 153, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Yang, Y.; Xiang, P.; Yu, F.; Zheng, F.; Liu, T.; Shi, Y.; Zhang, X.; Dong, M.; Cong, B.; et al. A novel approach for determination of paraquat based on dried blood spot (DBS) extraction and UHPLC-HRMS analysis. J. Pharm. Biomed. Anal. 2018, 159, 11–17. [Google Scholar] [CrossRef]
- Luo, X.H.; Zhang, J.; Zhang, Z.G.; Lin, Y.; Yu, Z.; Wang, X.; Lin, K. Urine Metabolic Changes of Acute Paraquat Poisoning Rats after Intervention with Curcumin. Latin Am. J. Pharm. 2018, 37, 131–138. [Google Scholar]
- Zhang, L.; Yan, J.; Chen, L.; Tu, C. Establishment of detection method for simultaneous determination of plasma concentrations of paraquat and diquat by high performance liquid chromatography. Adv. Drug React. J. 2018, 20, 103–109. [Google Scholar]
- Merritt, T.J.S.; Douglas, L.; Rzezniczak, T.Z.; Watterson, J.H. Rapid and simple analysis of paraquat in tissue homogenate by ultra-high performance liquid chromatography. Anal. Methods 2011, 3, 1428–1432. [Google Scholar] [CrossRef]
- Zhang, W.; Su, Y.; Shi, J.; Zhang, M.; Wu, B.; Chen, S.; Hu, S.; Rao, Z.; Zheng, J. Reversed-phase high performance liquid chromatography method for the determination of paraquat in whole blood. Anal. Methods 2014, 6, 6560–6564. [Google Scholar] [CrossRef]
- Chen, L.; Zou, J.; Xiao, H.; He, L.; Tong, R. Determination of paraquat in human serum by HPLC. Chin. J. Hosp. Pharm. 2010, 30, 2091–2094. [Google Scholar]
- Moreira, P.N.; de Pinho, P.G.; Baltazar, M.T.; Bastos, M.D.L.; Carvalho, F.; Dinis-Oliveira, R.J. Quantification of paraquat in postmortem samples by gas chromatography-ion trap mass spectrometry and review of the literature. Biomed. Chromatogr. 2012, 26, 338–349. [Google Scholar] [CrossRef]
- Wu, D.; Wu, C.; Deng, X.; Li, Y.; Li, Q.; Liu, X. Determination of Paraquat in Human Plasma Using Liquid-Liquid Extraction and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Agrochemicals 2021, 60, 440–445. [Google Scholar]
- Ma, J.; Li, H.; Zhang, J.; Yan, Y.; Ye, Q.; Shao, B. Determination of paraquat in serum by ultra performance liquid chromatography tandem mass spectrometry and toxicokinetics of paraquat in rat. Wei Sheng Yan Jiu J. Hyg. Res. 2018, 47, 993–997. [Google Scholar]
- Hu, L.; Hong, G.; Tang, Y.; Wang, X.; Wen, C.; Lin, F.; Lu, Z. Early Metabolome Profiling and Prognostic Value in Paraquat-Poisoned Patients: Based on Ultraperformance Liquid Chromatography Coupled To Quadrupole Time-of-Flight Mass Spectrometry. Chem. Res. Toxicol. 2017, 30, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Jayanta, S.; Leepipatpiboon, N. Hollow-fiber Microextraction Combined with Hydrophilic Interaction Liquid Chromatography Mass Spectrometry for Analytical Determination of High Polarity Herbicides in Water. Chiang Mai J. Sci. 2018, 45, 2381–2396. [Google Scholar]
- Fan, J.; Li, P.; Liu, H.; Zhao, R.; Zhao, Z.; Du, P.; Liu, L. Optimized liquid chromatography-electrospray quadrupole linear ion trap mass spectrometry method for the quantitation of paraquat in plasma: Application to a toxicokinetic study. Anal. Methods 2019, 11, 2756–2762. [Google Scholar] [CrossRef]
- Yoshioka, N.; Asano, M.; Kuse, A.; Matsuoka, T.; Akiyama, Y.; Mitsuhashi, T.; Nagasaki, Y.; Ueno, Y. Rapid and sensitive quantification of paraquat and diquat in human serum by liquid chromatography/time-of-flight mass spectrometry using atmospheric pressure photoionization. Forensic Toxicol. 2012, 30, 135–141. [Google Scholar] [CrossRef]
- Mao, Z.; Yu, Y.; Sun, H.; Wu, C.; Jiang, Q.; Chu, C.; Zhao, C.; Zhou, Y.; Zhang, J.; Cao, Y.; et al. Simultaneous determination of diquat and its two primary metabolites in rat plasma by ultraperformance liquid chromatography—tandem mass spectrometry and its application to the toxicokinetic study. Forensic Toxicol. 2022, 40, 332–339. [Google Scholar] [CrossRef]
- Vu, A.P.; Nguyen, T.N.; Do, T.T.; Doan, T.H.; Ha, T.H.; Ta, T.T.; Nguyen, H.L.; Hauser, P.C.; Nguyen, T.A.H.; Mai, T.D. Clinical screening of paraquat in plasma samples using capillary electrophoresis with contactless conductivity detection: Towards rapid diagnosis and therapeutic treatment of acute paraquat poisoning in Vietnam. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1060, 111–117. [Google Scholar] [CrossRef]
- Lanaro, R.; Costa, J.L.; Fernandes, L.C.R.; Resende, R.R.; Tavares, M.F.M. Detection of Paraquat in Oral Fluid, Plasma, and Urine by Capillary Electrophoresis for Diagnosis of Acute Poisoning. J. Anal. Toxicol. 2011, 35, 274–279. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, H.; Zhao, S.; Yang, J.; Chen, X.; Li, X. Simultaneous detection of paraquat and creatinine in urine by capillary electrophoresis. Chin. J. Lab. Med. 2013, 36, 791–795. [Google Scholar]
- Wang, X.; Du, Y.; Li, Q.; Wu, T.; Hu, H.; Xu, Y.; Zhang, H.; Pan, Y. Fabrication of uniform substrate based on silver nanoparticles decorated glycidyl methacrylate-ethylene dimethacrylate porous material for ultrasensitive surface-enhanced Raman scattering detection. J. Raman Spectrosc. 2014, 45, 47–53. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, X.; Wu, J.; Zhang, W.; Lu, X.; Sun, H.; Zhang, J.; Guo, L.; Xie, J. Quantification and toxicokinetics of paraquat in mouse plasma and lung tissues by internal standard surface-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2022, 414, 2371–2383. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Gao, H.; Liu, G.; Tian, Z.; Feng, J.; Guo, L.; Xie, J. Rapid on-site detection of paraquat in biologic fluids by iodide-facilitated pinhole shell-isolated nanoparticle-enhanced Raman spectroscopy. RSC Adv. 2016, 6, 59919–59926. [Google Scholar] [CrossRef]
- Junior, D.W.; Deroco, P.B.; Kubota, L.T. A copper-based metal-organic framework/reduced graphene oxide-modified electrode for electrochemical detection of paraquat. Microchim. Acta 2022, 189, 278. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.M.; Liu, F.; Wang, W.; Lian, K.Q.; Ma, L.; Shi, H.M.; Kang, W.J. Electrochemical Behavior of Paraquat on a Highly Ordered Biosensor Based on an Unmodified DNA-3D Gold Nanoparticle Composite and Its Application. Electrochim. Acta 2015, 153, 190–199. [Google Scholar] [CrossRef]
- El Kasmi, S.; Lahrich, S.; Farahi, A.; Zriouil, M.; Ahmamou, M.; Bakasse, M.; El Mhammedi, M.A. Electrochemical determination of paraquat in potato, lemon, orange and natural water samples using sensitive-rich clay carbon electrode. J. Taiwan Inst. Chem. Eng. 2016, 58, 165–172. [Google Scholar] [CrossRef]
- Sun, X.; Li, Z.; Cai, Y.; Wei, Z.; Fang, Y.; Ren, G.; Huang, Y. Electrochemical impedance spectroscopy for analytical determination of paraquat in meconium samples using an immunosensor modified with fullerene, ferrocene and ionic liquid. Electrochim. Acta 2011, 56, 1117–1122. [Google Scholar] [CrossRef]
- Shindo, K.; Kishikawa, N.; Ohyama, K.; Kuroda, N. Simultaneous Determination of Paraquat and Diquat in Human Plasma Using HPLC with Chemiluminescence Detection. Bunseki Kagaku 2015, 64, 581–587. [Google Scholar] [CrossRef]
- Song, Y.; Feng, X.-S. Sample Preparation and Analytical Methods for Steroid Hormones in Environmental and Food Samples: An Update Since 2012. Crit. Rev. Anal. Chem. 2023, 53, 69–87. [Google Scholar] [CrossRef]
- Usui, K.; Minami, E.; Fujita, Y.; Kobayashi, H.; Hanazawa, T.; Kamijo, Y.; Funayama, M. A fast paraquat quantitation method in human serum using probe electrospray ionization—tandem mass spectrometry for emergency settings. J. Pharmacol. Toxicol. Methods 2019, 100, 106610. [Google Scholar] [CrossRef]
- Wen, C.; Lin, F.; Huang, B.; Zhang, Z.; Wang, X.; Ma, J.; Lin, G.; Chen, H.; Hu, L. Metabolomics Analysis in Acute Paraquat Poisoning Patients Based on UPLC-Q-TOF-MS and Machine Learning Approach. Chem. Res. Toxicol. 2019, 32, 629–637. [Google Scholar] [CrossRef]
- Liu, W.; Li, S.; Wu, Y.K.; Yan, X.; Zhu, Y.M.; Jiang, F.Y.; Jiang, Y.; Zou, L.H.; Wang, T.T. Metabolic profiling of rats poisoned with paraquat and treated with Xuebijing using a UPLC-QTOF-MS/MS metabolomics approach. Anal. Methods 2020, 12, 4562–4571. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; Wu, P.; Pan, J.; Guo, P.; Liu, S. In vivo microcapillary sampling coupled with matrix-assisted laser desorption/ionization fourier transform ion cyclotron resonance mass spectrometry for real-time monitoring of paraquat and diquat in living vegetables. Food Chem. 2022, 388, 132998. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Hou, X.; Qiu, S.; Jiao, Y.; Luo, X.; Xian, Z.; Wang, T. Determination of Paraquat in Vegetables by Ultra Performance Liquid Chromatogra—phy—Quadrupole/Electrostatic Field Orbitrap High Resolution Mass Spectrometry. J. Instrum. Anal. 2019, 38, 1126–1131. [Google Scholar]
- Nardin, T.; Barnaba, C.; Abballe, F.; Trenti, G.; Malacarne, M.; Larcher, R. Fast analysis of quaternary ammonium pesticides in food and beverages using cation-exchange chromatography coupled with isotope-dilution high-resolution mass spectrometry. J. Sep. Sci. 2017, 40, 3928–3937. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, Z.; Lou, J.; Mao, Z.; Li, K.; Chu, C.; Hu, L.; Li, Z.; Deng, C.; Fan, H.; et al. Identification of Serum-Based Metabolic Feature and Characteristic Metabolites in Paraquat Intoxicated Mouse Models. Front. Physiol. 2020, 11, 65. [Google Scholar] [CrossRef]
- Ni, Y.; Su, M.; Qiu, Y.; Jia, W.; Du, X. ADAP-GC 3.0: Improved Peak Detection and Deconvolution of Co-eluting Metabolites from GC/TOF-MS Data for Metabolomics Studies. Anal. Chem. 2016, 88, 8802–8811. [Google Scholar] [CrossRef] [PubMed]
- Laghrib, F.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Electrochemical sensors for improved detection of paraquat in food samples: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110349. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.M.; Liu, Y.; Lian, K.Q.; Ma, L.; Kang, W.J. Characterization of a sensitive biosensor based on an unmodified DNA and gold nanoparticle composite and its application in diquat determination. Arab. J. Chem. 2018, 11, 655–661. [Google Scholar] [CrossRef]
- Mhammedi, M.A.E.; Bakasse, M.; Bachirat, R.; Chtaini, A. Square wave voltammetry for analytical determination of paraquat at carbon paste electrode modified with fluoroapatite. Food Chem. 2008, 110, 1001–1006. [Google Scholar] [CrossRef]
- Shawish, H.M.A.; Ghalwa, N.A.; Hamada, M.; Basheer, A.-H. Modified carbon paste electrode for potentiometric dibromide pesticide in water and urine samples. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 140–145. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Yao, L.; Dai, P.; Ouyang, L.; Zhu, L. A sensitive and reproducible SERS sensor based on natural lotus leaf for paraquat detection. Microchem. J. 2021, 160, 105728. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Li, L.-H.; Wang, Y.; Wang, H.; Xu, Z.-L.; Tian, Y.-X.; Sun, Y.-M.; Yang, J.-Y.; Shen, Y.-D. Ultrasensitive and rapid colorimetric detection of paraquat via a high specific VHH nanobody. Biosens. Bioelectron. 2022, 205, 114089. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Duan, Y.; Yi, W.; Zhang, S.; Liang, W.; Li, H.; Yan, H.; Wu, B.; Fu, S.; Zhang, J.; et al. A rapid and reliable immunochromatographic strip for detecting paraquat poinsoning in domestic water and real human samples. Environ. Pollut. 2022, 315, 120324. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.-M.; Lin, S.-T.; Yen, T.-H.; Wang, Y.-L.; Cheng, C.-M. Paper-based diagnostic devices for clinical paraquat poisoning diagnosis. Biomicrofluidics 2016, 10, 034118. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; Lee, Y.-T.; Chen, H.-Y.; Ko, C.-H.; Hong, C.-T.; Wen, J.-W.; Yen, T.-H.; Cheng, C.-M. A Paper-Based Analytical Device for Analysis of Paraquat in Urine and Its Validation with Optical-Based Approaches. Diagnostics 2021, 11, 6. [Google Scholar] [CrossRef]
- Li, C.-B.; Li, X.-H.; Wang, Z.; Jiang, C.-H.; Peng, A. Serum paraquat concentration detected by spectrophotometry in patients with paraquat poisoning. World J. Emerg. Med. 2011, 2, 179–184. [Google Scholar] [CrossRef]
- de Almeida, R.M.; Yonamine, M. Enzymatic-spectrophotometric determination of paraquat in urine samples: A method based on its toxic mechanism. Toxicol. Mech. Methods 2010, 20, 424–427. [Google Scholar] [CrossRef]
- Li, Y.; Yao, G.; Ma, C.; Gong, M.; Yu, C.; Jie, N. Determination Of Paraquat In Water, Rice, And Urine Samples By Resonance Light Scattering Technique With Dna. Anal. Lett. 2011, 44, 709–716. [Google Scholar] [CrossRef]
- Sun, S.; Li, F.; Liu, F.; Wang, J.; Peng, X. Fluorescence detecting of paraquat using host-guest chemistry with cucurbit [8] uril. Sci. Rep. 2014, 4, 3570. [Google Scholar] [CrossRef]
- Chen, C.; Luo, H.; Zhang, Q.; Yang, Y.; Xiang, Z.; Liu, S. Rapid Determination of Paraquat in Whole Blood and Urine by Matrix Assisted Laser Desorption Ionization-Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Instrum. Anal. 2020, 39, 1126–1130. [Google Scholar]
- Chen, C.; Huang, Y.; Wu, P.; Pan, J.; Liu, S.; Guo, P. Rapid Determination of Paraquat and Diquat in Vegetables by Matrix Assisted Laser Desorption Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Instrum. Anal. 2021, 40, 684–689. [Google Scholar]
- Wu, X.; Li, W.; Guo, P.; Zhang, Z.; Xu, H. Rapid Trace Detection and Isomer Quantitation of Pesticide Residues via Matrix-Assisted Laser Desorption/lonization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 3966–3974. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Chu, X.; Zhao, Z.-X.; He, X.-S.; Guo, Y.-L. Analysis of low molecular weight compounds by MALDI-FTICR-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Xu, X.; Lv, Y.; Lei, W.; Hu, K.; Ye, F.; Zhao, S. Sulfonic acid functionalized hierarchical porous covalent organic frameworks as a SALDI-TOF MS matrix for effective extraction and detection of paraquat and diquat. J. Colloid Interface Sci. 2021, 603, 172–181. [Google Scholar] [CrossRef]
| Analytical Methods | Analytes | Matrix | Pretreatment Methods | Adsorbent/ Extractant | Recovery | LODS | Ref. |
|---|---|---|---|---|---|---|---|
| UPLC–HRMS | PQ & DQ | Human urine | MSPE | Fe3O4@SiO2@poly(4-VB) | PQ: 86.7–104.3% DQ: 100.3–109.9% | PQ: 0.12 μg/L DQ: 0.14 μg/L | [33] |
| LC–MS/MS | PQ & DQ | Blood & urine | Protein precipitation | Acetonitrile | PQ: Blood: 87.32–94.96%/urine: 88.93–108.36% DQ: Blood: 80.28–91.54%/urine: 95.56–101.02% | PQ: 0.1 μg/mL DQ: 0.05 μg/mL | [14] |
| HPLC–HRMS | PQ & DQ | Blood | Protein precipitation | Acetonitrile: water = 3:1 (v:v) | PQ: 86–108% DQ: 88–96% | PQ: 5 ng/mL DQ: 10 ng/mL | [19] |
| HPLC–DAD | PQ & DQ | Human plasma | SPE | 2 mL of ammonium formate, 2 mL of methanol, and 1 mL of a mixture solution of acetonitrile, ethyl acetate, and formic acid (4:4:2, v/v/v) | PQ: 95.38–103.97% DQ: 94.79–98.40% | 0.01 μg/mL | [24] |
| HPLC | PQ & DQ | Plasma & urine | MDSPE | CoFe2O4@SiO2 MNPs | 87.5–98.7% | PQ: 4.5 μg/L DQ: 4.3 μg/L | [35] |
| HPLC–UV | PQ | Biological samples | LLME | Mixture of triethylamine and water | 90.0–92.3% | 0.2 μg/L | [30] |
| HPLC–MS | PQ | Urine | DSPE | magnetic single-walled carbon nanotubes | 92.89–108.9% | 0.94 μg/L | [34] |
| GC–MS | PQ | Plasma & urine | HS-SPME | Polydimethylsiloxane fiber | Plasma: 94.00–99.85% Urine: 95.00–100.34% | 0.01 μg/mL | [29] |
| UPLC–HRMS | PQ | Plasma & urine | Protein precipitation | Acetonitrile | Plasma: 98.54–100.90% Urine: 93.51–99.45% | Urine: 0.1 ng/Ml Plasma: 0.3 ng/mL | [13] |
| GC–MS | PQ & DQ | Serum & urine | Monolithic spin column extraction | 0.2 mL of a mixture of chloroform and methanol (9:1 v/v) (MonoSpin® C18 extraction column) | 51.3–106.1% | PQ: 0.1 μg/mL DQ: 0.025 μg/mL | [26] |
| HPLC–MS | PQ & DQ | Human urine | SPE | 1 mL of 5% methanol in deionized water (v/v) & 10% formic acid in acetonitrile (v/v) (Strata-X-CW 33 μm polymeric 3 mL weak cation cartridges) | PQ: 83.4–85.5% DQ: 77.7–94.2% | 1 ng/mL | [27] |
| UV-Vis Spectrometry | PQ | Plasma & urine | MSPE | Fe3O4@SiO2 NPs | Plasma: 93.6–102.4% Urine: 92.9–103.5% | 12.2 μg/L | [36] |
| UPLC–HRMS | PQ | human urine | MSPE | Amphiphilic carboxyl-functionalized magnetic polymer microspheres (Amphiphilic-MPs-COOH) | 84.5–103% | 0.1–1.6 μg/L | [32] |
| LC–MS | PQ | Tissue | Whirling agitated single-drop microextraction | 1-dodecanol | >91.21% | 4.81 ng/g | [31] |
| HPLC–UV | PQ | Human plasma | protein precipitation with organic solvent backwashing | Acetonitrile and methylene chloride | 91.9% | 0.01 μg/mL | [21] |
| HPLC–UV | PQ | The whole blood | Protein precipitation with hydrochloric acid | Acetonitrile | 87.9–106.7% | 0.026 μg/mL | [18] |
| HPLC–UV | PQ | Human plasma | Protein precipitation | Trichloroacetic acid-methanol (1:9) | n.d. | n.d. | [17] |
| Matrix | Analytical Column | Mobile Phase | LODs | LOQs | Ref. |
|---|---|---|---|---|---|
| Blood/Urine | Agilent ZORBAX SB-Aq column (100 mm × 2.1 mm, 1.8 μm) | A: 15 mM HFBA B: acetonitrile Flow rate: 0.30 mL/min Gradient elution | PQ: 100 ng/mL DQ: 50 ng/mL | PQ: 200 ng/mL DQ: 100 ng/mL | [14] |
| Urine | Capcell Pak ST column (Shiseido Co., Ltd., Japan; 150 mm × 2.0 mm, 2.6 μm) | A: 0.4%TFA in water B: acetonitrile Flow rate: 0.30 mL/min Isocratic elution | PQ: 0.94 ng/mL | PQ: 2.82 ng/mL | [34] |
| Urine | SIELC Obelisc R columna (150 mm × 2.1 mm, 5 μm) | A: ammonium formate in water (Ph = 3.7) B: acetonitrile Flow rate: 0.5 mL/min Isocratic elution | PQ: 0.12 ng/mL DQ: 0.14 ng/mL | n.d. | [33] |
| DBS (Dry Blood Spot) | Hypersil GOLD C-18 column (100 mm × 2.1 mm, 1.9 μm) | A: 20 mM ammonium acetate with 0.1% formic acid B: acetonitrile Flow rate: 0.3 mL/min Isocratic elution | PQ: 0.5 ng/mL | PQ: 0.5 ng/mL | [43] |
| Plasma/Urine | ACQUITY UPLC bridged ethyl hybrid (BEH) HILIC column (100 mm × 2.1 mm, 1.7 μm) | A: 0.5% formic acid in 40 mM ammonium formate B: acetonitrile Flow rate: 0.3 mL/min Gradient elution | Plasma: 0.3 ng/mL Urine: 0.1 ng/mL | Plasma: 0.8 ng/mL Urine: 0.3 ng/mL | [13] |
| Plasma/Urine | Kinetex™ HILIC column (50 mm × 2.10 mm, 2.6 μm) | A: 250 mm ammonium formate containing 0.8% formic acid in water B: acetonitrile Flow rate: 0.3 mL/min Gradient elution | n.d. | PQ: 0.01 ng/mL | [20] |
| Plasma | Hypersil Gold C18 Column (250 mm × 4.6 mm, 5 μm) | A: acetonitrile B: 75 mmol/L sodium heptanesulfonate water solution (including 0.1 mol/L phosphoric acid) Flow rate: 1.0 mL/min Gradient elution | n.d. | PQ: 50 ng/mL | [23] |
| Plasma/Urine | Ion Pac CS18 (250 mm × 2.0 mm, 6.0 μm) | A: 30 mM MSA B: formic acid:acetonitrile (3:100, v:v) Flow rate: 0.3 mL/min Gradient elution | PQ: 1 ng/mL DQ: 0.5 ng/mL | PQ: 0.3 ng/mL DQ: 0.2 ng/mL | [22] |
| Urine | Atlantis® HILIC Silica, (150 mm × 2.1 mm, 5 μm) | A: 250 mM ammonium formate in deionized water, pH 3.7 B: acetonitrile Flow rate: 0.4 mL/min Isocratic elution | PQ: 0.63 ng/mL DQ: 0.13 ng/mL | n.d. | [27] |
| Blood | Hypersil GOLDTMC18 (100 mm × 2.1 mm, 1.9 μm) | A: 0.1% formic acid B: methanol Flow rate: 0.3 mL/min Gradient elution | PQ: 5 ng/mL DQ: 10 μg/mL | PQ: 10 ng/mL DQ: 20 ng/mL | [19] |
| Brain Tissue | ZORBAX RX-C8 column (150 mm × 4.6 mm, 5 μm) | with a three-solvent system: 0.1% formic acid in water (A), 0.1% formic acid in methanol (B), 0.1% formic acid in acetonitrile (C) Flow rate: 0.3 mL/min Gradient elution | PQ: 0.1 ng/mL | PQ: 2 ng/mL | [31] |
| Single Q MS | QQQ MS | LIT–MS | TOF MS | Orbitrap MS | FTICR MS |
|---|---|---|---|---|---|
| Advantages: | |||||
| Relatively small size and low cost | Cascade function and strong qualitative ability | Multistage tandem MS | Faster scan speed than that of orbitrap | Wider dynamic range than TOF (4 order of magntude) | Capability of multi-level cascade |
| Robustness and ease of maintenance | Good quantitative ability | Higher sensitivity than that of traditional 3D IT | high mass upper limit (6000–10,000 u) | Fast positive–negative ionization switching | Very good qualitative ability |
| Commonly used, especially in GC–MS | Higher S/N than that of single Q MS | Durable and easy to miniaturize | Good resolution for high m/z ions and large molecules | Higher resolution than TOF MS | Highest resolution; best sensitivity compared with the other four MS analyzers |
| Varied scanning modes (MRM, SRM, neutral loss, etc.) | Unknown components with a low content can be analyzed | High sensitivity | Stable mass axis (1 week) that is not affected by the environment | Capability of being combined with other ionization sources for the detection of different polarity compounds | |
| Disadvantages: | |||||
| Low resolving power and disturbances from the isotope and the other m/z approximation ionic | Low resolution | Compared to QQQ, there is a lack of a characteristic group screening function | Mass axis needs to be calibrated frequently | Limited ion capacity | Bulk weight, high cost |
| Insufficient quantitative ability | High cost and the requirement of delicate maintenance compared single Q MS | Relatively low resolution | More expensive than Q MS | Incapability of the cascade alone | Limited data acquisition speed |
| Limited capability in terms of mass range (usually <4000 Da) | Lack of quantitative analysis ability for untargeted unknowns | Narrower range of quality analysis than that of TOF | Noisier TOF baselines than those of orbitrap due to spurious signals | Limited data acquisition speed in the high mass resolution setting | Maintenance is very expensive |
| Matrix | Analytes | Electrode | Technique | Linear Range (M) | Detection Limit (M) | Ref. |
|---|---|---|---|---|---|---|
| Human urine, serum, natural samples | PQ | DNA-3D GNP/GE | DPV | 7.0 × 10−9–1.5 × 10−6 | 2.0 × 10−10 | [63] |
| Potato, lemon, orange, and natural water samples | PQ | CCPE | DPASV | 5 × 10−7–240 × 10−7 | 1.63 × 10−9 | [64] |
| Meconium | PQ | Ab/C60-FC-IL-GCE | CVs | 3.89 × 10−11 to 4.0 × 10−8 | 9.0 × 10−12 | [65] |
| Human urine and grain | DQ | CA DNA-GNP/GE | DPV | 1.0 × 10−9 to 1.2 × 10−6 | 2.0 × 10−10 | [77] |
| Apple and potato | PQ | FAP-CPE | SWV | 5 × 10−8 to 7 × 10−5 | 3.5 × 10−9 | [78] |
| Water and urine samples | DQ | Dq-PT-2-NPOE-Na-TPB/CPE | Potentiometric titration methods | 3.8 × 10−6 to 1.0 × 10−3 | 9.0 × 10−7 | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Li, L.; Gao, L. Paraquat and Diquat: Recent Updates on Their Pretreatment and Analysis Methods since 2010 in Biological Samples. Molecules 2023, 28, 684. https://doi.org/10.3390/molecules28020684
Guo H, Li L, Gao L. Paraquat and Diquat: Recent Updates on Their Pretreatment and Analysis Methods since 2010 in Biological Samples. Molecules. 2023; 28(2):684. https://doi.org/10.3390/molecules28020684
Chicago/Turabian StyleGuo, Honghui, Ling Li, and Lina Gao. 2023. "Paraquat and Diquat: Recent Updates on Their Pretreatment and Analysis Methods since 2010 in Biological Samples" Molecules 28, no. 2: 684. https://doi.org/10.3390/molecules28020684
APA StyleGuo, H., Li, L., & Gao, L. (2023). Paraquat and Diquat: Recent Updates on Their Pretreatment and Analysis Methods since 2010 in Biological Samples. Molecules, 28(2), 684. https://doi.org/10.3390/molecules28020684






