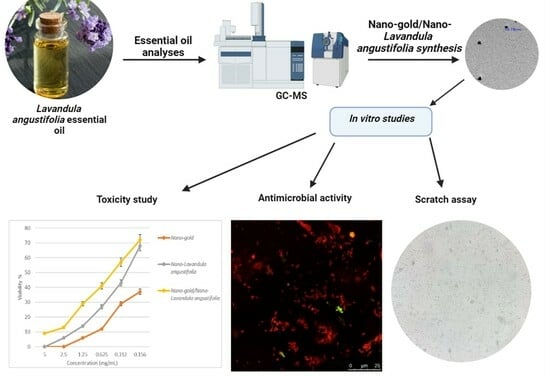
Nanoemulsion of Lavandula angustifolia Essential Oil/Gold Nanoparticles: Antibacterial Effect against Multidrug-Resistant Wound-Causing Bacteria
Abstract
:1. Introduction
2. Results and Discussions
2.1. Antibacterial Activity of Lavandula angustifolia Essential Oil
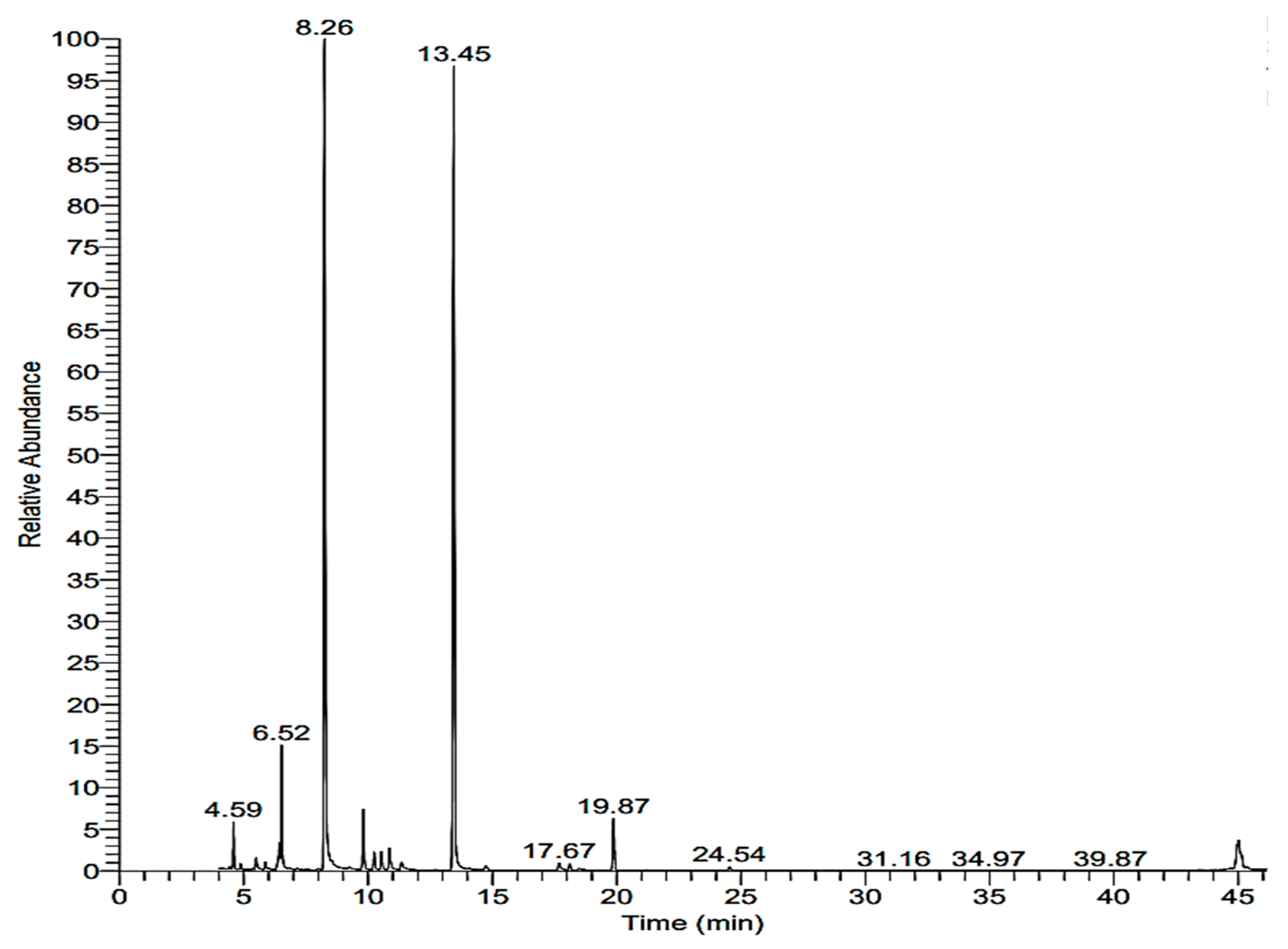
2.2. Chemical Analysis of Lavandula angustifolia
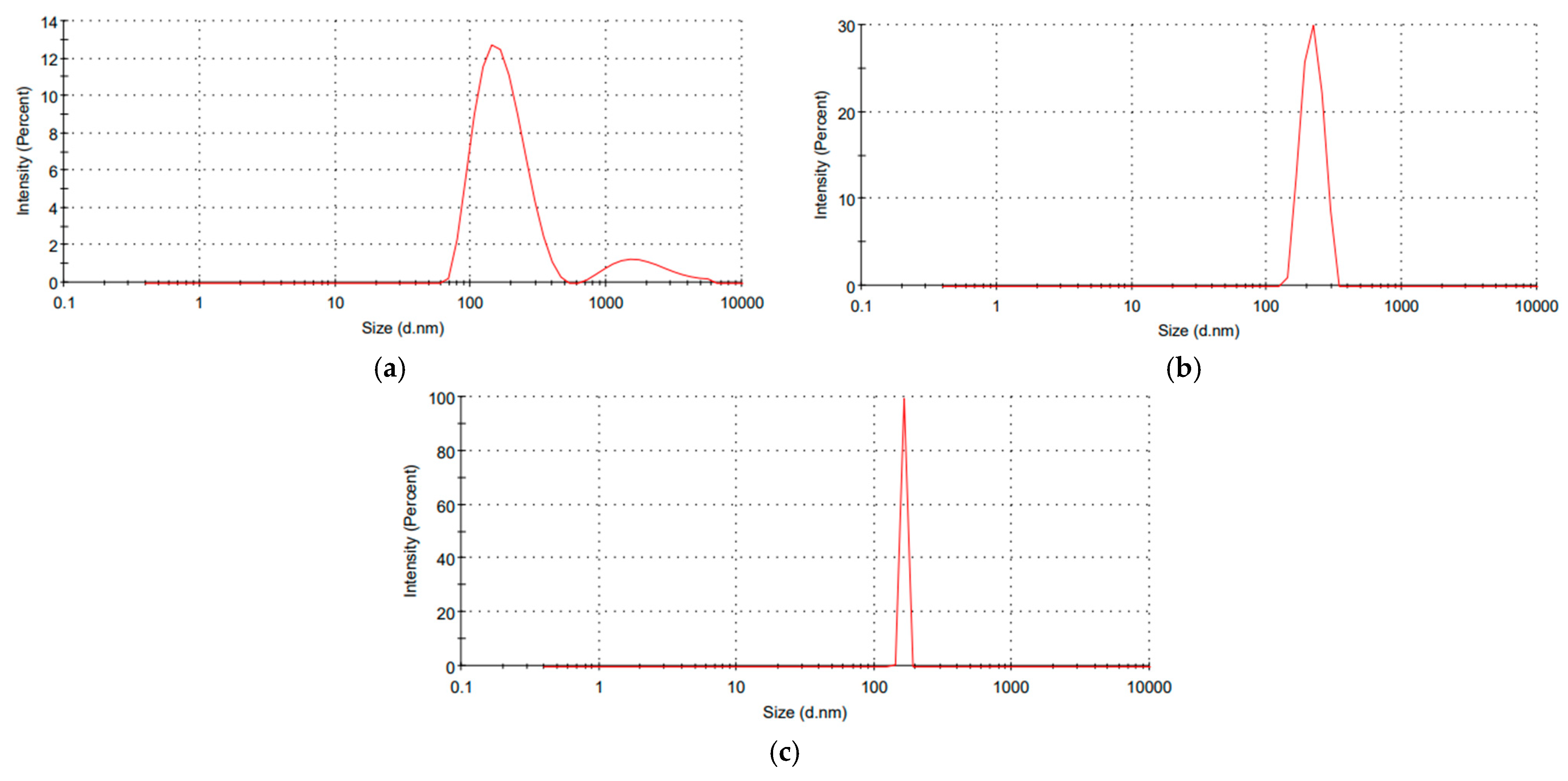
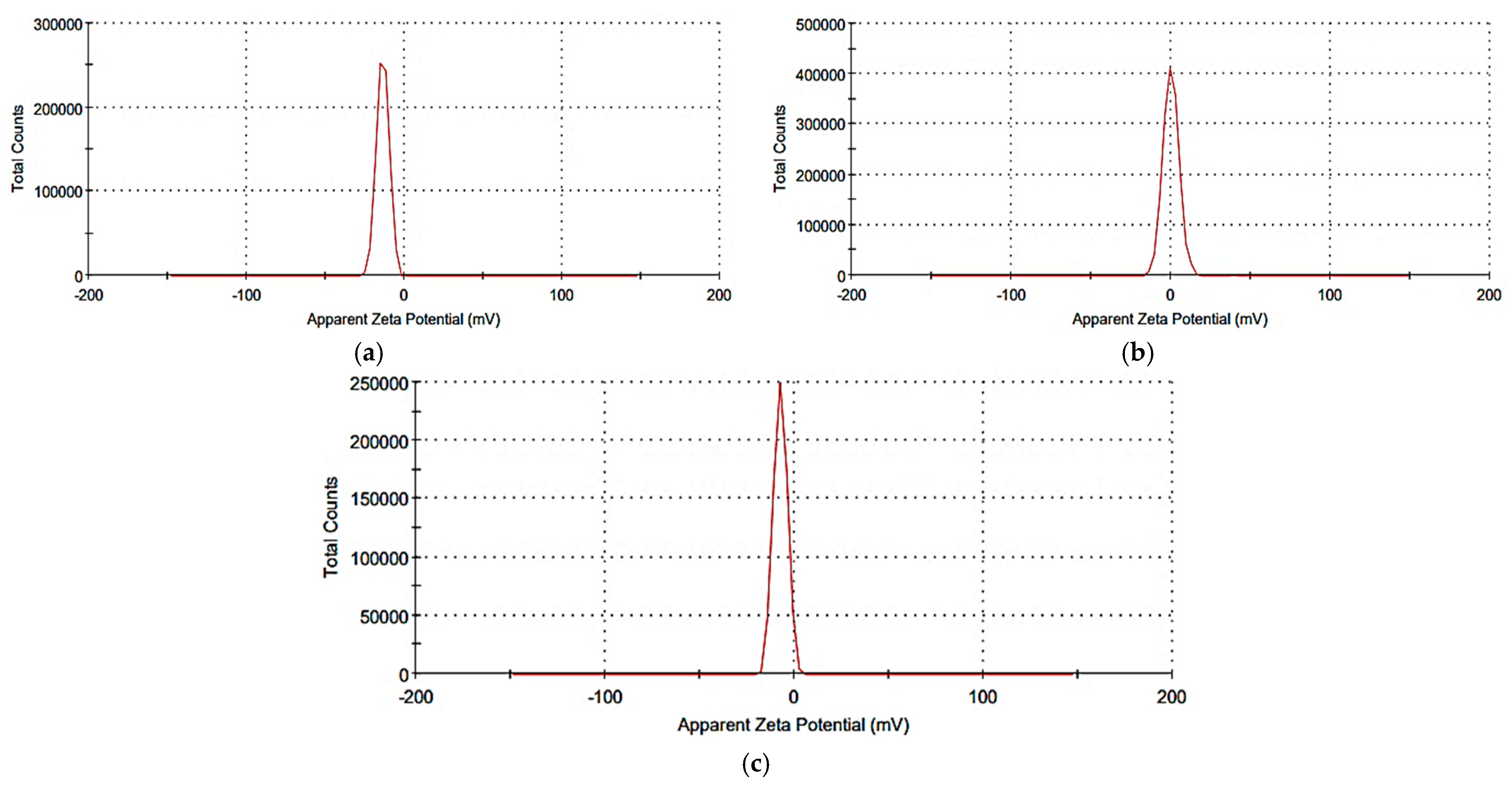
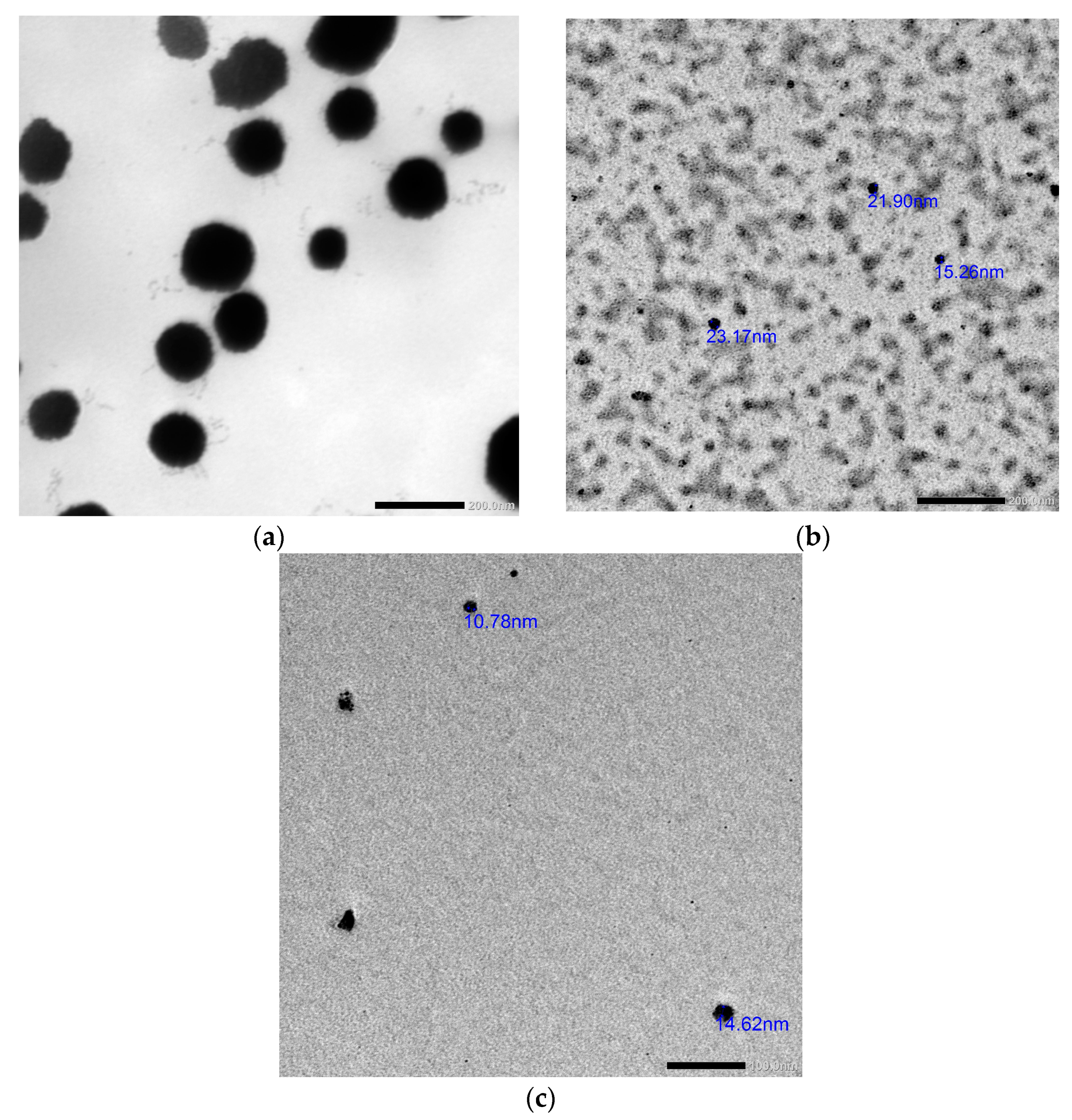
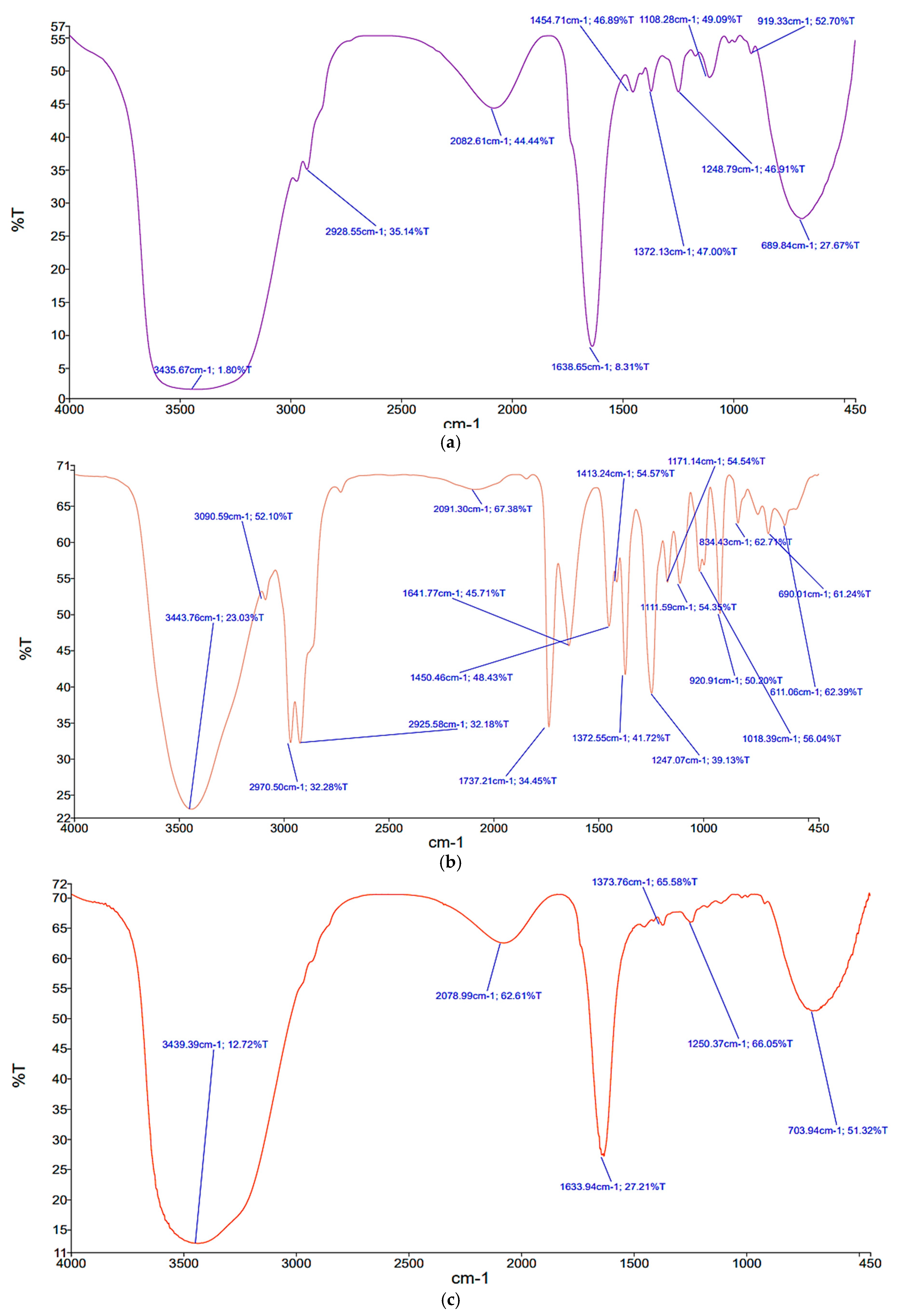
2.3. Synthesis and Characterization of the Prepared Nanosystems
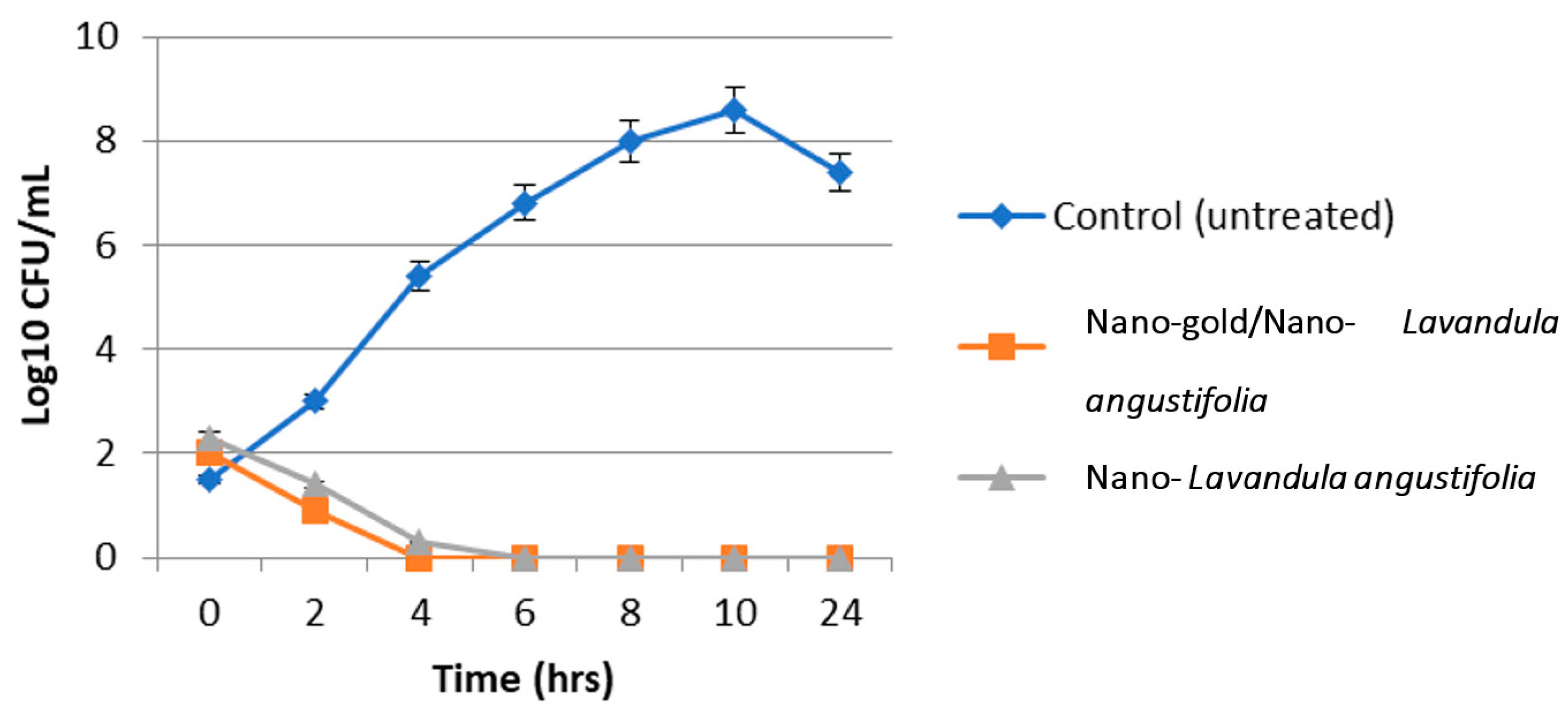
2.4. Antibacterial and Antibiofilm Activity of the Synthesized Nanosystems
2.5. Cytotoxic Effect of Nano-Gold/Nano-Lavandula angustifolia
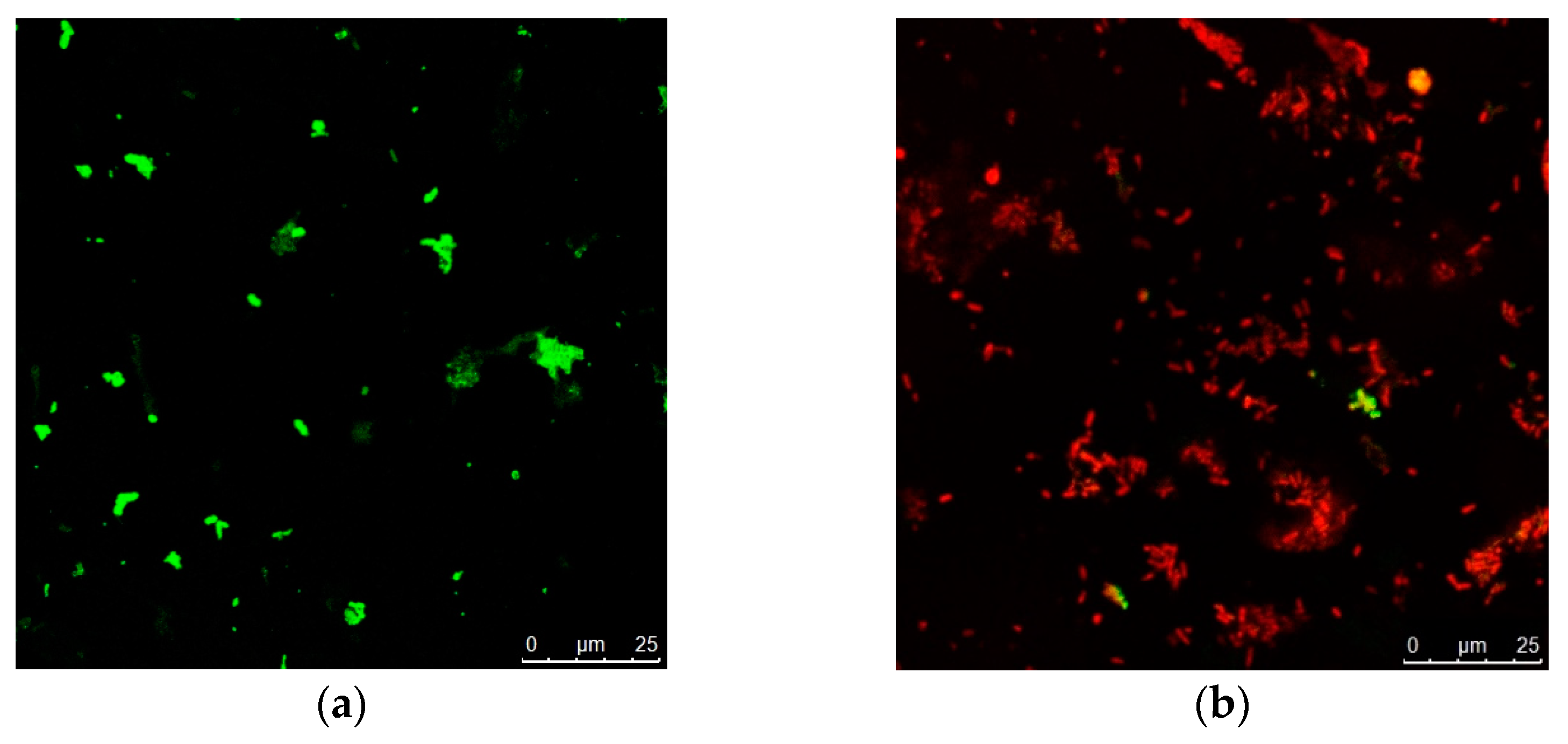
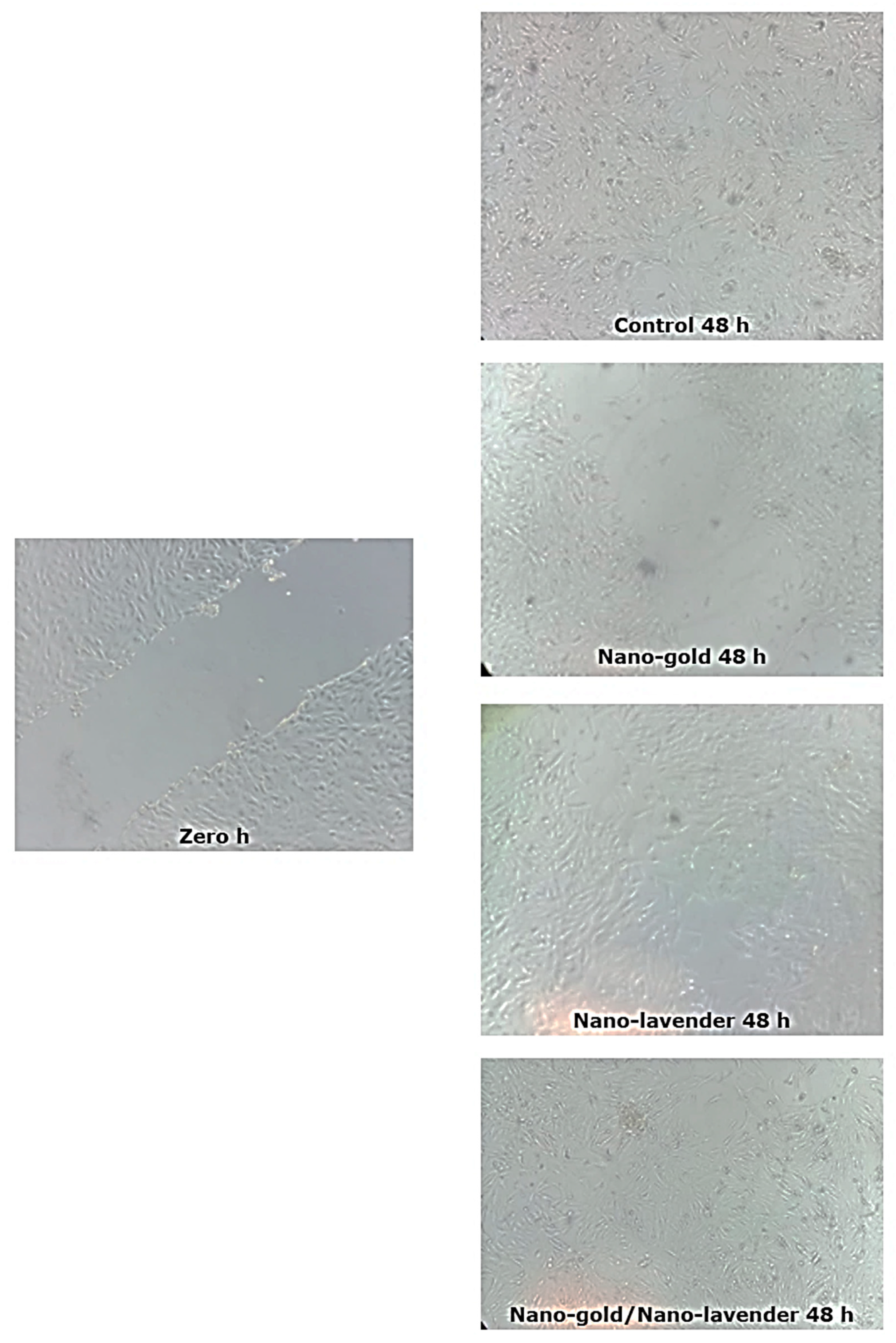
2.6. In Vitro Wound Healing Activity of Nano-Gold/Nano-Lavandula angustifolia
3. Materials and Methods
3.1. Chemicals
3.2. Microorganisms
3.3. Antibacterial Activity of the Tested Essential Oil (EO)
3.4. Chemical Characterization of Lavandula angustifolia Essential Oil
3.5. Essential Oil Nanoemulsion Formation
3.6. Synthesis of Gold Nanoparticles
3.7. Synthesis of Gold–EO Conjugated Nanoparticles
3.8. Characterization of the Prepared Nanosystems
3.9. Antibacterial and Antibiofilm Activity of the Prepared Nanosystems
3.10. Cytotoxic Effect
- AT = Mean absorbances of cells treated with different concentrations of each plant extract;
- AC = Mean absorbances of control untreated cells with culture medium only;
- Ab = Mean absorbances of cells treated with vehicle of plant extract (RPMI media without fetal bovine serum).
3.11. In Vitro Scratch Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound Microbiology and Associated Approaches to Wound Management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Who Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://tinyurl.com/kmva5da (accessed on 26 May 2018).
- Vyas, K.S.; Vasconez, H.C. Wound Healing: Biologics, Skin Substitutes, Biomembranes and Scaffolds. Healthcare 2014, 2, 356–400. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Alabdallah, N.M.; Al Jomaa, A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ. Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Moeini, A.; Masi, M.; Zonno, M.C.; Boari, A.; Cimmino, A.; Tarallo, O.; Vurro, M.; Evidente, A. Encapsulation of inuloxin A, a plant germacrane sesquiterpene with potential herbicidal activity, in β-cyclodextrins. Org. Biomol. Chem. 2019, 17, 2508–2515. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Bourhia, M.; Slighoua, M.; Ibnemoussa, S.; Bari, A.; Ullah, R.; Amaghnouje, A.; Di Cristo, F.; El Mzibri, M.; Calarco, A.; et al. Phytochemical Study on Antioxidant and Antiproliferative Activities of Moroccan Caralluma europaea Extract and Its Bioactive Compound Classes. Evid.-Based Complement. Altern. Med. 2020, 2020, 8409718. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Kusumawati, I.; Indrayanto, G. Natural antioxidants in cosmetics. Stud. Nat. Prod. Chem. 2013, 40, 485–505. [Google Scholar]
- Amaghnouje, A.; Mechchate, H.; Es-Safi, I.; Alotaibi, A.A.; Noman, O.M.; Nasr, F.A.; Fahd, A.; Al-Zharani, M.; Cerruti, P.; Calarco, A.; et al. Anxiolytic, antidepressant-like proprieties and impact on the memory of the hydro-ethanolic extract of Origanum majorana L. On mice. Appl. Sci. 2020, 10, 8420. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Mechchate, H.; Es-Safi, I.; Boukhira, S.S.; Aliqahtani, A.M.; Noman, O.; Nasr, A.; Conte, R.; Calarco, A.; Bousta, D. Subacute assessment of the toxicity and antidepressant-like effects of Origanum majorana L. polyphenols in Swiss albino mice. Molecules 2020, 25, 5653. [Google Scholar] [CrossRef]
- Marturano, V.; Bizzarro, V.; Ambrogi, V.; Cutignano, A.; Tommonaro, G.; Abbamondi, G.R.; Giamberini, M.; Tylkowski, B.; Carfagna, C.; Cerruti, P. Light-Responsive Nanocapsule-Coated Polymer Films for Antimicrobial Active Packaging. Polymers 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Cuttle, L.; Pearn, J.; McMillan, J.R.; Kimble, R.M. A review of first aid treatments for burn injuries. Burns 2009, 35, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Ovidi, E.; Masci, V.L.; Taddei, A.R.; Paolicelli, P.; Petralito, S.; Trilli, J.; Mastrogiovanni, F.; Tiezzi, A.; Casadei, M.A.; Giacomello, P.; et al. Chemical Investigation and Screening of Anti-Proliferative Activity on Human Cell Lines of Pure and Nano-Formulated Lavandin Essential Oil. Pharmaceuticals 2020, 13, 352. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro-and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef]
- Jianu, C.; Pop, G.; Gruia, A.T.; Horhat, F.G. Chemical composition and antimicrobial activity of essential oils of lavender (Lavandula angustifolia) and lavandin (Lavandula x intermedia) grown in Western Romania. Int. J. Agric. Biol. 2013, 15, 772–776. [Google Scholar]
- Ciocarlan, A.; Lupascu, L.; Aricu, A.; Dragalin, I.; Popescu, V.; Geana, E.-I.; Ionete, R.E.; Vornicu, N.; Duliu, O.G.; Hristozova, G.; et al. Chemical Composition and Assessment of Antimicrobial Activity of Lavender Essential Oil and Some By-Products. Plants 2021, 10, 1829. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Hui, L.; He, L.; Huan, L.; XiaoLan, L.; AiGuo, Z. Chemical composition of lavender essential oil and its antioxidant activity and inhibition against rhinitis-related bacteria. Afr. J. Microbiol. Res. 2010, 4, 309–313. [Google Scholar]
- Bhat, R.; Sharanabasava, V.; Deshpande, R.; Shetti, U.; Sanjeev, G.; Venkataraman, A. Photo-bio-synthesis of irregular shaped functionalized gold nanoparticles using edible mushroom Pleurotus florida and its anticancer evaluation. J. Photochem. Photobiol. B: Biol. 2013, 125, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, L.; Arunachalam, J. Microwave-Assisted Green Synthesis of Small Gold Nanoparticles Using Aqueous Garlic (Allium sativum) Extract: Their Application as Antibiotic Carriers. Int. J. Green Nanotechnol. 2012, 4, 163–173. [Google Scholar] [CrossRef]
- Chong, W.-T.; Tan, C.-P.; Cheah, Y.-K.; Lajis, A.F.B.; Dian, N.L.H.M.; Kanagaratnam, S.; Lai, O.-M. Optimization of process parameters in preparation of tocotrienol-rich red palm oil-based nanoemulsion stabilized by Tween80-Span 80 using response surface methodology. PLoS ONE 2018, 13, e0202771. [Google Scholar] [CrossRef]
- Pey, C.M.; Maestro, A.; Solé, I.; González, C.; Solans, C.; Gutierrez, J.M. Optimization of nano-emulsions prepared by low-energy emulsification methods at constant temperature using a factorial design study. Colloids Surf. Physicochem. Eng. Asp. 2006, 288, 144–150. [Google Scholar] [CrossRef]
- Scamehorn, J.F.; Comstock, M.J.; Osborne-Lee, I.W.; Schechter, R.S.; Kamrath, R.F.; Franses, E.I.; Meguro, K.; Muto, Y.; Sakurai, F.; Esumi, K.; et al. Phenomena in Mixed Surfactant Systems. Anal. Chem. 1986, 58, 1250A. [Google Scholar] [CrossRef]
- Bergström, M.; Eriksson, J.C. A Theoretical Analysis of Synergistic Effects in Mixed Surfactant Systems. Langmuir 2000, 16, 7173–7181. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, M.; Xu, J.; Wang, Q.; Fan, Z. Stabilization of water-in-octane nano-emulsion. Part I: Stabilized by mixed surfactant systems. Fuel 2010, 89, 2838–2843. [Google Scholar] [CrossRef]
- Izquierdo, P.; Esquena, J.; Tadros, T.F.; Dederen, J.C.; Feng, J.; Garcia-Celma, M.J.; Azemar, N.; Solans, C. Phase Behavior and Nano-emulsion Formation by the Phase Inversion Temperature Method. Langmuir 2004, 20, 6594–6598. [Google Scholar] [CrossRef]
- Morales, D.; Gutie´rrez, J.M.; Garcı´a-Celma, M.J.; Solans, Y.C. A Study of the Relation between Bicontinuous Microemulsions and Oil/Water Nano-emulsion Formation. Langmuir 2003, 19, 7196–7200. [Google Scholar] [CrossRef]
- Liu, W.; Sun, D.; Li, C.; Liu, Q.; Xu, J. Formation and stability of paraffin oil-in-water nano-emulsions prepared by the emulsion inversion point method. J. Colloid Interface Sci. 2006, 303, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Marinova, K.G.; Alargova, R.G.; Denkov, N.D.; Velev, O.D.; Petsev, D.N.; Ivanov, I.B.; Borwankar, R.P. Charging of Oil−Water Interfaces Due to Spontaneous Adsorption of Hydroxyl Ions. Langmuir 1996, 12, 2045–2051. [Google Scholar] [CrossRef]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for Food, Pharmaceutical and Cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Mandal, A.; Chakravorty, D.; Gopal, M.; Goswami, A. Evaluation of physicochemical properties, and antimicrobial efficacy of monoclinic sulfur-nanocolloid. J. Nanoparticle Res. 2013, 15, 1491. [Google Scholar] [CrossRef]
- Samfira, S.I.; Rodino, P.; Petrache, R.; Cristina, M.; Butu, M. Butnariu, Characterization and identity confirmation of essential oils by mid infrared absorption spectrophotometry. Dig. J. Nanomater. Biostructures 2015, 10, 557–566. [Google Scholar]
- Cossetin, L.F.; Garlet, Q.I.; Velho, M.C.; Gündel, S.; Ourique, A.F.; Heinzmann, B.M.; Monteiro, S.G. Development of nanoemulsions containing Lavandula dentata or Myristica fragrans essential oils: Influence of temperature and storage period on physical-chemical properties and chemical stability. Ind. Crops Prod. 2021, 160, 113115. [Google Scholar] [CrossRef]
- Palanisamy, N.K.; Ferina, N.; Amirulhusni, A.N.; Mohd-Zain, Z.; Hussaini, J.; Ping, L.J.; Durairaj, R. Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 1–7. [Google Scholar] [CrossRef]
- Warisnoicharoen, W.; Lansley, A.; Lawrence, M. Nonionic oil-in-water microemulsions: The effect of oil type on phase behaviour. Int. J. Pharm. 2000, 198, 7–27. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J. Nanobiotechnol. 2012, 10, 1–9. [Google Scholar] [CrossRef]
- Eskandari-Nojehdehi, M.; Jafarizadeh-Malmiri, H.; Rahbar-Shahrouzi, J. Optimization of processing parameters in green synthesis of gold nanoparticles using microwave and edible mushroom (Agaricus bisporus) extract and evaluation of their antibacterial activity. Nanotechnol. Rev. 2016, 5, 537–548. [Google Scholar] [CrossRef]
- Chianese, A.; Gravina, C.; Morone, M.V.; Ambrosino, A.; Formato, M.; Palma, F.; Foglia, F.; Nastri, B.M.; Zannella, C.; Esposito, A.; et al. Lavandula austroapennina: Assessment of the Antiviral Activity of Lipophilic Extracts from Its Organs. Viruses 2023, 15, 1648. [Google Scholar] [CrossRef] [PubMed]
- Bergen, J.M.; von Recum, H.A.; Goodman, T.T.; Massey, A.P.; Pun, S.H. Gold Nanoparticles as a Versatile Platform for Optimizing Physicochemical Parameters for Targeted Drug Delivery. Macromol. Biosci. 2006, 6, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Vairavel, M.; Devaraj, E.; Shanmugam, R. An eco-friendly synthesis of Enterococcus sp.–mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells. Environ. Sci. Pollut. Res. 2020, 27, 8166–8175. [Google Scholar] [CrossRef] [PubMed]
- Vilela, J.d.M.V.; Moghassemi, S.; Dadashzadeh, A.; Dolmans, M.-M.; Azevedo, R.B.; Amorim, C.A. Safety of Lavender Oil-Loaded Niosomes for In Vitro Culture and Biomedical Applications. Nanomaterials 2022, 12, 1999. [Google Scholar] [CrossRef]
- Bratovcic, A. Green Synthesis of Various Nanostructures Containing Essential Oil and Silver Nanoparticles: Nanocomposites, Nanoemulsions and Nanoencapsules. Int. J. Eng. Res. Appl. 2023, 13, 234–242. [Google Scholar]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Lyoussi, B.; Oumokhtar, B.; Abdellaoui, A. Phytochemistry, antioxidant and antibacterial activities of two Moroccan Teucrium polium L. subspecies: Preventive approach against nosocomial infections. Arab. J. Chem. 2020, 13, 3866–3874. [Google Scholar] [CrossRef]
- El Abdali, Y.; Beniaich, G.; Mahraz, A.M.; El Moussaoui, A.; Bin Jardan, Y.A.; Akhazzane, M.; Chebaibi, M.; Nafidi, H.-A.; Eloutassi, N.; Bourhia, M.; et al. Antibacterial, Antioxidant, and in silico NADPH Oxidase Inhibition Studies of Essential Oils of Lavandula dentata against Foodborne Pathogens. Evid.-Based Complement. Altern. Med. 2023, 2023, 9766002. [Google Scholar] [CrossRef]
- Elnahas, R.A.; Elwakil, B.H.; Elshewemi, S.S.; Olama, Z.A. Egyptian Olea europaea leaves bioactive extract: Antibacterial and wound healing activity in normal and diabetic rats. J. Tradit. Complement. Med. 2021, 11, 427–434. [Google Scholar] [CrossRef]
- Shahavi, M.H.; Hosseini, M.; Jahanshahi, M.; Meyer, R.L.; Darzi, G.N. Evaluation of critical parameters for preparation of stable clove oil nanoemulsion. Arab. J. Chem. 2019, 12, 3225–3230. [Google Scholar] [CrossRef]
- Hosseinzadeh, N.; Shomali, T.; Hosseinzadeh, S.; Raouf Fard, F.; Pourmontaseri, M.; Fazeli, M. Green synthesis of gold nanoparticles by using Ferula persica Willd. gum essential oil: Production, characterization and in vitro anti-cancer effects. J. Pharm. Pharmacol. 2020, 72, 1013–1025. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Berent, S.; Motyka, A.; Jamroz, P.; Kurcbach, K.; Sledz, W.; Pohl, P. Comparison of the characteristics of gold nanoparticles synthesized using aqueous plant extracts and natural plant essential oils of Eucalyptus globulus and Rosmarinus officinalis. Arab. J. Chem. 2019, 12, 4795–4805. [Google Scholar] [CrossRef]
- Dorgham, R.A.; Abd Al Moaty, M.N.; Chong, K.P.; Elwakil, B.H. Molasses-Silver Nanoparticles: Synthesis, Optimization, Characterization, and Antibiofilm Activity. Int. J. Mol. Sci. 2022, 23, 10243. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.; Elwakil, B.H.; Elshewemi, S.S.; El-Naggar, M.Y.; Bekhit, A.A.; Olama, Z.A. Novel Siwa propolis and colistin-integrated chitosan nanoparticles: Elaboration; in vitro and in vivo appraisal. Nanomedicine 2020, 15, 1269–1284. [Google Scholar] [CrossRef]
- El-Attar, A.A.; El-Wakil, H.B.; Hassanin, A.H.; Bakr, B.A.; Almutairi, T.M.; Hagar, M.; Elwakil, B.H.; Olama, Z.A. Silver/Snail Mucous PVA Nanofibers: Electrospun Synthesis and Antibacterial and Wound Healing Activities. Membranes 2022, 12, 536. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; Belardinelli, E.; Fabiano, A.; Santoni, T.; Di Stefano, R. Effect of different chitosan derivatives on in vitro scratch wound assay: A comparative study. Int. J. Biol. Macromol. 2015, 76, 236–241. [Google Scholar] [CrossRef]

| Tested Pathogens | Lavandula angustifolia EO | ||
|---|---|---|---|
| IZ (mm) | MIC (µg/mL) | MBC (µg/mL) | |
| P. mirabilis | 16.0 ± 1.0 | 64.0 | 256.0 |
| K. pneumoniae | 20.0 ± 2.0 | 32.0 | 128.0 |
| MRSA | 25.0 ± 2.0 | 16.0 | 128.0 |
| E. coli | 17.0 ± 5.0 | 64.0 | 256.0 |
| S. aureus | 28.0 ± 3.0 | 16.0 | 128.0 |
| A. baumannii | 17.0 ± 1.0 | 64.0 | 256.0 |
| RT (min) | Area % | Compound |
|---|---|---|
| 6.52 | 11.4 | Cyclohexanol, 2-methyl-5-(1-methylethenyl) |
| 8.26 | 60.2 | Linalool |
| 13.45 | 38.5 | 1,8-cineol |
| Nanosystems under Test | Measured Parameters | P. mirabilis |
|---|---|---|
| Lavandula angustifolia nanoemulsion | IZ (mm) | 30.0 ± 2.0 |
| MIC (µg/mL) | 128.0 | |
| MBC (µg/mL) | 512.0 | |
| MBEC (µg/mL) | 128.0 | |
| Gold nanoparticles | IZ (mm) | 20.0 ± 1.0 |
| MIC (µg/mL) | 256.0 | |
| MBC (µg/mL) | 512.0 | |
| MBEC (µg/mL) | 256.0 | |
| Nano-gold/nano-Lavandula angustifolia | IZ (mm) | 45.0 ± 3.0 |
| MIC (µg/mL) | 8.0 | |
| MBC (µg/mL) | 256.0 | |
| MBEC (µg/mL) | 16.0 |
| Sample | Gap Width (µm) | Closure % | |
|---|---|---|---|
| 0 h | 48 h | ||
| Control | 124.03 ± 30.0 | 36.29 ± 6.0 | 70.74 |
| Nano-Lavandula angustifolia | 163.12 ± 12.0 | 19.65 ± 2.0 | 87.95 |
| Nano-gold | 155.63 ± 25.0 | 110.63 ± 2.0 | 28.91 |
| Nano-Lavandula angustifolia/Nano-gold | 164.12 ± 10.0 | 5.28 ± 0.5 | 96.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadel, B.A.; Elwakil, B.H.; Fawzy, E.E.; Shaaban, M.M.; Olama, Z.A. Nanoemulsion of Lavandula angustifolia Essential Oil/Gold Nanoparticles: Antibacterial Effect against Multidrug-Resistant Wound-Causing Bacteria. Molecules 2023, 28, 6988. https://doi.org/10.3390/molecules28196988
Fadel BA, Elwakil BH, Fawzy EE, Shaaban MM, Olama ZA. Nanoemulsion of Lavandula angustifolia Essential Oil/Gold Nanoparticles: Antibacterial Effect against Multidrug-Resistant Wound-Causing Bacteria. Molecules. 2023; 28(19):6988. https://doi.org/10.3390/molecules28196988
Chicago/Turabian StyleFadel, Balqis A., Bassma H. Elwakil, Esraa E. Fawzy, Marwa M. Shaaban, and Zakia A. Olama. 2023. "Nanoemulsion of Lavandula angustifolia Essential Oil/Gold Nanoparticles: Antibacterial Effect against Multidrug-Resistant Wound-Causing Bacteria" Molecules 28, no. 19: 6988. https://doi.org/10.3390/molecules28196988
APA StyleFadel, B. A., Elwakil, B. H., Fawzy, E. E., Shaaban, M. M., & Olama, Z. A. (2023). Nanoemulsion of Lavandula angustifolia Essential Oil/Gold Nanoparticles: Antibacterial Effect against Multidrug-Resistant Wound-Causing Bacteria. Molecules, 28(19), 6988. https://doi.org/10.3390/molecules28196988