Ultrafine Co-Species Interspersed g-C3N4 Nanosheets and Graphene as an Efficient Polysulfide Barrier to Enable High Performance Li-S Batteries
Abstract
1. Introduction
2. Results and Discussion
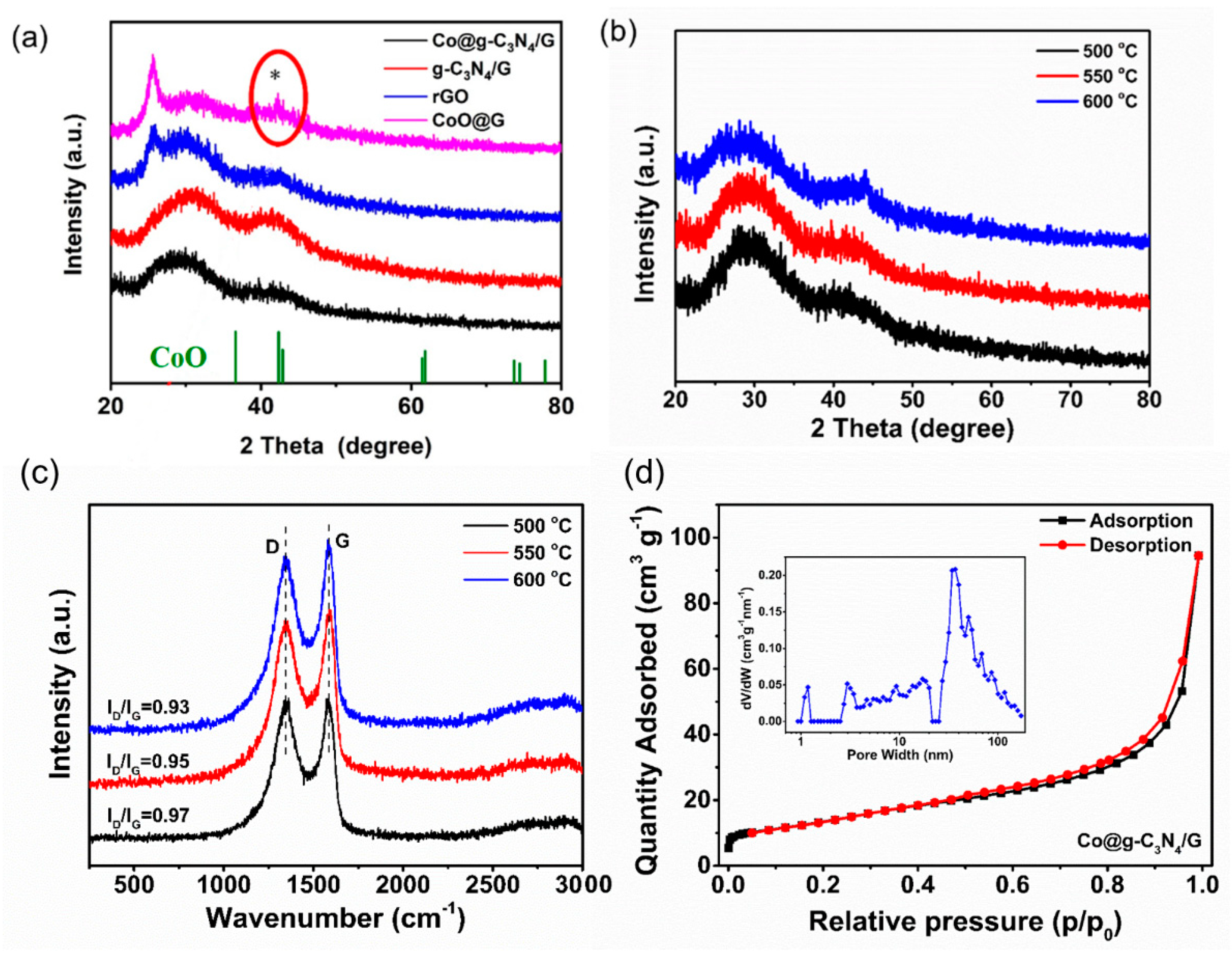
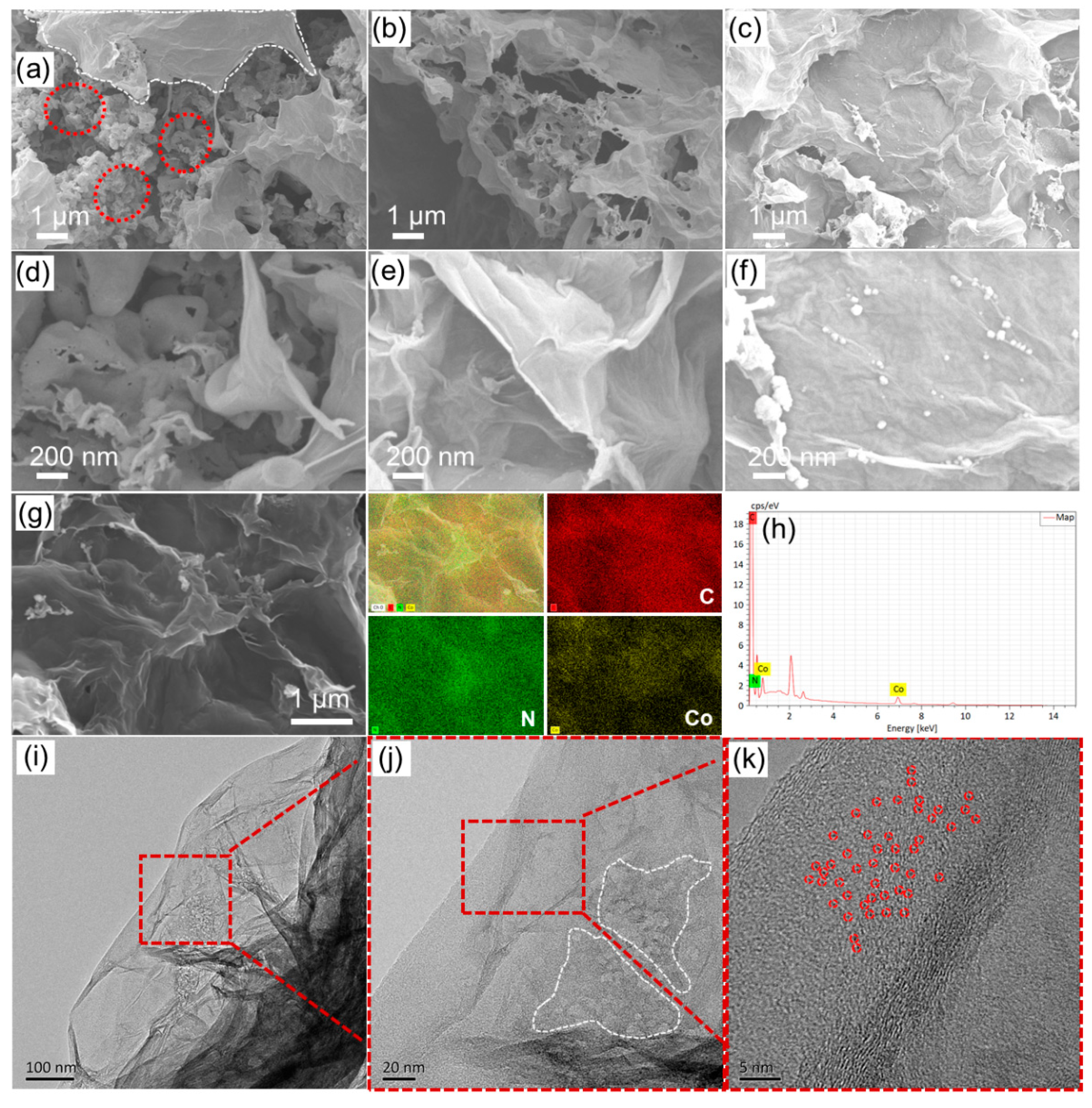
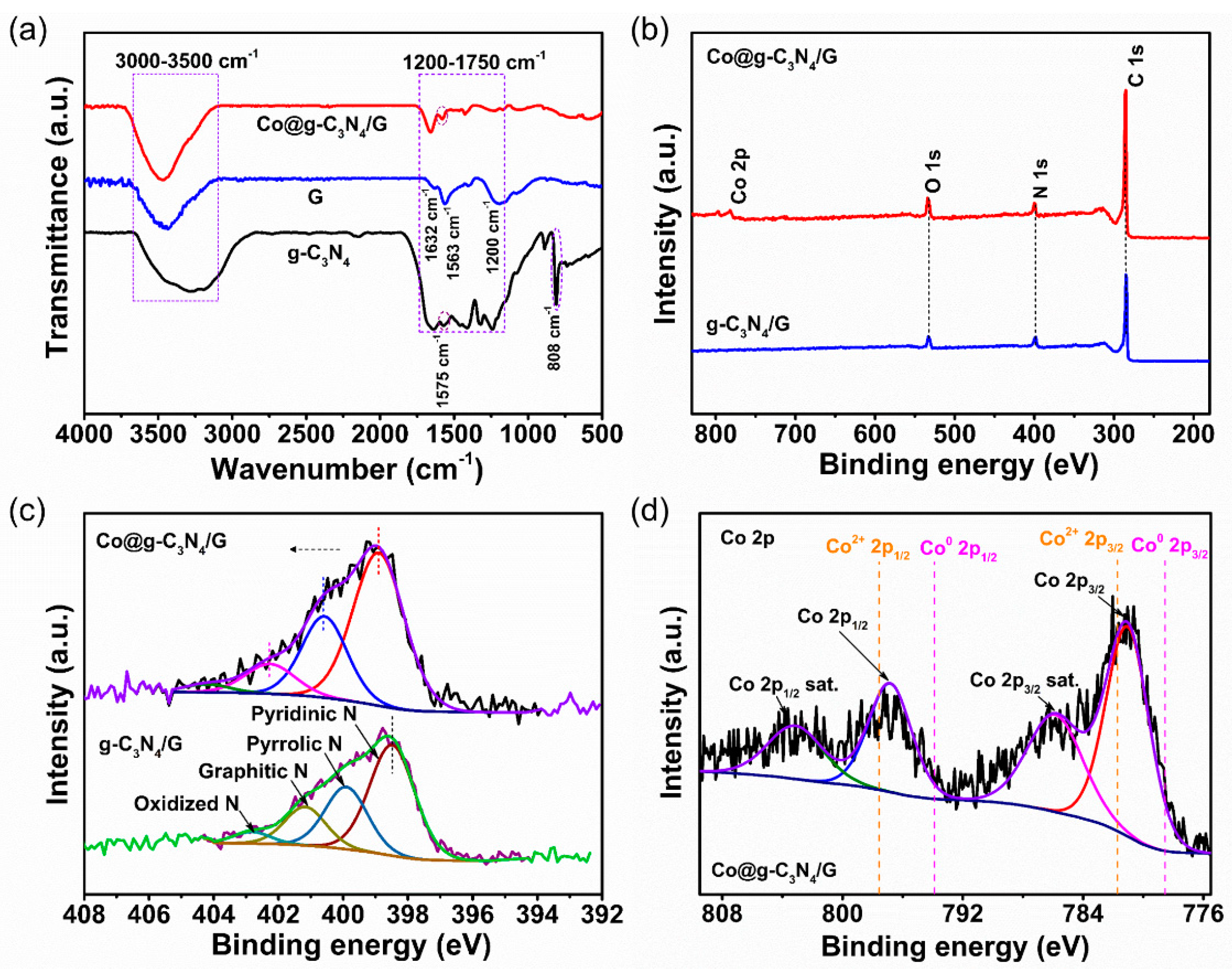
2.1. Synthesis and Characterization of the Co@g-C3N4/G Heterostructure Composites
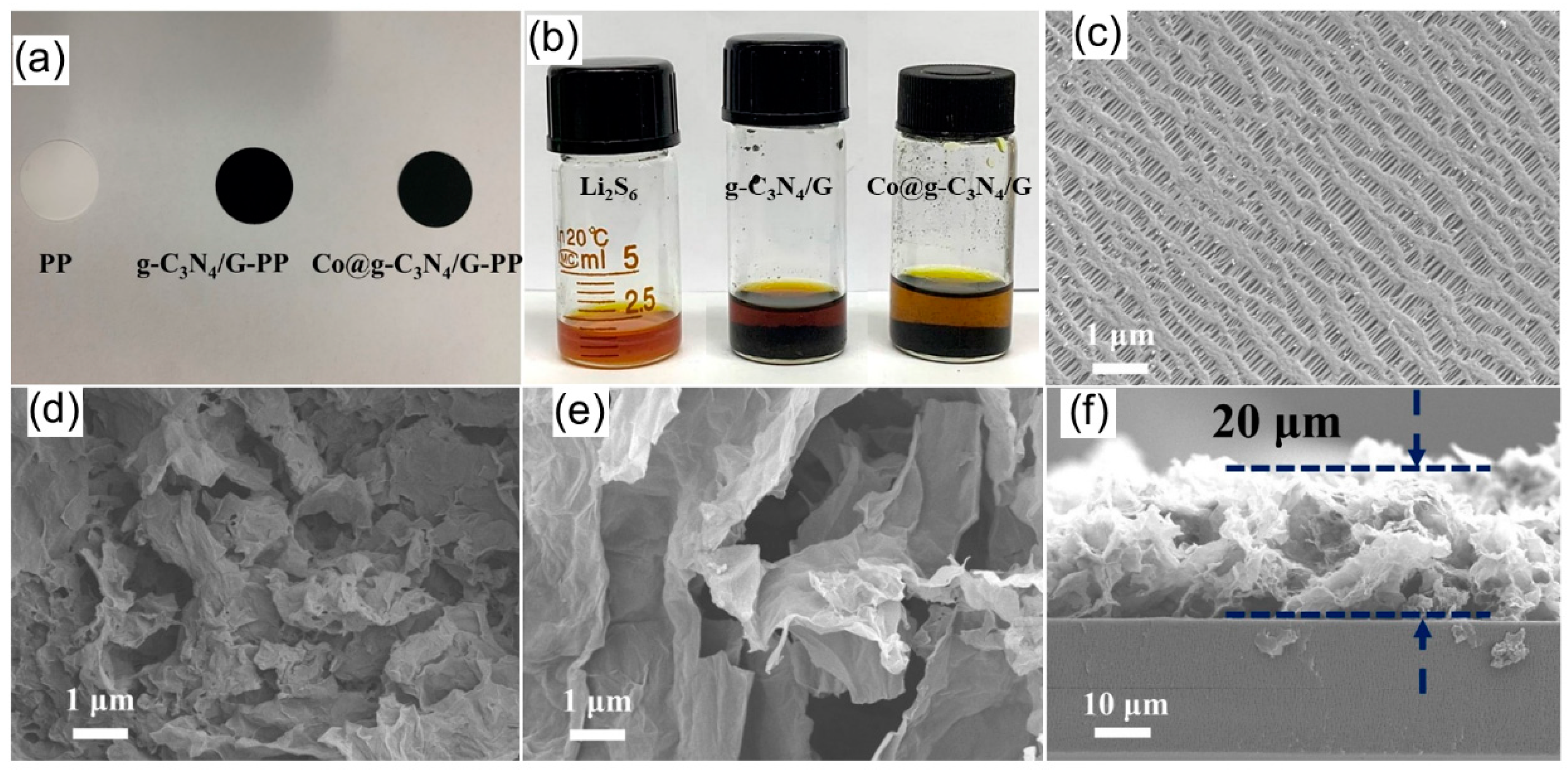
2.2. Visible Adsorption Experiments of Prepared Materials toward Li2S6 Solutions and Morphologies of g-C3N4/G- and Co@g-C3N4/G -PP Separators
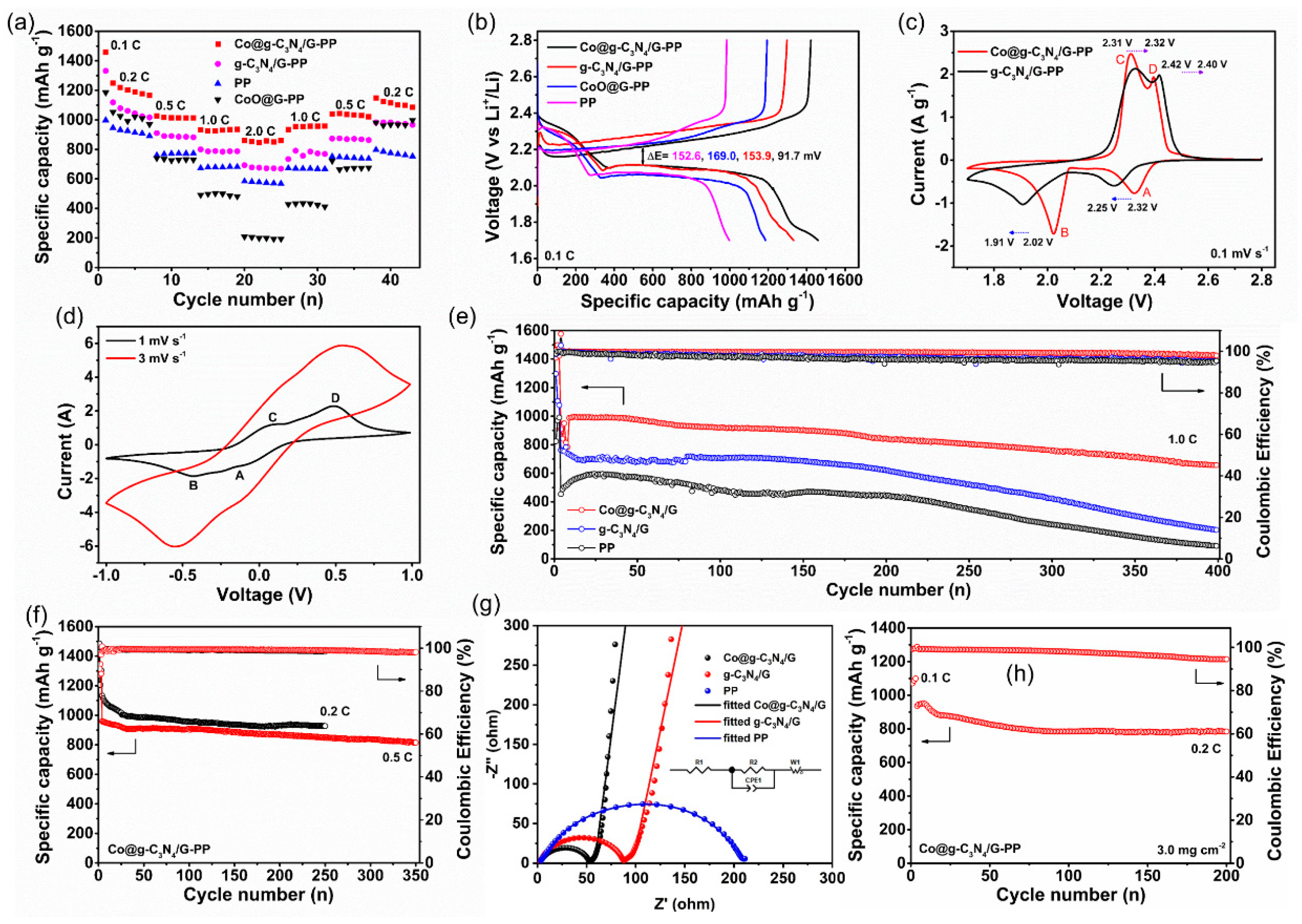
2.3. Electrochemical Performance of Li-S Batteries with the Modified Separators
3. Materials and Methods
3.1. Fabrication of Highly Dispersed Ultrafine Co-Species Interspersed g-C3N4 Nanosheets and Graphene Heterostructure (Co@g-C3N4/G)
3.2. Fabrication of the Co@g-C3N4/G-PP, g-C3N4/G-PP, and CoO@G-PP Separators
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Payandeh, S.; Strauss, F.; Mazilkin, A.; Kondrakov, A.; Brezesinski, T. Tailoring the LiNbO3 coating of Ni-rich cathode materials for stable and high-performance all-solid-state batteries. Nano Res. Energy 2022, 1, e9120016. [Google Scholar] [CrossRef]
- Zhao, W.X.; Gao, L.X.; Ma, X.Q.; Yue, L.C.; Zhao, D.L.; Li, Z.R.; Sun, S.J.; Luo, Y.S.; Liu, Q.; Sun, X.P.; et al. An exquisite branch-leaf shaped metal sulfoselenide composite endowing an ultrastable sodium-storage lifespan over 10,000 cycles. J. Mater. Chem. A 2022, 10, 16962–16975. [Google Scholar] [CrossRef]
- Yue, L.h.; Ma, C.Q.; Yan, S.H.; Wu, Z.G.; Zhao, W.X.; Liu, Q.; Luo, Y.L.; Zhong, B.H.; Zhang, F.; Liu, Y.; et al. Improving the intrinsic electronic conductivity of NiMoO4 anodes by phosphorous doping for high lithium storage. Nano Res. 2022, 15, 186–194. [Google Scholar] [CrossRef]
- Bhargav, A.; He, J.R.; Gupta, A.; Manthiram, A. Lithium-sulfur batteries: Attaining the critical metrics. Joule 2020, 4, 285–291. [Google Scholar] [CrossRef]
- Deng, D.; Xue, F.; Bai, C.; Lei, J.; Yuan, R.; Zheng, M.; Dong, Q. Enhanced adsorptions to polysulfides on graphene-supported BN nanosheets with excellent Li-S battery performance in a wide temperature range. ACS Nano 2018, 12, 11120–11129. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, G.Y.; Jiang, H.R.; Chen, Q.; Zhao, T.S. Achieving multiplexed functionality in a hierarchical MXene-based sulfur host for high-rate, high-loading lithium-sulfur batteries. Energy Storage Mater. 2020, 33, 147–157. [Google Scholar] [CrossRef]
- Luo, R.; Zhang, Z.; Xi, B.J.; Feng, J.K.; Xiong, S.L. Bimetal CoNi active sites on mesoporous carbon nanosheets to kinetically boost lithium-sulfur batteries. Small 2021, 17, 2100414. [Google Scholar] [CrossRef]
- Yu, Z.S.; Liu, M.L.; Guo, D.Y.; Wang, J.H.; Chen, X.; Li, J.; Jin, H.L.; Yang, Z.; Chen, X.A.; Wang, S. Radially inwardly aligned hierarchical porous carbon for ultra-long-life lithium-sulfur batteries. Angew. Chem. Int. Ed. 2020, 59, 6406. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Yen, Y.-J.; Tseng, Y.-H.; Chung, S.-H. Module-designed carbon-coated separators for high-loading, high-sulfur-utilization cathodes in lithium-sulfur batteries. Molecules 2022, 27, 228. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zou, K.; Qian, Y.; Deng, Y.; Zhang, L.; Chen, G. Insight to the synergistic effect of N-doping level and pore structure on improving the electrochemical performance of sulfur/N-doped porous carbon cathode for Li-S batteries. Carbon 2019, 144, 745–755. [Google Scholar] [CrossRef]
- Wei, W.L.; Liu, P. Rational porous design for carbon nanotubes derived from tubular polypyrrole as sulfur host for lithium-sulfur batteries. Micropor. Mesopor. Mat. 2021, 311, 110705. [Google Scholar] [CrossRef]
- Wang, J.X.; Jiang, K.L.; Shen, B.; Zhen, M.M. Synergetic effect of nitrogen/sulfur dual-doped hierarchically porous carbon networks for Li-S batteries. ACS Sustain. Chem. Eng. 2020, 8, 749. [Google Scholar] [CrossRef]
- Jo, S.-C.; Hong, J.-W.; Choi, I.-H.; Kim, M.-J.; Gon Kim, B.; Lee, Y.-J.; Young Choi, H.; Kim, D.; Kim, T.Y.; Baeg, K.-J.; et al. Multimodal capturing of polysulfides by phosphorus-doped carbon composites for flexible high-energy-density lithium-sulfur batteries. Small 2022, 18, 2202326. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Liu, J.F.; Cao, W.Q.; Li, X.L.; Zhang, C.H. 3D printing CO2-activated carbon nanotubes host to promote sulfur loading for high areal capacity lithium-sulfur batteries. Nano Res. 2022. [Google Scholar] [CrossRef]
- Han, W.J.; Li, Q.; Zhu, H.; Luo, D.; Qin, X.Y.; Li, B.H. Hierarchical porous graphene bubbles as host materials for advanced lithium sulfur battery cathode. Front. Chem. 2021, 9, 653476. [Google Scholar] [CrossRef]
- Cheng, Z.B.; Pan, H.; Chen, J.Q.; Meng, X.P.; Wang, R.H. Separator modified by cobalt-embedded carbon nanosheets enabling chemisorption and catalytic effects of polysulfides for high-energy-density lithium-sulfur batteries. Adv. Energy Mater. 2019, 9, 1901609. [Google Scholar] [CrossRef]
- Gao, X.G.; Huang, Y.; Li, X.; Gao, H.; Li, T.H. SnP0.94 nanodots confined carbon aerogel with porous hollow superstructures as an exceptional polysulfide electrocatalyst and “adsorption nest” to enable enhanced lithium-sulfur batteries. Chem. Eng. J. 2021, 420, 129724. [Google Scholar] [CrossRef]
- Jing, E.D.; Chen, L.; Xu, S.D.; Tian, W.Z.; Zhang, D.; Wang, N.; Bai, Z.C.; Zhou, X.X.; Liu, S.B.; Duan, D.H.; et al. Dual redox catalysis of VN/nitrogen-doped graphene nanocomposites for high-performance lithium-sulfur batteries. J. Energy Chem. 2022, 64, 574–582. [Google Scholar] [CrossRef]
- Pei, F.; Lin, L.; Fu, A.; Mo, S.; Ou, D.; Fang, X.; Zheng, N. A two-dimensional porous carbon-modified separator for high-energy-density Li-S batteries. Joule 2018, 2, 323–337. [Google Scholar] [CrossRef]
- Wang, F.; Ding, X.; Shi, R.; Li, M.; Lei, Y.; Lei, Z.; Jiang, G.; Xu, F.; Wang, H.; Jia, L.; et al. Facile synthesis of Ti4O7 on hollow carbon spheres with enhanced polysulfide binding for high performance lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 10494–10504. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, J.; Duan, S.R.; Zhang, J.; Wang, Q.; Zhang, Y.; Li, L.G.; Liu, H.T.; Xiao, Q.B.; Lin, H.Z. Anionic oxygen vacancies in Nb2O5-x/carbon hybrid host endow rapid catalytic behaviors for high-performance high areal loading lithium sulfur pouch cell. Chem. Eng. J. 2021, 417, 128172. [Google Scholar] [CrossRef]
- Xu, J.; Yang, L.K.; Cao, S.F.; Wang, J.W.; Ma, Y.M.; Zhang, J.J.; Lu, X.Q. Sandwiched cathodes assembled from CoS2-modified carbon clothes for high-performance lithium-sulfur batteries. Adv. Sci. 2021, 8, 2101019. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.B.; Chen, Y.L.; Yang, Y.S.; Zhang, L.J.; Pan, H.; Fan, X.; Xiang, S.C.; Zhang, Z.J. Metallic MoS2 nanoflowers decorated graphene nanosheet catalytically boosts the volumetric capacity and cycle life of lithium-sulfur batteries. Adv. Energy Mater. 2021, 11, 2003718. [Google Scholar] [CrossRef]
- Tan, T.X.; Chen, N.N.; Wang, Z.K.; Tang, Z.M.; Zhang, H.R.; Lai, Q.X.; Liang, Y.R. Thorn-like carbon nanofibers combined with molybdenum nitride nanosheets as a modified separator coating: An efficient chemical anchor and catalyst for Li-S batteries. ACS Appl. Energy Mater. 2022, 5, 6654. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Duan, H.; Deng, Y.; Chen, G. Fe3C/Fe nanoparticles embedded in N-doped porous carbon nanosheets and graphene: A thin functional interlayer for PP separator to boost performance of Li-S batteries. Chem. Eng. J. 2021, 415, 129001. [Google Scholar] [CrossRef]
- Tsao, Y.C.; Gong, H.X.; Chen, S.C.; Chen, G.; Liu, Y.Z.; Gao, T.Z.; Cui, Y.; Bao, Z.N. A nickel-decorated carbon flower/sulfur cathode for lean-electrolyte lithium-sulfur batteries. Adv. Energy Mater. 2021, 36, 2101449. [Google Scholar] [CrossRef]
- Shen, Z.H.; Jin, X.; Tian, J.M.; Li, M.; Yuan, Y.F.; Zhang, S.; Fang, S.S.; Fan, X.; Xu, W.G.; Lu, H.; et al. Cation-doped ZnS catalysts for polysulfide conversion in lithium-sulfur batteries. Nat. Catal. 2022, 5, 555–563. [Google Scholar] [CrossRef]
- Wang, Z.K.; Wang, Y.; Ji, H.Q.; Zhou, J.Q.; Qian, T.; Yan, C.L. Unity of opposites between soluble and insoluble lithium polysulfides in lithium-sulfur batteries. Adv. Mater. 2022, 34, e2203699. [Google Scholar] [CrossRef]
- He, L.; Yang, D.; Zhao, H.N.; Wei, L.Y.; Wang, D.S.; Wang, Y.Z.; Chen, G.; Wei, Y.J. Bipolar CoSe2 nanocrystals embedded in porous carbon nanocages as an efficient electrocatalyst for Li-S batteries. Chem. Eng. J. 2022, 440, 135820. [Google Scholar] [CrossRef]
- Pei, H.Y.; Yang, Q.; Waterhouse, G.I.N.; Guo, J.L.; Lu, S.Y. Self-supporting carbon nanofibers with Ni-single-atoms and uniformly dispersed Ni-nanoparticles as scalable multifunctional hosts for high energy density lithium-sulfur batteries. Small 2022, 27, 2202037. [Google Scholar] [CrossRef]
- Li, M.H.; Wang, H.; Wang, X.Y.; Ren, J.W.; Wang, R.F. Metallic FeCo clusters propelling the stepwise polysulfide conversion in lithium-sulfur batteries. J. Mater. Chem. 2022, 10, 21327–21335. [Google Scholar]
- Xiao, Z.B.; Li, Z.L.; Li, P.Y.; Meng, X.P.; Wang, R.H. Ultrafine Ti3C2 MXene nanodots-interspersed nanosheet for high-energy-density lithium-sulfur batteries. ACS Nano 2019, 13, 3608–3617. [Google Scholar] [PubMed]
- Zhang, Z.P.; Gao, X.J.; Dou, M.L.; Ji, J.; Wang, F. Biomass derived N-doped porous carbon supported single Fe atoms as superior electrocatalysts for oxygen reduction. Small 2017, 13, 1604290. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Y.X.; Zhang, K.Q.; Cheng, F.; Jiang, R.Y.; Liu, Y.Q.; Zhu, J.; Jin, Z.; Pang, H. Rapid construction of highly-dispersed cobalt nanoclusters embedded in hollow cubic carbon walls as an effective polysulfide promoter in high-energy lithium-sulfur batteries. Nano Res. 2022, 6, 5105. [Google Scholar] [CrossRef]
- Liu, R.Q.; Liu, Z.W.; Liu, W.H.; Liu, Y.J.; Lin, X.J.; Li, Y.; Huang, P. TiO2 and Co nanoparticle-decorated carbon polyhedral as efficient sulfur host for high-performance lithium-sulfur batteries. Small 2019, 12, 1804533. [Google Scholar] [CrossRef]
- Zhang, J.L.; Cheng, Y.; Chen, H.B.; Wang, Y.; Chen, Q.; Hou, G.Y.; Wen, M.; Tang, Y.P. MoP quantum dot-modified N,P-carbon nanotubes as a multifunctional separator coating for high-performance lithium-sulfur batteries. ACS Appl. Mater. Inter. 2022, 14, 16289–16299. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Meng, Z.; Yang, W.; Yan, X.; Guo, R.; Han, W. Facile synthesis of rGO/g-C3N4/CNT microspheres via an ethanol assisted spray-drying method for high-performance lithium-sulfur batteries. ACS Appl. Mater. Inter. 2019, 11, 819–827. [Google Scholar] [CrossRef]
- Wen, Z.; Wen, X.; Mao, S.; Mao, Z.; Kim, H.; Cui, S.; Lu, G.; Lu, X.; Chen, J. Crumpled nitrogen-doped graphene nanosheets with ultrahigh pore volume for high-performance supercapacitor. Adv. Mater. 2012, 24, 5610–5616. [Google Scholar] [CrossRef]
- Jiang, J.; Duan, D.L.; Ma, J.; Long, R.; Gao, C.; Xiong, Y.J. Van der waals heterostructures by single cobalt sites-anchored graphene and g-C3N4 nanosheets for photocatalytic syngas production with tunable CO/H2 ratio. Appl. Catal. B-Environ. 2021, 295, 120261. [Google Scholar] [CrossRef]
- Chen, A.M.; Liu, R.; Peng, X.; Chen, Q.F.; Wu, J.M. 2D hybrid nanomaterials for selective detection of NO2 and SO2 using “light on and off” strategy. ACS Appl. Mater. Inter. 2017, 9, 37191–37200. [Google Scholar]
- Gao, H.H.; Yang, H.C.; Xu, J.Z.; Li, J.X. Strongly coupled g-C3N4 nanosheets-Co3O4 quantum dots as 2D/0D heterostructure composite for peroxymonosulfate activation. Small 2018, 14, 1801355. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, S.X.; Duan, H.H.; Deng, Y.F.; Chen, G.H. A thin and multifunctional CoS@g-C3N4/Ketjen black interlayer deposited on polypropylene separator for boosting the performance of lithium-sulfur batteries. J. Colloid Interf. Sci. 2022, 1, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, W.; Shan, J.W.; Zhu, J.L.; Wu, S.; Li, F.; Liu, Z.G.; Li, Y.Y. High volumetric energy density Li-S batteries enabled by dense sulfur monolith cathodes with ultra-small-sized sulfur immobilizers. Chem. Eng. J. 2020, 401, 126076. [Google Scholar] [CrossRef]
- Cao, J.; Chen, C.; Zhao, Q.; Zhang, N.; Wang, X.Y.; Niu, Z.Q.; Chen, J. A flexible nanostructured paper of a reduced graphene oxide-sulfur composite for high-performance lithium-sulfur batteries with unconventional configurations. Adv. Mater. 2016, 28, 9629–9636. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Zhang, H.; Wang, M.M.; Han, G.Y. Hollow CoO nanoparticles embedded in N-doped mesoporous graphene for efficient oxygen reduction reaction. ChemistrySelect 2022, 7, e202200941. [Google Scholar] [CrossRef]
- Wan, B.; Guo, J.; Lai, W.; Wang, Y.; Liu, M.; Liu, H.; Wang, J.; Chou, S. Layered mesoporous CoO/reduced graphene oxide with strong interfacial coupling as a high-performance anode for lithium-ion batteries. J. Alloys Compd. 2021, 843, 156050. [Google Scholar] [CrossRef]
- Wang, S.Z.; Wang, J.L. Single atom cobalt catalyst derived from co-pyrolysis of vitamin B12 and graphitic carbon nitride for PMS activation to degrade emerging pollutants. Appl. Catal. B-Environ. 2023, 321, 122051. [Google Scholar] [CrossRef]
- Hu, J.S.; Zhang, P.F.; An, W.J.; Liu, L.; Liang, Y.H.; Cui, W.Q. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-Fenton reaction with enriched photocatalytic performance for removal of complex wastewater. Appl. Catal. B-Environ. 2019, 245, 130–142. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Huang, X.K.; Chen, N.; Qu, L.T. Three-dimensional graphitic carbon nitride functionalized graphene based high-performance supercapacitors. J. Mater. Chem. 2015, 3, 6761. [Google Scholar] [CrossRef]
- Hou, W.; Li, Y.; Ouyang, S.; Chen, H.; Ye, J.; Han, X.; Deng, Y. Bifunctional hydroxyl group over polymeric carbon nitride to achieve photocatalytic H2O2 production in ethanol aqueous solution with an apparent quantum yield of 52.8% at 420 nm. Chem. Commun. 2019, 55, 13279–13282. [Google Scholar] [CrossRef]
- Zhang, L.Q.; He, X.; Xu, X.W.; Liu, C.; Duan, Y.L.; Hou, L.Q.; Ma, C.; Yang, X.P.; Yang, F.; Cui, L.S.; et al. Highly active TiO2/g-C3N4/G photocatalyst with extended spectral response towards selective reduction of nitrobenzene. Appl. Catal. B-Environ. 2017, 203, 1–8. [Google Scholar] [CrossRef]
- Du, Z.Z.; Du, X.J.; Hu, W.; Chuang, C.H.; Xie, S.; Hu, A.; Yan, W.S.; Kong, X.H.; Wu, X.J.; Ji, H.X.; et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium-sulfur batteries. J. Am. Chem. Soc. 2019, 141, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Shi, C.S.; Sha, J.W.; Ma, L.Y.; Liu, E.Z.; Zhao, N.Q. Single-atom cobalt supported on nitrogen-doped three-dimensional carbon facilitating polysulfide conversion in lithium-sulfur batteries. ACS Appl. Mater. Inter. 2022, 14, 25337–25347. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Sun, Y.J.; Wu, J.H.; Jin, J.; Wei, C.H.; Shi, Z.X.; Wang, M.L.; Cai, J.S.; An, X.-T.; Wang, P.; et al. A dual-functional fibrous skeleton implanted with single-atomic Co-Nx dispersions for longevous Li-S full batteries. ACS Nano 2021, 15, 14105–14115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Zhou, P.; Li, H.; Gao, T.T.; Zhou, L.; Zhang, Y.L.; Xiao, N.; Xia, Z.H.; Wang, L.; Zhang, Q.H.; et al. A freestanding flexible single-atom cobalt-based multifunctional interlayer toward reversible and durable lithium-sulfur batteries. Small Methods 2020, 4, 1900701. [Google Scholar] [CrossRef]
- Yin, P.Q.; Yao, T.; Wu, Y.; Zheng, L.R.; Lin, Y.; Liu, W.; Ju, H.X.; Zhu, J.F.; Hong, X.; Deng, Z.X.; et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem. Int. Ed. 2016, 128, 10958–10963. [Google Scholar] [CrossRef]
- Wang, P.; Ren, Y.Y.; Wang, R.T.; Zhang, P.; Ding, M.J.; Li, C.X.; Zhao, D.Y.; Qian, Z.; Zhang, Z.W.; Zhang, L.Y.; et al. Atomically dispersed cobalt catalyst anchored on nitrogen-doped carbon nanosheets for lithium-oxygen batteries. Nat. Commun. 2020, 11, 1576. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Zhou, J.; Zhao, H.; Lin, N.; Zhu, L.; Zhu, Y.; Wang, G.; Qian, Y. Fully integrated hierarchical double-shelled Co9S8@CNT nanostructures with unprecedented performance for Li-S batteries. Nanoscale Horiz. 2019, 4, 182. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, W.Y.; Zhang, B.; Fu, L.; Wan, M.T.; Li, G.C.; Cai, Z.; Tu, S.B.; Duan, X.R.; Seh, Z.W.; et al. Manipulating redox kinetics of sulfur species using mott-schottky electrocatalysts for advanced lithium-sulfur batteries. Nano Lett. 2021, 21, 6656–6663. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, G.R.; Li, M.J.; Liu, R.P.; Li, H.B.; Li, T.Y.; Sun, M.Z.; Deng, Y.R.; Feng, M.; Chen, Z.W. Reinforced polysulfide barrier by g-C3N4/CNT composite towards superior lithium-sulfur batteries. J. Energy Chem. 2021, 53, 234–240. [Google Scholar] [CrossRef]
- Zhai, P.-Y.; Peng, H.-J.; Cheng, X.-B.; Zhu, L.; Huang, J.-Q.; Zhu, W.C.; Zhang, Q. Scaled-up fabrication of porous-graphene-modified separators for high-capacity lithium-sulfur batteries. Energy Storage Mater. 2017, 7, 56–63. [Google Scholar] [CrossRef]
- Tian, D.; Song, X.; Wang, M.; Wu, X.; Qiu, Y.; Guan, B.; Xu, X.; Fan, L.; Zhang, N.; Sun, K. MoN supported on graphene as a bifunctional interlayer for advanced Li-S batteries. Adv. Energy Mater. 2019, 9, 1901940. [Google Scholar] [CrossRef]
- Pan, H.; Cheng, Z.B.; Xiao, Z.B.; Li, X.J.; Wang, R.H. The fusion of imidazolium-based ionic polymer and carbon nanotubes: One type of new heteroatom-doped carbon precursors for high-performance lithium-sulfur batteries. Adv. Funct. Mater. 2017, 27, 1703936. [Google Scholar] [CrossRef]
- Guo, P.Q.; Sun, K.; Shang, X.N.; Liu, D.Q.; Wang, Y.R.; Liu, Q.M.; Fu, Y.J.; He, D.Y. Nb2O5/RGO nanocomposite modified separators with robust polysulfide traps and catalytic centers for boosting performance of lithium-sulfur batteries. Small 2019, 15, 1902363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Liu, D.B.; Muhammad, Z.; Wan, F.; Xie, W.; Wang, Y.J.; Song, L.; Niu, Z.Q.; Chen, J. Single nickel atoms on nitrogen-doped graphene enabling enhanced kinetics of lithium-sulfur batteries. Adv. Mater. 2019, 31, 1903955. [Google Scholar] [CrossRef]
- Wei, L.; Li, W.; Zhao, T.; Zhang, N.; Li, L.; Wu, F.; Chen, R. Cobalt nanoparticles shielded in N-doped carbon nanotubes for high areal capacity Li-S batteries. Chem. Commun. 2020, 56, 3007–3010. [Google Scholar] [CrossRef]
- Zhang, H.j.; Liu, Q.Z.; Ruan, S.J.; Ma, C.; Jia, X.F.; Qiao, W.M.; Ling, L.C.; Wang, J.T. In-situ construction of g-C3N4/carbon heterostructure on graphene nanosheet: An efficient polysulfide barrier for advanced lithium-sulfur batteries. Appl. Surf. Sci. 2022, 578, 152022. [Google Scholar] [CrossRef]
- Sun, W.H.; Lu, Y.-C.; Huang, Y.Q. An effective sulfur conversion catalyst based on MnCo2O4.5 modified graphitized carbon nitride nanosheets for high-performance Li-S batteries. J. Mater. Chem. A 2021, 9, 21184–21196. [Google Scholar] [CrossRef]
- Luo, M.; Bai, Y.; Sun, R.; Wang, Z.H.; Sun, W.; Lin, P.; Dai, X.; Sun, K.N. Enhanced performance of lithium-sulfur batteries with Co-doped g-C3N4 nanosheet-based separator. Ind. Eng. Chem. Res. 2021, 60, 1231–12405. [Google Scholar] [CrossRef]
- Ma, H.; Song, C.L.; Liu, N.; Zhao, Y.; Bakenov, Z. Nitrogen-deficient graphitic carbon nitride/carbon nanotube as polysulfide barrier of high-performance lithium-sulfur batteries. ChemElectroChem 2020, 7, 4906–4912. [Google Scholar] [CrossRef]
- Chen, M.F.; Zhao, X.M.; Li, Y.F.; Zeng, P.; Liu, H.; Yu, H.; Wu, M.; Li, Z.H.; Shao, D.S.; Miao, C.Q.; et al. Kinetically elevated redox conversion of polysulfides of lithium-sulfur battery using a separator modified with transition metals coordinated g-C3N4 with carbon-conjugated. Chem. Eng. J. 2020, 385, 123905–123916. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.W.; Chen, Y.X.; Li, Q.; Chen, C.T.; Zhong, B.H.; Guo, X.D.; Wu, Z.G.; Wang, G.K. Novel bifunctional separator with a self-assembled FeOOH/coated g-C3N4/KB bilayer in lithium-sulfur batteries. ACS Appl. Mater. Inter. 2020, 12, 57859–57869. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Wu, T.; Zhang, S.P.; Gu, S.; Jin, J.; Wen, Z.Y. Metal-organic-framework-derived N-C-Co film as a shuttle-suppressing interlayer for lithium sulfur battery. Chem. Eng. J. 2018, 334, 2356. [Google Scholar] [CrossRef]
- Jiang, R.J.; Jiang, M.; Huang, Z.Y.; Wang, J.; Kuang, Y.F.; Fu, C.P. Constructing light-weight polar boron-doped carbon nitride nanosheets with increased active sites and conductivity for high performance lithium-sulfur batteries. Int. J. Hydrogen Energy 2020, 45, 14940–14952. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liu, X.; Deng, Y. Ultrafine Co-Species Interspersed g-C3N4 Nanosheets and Graphene as an Efficient Polysulfide Barrier to Enable High Performance Li-S Batteries. Molecules 2023, 28, 588. https://doi.org/10.3390/molecules28020588
Wang S, Liu X, Deng Y. Ultrafine Co-Species Interspersed g-C3N4 Nanosheets and Graphene as an Efficient Polysulfide Barrier to Enable High Performance Li-S Batteries. Molecules. 2023; 28(2):588. https://doi.org/10.3390/molecules28020588
Chicago/Turabian StyleWang, Shanxing, Xinye Liu, and Yuanfu Deng. 2023. "Ultrafine Co-Species Interspersed g-C3N4 Nanosheets and Graphene as an Efficient Polysulfide Barrier to Enable High Performance Li-S Batteries" Molecules 28, no. 2: 588. https://doi.org/10.3390/molecules28020588
APA StyleWang, S., Liu, X., & Deng, Y. (2023). Ultrafine Co-Species Interspersed g-C3N4 Nanosheets and Graphene as an Efficient Polysulfide Barrier to Enable High Performance Li-S Batteries. Molecules, 28(2), 588. https://doi.org/10.3390/molecules28020588







