Fabry–Pérot Cavities with Suspended Palladium Membranes on Optical Fibers for Highly Sensitive Hydrogen Sensing
Abstract
:1. Introduction
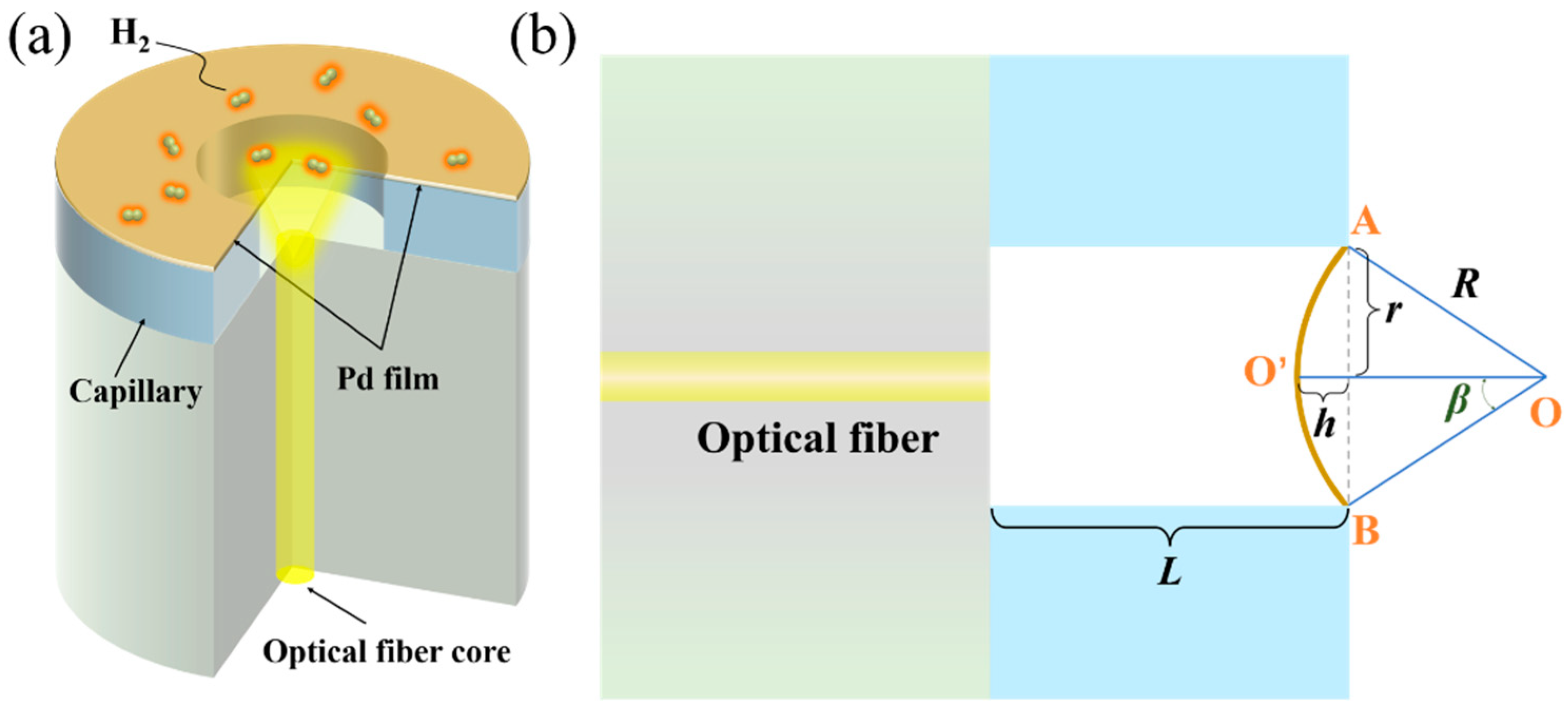
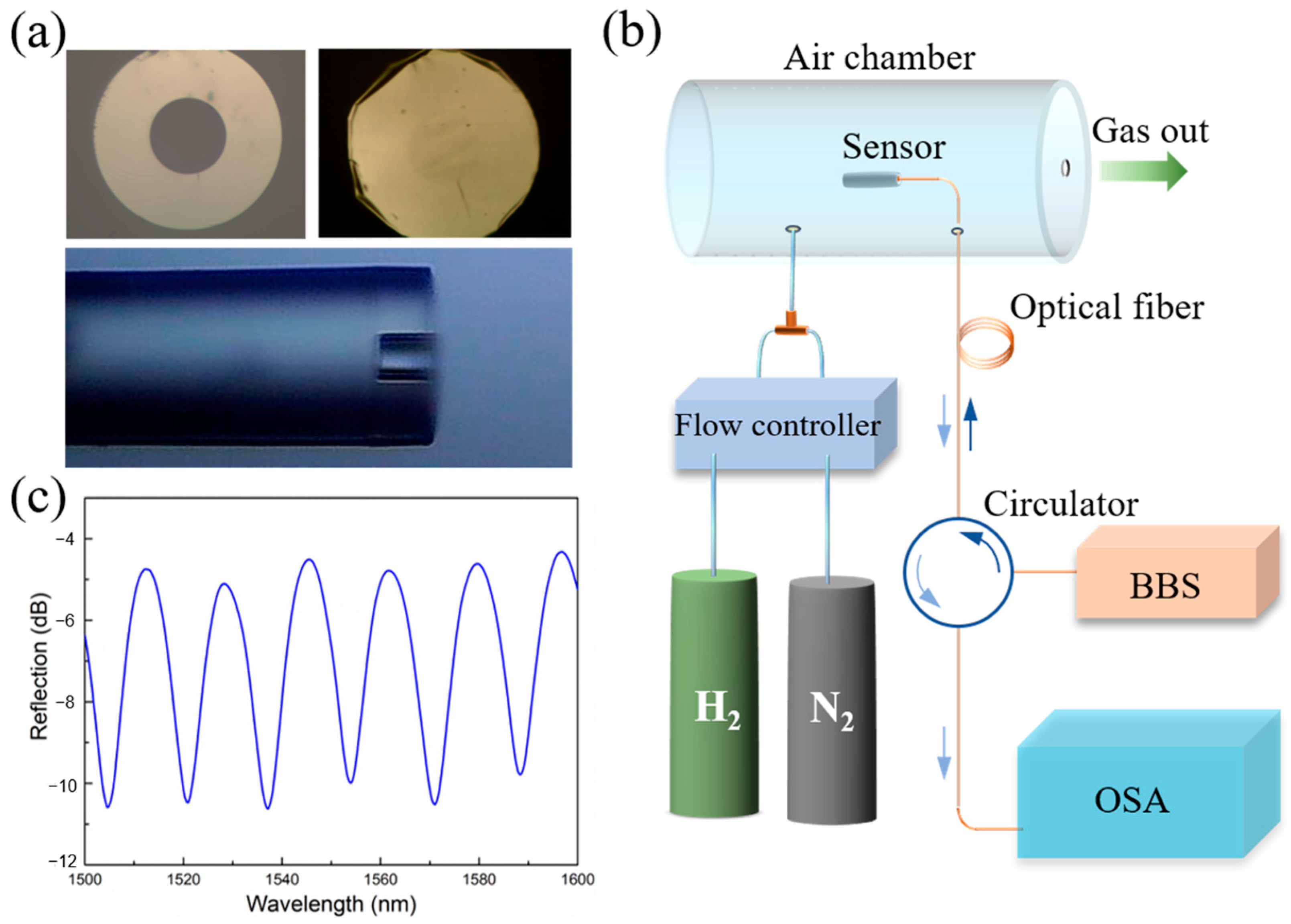
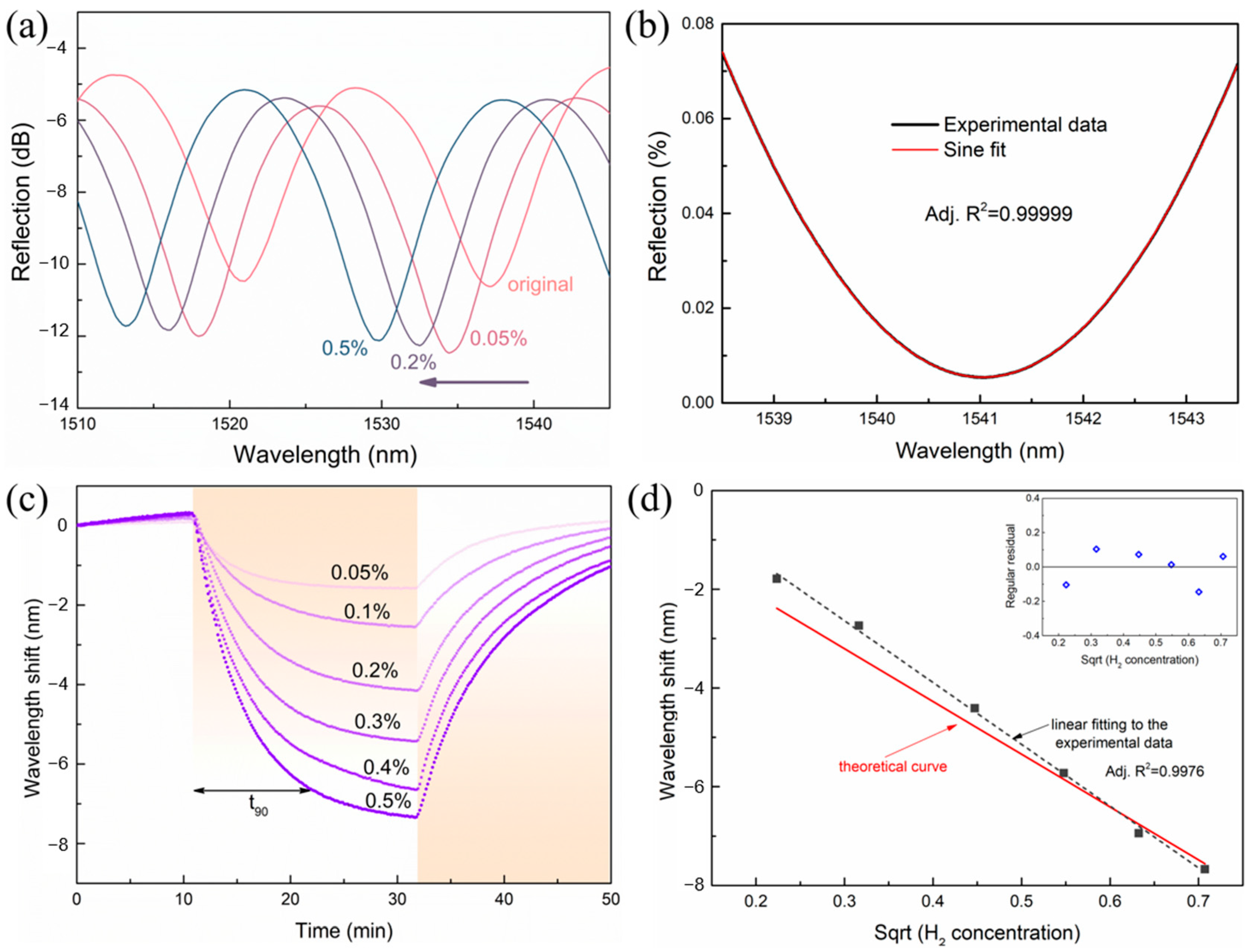
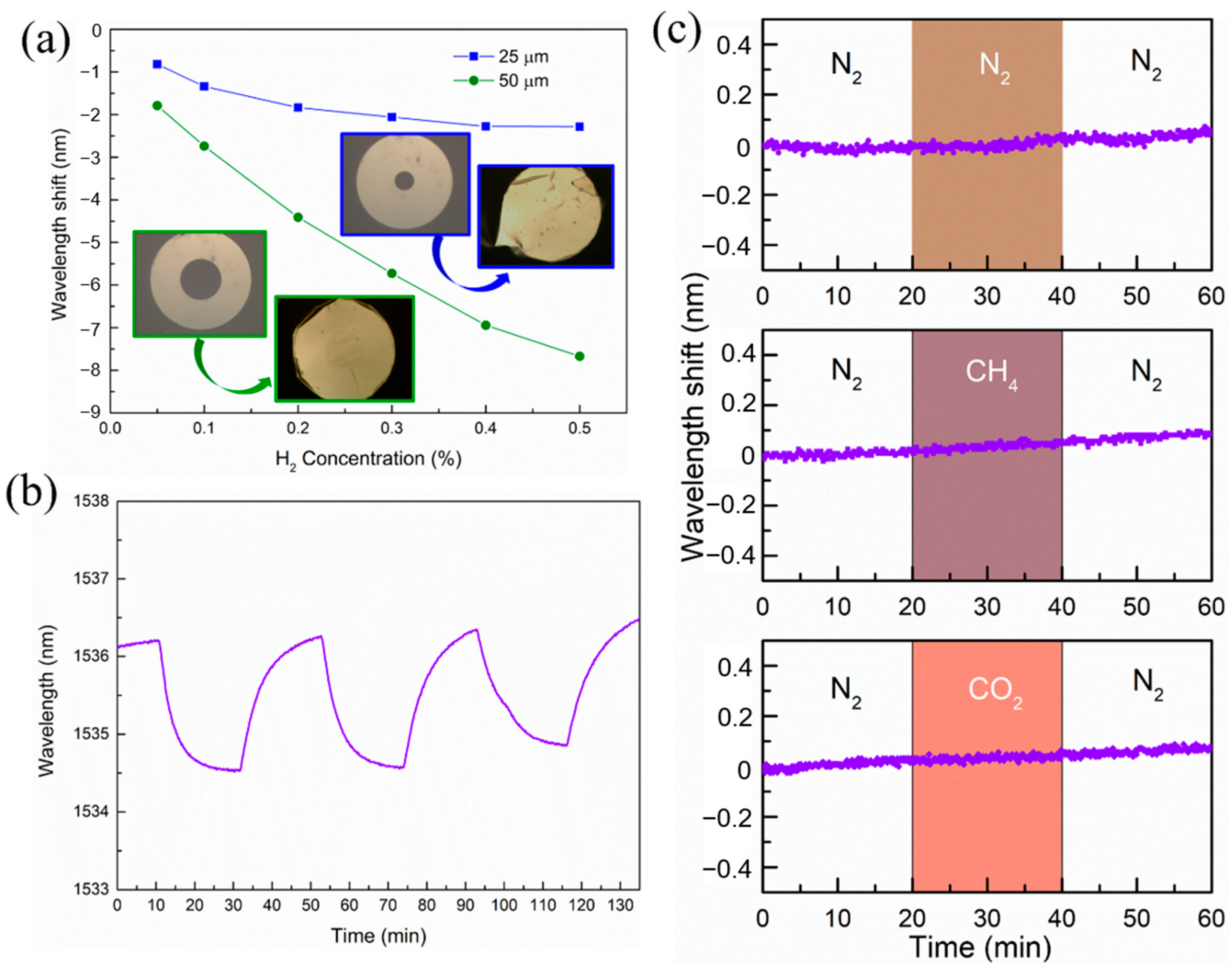
2. Results and Discussions
3. Materials and Methods
3.1. Fabrication of the Sensor
3.2. H2 Sensing Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Balta, M.Ö.; Balta, M.T. Development of a sustainable hydrogen city concept and initial hydrogen city projects. Energy Policy 2022, 166, 113015. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Hydrogen sensing with palladium-coated optical fibers. J. Appl. Phys. 1988, 64, 3706–3712. [Google Scholar] [CrossRef]
- Wadell, C.; Syrenova, S.; Langhammer, C. Plasmonic Hydrogen Sensing with Nanostructured Metal Hydrides. ACS Nano 2014, 8, 11925–11940. [Google Scholar] [CrossRef]
- Buttner, W.J.; Post, M.B.; Burgess, R.; Rivkin, C. An overview of hydrogen safety sensors and requirements. Int. J. Hydrog. Energy 2011, 36, 2462–2470. [Google Scholar] [CrossRef]
- TobišKa, P.; Hugon, O.; Trouillet, A.; Gagnaire, H. An integrated optic hydrogen sensor based on SPR on palladium. Sens. Actuators B Chem. 2001, 74, 168–172. [Google Scholar] [CrossRef]
- Ai, B.; Sun, Y.; Zhao, Y. Plasmonic Hydrogen Sensors. Small 2022, 18, 2107882. [Google Scholar] [CrossRef]
- LaConti, A.B.; Maget, H.J.R. Electrochemical Detection of H2, CO, and Hydrocarbons in Inert or Oxygen Atmospheres. J. Electrochem. Soc. 1971, 118, 506. [Google Scholar] [CrossRef]
- Langhammer, C.; Larsson, E.M.; Zhdanov, V.P.; Zorić, I. Asymmetric Hysteresis in Nanoscopic Single-Metal Hydrides: Palladium Nanorings. J. Phys. Chem. C 2012, 116, 21201–21207. [Google Scholar] [CrossRef]
- Seiyama, T.; Kagawa, S. A New Detector for Gaseous Components Using Semiconductive Thin Films. Anal. Chem. 1966, 38, 1502–1503. [Google Scholar] [CrossRef]
- Butler, M.A. Fiber Optic Sensor for Hydrogen Concentrations near the Explosive Limit. J. Electrochem. Soc. 1991, 138, 46–47. [Google Scholar] [CrossRef]
- Trouillet, A.; Marin, E.; Veillas, C. Fibre gratings for hydrogen sensing. Int. Conf. Opt. Fibre Sens. Int. Soc. Opt. Photonics 2005, 5855, 395. [Google Scholar]
- Kim, Y.H.; Kim, M.J.; Rho, B.S.; Park, M.S.; Jang, J.H.; Lee, B.H. Ultra Sensitive Fiber-Optic Hydrogen Sensor Based on High Order Cladding Mode. IEEE Sens. J. 2011, 11, 1423–1426. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors-a review. Sens. Actuators B Chem. 2011, B157, 329–352. [Google Scholar] [CrossRef]
- Sutapun, B.; Tabib-Azar, M.; Kazemi, A. Pd-coated elastooptic fiber optic Bragg grating sensors for multiplexed hydrogen sensing. Sens. Actuators B Chem. 1999, 60, 27–34. [Google Scholar] [CrossRef]
- Wang, M.; Yang, M.; Cheng, J.; Dai, J.; Yang, M.; Wang, D.N. Femtosecond laser fabricated micro Mach-Zehnder interferometer with Pd film as sensing materials for hydrogen sensing. Opt. Lett. 2012, 37, 1940–1942. [Google Scholar] [CrossRef]
- Ming, T.; Ping, L.; Li, C.; Liu, D.; Zhang, J. Femtosecond laser fabricated in-line micro multicavity fiber FP interferometers sensor. Opt. Commun. 2014, 316, 80–85. [Google Scholar]
- Zeakes, J.S.; Murphy, K.A.; Elshabini-Riad, A.; Claus, R.O. Modified extrinsic Fabry-Perot interferometric hydrogen gas sensor. IEEE Lasers Electro-Opt. Soc. Meet. 1994, 2, 235–236. [Google Scholar]
- Kim, Y.H.; Lee, C.; Ji, H.K.; Lee, Y.T.; Lee, B.H. High finesse interferometric hydrogen sensor based on fiber-optic Fabry-Perot cavity modulations. In Proceedings of the OFS International Conference on Optical Fiber Sensors, Beijing, China, 15–19 October 2012. [Google Scholar]
- Wang, M.; Yang, M.; Cheng, J.; Zhang, G.; Liao, C.R.; Wang, D.N. Fabry–Pérot Interferometer Sensor Fabricated by Femtosecond Laser for Hydrogen Sensing. IEEE Photonics Technol. Lett. 2013, 25, 713–716. [Google Scholar] [CrossRef]
- Nugroho, F.; Darmadi, I.; Cusinato, L.; Susarrey-Arce, A.; Schreuders, H.; Bannenberg, L.J.; Fanta, A.S.; Kadkhodazadeh, S.; Wagner, J.B.; Antosiewicz, T. Metal–polymer hybrid nanomaterials for plasmonic ultrafast hydrogen detection. Nat. Mater. 2019, 18, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, Z.; Ma, J.; Ma, C.; Guan, B.O.; Chen, K. Large-Area Ordered Palladium Nanostructures by Colloidal Lithography for Hydrogen Sensing. Molecules 2022, 27, 6100. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tang, M.L.; Hentschel, M.; Giessen, H.; Alivisatos, P. Nanoantenna-enhanced gas sensing in a single tailored nanofocus. Nat. Mater. 2011, 10, 631–636. [Google Scholar] [CrossRef]
- Iannuzzi, D.; Deladi, S.; Slaman, M.; Rector, J.H.; Schreuders, H.; Elwenspoek, M.C. A fiber-top cantilever for hydrogen detection. Sens. Actuators B Chem. 2007, 121, 706–708. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Y.; Bai, X.; Chen, K.; Guan, B.O. High-sensitivity and fast-response fiber-tip Fabry-Perot hydrogen sensor with suspended palladium-decorated graphene. Nanoscale 2019, 11, 15821–15827. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, X.; Liu, Q.; Zhang, Y.; Liang, Y.; Liu, Y.; Peng, W. The nanophotonic machinal cavity and its hydrogen sensing application. Sens. Actuators B Chem. 2022, 367, 132095. [Google Scholar] [CrossRef]
- Xiong, C.; Zhou, J.; Liao, C.; Zhu, M.; Wang, Y.; Liu, S.; Li, C.; Zhang, Y.; Zhao, Y.; Gan, Z.; et al. Fiber-Tip Polymer Microcantilever for Fast and Highly Sensitive Hydrogen Measurement. ACS Appl. Mater. Interfaces 2020, 12, 33163–33172. [Google Scholar] [CrossRef]
- Ma, J.; Jin, W.; Xuan, H.; Wang, C.; Ho, H.L. Fiber-optic ferrule-top nanomechanical resonator with multilayer graphene film. Opt. Lett. 2014, 39, 4769–4772. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.M.; Lee, E.; Noh, J.-S.; Joe, J.H.; Jung, B.; Lee, W. Hydrogen gas sensing performance of Pd–Ni alloy thin films. Thin Solid Film. 2010, 519, 880–884. [Google Scholar] [CrossRef]
- Nugroho, F.A.A.; Eklund, R.; Nilsson, S.; Langhammer, C. A fiber-optic nanoplasmonic hydrogen sensor via pattern-transfer of nanofabricated PdAu alloy nanostructures. Nanoscale 2018, 10, 20533–20539. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, F.; Ling, H.; Yu, B.; Peng, W.; Yu, Q. A compact hydrogen sensor based on the fiber-optic Fabry-Perot interferometer. Opt. Laser Technol. 2020, 124, 105995. [Google Scholar] [CrossRef]
- ISO26142; Hydrogen Detection Apparatus-Stationary Applications. ISO: Geneva, Switzerland, 2010.

| Sensitive Film | Detection Range | Sensitivity and Detection Limit | Response Time | Recovery Time | Working Temp. | Ref. |
|---|---|---|---|---|---|---|
| 100 μm Pd | 4–10% | 0. 144 pm/ppm at 8% CH (500 ppm detection limit) | 401 s | ≈500 s | RT | [32] |
| 2 μm Pd | 0.5–5% | (32 ppm detection limit) | ≈30 min | - | RT | [19] |
| 50 nm Pd and 2 nm Ni | 4% | 0.0175 pm/ppm at 4% CH | 50 s | - | RT | [20] |
| Multiple 20 nm Pd | 2–8% | ≈0.0019 pm/ppm at 8% CH | ≈2 min | ≈5 min | RT | [21] |
| 5.6 nm Pd and 3 nm MLG | 0.02–3% | 0.25 pm/ppm at 0.02% CH (20 ppm detection limit) | 18 s | - | RT | [26] |
| 30 nm Pd | 0.05–0.5% | 3.6 pm/ppm at 0.05% CH 1.5 pm/ppm at 0.5% CH (3.3 ppm detection limit) | 11 min | 11 min | RT | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Ma, J.; Li, C.; Ma, C.; Li, J.; Guan, B.-O.; Chen, K. Fabry–Pérot Cavities with Suspended Palladium Membranes on Optical Fibers for Highly Sensitive Hydrogen Sensing. Molecules 2023, 28, 6984. https://doi.org/10.3390/molecules28196984
Xu F, Ma J, Li C, Ma C, Li J, Guan B-O, Chen K. Fabry–Pérot Cavities with Suspended Palladium Membranes on Optical Fibers for Highly Sensitive Hydrogen Sensing. Molecules. 2023; 28(19):6984. https://doi.org/10.3390/molecules28196984
Chicago/Turabian StyleXu, Feng, Jun Ma, Can Li, Churong Ma, Jie Li, Bai-Ou Guan, and Kai Chen. 2023. "Fabry–Pérot Cavities with Suspended Palladium Membranes on Optical Fibers for Highly Sensitive Hydrogen Sensing" Molecules 28, no. 19: 6984. https://doi.org/10.3390/molecules28196984
APA StyleXu, F., Ma, J., Li, C., Ma, C., Li, J., Guan, B.-O., & Chen, K. (2023). Fabry–Pérot Cavities with Suspended Palladium Membranes on Optical Fibers for Highly Sensitive Hydrogen Sensing. Molecules, 28(19), 6984. https://doi.org/10.3390/molecules28196984









