Development of SDS-Modified PbO2 Anode Material Based on Ti3+ Self-Doping Black TiO2NTs Substrate as a Conductive Interlayer for Enhanced Electrocatalytic Oxidation of Methylene Blue
Abstract
:1. Introduction
2. Results and Discussion
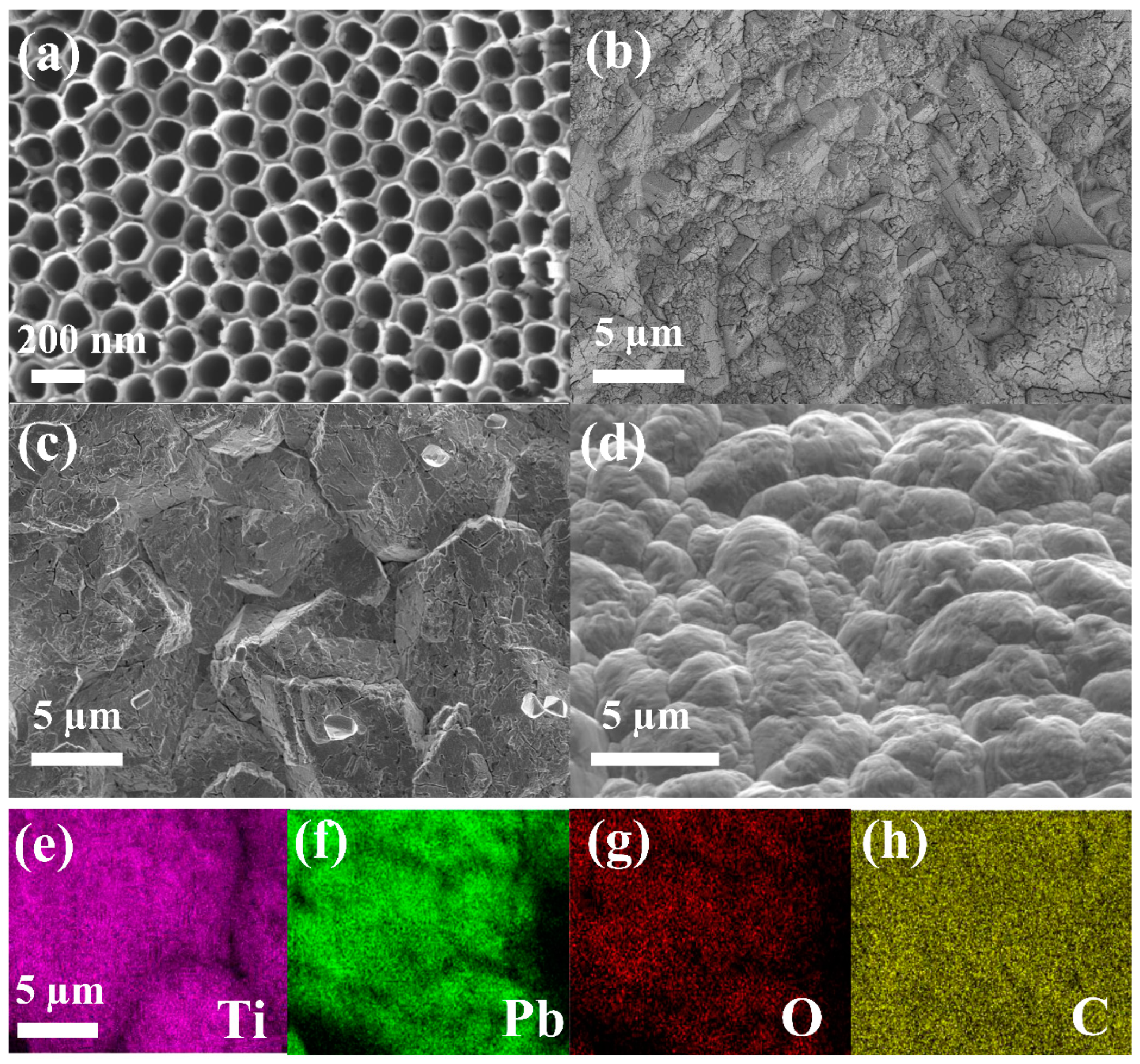
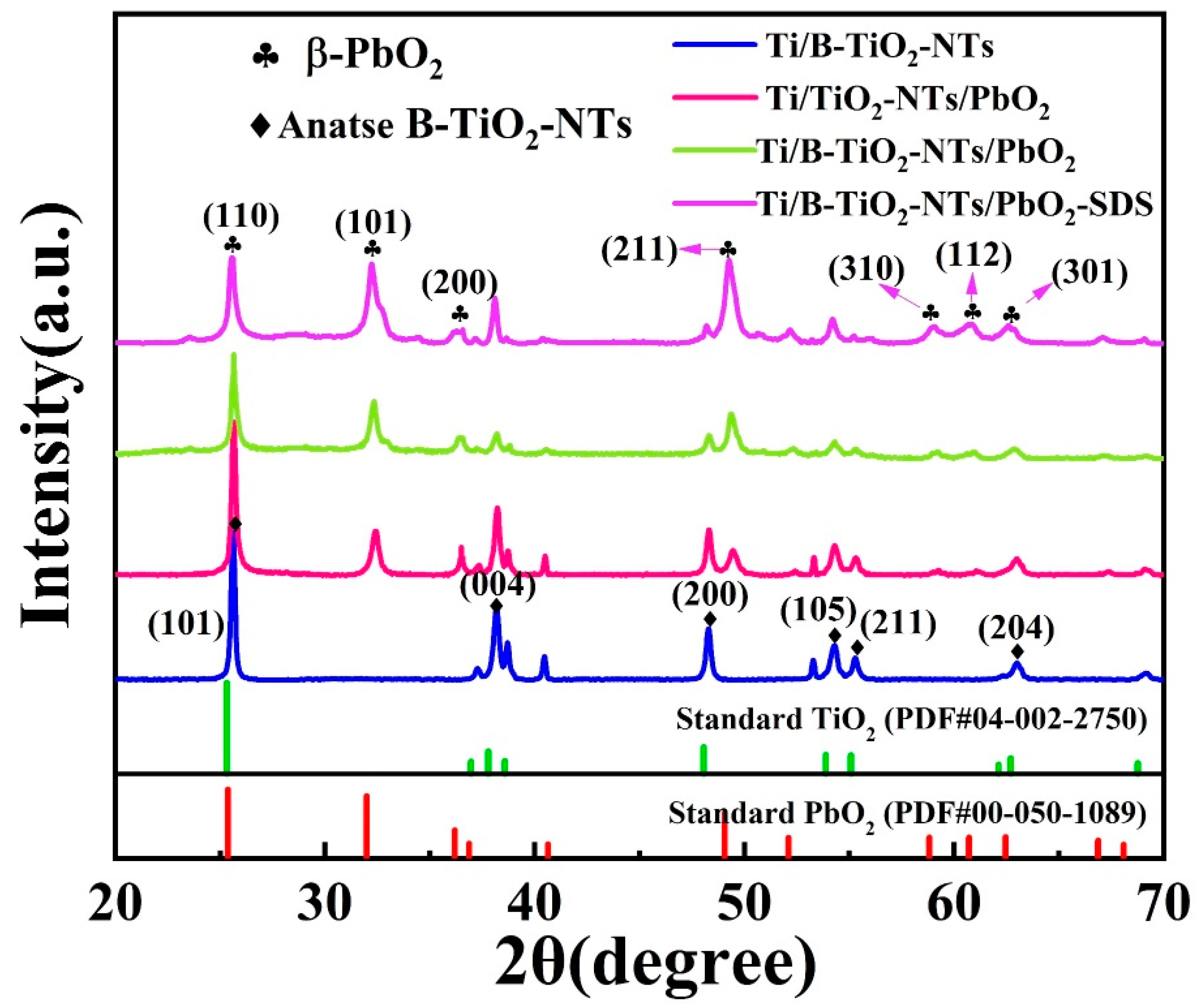
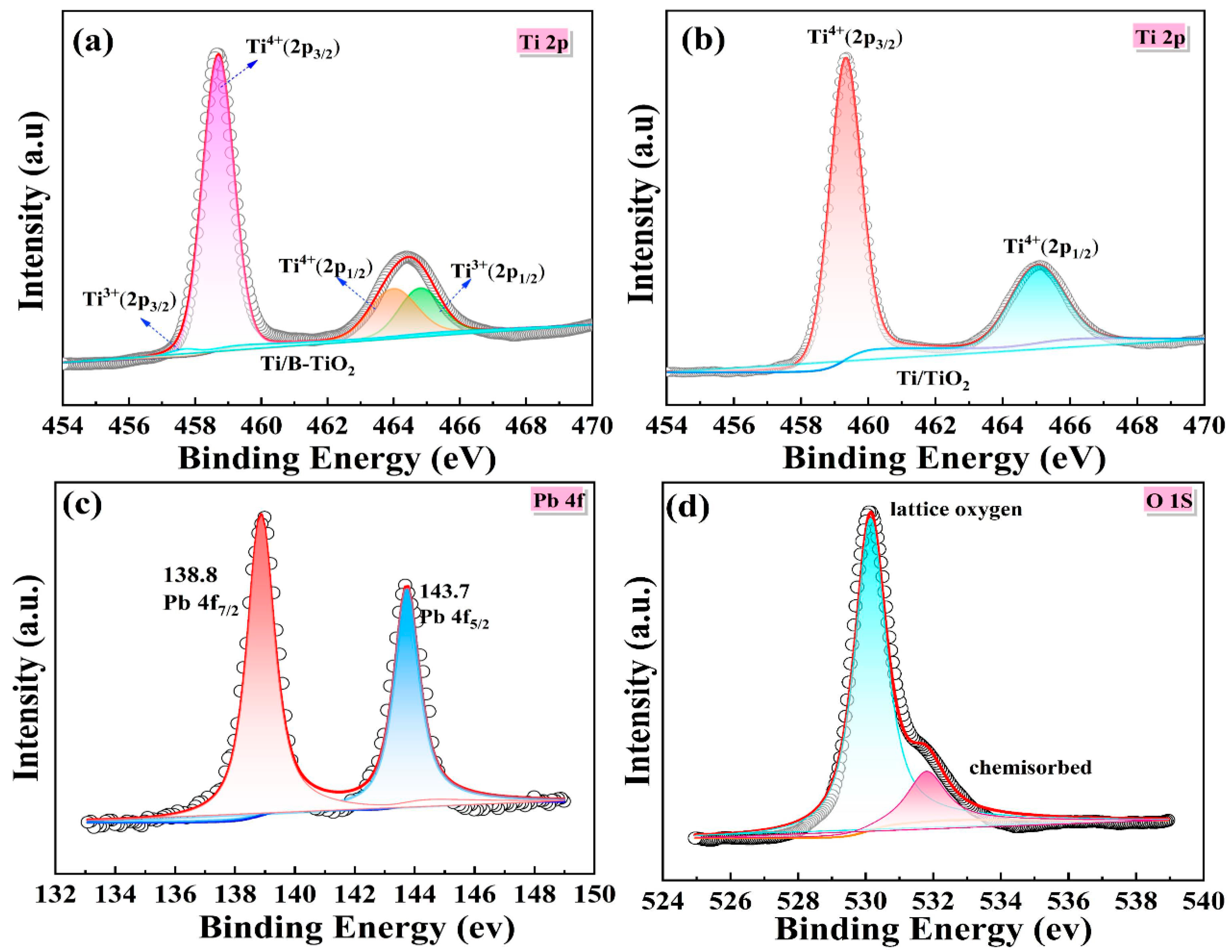
2.1. Morphology and Phase Analysis
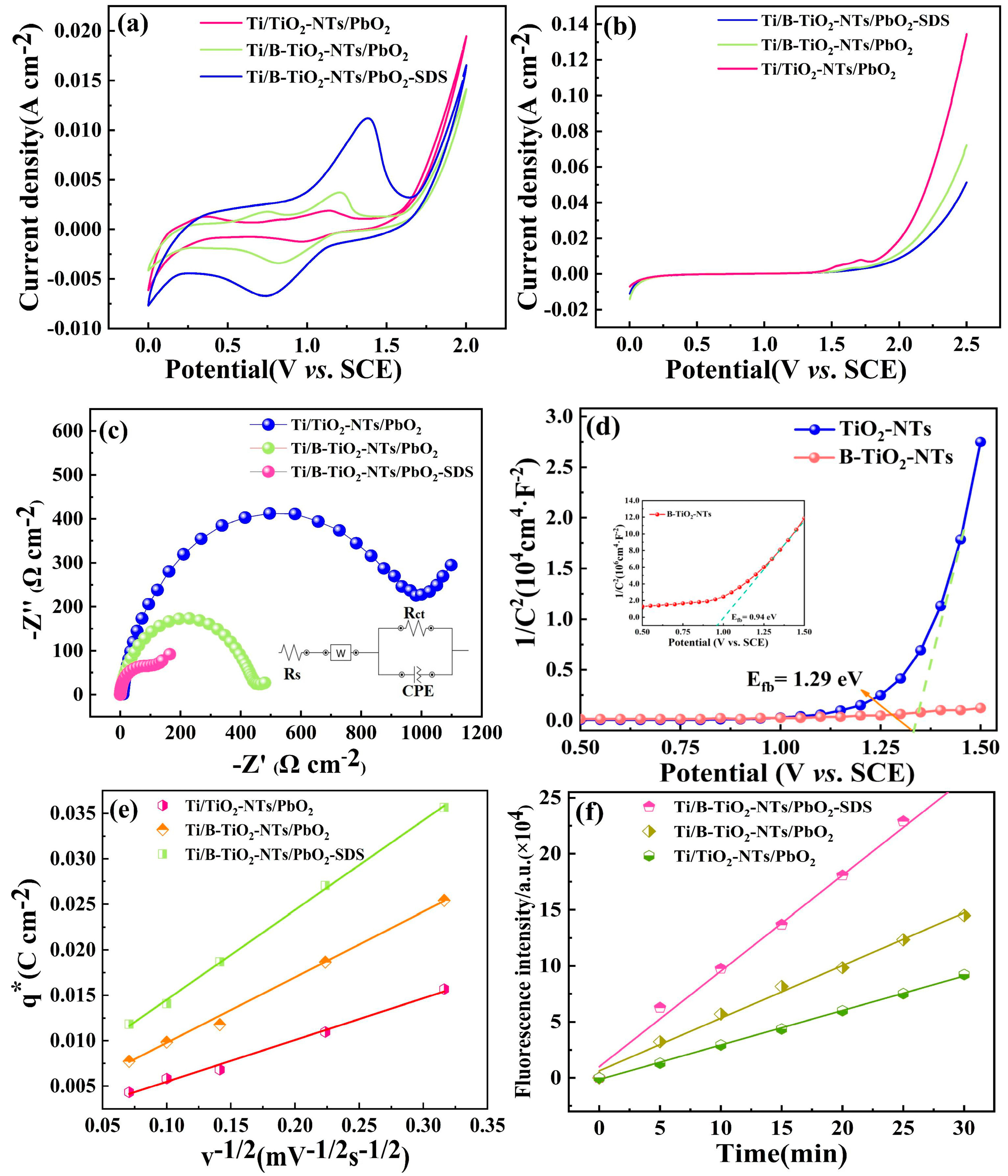
2.2. Electrochemical Characterization
2.3. Electrochemical Oxidation Degradation of Methylene Blue (MB)
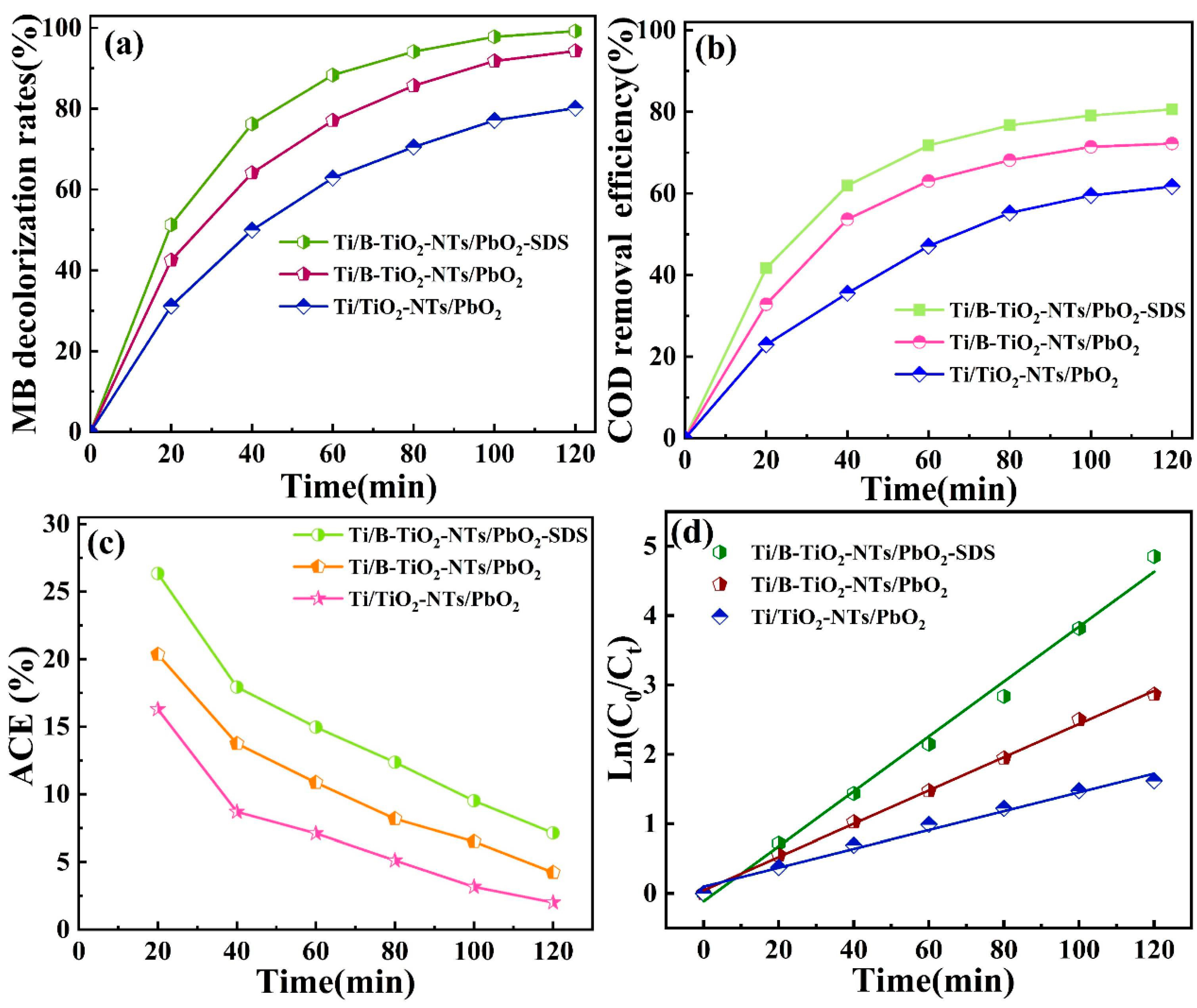
2.3.1. Effects of Different Anodes
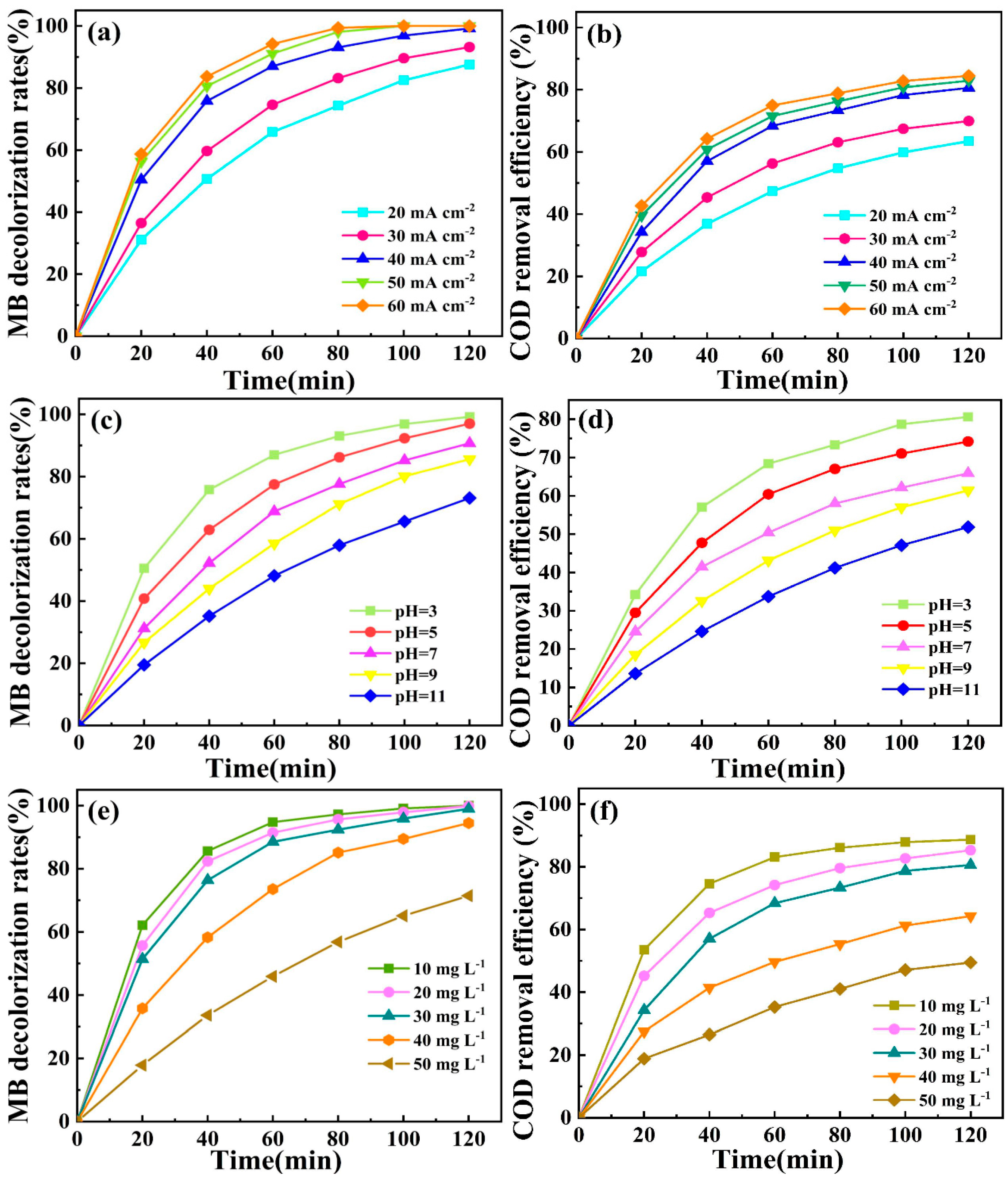
2.3.2. Effect of Current Density
2.3.3. Effect of Initial pH
2.3.4. Effect of Initial Concentration
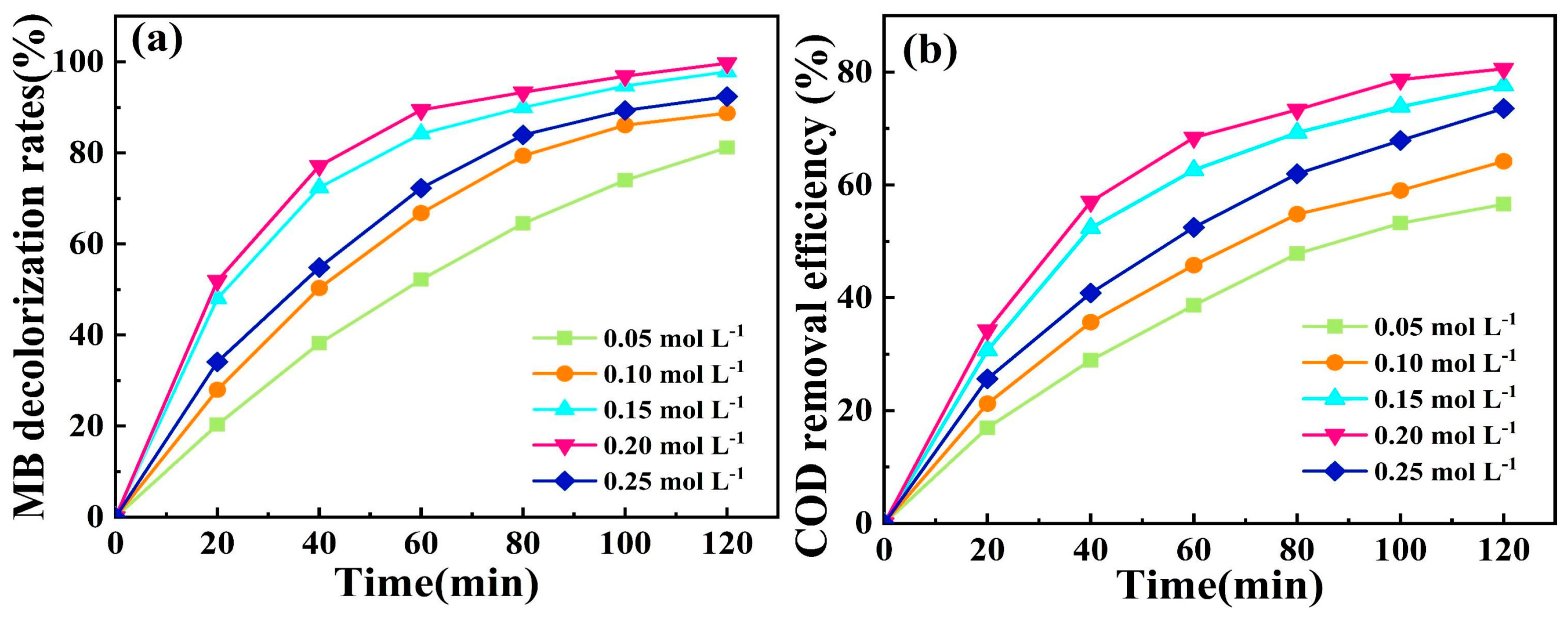
2.3.5. Effect of Electrolyte Concentration
2.4. Degradation Mechanism of MB Wastewater
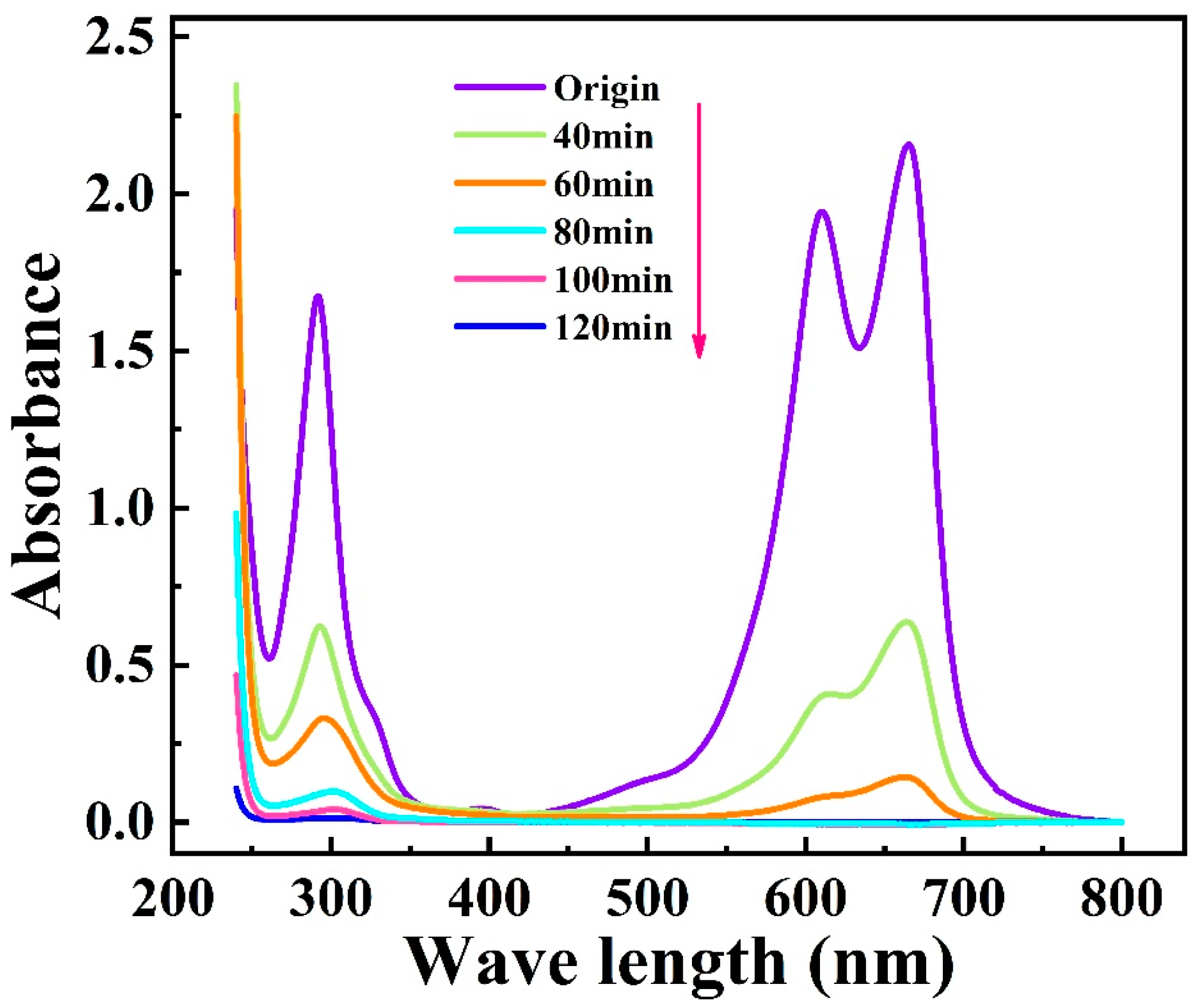
2.4.1. The UV-Vis Absorption Spectra Analysis
2.4.2. Trapping Experiments of Radicals
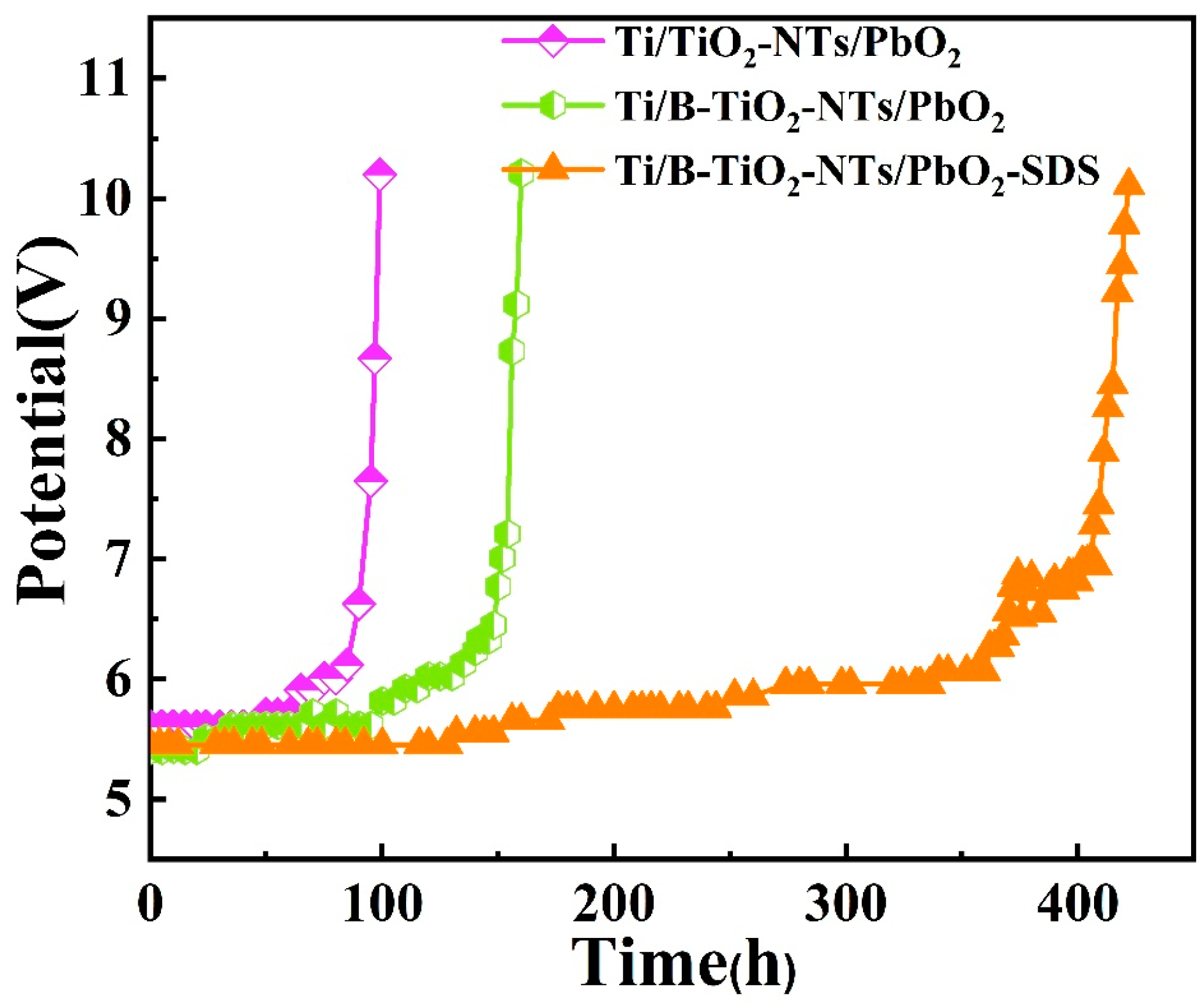
2.5. Electrode Stability and Recyclability
3. Experimental
3.1. Materials and Reagents
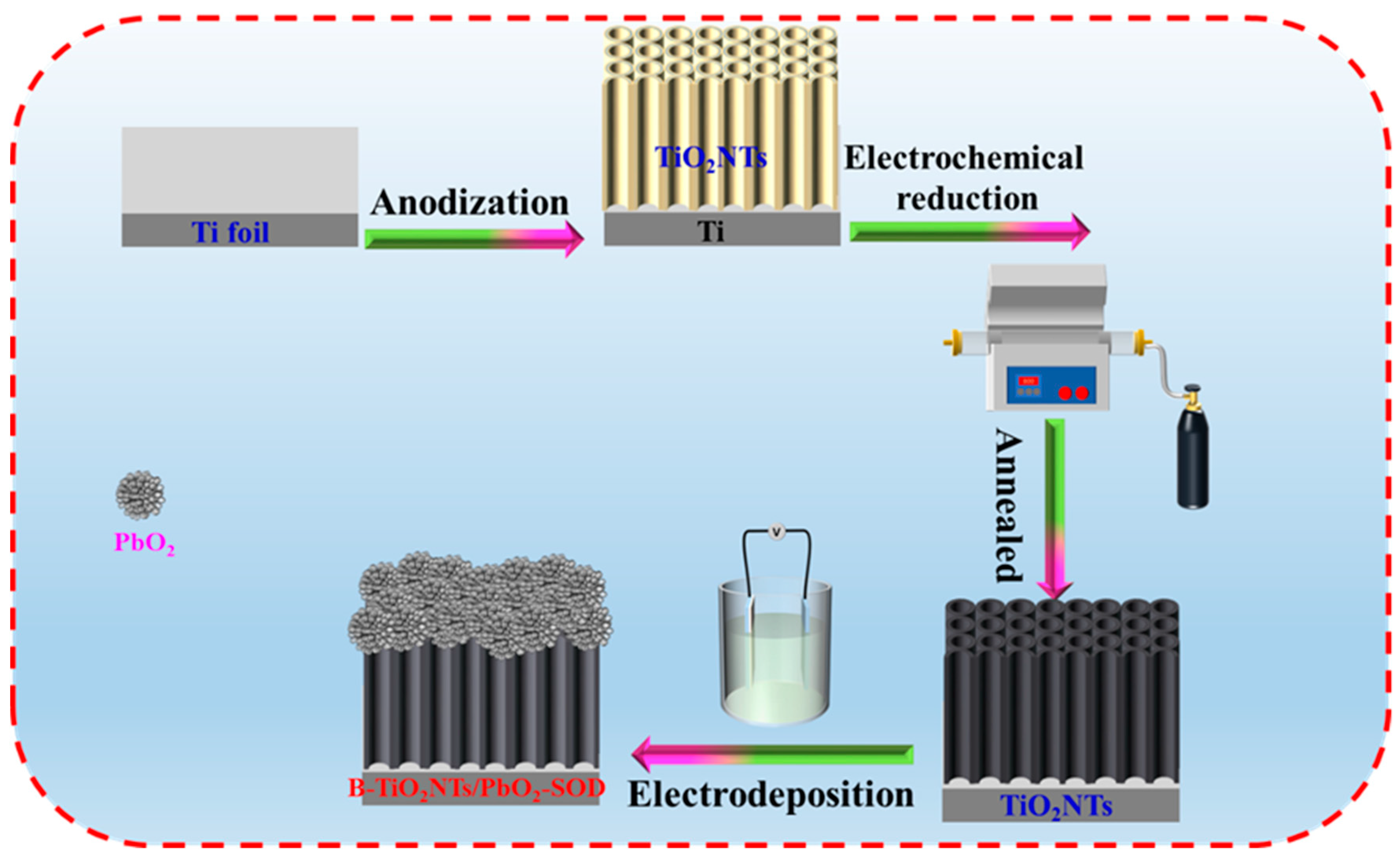
3.2. Preparation of Ti3+ Self-Doping Ti/B-TiO2-NTs/PbO2-SDS Electrode
- (1)
- Fabrication of the Ti3+ self-doping Ti/B-TiO2-NTs middle layer on the Ti substrate.
- (2)
- Deposition of the PbO2 coating on the middle layer
3.3. Characterization
3.4. Electrochemical Measurement
3.5. Electrocatalytic Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Man, S.; Bao, H.; Xu, K.; Yang, H.; Sun, Q.; Xu, L.; Yang, W.; Mo, Z.; Li, X. Preparation and characterization of Nd-Sb co-doped SnO2 nanoflower electrode by hydrothermal method for the degradation of norfloxacin. Chem. Eng. J. 2021, 417, 129266. [Google Scholar]
- Mameda, N.; Park, H.; Shah, S.S.A.; Lee, K.; Li, C.W.; Naddeo, V.; Choo, K.H. Highly robust and efficient Ti-based Sb-SnO2 anode with a mixed carbon and nitrogen interlayer for electrochemical 1, 4-dioxane removal from water. Chem. Eng. J. 2020, 393, 124794. [Google Scholar] [CrossRef]
- Hu, Z.W.; Guo, C.; Wang, P.; Guo, R.; Liu, X.W.; Tian, Y. Electrochemical degradation of methylene blue by Pb modified porous SnO2 anode. Chemosphere 2022, 305, 135447. [Google Scholar]
- Dos Santos, A.J.; Garcia-Segura, S.; Dosta, S.; Cano, I.G.; Martínez-Huitle, C.A.; Brillas, E. A ceramic electrode of ZrO2-Y2O3 for the generation of oxidant species in anodic oxidation. Assessment of the treatment of Acid Blue 29 dye in sulfate and chloride media. Sep. Purif. Technol. 2019, 228, 115747. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166, 603–643. [Google Scholar]
- Duan, X.; Zhao, C.; Liu, W.; Zhao, X.; Chang, L. Fabrication of a novel PbO2 electrode with a graphene nanosheet interlayer for electrochemical oxidation of 2- chlorophenol. Electrochim. Acta 2017, 240, 424–436. [Google Scholar] [CrossRef]
- Särkkä, H.; Bhatnagart, A.; Sillanpää, M. Recent developments of electro-oxidation in water treatment-A review. J. Electroanal. Chem. 2015, 754, 46–56. [Google Scholar]
- Shestakova, M.; Sillanpää, M. Electrode materials used for electrochemical oxi- dation of organic compounds in wastewater. Rev. Environ. Sci. Biotechnol. 2017, 16, 223–238. [Google Scholar]
- Xia, Y.; Dai, Q. Electrochemical degradation of methyldopa on a Fe doped PbO2 electrode: Electrode characterization, reaction kinetics and energy demands. J. Electrochem. Soc. 2017, 164, H877–H884. [Google Scholar] [CrossRef]
- Chen, S.X.; He, P.; Zhou, P.C.; Wang, X.J.; Xiao, F.; He, Q.H.; Li, J.; Jia, L.P.; Zhang, H.; Jia, B.; et al. Development of a novel graphitic carbon nitride and multiwall carbon nanotube co-doped Ti/PbO2 anode for electrocatalytic degradation of acetaminophen. Chemosphere 2021, 271, 129830. [Google Scholar]
- Li, S.D.; Wang, F.W.; Xu, M.; Wang, Y.Y.; Fang, W.Y.; Hu, Y.H. Fabrication and characteristics of a nanostructure PbO2 anode and its application for degradation of phenol. J. Electrochem. Soc. 2013, 160, E44–E48. [Google Scholar] [CrossRef]
- Bi, H.; Yu, C.; Gao, W.; Cao, P. Physicochemical characterisation of electrosynthesized lead dioxide coatings on Ti/SnO2-Sb substrates. Electrochimica. Acta 2013, 113, 446–453. [Google Scholar] [CrossRef]
- Wang, C.; Niu, J.F.; Yin, L.F.; Huang, J.X.; Hou, L.A. Electrochemical degradation of fluoxetine on nanotube array intercalated anode with enhanced electronic transport and hydroxyl radical production. Chem. Eng. J. 2018, 346, 662–671. [Google Scholar]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar]
- Chen, D.; Xiong, F.; Zhang, H.; Ma, C.; Cao, L.; Yang, J. Dimensional stable lead electrode modified by SDS for efficient degradation of bisphenol A. ACS Omega 2020, 5, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, X.; Huang, W.; Chen, R.; Zhang, W.; Li, H.; Lin, H. Hydrophobic networked PbO2 electrode for electrochemical oxidation of paracetamol drug and degradation mechanism kinetics. Chemosphere 2018, 193, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, A.Y.; Pei, D.N.; Yu, H.Q. Efficient electrochemical reduction of nitrobenzene by defect-engineered TiO2−x single crystals. Environ. Sci. Technol. 2016, 50, 5234–5242. [Google Scholar] [CrossRef]
- Pei, D.N.; Gong, L.; Zhang, A.Y.; Zhang, X.; Chen, J.J.; Mu, Y.; Yu, H.Q. Defective titanium dioxide single crystals exposed by high-energy {001} facets for efficient oxygen reduction. Nat. Commun. 2015, 6, 8696–8705. [Google Scholar] [CrossRef]
- Cerro-Lopez, M.; Meas-Vong, Y.; M’endez-Rojas, M.A.; Martínez-Huitle, C.A.; Quiroz, M.A. Formation and growth of PbO2 inside TiO2 nanotubes for environmental applications. Appl. Catal. B Environ. 2014, 144, 174–181. [Google Scholar]
- Dai, Q.; Zhou, J.; Weng, M.; Luo, X.; Feng, D.; Chen, J. Electrochemical oxidation metronidazole with Co modified PbO2 electrode: Degradation and mechanism. Separ. Purif. Technol. 2016, 166, 109–116. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Yan, W. Fabrication and characterization of PbO2 electrode modified with polyvinylidene fluoride (PVDF). Appl. Surf. Sci. 2016, 389, 278–286. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Jia, L.; Li, C.; Liu, H.; Wang, S.; Zhou, S.; Dong, F. Ti/PbO2-Sm2O3 composite based electrode for highly efficient electrocatalytic degradation of alizarin yellow R. J. Colloid Interface Sci. 2019, 533, 750–761. [Google Scholar] [PubMed]
- Duan, T.; Chen, Y.; Wen, Q.; Duan, Y. Enhanced electrocatalytic activity of nano-TiN composited Ti/Sb-SnO2 electrode fabricated by pulse electrodeposition for methylene blue decolorization. RSC Adv. 2014, 4, 57463–57475. [Google Scholar]
- Li, X.; Xu, H.; Yan, W. Effects of twelve sodium dodecyl sulfate (SDS) on electro-catalytic performance and stability of PbO2 electrode. J. Alloys Compd. 2017, 718, 386–395. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Z.; Wang, Y.; Mao, Y.; Cai, Z. Effective treatment of leachate concentrate using membrane distillation coupled with electrochemical oxidation. Sep. Purif. Technol. 2021, 267, 118679. [Google Scholar] [CrossRef]
- Gui, L.; Chen, Z.; Chen, B.; Song, Y.; Yu, Q.; Zhu, W.; Hu, Q.; Liu, Y.; Zheng, Z.; Lv, Z.; et al. Preparation and characterization of ZnO/PEG-Co(II)-PbO2 nanocomposite electrode and an investigation of the electrocatalytic degradation of phenol. J. Hazard. Mater. 2020, 399, 123018. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, G.; Guo, L.; Dai, Q.; Ma, X. Electrochemical oxidation of Acid Orange 7 azao dye using a PbO2 electrode: Parameter optization, reaction mechanism and toxicity evaluation. Chemosphere 2020, 241, 125010. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Dai, Q. Electrochemical degradation of antibiotic levofloxacin by PbO2 electrode: Kinetics, energy demands and reaction pathways. Chemosphere 2018, 205, 215–222. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Wu, J.; Niu, J. Insights into the electrochemical degradation of sulfamethoxazole and its metabolite by Ti/SnO2-Sb/Er-PbO2 anode. Chin. Chem. Lett. 2020, 31, 2673–2677. [Google Scholar] [CrossRef]
- Liu, Y.; Pang, D.; Wang, L.; Song, H.; Liu, R.; Hu, S.; Shen, Y.; Li, A.; Zhang, S. Electrochemically reduced phytic acid-doped TiO2 nanotubes for the efficient electrochemical degradation of toxic pollutants. J. Hazard. Mater. 2021, 414, 125600. [Google Scholar]
- Zhao, J.; Zhu, C.; Lu, J.; Hu, C.; Peng, S.; Chen, T. Electro-catalytic degradation of bisphenol A with modified Co3O4/β-PbO2/Ti electrode. Electrochim. Acta 2014, 118, 169–175. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Xu, Z.; Niu, J.; Hou, L.A. Electrochemical degradation of enrofloxacin by lead dioxide anode: Kinetics, mechanism and toxicity evaluation. Chem. Eng. J. 2017, 326, 911–920. [Google Scholar] [CrossRef]
- Duan, X.Y.; Wang, Q.; Tu, S.Q.; Wang, W.Y.; Sui, X.Y.; Chang, L.M. Electrocatalytic degradation of 2,4-dichlorophenol by a 3DG-PbO2 powdered anode: Experimental and theoretical insights. Sep. Purif. Technol. 2022, 282, 120003. [Google Scholar]
- Dong, H.; Hu, X.Y.; Zhang, Y.H.; Jiang, W.Q.; Zhang, X. Co/La modified Ti/PbO2 anodes for chloramphenicol degradation: Catalytic performance and reaction mechanism. Chemosphere 2021, 285, 131568. [Google Scholar] [CrossRef] [PubMed]
- Oturan, N.; Wu, J.; Zhang, H.; Sharma, V.K.; Oturan, M.A. Electrocatalytic destruction of the antibiotic tetracycline in aqueous medium by electrochemical advanced oxidation processes: Effect of electrode materials. Appl. Catal. B-Environ. 2013, 140–141, 92–97. [Google Scholar] [CrossRef]
- Liang, X.L.; Zhong, Y.H.; Zhu, S.Y.; Ma, L.Y.; Yuan, P.; Zhu, J.X.; He, H.P.; Jiang, Z. The contribution of vanadium and titanium on improving methylene bluedecolorization through heterogeneous UV-Fenton reaction catalyzed by theirco-doped magnetite. J. Hazard. Mater. 2012, 199–200, 247–254. [Google Scholar] [CrossRef]
- Yao, Y.W.; Zhao, C.M.; Zhao, M.M.; Wang, X. Electrocatalytic degradation of methylene blue on PbO2-ZrO2 nanocomposite electrodes prepared by pulse electrodeposition. J. Hazard. Mater. 2013, 263, 726–734. [Google Scholar] [CrossRef]
- Qiao, J.; Guo, Y.; Dong, H.; Guan, X.; Zhou, G.; Sun, Y. Activated peroxydislfate by sulfidated zero-valent iron for enhanced organic micropollutants removal from water. Chem. Eng. J. 2020, 396, 125301. [Google Scholar] [CrossRef]
- Yu, W.K.; Xu, M.; Liang, X.; Wang, J.Y.; Fang, W.Y.; Wang, F.W. Fabrication of Ce MOFs/Black-TNTs and CCM/Black-TNTs as high-efficiency photoelectrode for wastewater treatment and hydrogen production. Sep. Purif. Technol. 2022, 303, 122155. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Xu, M.; Zhu, C.; Fang, W.; Wei, Y. Electrocatalytic degradation of methylene blue on Co doped Ti/TiO2 nanotube/PbO2 anodes prepared by pulse electrodeposition. J. Electroanal. Chem. 2015, 759, 158. [Google Scholar] [CrossRef]
- Ansari, A.; Nematollahi, D. Convergent paired electrocatalytic degradation of p-dinitrobenzene by Ti/SnO2-Sb/β-PbO2 anode. A new insight into the electrochemical degradation mechanism. Appl. Catal. B Environ. 2020, 261, 118226. [Google Scholar] [CrossRef]
- Zou, C.J.; Ma, C.L.; Chen, F.; Shao, X.; Cao, L.M.; Yang, J. Crystal facet controlled stable PbO2 electrode for efficient degradation of tetracycline. J. Electroanal. Chem. 2022, 914, 116330. [Google Scholar]
- Shimi, A.K.; Parvathiraj, C.; Kumari, S.; Dalal, J.; Kumar, V.; Wabaidurd, S.M.; Alothman, Z.A. Green synthesis of SrO nanoparticles using leaf extract of Albizia julibrissin and its recyclable photocatalytic activity: An eco-friendly approach for treatment of industrial wastewater. Environ. Sci. Adv. 2022, 1, 849–861. [Google Scholar]
- Rohilla, S.; Gupta, A.; Kumar, V.; Kumari, S.; Petru, M.; Amor, N.; Noman, M.T.; Dalal, J. Excellent UV-Light Triggered Photocatalytic Performance of ZnO.SiO2 Nanocomposite for Water Pollutant Compound Methyl Orange Dye. Nanomaterials 2021, 11, 2548. [Google Scholar] [PubMed]
- Zhou, Y.; Li, Z.; Hao, C.; Zhang, Y.; Chai, S.; Han, G.; Xu, H.; Lu, J.; Dang, Y.; Sun, X.; et al. Electrocatalysis enhancement of α, β-PbO2 nanocrystals induced via rare earth Er(III) doping strategy: Principle, degradation application and electrocatalytic mechanism. Electrochim. Acta. 2020, 333, 135535. [Google Scholar]
- Ying, S.; Li, Y.D.; Yao, Y.W.; Xia, Y.; Jiao, M.Y.; Han, E.S. Electrodeposition and Catalytic Performance of Hydrophobic PbO2 Electrode Modified by Surfactant OP-10. ECS J. Solid State Sci. Technol. 2021, 10, 123005. [Google Scholar]
- Yang, C.; Shang, S.S.; Li, X.Y. Fabrication of sulfur-doped TiO2 nanotube array as a conductive interlayer of PbO2 anode for efficient electrochemical oxidation of organic pollutants. J. Hazard Mater. 2021, 258, 118035. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Gao, C.; Zhang, X.; Liang, X.; Hu, Y.; Wang, F. Development of SDS-Modified PbO2 Anode Material Based on Ti3+ Self-Doping Black TiO2NTs Substrate as a Conductive Interlayer for Enhanced Electrocatalytic Oxidation of Methylene Blue. Molecules 2023, 28, 6993. https://doi.org/10.3390/molecules28196993
Xu M, Gao C, Zhang X, Liang X, Hu Y, Wang F. Development of SDS-Modified PbO2 Anode Material Based on Ti3+ Self-Doping Black TiO2NTs Substrate as a Conductive Interlayer for Enhanced Electrocatalytic Oxidation of Methylene Blue. Molecules. 2023; 28(19):6993. https://doi.org/10.3390/molecules28196993
Chicago/Turabian StyleXu, Mai, Chunli Gao, Xiaoyan Zhang, Xian Liang, Yunhu Hu, and Fengwu Wang. 2023. "Development of SDS-Modified PbO2 Anode Material Based on Ti3+ Self-Doping Black TiO2NTs Substrate as a Conductive Interlayer for Enhanced Electrocatalytic Oxidation of Methylene Blue" Molecules 28, no. 19: 6993. https://doi.org/10.3390/molecules28196993
APA StyleXu, M., Gao, C., Zhang, X., Liang, X., Hu, Y., & Wang, F. (2023). Development of SDS-Modified PbO2 Anode Material Based on Ti3+ Self-Doping Black TiO2NTs Substrate as a Conductive Interlayer for Enhanced Electrocatalytic Oxidation of Methylene Blue. Molecules, 28(19), 6993. https://doi.org/10.3390/molecules28196993





