Scaffolds for Cartilage Tissue Engineering from a Blend of Polyethersulfone and Polyurethane Polymers
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphology Characterization of the Membranes before Degradation
2.2. Degradation Studies
2.2.1. SEM Imaging and pH Measurement during and after Degradation
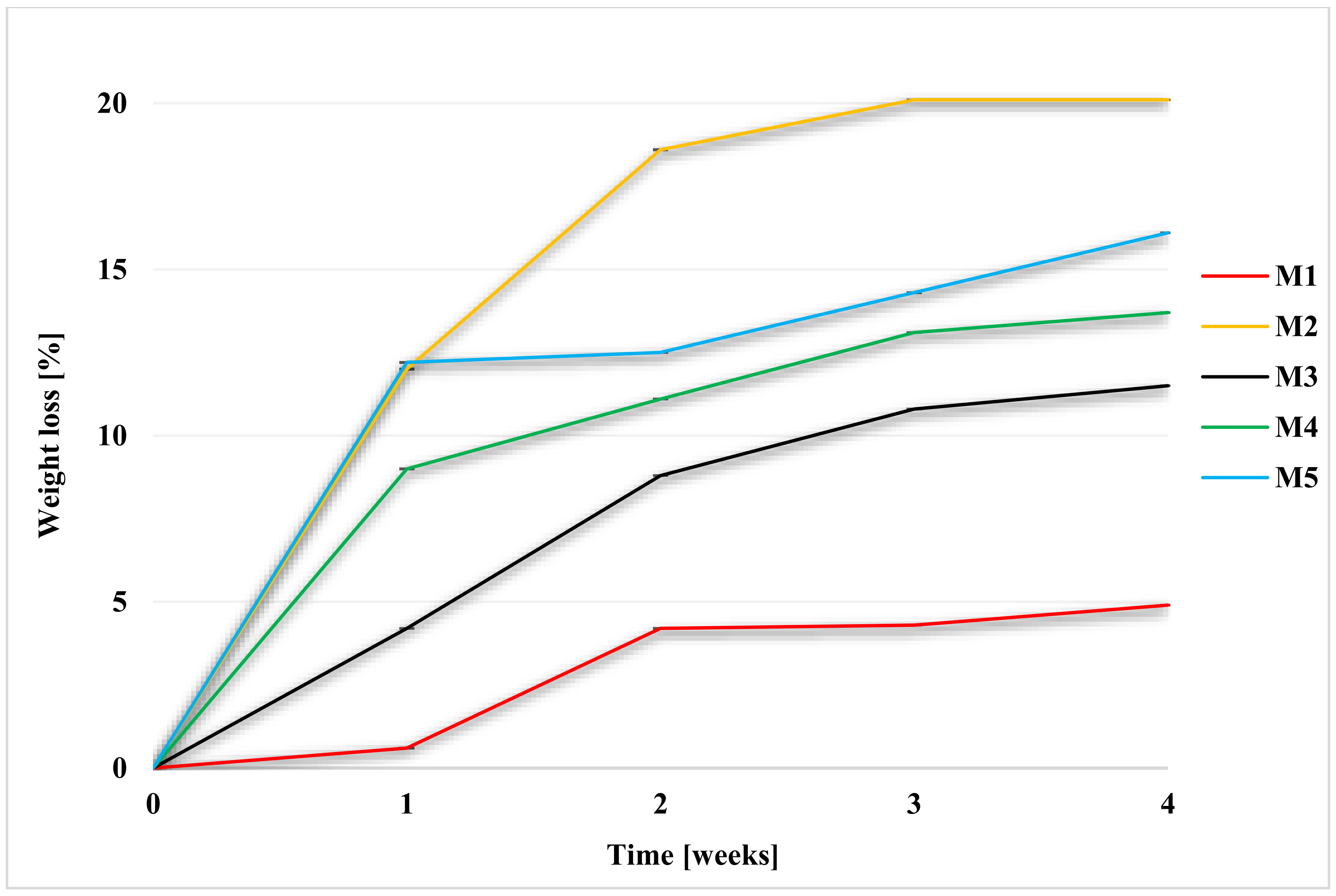
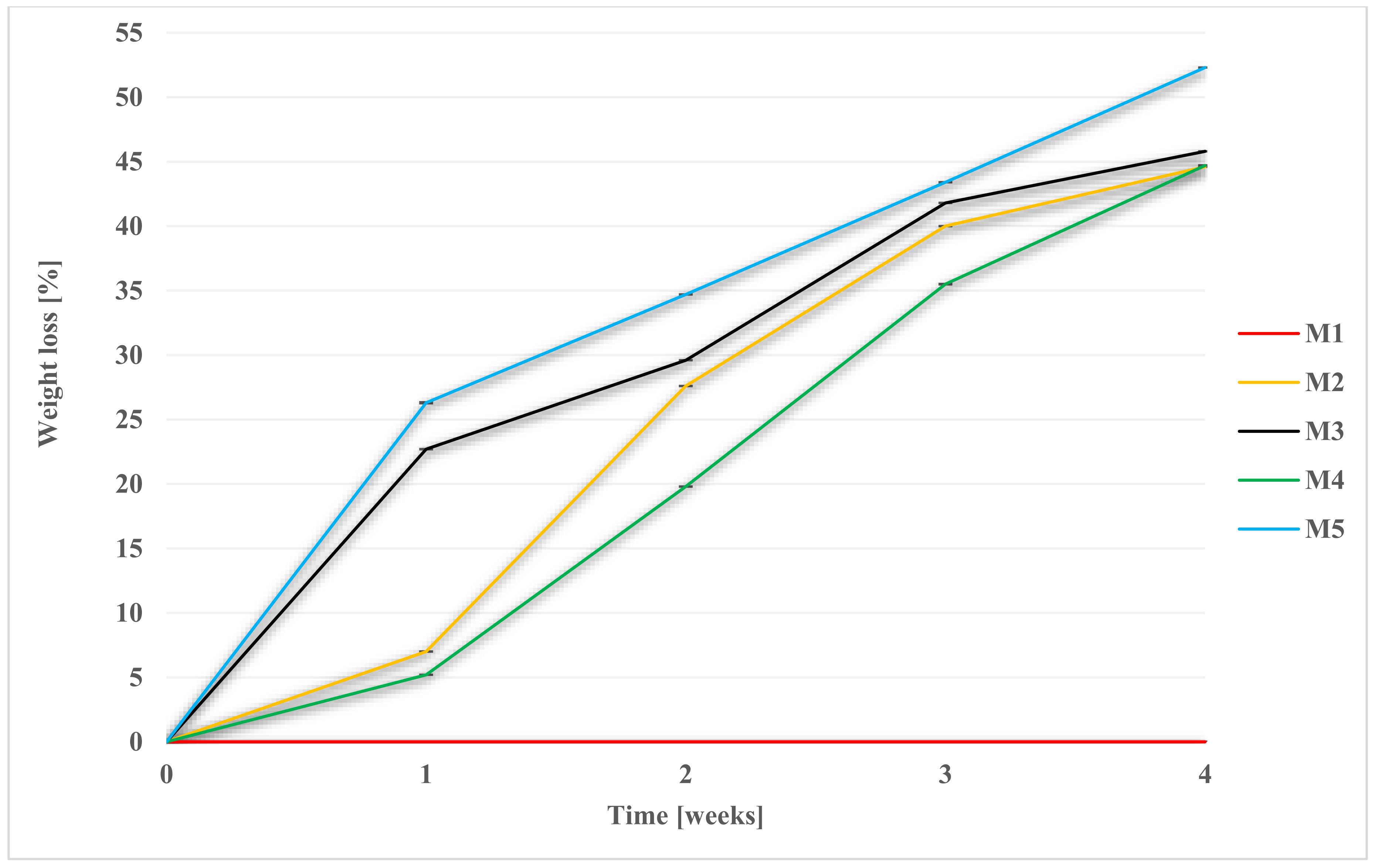
2.2.2. Weight Loss during Degradation
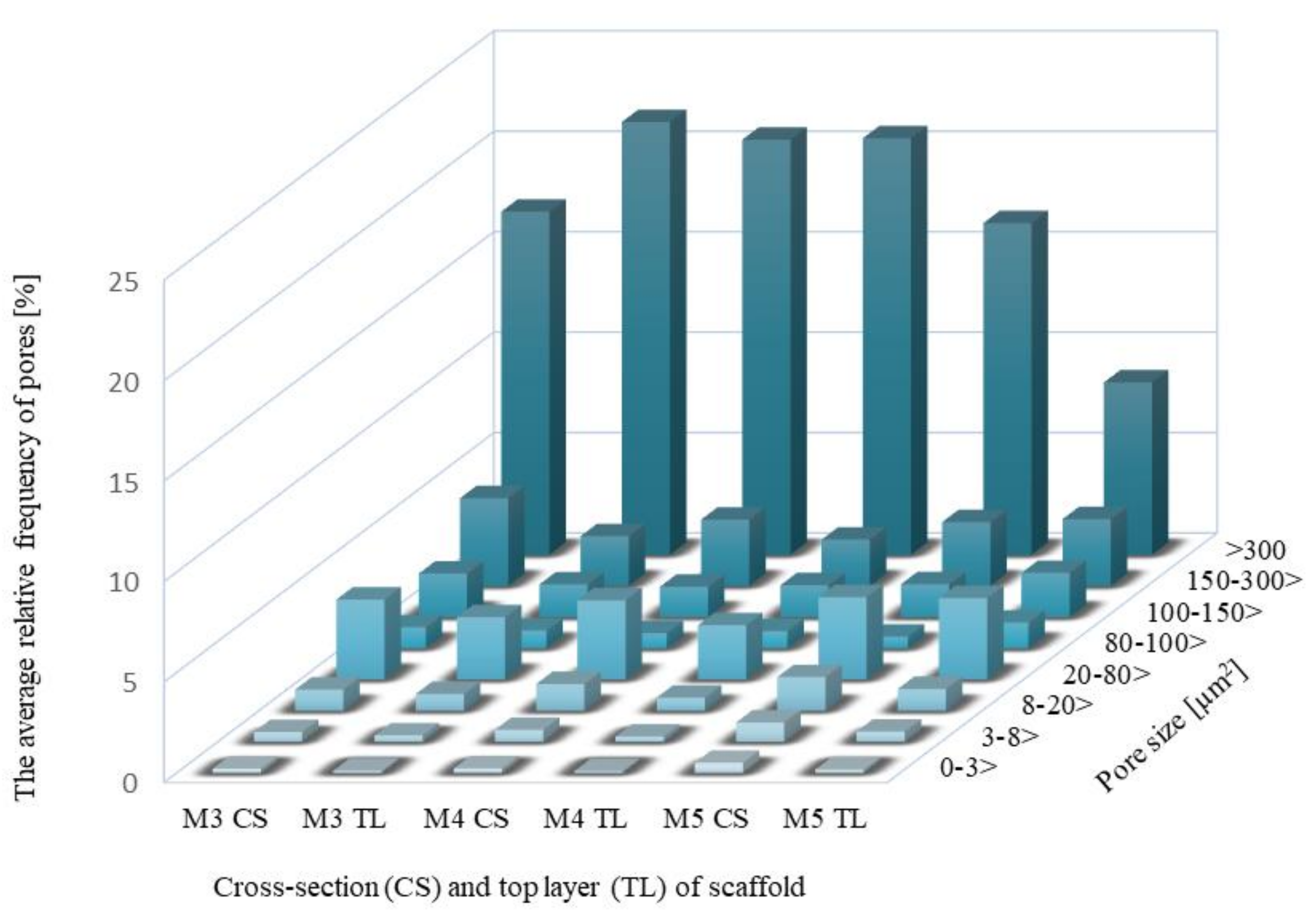
2.3. Estimate of Pores in Scaffolds by Computer Analysis of SEM Images
2.3.1. Pore Distributions and the Total Aera of Pores for Scaffolds before Degradation
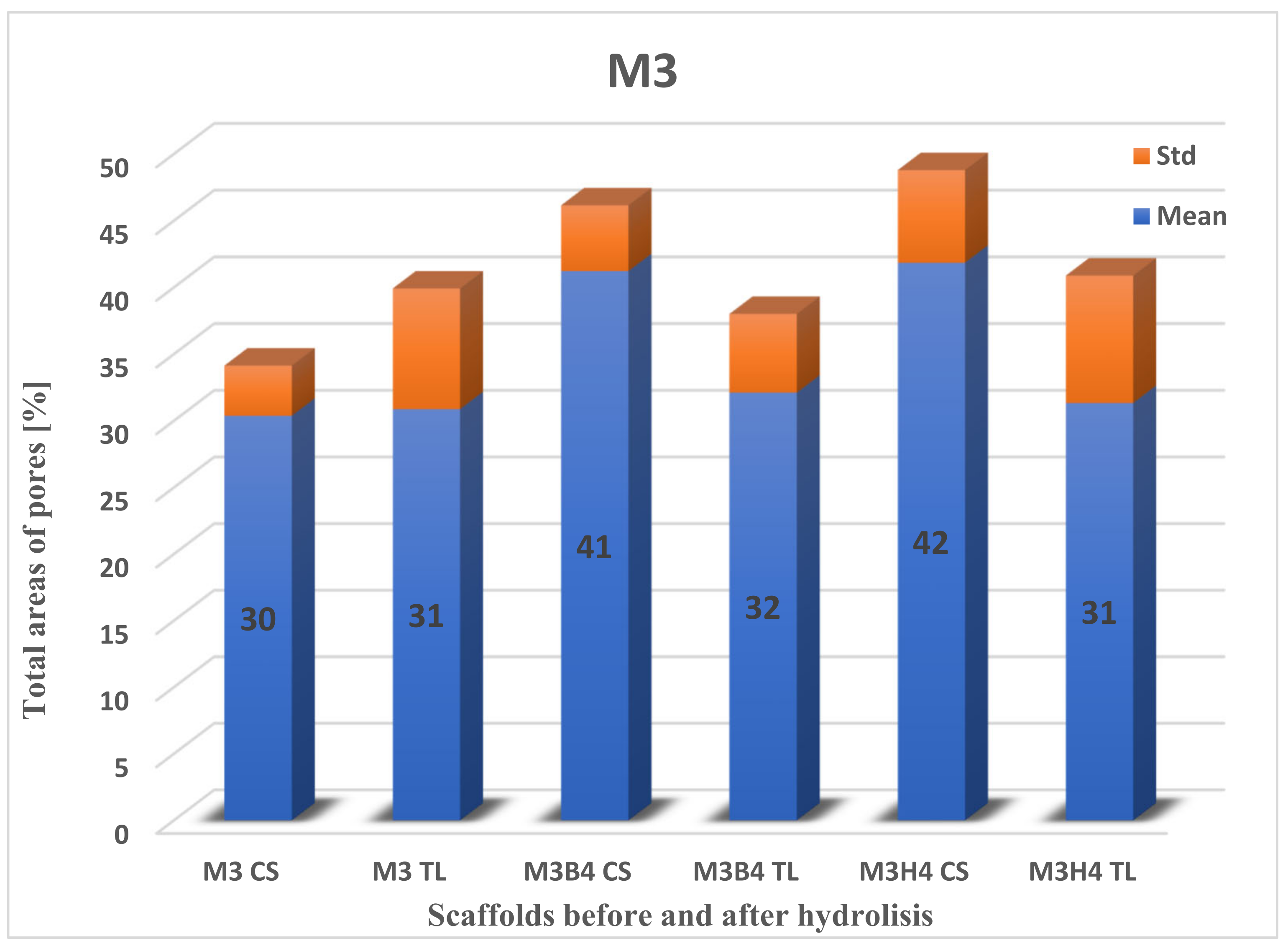
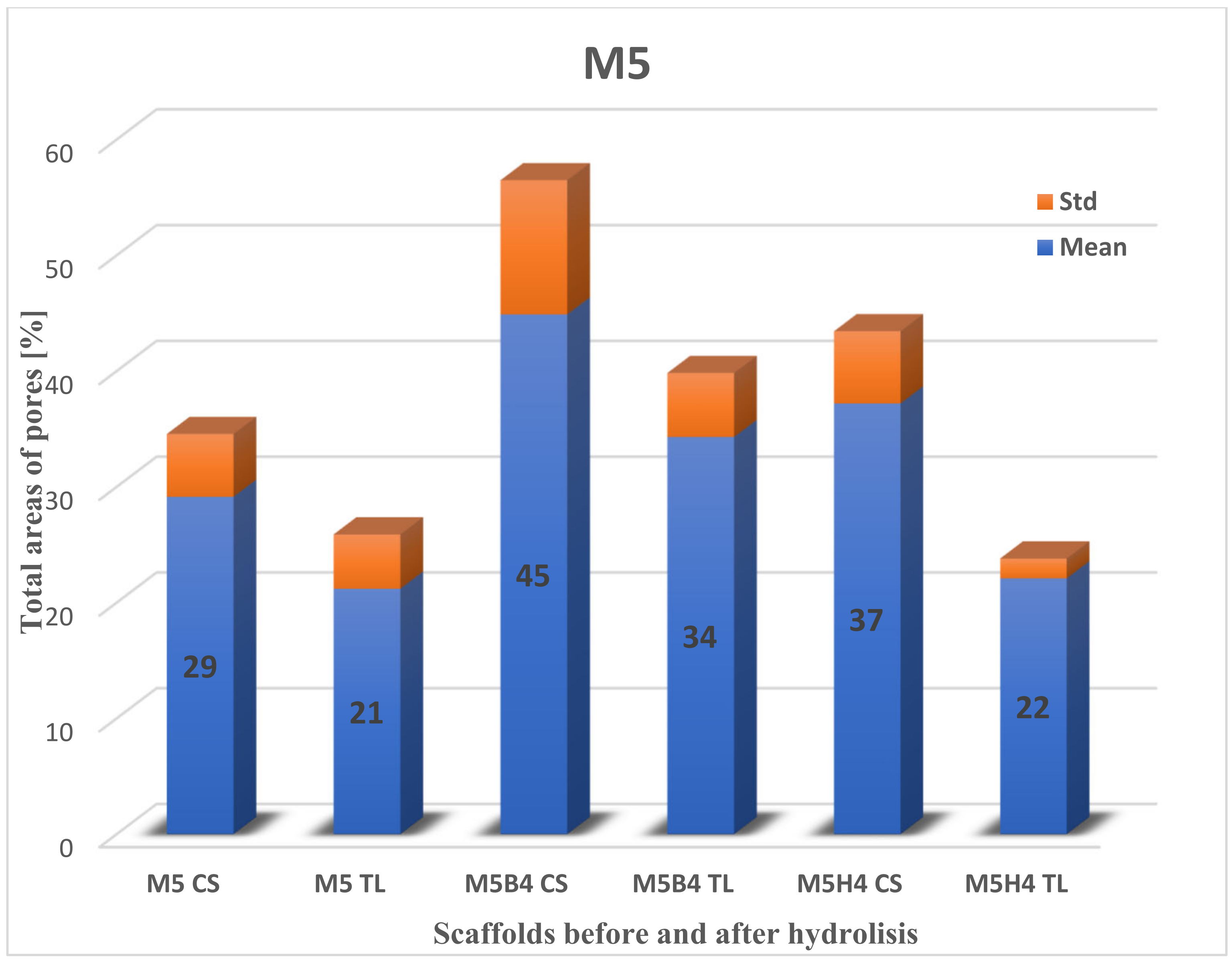
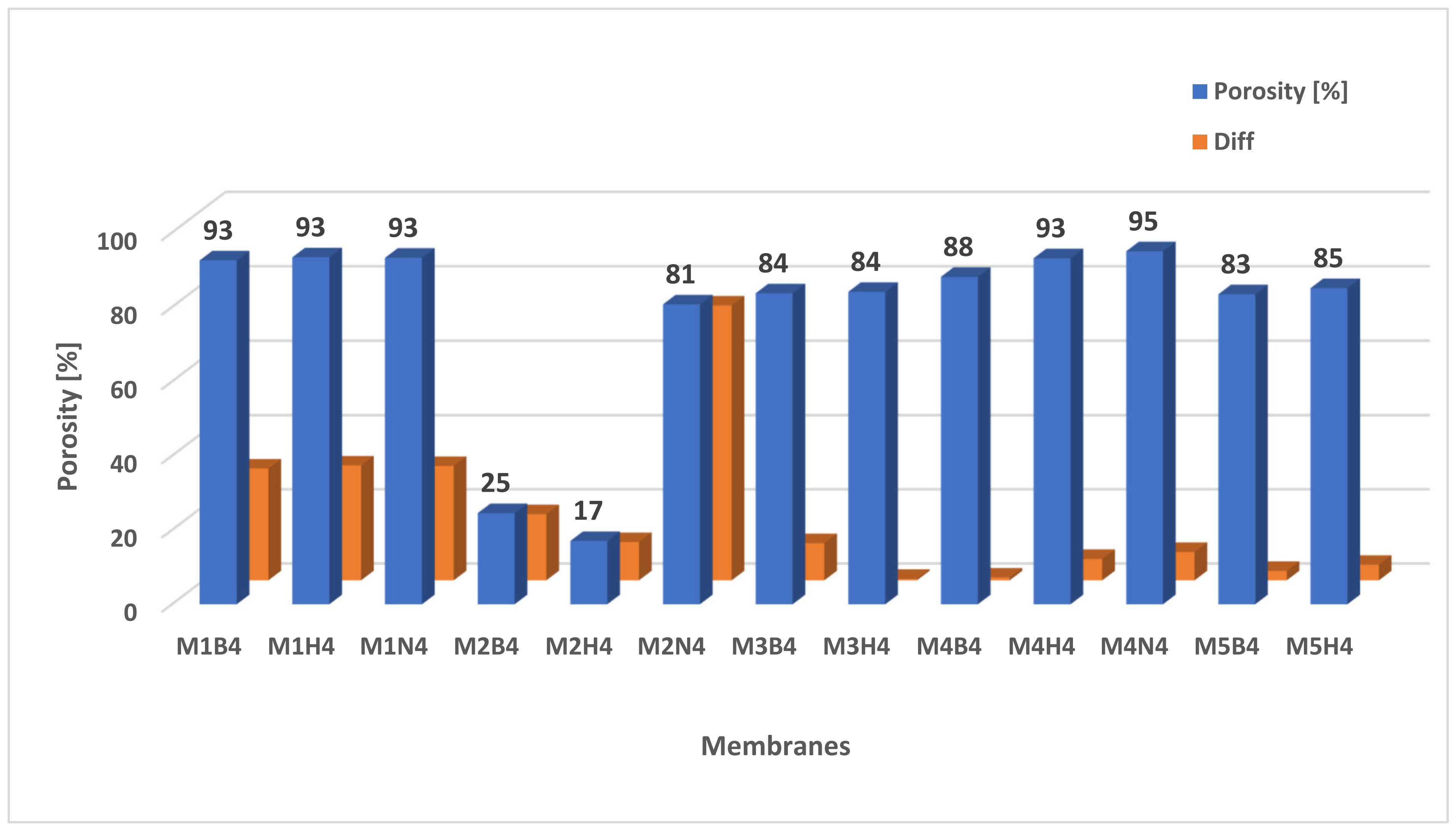
2.3.2. Total Area of Pores after Degradation
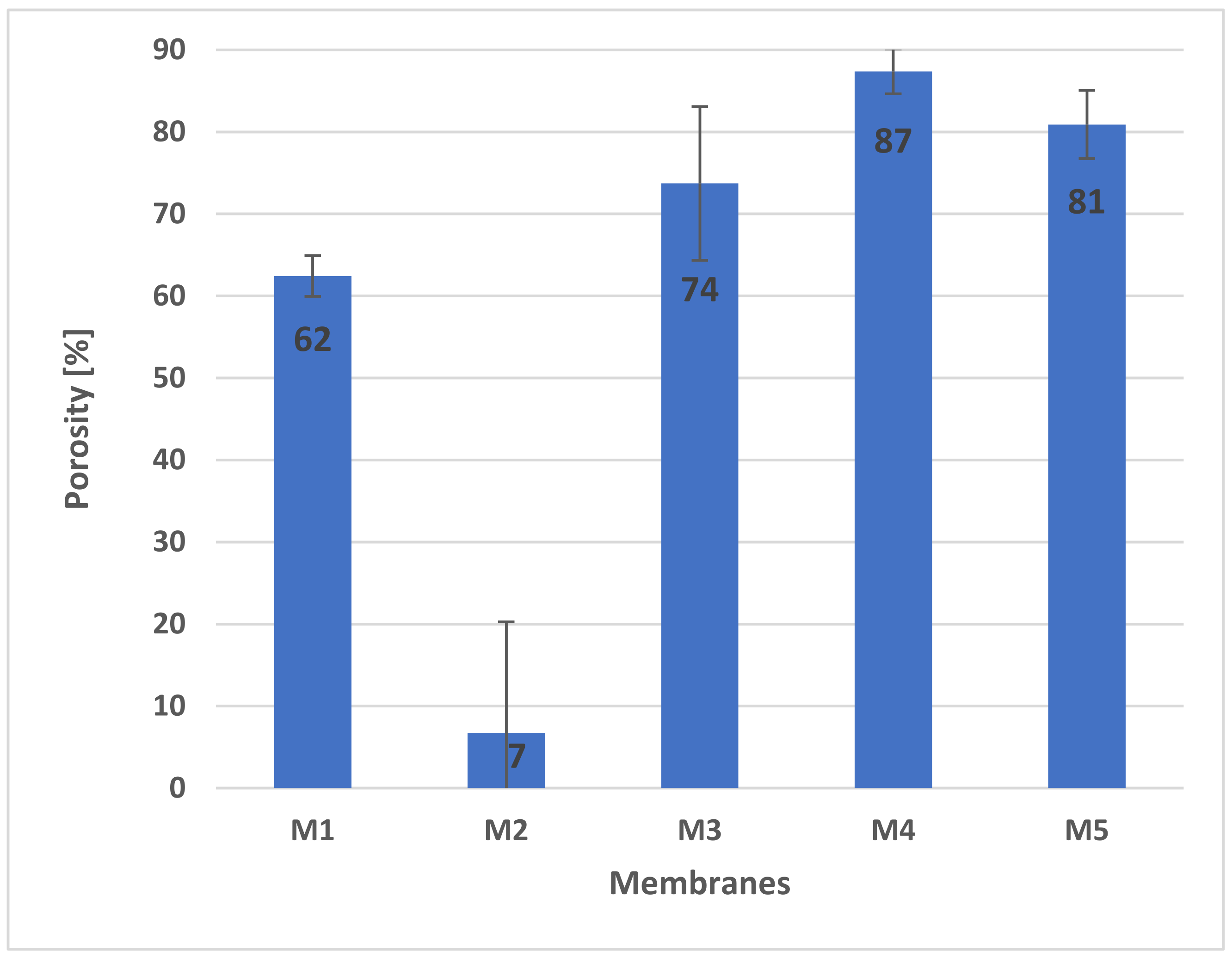
2.4. Porosity of Membranes
2.5. Wettability of Membranes
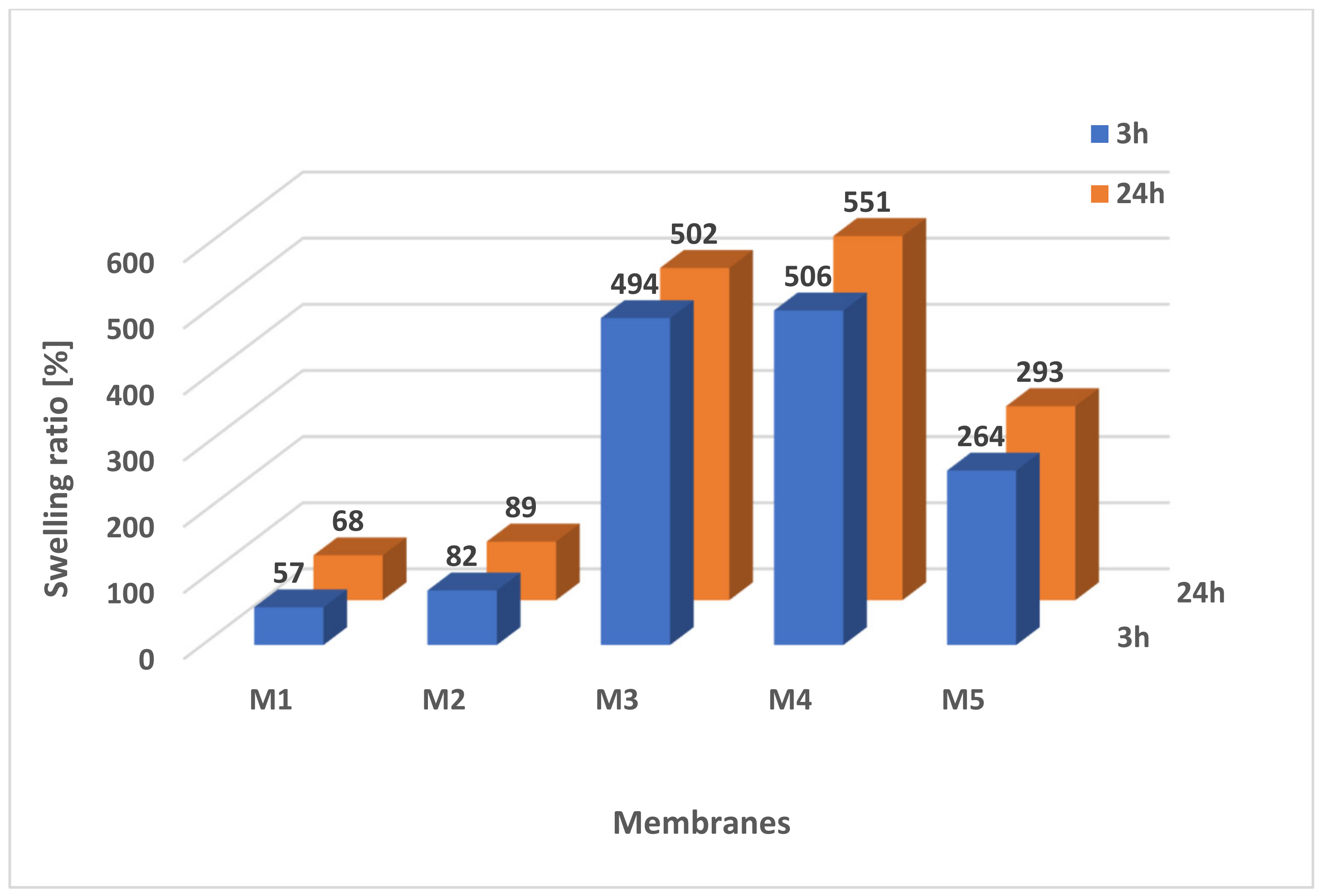
2.5.1. Swelling Ratio
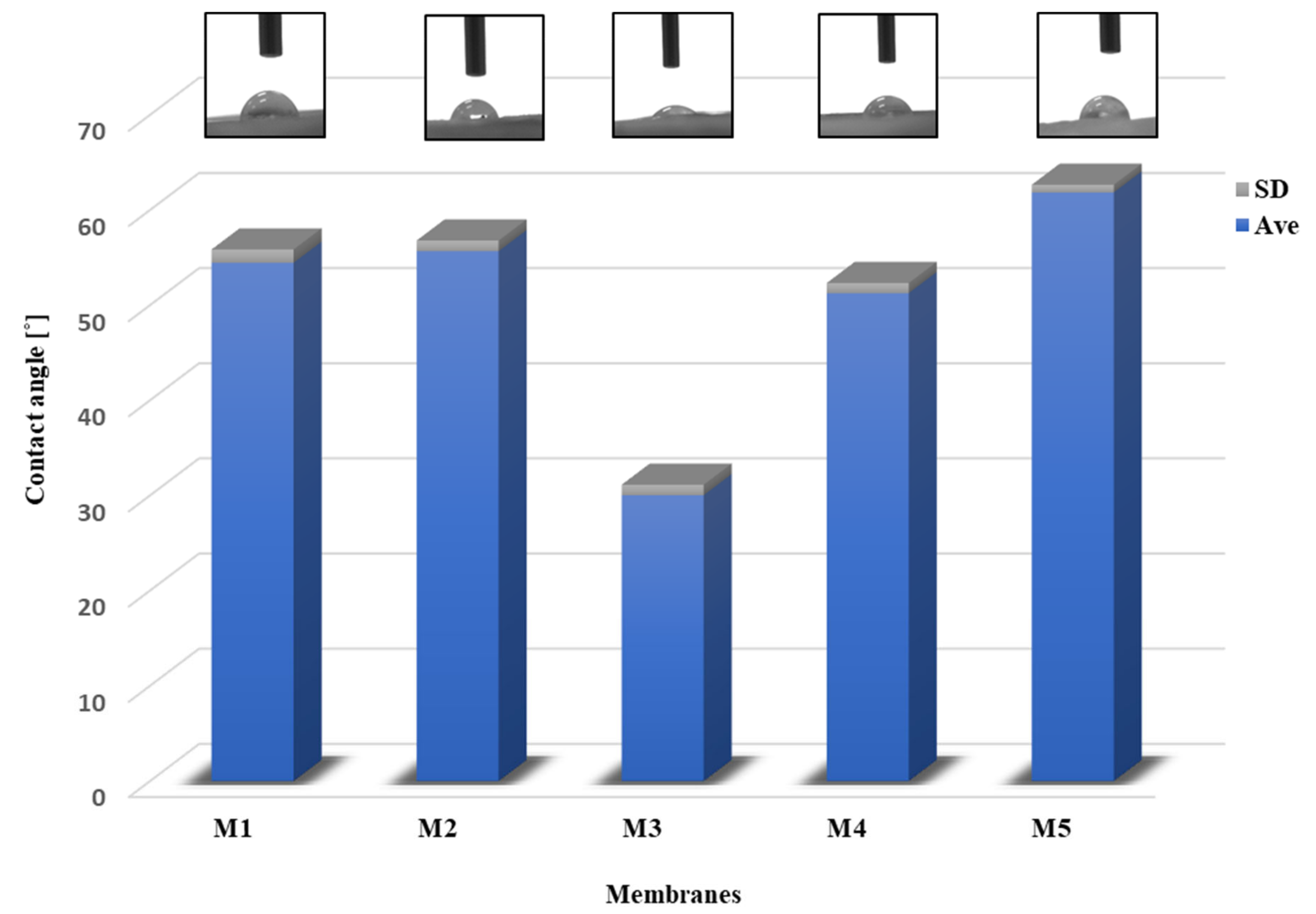
2.5.2. Contact Angle
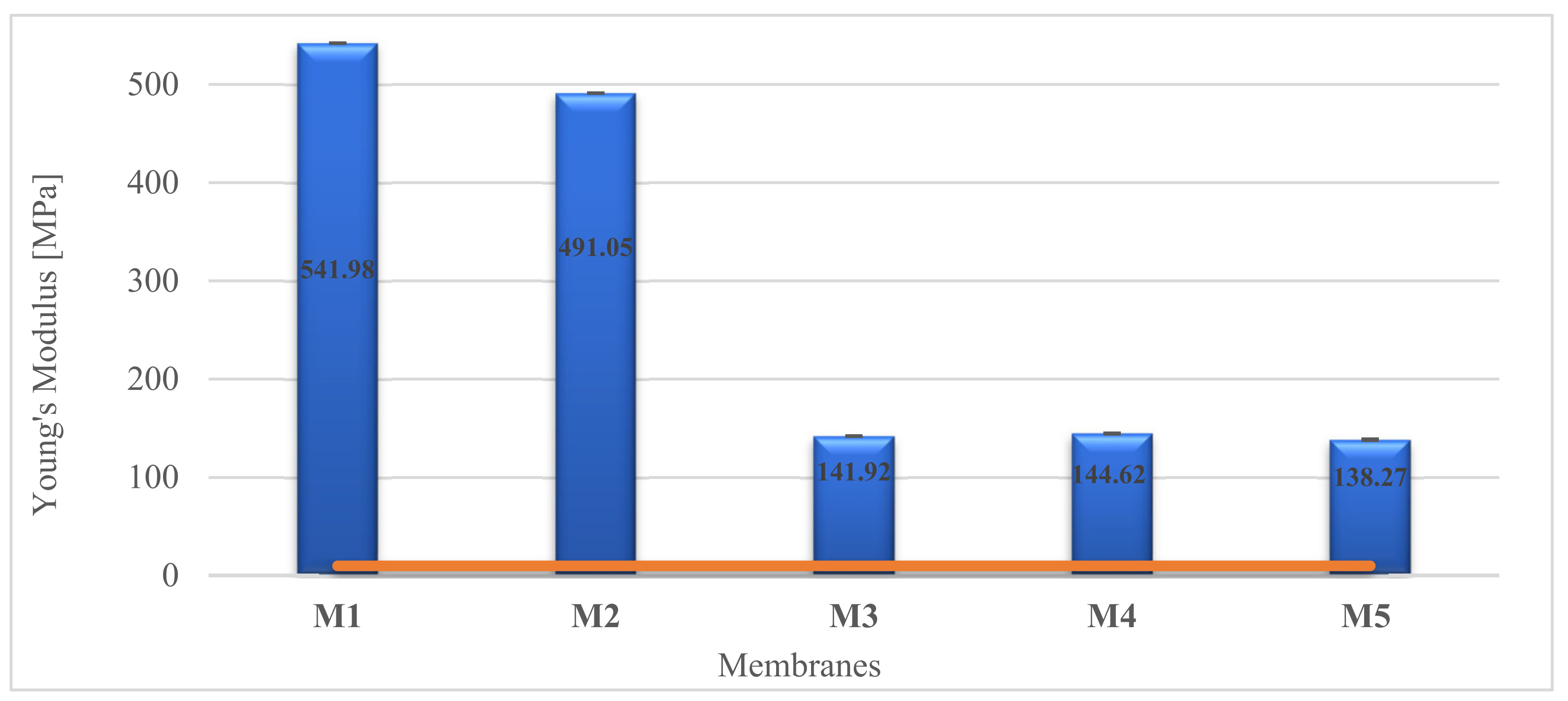
2.6. Mechanical Properties
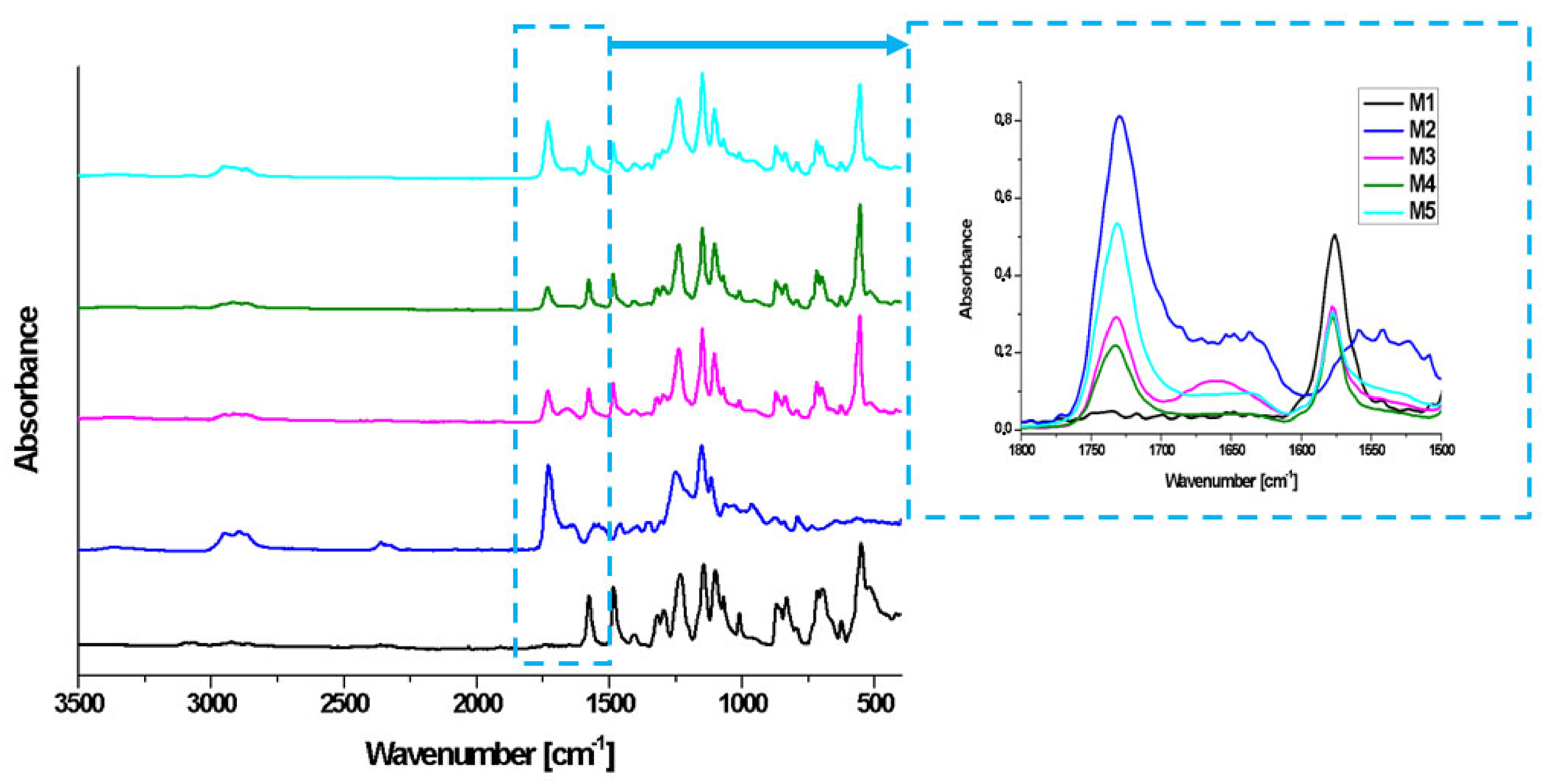
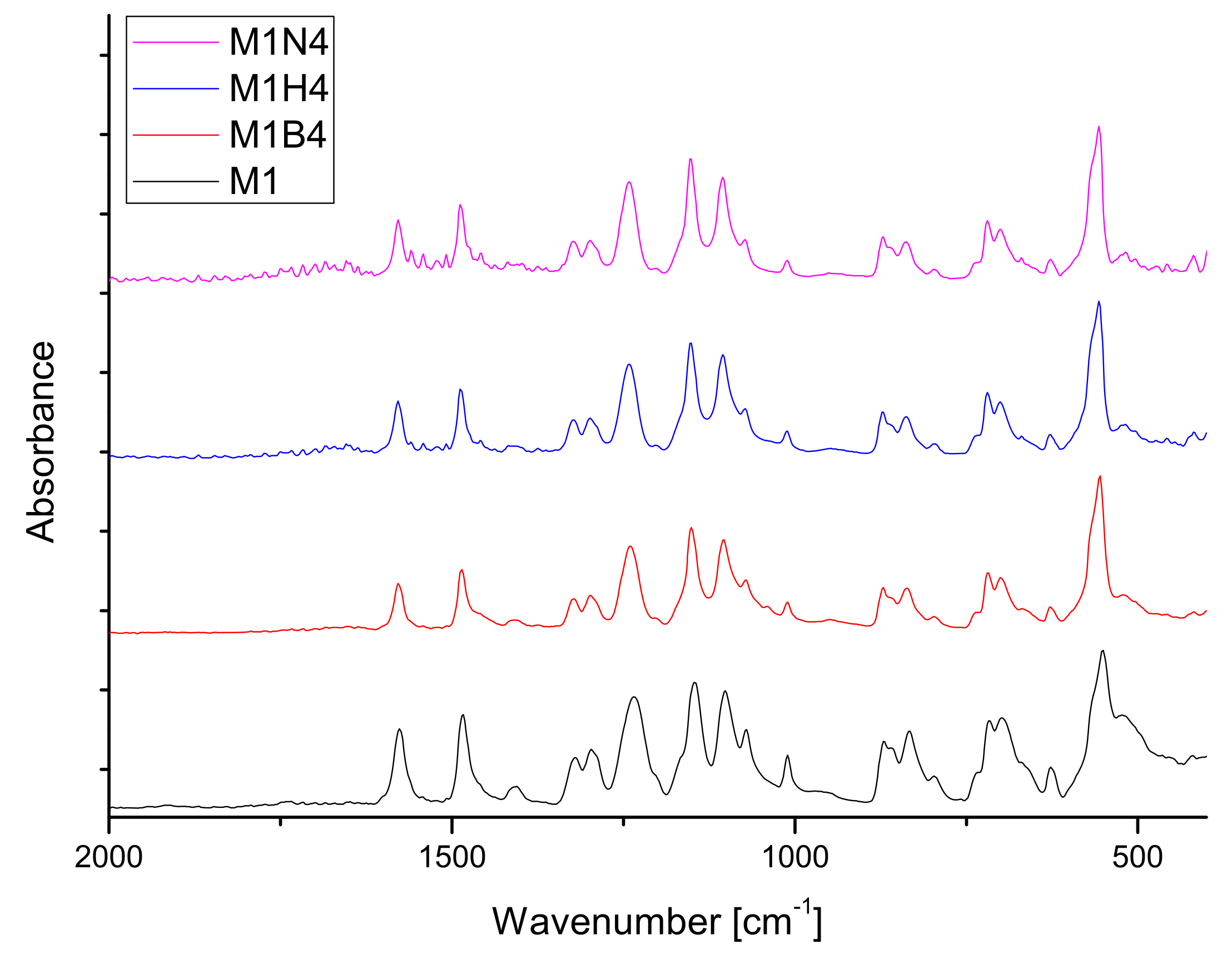
2.7. FT-IR Analysis
3. Materials and Methods
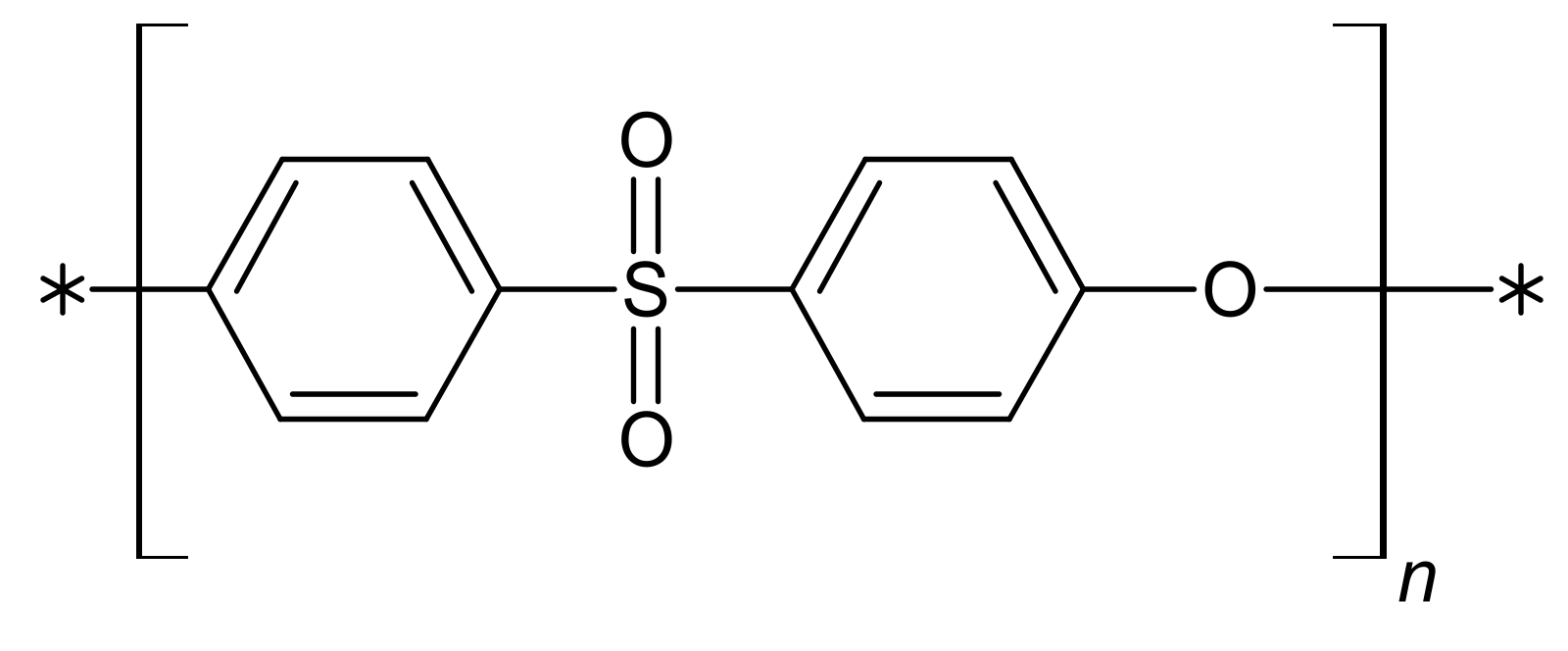
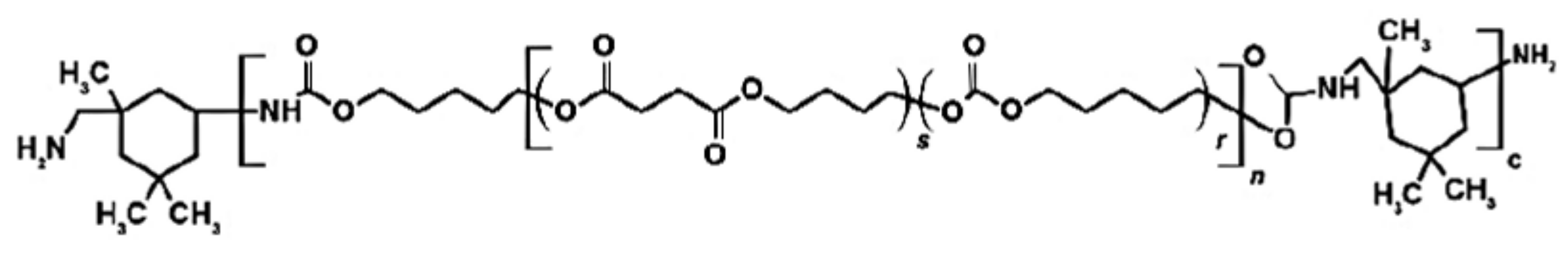
3.1. Materials
3.2. Preparation of the Reference Membranes M1 and M2
- M1: The PES polymer was dissolved in DMF and DMSO (mixed in a ratio of 1:1) to obtain 8.6 wt.% concentration. Then, the 2.5 wt.% PVP 10 kDa and 2.5 wt.% Pluronic F127 were added with constant stirring at room temperature until a solution was achieved.
- M2: The PUR polymer was dissolved in DMF and DMSO (mixed in a ratio of 1:1) to obtain 8.6 wt.% concentration. Then, the 2.5 wt.% PVP 10 kDa and 2.5 wt.% Pluronic F127 were added with constant stirring at room temperature until a solution was achieved.
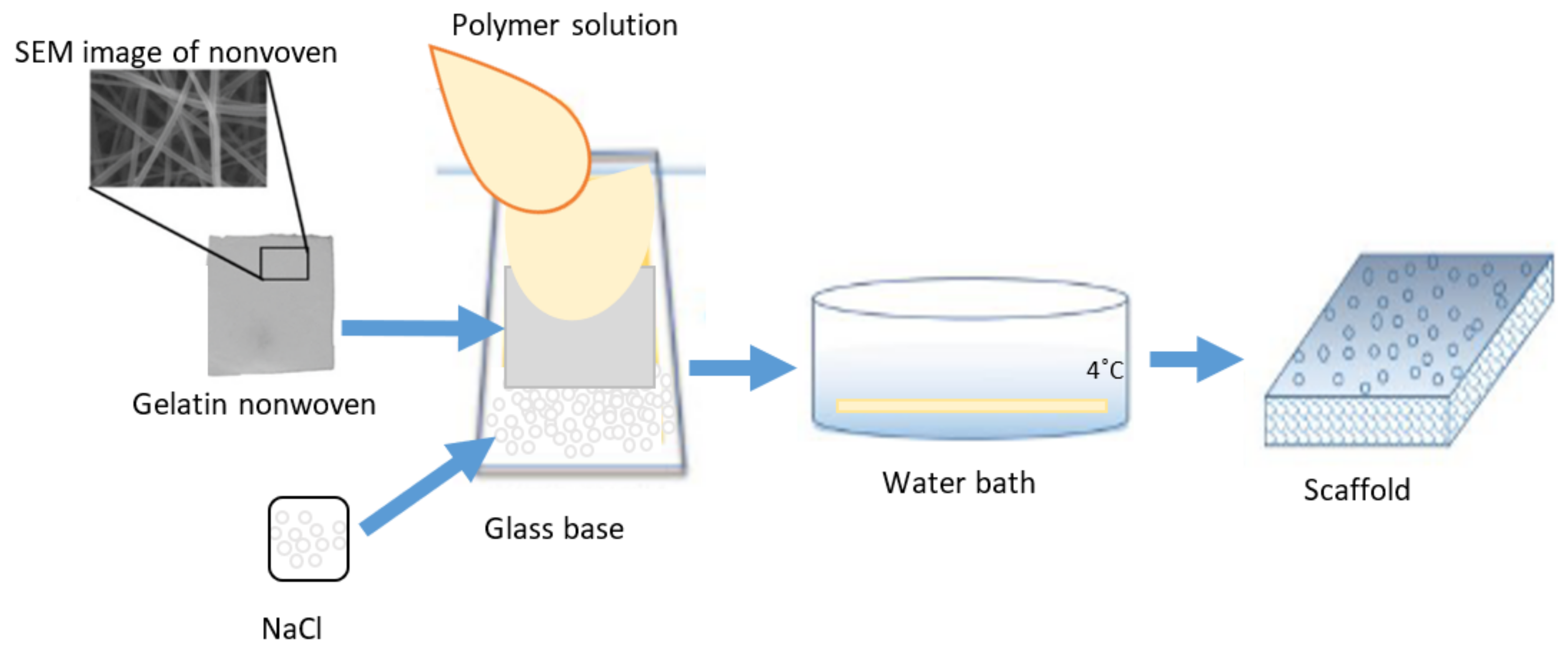
3.3. Preparation of the PUR-PES Scaffolds M3–M5 Using Gelatin Nonwoven
3.4. SEM Observation
3.5. Estimate of Pores in Scaffolds by MeMoExplorer™ Software
3.6. Degradation of Scaffolds
3.7. Porosity of Membranes
3.8. Swelling Ratio
3.9. Contact Angle Measurement
3.10. Mechanical Property
3.11. FT-IR
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications; Springer: Singapore, 2019; Volume 11, ISBN 0123456789. [Google Scholar]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Shin, Y.M.; Kim, K.; Shin, H. Tissue Engineering and Regenerative Medicine 2017: A Year in Review. Tissue Eng. Part B Rev. 2018, 24, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 615–623. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Krysiak, Z.J.; Łukowska, E.; Gruba, M.; Sikorska, W.; Kruk, A.; Dulnik, J.; Czubak, J.; Chwojnowski, A. Three-dimensional scaffolds for bioengineering of cartilage tissue. Biocybern. Biomed. Eng. 2022, 42, 494–511. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Sikorska, W.; Chwojnowski, A. Review of synthetic and hybrid scaffolds in cartilage tissue engineering. Membranes 2020, 10, 348. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Verma, S.K.; Modi, A.; Singh, A.K.; Teotia, R.; Bellare, J. Improved hemodialysis with hemocompatible polyethersulfone hollow fiber membranes: In vitro performance. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1286–1298. [Google Scholar] [CrossRef]
- Farina, M.; Alexander, J.F.; Thekkedath, U.; Ferrari, M.; Grattoni, A. Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond. Adv. Drug Deliv. Rev. 2019, 139, 92–115. [Google Scholar] [CrossRef]
- Kupikowska-Stobba, B.; Lewińska, D. Polymer microcapsules and microbeads as cell carriers for: In vivo biomedical applications. Biomater. Sci. 2020, 8, 1536–1574. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Vullings, J.; van de Loo, F.A.J. Osteoporosis and osteoarthritis are two sides of the same coin paid for obesity. Nutrition 2020, 70, 110486. [Google Scholar] [CrossRef]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Zaszczyńska, A.; Zabielski, K.; Sajkiewicz, P. Hydrogel, electrospun and composite materials for bone/cartilage and neural tissue engineering. Materials 2021, 14, 6899. [Google Scholar] [CrossRef]
- Collins, K.H.; Herzog, W.; MacDonald, G.Z.; Reimer, R.A.; Rios, J.L.; Smith, I.C.; Zernicke, R.F.; Hart, D.A. Obesity, metabolic syndrome, and musculoskeletal disease: Common inflammatory pathways suggest a central role for loss of muscle integrity. Front. Physiol. 2018, 9, 112. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef]
- Walter, S.G.; Ossendorff, R.; Schildberg, F.A. Articular cartilage regeneration and tissue engineering models: A systematic review. Arch. Orthop. Trauma Surg. 2019, 139, 305–316. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport. Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage—Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of damaged articular cartilage: Current approaches and future directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Mirza, U.; Shubeena, S.; Shah, M.S.; Zaffer, B. Microfracture: A technique for repair of chondral defects. J. Entomol. Zool. Stud. 2018, 6, 1092–1097. [Google Scholar]
- Brittberg, M. Symposium Scaffold based Autologous Chondrocyte Implantation: The Surgical Technique. Asian J. Arthrosc. 2019, 4, 23–26. [Google Scholar]
- Zhao, Z.; Fan, C.; Chen, F.; Sun, Y.; Xia, Y.; Ji, A.; Wang, D. Progress in Articular Cartilage Tissue Engineering: A Review on Therapeutic Cells and Macromolecular Scaffolds. Macromol. Biosci. 2019, 20, 1900278. [Google Scholar] [CrossRef]
- Brittberg, M.; Recker, D.; Ilgenfritz, J.; Saris, D.B.F. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am. J. Sport. Med. 2018, 46, 1343–1351. [Google Scholar] [CrossRef]
- Baranowski, M.; Wasyłeczko, M.; Kosowska, A.; Plichta, A.; Kowalczyk, S.; Chwojnowski, A.; Bielecki, W.; Czubak, J. Regeneration of Articular Cartilage Using Membranes of Polyester Scaffolds in a Rabbit Model. Pharmaceutics 2022, 14, 1016. [Google Scholar] [CrossRef]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Panadero, J.A.; Lanceros-Mendez, S.; Ribelles, J.L.G. Differentiation of mesenchymal stem cells for cartilage tissue engineering: Individual and synergetic effects of three-dimensional environment and mechanical loading. Acta Biomater. 2016, 33, 1–12. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Takahashi, T.; Ogasawara, T.; Asawa, Y.; Mori, Y.; Uchinuma, E.; Takato, T.; Hoshi, K. Three-Dimensional Microenvironments Retain Chondrocyte Phenotypes During Proliferation Culture. Tissue Eng. 2007, 13, 1583–1592. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Kalkan, R.; Nwekwo, C.W.; Adali, T. The Use of Scaffolds in Cartilage Regeneration. Eukaryot. Gene Expr. 2018, 28, 343–348. [Google Scholar] [CrossRef]
- Gadomska-Gajadhur, A.; Kruk, A.; Ruśkowski, P.; Sajkiewicz, P.; Dulnik, J.; Chwojnowski, A. Original method of imprinting pores in scaffolds for tissue engineering. Polym. Adv. Technol. 2021, 32, 355–367. [Google Scholar] [CrossRef]
- Nava, M.M.; Draghi, L.; Giordano, C.; Pietrabissa, R. The effect of scaffold pore size in cartilage tissue engineering. J. Appl. Biomater. Funct. Mater. 2016, 14, e233–e239. [Google Scholar] [CrossRef]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part A 2015, 21, 486–497. [Google Scholar] [CrossRef]
- Koh, Y.G.; Lee, J.A.; Kim, Y.S.; Lee, H.Y.; Kim, H.J.; Kang, K.T. Optimal mechanical properties of a scaffold for cartilage regeneration using finite element analysis. J. Tissue Eng. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef]
- Ahmadi, F.; Giti, R.; Mohammadi-Samani, S.; Mohammadi, F. Biodegradable Scaffolds for Cartilage Tissue Engineering. GMJ 2017, 6, 70–80. [Google Scholar] [CrossRef]
- Joshi, S.R.; Pendyala, G.S.; Shah, P.; Mopagar, V.P.; Padmawar, N.; Padubidri, M. Scaffolds—The Ground for Regeneration: A Narrative Review. J. Int. Soc. Prev. Community Dent. 2020, 10, 692–699. [Google Scholar] [PubMed]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Irawan, V.; Sung, T.C.; Higuchi, A.; Ikoma, T. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med. 2018, 15, 673–697. [Google Scholar] [CrossRef]
- Steinwachs, M.R.; Gille, J.; Volz, M.; Anders, S.; Jakob, R.; De Girolamo, L.; Volpi, P.; Schiavone-Panni, A.; Scheffler, S.; Reiss, E.; et al. Systematic Review and Meta-Analysis of the Clinical Evidence on the Use of Autologous Matrix-Induced Chondrogenesis in the Knee. Cartilage 2021, 13, 42S–56S. [Google Scholar] [CrossRef]
- Gobbi, A.; Whyte, G.P. Long-term Clinical Outcomes of One-Stage Cartilage Repair in the Knee with Hyaluronic Acid—Based Scaffold Embedded with Mesenchymal Stem Cells Sourced from Bone Marrow Aspirate Concentrate. Am. J. Sport. Med. 2019, 47, 1621–1628. [Google Scholar] [CrossRef]
- Chen, S.; Chen, W.; Chen, Y.; Mo, X.; Fan, C. Chondroitin sulfate modified 3D porous electrospun nano fiber scaffolds promote cartilage regeneration. Mater. Sci. Eng. C 2021, 118, 111312. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, X.; Cai, D.; Li, J.; Mu, Q.; Zhang, W.; Zhu, S. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 2017, 63, 64–75. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, T.H.; Kang, D.L.; Baek, S.Y.; Lee, Y.; Koh, Y.G.; Kim, Y. Il Chondrogenic differentiation of human ASCs by stiffness control in 3D fibrin hydrogel. Biochem. Biophys. Res. Commun. 2020, 522, 213–219. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-roitman, J.; Schroeder, A. Mini Review Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nourbakhsh, M.S. Electrospun polycaprolactone scaffolds for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 527–539. [Google Scholar] [CrossRef]
- Dao, T.T.; Vu, N.B.; Pham, L.H.; Bui, T.; Le, P.T.; Van Pham, P. In Vitro Production of Cartilage Tissue from Rabbit Bone Marrow-Derived Mesenchymal Stem Cells and Polycaprolactone Scaffold. In Tissue Engineering and Regenerative Medicine; Springer: Cham, Switzerland, 2017; Volume 1084, pp. 45–60. [Google Scholar] [CrossRef]
- Wen, Y.; Dai, N.; Hsu, S. Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater. 2019, 88, 301–313. [Google Scholar] [CrossRef]
- Hung, K.; Tseng, C.; Hsu, S. Synthesis and 3D Printing of Biodegradable Polyurethane Elastomer by a Water-Based Process for Cartilage Tissue Engineering Applications. Adv. Healthc. Mater. 2014, 3, 1578–1587. [Google Scholar] [CrossRef]
- Mahboudi, H.; Soleimani, M.; Enderami, S.E.; Kehtari, M.; Hanaee- Ahvaz, H.; Ghanbarian, H.; Bandehpour, M.; Nojehdehi, S.; Mirzaei, S.; Kazemi, B. The effect of nanofibre-based polyethersulfone (PES) scaffold on the chondrogenesis of human induced pluripotent stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1948–1956. [Google Scholar] [CrossRef]
- Plończak, M.; Czubak, J.; Hoser, G.; Chwojnowskl, A.; Kawiak, J.; Dudzińskp, K.; Czumińska, K. Repair of articular cartilage full thickness defects with cultured chondrocytes placed on polysulphonic membrane—Experimental studies in rabbits. Biocybern. Biomed. Eng. 2008, 28, 87–93. [Google Scholar]
- Irfan, M.; Idris, A. Overview of PES biocompatible/hemodialysis membranes: PES-blood interactions and modification techniques. Mater. Sci. Eng. C 2015, 56, 574–592. [Google Scholar] [CrossRef]
- Sikorska, W.; Przytulska, M.; Wasyłeczko, M.; Wojciechowski, C.; Kulikowski, J.L.; Chwojnowski, A. Influence of hydrolysis, solvent and pvp addition on the porosity of psf/pur blend partly degradable hollow fiber membranes evaluated using the memoexplorer software. Desalination Water Treat. 2021, 214, 114–119. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, K.; Kai, D.; Li, Z.; Loh, X.J. Polyester elastomers for soft tissue engineering. Chem. Soc. Rev. 2018, 47, 4545–4580. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Sikorska, W.; Przytulska, M.; Dulnik, J.; Chwojnowski, A. Polyester membranes as 3D scaffolds for cell culture. Desalination Water Treat. 2021, 214, 181–193. [Google Scholar] [CrossRef]
- Sikorska, W.; Wasyłeczko, M.; Przytulska, M.; Wojciechowski, C.; Rokicki, G.; Chwojnowski, A. Chemical degradation of PSF-PUR blend hollow fiber membranes-assessment of changes in properties and morphology after hydrolysis. Membranes 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, W.; Milner-Krawczyk, M.; Wasyłeczko, M.; Wojciechowski, C.; Chwojnowski, A. Biodegradation process of PSF-PUR blend hollow fiber membranes using escherichia coli Bacteria—Evaluation of changes in properties and porosity. Polymers 2021, 13, 1311. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.; Narayan, B.; Rastogi, A.; Srivastava, P. Design and evaluation of chitosan/poly (L-lactide)/pectin based composite scaffolds for cartilage tissue regeneration. Int. J. Biol. Macromol. 2018, 112, 909–920. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Setayeshmehr, M.; Esfandiari, E.; Rafieinia, M.; Hashemibeni, B.; Taheri-Kafrani, A.; Samadikuchaksaraei, A.; Kaplan, D.L.; Moroni, L.; Joghataei, M.T. Hybrid and composite scaffolds based on extracellular matrices for cartilage tissue engineering. Tissue Eng. Part B Rev. 2019, 25, 202–224. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Pan, Z.; Sun, H.; Wang, J.; Yu, D.; Zhu, S.; Dai, J.; Chen, Y.; Tian, N.; et al. The effects of lactate and acid on articular chondrocytes function: Implications for polymeric cartilage scaffold design. Acta Biomater. 2016, 42, 329–340. [Google Scholar] [CrossRef]
- Lin, H.; Beck, A.M.; Fritch, M.R.; Tuan, R.S.; Deng, Y.; Kilroy, E.J.; Tang, Y.; Alexander, P.G. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. Tissue Eng. Regen. Med. 2019, 13, 1418–1429. [Google Scholar] [CrossRef]
- Safinsha, S.; Ali, M.M. Composite scaffolds in tissue engineering. Mater. Today Proc. 2020, 24, 2318–2329. [Google Scholar] [CrossRef]
- Bistolfi, A.; Ferracini, R.; Galletta, C.; Tosto, F.; Sgarminato, V.; Digo, E.; Vernè, E.; Massè, A. Regeneration of articular cartilage: Scaffold used in orthopedic surgery. A short handbook of available products for regenerative joints surgery. Clin. Sci. Res. Rep. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Tan, R.Y.H.; Lee, C.S.; Pichika, M.R.; Cheng, S.F.; Lam, K.Y. PH Responsive Polyurethane for the Advancement of Biomedical and Drug Delivery. Polymers 2022, 14, 1672. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Cauich-Rodríguez, V.; Chan-Chan, L.H.; Hernandez-Sanchez, F.; Cervantes-Uc, J.M. Degradation of Polyurethanes for Cardiovascular Applications. Adv. Biomater. Sci. Biomed. Appl. 2013, 8, 51–82. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Kitahara, S. Effect of Surface Hydrolysis of PLA Film on Apatite Formation in Vitro. J. Oral Tissue Eng. 2008, 6, 77–87. [Google Scholar]
- Shuai, C.; Yu, L.; Yang, W.; Peng, S.; Zhong, Y.; Feng, P. Phosphonic acid coupling agent modification of HAP nanoparticles: Interfacial effects in PLLA/HAP bone scaffold. Polymers 2020, 12, 199. [Google Scholar] [CrossRef]
- Woźniak-Budych, M.J. Polymeric membranes for biomedical applications. Membr. Technol. Acad. Ind. 2022, 15, 111–140. [Google Scholar] [CrossRef]
- Jakutowicz, T. Treatment of articular cartilage damage in rabbits using chondrocyte transplants auto—And allogeneic—A comparati. Ph.D. Thesis, Medical Center for Postgraduate Education, Otwock, Poland, 2016. [Google Scholar]
- Przytulska, M.; Kulikowski, J.L.; Wasyłeczko, M.; Chwojnowski, A.; Piętka, D. The evaluation of 3D morphological structure of porous membranes based on a computer-aided analysis of their 2D images. Desalin. Water Treat. 2018, 128, 11–19. [Google Scholar] [CrossRef]
- Sun, L.; Guo, J.; Chen, H.; Zhang, D.; Shang, L.; Zhang, B.; Zhao, Y. Tailoring Materials with Specific Wettability in Biomedical Engineering. Adv. Sci. 2021, 8, 2100126. [Google Scholar] [CrossRef]
- Lee, S.S.; Santschi, M.; Ferguson, S.J. A Biomimetic Macroporous Hybrid Scaffold with Sustained Drug Delivery for Enhanced Bone Regeneration. Biomacromolecules 2021, 22, 2460–2471. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Massah, F. Improvement of hydrophilicity and cell attachment of polycaprolactone scaffolds using green synthesized carbon dots. Mater. Today Sustain. 2021, 13, 100075. [Google Scholar] [CrossRef]
- Aljanabi, A.A.A.; Mousa, N.E.; Aljumaily, M.M.; Majdi, H.S.; Yahya, A.A.; AL-Baiati, M.N.; Hashim, N.; Rashid, K.T.; Al-Saadi, S.; Alsalhy, Q.F. Modification of Polyethersulfone Ultrafiltration Membrane Using Poly(terephthalic acid-co-glycerol-g-maleic anhydride) as Novel Pore Former. Polymers 2022, 14, 3408. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Kuzmenko, D.; Gitis, V. Impact of chemical cleaning on properties and functioning of polyethersulfone membranes. J. Membr. Sci. 2007, 305, 176–184. [Google Scholar] [CrossRef]
- Shams, M.; Karimi, M.; Jahangir, V.; Mohammadian, M.; Salimi, A. Surface modification of nanofibrous polyethersulfone scaffolds with fluorapatite nanoparticles toward improved stem cell behavior and osteogenic activity in vitro. Surf. Interfaces 2023, 36, 102512. [Google Scholar] [CrossRef]
- Chen, Y.; Etxabide, A.; Seyfoddin, A.; Ramezani, M. Fabrication and characterisation of poly (vinyl alcohol)/chitosan scaffolds for tissue engineering applications. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Alosaimi, A.M.; Alorabi, R.O.; Katowah, D.F.; Al-thagafi, Z.T.; Alsolami, E.S.; Hussein, M.A.; Qutob, M.; Rafatullah, M. Review on Biomedical Advances of Hybrid Nanocomposite Biopolymeric Materials. Bioengineering 2023, 10, 279. [Google Scholar] [CrossRef]
- Lai, Y.S.; Chen, W.C.; Huang, C.H.; Cheng, C.K.; Chan, K.K.; Chang, T.K. The effect of graft strength on knee laxity and graft in-situ forces after posterior cruciate ligament reconstruction. PLoS ONE 2015, 10, e0127293. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Mazurek-Budzyńska, M.; Behl, M.; Razzaq, M.Y.; Nöchel, U.; Rokicki, G.; Lendlein, A. Hydrolytic stability of aliphatic poly(carbonate-urea-urethane)s: Influence of hydrocarbon chain length in soft segment. Polym. Degrad. Stab. 2019, 161, 283–297. [Google Scholar] [CrossRef]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Membr. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Cao, X.Y.; Tian, N.; Dong, X.; Cheng, C.K. Polylactide composite pins reinforced with bioresorbable continuous glass fibers demonstrating bone-like apatite formation and spiral delamination degradation. Polymers 2019, 11, 812. [Google Scholar] [CrossRef]
- Denis, P.; Dulnik, J.; Sajkiewicz, P. Electrospinning and Structure of Bicomponent Polycaprolactone/Gelatin Nanofibers Obtained Using Alternative Solvent System. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 354–364. [Google Scholar] [CrossRef]
- Dulnik, J.; Ko, D.; Denis, P.; Sajkiewicz, P. The effect of a solvent on cellular response to PCL/gelatin and PCL/collagen electrospun nano fibres. Eur. Polym. J. 2018, 104, 147–156. [Google Scholar] [CrossRef]
- Chrzanowski, W.; Ali, E.; Neel, A.; Andrew, D.; Campbell, J. Effect of surface treatment on the bioactivity of nickel—Titanium. Acta Biomater. 2008, 4, 1969–1984. [Google Scholar] [CrossRef]
- Ho, S.T.; Hutmacher, D.W. A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials 2006, 27, 1362–1376. [Google Scholar] [CrossRef]
- Park, H.J.; Min, K.D.; Lee, M.C.; Kim, S.H.; Lee, O.J.; Ju, H.W.; Moon, B.M.; Lee, J.M.; Park, Y.R.; Kim, D.W.; et al. Fabrication of 3D porous SF/β-TCP hybrid scaffolds for bone tissue reconstruction. J. Biomed. Mater. Res. Part A 2016, 104, 1779–1787. [Google Scholar] [CrossRef]
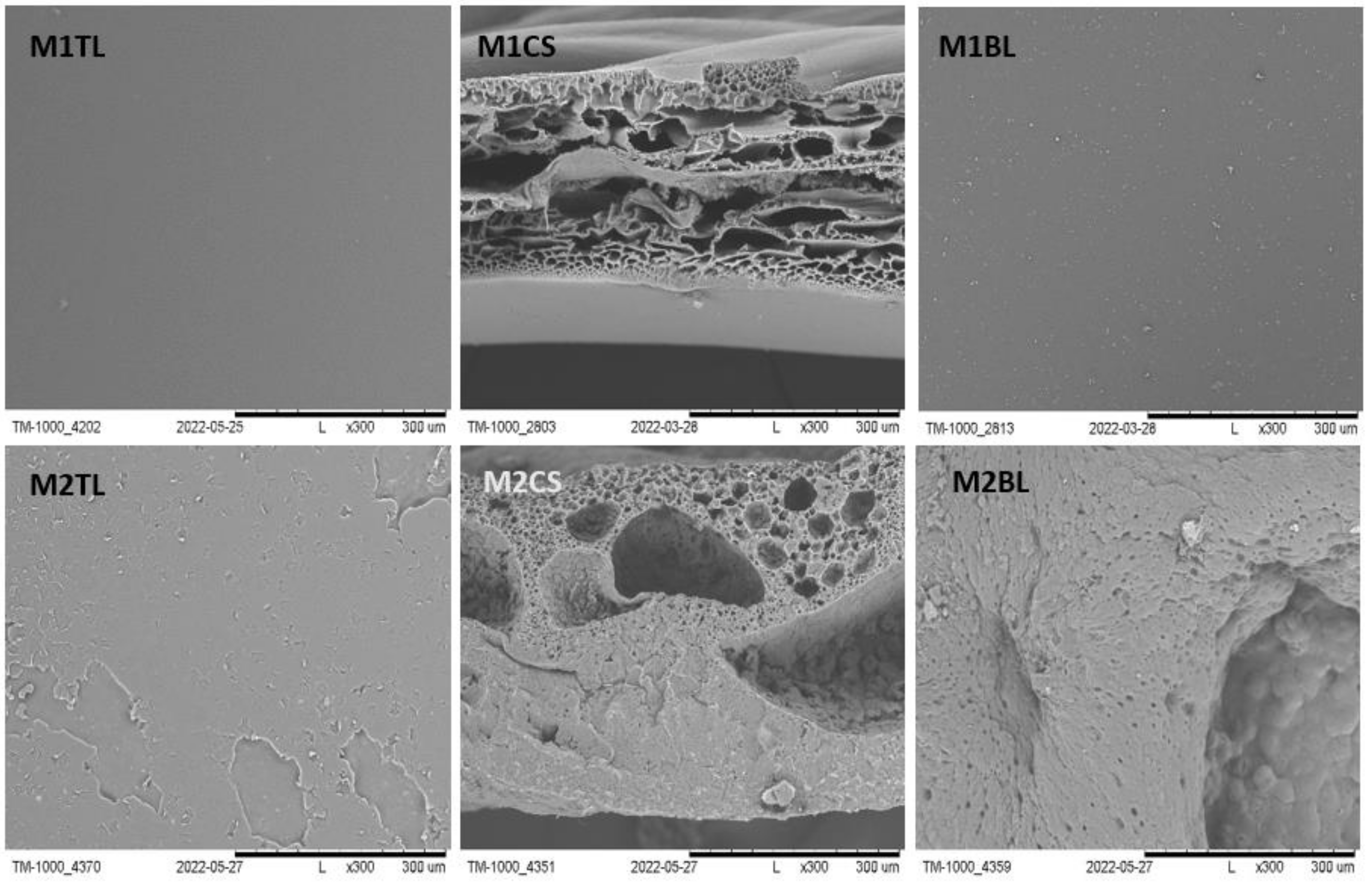
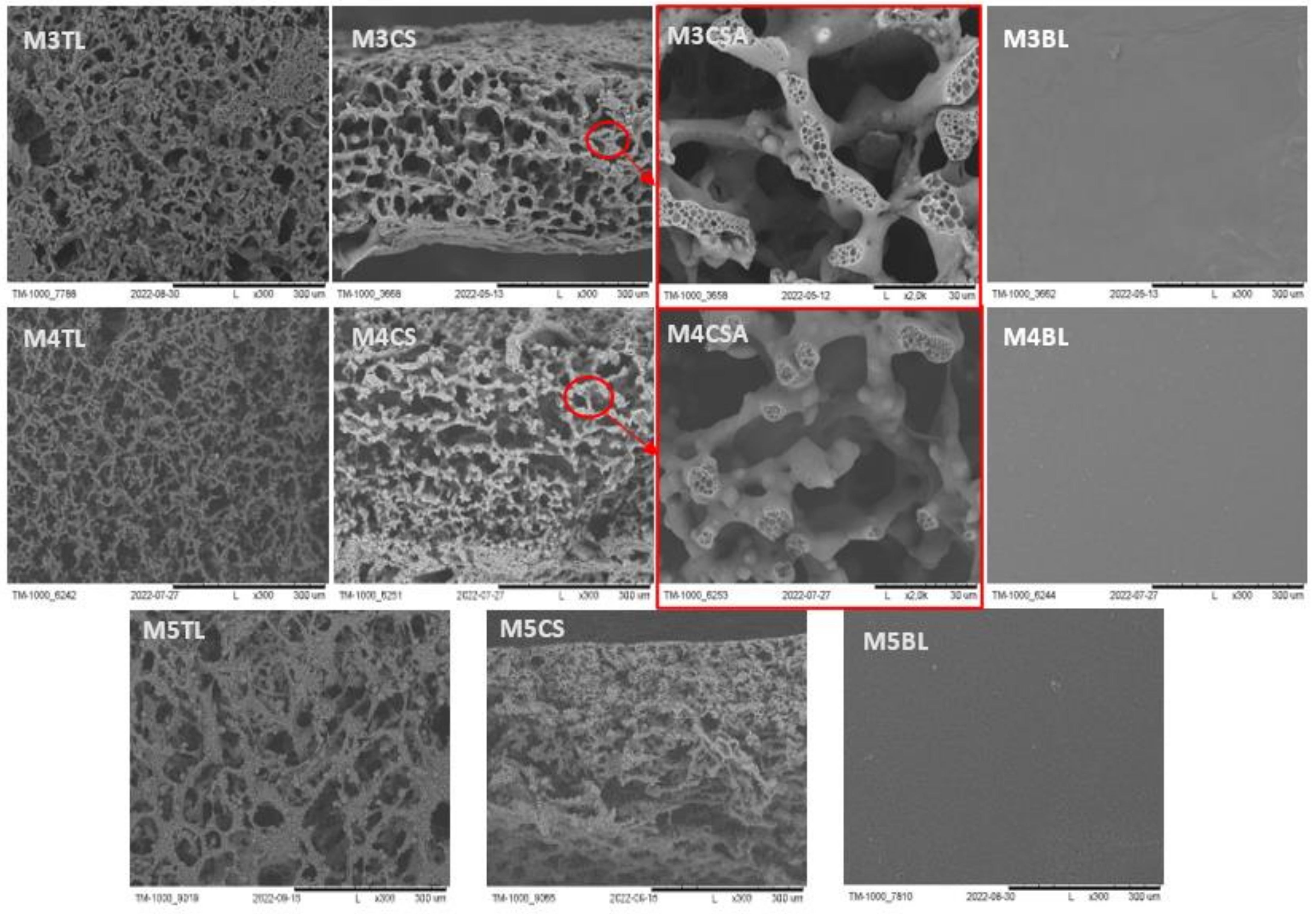

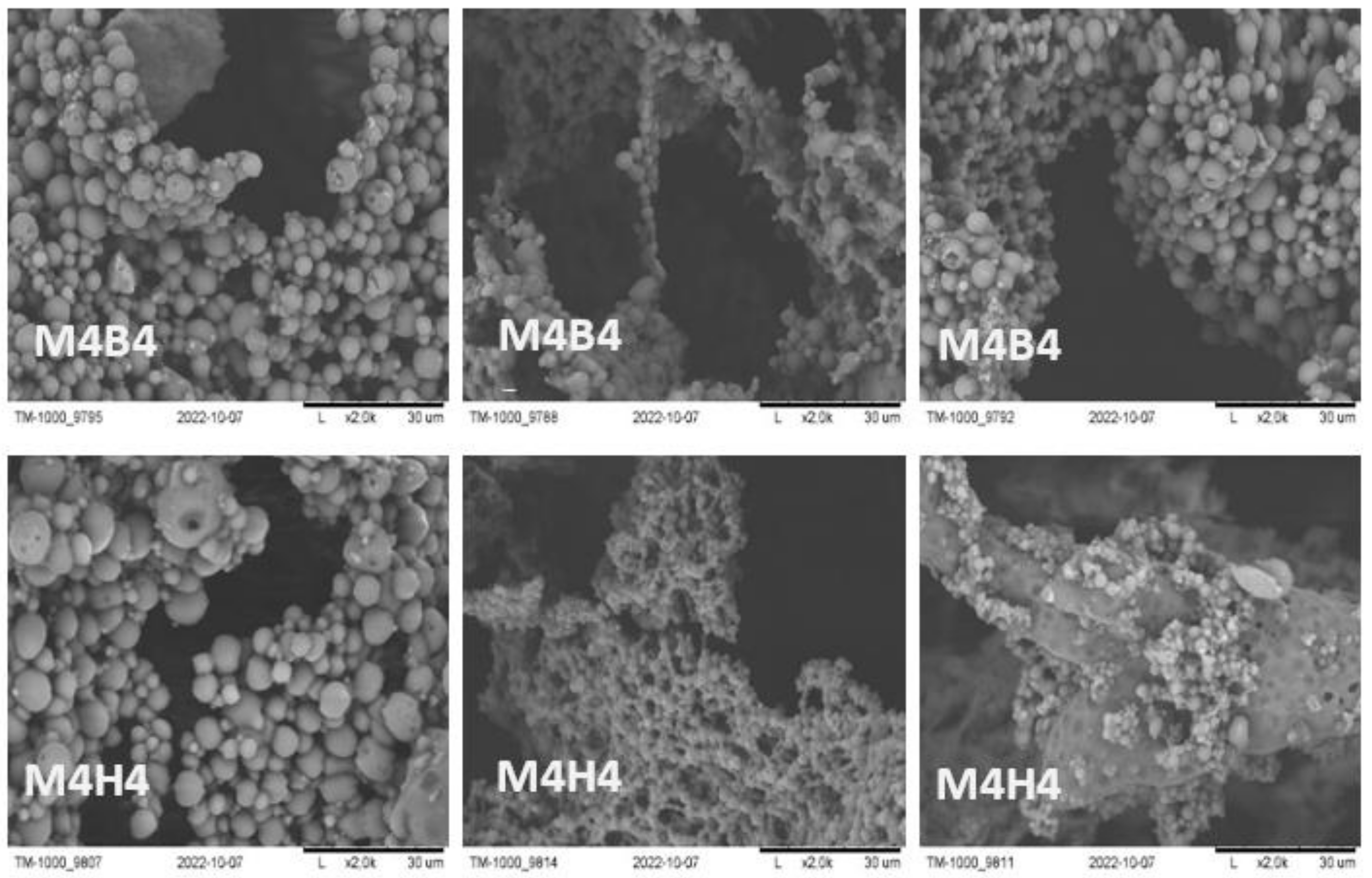




| Fluid | SBF | HBSS | NaOH | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Membranes | M1 | M2 | M3 | M4 | M5 | M1 | M2 | M3 | M4 | M5 | M1 | M2 | M3 | M4 | M5 | |
| Initial pH | 7.31 | 7.54 | 13.50 | |||||||||||||
| Average pH after 2 weeks | 7.42 | 7.47 | 7.67 | 7.05 | 7.30 | 7.39 | 7.26 | 7.10 | 7.58 | 6.98 | 13.80 | 13.89 | 13.99 | 13.78 | 13.84 | |
| Average pH after 4 weeks | 7.68 | 7.66 | 7.35 | 7.47 | 7.50 | 7.37 | 7.49 | 6.87 | 7.24 | 7.84 | 13.52 | 13.47 | 13.77 | 13.57 | 13.45 | |
| Scaffold | Difference in HBSS Medium [%] | Difference in SBF Medium [%] |
|---|---|---|
| M3 CS | 11.47 | 10.84 |
| M3 TL | 1.01 | 1.23 |
| M4 CS | 18.89 | 5.80 |
| M4 TL | 17.36 | 4.33 |
| M5 CS | 8.06 | 15.75 |
| M5 TL | 0.89 | 13.10 |
| Scaffold | Total Area of Pores > 300 µm2 before and after Degradation | ||
|---|---|---|---|
| Before Degradation | After Degradation in HBSS | After Degradation in SBF | |
| M3 CS | 17.08 ± 5.61 | 32.11 ± 9.11 | 31.39 ± 6.26 |
| M4 CS | 20.66 ± 11.22 | 44.75 ± 10.46 | 24.82 ± 13.68 |
| M5 CS | 16.51 ± 7.72 | 27.33 ± 7.72 | 36.50 ± 9.12 |
| Scaffold | PES:PUR Weight Ratios |
|---|---|
| M3 | 1:1 |
| M4 | 2:1 |
| M5 | 1:2 |
| j | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Size µm2 | 0–3 | 3–8 | 8–20 | 20–80 | 80–100 | 100–150 | 150–300 | >300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasyłeczko, M.; Remiszewska, E.; Sikorska, W.; Dulnik, J.; Chwojnowski, A. Scaffolds for Cartilage Tissue Engineering from a Blend of Polyethersulfone and Polyurethane Polymers. Molecules 2023, 28, 3195. https://doi.org/10.3390/molecules28073195
Wasyłeczko M, Remiszewska E, Sikorska W, Dulnik J, Chwojnowski A. Scaffolds for Cartilage Tissue Engineering from a Blend of Polyethersulfone and Polyurethane Polymers. Molecules. 2023; 28(7):3195. https://doi.org/10.3390/molecules28073195
Chicago/Turabian StyleWasyłeczko, Monika, Elżbieta Remiszewska, Wioleta Sikorska, Judyta Dulnik, and Andrzej Chwojnowski. 2023. "Scaffolds for Cartilage Tissue Engineering from a Blend of Polyethersulfone and Polyurethane Polymers" Molecules 28, no. 7: 3195. https://doi.org/10.3390/molecules28073195
APA StyleWasyłeczko, M., Remiszewska, E., Sikorska, W., Dulnik, J., & Chwojnowski, A. (2023). Scaffolds for Cartilage Tissue Engineering from a Blend of Polyethersulfone and Polyurethane Polymers. Molecules, 28(7), 3195. https://doi.org/10.3390/molecules28073195








