Obtaining 2,3-Dihydrobenzofuran and 3-Epilupeol from Ageratina pichinchensis (Kunth) R.King & Ho.Rob. Cell Cultures Grown in Shake Flasks under Photoperiod and Darkness, and Its Scale-Up to an Airlift Bioreactor for Enhanced Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Cell Cultures in Shake Flasks under Photoperiod and Absolute Darkness
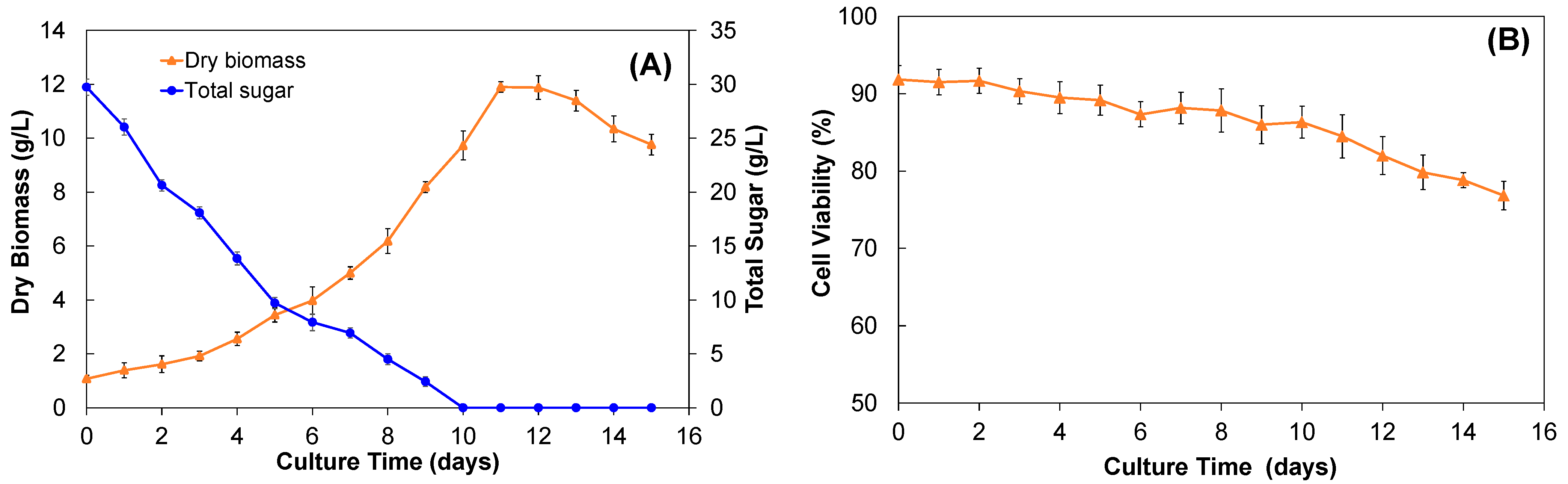
2.1.1. Yield of Biomass
2.1.2. Sugar Consumption, Cell Viability, and pH
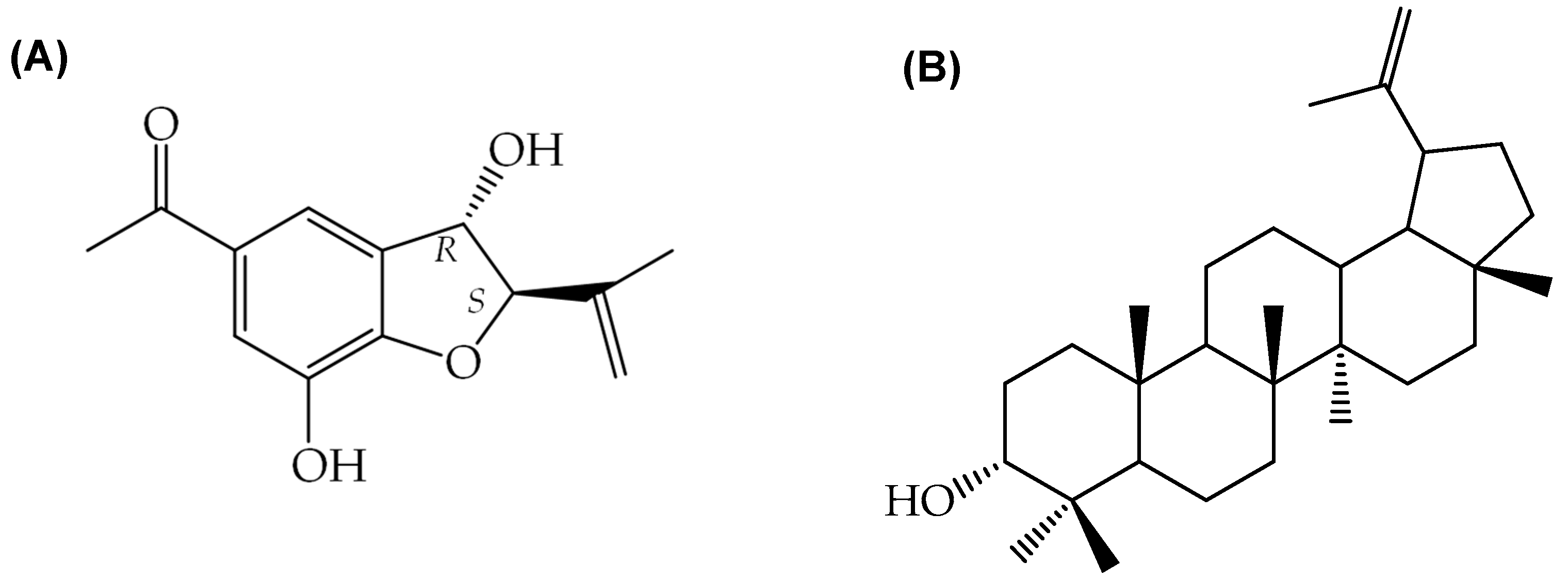
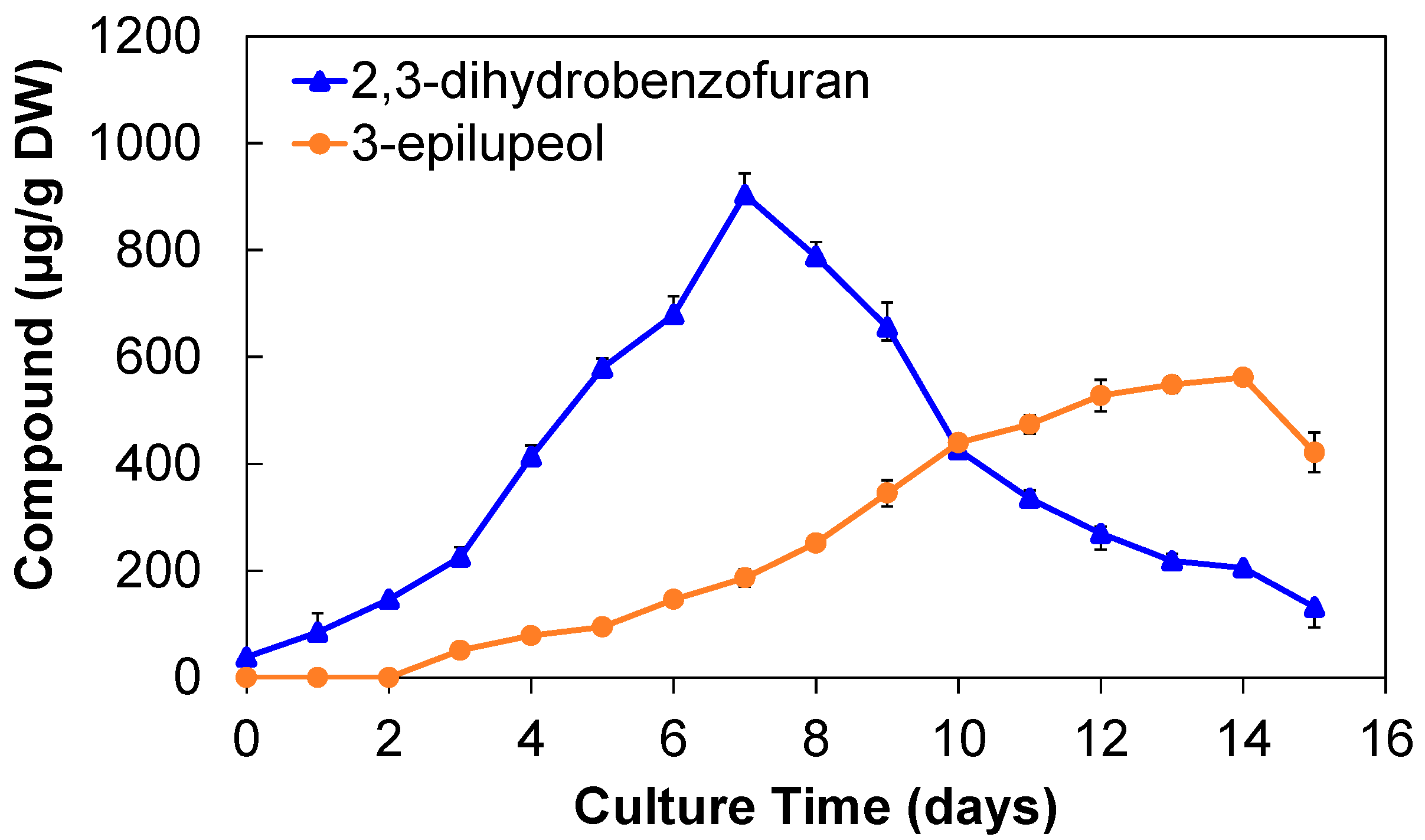
2.1.3. Production of 2,3-Dihydrobenzofuran and 3-Epilupeol
2.2. Cell Culture in an Airlift Bioreactor under Photoperiod Conditions
2.2.1. Growth Kinetics and Biomass Yield
2.2.2. Production of 2,3-Dihydrobenzofuran and 3-Epilupeol
3. Materials and Methods
3.1. Obtaining Plant Material
3.2. Cell Culture in Shake Flask under Photoperiod and Absolute Darkness Conditions
3.3. Bioreactor
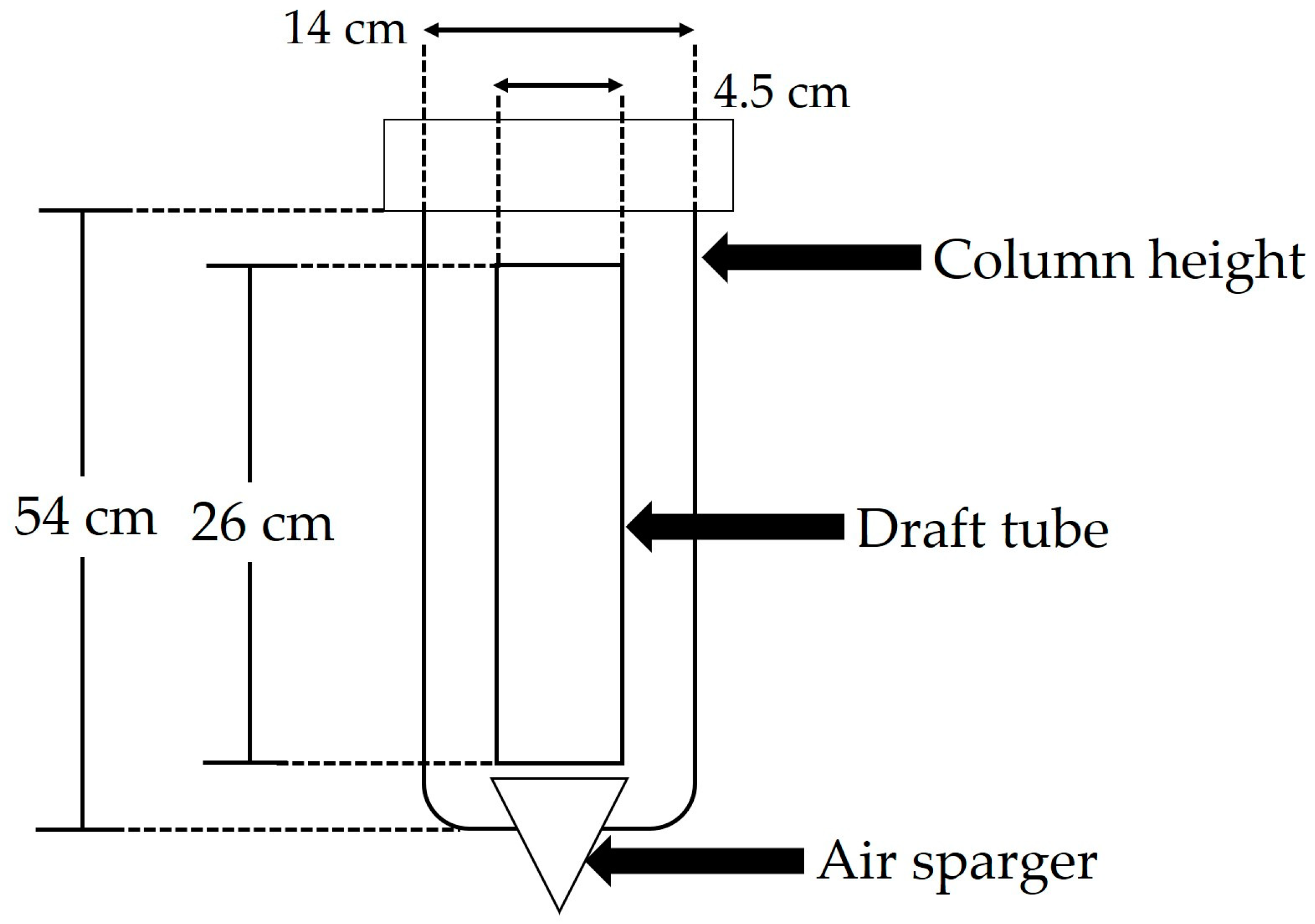
3.3.1. Bioreactor Characteristics
3.3.2. Cell Cultures and Bioreactor Operating Conditions
3.4. Cell Viability in Shake Flask and Bioreactor
3.5. Sugar Quantification in Shake Flask and Bioreactor
3.6. Extraction of 2,3-Dihydrobenzofuran and 3-Epilupeol of Cell Cultures
3.7. Quantification of 2,3-Dihydrobenzofuran and 3-Epilupeol by GC-MS
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Borges, C.V.; Minatel, I.O.; Gomez-Gomez, H.A.; Pereira, L.G.P. Medicinal plants: Influence of environmental factors on the content of secondary metabolites. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 259–277. [Google Scholar]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Takshak, S.; Agrawal, S.B. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J. Photochem. Photobiol 2019, 193, 51–88. [Google Scholar] [CrossRef]
- Mishra, T. Climate change and production of secondary metabolites in medicinal plants: A review. Int. J. Herb. Med. 2016, 4, 27–30. [Google Scholar]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol 2015, 3, 293–304. [Google Scholar]
- Faehnrich, B.; Franz, C.; Nemaz, P.; Kaul, H.-P. Medicinal plants and their secondary metabolites—State of the art and trends in breeding, analytics and use in feed supplementation—With special focus on German chamomile. J. Appl. Bot. Food Qual. 2021, 94, 61–74. [Google Scholar]
- Soni, U.; Brar, S.; Gauttam, V.K. Effect of seasonal variation on secondary metabolites of medicinal plants. Int. J. Pharm. Sci. Res. 2015, 6, 3654–3662. [Google Scholar]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–504. [Google Scholar]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plant. 2020, 18, 100255. [Google Scholar] [CrossRef]
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef]
- Kumar, M.; Radha, S.P.; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; Pradhan, P.C.; et al. Beneficial role of antioxidant secondary metabolites from medicinal plants in maintaining oral health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef]
- Bahmani, M.; Golshahi, H.; Saki, K.; Rafieian-Kopaei, M.; Delfan, B.; Mohammadi, T. Medicinal plants and secondary metabolites for diabetes mellitus control. Asian Pac. J. Trop. Dis. 2014, 4, S687–S692. [Google Scholar] [CrossRef]
- Sholikahah, E.N. Indonesian medicinal plants as sources of secondary metabolites for pharmaceutical industry. J. Med. Sc. 2016, 48, 226–239. [Google Scholar]
- Kumari, P.; Kumari, C.; Singh, P.S. Phytochemical screening of selected medicinal plants for secondary metabolites. Int. J. Life Sci. Res. 2017, 4, 1151–1157. [Google Scholar] [CrossRef]
- Bernstein, N.; Akram, M.; Daniyal, M.; Koltai, H.; Fridlender, M.; Gorelick, J. Antiinflammatory potential of medicinal plants: A source for therapeutic secondary metabolites. Adv. Agron. 2018, 150, 131–183. [Google Scholar]
- Twilley, D.; Rademan, S.; Lall, N. A review on traditionally used South African medicinal plants, their secondary metabolites and their potential development into anticancer agents. J. Ethnopharmacol. 2020, 261, 113101. [Google Scholar] [CrossRef]
- Velu, G.; Palanichamy, V.; Rajan, A.P. Phytochemical and pharmaceutical importance of plant secondary metabolites in modern medicine. In Bioorganic Phase in Natural Foods: An Overview; Roopan, S., Madhumitha, G., Eds.; Springer: Cham, Switzerland, 2018; Chapter 8. [Google Scholar]
- Maridass, M. Survey of phytochemical diversity of secondary metabolism in selected wild medicinal plants. Ethnobot. Leafl. 2010, 14, 616–625. [Google Scholar]
- Rungsung, W.; Ratha, K.K.; Dutta, S.; Ditix, A.K.; Hazra, J. Secondary metabolites of plants in drugs discovery. World J. Pharm. Res. 2015, 7, 604–613. [Google Scholar]
- Sree, N.V.; Udayasri, P.; Kumar, Y.A.; Babu, B.R.; Kumar, Y.P.; Varma, M.V. Advancements in the production of secondary metabolites. J. Nat. Prod. 2010, 3, 112–123. [Google Scholar]
- Cardoso, J.C.; Oliveira, M.E.B.S.; Cardoso, F.C.I. Advances and challenges on the in vitro production of secondary metabolites from medicinal plants. Hortic. Bras. 2019, 37, 124–132. [Google Scholar] [CrossRef]
- Tripathi, L.; Tripathi, J.N. Role of biotechnology in medicinal plants. Trop. J. Pharm. Res. 2003, 2, 243–253. [Google Scholar] [CrossRef]
- Gandhi, S.G.; Mahajan, V.; Bedi, Y.S. Changing trends in biotechnology of secondary metabolism in medicinal and aromatic plants. Planta 2014, 2, 303–317. [Google Scholar] [CrossRef]
- Yoshimatsu, Y. Tissue culture of medicinal plants: Micropropagation, transformation and production of useful secondary metabolites. Stud. Nat. Prod. Chem. 2008, 34, 647–752. [Google Scholar]
- Chandana, B.C.; Nagaveni, H.C.; Kumari; Lakshmana, D.; Shashikala, S.K.; Heena, M.S. Role of plant tissue culture in micropropagation, secondary production and conservation of some endangered medicinal crops. J. Pharmacog. Phytochem. 2018, 3, 246–251. [Google Scholar]
- Pérez-González, M.Z.; Jiménez-Arellanes, M.A. Biotechnological processes to obtain bioactive secondary metabolites from some Mexican medicinal plants. Appl. Microbiol. Biotechnol. 2021, 105, 6257–6274. [Google Scholar] [CrossRef] [PubMed]
- Smetanska, I. Production of secondary metabolites using plant cell cultures. Adv. Biochem. Eng. Biotechnol. 2008, 111, 187–228. [Google Scholar] [PubMed]
- Vanisree, M.; Lee, C.-Y.; Lo, S.-F.; Nalawade, S.M.; Lin, C.Y.; Tsay, H.-S. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot. Bull. Acad. Sin. 2008, 45, 1–22. [Google Scholar]
- Kolowe, M.; Gaurav, V.; Roberts, S.C. Pharmaceutical active natural products synthesis and supply via plant cell culture technology. Mol. Pharm. 2008, 5, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Weathers, P.; Towler, M.J.; Xu, J. Bench to batch: Advances in plants cell culture for producing useful products. Appl. Microbiol. Biotechnol. 2010, 85, 1339–1351. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Weber, J. Bioreactors for plants cells: Hardware configuration and internal environment optimization as tools for wider commercialization. Biotechnol. Lett. 2014, 36, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Murthy, H.N.; Hahn, E.-J.; Paek, K.-Y. Enhanced production of caftaric acid, chlorogenic acid and cichoric acid in suspension cultures of Echinaceae purpureae by the manipulation of incubation temperature and photoperiod. Biochem. Eng. J. 2007, 36, 301–303. [Google Scholar] [CrossRef]
- Bong, F.B.; Subramaniam, S.; Chew, B.L. Effects of light illumination and subculture frequency on biomass production in cell suspension cultures of Clinacanthus nutans. Malays. Appl. Biol. 2021, 50, 197–204. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Danial, N.; Matter, M.A.; Rady, M.R. Effect of light and methyl jasmonate on the accumulation of anticancer compound in cell suspension cultures of Catharanths roseus. Egypt. Pharm. J. 2022, 20, 294–302. [Google Scholar]
- Khosroushahi, A.Y.; Valizadeh, M.; Ghasempour, A.; Khosrowshahli, M.; Naghdibadi, H.; Dadpour, M.R.; Omidi, Y. Improved taxol production by combination of inducing factors in suspension cell culture of Taxus baccata. Cell Biol. Int. 2006, 30, 262–269. [Google Scholar] [CrossRef]
- Donezz, D.; Kim, K.-H.; Antonie, S.; Conreux, A.; De Luca, V.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and viniferins by an elicited grapevine cell culture in a 2 L stired reactor. Process Biochem. 2011, 46, 1056–1062. [Google Scholar] [CrossRef]
- Nair, A.J.; Sudhakaran, P.R.; Rao, M.; Ramakrishna, S.V. Berberine synthesis by callus and cell suspension cultures of Coscinium fenestratum. Plant Cell Tissue Organ Cult. 1992, 29, 7–10. [Google Scholar] [CrossRef]
- Khan, T.; Krupadanam, D.; Anwar, S.Y. The role of phytohormone on the production of berberine in the calli cultures of an endangered medicinal plant, turmeric (Coscinium fenestratum I.). Afr. J. Biotechnol. 2008, 7, 3244–3246. [Google Scholar]
- Nazir, R.; Kumar, V.; Gupta, S.; Dwivedi, P.; Pandey, D.K.; Dey, A. Biotechnological strategies for the sustainable production of diosgenin from Dioscorea spp. Appl. Microbiol. Biotechnol. 2021, 105, 569–585. [Google Scholar] [CrossRef]
- Mekky, H.; Al-Sabahi, J.; Abdel-Kreem, M.F.M. Potentiating biosynthesis of the anticancer alkaloids vincristine and vinblastine in callus cultures of Catharanthus roseus. S. Afr. J. Bot. 2018, 114, 29–31. [Google Scholar] [CrossRef]
- Ataei-Azimi, A.; Hashemloian, B.D.; Ebrahimzadeh, H.; Majd, A. High in vitro production of ant-canceric indole alkaloids from periwinkle (Catharantus roseus) tissue culture. Afr. J. Biotechnol. 2008, 7, 2834. [Google Scholar]
- Valdiani, A.; Hansen, O.K.; Nielsen, U.B.; Johannsen, V.K.; Shariat, M.S.; Georgiev, M.I.; Omidvar, V.; Ebrahimi, M.; Dinanai, E.T.; Abiri, R. Bioreactor-based advances in plant tissue and cell culture: Challenges and prospects. Crit. Rev. Biotechnol. 2018, 39, 20–34. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Mukta, S.; Ahmed, S.R.; Afrin, D. Plant tissue culture- the alternative and efficient way to extract plant secondary metabolites. J. Sylhet Agril. Univ. 2017, 4, 1–13. [Google Scholar]
- Nartop, P. Engineering of biomass accumulation and secondary metabolite production in plant cell and tissue cultures. In Plant Metabolites and Regulation under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V., Tripathi, D., Alam, P., Alyemeni, M.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 9; pp. 169–194. [Google Scholar]
- BDMTM. Atlas de las Plantas de la Medicina Tradicional Mexicana. 1994. Available online: http://www.medicinatradicionalmexicana.unam.mx/index.html (accessed on 20 October 2022).
- WFO. Ageratina pichinchensis (Kunth) R.M.King & H.Rob. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0000122234 (accessed on 20 October 2022).
- Navarro, V.M.; González, A.; Fuentes, M.; Avilez, M.; Ríos, M.Y.; Zepeda, G. Antifungal activities of nine traditional Mexican medicinal plants. J. Ethnopharmacol. 2003, 87, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Torres-Barajas, L.; Rojas-Vera, J.; Morales-Méndez, A.; Rojas-Fermín, L.; Lucena, M.; Buitrago, A. Chemical composition and evaluation of antibacterial activity of essential oils of Ageratina jahnii and Ageratina pichinchensis collected in Mérida, Venezuela. Bol. Latinoam. Caribe Plantas Med. Aromat. 2013, 12, 92–98. [Google Scholar]
- Sánchez-Mendoza, M.E.; Reyes-Trejo, B.; Sánchez-Gómez, P.; Rodríguez-Silverio, J.; Castillo-Henkel, C.; Cervantes-Cuevas, H.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer chromene from Eupatorium aschenbornianum: Role of nitric oxide, prostaglandins and sulfydryls. Fitoterapia 2010, 81, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mendoza, M.E.; Rodriguez-Silverio, J.; Rivero-Cruz, J.F.; Rocha-González, H.I.; Pineda-Farías, J.B.; Arrieta, J. Antinociceptive effect and gastroprotective mechanisms of 3,5-diprenyl-4-hydroxyacetophenone from Ageratina pichinchensis. Fitoterapia 2013, 87, 11–19. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; González-Cortazar, M.; Alonso-Cortés, D.; Jiménez-Ferrer, E.; Nicasio-Torres, P.; Aguilar- Santamaría, L.; Tortoriello, J. Pharmacological and chemical study to identify wound-healing active compounds in Ageratina pichinchensis. Planta Med. 2013, 79, 622–627. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa-Álvarez, A.; Ramos-Mora, A.; Alonso-Cortés, D.; Jiménez-Ferrer, J.E.; Huerta-Reyes, M.E.; Tortoriello, J. Effect on the wound healing process and in vitro cell proliferation by the medicinal mexican plant Ageratina pichinchensis. Planta Med. 2011, 77, 979–983. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; Díaz-García, E.R.; Tortoriello, J. Pharmacological effect of Ageratina pichinchensis on wound healing in diabetic rats and genotoxicity evaluation. J. Ethnopharmacol. 2014, 156, 222–227. [Google Scholar] [CrossRef]
- Aguilar-Guadarrama, B.; Navarro, V.; León-Rivera, I.; Ríos, M.Y. Active compounds against tinea pedis dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 16, 1559–1565. [Google Scholar] [CrossRef]
- Dong, R.; Yuan, J.; Wu, S.; Huang, J.; Xu, X.; Wu, Z.; Gao, H. Anti-inflammation furanoditerpenoids from Caesalpinia minax Hance. Phytochemistry 2015, 117, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Kanwar, R.K.; Burrow, H.; Baratchi, S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr. Med. Chem. 2009, 16, 2373–2394. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, C.; Batra, S.; Vargo, M.A.; Voss, O.H.; Gavrilin, M.A.; Wewers, M.D.; Guttridge, D.C.; Grotewold, E.; Doseff, A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kB through the suppression of p65 phosphorylation. J. Immunol. 2007, 179, 7121–7127. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Franzblau, S.G.; Ukiva, M.; Okuda, H.; Zhang, F.; Suzuki, T.; Kimura, Y. Antitubercular activity of triterpenoids from Asteraceae flowers. Biol. Pharm. Bull. 2005, 28, 158–160. [Google Scholar] [CrossRef]
- Romero-Estrada, A.; Maldonado-Magaña, A.; González-Christen, J.; Marquina-Bahena, S.; Garduño-Ramírez, M.L.; Rodríguez-López, V.; Alvarez, L. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera Copallifera. BMC Complem. Altern. Med. 2016, 16, 422–432. [Google Scholar] [CrossRef]
- Sánchez-Ramos, M.; Marquina-Bahena, S.; Romero-Estrada, A.; Bernabé-Antonio, A.; Cruz-Sosa, F.; González-Christen, J.; Acevedo-Fernández, J.J.; Perea-Arango, I.; Álvarez, L. Establishment and phytochemical analysis of a callus culture from Ageratina pichinchensis (Asteraceae) and its anti-inflammatory activity. Molecules 2018, 23, 1258. [Google Scholar] [CrossRef]
- Sánchez-Ramos, M.; Alvarez, L.; Romero-Estrada, A.; Bernabé-Antonio, A.; Marquina-Bahena, S.; Cruz-Sosa, F. Establishment of a cell suspension culture of Ageratina pichinchensis (Kunth) for the improved production of anti-inflammatory compounds. Plants 2020, 9, 1398. [Google Scholar] [CrossRef]
- Ling, O.S.; Kiong, A.L.P.; Hussein, S. Establishment and optimization of growth parameters for cell suspension cultures of Ficus deltoideia. Am. Eurasian J. Sustain. Agric. 2008, 2, 38–49. [Google Scholar]
- Açikgöz, M.A. Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind. Crops Prod. 2020, 148, 112278. [Google Scholar] [CrossRef]
- Corchete, P.; Almagro, L.; Gabaldón, J.A.; Pedreño, M.A.; Palazón, J. Phenylpropanoids in Silybum marianum cultures treated whit cyclodextrins coated with magnetic nanoparticles. Appl. Microbiol. Biotechnol. 2022, 106, 2393–2401. [Google Scholar] [CrossRef]
- Tahizadeh, M.; Nasibi, F.; Kalantari, K.M.; Benakahani, F. Callogenesis optimization and cell suspension culture establishment of Dracocephalum polychaetum Bornm. and Dracocephalum kotschyi Bois.: An in vitro approach for secondary metabolite production. S. Afr. J. Bot. 2020, 132, 79–86. [Google Scholar] [CrossRef]
- Malik, S.; Bhushan, S.; Sharma, M.; Ahuja, P.S. Physico-chemical factors influencing the shikonin derivates production in cell suspension cultures of Arnebia eucroma (Royle) Johnston, a medicinally important plant species. Cell Biol. Int. 2011, 35, 153–158. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Zhou, L.-G.; Wu, J.-Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol. 2010, 87, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Shindo, C.; Kato, M.; Yokota, E.; Sakano, K.; Ashihara, H.; Shimmen, T. Regulation of cytoplasmic pH under extreme acid conditions in suspension culture cells of Catharanthus roseus: A possible role of inorganic phosphate. Plant Cell Physiol. 2000, 41, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Trujillo, A.; Cruz-Sosa, F.; Luria-Pérez, R.; Gutiérrez-Rebolledo, G.A.; Román-Guerrero, A.; Burrola-Aguilar, C.; Zepeda-Gómez, C.; Estrada-Zúñiga, M.E. Arnica montana cell culture establishment, ands assessment of its cytotoxic, antibacterial, α-amylase inhibitor, and antioxidant in vitro bioactivities. Plants 2021, 10, 2300. [Google Scholar] [CrossRef]
- Pérez-González, M.Z.; Nieto-Trujillo, A.; Gutiérrez-Rebolledo, G.A.; García-Martínez, I.; Estrada-Zúñiga, M.E.; Bernabé-Antonio, A.; Jiménez-Arellanes, M.A.; Cruz-Sosa, F. Lupeol acetate production and antioxidant activity of a cell suspension culture from Cnidoscolus chayamansa leaves. S. Afr. J. Bot. 2019, 125, 30–38. [Google Scholar] [CrossRef]
- Sajid, Z.A.; Aftab, F. An efficient methos for the cell suspension culture in potato (Solanum tuberosum L.). Pak. J. Bot. 2016, 48, 1993–1997. [Google Scholar]
- Shyam, C.; Tripathi, M.K.; Tiwari, S.; Ahuja, A.; Tripathi, N.; Gupta, N. In vitro regeneration from callus and cell suspension cultures in Indian mustard [Brassica juncea (Linn.) Czern & coss]. Int. J. Agric. Technol. 2021, 17, 1095–1112. [Google Scholar]
- Songserm, P.; Klanrit, P.; Klanrit, P.; Phetcharaburanin, J.; Thanonkeo, P.; Apiraksakorn, J.; Phomphrai, K.; Klanrit, P. Antioxidant and anticancer potential of bioactive compounds from Rhinacanthus nasutus cell suspension culture. Plants 2022, 11, 1994. [Google Scholar] [CrossRef]
- Fouad, A.; Hegazy, A.E.; Azab, E.; Khojah, E.; Kapiel, T. Boosting of antioxidants and alkaloids in Catharanthus roseus suspension cultures using silver nanoparticles with expression of CrMPK3 and STR genes. Plants 2021, 10, 2202. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Zhong, Y.; Huang, Z.; Yan, H.; Yuanda, L.V.; Jiang, B.; Zhong, G. Citrus cell suspension culture establishment, maintenance, efficient transformation and regeneration to complete transgenic plant. Plants 2021, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Bernabé-Antonio, A.; Sánchez-Sánchez, A.; Romero-Estrada, A.; Meza-Contreras, J.C.; Silva-Guzmán, J.A.; Fuentes-Talavera, F.J.; Hurtado-Díaz, I.; Alvarez, L.; Cruz-Sosa, F. Establishment of a cell suspension culture of Eysenhardtia platycarpa: Phytochemical screening of extracts and evaluation of antifungal activity. Plants 2021, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Lertphadungkit, P.; Suksiriworapong, J.; Satitpatipan, V.; Sirikantaramas, S.; Wongrapanich, A.; Bunsupa, S. Enhanced production of bryonolic acid in Trichosanthes cucumerina L. (Thai cultivar) cell cultures by elicitors and their biological activities. Plants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M.; Ali, S. Sucrose induced osmotic stress and photoperiod regimes enhanced the biomass and production of antioxidant secondary metabolites in shake-flask suspension cultures of Prunella vulgaris L. Plant Cell Tissue Organ Cult. 2015, 124, 573–581. [Google Scholar] [CrossRef]
- Ali, H.; Khan, M.A.; Khan, R.S. Impacts of hormonal elicitors and photoperiod regimes on elicitation of bioactive secondary volatiles in cell cultures of Ajuga bracteosa. J. Photochem. Photobiol. 2018, 183, 242–250. [Google Scholar] [CrossRef]
- Kumar, S.S.; Arya, M.; Mahadevappa, P.; Giridhar, P. Influence of photoperiod on growth, bioactive compounds and antioxidant activity in callus cultures of Basella rubra L. J. Photochem. Photobiol. 2020, 209, 111937. [Google Scholar] [CrossRef]
- Khan, T.; Abbasi, B.H.; Khan, M.A. The interplay between light, plant growth regulators and elicitors on growth and secondary metabolism in cell cultures of Fagonia indica. J. Photochem. Photobiol. 2018, 185, 153–160. [Google Scholar] [CrossRef]
- Zahir, A.; Ahmad, W.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. In vitro cultures of Linum usitatissimum L.: Synergistic effects of mineral nutrients and photoperiod regimes on growth and biosynthesis of lignans and neolignans. J. Photochem. Photobiol. 2018, 187, 141–150. [Google Scholar] [CrossRef]
- Chan, L.K.; Koay, S.S.; Boey, P.L.; Bhatt, A. Effects of abiotic stress on biomass and anthocyanin production in cell cultures of Melastoma malabathricum. Biol. Res. 2010, 43, 127–135. [Google Scholar] [CrossRef]
- Anjum, S.; Abbasi, B.H.; Doussot, J.; Favre-Réguillon, A.; Hano, C. Effects of photoperiod regimes and ultraviolet-C radiations on biosynthesis of industrially important lignans and neolignans in cell cultures of Linum usitatissimum L. (Flax). J. Photochem. Photobiol. 2017, 167, 216–227. [Google Scholar] [CrossRef]
- Aly, U.I.; El-Shabrawi, H.M.; Hanafy, M. Impact of culture conditions on alkaloid production from undifferentiated cell suspension cultures of Egyptian Henbane. Aust. J. Basic. Appl. Sci. 2010, 4, 4717–4725. [Google Scholar]
- Hennayake, C.K.; Takagi, S.; Nishimura, K.; Kanechi, M.; Uno, Y.; Inagaki, N. Differential expression of anthocyanin biosynthesis genes in suspension culture cells of Rosa hybrida cv. Charleston. Plant Biotechnol. J. 2006, 23, 379–385. [Google Scholar] [CrossRef]
- López-Laredo, A.R.; Ramírez-Flores, F.D.; Sepúlveda-Jiménez, G.; Trejo-Tapia, G. Comparison of metabolite levels in callus of Tecoma stans (L.) Juss. Ex Kunth. cultured in photoperiod and darkness. Vitr. Cell Dev. Biol. Plant 2009, 45, 550–558. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, B.; Jha, S. Establishment of forskolin yielding transformed cell suspension cultures of Coleus forskohlii as controlled by different factors. J. Biotechnol. 2000, 76, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, S.; Sharifi, M.; Yousefzadi, M.; Beshamgan, E. Effect of some phytohormones on podophyllotoxin production in cell and plantlets cultures of Linum album. J. Med. Plant By Prod. 2013, 1, 83–89. [Google Scholar]
- Andi, S.A.; Gholami, M.; Fors, C.M.; Maskani, F. The effect of light, phenylalanine and methyl jasmonate, alone or in combination, on growth and secondary metabolism in cell suspension cultures on Vitis vinifera. J. Photochem. Photobiol. 2019, 199, 111625. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Pais, M.S.S. Achillea millefolium (yarrow) cell suspension cultures: Establishment and growth and growth conditions. Biotechnol. Lett. 1991, 13, 63–68. [Google Scholar] [CrossRef]
- Beigmohamadi, M.; Movafeghi, A.; Sharafi, A.; Jafari, S.; Danafar, H. Cell suspension culture of Plumbago europaea L. Towards production of plumbagin. Iran. J. Biotech. 2019, 17, e2169. [Google Scholar] [CrossRef]
- Arias, J.P.; Zapata, K.; Rojano, B.; Arias, M. Effect of light wavelength on cell growth, content of phenolic compounds and antioxidant activity in cell suspension cultures of Thevetia peruviana. J. Photochem. Photobiol. 2016, 163, 87–91. [Google Scholar] [CrossRef]
- Kaewpintong, K.; Shotipruk, A.; Powtongsook, S.; Pavasant, P. Photoautotrophic high-density cultivation of vegetable cells of Haematococcus pluvialis in airlift bioreactor. Bioresour. Technol. 2007, 98, 288–295. [Google Scholar] [CrossRef]
- Navia-Osorio, A.; Garden, H.; Palazón, J.; Alfermann, A.W.; Piñol, M.T. Production of paclitaxel and baccatin III a 20-L airlift bioreactor by a cell suspension of Taxus wallichiana. Planta Med. 2002, 68, 336–340. [Google Scholar] [CrossRef]
- Navia-Osorio, A.; Garde, H.; Cudisó, R.M.; Palazóm, J.; Alfermann, A.W.; Piñol, M.T. Taxol® and baccatin III production in suspension cultures of Taxus baccata and Taxus wallichiana in an airlift bioreactor. J. Plant Physiol. 2002, 159, 97–102. [Google Scholar] [CrossRef]
- Salazar-Magallón, J.A.; de la Peña, A.H. Production of antifungal saponins in an airlift bioreactor with a cell line transformed from Solanum chrysotrichum and its activity against strawberry phytopathogens. Prep. Biochem. Biotechnol. 2020, 50, 204–214. [Google Scholar] [CrossRef]
- Woragidbumrung, K.; Sae-Tang, P.; Yao, H.; Han, J.; Chauvatcharin, S.; Zhong, J.-J. Impact of conditioned medium on cell cultures of Panax notoginseng in an airlift bioreactor. Process Biochem. 2001, 37, 209–213. [Google Scholar] [CrossRef]
- Ahmadi-Sakha, S.; Sharifi, M.; Niknam, V. Bioproduction of phenylethanoid glycosides by plant cell culture of Scrophularia striata Boiss.: From shake-flasks to bioreactor. Plant Cell Tissue Organ Cult. 2015, 124, 275–281. [Google Scholar] [CrossRef]
- Ahmadi-Sakha, S.; Sharifi, M.; Niknam, V.; Ahmadian-Chashmi, N. Phenol compounds profiling in shake flask and bioreactor system cell cultures of Scrophularia striata Boiss. Vitr. Cell. Dev. Biol. 2018, 54, 444–453. [Google Scholar] [CrossRef]
- Fulzele, D.P.; Heble, M.R. Large-scale cultivation of Catharanthus roseus cells: Production of ajmalicine in a 20L airlift bioreactor. J. Biotechnol. 1994, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Garg, S.; Singh, J.; Kumar, M. Enhanced production of napthoquinone metabolie (shikonin) from cell suspension culture of Arnebia sp. and its up-scaling through bioreactor. 3 Biotech 2014, 4, 263–273. [Google Scholar] [CrossRef]
- Han, J.-E.; Lee, H.; Ho, T.-T.; Park, S.-Y. Brazzein protein production in transgenic carrot cells using air-lift bioreactor culture. Plant Biotechnol. Rep. 2022, 16, 161–171. [Google Scholar] [CrossRef]
- Han, J.; Zhong, J.-J. High density cell culture of Panax notoginseng for production of ginseng saponin and polysaccharide in an airlift bioreactor. Biotechnol. Lett. 2002, 24, 1927–1930. [Google Scholar] [CrossRef]
- Kim, D.I.; Pedersen, H. Cultivation of Thalictrum rugosum cell suspension in an improved airlift bioreactor: Stimulatory effect of carbon dioxide and ethylene on alkaloid production. Biotechnol. Bioeng. 1991, 38, 331–339. [Google Scholar] [CrossRef]
- Kintzios, S.; Kollias, H.; Stratouris, E.; Makri, O. Scale-up micropropagation of sweet basil (Ocimum basilicum L.) in an airlift bioreactor and accumularion of rosmarinc acid. Biotechnol. Lett. 2004, 26, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Meghana, R.; Kush, A. Squalene production in the cell suspension cultures of Indian sandalwood (Santalum album L.) in shake flasks and air lift bioreactor. Plant Cell Tissue Organ Cult. 2018, 135, 155–167. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Palomares, L.A.; Ramírez, O.T. Bioreactor scale-up. In Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology; The Wiley Biotechnology Series; Flickinger, M.C., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2009; pp. 183–201. [Google Scholar]
- Rodríguez-Monroy, M.; Galindo, E. Broth rheology, growth and metabolite production of Beta vulgaris suspension culture: A comparative study between cultures grown in flasks and in a stirred tank. Enzym. Microb. Technol. 1999, 24, 687–693. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]



| Culture System | Culture Time (Day) | 2,3-Dihydrobenzofuran (µg/g DW) | Culture Time (Day) | 3-Epilupeol (µg/g DW) |
|---|---|---|---|---|
| Callus culture in jars/photoperiod * | 30 | 650.00 ± 11.00 | 30 | 201.10 ± 15.00 |
| Cell culture in flasks/photoperiod | 8 | 495.04 ± 22.85 | 16 | 414.24 ± 31.56 |
| Cell culture in flasks/absolute darkness | 8 | 315.44 ± 16.72 | 16 | 395.14 ± 13.32 |
| Cell culture in airlift bioreactor/photoperiod | 7 | 903.02 ± 41.06 | 14 | 561.63 ± 10.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ramos, M.; Marquina-Bahena, S.; Alvarez, L.; Bernabé-Antonio, A.; Cabañas-García, E.; Román-Guerrero, A.; Cruz-Sosa, F. Obtaining 2,3-Dihydrobenzofuran and 3-Epilupeol from Ageratina pichinchensis (Kunth) R.King & Ho.Rob. Cell Cultures Grown in Shake Flasks under Photoperiod and Darkness, and Its Scale-Up to an Airlift Bioreactor for Enhanced Production. Molecules 2023, 28, 578. https://doi.org/10.3390/molecules28020578
Sánchez-Ramos M, Marquina-Bahena S, Alvarez L, Bernabé-Antonio A, Cabañas-García E, Román-Guerrero A, Cruz-Sosa F. Obtaining 2,3-Dihydrobenzofuran and 3-Epilupeol from Ageratina pichinchensis (Kunth) R.King & Ho.Rob. Cell Cultures Grown in Shake Flasks under Photoperiod and Darkness, and Its Scale-Up to an Airlift Bioreactor for Enhanced Production. Molecules. 2023; 28(2):578. https://doi.org/10.3390/molecules28020578
Chicago/Turabian StyleSánchez-Ramos, Mariana, Silvia Marquina-Bahena, Laura Alvarez, Antonio Bernabé-Antonio, Emmanuel Cabañas-García, Angélica Román-Guerrero, and Francisco Cruz-Sosa. 2023. "Obtaining 2,3-Dihydrobenzofuran and 3-Epilupeol from Ageratina pichinchensis (Kunth) R.King & Ho.Rob. Cell Cultures Grown in Shake Flasks under Photoperiod and Darkness, and Its Scale-Up to an Airlift Bioreactor for Enhanced Production" Molecules 28, no. 2: 578. https://doi.org/10.3390/molecules28020578
APA StyleSánchez-Ramos, M., Marquina-Bahena, S., Alvarez, L., Bernabé-Antonio, A., Cabañas-García, E., Román-Guerrero, A., & Cruz-Sosa, F. (2023). Obtaining 2,3-Dihydrobenzofuran and 3-Epilupeol from Ageratina pichinchensis (Kunth) R.King & Ho.Rob. Cell Cultures Grown in Shake Flasks under Photoperiod and Darkness, and Its Scale-Up to an Airlift Bioreactor for Enhanced Production. Molecules, 28(2), 578. https://doi.org/10.3390/molecules28020578









