Research Progress of Dihydroquercetin in the Treatment of Skin Diseases
Abstract
1. Introduction
2. Classification of Skin Diseases
2.1. Skin Cancer
2.2. Atopic Dermatitis
2.3. Psoriasis
2.4. Skin Acne
2.5. Chloasma
3. Skin Dressing
4. Application of Dihydroquercetin in the Treatment of Skin Diseases
4.1. Treatment of Skin Cancer
4.2. Treatment of Psoriasis
4.3. Treatment of Skin Inflammation
4.4. Delay Skin Aging
4.5. Treatment of Skin Acne
4.6. Dihydroquercetin Promotes Wound Healing
4.7. Dihydroquercetin Treatment of Skin Burns
4.8. Treatment of Skin Ulcer and Chloasma
5. Dihydroquercetin Loaded Wound Dressing
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nigat, T.D.; Sitote, T.M.; Gedefaw, B.M. Fungal skin disease classification using the convolutional neural network. J. Healthc. Eng. 2023, 2023, 6370416. [Google Scholar] [CrossRef]
- Inthiyaz, S.; Altahan, B.R.; Ahammad, S.H.; Rajesh, V.; Kalangi, R.R.; Smirani, L.K.; Hossain, M.A.; Rashed, A.N.Z. Skin disease detection using deep learning. Adv. Eng. Softw. 2023, 175, 103361. [Google Scholar] [CrossRef]
- Miguel, L.; Jorge, M.; Rocha, B.; Miot, H.A. Incidence of skin diseases diagnosed in a public institution: Comparison between 2003 and 2014. An. Bras. Dermatol. 2017, 92, 423–425. [Google Scholar] [CrossRef]
- Ding, Q.; Chen, K.; Liu, X.; Ding, C.; Zhao, Y.; Sun, S.; Zhang, Y.; Zhang, J.; Liu, S.; Liu, W. Modification of taxifolin particles with an enteric coating material promotes repair of acute liver injury in mice through modulation of inflammation and autophagy signaling pathway. Biomed. Pharmacother. 2022, 152, 113242. [Google Scholar] [CrossRef]
- Ding, C.; Zhao, Y.; Chen, X.; Zheng, Y.; Liu, W.; Liu, X. Taxifolin, a novel food, attenuates acute alcohol-induced liver injury in mice through regulating the nf-kappab-mediated inflammation and pi3k/akt signalling pathways. Pharm. Biol. 2021, 59, 868–879. [Google Scholar] [CrossRef]
- Wang, X.D.; Meng, M.X.; Gao, L.B.; Liu, T.; Xu, Q.; Zeng, S. Permeation of astilbin and taxifolin in caco-2 cell and their effects on the p-gp. Int. J. Pharm. 2009, 378, 1–8. [Google Scholar] [CrossRef]
- Petitjean, C.; Benateau, H.; Veyssiere, A.; Morello, R.; Dompmartin, A.; Garmi, R. Interest of frozen section procedure in skin tumors other than melanoma. J. Plast. Reconstr. Aesthet. Surg. 2023, 84, 377–384. [Google Scholar] [CrossRef]
- Wekha, G.; Ebiju, I.; Ayesiga, I.; Kiyimba, B.; Muhumuza, J. A rare presentation of a giant malignant melanoma in a 67 year old female ugandan: A case report. Int. J. Surg. Case Rep. 2023, 109, 108569. [Google Scholar] [CrossRef]
- Thiessen, C.; Ling, I.; Horvai, A.; Kang, S. Fever, skin nodules, and hyperintense intramuscular leg lesions in a liver transplant patient. Am. J. Transplant. 2022, 22, 658–661. [Google Scholar] [CrossRef]
- Hiroyasu, S.; Hiroyasu, A.; Granville, D.J.; Tsuruta, D. Pathological functions of granzyme b in inflammatory skin diseases. J. Dermatol. Sci. 2021, 104, 76–82. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, S.; Lou, Z.; Gao, J. The macrophage polarization in inflammatory dermatosis and its potential drug candidates. Biomed. Pharmacother. 2023, 161, 114469. [Google Scholar] [CrossRef]
- Aslan Kayıran, M.; Wang, J.V.; Karadag, A.S. Papulosquamous annular diseases. Clin. Dermatol. 2022, 40, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Alexiades, M.; Kothare, A.; Goldberg, D.; Dover, J.S. Novel 1726 nm laser demonstrates durable therapeutic outcomes and tolerability for moderate-to-severe acne across skin types. J. Am. Acad. Dermatol. 2023, 89, 703–710. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Wang, B.; Shi, W.; Hu, X.; Wang, Y.; Guo, Y.; Xie, H.; Xiao, W.; Li, J. Integrated omics reveal the molecular characterization and pathogenic mechanism of rosacea. J. Investig. Dermatol. 2023, 02408-9. [Google Scholar] [CrossRef]
- Tan, Y.; Bai, Y.; Li, F.; Chen, J.; Cheng, Q.; Wang, J.; Li, J.; Lei, X. Evaluation of ala-pdt combined with antibiotics for the treatment of skin ulcers with sinus tract formation: A pilot study. Photodiagnosis Photodyn. Ther. 2020, 31, 101802. [Google Scholar] [CrossRef] [PubMed]
- Konisky, H.; Balazic, E.; Jaller, J.A.; Khanna, U.; Kobets, K. Tranexamic acid in melasma: A focused review on drug administration routes. J. Cosmet. Dermatol. 2023, 22, 1197–1206. [Google Scholar] [CrossRef]
- Iida, M.; Tazaki, A.; Deng, Y.; Chen, W.; Yajima, I.; Kondo-Ida, L.; Hashimoto, K.; Ohgami, N.; Kato, M. A unique system that can sensitively assess the risk of chemical leukoderma by using murine tail skin. Chemosphere 2019, 235, 713–718. [Google Scholar] [CrossRef]
- Sun, J.; Lv, X.; Zhang, Y. Application of the “hand as foot” teaching method in the pigmented nevus. Asian J. Surg. 2022, 45, 830–831. [Google Scholar] [CrossRef]
- Requena, C.; Manrique, E.; Nagore, E. Update on lentigo maligna: Diagnostic signs and treatment. Actas. Dermosifiliogr. 2023, 114, 413–424. [Google Scholar] [CrossRef]
- Sugawara-Mikami, M.; Ishii, N.; Yamazaki, M.; Kambara, T.; Sasaki, H.; Tachikawa, N.; Yotsu, R. Skin manifestations of suspected COVID-19: Complications of the disease or reactivation of latent viral infections? JAAD Case Rep. 2021, 12, 15–17. [Google Scholar] [CrossRef]
- Mazzei, R.; Leonti, M.; Spadafora, S.; Patitucci, A.; Tagarelli, G. A review of the antimicrobial potential of herbal drugs used in popular italian medicine (1850s–1950s) to treat bacterial skin diseases. J. Ethnopharmacol. 2020, 250, 112443. [Google Scholar] [CrossRef]
- Ruocco, E.; Donnarumma, G.; Baroni, A.; Tufano, M.A. Bacterial and viral skin diseases. Dermatol. Clin. 2007, 25, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Keith, A.; Hnilica, A.P.P. Chapter 4—Fungal skin diseases. In Small Animal Dermatology, 4th ed.; Hnilica, K.A., Patterson, A.P., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2017; pp. 94–131. [Google Scholar]
- Thakur, D.; Fatima, T.; Sharma, P.; Hasan, M.R.; Malhotra, N.; Khanuja, M.; Shukla, S.K.; Narang, J. High-performance biosensing systems for diagnostics of sexually transmitted disease—A strategic review. Process Biochem. 2023, 126, 223–237. [Google Scholar] [CrossRef]
- He, B.; Wang, W.; Dai, W.; Ali, K.; Yang, Q.; Xiang, T.; Ye, E.; Wu, Y.; Bu, Z. A rare case of condyloma acuminatum caused by hpv73 and hpv33 infection. J. Infect. Public Health 2022, 15, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Canseco, C.; Salinas, R. Aminophylline in the treatment of allergic dermatosis: Ii. Urticaria; Its comparative value in relation to antihistaminics and acth. J. Allergy 1953, 24, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Jin, C.; Yang, L.; Pan, Z. 326 analysis of thyroid function in 46 hospitalized patients with severe allergic dermatosis. J. Investig. Dermatol. 2017, 137, S55. [Google Scholar] [CrossRef]
- Yang, T.B.; Kim, B.S. Pruritus in allergy and immunology. J. Allergy. Clin. Immunol. 2019, 144, 353–360. [Google Scholar] [CrossRef]
- Clarke, K.J. Myiasis (fly disease) and insectal disease generally are causing mental illness. Med. Hypotheses 2013, 81, 360–365. [Google Scholar] [CrossRef]
- Resende, A.B.L.; Monteiro, G.P.; Ramos, C.C.; Lopes, G.S.; Broekman, L.A.; De Souza, J.M. Integrating the autoimmune connective tissue diseases for the medical student: A classification proposal based on pathogenesis and clinical phenotype. Heliyon 2023, 9, e16935. [Google Scholar] [CrossRef]
- Yang, M.; Wu, H.; Zhao, M.; Chang, C.; Lu, Q. The pathogenesis of bullous skin diseases. J. Transl. Autoimmun. 2019, 2, 100014. [Google Scholar] [CrossRef]
- Bayart, C.; Brandling-Bennett, H.A. 92—Congenital and hereditary disorders of the skin. In Avery’s Diseases of the Newborn, 11th ed.; Gleason, C.A., Sawyer, T., Eds.; Elsevier: Philadelphia, PA, USA, 2024; pp. 1332–1346. [Google Scholar]
- Liu, B.; Czajka, A.; Malik, A.N.; Hussain, K.; Jones, P.M.; Persaud, S.J. Equilibrative nucleoside transporter 3 depletion in β-cells impairs mitochondrial function and promotes apoptosis: Relationship to pigmented hypertrichotic dermatosis with insulin-dependent diabetes. Biochim. Biophys. Acta (Bba)-Mol. Basis Dis. 2015, 1852, 2086–2095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aşkın, Ö.; Uzunçakmak, T.K.Ü.; Altunkalem, N.; Tüzün, Y. Vitamin deficiencies/hypervitaminosis and the skin. Clin. Dermatol. 2021, 39, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Manfredini, E. Rare xanthomatosis in hyperlipidemia case. J. Am. Coll. Cardiol. 2021, 77, 2956. [Google Scholar] [CrossRef]
- Ashique, S.; Afzal, O.; Hussain, A.; Zeyaullah, M.; Altamimi, M.A.; Mishra, N.; Ahmad, M.F.; Dua, K.; Altamimi, A.S.A.; Anand, K. It’s all about plant derived natural phytoconstituents and phytonanomedicine to control skin cancer. J. Drug Deliv. Sci. Technol. 2023, 84, 104495. [Google Scholar] [CrossRef]
- Chandra, J.; Hasan, N.; Nasir, N.; Wahab, S.; Thanikachalam, P.V.; Sahebkar, A.; Ahmad, F.J.; Kesharwani, P. Nanotechnology-empowered strategies in treatment of skin cancer. Environ. Res. 2023, 235, 116649. [Google Scholar] [CrossRef] [PubMed]
- Monshi, B.; Vujic, M.; Kivaranovic, D.; Sesti, A.; Oberaigner, W.; Vujic, I.; Ortiz-Urda, S.; Posch, C.; Feichtinger, H.; Hackl, M.; et al. The burden of malignant melanoma—Lessons to be learned from austria. Eur. J. Cancer. 2016, 56, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Homey, B.; Steinhoff, M.; Ruzicka, T.; Leung, D.Y.M. Cytokines and chemokines orchestrate atopic skin inflammation. J. Allergy. Clin. Immunol. 2006, 118, 178–189. [Google Scholar] [CrossRef]
- Leung, D.Y.M. Pathogenesis of atopic dermatitis. J. Allergy. Clin. Immunol. 1999, 104, S99–S108. [Google Scholar] [CrossRef]
- Werfel, T.; Allam, J.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.; Wollenberg, A.; et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J. Allergy. Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef]
- Chan, T.C.; Lee, M.; Huang, W.; Chang, W.; Krueger, J.G.; Tsai, T. Capsaicin attenuates imiquimod-induced epidermal hyperplasia and cutaneous inflammation in a murine model of psoriasis. Biomed. Pharmacother. 2021, 141, 111950. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schon, M.P. Animal models of psoriasis. Clin. Dermatol. 2007, 25, 596–605. [Google Scholar] [CrossRef]
- Baliwag, J.; Barnes, D.H.; Johnston, A. Cytokines in psoriasis. Cytokine 2015, 73, 342–350. [Google Scholar] [CrossRef]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef]
- Gollnick, H. Current concepts of the pathogenesis of acne: Implications for drug treatment. Drugs 2003, 63, 1579–1596. [Google Scholar] [CrossRef] [PubMed]
- Endres, L.; Tit, D.M.; Bungau, S.; Pascalau, N.A.; Todan, L.M.; Bimbo-Szuhai, E.; Iancu, G.M.; Negrut, N. Incidence and clinical implications of autoimmune thyroiditis in the development of acne in young patients. Diagnostics 2021, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.M.; Alexis, A.; Baldwin, H.; Bettoli, V.; Del, R.J.; Dirschka, T.; Dreno, B.; Gold, L.S.; Harper, J.; Ko, J.Y.; et al. The personalized acne treatment tool—Recommendations to facilitate a patient-centered approach to acne management from the personalizing acne: Consensus of experts. JAAD Int. 2023, 12, 60–69. [Google Scholar] [CrossRef]
- He, Y.T.; Hao, Y.Y.; Yu, R.X.; Zhang, C.; Chen, B.Z.; Cui, Y.; Guo, X.D. Hydroquinone cream-based polymer microneedle roller for the combined treatment of large-area chloasma. Eur. J. Pharm. Biopharm. 2023, 185, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Trinca, R.B.; Westin, C.B.; Da Silva, J.A.F.; Moraes, Â.M. Electrospun multilayer chitosan scaffolds as potential wound dressings for skin lesions. Eur. Polym. J. 2017, 88, 161–170. [Google Scholar] [CrossRef]
- Rossi, S.; Faccendini, A.; Bonferoni, M.C.; Ferrari, F.; Sandri, G.; Del Fante, C.; Perotti, C.; Caramella, C.M. “sponge-like” dressings based on biopolymers for the delivery of platelet lysate to skin chronic wounds. Int. J. Pharm. 2013, 440, 207–215. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Mori, M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Riva, F.; Tenci, M.; Del Fante, C.; Nicoletti, G.; Caramella, C. Sponge-like dressings based on the association of chitosan and sericin for the treatment of chronic skin ulcers. Ii. Loading of the hemoderivative platelet lysate. J. Pharm. Sci. 2016, 105, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Ozel, C.; Apaydin, E.; Sariboyaci, A.E.; Tamayol, A.; Avci, H. A multifunctional sateen woven dressings for treatment of skin injuries. Colloids Surf. B Biointerfaces 2023, 224, 113197. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, J.; Liu, X.; Wang, Y.; Ji, J.; He, Q. Dietary flavonoids intake and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Clin. Nutr. 2014, 33, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Oi, N.; Chen, H.; Ok, K.M.; Lubet, R.A.; Bode, A.M.; Dong, Z. Taxifolin suppresses uv-induced skin carcinogenesis by targeting egfr and pi3k. Cancer Prev. Res. 2012, 5, 1103–1114. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, M.S.; Kim, H.S.; Park, W.S.; Kim, S.Y.; Jang, J.J.; Rhim, K.J.; Jang, J.; Lee, H.K.; Park, J.Y.; et al. Somatic mutations of fas (apo-1/cd95) gene in cutaneous squamous cell carcinoma arising from a burn scar. J. Investig. Dermatol. 2000, 114, 122–126. [Google Scholar] [CrossRef][Green Version]
- Zhou, W.; Guo, Z. Taxifolin inhibits the scar cell carcinoma growth by inducing apoptosis, cell cycle arrest and suppression of pi3k/akt/mtor pathway. J. BUON 2019, 24, 853–858. [Google Scholar]
- Di, T.; Zhai, C.; Zhao, J.; Wang, Y.; Chen, Z.; Li, P. Taxifolin inhibits keratinocyte proliferation and ameliorates imiquimod-induced psoriasis-like mouse model via regulating cytoplasmic phospholipase a2 and ppar-γ pathway. Int. Immunopharmacol. 2021, 99, 107900. [Google Scholar] [CrossRef]
- Bito, T.; Roy, S.; Sen, C.K.; Shirakawa, T.; Gotoh, A.; Ueda, M.; Ichihashi, M.; Packer, L. Flavonoids differentially regulate ifnγ-induced icam-1 expression in human keratinocytes: Molecular mechanisms of action. FEBS Lett. 2002, 520, 145–152. [Google Scholar] [CrossRef]
- Yuan, X.; Li, N.; Zhang, M.; Lu, C.; Du, Z.; Zhu, W.; Wu, D. Taxifolin attenuates imq-induced murine psoriasis-like dermatitis by regulating t helper cell responses via notch1 and jak2/stat3 signal pathways. Biomed. Pharmacother. 2020, 123, 109747. [Google Scholar] [CrossRef]
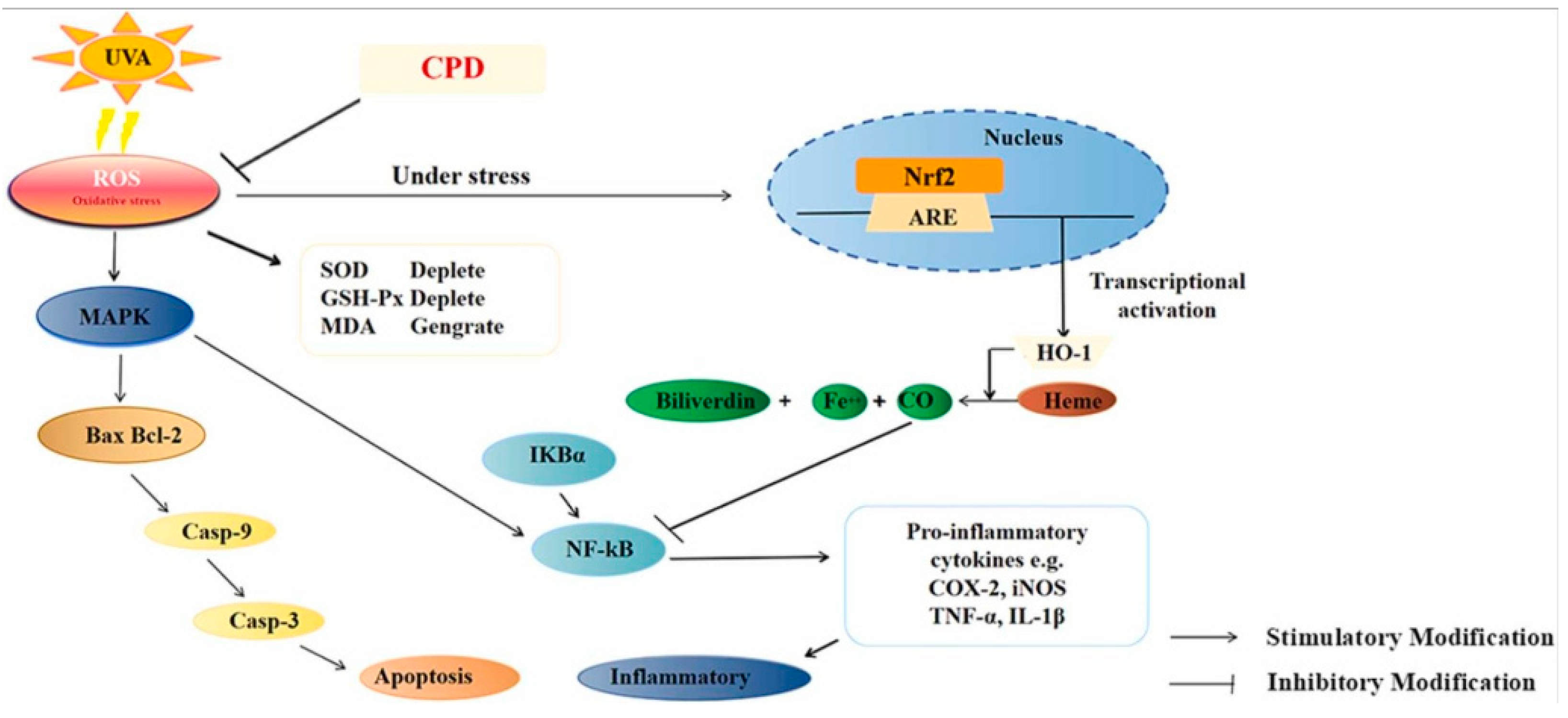
- Zhang, J.; Zheng, Y.; Hong, B.; Ma, L.; Zhao, Y.; Zhang, S.; Sun, S.; Ding, Q.; Wang, Y.; Liu, W.; et al. Dihydroquercetin composite nanofibrous membrane prevents uva radiation-mediated inflammation, apoptosis and oxidative stress by modulating mapks/nrf2 signaling in human epidermal keratinocytes. Biomed. Pharmacother. 2022, 155, 113727. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Eum, J.Y.; Park, S.H.; Kang, M.H.; Park, K.H.; Choi, S.E.; Lee, M.W.; Kang, K.H.; Oh, C.H.; Choi, Y.W. Pep-1 peptide-conjugated elastic liposomal formulation of taxifolin glycoside for the treatment of atopic dermatitis in nc/nga mice. Int. J. Pharm. 2010, 402, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Choi, S.E.; Jeong, M.S.; Park, K.H.; Moon, N.J.; Joo, S.S.; Lee, C.S.; Choi, Y.W.; Li, K.; Lee, M.K.; et al. Effect of taxifolin glycoside on atopic dermatitis-like skin lesions in nc/nga mice. Phytother. Res. 2010, 24, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Kotland, A.; Henes, B.; Poigny, S.; Wandrey, F. Deciphering the phytochemical profile of an alpine rose (Rhododendron ferrugineum L.) Leaf extract for a better understanding of its senolytic and skin-rejuvenation effects. Cosmetics 2022, 9, 37. [Google Scholar] [CrossRef]
- Irmanida Batubara, H.K.A.T. Anti-acne and tyrosinase inhibition properties of taxifolin and some flavanonol rhamnosides from kempas (koompassia malaccensis). Wood Res. J. 2010, 1, 45–49. [Google Scholar]
- Zhang, J.; Chen, K.; Ding, C.; Sun, S.; Zheng, Y.; Ding, Q.; Hong, B.; Liu, W. Fabrication of chitosan/pvp/dihydroquercetin nanocomposite film for in vitro and in vivo evaluation of wound healing. Int. J. Biol. Macromol. 2022, 206, 591–604. [Google Scholar] [CrossRef]
- Smith, K.; Collier, A.; Townsend, E.M.; O’Donnell, L.E.; Bal, A.M.; Butcher, J.; Mackay, W.G.; Ramage, G.; Williams, C. One step closer to understanding the role of bacteria in diabetic foot ulcers: Characterising the microbiome of ulcers. BMC Microbiol. 2016, 16, 54. [Google Scholar] [CrossRef]
- Shi, W.; Wu, Y.; Bian, D. P75ntr silencing inhibits proliferation, migration, and extracellular matrix deposition of hypertrophic scar fibroblasts by activating autophagy through inhibiting the pi3k/akt/mtor pathway. Can. J. Physiol. Pharmacol. 2021, 99, 349–359. [Google Scholar] [CrossRef]
- Shubina, V.S.; Shatalin, I. [Effect of the liposomal form of taxifolin complexes with metals of variable valence on skin regeneration after chemical burn]. Tsitologiia 2012, 54, 251–260. [Google Scholar]
- Shubina, V.S.; Shatalin, Y.V. Skin regeneration after chemical burn under the effect of taxifolin-based preparations. Bull. Exp. Biol. Med. 2012, 154, 152–157. [Google Scholar] [CrossRef]
- Moon, S.H.; Lee, C.M.; Nam, M.J. Cytoprotective effects of taxifolin against cadmium-induced apoptosis in human keratinocytes. Hum. Exp. Toxicol. 2019, 38, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhou, M.; Fu, H. Study on mussel-inspired tough ta/pani@cncs nanocomposite hydrogels with superior self-healing and self-adhesive properties for strain sensors. Compos. Part B Eng. 2020, 201, 108356. [Google Scholar] [CrossRef]
- Shevelev, A.B.; La Porta, N.; Isakova, E.P.; Martens, S.; Biryukova, Y.K.; Belous, A.S.; Sivokhin, D.A.; Trubnikova, E.; Zylkova, M.; Belyakova, A.; et al. In vivo antimicrobial and wound-healing activity of resveratrol, dihydroquercetin, and dihydromyricetin against staphylococcus aureus, pseudomonas aeruginosa, and candida albicans. Pathogens 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Terekhov, R.P.; Selivanova, I.A.; Anurova, M.N.; Zhevlakova, A.K.; Nikitin, I.D.; Cong, Z.; Ma, S.; Yang, F.; Dong, Z.; Liao, Y. Comparative study of wound-healing activity of dihydroquercetin pseudopolymorphic modifications. Bull. Exp. Biol. Med. 2021, 170, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Sajadi-Javan, Z.S.; Varshosaz, J.; Mirian, M.; Manshaei, M.; Aminzadeh, A. Thermo-responsive hydrogels based on methylcellulose / persian gum loaded with taxifolin enhance bone regeneration: An in vitro/in vivo study. Cellulose 2022, 29, 2413–2433. [Google Scholar] [CrossRef]
- Camila Araujo Scharf Pinto, M.F.Z.D. The use of pycnogenol in the treatment of melasma. Surgical and Cosmetic Dermatology 2015, 3, 218–222. [Google Scholar] [CrossRef]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sanchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural product-based nanomedicines for wound healing purposes: Therapeutic targets and drug delivery systems. Int. J. Nanomed. 2018, 13, 5023–5043. [Google Scholar] [CrossRef]
- Ding, Q.; Ding, C.; Liu, X.; Zheng, Y.; Zhao, Y.; Zhang, S.; Sun, S.; Peng, Z.; Liu, W. Preparation of nanocomposite membranes loaded with taxifolin liposome and its mechanism of wound healing in diabetic mice. Int. J. Biol. Macromol. 2023, 241, 124537. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Valachova, K.; Omer, A.M.; El-Shafeey, M.; Mohy, E.M.; Soltes, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef]
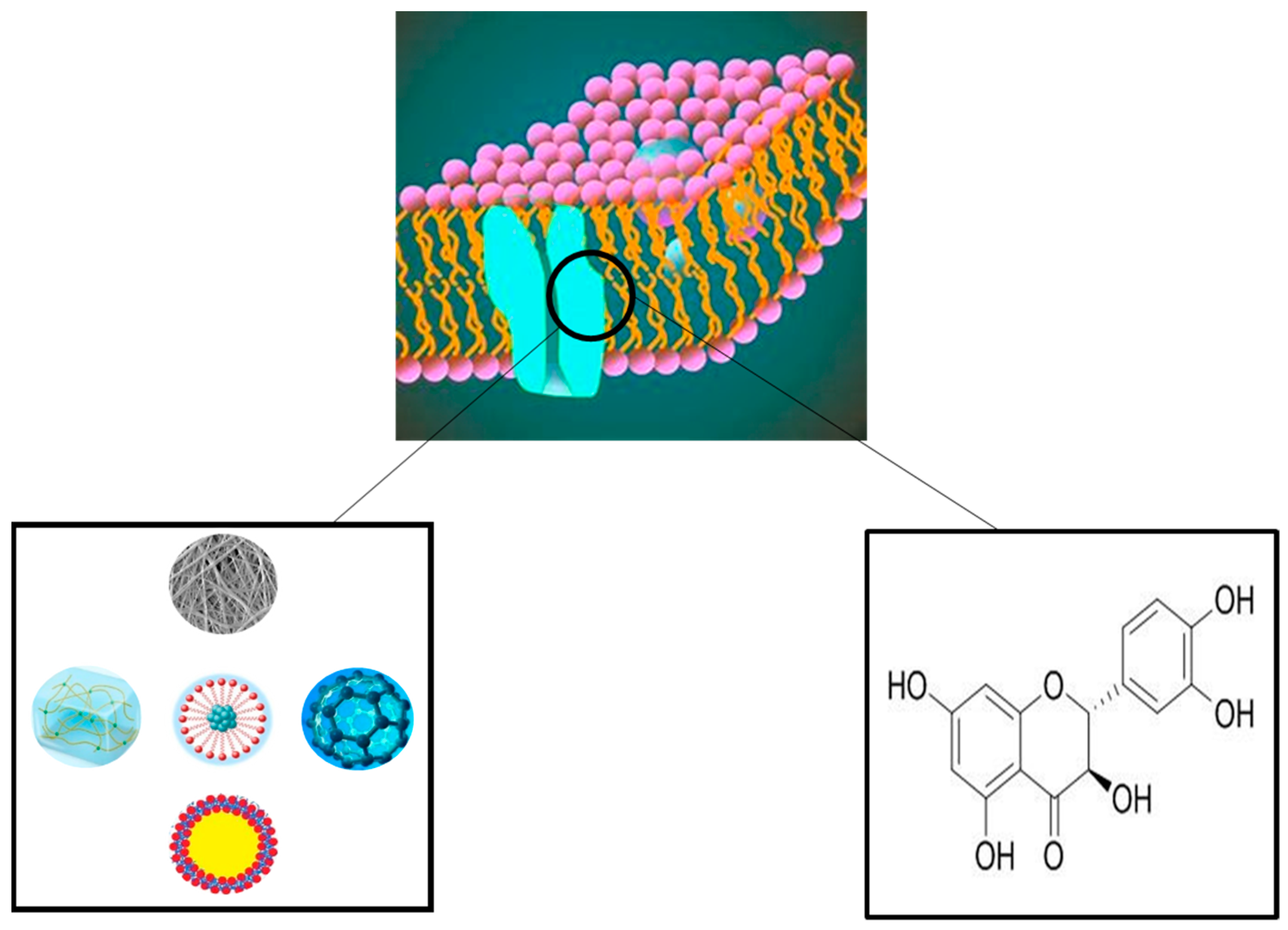
| Serial Number | Skin Diseases | Represents Disease | References |
|---|---|---|---|
| 1 | Skin cancer | Basal cell carcinoma of the skin, squamous cell carcinoma, malignant melanoma, nodule | [7,8,9] |
| 2 | Inflammatory dermatosis | Psoriasis, atopic dermatitis, autoimmune vesicular disease, and alopecia areata | [10,11] |
| 3 | Erythematopapular scaling dermatosis | Psoriasis, subcorneal pustular skin disease, pityriasis rosea, seborrheic dermatitis, and nummular dermatitis | [12] |
| 4 | Skin and accessory organ diseases | Acne, rosacea, skin ulcers, etc | [13,14,15] |
| 5 | Pigment disorder is a skin disease | Chloasma, vitiligo, pigmented nevus, freckle nevus, etc. | [16,17,18,19] |
| 6 | Viral skin diseases | Herpes simplex, herpes zoster, warts, molluscum contagiosum, hand, foot, and mouth disease | [20] |
| 7 | Bacterial dermatosis | Impetigo, folliculitis, erysipelas, and leprosy | [21,22] |
| 8 | Fungal dermatosis | Tinea corporis, tinea pedis, onychomycosis, pityriasis versicolor, malassezia folliculitis, etc | [23] |
| 9 | sexually transmitted disease | Syphilis, gonorrhea, and condyloma acuminatum | [24,25] |
| 10 | allergic skin diseases | Contact dermatitis, eczema, urticaria, drug reaction | [26,27] |
| 11 | Neurofunctional dermatosis | Prurigo nodosa, neurodermatitis, and parasitic paranoia | [28,29] |
| 12 | Connective tissue diseases | Lupus erythematosus, scleroderma, and dermatomyositis | [30] |
| 13 | Bullous skin diseases | Pemphigus, bullous pemphigoid, cicatricial pemphigoid, herpetic dermatitis, linear IgA bullous dermatosis, acquired epidermolysis bullosa, pemphigoid pregnancy | [31] |
| 14 | Genodermatoses | Ichthyosis vulgaris, keratosis folliculi, albinism, hirsutism, epidermolysis bullosa, tinea versicolor | [32,33] |
| 15 | Nutrition and disordered metabolic dermatosis | Vitamin deficiency | [34,35] |
| Biological Activity | Experimental Model | Mechanism |
|---|---|---|
| Anti-inflammatory | C57BL/6 mice | Regulating the TLR4/NF-κB axis to prevent inflammation and apoptosis. |
| RAW 264.7 cells | Regulates the expression of iNOS, VEGF, COX-2, and TNF-α and affects the MAPK signaling pathway. | |
| SD rat | Inhibition of microglial pyroptosis via the PI3K/Akt signaling pathway | |
| DMM rats and chondrocytes | Activating the Nrf2 pathway inhibits inflammation, alleviates apoptosis, and reduces ECM degradation to reshape the articular cartilage microenvironment. | |
| BV2 cell line | Upregulates pAMPK levels and activates the Nrf2/HO-1 signaling pathway | |
| Antibacterial | E. coli and Staphylococcus aureus | Destroy the integrity of bacterial cell walls and membranes, inhibit bacterial biofilm formation, cause stress, and lead to increased superoxide dismutase and alkaline phosphatase activities in bacteria |
| Antioxidants | Westal rat | Reduce oxidative stress and reduce pro-inflammatory cytokine levels |
| glutamatergic neurons | Inhibition of basal and OGD-induced mitochondrial ROS production in GABAergic neurons. | |
| Antiviral | HAV stock: CF 53 | Reduce the infectivity and antigenicity of HAV |
| Dressing Type | Experimental Model | Mechanism |
|---|---|---|
| Nanofiber membrane | Diabetic mice | It Inhibits the activation of κBα (IκBα)/nuclear factor κB (NF-κB) signaling pathway and increases the expression of CD and VEGF in skin tissue |
| Human skin keratinocytes | Prevent oxidative stress, inflammation, and apoptosis induced by UVA radiation-induced MAPK/Nrf2 signaling pathway | |
| Mice | The PI3K/AKT/MR signaling pathway is significantly inhibited to activate autophagy and promote skin repair. | |
| Female Wistar rats | Good antioxidant, antibacterial, and anti-inflammatory activity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Qiu, D.; Yang, T.; Su, J.; Liu, C.; Su, X.; Li, A.; Sun, P.; Li, J.; Yan, L.; et al. Research Progress of Dihydroquercetin in the Treatment of Skin Diseases. Molecules 2023, 28, 6989. https://doi.org/10.3390/molecules28196989
Liu Z, Qiu D, Yang T, Su J, Liu C, Su X, Li A, Sun P, Li J, Yan L, et al. Research Progress of Dihydroquercetin in the Treatment of Skin Diseases. Molecules. 2023; 28(19):6989. https://doi.org/10.3390/molecules28196989
Chicago/Turabian StyleLiu, Ziyang, Dengjun Qiu, Tong Yang, Jingxu Su, Chengyuan Liu, Xinyue Su, Anning Li, Pingping Sun, Jianguo Li, Li Yan, and et al. 2023. "Research Progress of Dihydroquercetin in the Treatment of Skin Diseases" Molecules 28, no. 19: 6989. https://doi.org/10.3390/molecules28196989
APA StyleLiu, Z., Qiu, D., Yang, T., Su, J., Liu, C., Su, X., Li, A., Sun, P., Li, J., Yan, L., Ding, C., & Zhang, S. (2023). Research Progress of Dihydroquercetin in the Treatment of Skin Diseases. Molecules, 28(19), 6989. https://doi.org/10.3390/molecules28196989








