Microplastics’ Shape and Morphology Analysis in the Presence of Natural Organic Matter Using Flow Imaging Microscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Microplastics by the FlowCam
2.2. Effect of Humic Acid (HA) on the Analysis of PE Microplastics
2.3. Effect of Bovine Serum Albumin (BSA) on the Analysis of PE Microplastics
3. Materials and Methods
3.1. Microplastics and NOMs
3.2. FlowCam Apparatus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, D.; Allen, S.; Abbasi, S.; Baker, A.; Bergmann, M.; Brahney, J.; Butler, T.; Duce, R.A.; Eckhardt, S.; Evangeliou, N.; et al. Microplastics and nanoplastics in the marine-atmosphere environment. Nat. Rev. Earth Environ. 2022, 3, 393–405. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, E.; Singh, S.; Pandey, A.; Bhargava, P.C. Micro- and nano-plastics (MNPs) as emerging pollutant in ground water: Environmental impact, potential risks, limitations and way forward towards sustainable management. Chem. Eng. J. 2023, 459, 141568. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Raveendran, S.; Singh, S.; Pillai, S. Environmental Impacts of Microplastics and Nanoplastics: A Current Overview. Front. Microbiol. 2021, 12, 768297. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Narvaez, O.M.; Goonetilleke, A.; Perez, L.; Bandala, E.R. Engineered technologies for the separation and degradation of microplastics in water: A review. Chem. Eng. J. 2021, 414, 128692. [Google Scholar] [CrossRef]
- Skaf, D.W.; Punzi, V.L.; Rolle, J.T.; Kleinberg, K.A. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chem. Eng. J. 2020, 386, 123807. [Google Scholar] [CrossRef]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 123807. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; Volume 10. [Google Scholar]
- Kim, M.-J.; Na, S.-H.; Batool, R.; Byun, I.-S.; Kim, E.-J. Seasonal variation and spatial distribution of microplastics in tertiary wastewater treatment plant in South Korea. J. Hazard. Mater. 2022, 438, 129474. [Google Scholar] [CrossRef]
- Junaid, M.; Siddiqui, J.A.; Liu, S.; Lan, R.; Abbas, Z.; Chen, G.; Wang, J. Adverse multigeneration combined impacts of micro(nano)plastics and emerging pollutants in the aquatic environment. Sci. Total. Environ. 2023, 882, 163679. [Google Scholar] [CrossRef]
- Sipe, J.M.; Bossa, N.; Berger, W.; von Windheim, N.; Gall, K.; Wiesner, M.R. From bottle to microplastics: Can we estimate how our plastic products are breaking down? Sci. Total. Environ. 2022, 814, 152460. [Google Scholar] [CrossRef]
- Wei, L.; Yue, Q.; Chen, G.; Wang, J. Microplastics in rainwater/stormwater environments: Influencing factors, sources, transport, fate, and removal techniques. TrAC Trends Anal. Chem. 2023, 165, 117147. [Google Scholar] [CrossRef]
- Vega-Herrera, A.; Llorca, M.; Borrell-Diaz, X.; Redondo-Hasselerharm, P.E.; Abad, E.; Villanueva, C.M.; Farré, M. Polymers of micro(nano) plastic in household tap water of the Barcelona Metropolitan Area. Water Res. 2022, 220, 118645. [Google Scholar] [CrossRef] [PubMed]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y. Treatment characteristics of microplastics at biological sewage treatment facilities in Korea. Mar. Pollut. Bull. 2018, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Ding, T.; Peng, C.; Naz, I.; Sun, H.; Li, J.; Liu, J. Micro- and nanoplastics in wastewater treatment plants: Occurrence, removal, fate, impacts and remediation technologies—A critical review. Chem. Eng. J. 2021, 423, 130205. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Maliwan, T.; Pungrasmi, W.; Lohwacharin, J. Effects of microplastic accumulation on floc characteristics and fouling behavior in a membrane bioreactor. J. Hazard. Mater. 2020, 411, 124991. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Liu, X.; Deng, Q.; Zheng, Y.; Wang, D.; Ni, B.-J. Microplastics aging in wastewater treatment plants: Focusing on physicochemical characteristics changes and corresponding environmental risks. Water Res. 2022, 221, 118780. [Google Scholar] [CrossRef]
- Kook, H.; Cha, M.; Park, C. Transport of emerging organic ultraviolet (UV) filters in ceramic membranes: Role of polyethylene (PE) microplastics. Chemosphere 2022, 309, 136570. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, C.; Dai, Y.; Li, Y.; Jiao, S.; Ma, X.; Liu, X.; Wang, L. Slower antibiotics degradation and higher resistance genes enrichment in plastisphere. Water Res. 2022, 222, 118920. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, C.; He, D.; Sun, J.; Zhang, A.; Li, J.; Pan, X. Interactions between polypropylene microplastics (PP-MPs) and humic acid influenced by aging of MPs. Water Res. 2022, 222, 118921. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, S.; Junaid, M.; Gao, D.; Ai, W.; Chen, G.; Wang, J. Di-(2-ethylhexyl) phthalate exacerbated the toxicity of polystyrene nanoplastics through histological damage and intestinal microbiota dysbiosis in freshwater Micropterus salmoides. Water Res. 2022, 219, 118608. [Google Scholar] [CrossRef]
- Dronjak, L.; Exposito, N.; Rovira, J.; Florencio, K.; Emiliano, P.; Corzo, B.; Schuhmacher, M.; Valero, F.; Sierra, J. Screening of microplastics in water and sludge lines of a drinking water treatment plant in Catalonia, Spain. Water Res. 2022, 225, 119185. [Google Scholar] [CrossRef]
- Cunsolo, S.; Williams, J.; Hale, M.; Read, D.S.; Couceiro, F. Optimising sample preparation for FTIR-based microplastic analysis in wastewater and sludge samples: Multiple digestions. Anal. Bioanal. Chem. 2021, 413, 3789–3799. [Google Scholar] [CrossRef]
- Schrank, I.; Möller, J.N.; Imhof, H.K.; Hauenstein, O.; Zielke, F.; Agarwal, S.; Löder, M.G.; Greiner, A.; Laforsch, C. Microplastic sample purification methods—Assessing detrimental effects of purification procedures on specific plastic types. Sci. Total. Environ. 2022, 833, 154824. [Google Scholar] [CrossRef]
- Lee, J.; Wang, J.; Oh, Y.; Jeong, S. Highly efficient microplastics removal from water using in-situ ferrate coagulation: Performance evaluation by micro-Fourier-transformed infrared spectroscopy and coagulation mechanism. Chem. Eng. J. 2023, 451, 138556. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Analysis and Prevention of Microplastics Pollution in Water: Current Perspectives and Future Directions. ACS Omega 2019, 4, 6709–6719. [Google Scholar] [CrossRef]
- Primpke, S.; Dias, P.A.; Gerdts, G. Automated identification and quantification of microfibres and microplastics. Anal. Methods 2019, 11, 2138–2147. [Google Scholar] [CrossRef]
- Kook, H.; Park, C. Engineered Approaches to Facile Identification of Tiny Microplastics in Polymeric and Ceramic Membrane Filtrations for Wastewater Treatment. Membranes 2022, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Pedrotti, M.; Petit, S.; Eyheraguibel, B.; Kerros, M.; Elineau, A.; Ghiglione, J.; Loret, J.; Rostan, A.; Gorsky, G. Pollution by anthropogenic microfibers in North-West Mediterranean Sea and efficiency of microfiber removal by a wastewater treatment plant. Sci. Total. Environ. 2020, 758, 144195. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.; Kerpen, J.; Prediger, J.; Barkmann, L.; Müller, L. Determination of the microplastics emission in the effluent of a municipal waste water treatment plant using Raman microspectroscopy. Water Res. X 2019, 2, 100014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, M.; Lian, E.; Xia, L.; Asimakopoulos, A.G.; Luo, S.; Wang, L. Identification of Poly(ethylene terephthalate) Nanoplastics in Commercially Bottled Drinking Water Using Surface-Enhanced Raman Spectroscopy. Environ. Sci. Technol. 2023, 57, 8365–8372. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef]
- Renner, G.; Schmidt, T.C.; Schram, J. Automated rapid & intelligent microplastics mapping by FTIR microscopy: A Python–based workflow. MethodsX 2019, 7, 100742. [Google Scholar] [CrossRef]
- Chakraborty, I.; Banik, S.; Biswas, R.; Yamamoto, T.; Noothalapati, H.; Mazumder, N. Raman spectroscopy for microplastic detection in water sources: A systematic review. Int. J. Environ. Sci. Technol. 2022, 20, 10435–10448. [Google Scholar] [CrossRef]
- Luo, Y.; Gibson, C.T.; Tang, Y.; Naidu, R.; Fang, C. Characterising microplastics in shower wastewater with Raman imaging. Sci. Total. Environ. 2022, 811, 152409. [Google Scholar] [CrossRef]
- Nava, V.; Frezzotti, M.L.; Leoni, B. Raman Spectroscopy for the Analysis of Microplastics in Aquatic Systems. Appl. Spectrosc. 2021, 75, 1341–1357. [Google Scholar] [CrossRef]
- Do, T.; Park, Y.; Lim, B.; Kim, S.; Chae, M.-Y.; Chun, C.-H. Effect of the first-flush phenomenon on the quantification of microplastics in rainwater. Mar. Pollut. Bull. 2023, 187, 114559. [Google Scholar] [CrossRef]
- Ni, B.-J.; Thomas, K.V.; Kim, E.-J. Microplastics and nanoplastics in urban waters. Water Res. 2023, 229, 119473. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Hyeon, Y.; Kim, S.; Ok, E.; Park, C. A fluid imaging flow cytometry for rapid characterization and realistic evaluation of microplastic fiber transport in ceramic membranes for laundry wastewater treatment. Chem. Eng. J. 2023, 454, 140028. [Google Scholar] [CrossRef]
- Kaile, N.; Lindivat, M.; Elio, J.; Thuestad, G.; Crowley, Q.G.; Hoell, I.A. Preliminary Results From Detection of Microplastics in Liquid Samples Using Flow Cytometry. Front. Mar. Sci. 2020, 7, 552688. [Google Scholar] [CrossRef]
- Mondal, T.; Jho, E.H.; Hwang, S.K.; Hyeon, Y.; Park, C. Responses of earthworms exposed to low-density polyethylene microplastic fragments. Chemosphere 2023, 333, 138945. [Google Scholar] [CrossRef] [PubMed]
- Ayala, Z.R.; Judge, S.; Anglès, S.; Greenfield, D.I. A comparison between the FlowCam 8100, microscopy, and sandwich hybridization assay for quantifying abundances of the saxitoxin-producing dinoflagellate, Alexandrium catenella. Harmful Algae 2023, 125, 102423. [Google Scholar] [CrossRef]
- Roache-Johnson, K.H.; Stephens, N.R. FlowCam 8400 and FlowCam Cyano Phytoplankton Classification and Viability Staining by Imaging Flow Cytometry. In Spectral and Imaging Cytometry: Methods in Molecular Biology; Humana: New York, NY, USA, 2023; pp. 219–244. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Xue, J.; Yang, Y.; Wu, H. Applicability of flow imaging microscopy (FlowCAM) as a ballast water investigation tool. Reg. Stud. Mar. Sci. 2023, 60, 102821. [Google Scholar] [CrossRef]
- Owen, B.M.; Hallett, C.S.; Cosgrove, J.J.; Tweedley, J.R.; Moheimani, N.R. Reporting of methods for automated devices: A systematic review and recommendation for studies using FlowCam for phytoplankton. Limnol. Oceanogr. Methods 2022, 20, 400–427. [Google Scholar] [CrossRef]
- Wang, X.; Fan, W.; Dong, Z.; Liang, D.; Zhou, T. Interactions of natural organic matter on the surface of PVP-capped silver nanoparticle under different aqueous environment. Water Res. 2018, 138, 224–233. [Google Scholar] [CrossRef]

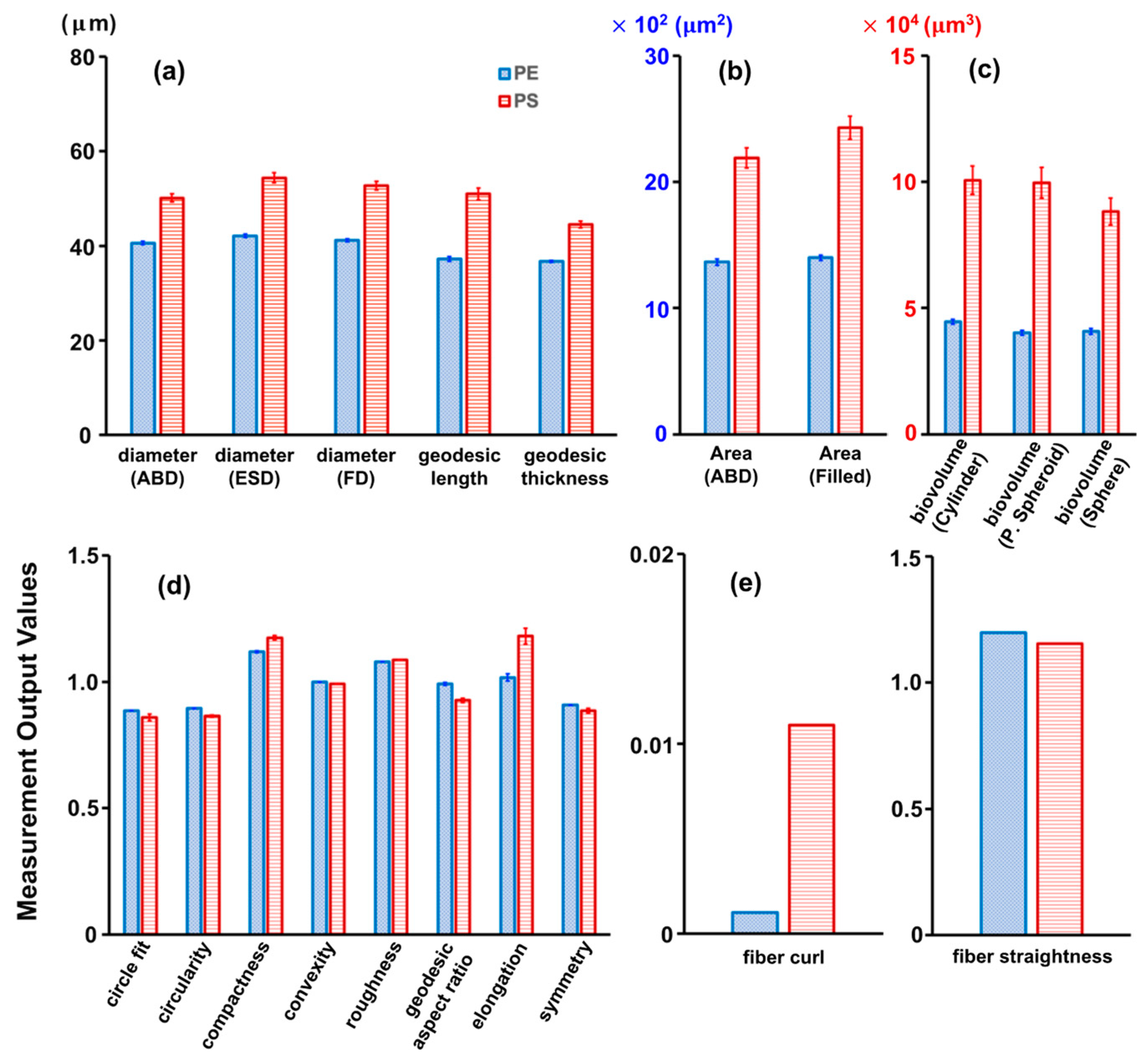
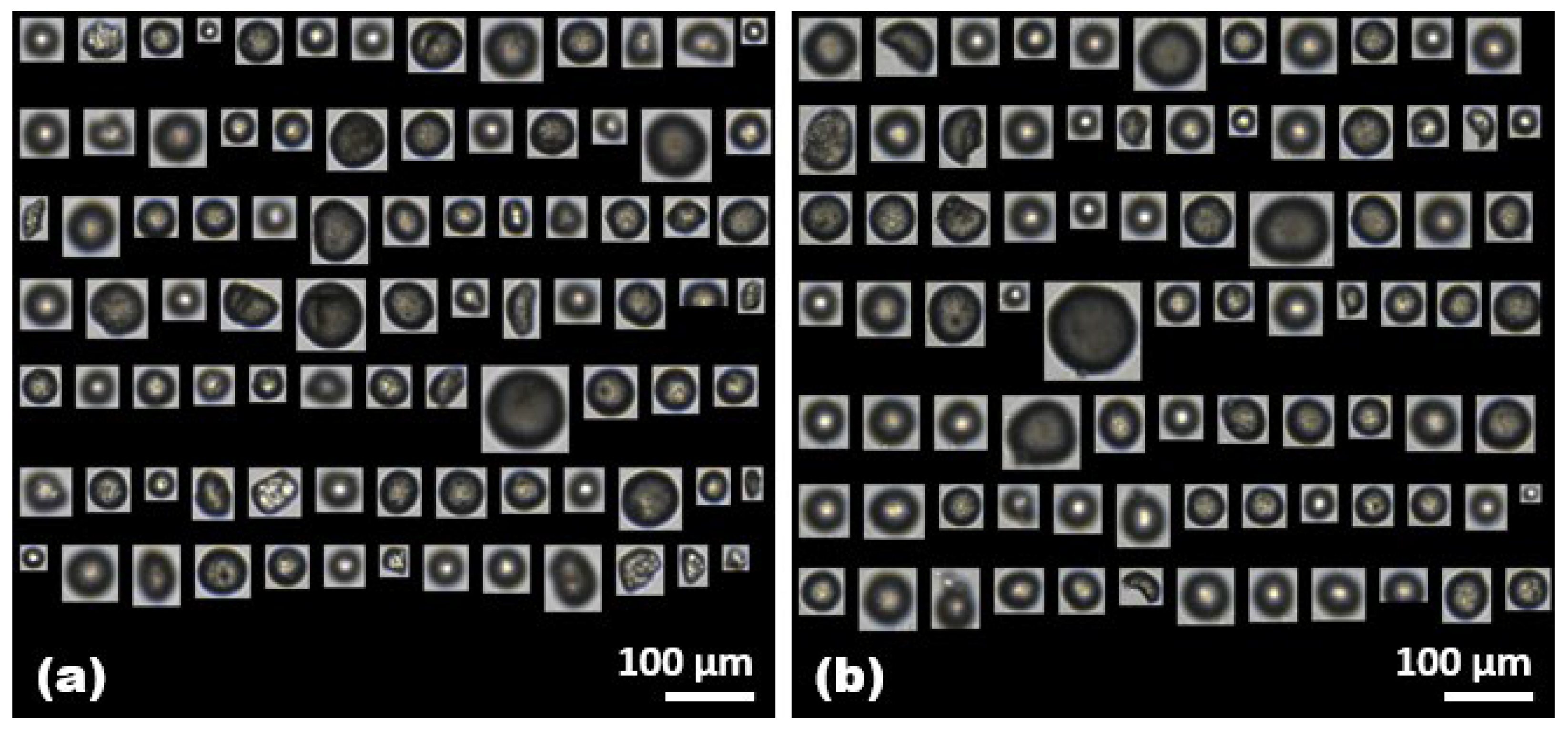
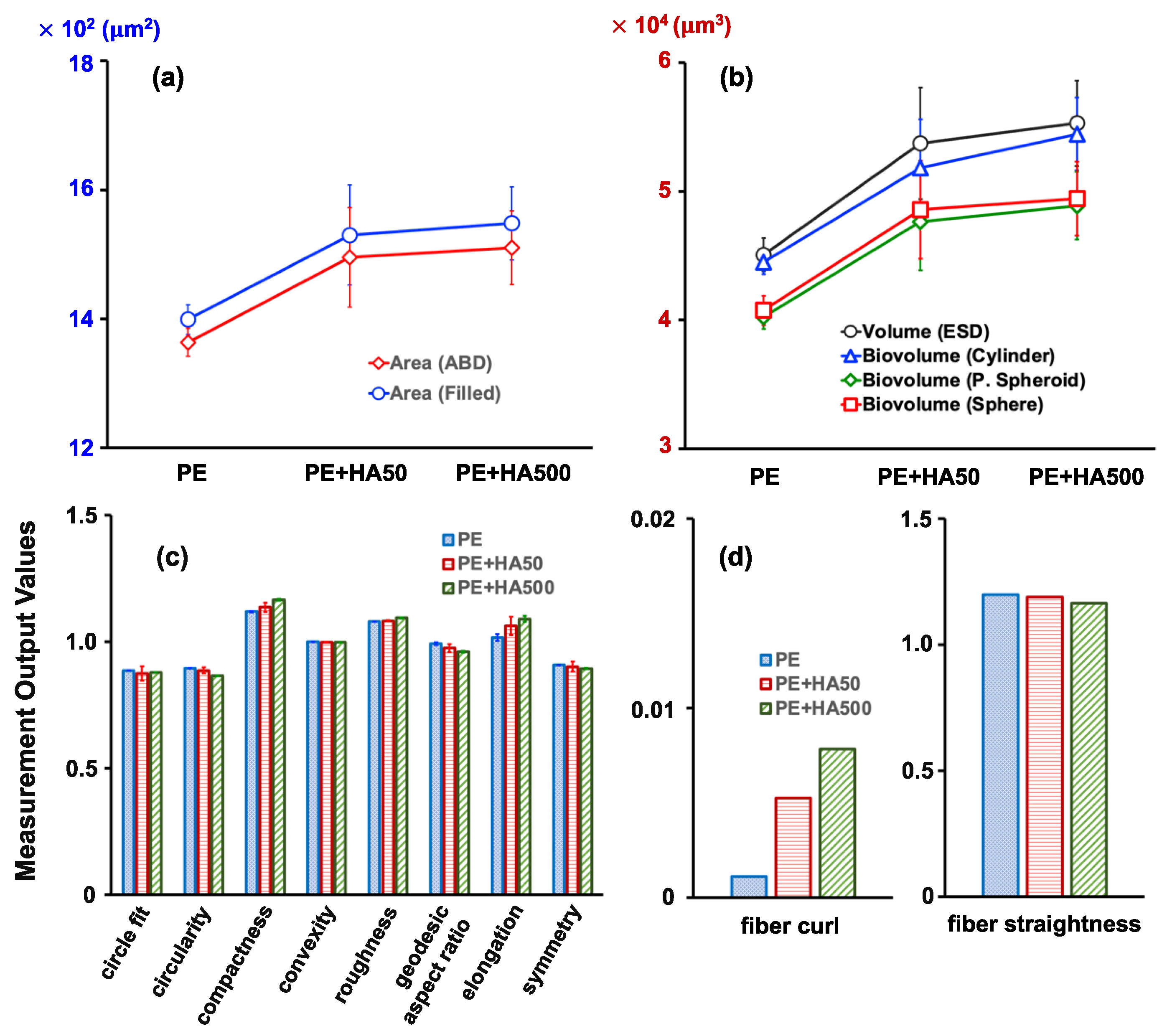



| Trial 1 (Number/mL) | Trial 2 (Number/mL) | Trial 3 (Number/mL) | Average (Number/mL) | |
|---|---|---|---|---|
| PE | 798 ± 70 | 882 ± 22 | 843 ± 70 | 841 ± 42 |
| PS | 236 ± 17 | 334 ± 18 | 285 ± 9 | 285 ± 49 |
| Length (Geodesic) (μm) | Length (Feret) (μm) | Perimeter (μm) | Convex Perimeter (μm) | |
|---|---|---|---|---|
| PE | 37.24 ± 0.50 | 44.56 ± 0.35 | 147.88 ± 1.25 | 137.06 ± 1.05 |
| PE with HA50 | 39.12 ± 1.00 | 46.32 ± 0.96 | 153.44 ± 3.35 | 141.93 ± 3.21 |
| PE with HA500 | 40.25 ± 0.73 | 46.55 ± 0.88 | 156.24 ± 2.65 | 142.92 ± 2.54 |
| Length (Geodesic) (μm) | Length (Feret) (μm) | Perimeter (μm) | Convex Perimeter (μm) | |
|---|---|---|---|---|
| PE | 37.24 ± 0.50 | 44.56 ± 0.35 | 147.88 ± 1.25 | 137.06 ± 1.05 |
| PE with BSA50 | 37.99 ± 0.29 | 44.74 ± 0.15 | 148.32 ± 0.12 | 137.09 ± 0.05 |
| PE with BSA500 | 40.12 ± 0.75 | 46.98 ± 0.63 | 156.05 ± 1.15 | 144.40 ± 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Hyeon, Y.; Park, C. Microplastics’ Shape and Morphology Analysis in the Presence of Natural Organic Matter Using Flow Imaging Microscopy. Molecules 2023, 28, 6913. https://doi.org/10.3390/molecules28196913
Kim S, Hyeon Y, Park C. Microplastics’ Shape and Morphology Analysis in the Presence of Natural Organic Matter Using Flow Imaging Microscopy. Molecules. 2023; 28(19):6913. https://doi.org/10.3390/molecules28196913
Chicago/Turabian StyleKim, Soyoun, Yejin Hyeon, and Chanhyuk Park. 2023. "Microplastics’ Shape and Morphology Analysis in the Presence of Natural Organic Matter Using Flow Imaging Microscopy" Molecules 28, no. 19: 6913. https://doi.org/10.3390/molecules28196913
APA StyleKim, S., Hyeon, Y., & Park, C. (2023). Microplastics’ Shape and Morphology Analysis in the Presence of Natural Organic Matter Using Flow Imaging Microscopy. Molecules, 28(19), 6913. https://doi.org/10.3390/molecules28196913







