Investigating the Structural Evolution and Catalytic Activity of c-Co/Co3Mo Electrocatalysts for Alkaline Hydrogen Evolution Reaction
Abstract
:1. Introduction
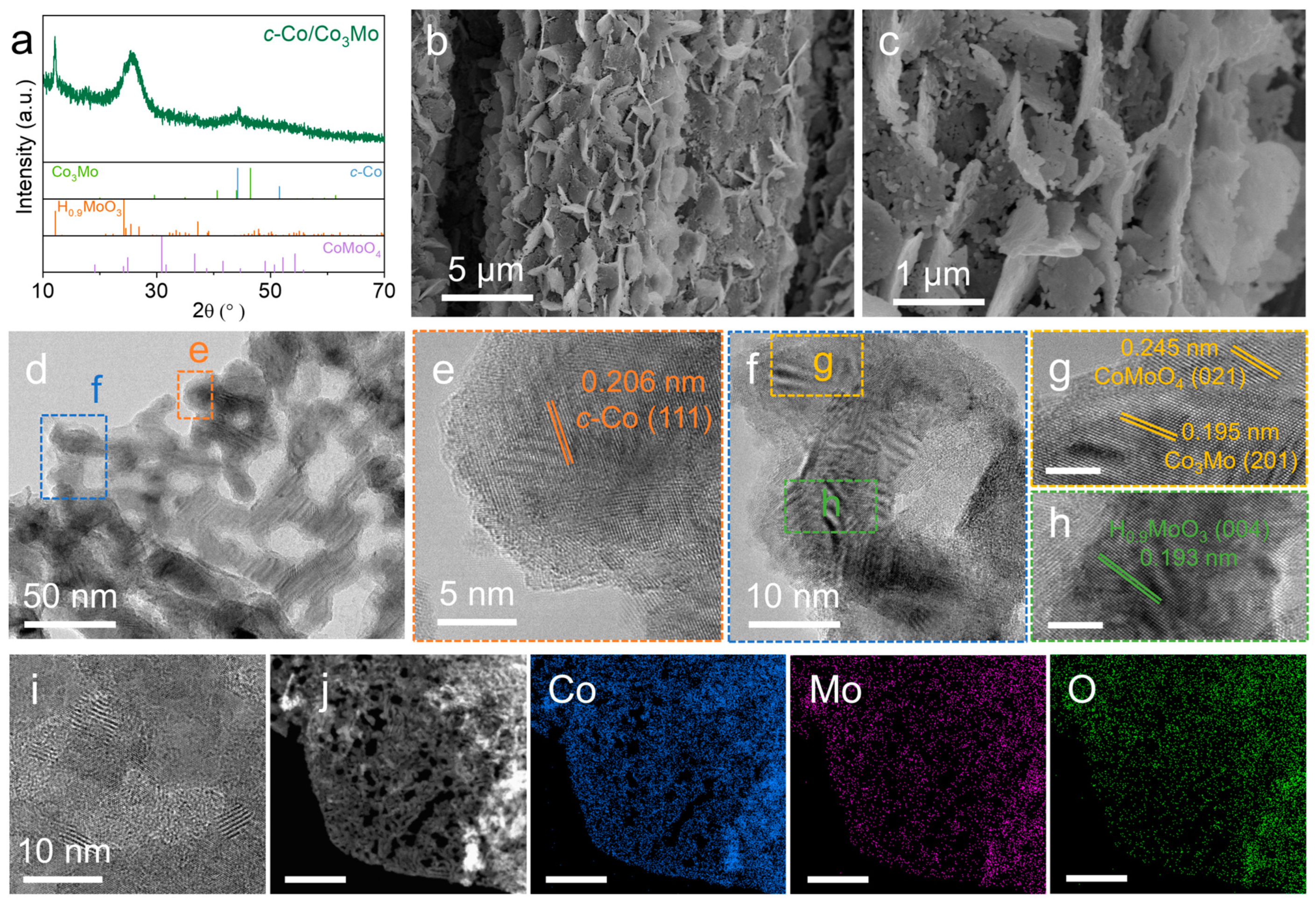
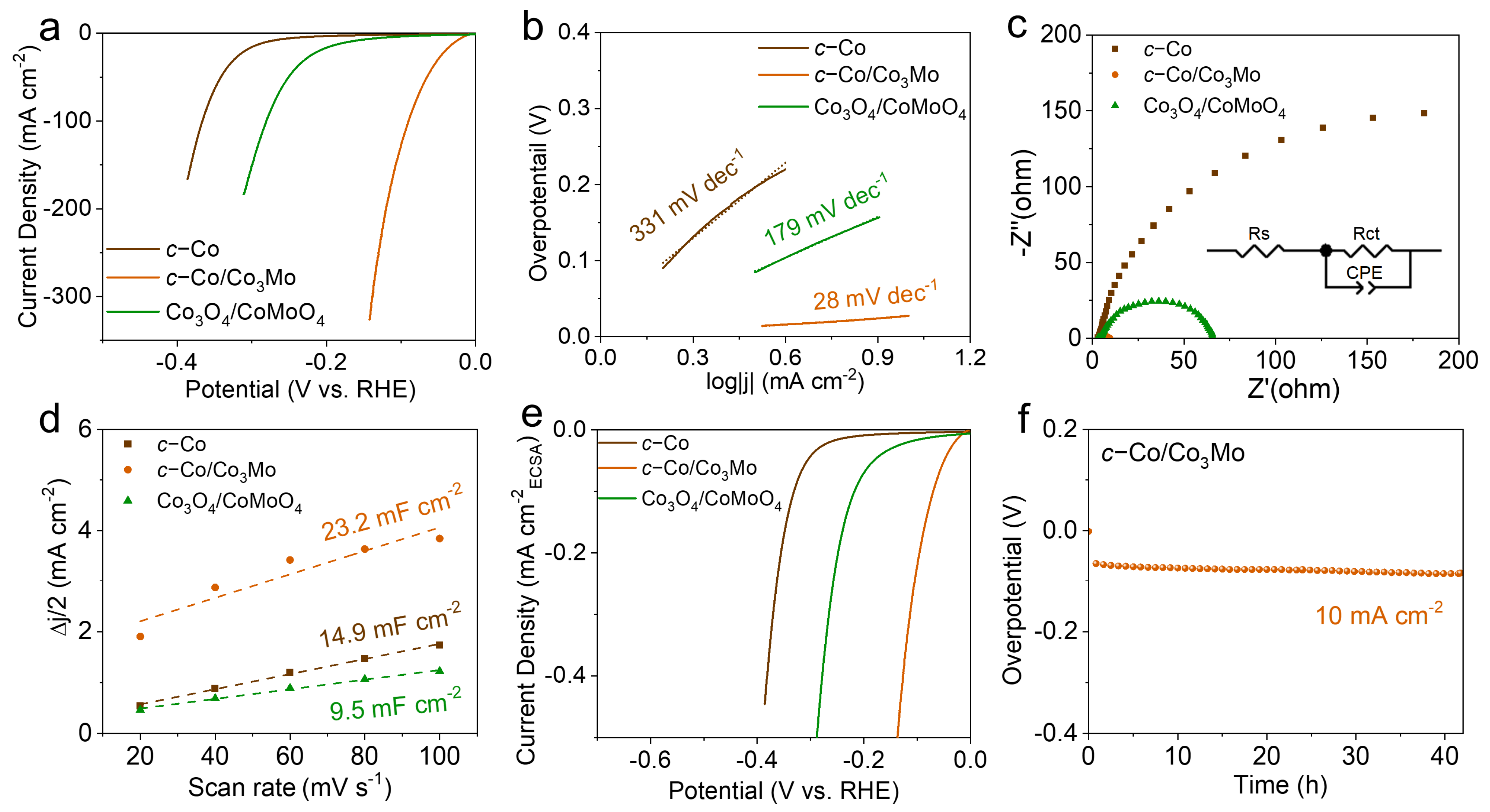
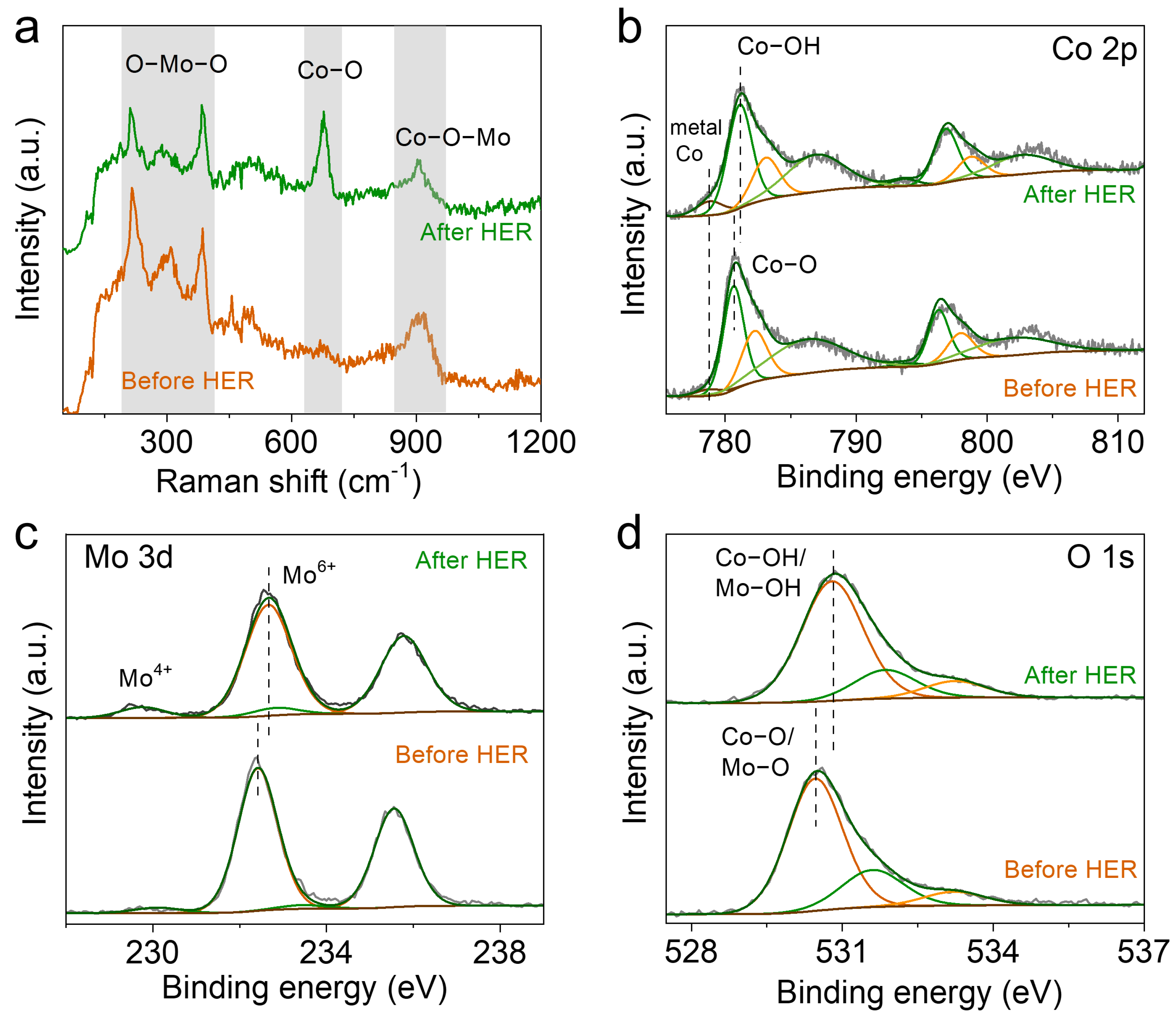
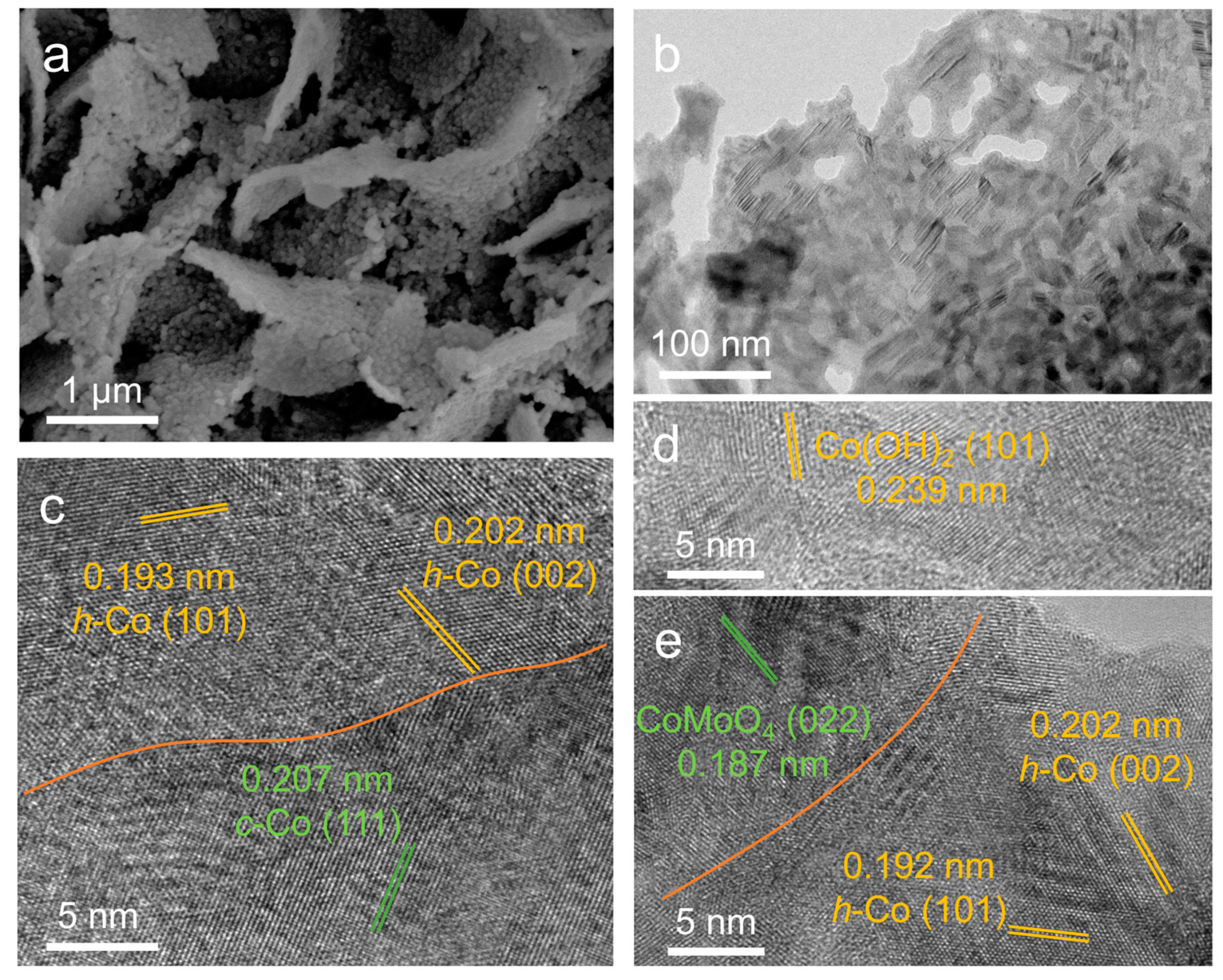
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of the Co(OH)2/CoMoO4 Precursor on Carbon Cloth
3.3. Synthesis of c-Co/Co3Mo on Carbon Cloth
3.4. Synthesis of c-Co on Carbon Cloth
3.5. Synthesis of Co3O4/CoMoO4 on Carbon Cloth
3.6. Preparation of 20% Pt/C Electrode on Carbon Cloth
3.7. Characterizations
3.7.1. Scanning Electron Microscopy (SEM)
3.7.2. Transmission Electron Microscopy (TEM) with Energy Dispersive X-ray Spectroscopy (EDX)
3.7.3. Powder X-ray Diffraction (XRD)
3.7.4. X-ray Photoelectron Spectroscopy (XPS)
3.7.5. Raman Spectroscopy
3.8. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, L.N.; Shi, L.; Liu, Q.; Huang, Y.L.; Yan, W.S.; Liang, X.; Zhao, X.; Chen, H.; Zou, X.X. Structurally Robust Honeycomb Layered Strontium Iridate as an Oxygen Evolution Electrocatalyst in Acid. ACS Catal. 2023, 13, 7322–7330. [Google Scholar] [CrossRef]
- Ran, L.; Li, Z.W.; Ran, B.; Cao, J.Q.; Shao, T.; Song, Y.R.; Leung, M.K.H.; Sun, L.C.; Hou, J.G. Engineering Single-Atom Active Sites on Covalent Organic Frameworks for Boosting CO2 Photoreduction. J. Am. Chem. Soc. 2022, 144, 17097–17109. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, L.; Chen, H.; Liang, X.; Tian, B.; Zhang, K.; Zou, Y.; Zou, X. A Highly Active, Long-Lived Oxygen Evolution Electrocatalyst Derived from Open-Framework Iridates. Adv. Mater. 2023, 35, 2208539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Zhu, S.S.; Liu, Y.Y.; Zhang, X.; Wang, J.J.; Braun, A. Covalent S-O Bonding Enables Enhanced Photoelectrochemical Performance of Cu2S/Fe2O3 Heterojunction for Water Splitting. Small 2021, 17, 2100320. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Shi, R.; Li, Z.H.; Zhao, J.Q.; Su, C.L.; Zhang, T.R. Triphase Photocatalytic CO2 Reduction over Silver-Decorated Titanium Oxide at a Gas–Water Boundary. Angew. Chem. 2022, 61, e202200802. [Google Scholar] [CrossRef]
- Miao, Z.Y.; Huang, Y.; Xin, J.P.; Su, X.W.; Sang, Y.H.; Liu, H.; Wang, J.J. High Performance Symmetric Supercapacitor Constructed Using Carbon Cloth Boosted by Engineering Oxygen-containing Functional Groups. ACS Appl. Mater. Interfaces 2019, 11, 18044–18050. [Google Scholar] [CrossRef]
- Wang, Z.J.; Li, M.X.; Yu, J.H.; Ge, X.B.; Liu, Y.H.; Wang, W.H. Low-Iridium-Content IrNiTa Metallic Glass Films as Intrinsically Active Catalysts for Hydrogen Evolution Reaction. Adv. Mater. 2020, 32, 1906384. [Google Scholar] [CrossRef]
- Yi, P.; Song, Y.Y.; Li, C.Y.; Liu, R.Z.; Sun, J.K. Heterostructured Mn-doped NiSx/NiO/Ni3N Nanoplate Arrays as Bifunctional Electrocatalysts for Energy-Saving Hydrogen Production and Urea Degradation. Appl. Surf. Sci. 2023, 619, 156789. [Google Scholar] [CrossRef]
- Chiou, T.W.; Hsu, I.J.; Li, W.L.; Tung, C.Y.; Yang, Z.Q.; Lee, J.F.; Lin, T.W. Fluoride-incorporated cobalt-based electrocatalyst towards enhanced hydrogen evolution reaction. Chem. Commun. 2022, 58, 2746–2749. [Google Scholar] [CrossRef]
- Feng, J.X.; Xu, H.; Ye, S.H.; Ouyang, G.; Tong, Y.X.; Li, G.R. Silica-polypyrrole hybrids as high-performance metal-free electrocatalysts for the hydrogen evolution reaction in neutral media. Angew. Chem. Int. Ed. Engl. 2017, 56, 8120–8124. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Zhao, Y.Y.; Zhai, P.L.; Wang, C.; Gao, J.F.; Sun, L.C.; Hou, J.G. Triggering Lattice Oxygen Activation of Single-Atomic Mo Sites Anchored on Ni–Fe Oxyhydroxides Nanoarrays for Electrochemical Water Oxidation. Adv. Mater. 2022, 34, 2202523. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, L.W.; Liu, X.L.; Tan, T.; Liu, H.; Wang, J.J. Precisely engineering the electronic structure of active sites boosts the activity of iron-nickel selenide on nickel foam for highly efficient and stable overall water splitting. Appl. Catal. B Environ. 2021, 299, 120678. [Google Scholar] [CrossRef]
- Yao, Z.C.; Tang, T.; Jiang, Z.; Wang, L.; Hu, J.S.; Wan, L.J. Electrocatalytic Hydrogen Oxidation in Alkaline Media: From Mechanistic Insights to Catalyst Design. ACS Nano 2022, 16, 5153–5183. [Google Scholar] [CrossRef] [PubMed]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 0003. [Google Scholar] [CrossRef]
- Su, L.X.; Chen, J.X.; Yang, F.L.; Li, P.; Jin, Y.M.; Luo, W.; Chen, S.L. Electric-Double-Layer Origin of the Kinetic pH Effect of Hydrogen Electrocatalysis Revealed by a Universal Hydroxide Adsorption-Dependent Inflection-Point Behavior. J. Am. Chem. Soc. 2023, 145, 12051–12058. [Google Scholar] [CrossRef]
- Zhai, W.; Ma, Y.; Chen, D.; Ho, J.C.; Dai, Z.; Qu, Y. Recent progress on the long-term stability of hydrogen evolution reaction electrocatalysts. InfoMat 2022, 4, e12357. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, P.; Sheng, W. Hydrogen Evolution and Oxidation: Mechanistic Studies and Material Advances. Adv. Mater. 2019, 31, 1808066. [Google Scholar] [CrossRef]
- Cui, Z.B.; Jiao, W.S.; Huang, Z.Y.; Chen, G.Z.; Zhang, B.; Han, Y.H.; Huang, W. Design and Synthesis of Noble Metal-Based Alloy Electrocatalysts and Their Application in Hydrogen Evolution Reaction. Small 2023, 19, 2301465. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.; Gao, M.R.; Yu, S.H. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Chen, L.L.; Yang, S.S.; Qian, K.; Wei, W.; Sun, C.; Xie, J.M. In situ growth of N-doped carbon coated CoNi alloy with graphene decoration for enhanced HER performance. J. Energy Chem. 2019, 29, 129–135. [Google Scholar] [CrossRef]
- Qiu, T.J.; Cheng, J.Q.; Liang, Z.B.; Tabassum, H.; Shi, J.M.; Tang, Y.Q.; Guo, W.H.; Zheng, L.R.; Gao, S.; Xu, S.Z.; et al. Unveiling the nanoalloying modulation on hydrogen evolution activity of ruthenium-based electrocatalysts encapsulated by B/N co-doped graphitic nanotubes. Appl. Catal. B Environ. 2022, 316, 121626. [Google Scholar] [CrossRef]
- Kuang, P.Y.; Ni, Z.R.; Zhu, B.C.; Lin, Y.; Yu, J.G. Modulating the d-Band Center Enables Ultrafine Pt3Fe Alloy Nanoparticles for pH-Universal Hydrogen Evolution Reaction. Adv. Mater. 2023, 2303030. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.P.; Xiao, B.B.; Huang, M.; Yan, L.H.; Wang, Z.P.; Huang, Y.C.; Shen, S.J.; Zhang, Q.H.; Gu, L.; Zhong, W.W. Realizing Negatively Charged Metal Atoms through Controllable d-Electron Transfer in Ternary Ir1−xRhxSb Intermetallic Alloy for Hy-drogen Evolution Reaction. Adv. Energy Mater. 2022, 12, 2200. [Google Scholar]
- Chen, Y.H.; Yue, K.H.; Zhao, J.W.; Cai, Z.Y.; Wang, X.Y.; Yan, Y. Effective modulating of the Mo dissolution and polymeriza-tion in Ni4Mo/NiMoO4 heterostructure via metal-metal oxide-support interaction for boosting H2 production. Chem. Eng. J. 2023, 466, 143097. [Google Scholar] [CrossRef]
- Faber, M.S.; Jin, S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 2014, 7, 3519–3542. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Huang, Y.; Jiang, L.W.; Meng, C.; Yin, Z.H.; Liu, H.; Wang, J.J. Modulating the electronic structure of CoS2 by Sn doping boosting urea oxidation for efficient alkaline hydrogen production. J. Colloid Interface Sci. 2023, 642, 574. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Shi, Y.; Zhou, W.; Yu, Y.; Zhang, B. Unveiling the In Situ Dissolution and Polymerization of Mo in Ni4Mo Alloy for Promoting Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 7051–7055. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Liu, J.; Zhong, C.; Tu, Y.; Li, P.; Du, L.; Chen, S.; Cui, Z. OH spectator at IrMo intermetallic narrowing activity gap between alkaline and acidic hydrogen evolution reaction. Nat. Commun. 2022, 13, 5497. [Google Scholar] [CrossRef]
- Jiang, L.W.; Huang, Y.; Zou, Y.; Meng, C.; Xiao, Y.; Liu, H.; Wang, J.J. Boosting the Stability of Oxygen Vacancies in α-Co(OH)2 Nanosheets with Coordination Polyhedrons as Rivets for High-Performance Alkaline Hydrogen Evolution Electrocatalyst. Adv. Energy Mater. 2022, 12, 2202351. [Google Scholar] [CrossRef]
- Jin, Q.; Ren, B.; Li, D.; Cui, H.; Wang, C. In situ promoting water dissociation kinetic of Co based electrocatalyst for unprecedentedly enhanced hydrogen evolution reaction in alkaline media. Nano Energy 2018, 49, 14–22. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Y.T.; Yao, R.Q.; Wan, W.B.; Ge, X.; Zhang, W.; Wen, Z.; Lang, X.Y.; Zheng, W.T.; Jiang, Q. Spontaneously Separated Intermetallic Co3Mo from Nanoporous Copper as Versatile Electrocatalysts for Highly Efficient Water Splitting. Nat. Commun. 2020, 11, 2940. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ge, Y.; Feng, Q.; Zhuang, P.; Chu, H.; Cao, Y.; Smith, W.R.; Dong, P.; Ye, M.; Shen, J. Nesting Co3Mo binary alloy nanoparticles onto molybdenum oxide nanosheet arrays for superior hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2019, 11, 9002–9010. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, 4998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xing, Y.; Xu, S.; Lu, Y.; Sun, S.; Jiang, D. Interfacing Co3Mo with CoMoOx for synergistically boosting electrocatalytic hydrogen and oxygen evolution reactions. Chem. Eng. J. 2022, 431, 133240. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.X.; Jiang, L.; Lin, H.; An, Y.M.; Zhou, D.; Leung, M.K.H.; Yang, S.H. Nanohybridization of MoS2 with Layered Double Hydroxides Efficiently Synergizes the Hydrogen Evolution in Alkaline Media. Joule 2017, 1, 383–393. [Google Scholar] [CrossRef]
- Wen, S.T.; Huang, J.; Li, T.T.; Chen, W.; Chen, G.L.; Zhang, Q.; Zhang, X.H.; Qian, Q.Y.; Ostrikov, K. Multiphase nanosheet-nanowire cerium oxide and nickel-cobalt phosphide for highly-efficient electrocatalytic overall water splitting. Appl. Catal. B Environ. 2022, 316, 121678. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, H.; Sun, L.; Jia, F.; Liu, B. Ordered Mesoporous Intermetallic Trimetals for Efficient and pH-Universal Hydrogen Evolution Electrocatalysis. Adv. Energy Mater. 2022, 12, 2201478. [Google Scholar] [CrossRef]
- Shah, A.H.; Zhang, Z.; Huang, Z.; Wang, S.; Zhong, G.; Wan, C.; Alexandrova, A.N.; Huang, Y.; Duan, X. The role of alkali metal cations and platinum-surface hydroxyl in the alkaline hydrogen evolution reaction. Nat. Catal. 2022, 5, 923–933. [Google Scholar] [CrossRef]
- Wang, J.; Xin, S.; Xiao, Y.; Zhang, Z.; Li, Z.; Zhang, W.; Li, C.; Bao, R.; Peng, J.; Yi, J.; et al. Manipulating the Water Dissociation Electrocatalytic Sites of Bimetallic Nickel-Based Alloys for Highly Efficient Alkaline Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202202518. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Kim, T.; Lee, J.; Choi, S.I.; Lee, K. Morphology-Controlled Metal Sulfides and Phosphides for Electrochemical Water Splitting. Adv. Mater. 2019, 31, e1806682. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhang, W.; Shi, Z.; Yang, L.; Tang, Y. Structural Design and Electronic Modulation of Transition-Metal-Carbide Electrocatalysts toward Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, 1802880. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Niu, X.; Zhang, K.; Wang, Z.; Cui, Y.; Wang, R. Chemical Interactions and Mechanisms of Different pH Regulators on Copper and Cobalt Removal Rate of Copper Film CMP for GLSI. ECS J. Solid State Sci. Technol. 2019, 8, 99–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Huang, Z.; Ren, L.; Qi, X.; Wei, X.; Zhong, J. Facile hydrothermal synthesis of NiMoO4@CoMoO4 hierarchical nanospheres for supercapacitor applications. Phys. Chem. Chem. Phys. 2015, 17, 20795–20804. [Google Scholar] [CrossRef]
- Lin, B.; Chen, J.; Yang, R.; Mao, S.; Qin, M.; Wang, Y. Multi-hierarchical cobalt-based electrocatalyst towards high rate H2 production. Appl. Catal. B Environ. 2022, 316, 121666. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Salgueiriño, V. Thermodynamic CoO–Co3O4 crossover using Raman spectroscopy in magnetic octahedron-shaped nanocrystals. J. Raman Spectrosc. 2017, 48, 837–841. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, L.W.; Liu, H.; Wang, J.J. Electronic structure regulation and polysulfide bonding of Co-doped (Ni, Fe)1+xS enable highly efficient and stable electrocatalytic overall water splitting. Chem. Eng. J. 2022, 441, 136121. [Google Scholar] [CrossRef]
- Men, Y.N.; Jia, S.F.; Li, P.; Tan, Y.; Wang, J.B.; Zhao, P.P.; Cheng, G.Z.; Chen, S.L.; Luo, W. Boosting alkaline hydrogen evolution electrocatalysis through electronic communicating vessels on Co2P/Co4N heterostructure catalyst. Chem. Eng. J. 2022, 433, 133831. [Google Scholar] [CrossRef]
- Jiang, L.W.; Huang, Y.; Chen, B.B.; Zhou, J.Q.; Liu, H.; Wang, J.J. Electrochemically induced in-situ generated Co(OH)2 nanoplates to promote the Volmer process toward efficient alkaline hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 8497–8506. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, S.; Xu, S.; Yang, X.; Bian, J.; Lv, C.; Yu, Z.; He, T.; Huang, Z.; Boukhvalov, D.W.; et al. Modulation of Volmer step for efficient alkaline water splitting implemented by titanium oxide promoting surface reconstruction of cobalt carbonate hydroxide. Nano Energy 2021, 82, 105732. [Google Scholar] [CrossRef]
- Yin, Z.H.; Huang, Y.; Jiang, L.W.; Meng, C.; Wu, Y.Z.; Liu, H.; Wang, J.J. Revealing the In Situ Evolution of Tetrahedral NiMoO4 Micropillar Array for Energy-Efficient Alkaline Hydrogen Production Assisted by Urea Electrolysis. Small Struct. 2023, 4, 2300028. [Google Scholar] [CrossRef]
- Gao, W.Q.; Zou, Y.; Zang, Y.M.; Zhao, X.L.; Zhou, W.J.; Dai, Y.; Liu, H.; Wang, J.J.; Ma, Y.D.; Sang, Y.H. Magnetic-field-regulated Ni-Fe-Mo ternary alloy electrocatalysts with enduring spin polarization enhanced oxygen evolution reaction. Chem. Eng. J. 2023, 455, 140821. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, W.; Shen, K.; Zhang, Q.; Zhao, R.; Xiang, H.; Wu, J.; Li, X.; Yang, N. Electrochemically Reconstructed Cu-FeOOH/Fe3O4 Catalyst for Efficient Hydrogen Evolution in Alkaline Media. Adv. Energy Mater. 2022, 12, 2200077. [Google Scholar] [CrossRef]
- Wu, Z.P.; Zhang, H.B.; Zuo, S.W.; Wang, Y.; Zhang, S.L.; Zhang, J.; Zang, S.Q.; Lou, X.W. Manipulating the Local Coordination and Electronic Structures for Efficient Electrocatalytic Oxygen Evolution. Adv. Mater. 2021, 33, 2103004. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Jiang, L.-W.; Wang, J.-J. Investigating the Structural Evolution and Catalytic Activity of c-Co/Co3Mo Electrocatalysts for Alkaline Hydrogen Evolution Reaction. Molecules 2023, 28, 6986. https://doi.org/10.3390/molecules28196986
Chen L, Jiang L-W, Wang J-J. Investigating the Structural Evolution and Catalytic Activity of c-Co/Co3Mo Electrocatalysts for Alkaline Hydrogen Evolution Reaction. Molecules. 2023; 28(19):6986. https://doi.org/10.3390/molecules28196986
Chicago/Turabian StyleChen, Long, Li-Wen Jiang, and Jian-Jun Wang. 2023. "Investigating the Structural Evolution and Catalytic Activity of c-Co/Co3Mo Electrocatalysts for Alkaline Hydrogen Evolution Reaction" Molecules 28, no. 19: 6986. https://doi.org/10.3390/molecules28196986
APA StyleChen, L., Jiang, L.-W., & Wang, J.-J. (2023). Investigating the Structural Evolution and Catalytic Activity of c-Co/Co3Mo Electrocatalysts for Alkaline Hydrogen Evolution Reaction. Molecules, 28(19), 6986. https://doi.org/10.3390/molecules28196986






