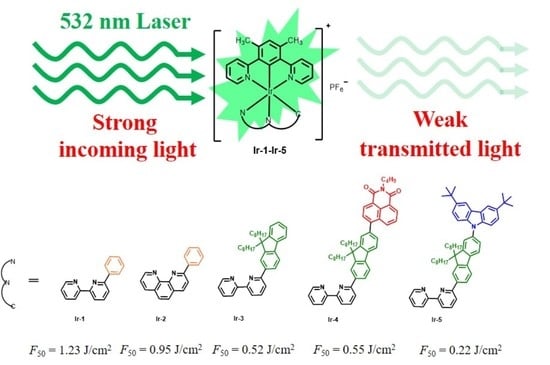
Synthesis, Photophysics and Tunable Reverse Saturable Absorption of Bis-Tridentate Iridium(III) Complexes via Modification on Diimine Ligand
(This article belongs to the Section Materials Chemistry)
Abstract
1. Introduction
2. Results and Discussion
2.1. Design and Synthesis
2.2. Ground State UV-Vis Absorption
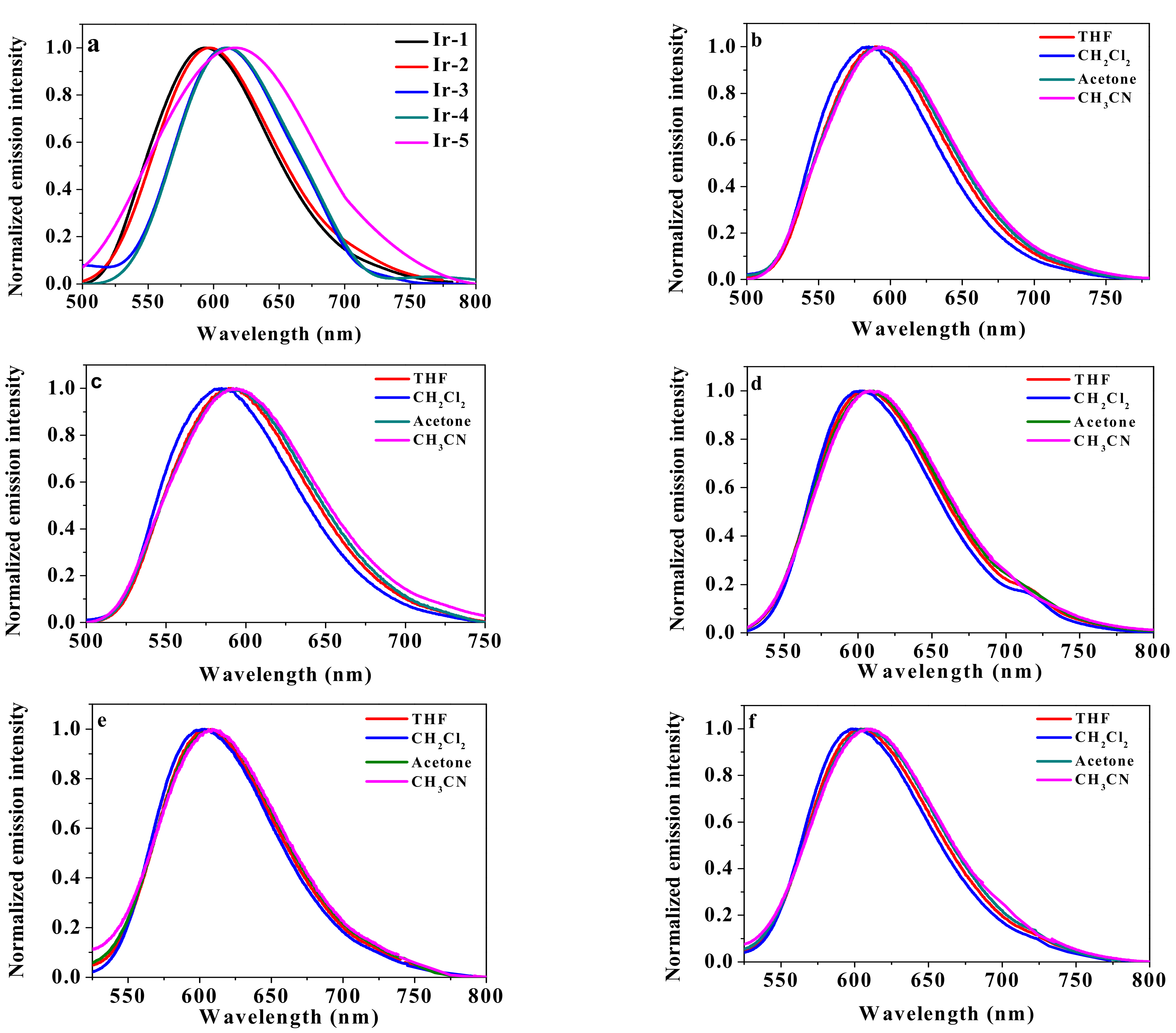
2.3. Photoluminescence
2.4. DFT Calculations
2.5. Transient Absorption
2.6. Optical Power Limiting Properties
3. Experimental Section
3.1. Computational Methods
3.2. Preparation of Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tsuboyama, A.; Iwawaki, H.; Furugori, M.; Mukaide, T.; Kamatani, J.; Igawa, S.; Moriyama, T.; Miura, S.; Takiguchi, T.; Okada, S.; et al. Homoleptic Cyclometalated Iridium Complexes with Highly Efficient Red Phosphorescence and Application to Organic Light-Emitting Diode. J. Am. Chem. Soc. 2003, 125, 12971–12979. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.T.; Slinker, J.D.; Lowry, M.S.; Cox, M.P.; Bernhard, S.; Malliaras, G.G. Improved Turn-on Times of Iridium Electroluminescent Devices by Use of Ionic Liquids. Chem. Mater. 2005, 17, 3187–3190. [Google Scholar] [CrossRef]
- Du, B.-S.; Lin, C.-H.; Chi, Y.; Hung, J.-Y.; Chung, M.-W.; Lin, T.-Y.; Lee, G.-H.; Wong, K.-T.; Chou, P.-T.; Hung, W.-Y.; et al. Diphenyl(1-Naphthyl)Phosphine Ancillary for Assembling of Red and Orange-Emitting Ir(III) Based Phosphors; Strategic Synthesis, Photophysics, and Organic Light-Emitting Diode Fabrication. Inorg. Chem. 2010, 49, 8713–8723. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-Y.; Zhou, G.-J.; Yu, X.-M.; Kwok, H.-S.; Lin, Z. Efficient Organic Light-Emitting Diodes Based on Sublimable Charged Iridium Phosphorescent Emitters. Adv. Funct. Mater. 2007, 17, 315–323. [Google Scholar] [CrossRef]
- Zhao, W.; Castellano, F.N. Upconverted Emission from Pyrene and Di- Tert -Butylpyrene Using Ir(Ppy) 3 as Triplet Sensitizer. J. Phys. Chem. A 2006, 110, 11440–11445. [Google Scholar] [CrossRef]
- Rachford, A.A.; Ziessel, R.; Bura, T.; Retailleau, P.; Castellano, F.N. Boron Dipyrromethene (Bodipy) Phosphorescence Revealed in [Ir(Ppy) 2 (Bpy-C≡C-Bodipy)] +. Inorg. Chem. 2010, 49, 3730–3736. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, S.; Zhou, G.; Wong, W.-Y.; Roy, V.A.L. Ambipolar Organic Light-Emitting Electrochemical Transistor Based on a Heteroleptic Charged Iridium(III) Complex. Appl. Phys. Lett. 2013, 102, 083301. [Google Scholar] [CrossRef]
- Belmore, K.A.; Vanderpool, R.A.; Tsai, J. Cherng.; Khan, M.A.; Nicholas, K.M. Transition-Metal-Mediated Photochemical Disproportionation of Carbon Dioxide. J. Am. Chem. Soc. 1988, 110, 2004–2005. [Google Scholar] [CrossRef]
- Matt, B.; Moussa, J.; Chamoreau, L.-M.; Afonso, C.; Proust, A.; Amouri, H.; Izzet, G. Elegant Approach to the Synthesis of a Unique Heteroleptic Cyclometalated Iridium(III)-Polyoxometalate Conjugate. Organometallics 2012, 31, 35–38. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Li, C. Theoretical Study of Structure, Stability, and the Hydrolysis Reactions of Small Iridium Oxide Nanoclusters. J. Phys. Chem. A 2012, 116, 9985–9995. [Google Scholar] [CrossRef]
- Liu, R.; Dandu, N.; Chen, J.; Li, Y.; Li, Z.; Liu, S.; Wang, C.; Kilina, S.; Kohler, B.; Sun, W. Influence of Different Diimine (N ∧ N) Ligands on the Photophysics and Reverse Saturable Absorption of Heteroleptic Cationic Iridium(III) Complexes Bearing Cyclometalating 2-{3-[7-(Benzothiazol-2-Yl)Fluoren-2-Yl]Phenyl}pyridine (C ∧ N) Ligands. J. Phys. Chem. C 2014, 118, 23233–23246. [Google Scholar] [CrossRef]
- Yao, C.; Tian, Z.; Jin, D.; Zhao, F.; Sun, Y.; Yang, X.; Zhou, G.; Wong, W.-Y. Platinum( ii ) Acetylide Complexes with Star- and V-Shaped Configurations Possessing Good Trade-off between Optical Transparency and Optical Power Limiting Performance. J. Mater. Chem. C 2017, 5, 11672–11682. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, S.; Lu, J.; Shi, H.; Zhu, H. Pt(II) Diimine Complexes Bearing Difluoro-Boron-Dipyrromethene Acetylide Ligands: Synthesis, Photophysics, Aggregation Included Emission and Optical Power Limiting Properties. Dyes Pigments 2017, 147, 291–299. [Google Scholar] [CrossRef]
- You, Y.; Nam, W. Photofunctional Triplet Excited States of Cyclometalated Ir(Iii) Complexes: Beyond Electroluminescence. Chem. Soc. Rev. 2012, 41, 7061. [Google Scholar] [CrossRef]
- Li, Y.; Dandu, N.; Liu, R.; Hu, L.; Kilina, S.; Sun, W. Nonlinear Absorbing Cationic Iridium(III) Complexes Bearing Benzothiazolylfluorene Motif on the Bipyridine (N∧N) Ligand: Synthesis, Photophysics and Reverse Saturable Absorption. ACS Appl. Mater. Interfaces 2013, 5, 6556–6570. [Google Scholar] [CrossRef]
- Hirata, S.; Totani, K.; Yamashita, T.; Adachi, C.; Vacha, M. Large Reverse Saturable Absorption under Weak Continuous Incoherent Light. Nat. Mater. 2014, 13, 938–946. [Google Scholar] [CrossRef]
- Dini, D.; Calvete, M.J.F.; Hanack, M. Nonlinear Optical Materials for the Smart Filtering of Optical Radiation. Chem. Rev. 2016, 116, 13043–13233. [Google Scholar] [CrossRef]
- Yuan, G.-J.; Shao, D.-S.; Hu, B.-W.; Liu, W.-L.; Ren, X.-M. A Rotorlike Supramolecular Assembly, {[K(18-Crown-6)]PbI 3 } ∞, with a Reversible Breaking-Symmetry Phase Transition near Room Temperature. Inorg. Chem. 2020, 59, 980–983. [Google Scholar] [CrossRef]
- Tang, M.; Zhu, S.; Liu, R.; Wang, J.; Zhang, Z.; Zhu, H. Synthesis, Characterization and Optical Properties of Novel Ir(III) Complexes Bearing N-Heterocycle Substituents. J. Organomet. Chem. 2019, 880, 363–367. [Google Scholar] [CrossRef]
- Lu, J.; Pan, Q.; Zhu, S.; Liu, R.; Zhu, H. Ligand-Mediated Photophysics Adjustability in Bis-Tridentate Ir(III) Complexes and Their Application in Efficient Optical Limiting Materials. Inorg. Chem. 2021, 60, 12835–12846. [Google Scholar] [CrossRef]
- Liu, B.; Jabed, M.A.; Kilina, S.; Sun, W. Synthesis, Photophysics, and Reverse Saturable Absorption of Trans -Bis-Cyclometalated Iridium (III) Complexes (C^N^C)Ir(R-Tpy) + (Tpy = 2,2′:6′,2″-Terpyridine) with Broadband Excited-State Absorption. Inorg. Chem. 2020, 59, 8532–8542. [Google Scholar] [CrossRef] [PubMed]
- Kuei, C.-Y.; Liu, S.-H.; Chou, P.-T.; Lee, G.-H.; Chi, Y. Room Temperature Blue Phosphorescence: A Combined Experimental and Theoretical Study on the Bis-Tridentate Ir( iii ) Metal Complexes. Dalton Trans. 2016, 45, 15364–15373. [Google Scholar] [CrossRef] [PubMed]
- Whittle, V.L.; Williams, J.A.G. A New Class of Iridium Complexes Suitable for Stepwise Incorporation into Linear Assemblies: Synthesis, Electrochemistry, and Luminescence. Inorg. Chem. 2008, 47, 6596–6607. [Google Scholar] [CrossRef] [PubMed]
- Kuei, C.-Y.; Tsai, W.-L.; Tong, B.; Jiao, M.; Lee, W.-K.; Chi, Y.; Wu, C.-C.; Liu, S.-H.; Lee, G.-H.; Chou, P.-T. Bis-Tridentate Ir(III) Complexes with Nearly Unitary RGB Phosphorescence and Organic Light-Emitting Diodes with External Quantum Efficiency Exceeding 31%. Adv. Mater. 2016, 28, 2795–2800. [Google Scholar] [CrossRef] [PubMed]
- Gildea, L.F.; Batsanov, A.S.; Williams, J.A.G. Bright Orange/Red-Emitting Rhodium (Iii) and Iridium(Iii) Complexes: Tridentate N^C^N-Cyclometallating Ligands Lead to High Luminescence Efficiencies. Dalton Trans. 2013, 42, 10388. [Google Scholar] [CrossRef]
- Guo, F.; Sun, W.; Liu, Y.; Schanze, K. Synthesis, Photophysics, and Optical Limiting of Platinum (II) 4‘-Tolylterpyridyl Arylacetylide Complexes. Inorg. Chem. 2005, 44, 4055–4065. [Google Scholar] [CrossRef]
- Shao, P.; Li, Y.; Sun, W. Cyclometalated Platinum (II) Complex with Strong and Broadband Nonlinear Optical Response. J. Phys. Chem. A 2008, 112, 1172–1179. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, B.; Shao, P.; Li, Y.; Sun, W. Synthesis and Photophysics of Platinum (II) 6-Phenyl-4-(9,9-Dihexylfluoren-2-Yl)-2,2′-Bipyridine Complexes with Phenothiazinyl Acetylide Ligand. J. Phys. Chem. A 2010, 114, 7055–7062. [Google Scholar] [CrossRef]
- Wu, S.-H.; Ling, J.-W.; Lai, S.-H.; Huang, M.-J.; Cheng, C.H.; Chen, I.-C. Dynamics of the Excited States of [Ir(Ppy) 2 Bpy] + with Triple Phosphorescence. J. Phys. Chem. A 2010, 114, 10339–10344. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, S.; Shi, M.; Wang, C.; Yu, M.; Li, L.; Li, F.; Yi, T.; Huang, C. Series of New Cationic Iridium(III) Complexes with Tunable Emission Wavelength and Excited State Properties: Structures, Theoretical Calculations, and Photophysical and Electrochemical Properties. Inorg. Chem. 2006, 45, 6152–6160. [Google Scholar] [CrossRef]
- Zhu, Z.-L.; Chen, W.-C.; Ni, S.-F.; Yan, J.; Wang, S.F.; Fu, L.-W.; Tsai, H.-Y.; Chi, Y.; Lee, C.-S. Constructing Deep-Blue Bis-Tridentate Ir( iii ) Phosphors with Fluorene-Based Dianionic Chelates. J. Mater. Chem. C 2021, 9, 1318–1325. [Google Scholar] [CrossRef]
- Tai, W.-S.; Gnanasekaran, P.; Chen, Y.-Y.; Hung, W.-Y.; Zhou, X.; Chou, T.-C.; Lee, G.-H.; Chou, P.-T.; You, C.; Chi, Y. Rational Tuning of Bis-Tridentate Ir(III) Phosphors to Deep-Blue with High Efficiency and Sub-Microsecond Lifetime. ACS Appl. Mater. Interfaces 2021, 13, 15437–15447. [Google Scholar] [CrossRef]
- Zhu, S.; Pan, Q.; Li, Y.; Liu, W.; Liu, R.; Zhu, H. Fluorene-Decorated Ir (III) Complexes: Synthesis, Photophysics and Tunable Triplet Excited State Properties in Aggregation. Dalton Trans. 2022, 51, 13322–13330. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Luo, Z.-D.; Pan, Y.; Kumar Singh, A.; Trivedi, M.; Kumar, A. Recent Developments in Luminescent Coordination Polymers: Designing Strategies, Sensing Application and Theoretical Evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, L.; Liu, D.; Wang, J.; Sakiyama, H.; Muddassir, M.; Nezamzadeh-Ejhieh, A.; Liu, J. Series of Highly Stable Cd (ii)-Based MOFs as Sensitive and Selective Sensors for Detection of Nitrofuran Antibiotic. CrystEngComm 2021, 23, 8043–8052. [Google Scholar] [CrossRef]
- Büldt, L.A.; Guo, X.; Vogel, R.; Prescimone, A.; Wenger, O.S. A Tris(Diisocyanide)Chromium(0) Complex Is a Luminescent Analog of Fe(2,2′-Bipyridine)32+. J. Am. Chem. Soc. 2017, 139, 985–992. [Google Scholar] [CrossRef]
- Lu, T.; Wang, C.; Lystrom, L.; Pei, C.; Kilina, S.; Sun, W. Effects of Extending the π-Conjugation of the Acetylide Ligand on the Photophysics and Reverse Saturable Absorption of Pt(ii) Bipyridine Bisacetylide Complexes. Phys. Chem. Chem. Phys. 2016, 18, 28674–28687. [Google Scholar] [CrossRef]
- Hongbing, Z.; Wenzhe, C.; Minquan, W.; Chunlin, Z. Optical Limiting Effects of Multi-Walled Carbon Nanotubes Suspension and Silica Xerogel Composite. Chem. Phys. Lett. 2003, 382, 313–317. [Google Scholar] [CrossRef]
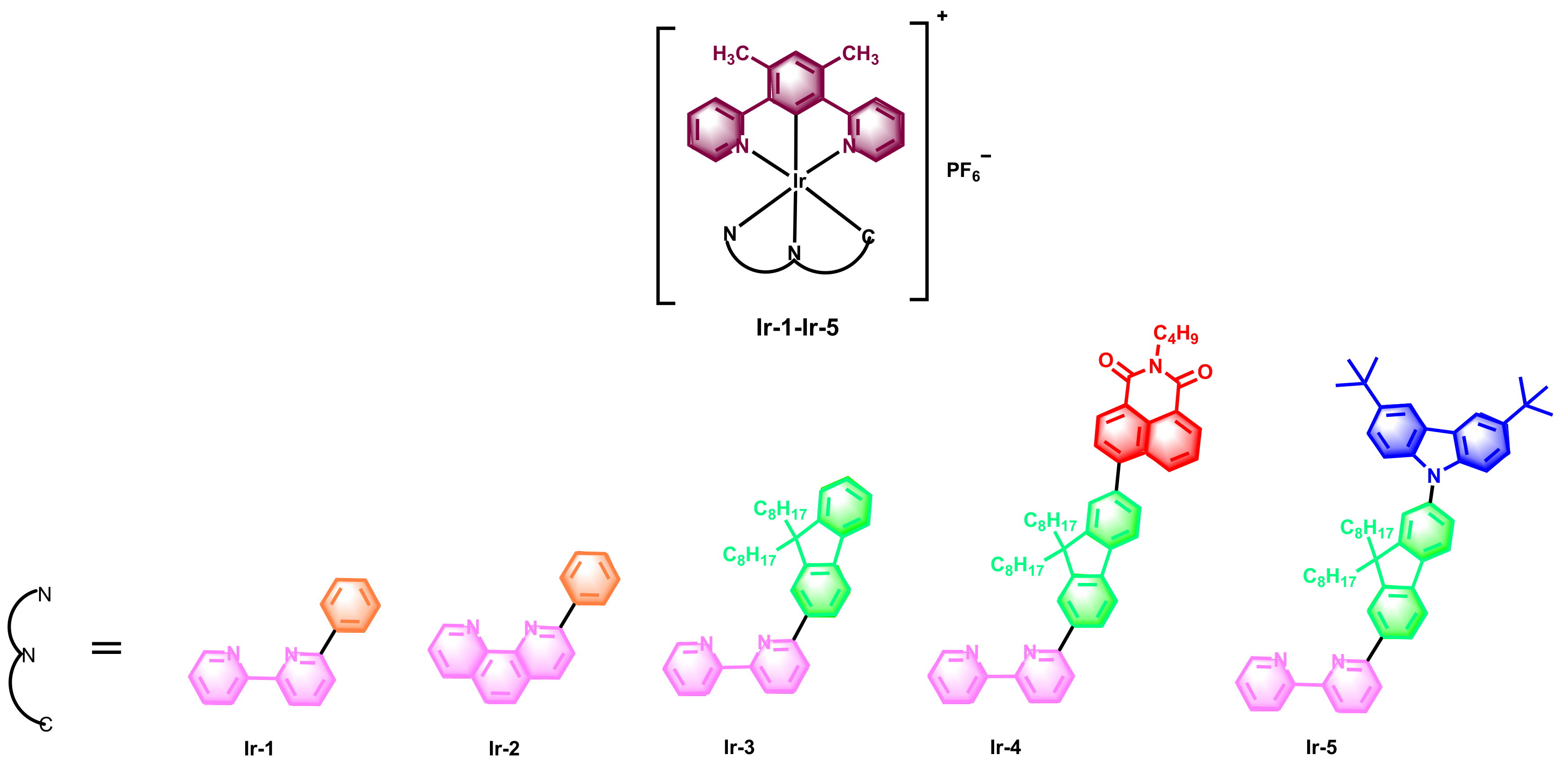
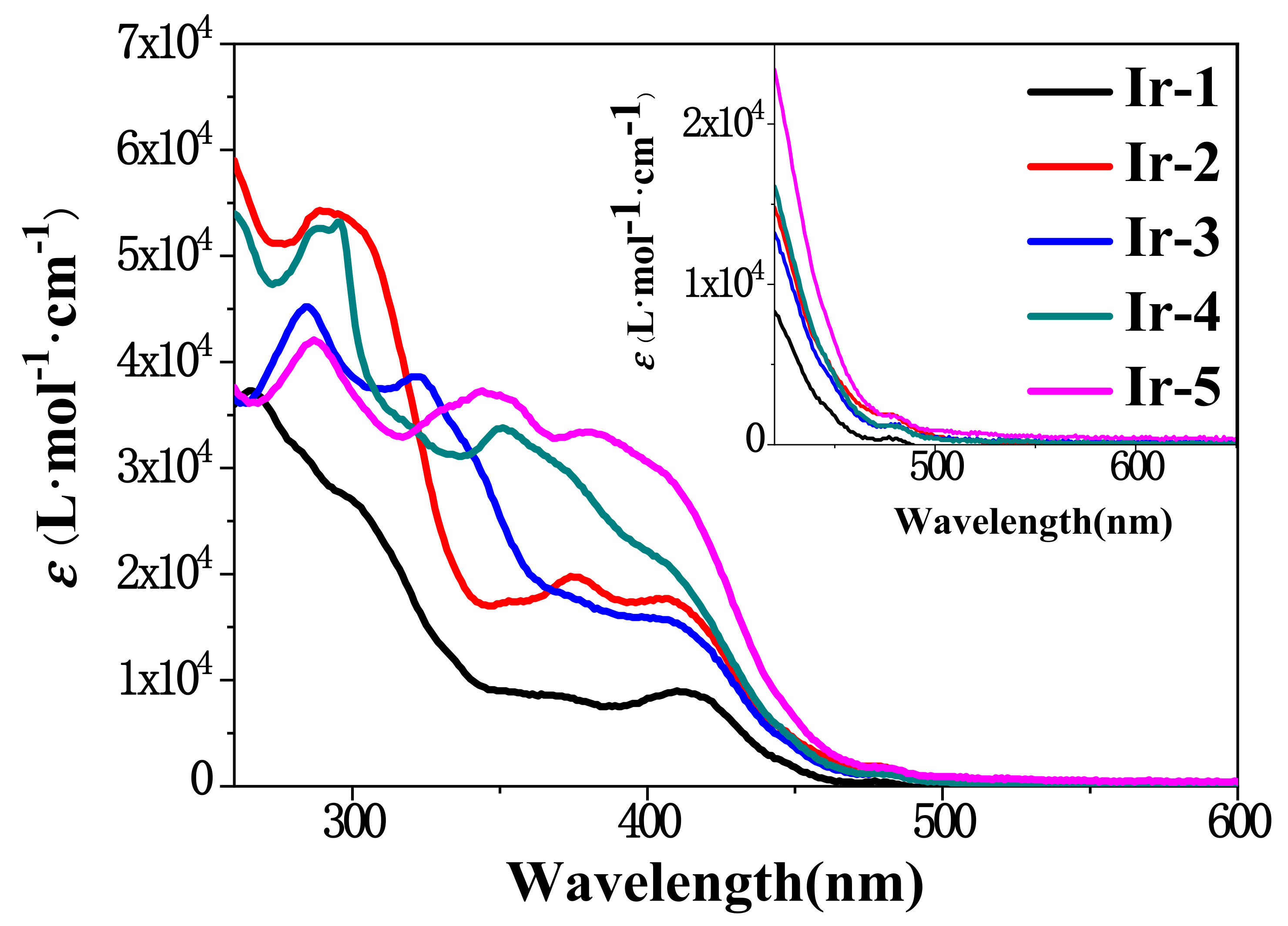
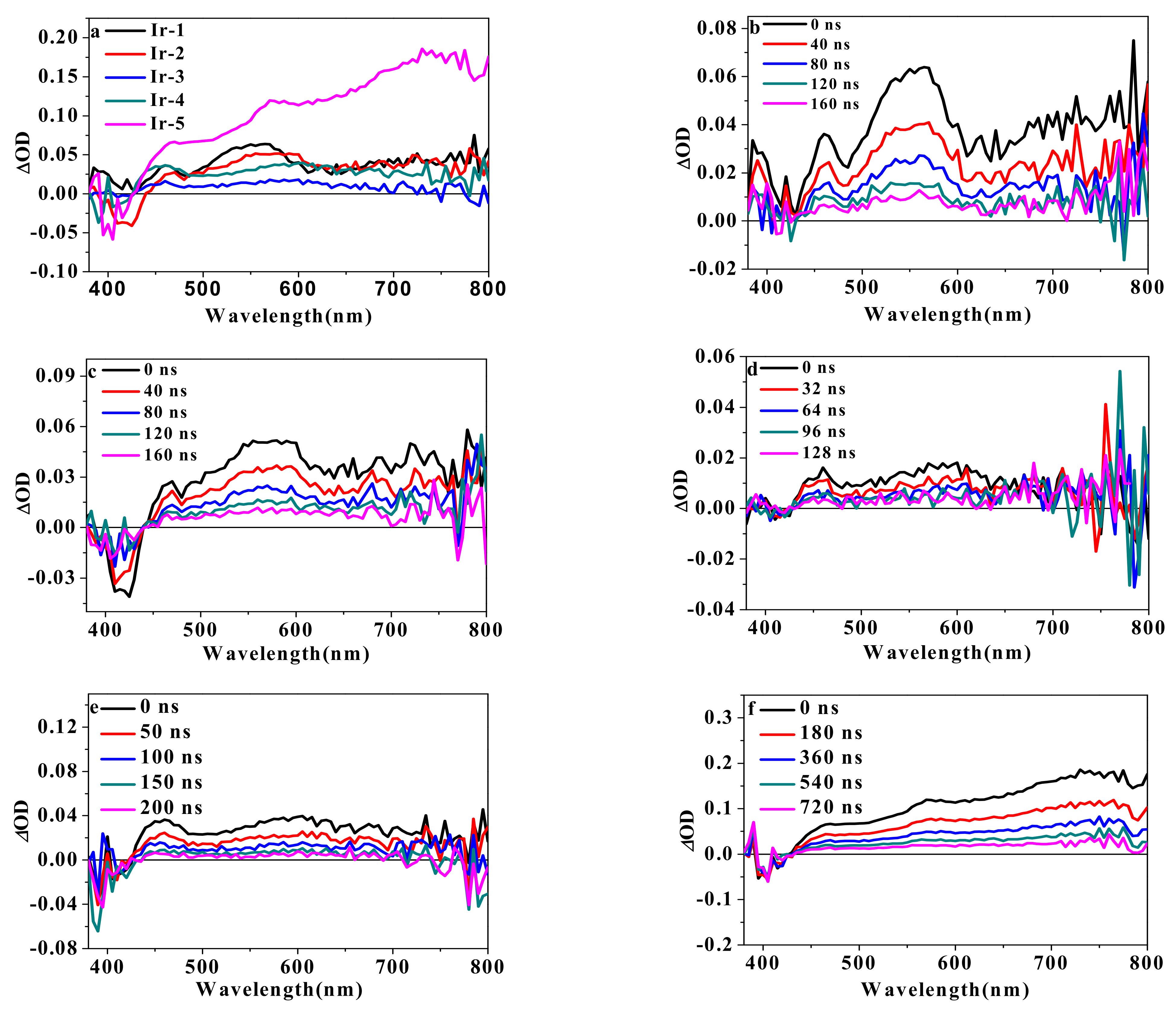
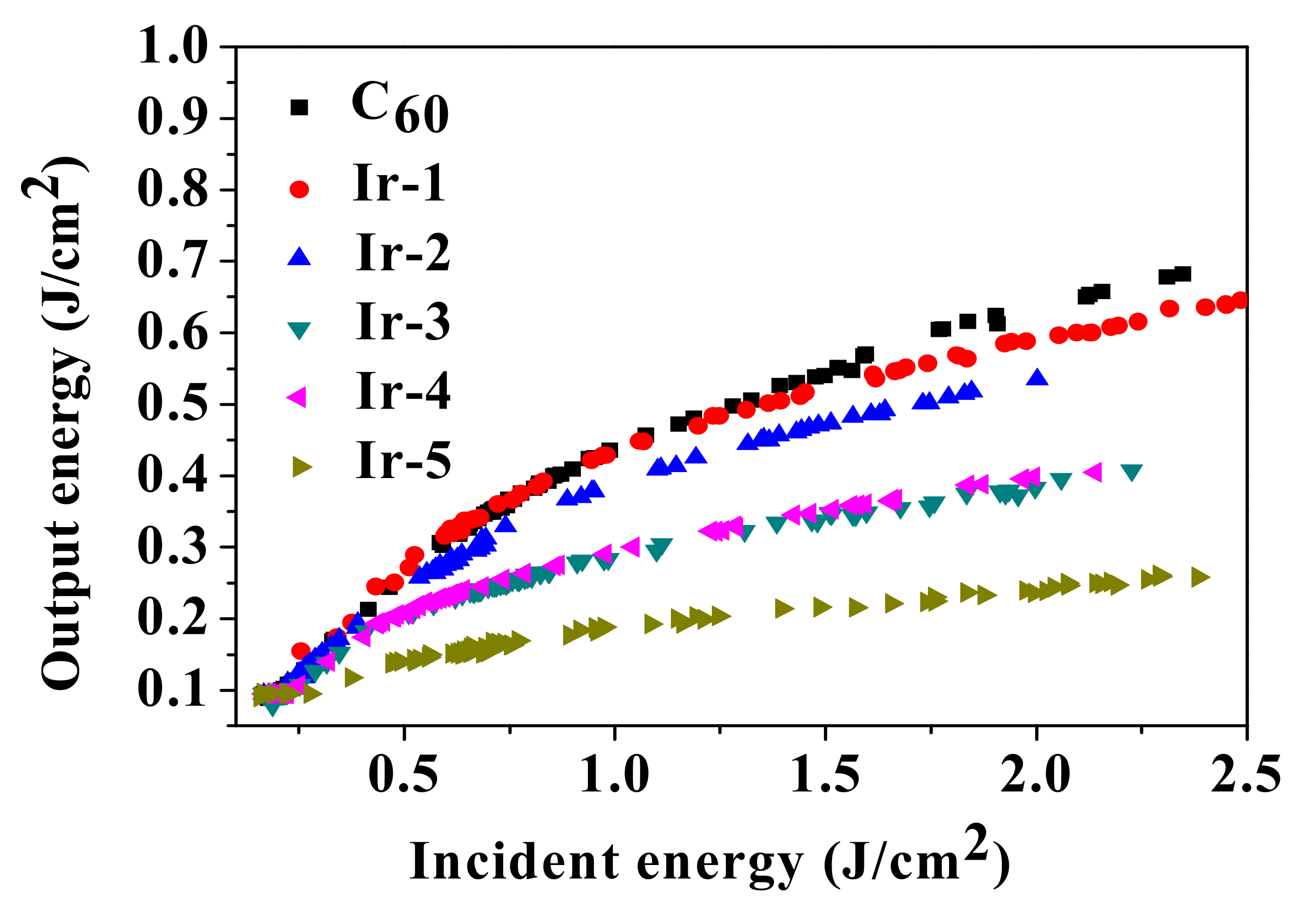
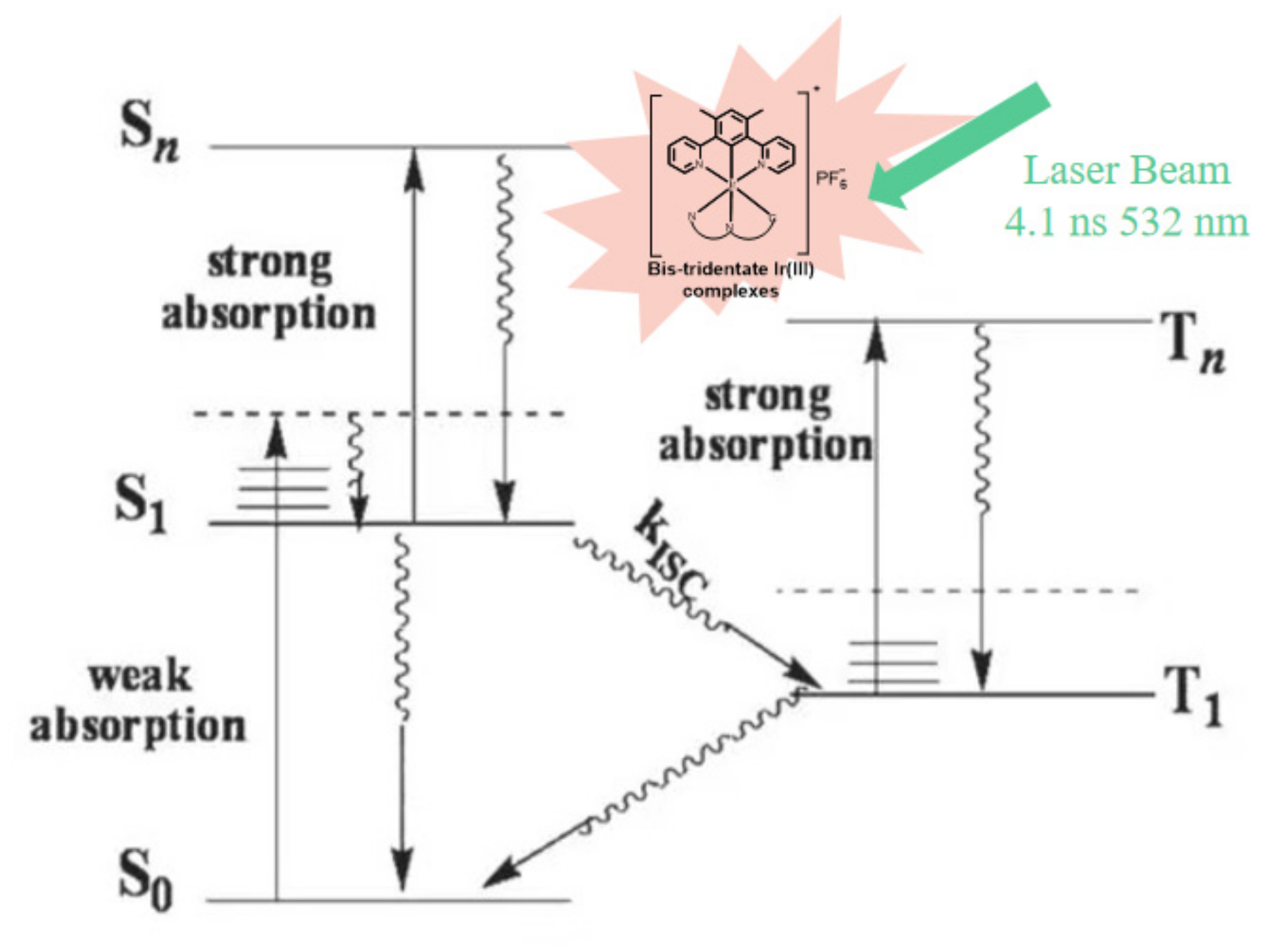
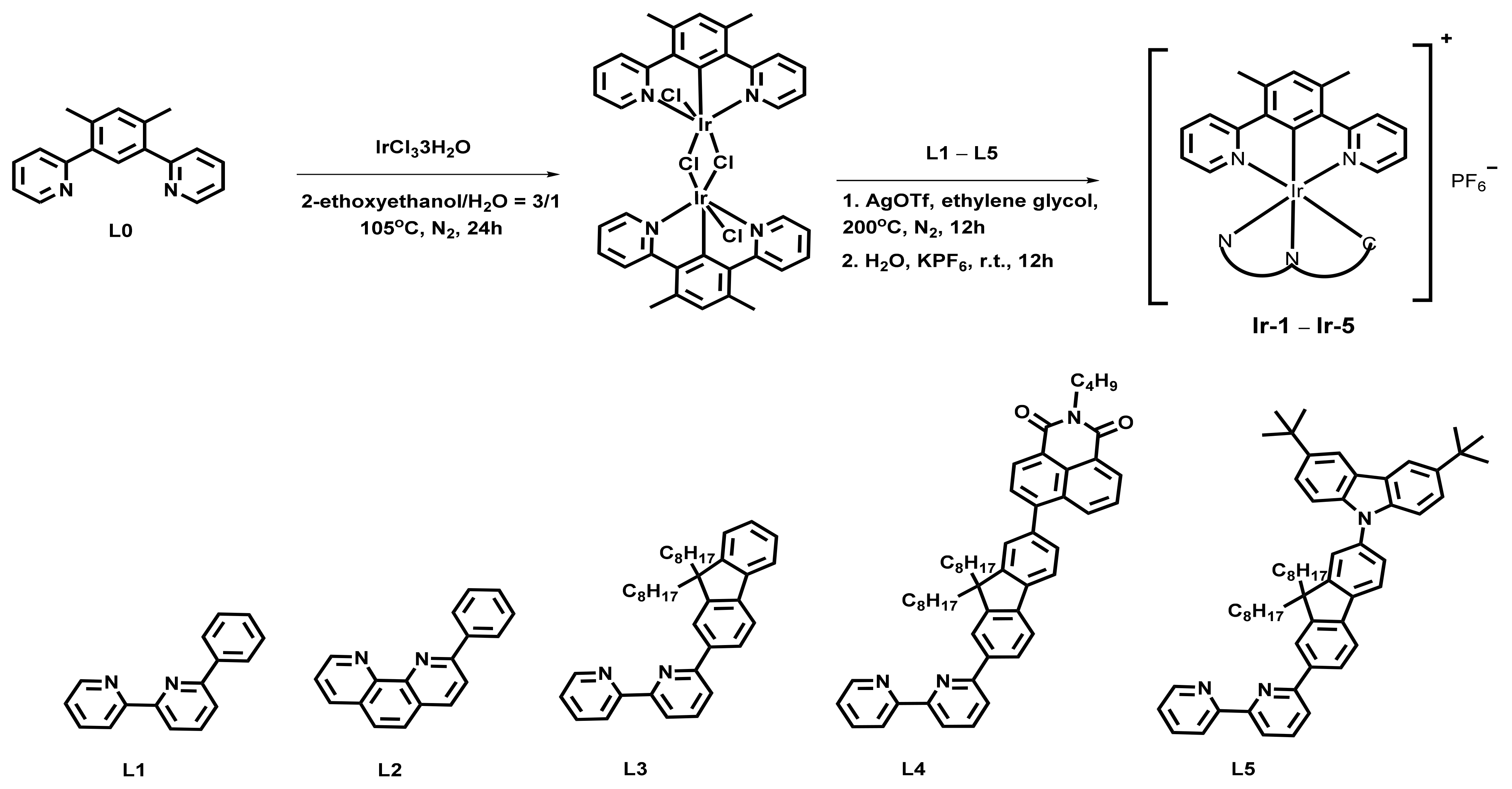
| Complex | λabs a/nm (ε/104 L·mol−1·cm−1) | λem b/nm (τem/ns), Φem | λT1-Tn/nm c (log εT1-Tn, τTA/ns); ΦT |
|---|---|---|---|
| Ir-1 | 273 (5.36), 348 (4.15), 409 (2.46), 468 (0.65) | 594 (111); 0.34 | 530 (4.91, 69), 565 (5.11, 70), 0.23 |
| Ir-2 | 276 (5.68), 359 (4.22), 421 (1.73), 455 (0.55) | 596 (105); 0.13 | 560 (4.18, 86), 590 (4.57, 78), 0.26 |
| Ir-3 | 276 (4.51), 362 (6.13), 430 (2.79), 480 (0.75) | 609 (135); 0.29 | 410 (4.07, 74), 530 (4.51, 95), 605 (4.77, 56); 0.18 |
| Ir-4 | 273 (6.36), 348 (5.15), 409 (2.96), 468 (0.65) | 610 (241); 0.18 | 460 (4.91, 198), 530 (5.11, 179), 560 (5.12,94); 0.23 |
| Ir-5 | 256 (5.79), 345 (4.52), 409 (2.47), 465 (0.65) | 613 (341); 0.24 | 530 (4.09, 341), 570 (4.25, 354), 653 (4.26, 352); 0.35 |
| λem/nm (Φem) | ||||
|---|---|---|---|---|
| THF | CH2Cl2 | Acetone | CH3CN | |
| Ir-1 | 589 (0.29) | 584 (0.29) | 594 (0.34) | 594 (0.30) |
| Ir-2 | 589 (0.19) | 585 (0.13) | 594 (0.13) | 596 (0.17) |
| Ir-3 | 604 (0,23) | 602 (0.28) | 607 (0.29) | 609 (0.14) |
| Ir-4 | 603 (0.18) | 603 (0.24) | 609 (0.18) | 610 (0.12) |
| Ir-5 | 604 (0.24) | 601 (0.26) | 609 (0.24) | 613 (0.24) |
| State | E/eV | λ a/nm | λ b/nm | f | Main Configurations | Assignment | |
|---|---|---|---|---|---|---|---|
| Ir-1 | S1 | 2.3865 | 525 | 519.52 | 0.0001 | HOMO→LUMO 98.24% | 1MLCT/1LLCT |
| T1 | 2.3406 | 594 | 529.71 | 0 | HOMO→LUMO 97.09% | 3MLCT/3LLCT | |
| Ir-2 | S1 | 2.4874 | 524 | 498.46 | 0.0022 | HOMO→LUMO 97.53% | 1MLCT/1LLCT |
| T1 | 2.3722 | 595 | 522.65 | 0 | HOMO-1→UMO 83.18% | 3MLCT/3LLCT | |
| Ir-3 | S1 | 2.3197 | 527 | 534.49 | 0.0042 | HOMO-1→LUMO 5.42% HOMO→LUMO 92.32% | 1MLCT/1LLCT |
| T1 | 2.1172 | 609 | 585.59 | 0 | HOMO-1→LUMO 7.33% HOMO→LUMO 88.81% | 3MLCT/3LLCT | |
| Ir-4 | S1 | 2.2264 | 530 | 556.87 | 0.0052 | HOMO-1→LUMO 12.43% HOMO→LUMO 85.96% | 3MLCT/3LLCT |
| T1 | 2.0842 | 610 | 594.89 | 0 | HOMO-1→LUMO 29.67% HOMO→LUMO 63.21% | 3MLCT/3LLCT | |
| Ir-5 | S1 | 1.4610 | 538 | 588.64 | 0.0024 | HOMO→ LUMO 99.57% | 3MLCT/3LLCT |
| T1 | 1.4406 | 613 | 611.64 | 0 | HOMO→ LUMO 98.17% | 3MLCT/3LLCT |
| HOMO-1 | HOMO | LUMO | |
|---|---|---|---|
| Ir-1 |  −8.06 eV |  −7.90 eV | 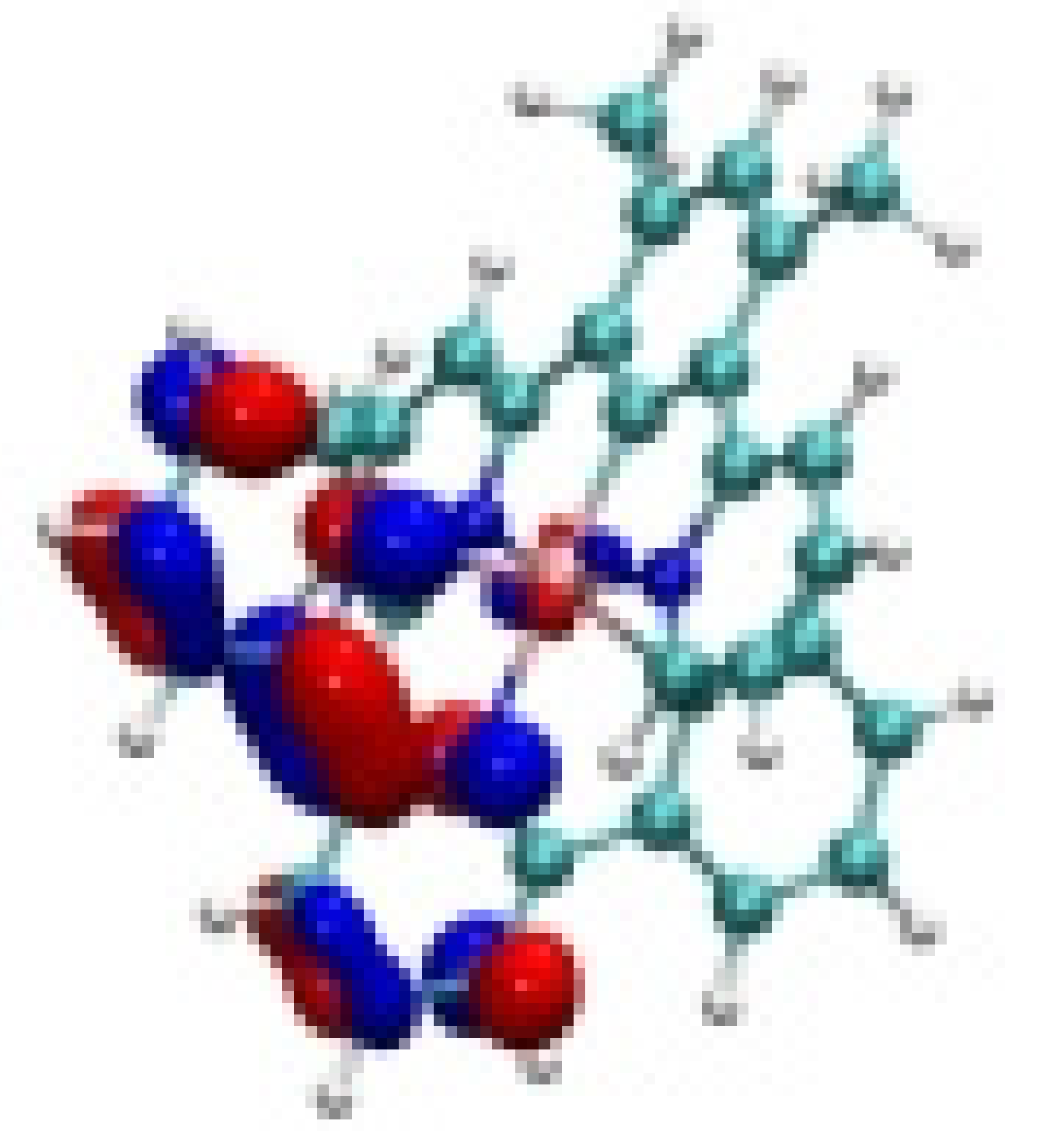 −4.82 eV |
| Ir-2 | 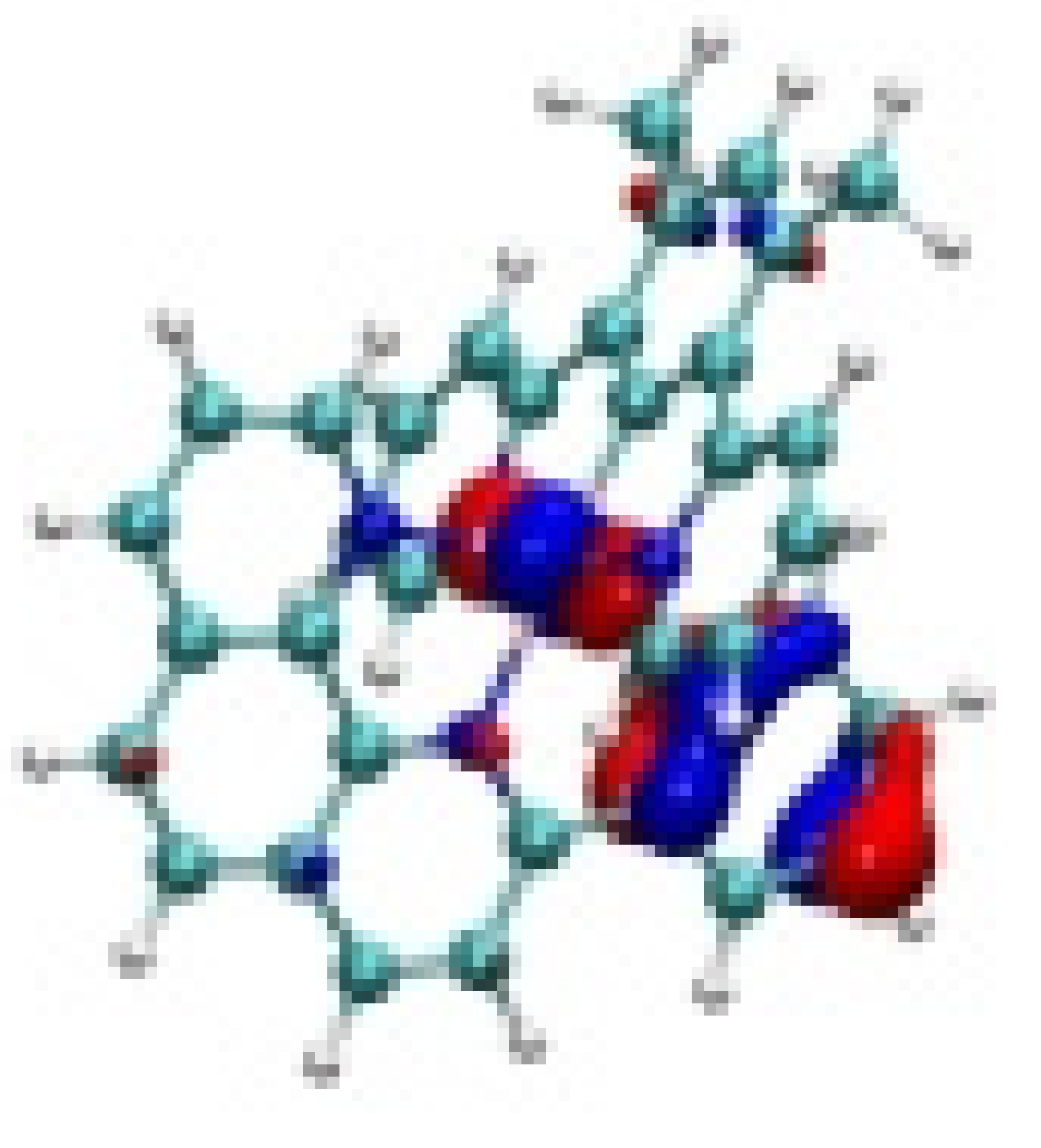 −8.05 eV |  −7.88 eV | 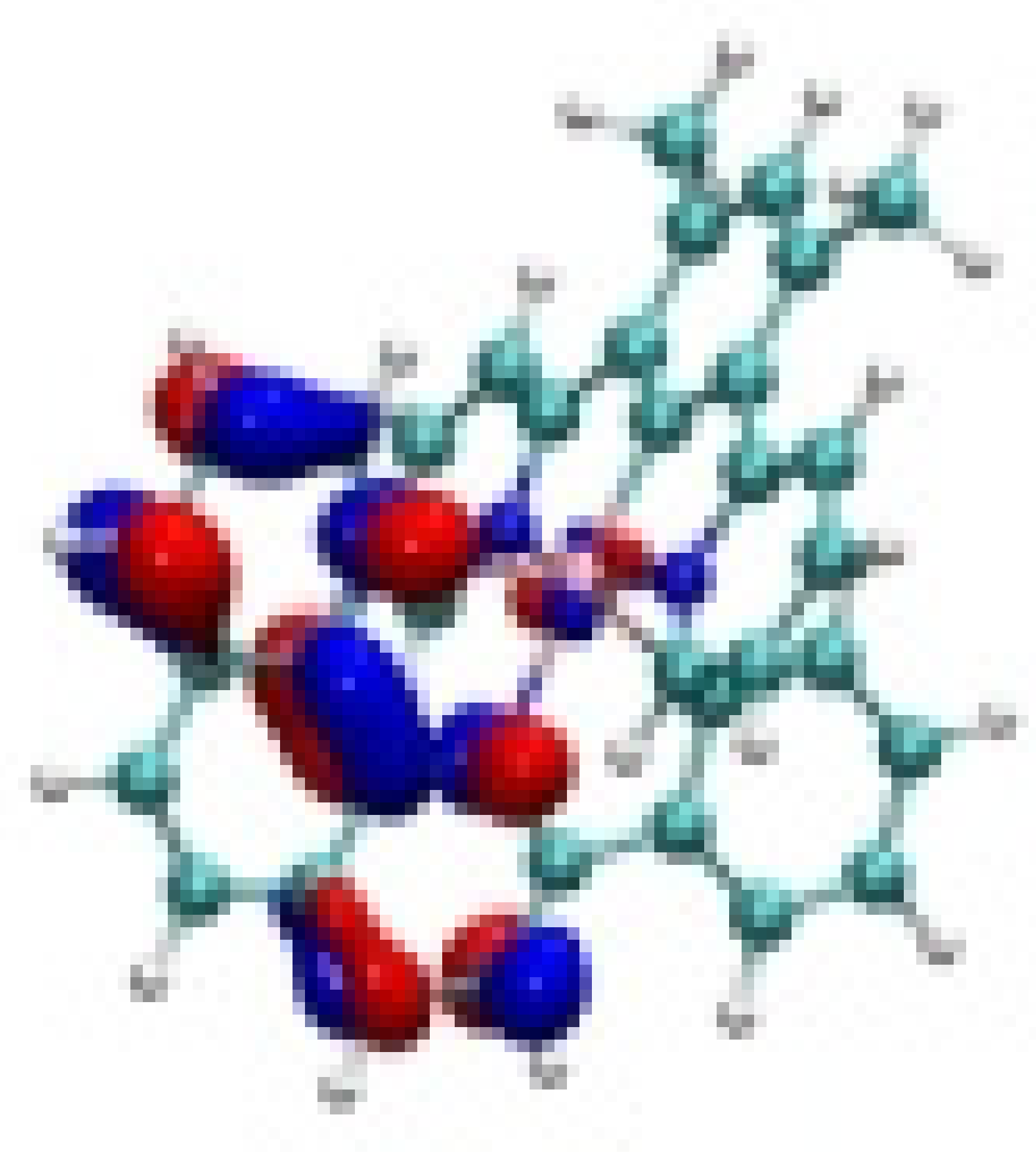 −4.71 eV |
| Ir-3 | 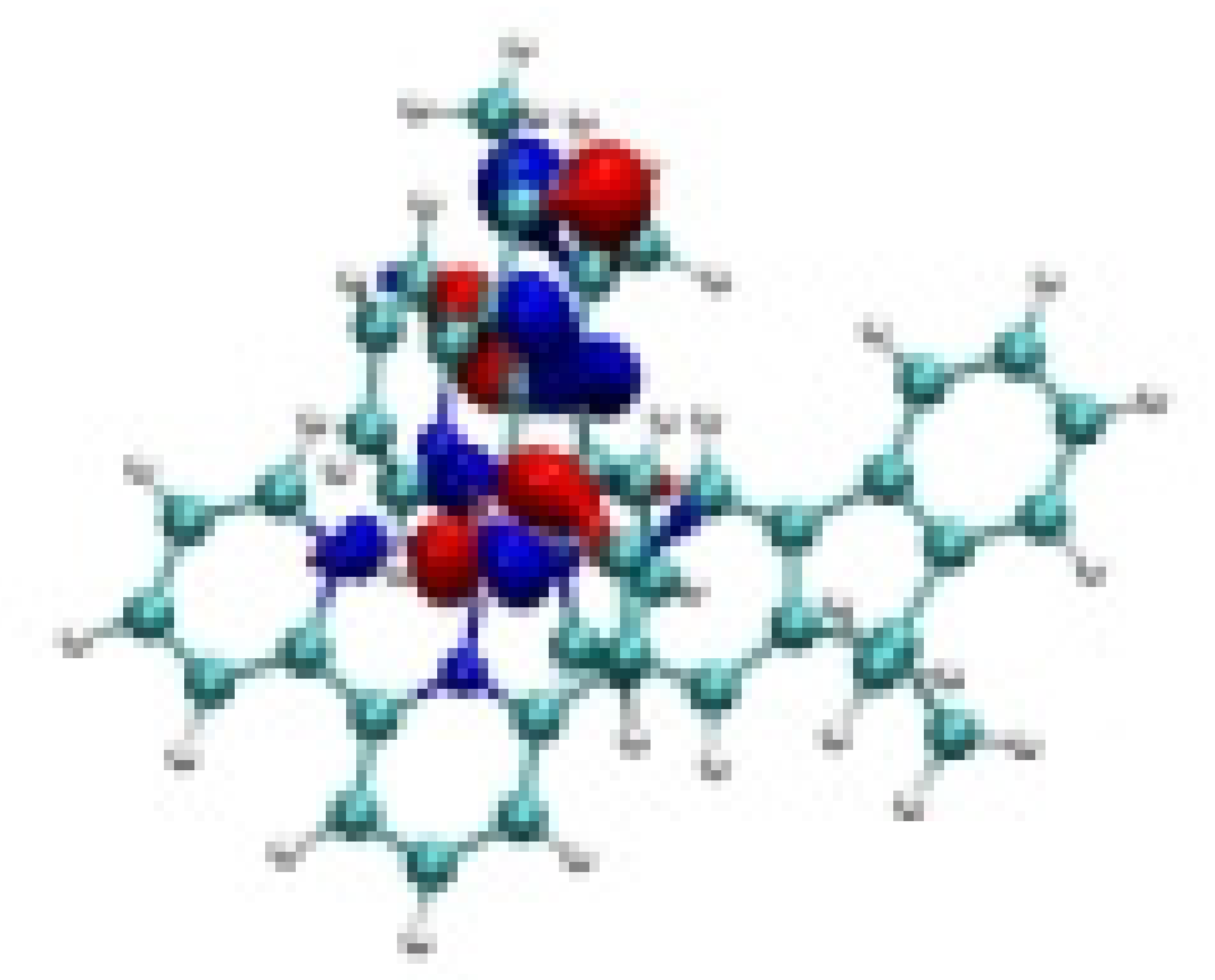 −7.81 eV | 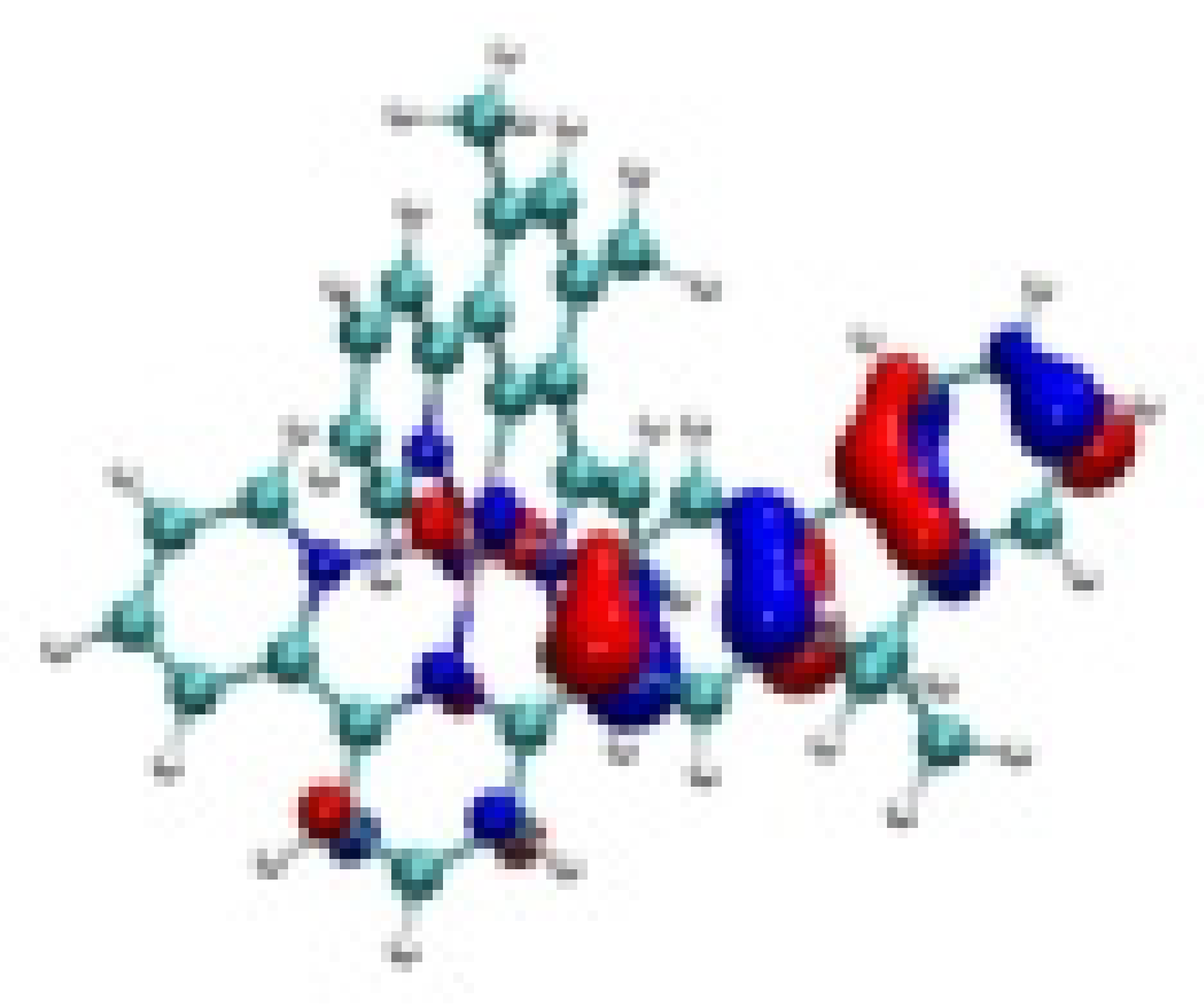 −7.54 eV | 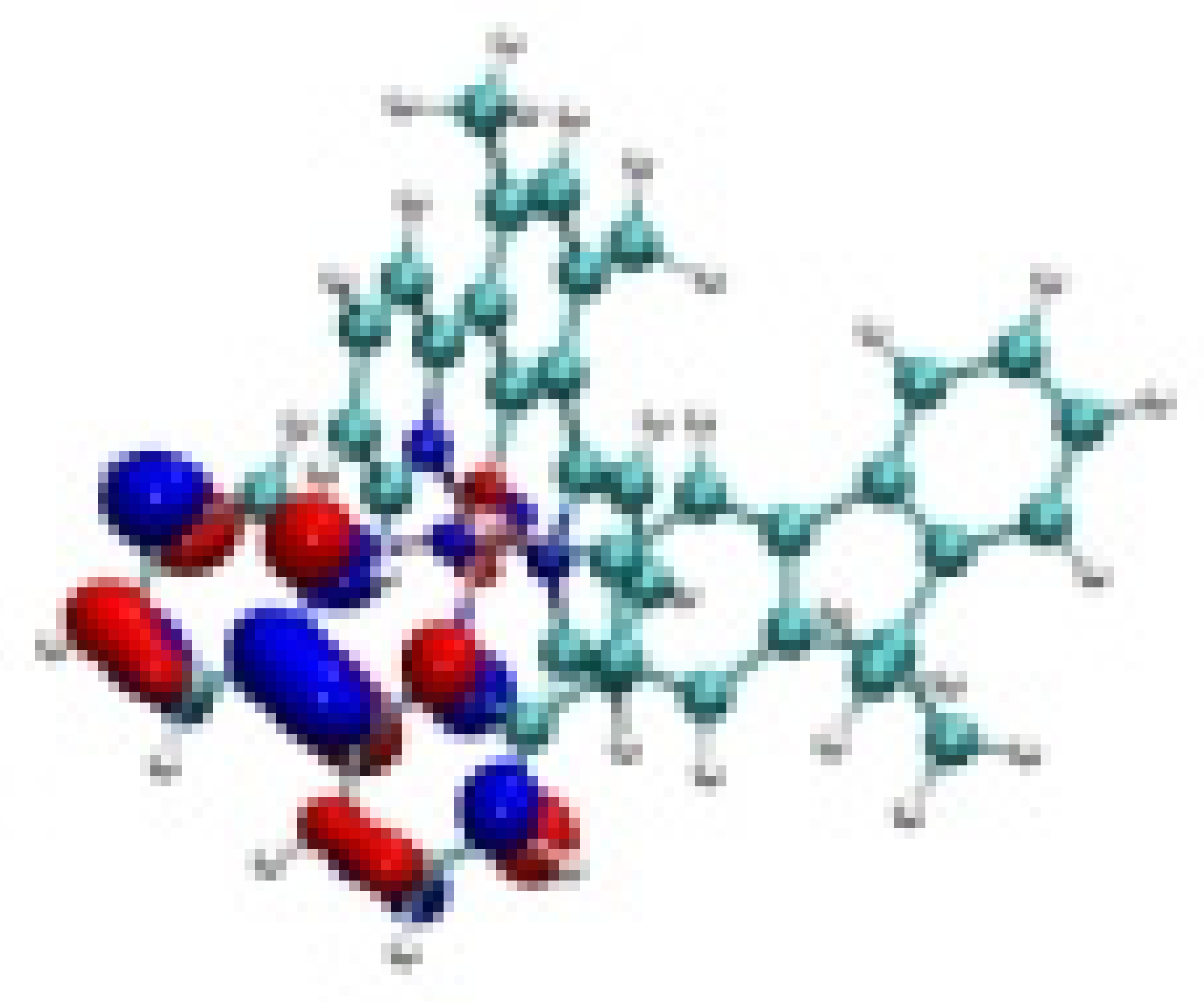 −4.70 eV |
| Ir-4 | 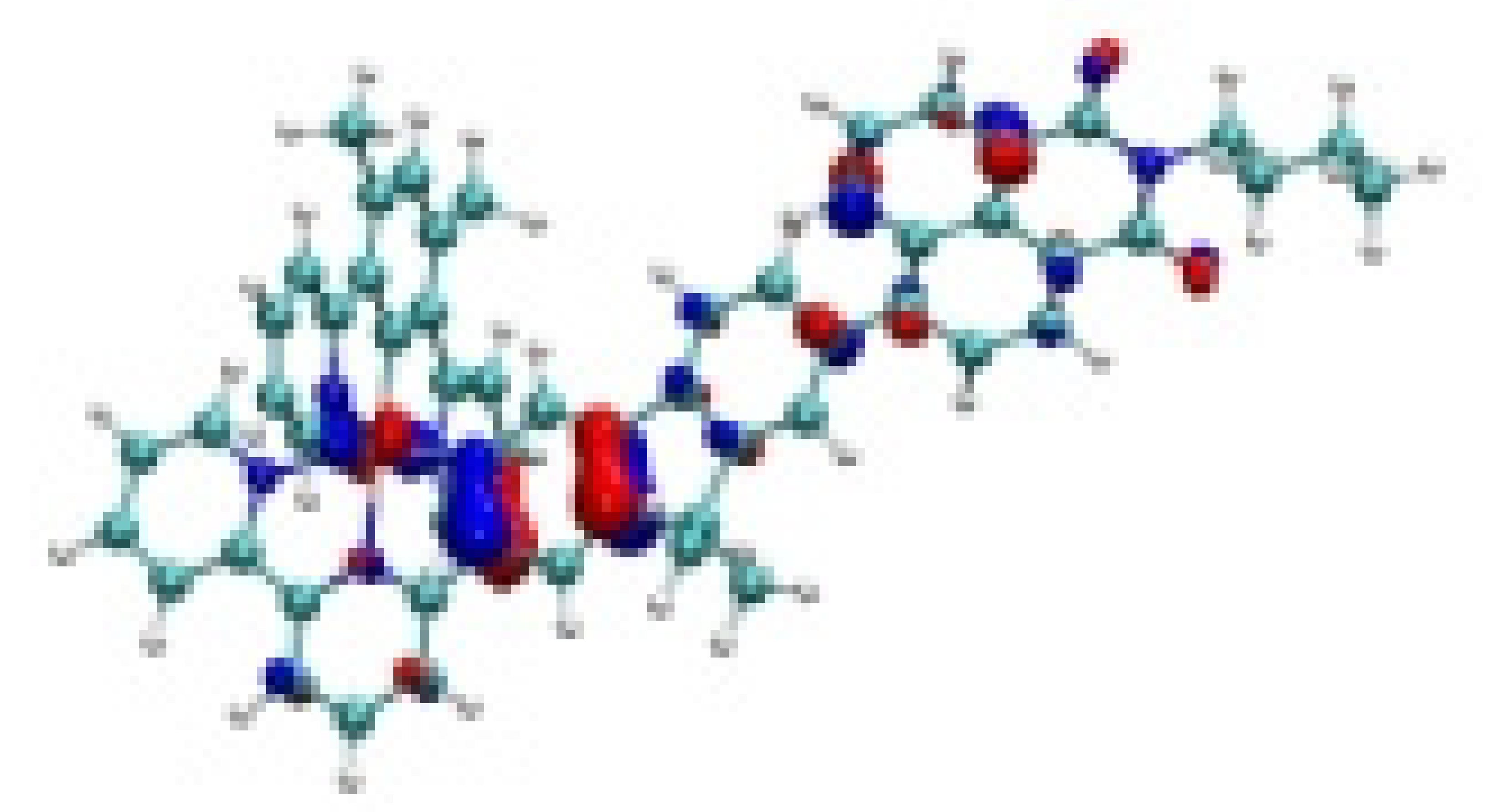 −7.82 eV | 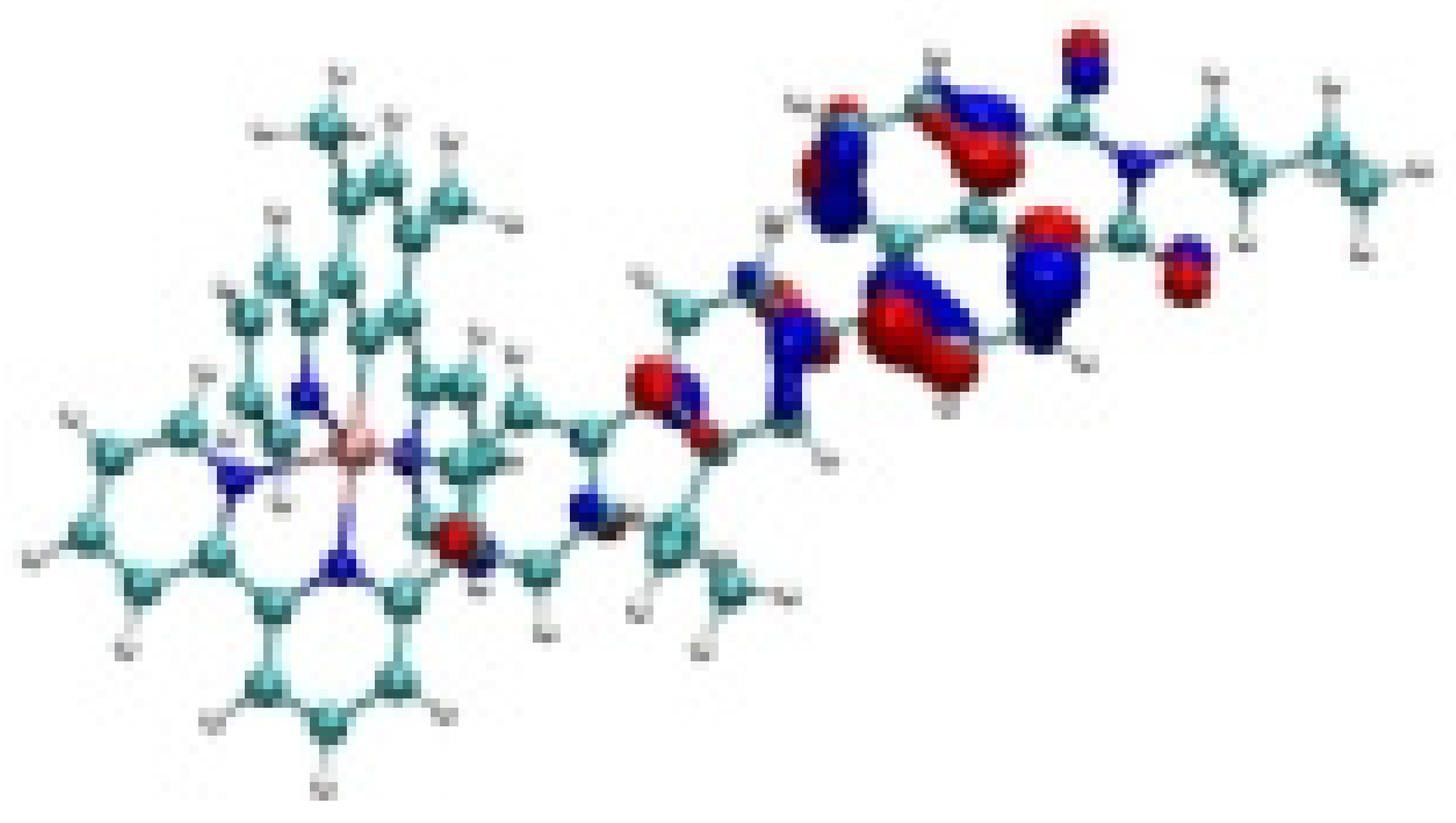 −7.33 eV |  −4.78 eV |
| Ir-5 |  −6.87 eV |  −6.39 eV |  −4.69 eV |
| Complex | Ir-1 | Ir-2 | Ir-3 | Ir-4 | Ir-5 |
|---|---|---|---|---|---|
| F50 a(J/cm2) | 1.23 | 0.95 | 0.52 | 0.55 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Jiang, Z.; Tang, M.; Jiang, X.; Tu, H.; Zhu, S.; Liu, R.; Zhu, H. Synthesis, Photophysics and Tunable Reverse Saturable Absorption of Bis-Tridentate Iridium(III) Complexes via Modification on Diimine Ligand. Molecules 2023, 28, 566. https://doi.org/10.3390/molecules28020566
Li G, Jiang Z, Tang M, Jiang X, Tu H, Zhu S, Liu R, Zhu H. Synthesis, Photophysics and Tunable Reverse Saturable Absorption of Bis-Tridentate Iridium(III) Complexes via Modification on Diimine Ligand. Molecules. 2023; 28(2):566. https://doi.org/10.3390/molecules28020566
Chicago/Turabian StyleLi, Guochang, Zhao Jiang, Meng Tang, Xiaoli Jiang, Houfu Tu, Senqiang Zhu, Rui Liu, and Hongjun Zhu. 2023. "Synthesis, Photophysics and Tunable Reverse Saturable Absorption of Bis-Tridentate Iridium(III) Complexes via Modification on Diimine Ligand" Molecules 28, no. 2: 566. https://doi.org/10.3390/molecules28020566
APA StyleLi, G., Jiang, Z., Tang, M., Jiang, X., Tu, H., Zhu, S., Liu, R., & Zhu, H. (2023). Synthesis, Photophysics and Tunable Reverse Saturable Absorption of Bis-Tridentate Iridium(III) Complexes via Modification on Diimine Ligand. Molecules, 28(2), 566. https://doi.org/10.3390/molecules28020566